Abstract
During the fermentation of ripened pu-erh tea (RPT), the composition of lipids and other compounds changes significantly. In this study, we conducted industrial fermentation of RPT and observed that the levels of water extract, tea polyphenols, free amino acids, catechins, caffeine, rutin, theophylline, luteolin, and myricetin decreased, while the level of soluble sugar increased. Additionally, the levels of gallic acid, quercetin, ellagic acid, and kaempferol first increased and then decreased during fermentation. We identified a total of 731 lipids, which were classified into seven categories using a lipomics method. Among these lipids, 85 with relatively high contents decreased, while 201 lipids with low contents increased after fermentation. This led to an overall decrease in the sum contents of lipids and dominant lipids, including glycerophospholipids and saccharolipids. We also detected 33 medium- and long-chain fatty acids, with α-linolenic acid (881.202 ± 12.13–1322.263 ± 19.78 μg/g), palmitic acid (797.275 ± 19.56–955.180 ± 30.49 μg/g), and linoleic acid (539.634 ± 15.551–706.869 ± 12.14 μg/g) being the predominant ones. Coenzymes Q9 (62.76–63.57 μg/g) and Q10 (50.82–59.33 μg/g) were also identified in the fermentation process. Our findings shed light on the changes in lipids during the fermentation of RPT and highlight the potential bio-active compounds, such as α-linolenic acid, linoleic acid, Coenzymes Q9, and Q10, in ripened pu-erh tea. This contributes to a better understanding of the fermentation mechanism for RPT.
Keywords: α-linolenic acid, Coenzyme Q9, Coenzyme Q10, Lipomics, Ripened pu-erh tea
Graphical abstract
Highlights
-
•
Two batches of industrial fermentation of ripen pu-erh tea were developed.
-
•
731 lipid molecules were identified in fermented tea leaves.
-
•
The dominant lipids were decreased during fermentation.
-
•
Coenzyme Q9 and Q10 were important bio-active compounds of ripen pu-erh tea.
Abbreviations
- RPT
Ripened pu-erh tea
- RM
Raw materials
- IF
Intermediate fermenting tea leaves
- FF
Final fermented tea leaves
- LC-MS/MS
Liquid chromatography-tandem mass spectrometry
- GC–MS
Gas chromatography-mass spectrometry
- HPLC
High-performance liquid chromatography
- FC
Fold change
- VIP
Variable importance in projection
- p
p-value
- PCA
Principal component analysis
- ANOVA
Analysis of Variance
- QC
Quality control
- Coenzyme Q8
Co Q8
- Coenzyme Q9
CoQ9
- Coenzyme Q10
CoQ10
1. Introduction
Tea made from the new shoots of the Camellia sinensis (L.) O. Kuntze plant is the most popular aromatic beverage globally (Samynathan et al., 2021). Lipids make up about 8% of the weight of dried tea, with the majority being glycerophospholipids and saccharolipids (Xiaocun, 2003). As far back as 1978, it was suggested that the breakdown of membrane lipids by enzymes initiates the formation of volatile carbonyl compounds, which partly contribute to the flavor of black tea (Selvendran et al., 1978). Saturated and unsaturated fatty acids have been reported to be tea aroma precursors, which produce alcohols, aldehydes, and lactones (Yang et al., 2013). Unsaturated fatty acids, such as linolenic acid, linoleic acid, oleic acid, and palmitoleic acid, act as precursors for aroma compounds containing six to ten carbons, including (E)-2-hexanal (leafy) and (Z)-3-hexanol (leafy), which contribute fresh and greenish odors in tea infusion (Ho et al., 2015). Lipid degradation can also produce cyclic aroma molecules, such as methyl jasmonate, cis-jasmone, and jasmine lactones, which have been identified as important fatty acid derivatives of the jasmine-like aroma of semi-fermented teas (Ho et al., 2015; Yang et al., 2013). Therefore, lipids are hydrophobic compounds implicated in the quality of tea flavor. The transformation of lipids in tea leaves during the postharvest stages varies by tea variety, making it both scientifically and commercially important to understand lipid transformations during tea production (Huang et al., 2023).
Numerous studies have examined lipid profiles in tea production using an ultrahigh-performance supercritical fluid chromatography/mass spectrometry-based lipidomics approach, which is one of the fastest analytical methods covering a wide range of lipid categories (Wolrab et al., 2022). In tea leaves, major lipids fall into eight chemical categories: fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, sterol lipids, prenol lipids, saccharolipids, and polyketides (Huang et al., 2023). Li et al. identified 192 lipid species covering 17 subclasses, which change dynamically during black tea production and are involved in chlorophyll degradation and metabolic pathways of glycoglycerolipids and other extra-plastidial membrane lipids (Li et al., 2017). Significant variations in lipid composition are also observed during green tea manufacture, particularly during the fixation stage. A study detected 283 lipid species covering 20 subclasses, mainly associated with chlorophyll decomposition, phosphatidic acid reduction, and glycolipid degradation, which potentially contribute to tea color and aroma quality (Li et al., 2021). Chen et al. discovered that fatty acids are essential precursors for black tea flavor formation (Chen et al., 2021). Additionally, Peng et al. investigated the effects of baking and storage on chemical constituents in fresh, strong, and aged-scent types of Foshou oolong teas. They found that the content of most lipids increases after baking and storage (Peng et al., 2022).
Ripened pu-erh tea (Pu-erh Shucha, RPT) is a well-known traditional Chinese tea. It has been shown to provide multiple health benefits, including antioxidative, antimutagenic, antimicrobial, laxative, and neuroprotective activities. Additionally, it has effects in controlling or preventing hypercholesterolemia, hyperglycemia, obesity, diabetes, osteoporosis, and Alzheimer's disease (Jia et al., 2022; Wang et al., 2022a). This type of tea is popular in Southeast Asia and is increasingly gaining popularity in the Western world. RPT is produced by spontaneous fermentation under high humidity of sun-dried green tea produced from C. sinensis var. assamica (JW Masters) Kitamura in Yunnan, China. The spontaneous fermentation is the core process in forming the health benefits of RPT(Wang et al., 2022b), and this fermentation results in a multitude of chemical changes and transformations of sun-dried green tea leaves (Lv et al., 2013). The chemical compounds in RPT, including amino acids, carbohydrates, minerals, phenolic compounds, phenolic pigments, and purine alkaloids (caffeine, theobromine) and their changes during spontaneous fermentation (Wang et al., 2022a), as well as volatile components, have been well reviewed recently (Wang et al., 2022b).
