Abstract
Purpose
Sex-based differences in lumbar spine's fat content in adults are minimal, but significant variations exist in diffusion-weighted imaging (DWI) signal characteristics. This study aimed to investigate fat content’s impact on DWI performance in lumbar spine and potential sex differences.
Methods
A retrospective analysis was conducted on upper abdominal MRI examinations in asymptomatic adult. The lumbar 1 vertebral apparent diffusion coefficient (ADC) values and fat fraction were measured. Using DWI images (b = 800 s/mm2), the lumbar 1 vertebral signal was categorized into high and iso-low signal groups. A univariate and multivariate analysis was conducted to investigate the influence of fat fraction on DWI performance. Finally, the participants were divided into three groups to analyze sex differences in the effect of fat content on DWI performance.
Results
202 subjects, 99 men were included. Fat content significantly influenced lumbar spine DWI signal in both sexes (p < 0.05). The effect on ADC values was significant only in women (p < 0.001). Women demonstrated a significantly higher proportion of high DWI signal than men in the low (p = 0.002) and middle (p = 0.012) fat content groups. Additionally, women had higher ADC values in the low fat group (p = 0.004) but lower values in the high fat group (p = 0.004).
Conclusion
Fat content significantly impacts the DWI signal of lumbar spine, with a slight sex difference observed. These sex differences suggest that DWI signals may provide valuable information about the bone marrow beyond fat content.
Keywords: Bone marrow, Diffusion-weighted imaging (DWI), Apparent diffusion coefficient (ADC), Fat fraction, Sex
Highlights
-
●
Investigated impact of fat content on lumbar spine DWI signals.
-
●
Revealed significant influence of fat content on DWI signals in both genders.
-
●
Women exhibited higher prevalence of high DWI signals in low and middle fat content groups.
-
●
Suggested potential sex differences in DWI signals related to bone marrow status.
-
●
DWI signals may provide additional insights for bone marrow beyond fat content.
1. Background
Diffusion-weighted imaging (DWI), a magnetic resonance imaging (MRI) technique, reflects the water composition and diffusivity within tissue microenvironments. Currently, it demonstrates high value in clinical practice for assessing bone marrow disorders [1], [2], [3], [4], [5], [6]. However, there are considerable variations in the apparent diffusion coefficient (ADC) values of the spine and pelvis among healthy adults [7], [8], [9], [10], [11]. Several studies have shown that the ADC values of bone marrow in healthy adults are influenced by age and sex [7], [8], [9], [11], [12], [13], [14]. Specifically, a significant negative correlation has been observed between the ADC values of the lumbar spine and age in adult women, but not in men [7], [8]. Notably, fat content gradually increases with age [15], [16]. Additionally, a significant negative correlation has revealed between ADC values and fat fraction in the spine for women, but not for men [12].
There is an increasing amount of literature dedicated to investigating the disparities in DWI signals of midshaft bone among healthy individuals [7], [9], [10], [17], [18]. The study showed that a large proportion of women under the age of 50 have a high DWI signal in the lumbar spine, whereas women over the age of 50 and adult men have an iso-low signal [7]. Moreover, these studies have shown that the DWI signal of bone marrow is influenced by age, menstrual status, hematopoietic function, and estrogen levels in women [10], [13], [18], as these factors can impact fat content in bone marrow. In addition, prior to reaching the age of 50, adult men have 10 % more fat in their lumbar spine compared to women [16]. However, the disparity in the fat fraction of the bone marrow between the sexes is relatively small at this stage, while the DWI signal characteristics exhibits significant variation. Despite these findings, no previous studies have specifically investigated the effect of fat fraction on bone marrow DWI signals and potential sex differences.
This study aims to address these gaps with two objectives. Firstly, to investigate the influence of fat fraction on DWI signals in the lumbar spine, and secondly, to determine whether there are differences between the sexes.
2. Materials and methods
2.1. Participants
This research analyzed the radiological data of adult patients who were admitted to our hospital between January 2021 and June 2022 and underwent MRI of the upper abdomen.
Inclusion criteria were as follows: (a) normal lumbar spine development, (b) age ≥ 18 years.
Exclusion criteria were as follows: (a) history of lumbar fracture, surgery, and chemoradiotherapy; (b) history of malignant hematological disorders and tumors; (c) history of hormonal drug use; (d) history of recent infection within one month; (e) history of cirrhosis; (f) obvious artifacts in MRI. The study enrolled 202 participants, and Fig. 1 shows the flowchart of the study.