In a previous study, 90 lipids or lipid-like molecules were identified in fermenting samples of pu-erh tea through untargeted metabolomics analysis. This included 56 glycerophospholipids and 18 fatty acyls(Zhao et al., 2019). In another untargeted metabolomics analysis, 108 lipids and lipid-like molecules were identified in tea leaves that changed differentially after fermentation by Aspergillus niger, A. tamarii, and A. fumigatus, comprising glycerophospholipids (41 metabolites), fatty acyls (20 metabolites), prenol lipids (15 metabolites), glycerolipids (14 metabolites), sterol lipids (eight metabolites), steroids and steroid derivatives (six metabolites), and sphingolipids (four metabolites) (Ma et al., 2021). Recently, Li et al. (2023) detected 485 individual lipid species covering 26 subclasses, with 362 species significantly altered during RPT fermentation (Li et al., 2023). These studies demonstrated that owing to the complex and dynamic of the microbiome participating in spontaneous fermentation, lipids in RPT fermentation are complex and warrant further study. In the present study, we analyzed two batches of industrially fermented RPT, collecting 18 samples of raw materials (RM), intermediate fermenting tea leaves (IF), and final fermented tea leaves (FF). We used liquid chromatography-tandem mass spectrometry (LC-MS/MS) to analyze the lipid composition and changes during fermentation. Additionally, we measured the changes in fatty acids and characteristic components in tea leaves during fermentation using gas chromatography-mass spectrometry (GC-MS), high-performance liquid chromatography (HPLC), and spectrophotometry.
2. Materials and methods
2.1. Materials and chemical standards
The reference compounds catechin-3-gallate (CG), gallocatechin-3-gallate (GCG), epigallocatechin (EGC), catechin (C), epicatechin (EC), epicatechin-3-gallate (ECG), epigallocatechin-3-gallate (EGCG), myricetin, luteolin, rutin, taxifolin, quercetin, gallic acid, ellagic acid, kaempferol, caffeine, and theophylline were obtained from Chengdu Manst Biotechnology Co., Ltd. (Chengdu, China). Acetonitrile and methanol for HPLC analysis were purchased from Beijing Mirida Technology Co., Ltd. in Beijing, China. MS-grade methanol and acetonitrile, and HPLC-grade 2-propanol were purchased from Thermo Fisher Co., Ltd. HPLC-grade formic acid and HPLC-grade ammonium formate were purchased from Sigma Co., Ltd. Methanol, dichloromethane, 2,2,4-trimethylpentane, and hexane for GC-MS were purchased from ANPEL Laboratory Technologies Inc in Shanghai, China. Sulfuric acid, sodium chloride, and chloroform were purchased from Sinopharm in Beijing, China. Ultra-pure water was prepared in-house using a Milli-Q water purification system from Millipore in Bedford, MA, USA. Other chemicals and reagents were of analytical grade.
All lipid standards were purchased from Avanti Polar Lipids in Alabaster, Alabama, USA. The standards include triacylglycerol (d7-TG-15:0/18:1/15:0), diacylglycerol (d7-DG-15:0/18:1), monoacylglycerol (d7-MAG-18:1), phosphatidylcholine (d7-PC-15:0/18:1), phosphatidylethanolamine (d7-PE-15:0/18:1), phosphatidylserine (d7-PS-15:0/18:1), phosphatidylinositol (d7-PI-15:0/18:1), phosphatidic acid (d7-PA-15:0/18:1), phosphatidylglycerol (d7-PG-15:0/18:1), cholesterol ester (CE-17:0), sphingomyelin (d9-SM-d18:1/12:0), ceramide (d7-Cer-d18:1/17:0), lysophosphatidylethanolamine (d7-Lyso PE-18:1), and lysophosphatidylcholine (d7-Lyso PC-18:1). The internal standard concentrations were prepared at 100 μg/mL. A mixed solution with a concentration of 1 μg/mL was prepared in MeOH and stored at −20 °C until further use. Additionally, thirty-seven fatty acid standards were purchased from ANPEL Laboratory Technologies Inc. in Shanghai, China. Fatty acid standards were prepared as 10 mg/mL or 5 mg/mL solutions using chloroform.
2.2. Fermentation of Pu-erh tea and sample collection
Two batches of industrially fermented RPT (F1 and F2) were produced at Yunnan Xiaguan Tuocha Tea (Group) Co., Ltd. from July to September 2020, according to the traditional spontaneous fermentation methods. Briefly, 15 tons of sun-dried green tea leaves with a moisture content of 8.3% were used as raw material and showered with spring water to increase moisture to approximately 40%. The tea mass was then piled to a height of approximately 1.0 m, allowing microorganisms from the raw material, fermentation room, and water to grow and reproduce. After approximately 7–10 days, the piled tea mass was broken down, showered with water, mixed, and piled up again. This repiling and mixing was repeated eight times. Tea leaves from the raw material (RM), intermediate fermenting (IF), and final fermented (FF) stages were collected using a five-point sampling method. Three repetitions comprising a total of 18 samples were performed (Table S1). The collected tea leaves were freeze-dried and subjected to measurement of tea chemical composition, sensory evaluation, lipomics analysis, and GC-MS analysis.
2.3. Determination of characteristic compositions of tea leaves and sensory evaluation
The contents of 17 characteristic compounds in tea leaves, including catechins (EGCG, GCG, EGC, CG, EC, ECG, C), myricetin, luteolin, rutin, taxifolin, quercetin, gallic acid, ellagic acid, kaempferol, caffeine, and theophylline, were measured using HPLC methods described previously (Nian et al., 2019). The measurements were conducted using an Agilent 1200 series HPLC system (Agilent Technologies, Santa Clara, CA, USA) and separated in an analytical Agilent Poroshell 120 EC-C 18 column (4.6 mm × 100 mm, 2.7 μm, Agilent Technologies, Santa Clara, CA, USA). Contents of polyphenols, free amino acids, and soluble sugars and water in tea leaves were determined using a spectrophotometric method based on FeSO4, ninhydrin, and anthrone, respectively, as described in a previous report (Wang, Peng and Gong, 2011). The Sensory evaluation of tea leaves was performed according to the methodology in GB/T 23776-2018 (China, 2018).
2.4. Lipomics analysis of tea leaves
Lipid profiles of tea leaves were analyzed at Shanghai Applied Protein Technology Co., Ltd. (Shanghai, China) through a non-targeted lipomic analysis platform based on UPLC-Orbitrap mass spectrometry, LipidSearch software), and isotopic internal standards of 13 lipid molecules (Cer, LPC, PC, LPE, PE, PI, PS, PA, PG, SM, Chol Ester, DG, TG). Lipids were extracted according to the methyl tert-butyl ether (MTBE) method. Initially, the samples were spiked with internal lipid standard, followed by homogenization with 200 μL water and 240 μL methanol. Subsequently, 800 μL of MTBE was added, and the mixture was subjected to ultrasound and then incubated at room temperature. After centrifugation, the upper layer was obtained and dried under nitrogen before undergoing LC-MS/MS analysis. Quality control (QC) samples were used to ensure accurate analysis.
Lipid extracts were re-dissolved in 200 μL of 90% (v/v) isopropanol/acetonitrile, centrifuged at 14000 g for 15 min, and finally, 3 μL of sample was injected. Injected samples were separated by reverse-phase chromatography using a CSH C18 column (1.7 μm, 2.1 mm × 100 mm, Waters). Solvent A was acetonitrile water (6:4, v/v) with 0.1% (v/v) formic acid and 0.1 mM ammonium formate. Solvent B was acetonitrile–isopropanol (1:9, v/v) with 0.1% (v/v) formic acid and 0.1 mM ammonium formate. The initial mobile phase was 30% solvent B at a flow rate of 300 μL/min. This was held for 2 min and then linearly increased to 100% solvent B over 23 min, followed by equilibrating at 5% solvent B for 10 min.
Mass spectra were acquired using a Q-Exactive Plus (Thermo Scientific) in positive and negative mode, respectively. Full MS/data-dependent MS2 (dd-MS2) scan mode within a mass range from m/z 200 up to 1800 was utilized. Resolutions of 70,000 and 17,500 were chosen for full-scan spectra and fragment spectra, respectively. Source parameters were as follows: ion spray voltage, 3.0 kV; auxiliary gas heater temperature, 300 °C; auxiliary gas flow rate, 15 arb; sheath gas flow rate, 45 arb; capillary temperature, 350 °C; and the S-Lens RF Level was set at 50%.
The data underwent peak picking and lipid identification using LipidSearch software(Thermo Scientific). The mass tolerance for both precursor and fragment was set to 5 ppm, and the production threshold was set at 5%. Preprocessed lipidomics data files were imported into MetaboAnalyst 5.0 (Pang et al., 2021, 2022) for multivariate statistical analysis, including principal component analysis (PCA), (orthogonal) partial least-squares-discriminant analysis (OPLS-DA), and volcano plot analysis. Lipids showing differential changes between raw material and fermented tea leaves were selected using the criteria p < 0.05, fold change (FC) > 2.0 or < 0.5, and variable importance in projection (VIP) > 1.0. Furthermore, lipid metabolic pathway analysis based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database was also performed.
2.5. GC-MS analysis of medium- and long-chain fatty acids
A GC-MS approach for measuring 37 medium- and long-chain fatty acids—butyric acid, hexanoic acid, octanoic acid, decanoic acid, undecanoic acid, lauric acid, tridecanoic acid, myristic acid, myristoleic acid, pentadecanoic acid, pentadecenoic acid, palmitic acid, palmitoleic acid, heptadecanoic acid, heptadecenoic acid, stearic acid, elaidic acid, oleic acid, linoelaidic acid, linoleic acid, arachidic acid, gamma linolenic acid, 11-eicosenoic acid, linolenic acid, heneicosanoic acid, 11,14-eicosadienoic acid, behenic acid, cis-8,11,14-eicosatrienoic acid ester, erucic acid, 11,14,17-eicosatrienoic acid, tricosanoic acid, arachidonic acid, docosadienoic acid, lignoceric acid, eicosapentaenoic acid, nervonic acid, and docosahexaenoic acid—was developed at Sanshu Biotechnology Co., Ltd. (www.sanshubio.com, Nantong, Jiangsu, China) according to methods described in a previous report (Li et al., 2015).
2.6. Statistical analysis
The data were analyzed using the SPSS 20.0 software package (SPSS Inc, Chicago, IL, USA). The results are expressed as mean ± standard deviation (n = 3). A one-way Analysis of Variance (ANOVA) was used to identify statistical differences followed by the least-significant difference method for paired data, with p < 0.05 indicating statistical significance. Heatmap analysis was performed using TBtools software (version 1.0971) (Chen et al., 2020).
3. Results and discussion
3.1. Change in sensory quality and characteristic compounds during fermentation of RPT
The sensory evaluation showed that the color of tea leaves changed during the fermentation process. The tea leaves in the raw material (RM) were green; which turned orange-red in intermediate fermenting samples (IF), and then brown in the final fermented samples (FF). Infused tea leaves of the RM were yellow-green, transforming to brown in IF and then reddish-brown in FF. Tea infusions of RM were yellow, transforming to orange-yellow in IF and then reddish-brown in FF. The infusions of RM tasted astringent and slightly bitter, becoming slightly sour in IF, then mellow after fermentation. The aroma of tea leaves changed from fresh (RM) to fungal flavor (IF), and then stale flavor (FF) (Fig. 1A).
Fig. 1.
Sensory evaluation of tea samples (A), and change in contents of characteristic tea compounds (B).
The sensory characteristics of tea change along with its chemical composition. In this work, 21 characteristic components of tea were measured by HPLC or spectrophotometric methods. The contents of water extract, tea polyphenols, free amino acids, catechins, caffeine, and rutin were significantly decreased by fermentation (p < 0.05), while the soluble sugar content was significantly increased by fermentation (p < 0.05) (Fig. 1B). The levels of gallic acid, quercetin, ellagic acid, and kaempferol initially increased and then decreased during fermentation. For example, the gallic acid contents were 2.39 ± 0.5 mg/g (RM1) and 2.46 ± 0.6 mg/g (RM2), which increased to 32.54 ± 4.7 mg/g (IF1), and 42.34 ± 4.9 mg/g (IF2) and then decreased to 15.39 ± 2.2 mg/g (FF1) and 18.86 ± 2.2 mg/g (FF2), respectively. The levels of ellagic acid were 0.71 ± 0.1 mg/g and 0.69 ± 0.2 mg/g in RM, increasing to 1.22 ± 1.5 mg/g and 1.0 ± 0.2 mg/g, respectively, in FF. The increase in gallic acid and ellagic acid may be responsible for the sour taste in infusions of IF, consistent with our previous study (Mingli et al., 2020). Both sensory quality and changes in characteristic components were similar to those reported for the general fermentation of RPT (Zhao et al., 2019), and demonstrated these two fermentations were well-developed.