Fig. 1.
Flowchart of inclusion and exclusion.
2.2. MR examination
An upper abdomen MRI was performed with an eight-channel phased-array body coil and breath gating on a 1.5-T magnetic resonance scanner (Optima MR360, GE Healthcare).
The parameters of breath-triggered axial position diffusion-weighted imaging: repetition time (TR) = 6600 ms, echo time (TE) = 75 ms, layer thickness = 6 mm, spacing = 1.2 mm, field of view (FOV) = 400 × 400 mm, matrix = 256 × 256, number of excitations (NEX) = 4, and b-values were taken as 0 and 800 s/mm2. Quantitative ADC maps were automatically generated using the MR system.
The parameters of two-point Dixon (liver imaging with volume acceleration-flexible, LAVA-Flex): TR = 5.9 ms, TE = 3.1 ms, flip angle = 15, layer thickness = 6 mm, layer spacing = − 4.5 mm, FOV = 420 × 336 mm, matrix = 256 × 192.
2.3. Image evaluation
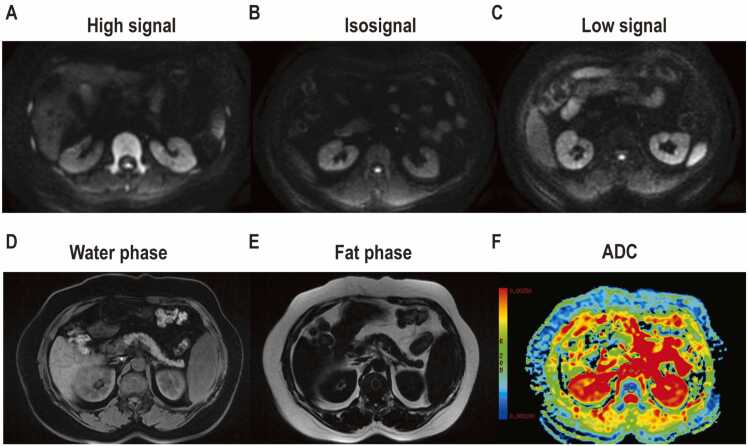
The lumbar 1 vertebra was chosen for investigation in this study. This is because previous studies have reported consistent DWI signals in the mid-shaft bone [7], [10], [13]. Additionally, the lower occurrence of age-related degenerative changes such as Schmorl’s nodes, disc herniation, and endplate inflammation in this specific region was considered as these factors could impact the correlation between fat content and DWI signals. The middle level of the lumbar 1 vertebra was identified against the coronal T2WI on the Tomorrow Medical Network PACS system. The DWI signals of the lumbar 1 vertebral and adjacent erector spinae muscle at this level was compared, and the signals of the bone marrow were classified as high and iso-low signal groups. The high signal group had a higher signal intensity to adjacent erector spinae muscle, while the iso-low signal group had an equal or lower signal intensity. An elliptical area of interest, measuring 150 mm2 ± 25 mm2, was delineated in the center of the L1 vertebra. The signal intensity (SI) of the water and fat phases of the bone marrow in the lumbar 1 vertebrae was measured, and calculation of the fat fraction was performed using the following formula: (fat fraction = SI fat/(SI water + SI fat) × 100 %). Moreover, at the GE ADW4.6 workstation, an elliptical area of interest was outlined at the mid-level of the lumbar 1 vertebrae, with an area of 150 mm2 ± 25 mm2, and the ADC values of the bone marrow were measured. A graphical illustration is illustrated in Fig. 2.
Fig. 2.
Schematic representations of Diffusion-weighted imaging (DWI) signals, fat fraction and apparent diffusion coefficient (ADC) measurements of the lumbar 1 vertebral body: (A) high signal; (B) isosignal; (C) low signal; ROI placement on (D) water phase; (E) fat phase; (F) ADC map.
Both L.H and Z.H, each with 10 years of clinical experience in interpreting MR images, performed DWI signal evaluations, measured ADC values, and assessed the fat fraction of the lumbar spine to test inter-individual reliability. Finally, the results of L.H, an experienced musculoskeletal physician, were used for subsequent statistical analysis.