3.2. Composition of lipids in fermenting tea leaves
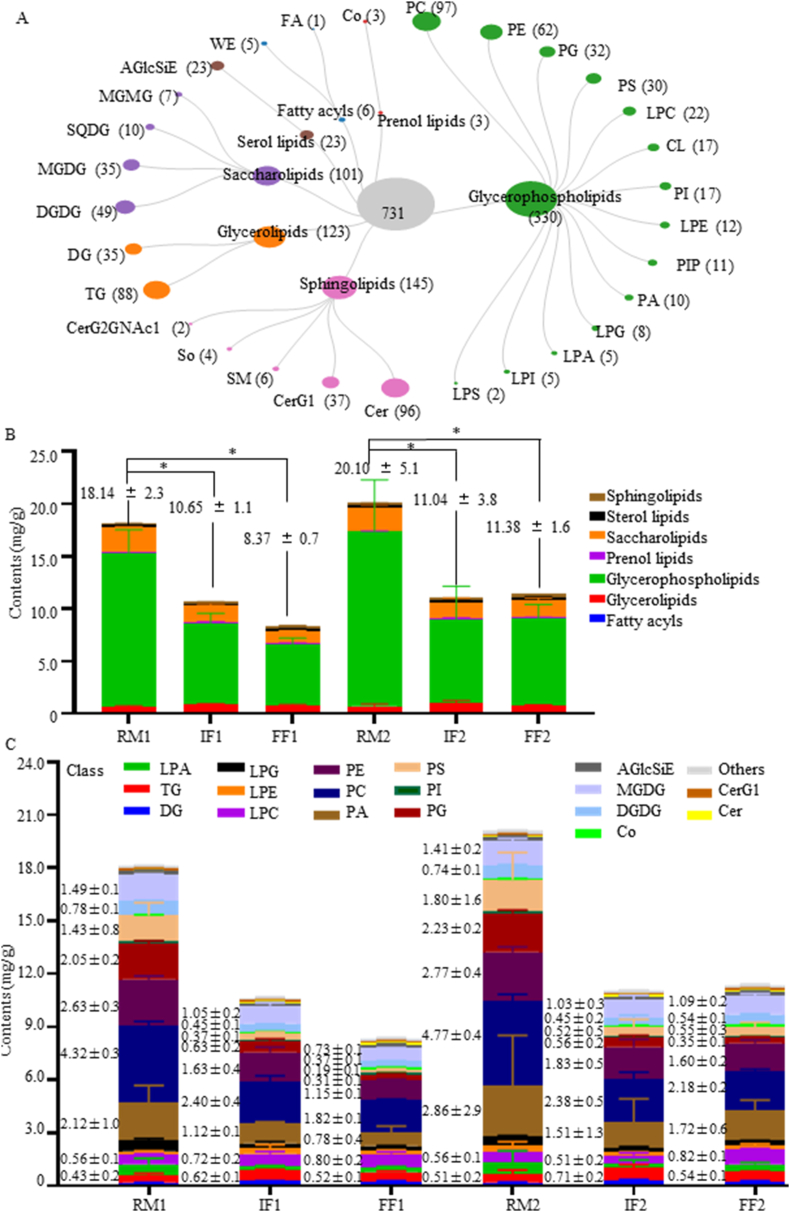
The lipids extracted from tea leaves in RM, IF, and FF were analyzed using an LC-MS/MS-based metabolomics approach. A total of 731 lipid molecules were identified (Fig. 2A, Supplemental data 1 sheet 1) and classified into seven categories: glycerophospholipids (GPs, 330 molecules), sphingolipids (SPs, 145 molecules), glycerolipids (GLs, 123 molecules), saccharolipids (SLs, 101 molecules), sterol lipids (STs, 23 molecules), fatty acyls (FAs, six molecules), and prenol lipids (PLs, three molecules). Recently, Li et al. (2023) reported detected 485 lipid species across seven major classes: GPs (158 species), GLs (113 species), FAs (68 species), SPs (54 species), glycoglycerolipids (51 species), STs (24 species), and PLs (17 species) (Li et al., 2023). Glycoglycerolipids belonged to SLs, therefore, the categories of lipids identified in our work were the same as those identified by Li et al. (2023). In comparison with the report of Li et al. (2023), more molecules belonged to GPs, SPs, GLs, and SLs, while fewer FAs and PLs were identified in this work.
Fig. 2.
Categories and classes of lipids identified (A), and the sum contents of lipids in each category (B) and class (C).
The sum contents of lipids in RM were 18.14 ± 2.28 mg/g (RM1) and 23.86 ± 5.60 mg/g (RM2), which decreased to 10.65 ± 1.05 mg/g (IF1) and 11.04 ± 3.74 mg/g (IF2) (p < 0.05), and then to 8.37 ± 0.07 mg/g (FF1) and 11.38 ± 1.55 mg/g (FF2) following fermentation. Glycerophospholipids were the dominant lipids, with a sum content of 14.73 ± 2.19 mg/g (RM1) and 20.46 ± 5.60 mg/g (RM2); these sum levels were reduced to 7.75 ± 0.90 mg/g (IF1) and then 8.0 ± 3.15 mg/g (FF1), and 5.82 ± 0.59 mg/g (IF2) and then 8.25 ± 1.38 mg/g (FF2), respectively, during fermentation. Tea leaves contained 1.15 ± 0.05 to 2.37 ± 0.10 mg/g saccharolipids and 0.60 ± 0.13 to 1.00 ± 0.23 mg/g glycerolipids (Fig. 2B). These results confirm that tea lipids are mainly glycerophospholipids and saccharolipids, both of which decrease during fermentation (Xiaocun, 2003).
The lipid molecules were classified into 29 subclasses. The majority of lipids were phosphatidylcholine (PC, 97 lipids), ceramides (Cer, 96), triglyceride (TG, 88 lipids), phosphatidylethanolamine (PE, 62 lipids), di galactosyl diacylglycerol (DGDG, 49 lipids), simple Glc series (CerG1, 37 lipids), diglyceride (DG, 35 lipids), monogalactosyldiacylglycerol (MGDG, 35 lipids), phosphatidylglycerol (PG, 32 lipids), or phosphatidylserine (PS, 30 lipids) (Fig. 2A). Li et al. (2023) identified 26 subclasses of lipids, including 88 TG, 41 free fatty acids (FFA), 34 hexosylceramide, and 37 PC lipids (Li et al., 2023). Both studies identified TG, PE, DG, Cer, DGDG, SQDG, and MGDG as principal lipids in RPT; however, we identified more PC and Cer, while Li et al. (2023) reported more FFA, HexCer, and FAHFA in RPT. This difference in lipid identification may result from differences in the raw material and fermentation. This work and the study of Li et al. (2023) both identified a more complex lipid composition during the fermentation of RPT than previous studies on the manufacturing processes of black tea (Li et al., 2017), green tea (Li et al., 2021), and purple-leaf tea (Chen et al., 2021), Which identified 192, 283, and 291 lipid species, respectively. We suggest that the complexity of lipid compositions in RPT results from multiple microbiomes participating in fermentation.
In the class level, the predominant lipids in RM were PC (4.32 ± 0.3 mg/g, 4.77 ± 0.4 mg/g), PE (2.63 ± 0.3 mg/g, 2.77 ± 0.4 mg/g), PA (2.12 ± 1.0 mg/g, 2.86 ± 2.9 mg/g), PG (2.05 ± 0.2 mg/g, 2.23 ± 0.2 mg/g), MGDG (1.49 ± 0.1 mg/g, 1.41 ± 0.2 mg/g), and PS (1.43 ± 0.8 mg/g, 1.80 ± 1.6 mg/g). Which changed to PC (2.40 ± 0.4 mg/g, 2.38 ± 0.5 mg/g), PE (1.63 ± 0.4 mg/g, 1.83 ± 0.5 mg/g), PA (1.12 ± 0.1 mg/g,1.51 ± 1.3 mg/g), and MGDG (1.05 ± 0.2, 1.41 ± 0.2 mg/g). After fermentation, the dominant lipids were PC (1.82 ± 0.1 mg/g, 2.18 ± 0.2 mg/g), PE (1.15 ± 0.1 mg/g, 1.60 ± 0.2 mg/g), PA (0.78 ± 0.4 mg/g,1.72 ± 0.6 mg/g), and MGDG (0.73 ± 0.1 mg/g, 1.09 ± 0.2 mg/g) (Fig. 2C).
3.3. Changes in lipids during RPT fermentation
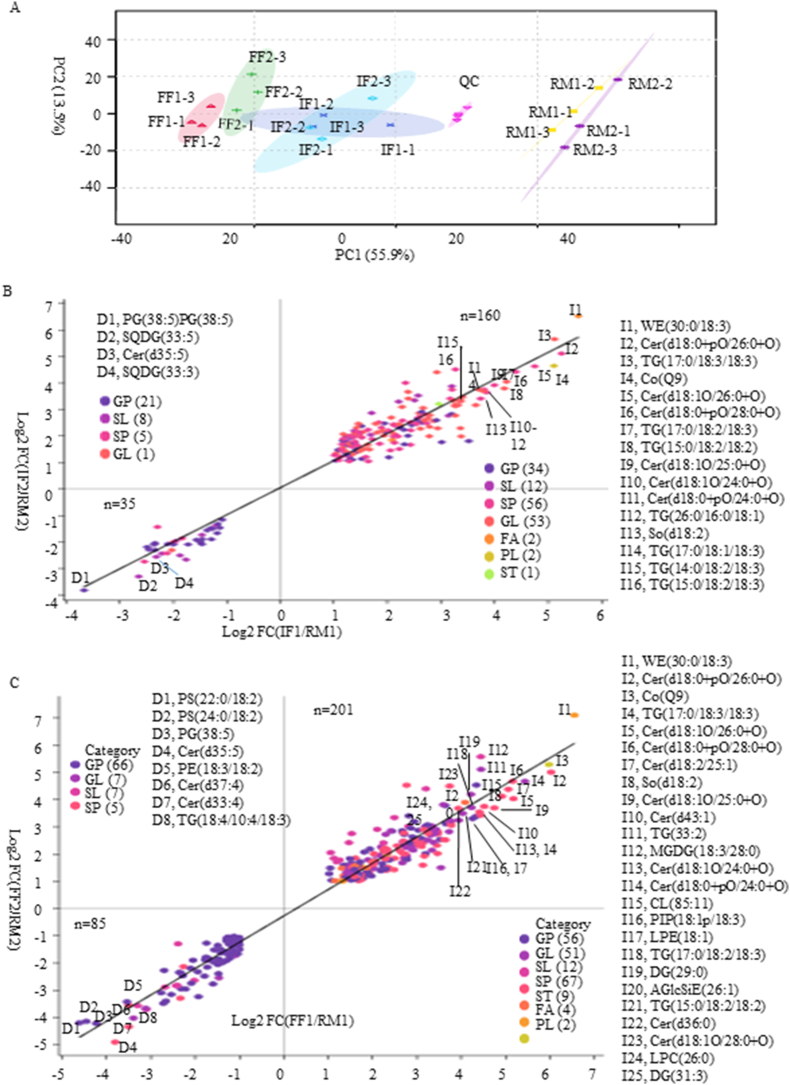
In a PCA score plot showing 69.4% of the variables, the QC samples clustered together, indicating good stability and reproducibility in the lipomics analysis. Differential clustering of RM, IF, and FF indicated significant changes in lipid profiles during the fermentation of RPT (Fig. 3A), which is consistent with a study by Li et al. (2023). The levels of 35 lipids, including GPs (21 lipids), SLs (eight lipids), SPs (five lipids), and GL (one lipid), were significantly decreased in both IF1/RM1 and IF2/RM2 (VIP >1.0, p < 0.05 and FC < 0.5). Specifically, the levels of PG (38:5), SQDG (33:5), Cer (d35:5), and SQDG (33:3) decreased more than fivefold (Fig. 3B–Supplemental data 2 sheet 1). Additionally, the levels of 160 lipids, including SPs (56 lipids), GLs (53 lipids), GPs (34 lipids), SLs (12 lipids), FAs (two lipids), PLs (two lipids), and ST (one lipid), were significantly increased in both IF1/RM1 and IF2/RM2 comparisons (VIP >1.0, p < 0.05 and FC > 2.0). Among these, the levels of 16 lipids increased more than 10-fold, e.g., WE (30:0/18:3), Cer (d18:0+pO/26:0+O), TG (17:0/18:3/18:3), Coenzyme Q9 (CoQ9), Cer (d18:1O/26:0+O), Cer (d18:0+pO/28:0+O), TG (17:0/18:2/18:3), TG (15:0/18:2/18:2), Cer (d18:1O/25:0+O) (Fig. 3B–Supplemental data 2 sheet 1).
Fig. 3.
PCA analysis of samples based on lipids (A), significantly changed lipids in comparisons of IF1/RM1 and IF2/RM2 (B), FF1/RM1, and FF2/RM2 (C).
In comparing tea leaves of FF to those of RM, the contents of 85 lipids, comprising GPs (66 lipids), GLs (seven lipids), SLs (seven lipids), and SPs (five lipids), were decreased significantly in both FF1/RM1 and FF2/RM2 comparisons (VIP >1.0, p < 0.05 and FC < 0.5). Among these, the levels of eight lipids were decreased by more than 10-fold: PS (22:0/18:2), PS (24:0/18:2), PG (38:5), Cer (d35:5), PE (18:3/18:2), Cer (d37:4), Cer (d33:4), and TG (18:4/10:4/18:3) (Fig. 3C–Supplemental data 2 sheet 2). The levels of 201 lipids comprising SPs (67 lipids), GPs (56 lipids), GLs (51 lipids), SLs (12 lipids), STs (nine lipids), FAs (four lipids), and PLs (two lipids) were increased significantly in comparisons of both FF1/RM1 and FF2/RM2 (VIP >1.0, p < 0.05 and FC > 2). Among these, the levels of 25 lipids increased more than 10-fold, e.g., WE (30:0/18:3), Cer (d18:0+pO/26:0+O), CoQ9, and TG (17:0/18:3/18:3), Cer (d18:1O/26:0+O) (Fig. 3C–Supplemental data 2 sheet 2).
3.4. Fermentation decreases the most highly abundant lipids and increases less abundant lipids
In both fermentations, the total lipid content decreased, while the relative levels of 201 lipids increased. To investigate this change, we compared the contents of 57 lipids with amounts greater than 0.1 mg/g in any sample using a heat map (Fig. 4A, Supplemental data 3). Among them, levels of 52 lipids decreased, while the contents of LPC (18:2), LPC (16:0), MGDG (16:0/18:3), TG (18:3/18:2/18:3), and TG (18:3/18:2/18:2) increased. The decrease of 52 lipids with amounts greater than 0.1 mg/g resulted in a decrease in the total lipid content. The sum contents of these 57 lipids were 14.04 ± 1.9 mg/g (RM1) 15.62 ± 4.2 mg/g (RM2), which decreased to 5.83 ± 0.5 mg/g (FF1), and 8.13 ± 1.2 mg/g (FF2) after fermentation (Fig. 4B). Therefore, this analysis revealed a decrease in the most highly abundant lipids.
Fig. 4.
Changes in lipids with contents greater than 0.1 mg/g in any sample (A); Sum contents of lipids with contents greater than 0.1 mg/g in any sample (B).