2.4. Statistical analysis
All analyzes were performed using SPSS Statistics version 21. The Shapiro-Wilk test was used to assess the normality of the measures. Age and body mass index (BMI) were compared between sexes using the Mann-Whitney U tests. The chi-squared test was applied to evaluate the differences in smoking between sexes. The kappa coefficient was used to evaluate the inter-observer reliability of DWI signal. The inter-observer reliability of the ADC values and fat fraction measurements were evaluated using intraclass correlation coefficients (ICC). Binary logistic regression was employed to examine the independent variables possibly influencing the lumbar DWI signal, including age, fat fraction, BMI, smoking, and menstrual status. Additionally, multiple linear regression was used to analyze the factors that impact the ADC values of the lumbar. Factors with p < 0.1 were entered into the multi-factor models analysis using the enter selection method. The chi-squared test was used to assess differences in DWI signals between sexes in the different fat content groups. In addition, the independent samples t-test was used to compare ADC values between the sexes. A significance level of p < 0.05 was considered statistically significant.
3. Results
3.1. Baseline information
Baseline information was presented in Table 1.
Table 1.
The baseline information.
| Men (n = 99) | Women (n = 103) | P | |
|---|---|---|---|
| Age (y) | 49.00(38.00, 58.00) | 52.00(40.00, 59.00) | 0.534 |
| BMI (kg/m2) | 23.10(20.80, 24.80) | 22.60(21.00, 24.50) | 0.857 |
| Smoking (Y/N) | 31/68 | 0/103 | <0.001 |
| Menstrual status (Pre/Post) | NA | 44/59 | NA |
Note. BMI: Body mass index; NA: Not applicable.
3.2. Interobserver agreement
The kappa coefficient value for DWI signal of the lumbar spine by different observers was 0.842 (95 % CI: 0.765, 0.918). The ICC for ADC values and a fat fraction of lumbar bone marrow were 0.713 (95 % CI: 0.637, 0.774) and 0.859 (95 % CI: 0.819, 0.892), respectively. The consistency of measurement and assessment between different observers was good to very good.
3.3. The influence of fat content on the DWI signals
In men, univariate analysis revealed a significant influence of fat fraction (p = 0.008) on DWI signals in the lumbar spine, with no significant impact found for age, BMI, and smoking (all p > 0.05).
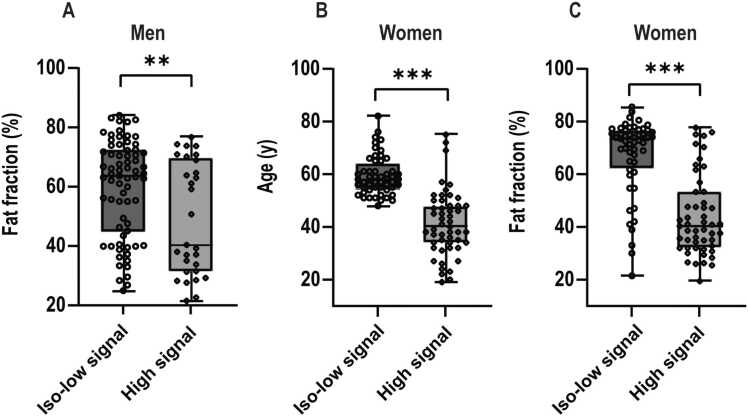
In women, univariate analysis demonstrated that age, fat fraction, and menstrual status had a significant effect on DWI signals (all p < 0.001), while BMI did not show a significant impact (p = 0.334). A multivariate analysis revealed that both age and fat fraction were independent risk factors for DWI signals (all p < 0.05), whereas menstrual status was not (p = 0.242). Details are shown in Table 2 and Fig. 3.
Table 2.
The factors influencing DWI signals in lumbar spine.
| Variables | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| High signal (M/W= 29/51) | Iso-low signal (M/W= 70/52) | P | OR(95 % CI) | P | ||
| Men | Age (y) | 48.07 ± 14.61 | 49.86 ± 13.55 | 0.557 | - | - |
| Fat fraction (%) | 40.28(31.32, 69.48) | 63.83(44.65, 72.25) | 0.008 | - | - | |
| BMI (kg/m2) | 22.48 ± 2.53 | 23.11 ± 2.82 | 0.292 | - | - | |
| Smoking (Y/N) | 8/21 | 23/47 | 0.607 | - | - | |
| Women | Age (y) | 40.00(34.00, 48.00) | 58.00(54.25, 64.00) | <0.001 | 0.90(0.80, 0.99) | 0.046 |
| Fat fraction (%) | 40.28(31.97, 53.27) | 73.11(62.44, 76.64) | <0.001 | 0.95(0.92, 0.99) | 0.005 | |
| BMI (kg/m2) | 22.50(20.30, 23.90) | 23.15(21.38, 25.48) | 0.334 | - | - | |
| Menstrual status (Pre/Post) |
39/12 | 5/47 | <0.001 | 2.97(0.48, 18.31) | 0.242 | |
Note. BMI: Body mass index; NA: Not applicable.