After fermentation, the levels of 201 lipids increased, with 25 lipids increasing more than 10-fold. Their contents increased from 0.01 to 10.08 μg/g in raw material (RM) to 0.08–63.57 μg/g in fermented material (FF) (Table S2). For example, CoQ9 contents were 2.10 ± 0.6 μg/g (RM1) and 2.90 ± 1.2 μg/g (RM2), which increased to 62.72 ± 12.5 μg/g (FF1) and 63.57 ± 11.8 μg/g (FF2); the contents of LPE (18:1) were 6.04 ± 1.2 μg/g (RM1) and 10.08 ± 4.0 μg/g (RM2), which increased to 53.25 ± 10.1 μg/g (FF1) and 59.62 ± 21.2 μg/g (FF2); the contents of Cer (d18:1O/24:0+O) were 2.09+±0.8 μg/g (RM1) and 2.71 ± 0.3 μg/g (RM2), which increased to 21.47 ± 8.1 μg/g (FF1) and 16.86 ± 3.9 μg/g (FF2). This demonstrated that the contents of most of the increased lipids were low. In summary, during the fermentation of RPT, the content of most highly abundant lipids in tea leaves was decreased, while that of many less abundant lipids was increased, resulting in a decrease in the total contents of lipids.
3.5. Changes in medium- and long-chain fatty acids
In previous work, Li et al. (2023) identified 41 FFAs; however, in this work, only FA (18:4) was detected, suggesting that our LC-MS-based lipidomics method cannot detect FFAs. Therefore, a GC-MS protocol for the measurement of 37 medium- and long-chain FFAs was developed, and 33 of these were detected in tea leaves. Among them, the majority of FFAs were α-linolenic acid (18:3n-3) (881.202 ± 12.13–1322.263 ± 19.78 μg/g), palmitic acid (C16:0) (797.275 ± 19.56–955.180 ± 30.49 μg/g), and linoleic acid (C18:2n6c) (539.634 ± 15.551–706.869 ± 12.14 μg/g) (Fig. 5A and B); levels of other FFAs ranged from 0.005 ± 0.01–349.139 ± 28.35 μg/g (Table S2). These results are consistent with the previous study that fatty acids from dark tea include α-linolenic acid, linolenic acid, dodecanamide, linoleamide, and stearamide (Huang et al., 2023). During both fermentations, the contents of α-linolenic acid and linoleic acid were significantly decreased (p < 0.05); while the content of palmitic acid was decreased in fermentation 2, but did not change significantly in fermentation 1. Wu et al. (2024) found in mature tea leaves, the proportion of long-chain PUFAs (represented by α-linolenic acid and linoleic acid) was higher than the proportion of MUFAs (represented by cis-9-octadecenoic acid) and SFAs (represented by palmitic acid)(Wu et al., 2024). In comparison with the report of Wu et al. (2024), the majority of FFAs were α-linolenic acid (18:3n-3), palmitic acid (C16:0), and linoleic acid in this work. Linoleic acid (18:2n-6, LA) and α-linolenic acid (18:3n-3, ALA) are long-chain omega-6 and omega-3 polyunsaturated fatty acids, respectively. In human tissues, LA is converted mainly to arachidonic acid and ALA is converted into docosahexaenoic acid (Sanders, 2017). Linoleic acid is an essential nutrient that has a specific role in the maintenance of the water permeability barrier as a component of acylglycosyl ceramides and in the transport of cholesterol in blood (Sanders, 2017). It constitutes the predominant proportion of dietary polyunsaturated fatty acids, and increased dietary intake or tissue levels of linoleic acid are associated with a reduced incidence of cardiovascular diseases and metabolic syndrome or type 2 diabetes (Marangoni et al., 2020). Zhang et al. (2020) discovered that higher levels of linoleic acid are associated with a reduced risk of stroke, especially ischemic stroke (Zhang et al., 2020). Nava-Lauson et al. (2023) suggested that linoleic acid treatment could enhance adoptive T-cell therapy in tumor treatment (Nava-Lauson et al., 2023). Pharmacological research shows that ALA has anti-metabolic syndrome, anticancer, anti-inflammatory, anti-oxidant, anti-obesity, and neuroprotection properties. Additionally, it improves the regulation of the intestinal flora (Yuan et al., 2022). Our GC-MS analysis identified LA and ALA in RPT, which may be responsible for its bio-activity.
Fig. 5.
Contents of α-linolenic acid, palmitic acid, and linoleic acid in tea leaves (A, B); contents of CoQ8, CoQ9, and CoQ10 in tea leaves (C, D).
3.6. Detection of CoQ9 and CoQ10 in RPT
In this work, CoQ8, CoQ9, and CoQ10 were detected in RM with contents of 4.70–5.99, 2.10–2.90, and 21.62–25.18 μg/g, respectively; contents of CoQ9 and CoQ10 increased to 38.72–44.00 and 35.99–37.29 μg/g, respectively, in IF and then increased to 62.76–63.57 and 50.82–59.33 μg/g, respectively, in FF tea leaves; the contents of CoQ8 showed no significant difference at the different stages of fermentation (Fig. 5C and D). Interestingly, the contents of CoQ10 in RPT were higher than those in vegetables (0.1–2.3 μg/g), fruits (0.1–2.2 μg/g), cereals (2.1–4.7 μg/g), fish (0.3–27 μg/g), and even meats (2.9–41 μg/g), except for pork heart (203 μg/g) (Yubero-Serrano et al., 2020). Considering that microorganisms can produce CoQ10, and microbial fermentation remains the most effective method for industrial production of CoQ10 (Lee et al., 2017), we assume that the enrichment of CoQ10 in RPT results from microbial fermentation during manufacturing.
Coenzyme Q10 (CoQ10) is the main form of endogenous coenzyme Q in the human body's mitochondrial respiratory chain. It is found in all tissues and cells, particularly in the heart (Amar-Yuli et al., 2009). The primary physiological functions of CoQ10 are (1) as a cofactor in the production of adenosine triphosphate, (2) as an antioxidant, (3) as an influence on the expression of genes involved in cellular signaling and metabolism, and (4) in the modulation of the mechanical and permeability of lipid membranes (similar to cholesterol) (Katzinger et al., 2020). As an anti-inflammatory and antioxidant agent able to prevent damage induced by free radicals and activation of inflammatory signaling pathways, CoQ10 is used in clinical applications for migraine, neurodegenerative diseases (including Parkinson's and Alzheimer's diseases), cancer, and degenerative muscle disorders (such as multiple sclerosis and chronic fatigue syndrome) (Testai et al., 2021). CoQ10 has an excellent safety record and is well tolerated even in high doses for extended periods, with limited side effects. As a result, it is one of the most widely used dietary and nutritional supplements on the market (Raizner, 2019; Yubero-Serrano et al., 2020). Lekli et al. reported that CoQ9 by itself, or after being converted into CoQ10, reduces myocardial ischemia/reperfusion-induced injury (Lekli et al., 2008). Therefore, RPT contains CoQ9 and CoQ10, important bio-active compounds responsible for its health benefits.
Many studies have reported that RPT (Ripened Pu-erh Tea) possesses various bio-activities, including antioxidative, antimutagenic, antimicrobial, laxative, and neuroprotective activities. It also plays a role in controlling or preventing hypercholesterolemia, hyperglycemia, obesity, diabetes, osteoporosis, and Alzheimer's disease. However, the specific bio-active compounds present in RPT are not yet clear (Wang et al., 2021). Contents of theabrownin, caffeine, polysaccharides, and gallic acid in RPT were higher than those in raw pu-erh tea, indicating that they are important bio-active compounds in RPT (Wang et al., 2021). Zhang et al. (2011) isolated (3,4-dihydroxy benzoyl)-3,4-dihydroxy benzamide from the RPT and showed that it can prevent H2O2-induced cell death of human microvascular endothelial cells (Zhang et al., 2011); Su et al. (2016) isolated teadenol A from RPT, which showed antioxidant activity (Su et al., 2016). The fermentation process leads to multiple chemical changes and transformations, resulting in a complex composition of bioactive compounds. In this work, α-linolenic acid, linoleic acid, coenzyme Q9, and coenzyme Q10 were identified in RPT at relatively high levels, suggesting these as bio-active compounds in RPT. Their role in producing the healthful benefits of RPT warrants further evaluation.
4. Conclusions
In this study, the changes in chemical compounds and lipids in Pu-erh tea during two industrial fermentation processes were measured. After fermentation, the levels of water extract, tea polyphenols, free amino acids, catechins, caffeine, rutin, theophylline, luteolin, and myricetin decreased, while the level of soluble sugar increased. Additionally, 731 lipid molecules and 33 medium- and long-chain FFAs were found in the tea samples. Following fermentation, the content of 85 abundant lipids decreased significantly, while 201 less abundant lipids increased significantly, resulting in an overall decrease in total lipid content. Moreover, linoleic acids (881.202–1322.263 μg/g), linolenic acids (539.634–706.869 μg/g), CoQ9 (62.76–63.57 μg/g), and CoQ10 (50.82–59.33 μg/g) were detected in the final fermented tea leaves, indicating their importance as bioactive lipids in RPT. This research provides insight into the lipid composition and changes during the fermentation of RPT, enhancing our understanding of the fermentation mechanism and bioactive compounds of RPT.
Ethical statement and sensory consent
All members volunteered to participate in the sensory evaluation and agreed to its publication. In addition, appropriate protocols were used to protect the rights and privacy of all participants during the execution of the research.
Consent to publish
All authors agree to publish.
CRediT authorship contribution statement
Qiu-yue Chen: Data curation, Validation, Formal analysis, Writing – original draft. Ming-li Liu: Data curation, Formal analysis, Validation, Methodology. Ruo-yu Li: Data curation, Formal analysis, Validation. Bin Jiang: Formal analysis, Investigation, Validation. Kun-yi Liu: Formal analysis, Validation. Yan-qin Xiao: Formal analysis, Software. Qi Wang: Formal analysis, Validation. Teng Wang: Formal analysis, Software. Lian-qin Zhao: Resources. Wei-tao Wang: Resources. Zhi-wei Liu: Software, Validation. Li-jiao Chen: Software, Validation. Yan Ma: Supervision, Funding acquisition, Project administration, Resources. Ming Zhao: Supervision, Funding acquisition, Project administration, Resources, Writing – review & editing, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 32160728and 31760225).
Handling Editor: Dr. Siyun Wang
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2024.100831.
Contributor Information
Yan Ma, Email: mayan202@163.com.
Ming Zhao, Email: zhaoming02292002@aliyun.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Amar-Yuli I., Aserin A., Garti N. In: Designing Functional Foods. Mcclements D.J., Decker E.A., editors. Woodhead Publishing; 2009. 26 - coenzyme Q10: functional benefits, dietary uptake and delivery mechanisms; pp. 676–700. [Google Scholar]
- Chen C., Chen H., Zhang Y., Thomas H.R., Frank M.H., He Y., Xia R. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol. Plant. 2020;13(8):1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- Chen X., Wang P., Wei M., Lin X., Gu M., Fang W., Ye N. Lipidomics analysis unravels changes from flavor precursors in different processing treatments of purple-leaf tea. J. Sci. Food Agric. 2021 doi: 10.1002/jsfa.11721. [DOI] [PubMed] [Google Scholar]
- Ho C., Zheng X., Li S. Tea aroma formation. Food Sci. Hum. Wellness. 2015;4(1):9–27. doi: 10.1016/j.fshw.2015.04.001. [DOI] [Google Scholar]
- Huang F., Yang P., Bai S., Liu Z., Li J., Huang J., Xiong L. Lipids: a noteworthy role in better tea quality. Food Chem. 2023;137071 doi: 10.1016/j.foodchem.2023.137071. [DOI] [PubMed] [Google Scholar]
- Jia W., Rajani C., Lv A., Fan T., Zheng X. Pu-erh tea: a review of a healthful brew. Journal of Traditional Chinese Medical Sciences. 2022;9(2):95–99. doi: 10.1016/j.jtcms.2022.04.005. [DOI] [Google Scholar]
- Katzinger J., Mischley L., Allen J. In: Textbook of Natural Medicine. fifth ed. Pizzorno J.E., Murray M.T., editors. Churchill Livingstone; 2020. 68 - coenzyme Q10; pp. 526–536. [Google Scholar]
- Lee S.Q., Tan T.S., Kawamukai M., Chen E.S. Cellular factories for coenzyme Q(10) production. Microb. Cell Factories. 2017;16(1):39. doi: 10.1186/s12934-017-0646-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekli I., Das S., Das S., Mukherjee S., Bak I., Juhasz B., Das D.K. Coenzyme Q9 provides cardioprotection after converting into coenzyme Q10. J. Agric. Food Chem. 2008;56(13):5331–5337. doi: 10.1021/jf800035f. [DOI] [PubMed] [Google Scholar]
- Li S.S., Yuan R.Y., Chen L.G., Wang L.S., Hao X.H., Wang L.J., Du H. Systematic qualitative and quantitative assessment of fatty acids in the seeds of 60 tree peony (Paeonia section Moutan DC.) cultivars by GC-MS. Food Chem. 2015;173:133–140. doi: 10.1016/j.foodchem.2014.10.017. [DOI] [PubMed] [Google Scholar]
- Li J., Hua J., Zhou Q., Dong C., Wang J., Deng Y., Jiang Y. Comprehensive lipidome-wide profiling reveals dynamic changes of tea lipids during manufacturing process of black tea. J. Agric. Food Chem. 2017;65(46):10131–10140. doi: 10.1021/acs.jafc.7b03875. [DOI] [PubMed] [Google Scholar]
- Li J., Hua J., Yuan H., Deng Y., Zhou Q., Yang Y., Jiang Y. Investigation on green tea lipids and their metabolic variations during manufacturing by nontargeted lipidomics. Food Chem. 2021;339 doi: 10.1016/j.foodchem.2020.128114. [DOI] [PubMed] [Google Scholar]
- Li J., Yuan H., Rong Y., Qian M.C., Liu F., Hua J., Jiang Y. Lipid metabolic characteristics and marker compounds of ripened Pu-erh tea during pile fermentation revealed by LC-MS-based lipidomics. Food Chem. 2023;404 doi: 10.1016/j.foodchem.2022.134665. [DOI] [PubMed] [Google Scholar]
- Lv H., Zhang Y., Lin Z., Liang Y. Processing and chemical constituents of Pu-erh tea: a review. Food Res. Int. 2013;53(2):608–618. doi: 10.1016/j.foodres.2013.02.043. [DOI] [Google Scholar]
- Ma Y., Ling T.J., Su X.Q., Jiang B., Nian B., Chen L.J., Zhao M. Integrated proteomics and metabolomics analysis of tea leaves fermented by Aspergillus niger, Aspergillus tamarii and Aspergillus fumigatus. Food Chem. 2021;334 doi: 10.1016/j.foodchem.2020.127560. [DOI] [PubMed] [Google Scholar]
- Marangoni F., Agostoni C., Borghi C., Catapano A.L., Cena H., Ghiselli A., Poli A. Dietary linoleic acid and human health: focus on cardiovascular and cardiometabolic effects. Atherosclerosis. 2020;292:90–98. doi: 10.1016/j.atherosclerosis.2019.11.018. [DOI] [PubMed] [Google Scholar]
- Nava Lauson C.B., Tiberti S., Corsetto P.A., Conte F., Tyagi P., Machwirth M., Manzo T. Linoleic acid potentiates CD8+ T cell metabolic fitness and antitumor immunity. Cell Metabol. 2023;35(4):633–650. doi: 10.1016/j.cmet.2023.02.013. [DOI] [PubMed] [Google Scholar]
- Pang Z., Chong J., Zhou G., de Lima Morais D.A., Chang L., Barrette M., Xia J. MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021;49(W1):W388–W396. doi: 10.1093/nar/gkab382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Z., Zhou G., Ewald J., Chang L., Hacariz O., Basu N., Xia J. Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022;17(8):1735–1761. doi: 10.1038/s41596-022-00710-w. [DOI] [PubMed] [Google Scholar]
- Peng J., Dai W., Lu M., Yan Y., Zhang Y., Chen D., Lin Z. New insights into the influences of baking and storage on the nonvolatile compounds in oolong tea: a nontargeted and targeted metabolomics study. Food Chem. 2022;375 doi: 10.1016/j.foodchem.2021.131872. [DOI] [PubMed] [Google Scholar]
- Raizner A.E. Coenzyme Q(10) Methodist Debakey Cardiovasc J. 2019;15(3):185–191. doi: 10.14797/mdcj-15-3-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samynathan R., Thiruvengadam M., Nile S.H., Shariati M.A., Rebezov M., Mishra R.K., Lorenzo J.M. Recent insights on tea metabolites, their biosynthesis and chemo-preventing effects: a review. Crit. Rev. Food Sci. Nutr. 2021:1–20. doi: 10.1080/10408398.2021.1984871. [DOI] [PubMed] [Google Scholar]
- Sanders T.A.B. In: Vegetarian and Plant-Based Diets in Health and Disease Prevention. Mariotti F., editor. Academic Press; 2017. 37 - polyunsaturated fatty acid status in vegetarians; pp. 667–681. [Google Scholar]
- Selvendran R.R., Reynolds J., Galliard T. Production of volatiles by degradation of lipids during manufacture of black tea. Phytochemistry. 1978;17(2):233–236. doi: 10.1016/S0031-9422(00)94152-9. [DOI] [Google Scholar]
- Su X., Zhang G., Ma Y., Chen M., Chen S., Duan S., Zhao M. Isolation, identification, and biotransformation of teadenol A from solid state fermentation of Pu-erh tea and in vitro antioxidant activity. Appl. Sci. 2016;6(6):161. doi: 10.3390/app6060161. [DOI] [Google Scholar]
- Testai L., Martelli A., Flori L., Cicero A., Colletti A. Coenzyme Q(10): clinical applications beyond cardiovascular diseases. Nutrients. 2021;13(5) doi: 10.3390/nu13051697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Qiu Y., Gan R., Zhu F. Chemical constituents and biological properties of Pu-erh tea. Food Res. Int. 2021;110899 doi: 10.1016/j.foodres.2021.110899. [DOI] [PubMed] [Google Scholar]
- Wang C., Li J., Wu X., Zhang Y., He Z., Zhang Y., Liu Z. Pu-erh tea unique aroma: volatile components, evaluation methods and metabolic mechanism of key odor-active compounds. Trends Food Sci. Technol. 2022;124:25–37. doi: 10.1016/j.tifs.2022.03.031. [DOI] [Google Scholar]
- Wang S., Qiu Y., Gan R., Zhu F. Chemical constituents and biological properties of Pu-erh tea. Food Res. Int. 2022;154 doi: 10.1016/j.foodres.2021.110899. [DOI] [PubMed] [Google Scholar]
- Wolrab D., Peterka O., Chocholoušková M., Holčapek M. Ultrahigh-performance supercritical fluid chromatography/mass spectrometry in the lipidomic analysis. TrAC, Trends Anal. Chem. 2022;149 doi: 10.1016/j.trac.2022.116546. [DOI] [Google Scholar]
- Wu Q.Y., Zhou Z.W., He J.H., Zhao S.H., Ruan S.L., Liu X.C., Sun Y. Analysis of aroma precursors in Jinmudan fresh tea leaves and dynamic change of fatty acid volatile during black tea processing. Food Chem. X. 2024;21 doi: 10.1016/j.fochx.2024.101155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiaocun W. third ed. China Agriculture Press; 2003. Tea Biochemistry. [Google Scholar]
- Yang Z., Baldermann S., Watanabe N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013;53(2):585–599. doi: 10.1016/j.foodres.2013.02.011. [DOI] [Google Scholar]
- Yuan Q., Xie F., Huang W., Hu M., Yan Q., Chen Z., Liu L. The review of alpha-linolenic acid: sources, metabolism, and pharmacology. Phytother Res. 2022;36(1):164–188. doi: 10.1002/ptr.7295. [DOI] [PubMed] [Google Scholar]
- Yubero-Serrano E.M., Gutierrez-Mariscal F.M., Garcia-Rios A., Delgado-Lista J., Pérez-Martinez P., Camargo A., Lopez-Miranda J. In: Aging. second ed. Preedy V.R., Patel V.B., editors. Academic Press; 2020. Chapter 16 - coenzyme Q10 as an antioxidant in the elderly; pp. 165–171. [Google Scholar]
- Zhang L., Ma Z.Z., Che Y.Y., Li N., Tu P.F. Protective effect of a new amide compound from Pu-erh tea on human micro-vascular endothelial cell against cytotoxicity induced by hydrogen peroxide. Fitoterapia. 2011;82(2):267–271. doi: 10.1016/j.fitote.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Zhang W., Zhou F., Huang H., Mao Y., Ye D. Biomarker of dietary linoleic acid and risk for stroke: a systematic review and meta-analysis. Nutrition. 2020;79–80 doi: 10.1016/j.nut.2020.110953. [DOI] [PubMed] [Google Scholar]
- Zhao M., Su X.Q., Nian B., Chen L.J., Zhang D.L., Duan S.M., Ma Y. Integrated meta-omics approaches to understand the microbiome of spontaneous fermentation of traditional Chinese Pu-erh tea. mSystems. 2019;4(6) doi: 10.1128/mSystems.00680-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.