Fig. 3.
The factors affect Diffusion-weighted imaging (DWI) signals of the lumbar spine in men and women. The subplots illustrate the differences in fat fraction in men (A), age in women (B) and fat fraction in women (C) between the high and iso-low signal groups. Asterisks denote the significance level: * p < 0.05, ** p < 0.01, *** p < 0.001.
3.4. The influence of fat content on the ADC values
In men, univariate analysis showed that BMI was a significant factor impact on the ADC values in lumbar spine (p = 0.029). However, age, fat fraction and smoking were not (all p > 0.05).
In women, univariate analysis showed that age, fat fraction, BMI and menstrual status significantly influenced ADC values (all p < 0.001). Furthermore, multivariate analysis confirmed that fat fraction and BMI were independent risk factors for ADC values (all p < 0.001), whereas age and menstrual status were not (both p > 0.05). Details are shown in Table 3.
Table 3.
The factors influencing ADC values in lumbar spine.
| Variables | Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|
| β | P | β | P | ||
| Men | Age (y) | − 0.13 | 0.200 | - | - |
| Fat fraction (%) | − 0.07 | 0.479 | - | - | |
| BMI (kg/m2) | − 0.22 | 0.029 | - | - | |
| Smoking (Y/N) | 0.05 | 0.655 | - | - | |
| Women | Age (y) | − 0.53 | < 0.001 | − 0.09 | 0.511 |
| Fat fraction (%) | − 0.60 | < 0.001 | − 0.39 | < 0.001 | |
| BMI (kg/m2) | − 0.32 | < 0.001 | − 0.25 | < 0.001 | |
| Menstrual status (Pre/Post) |
0.53 | < 0.001 | 0.23 | 0.067 | |
Note. BMI: Body mass index; NA: Not applicable.
3.5. Sex differences in the influence of fat fraction on DWI signals
Participants were divided into three groups based on the tertiles of fat fraction. The low group (< 42.3 %) comprised 31 men and 36 women. The middle group (42.3–70.1 %) included 38 men and 30 women. The high group (> 70.1 %) consisted of 30 men and 37 women.
The proportion of high DWI signal in the lumbar spine was significantly higher in women compared to men in the low (p = 0.002) and middle (p = 0.012) content groups, while no significant differences between sexes (p = 0.688) in the high content group. Details are shown in Table 4.
Table 4.
Sex differences in DWI signals in various fat content groups.
| Fat content | Low content group |
Middle content group |
High content group |
|||
|---|---|---|---|---|---|---|
| High signal | iso-low signal | High signal | iso-low signal | High signal | iso-low signal | |
| Men n (%) |
15(48.39 %) | 16(51.61 %) | 8(21.05 %) | 30(78.95 %) | 6(20.00 %) | 24(80.00 %) |
| Women n (%) |
30(83.33 %) | 6(16.67 %) | 15(50.00 %) | 15(50.00 %) | 6(16.22 %) | 31(83.78 %) |
| χ2 | 9.224 | 6.276 | 0.161 | |||
| P | 0.002 | 0.012 | 0.688 | |||
Note. DWI: Diffusion-weighted imaging.
3.6. Sex differences in the influence of fat fraction on ADC values
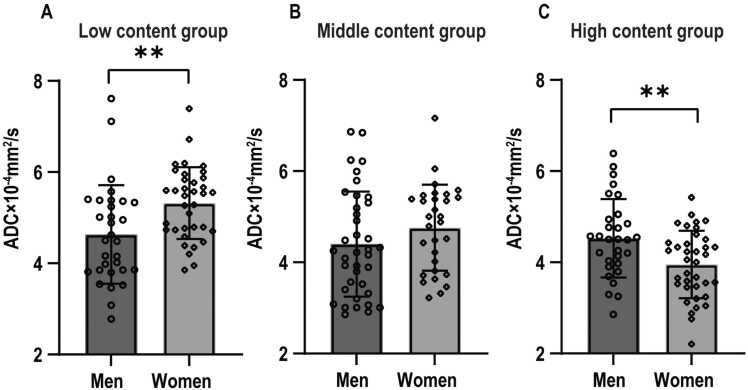
In the low content group, the ADC values of the lumbar spine were significantly higher in women than in men (p = 0.004). No significant difference was observed in the middle content group (p = 0.173). Conversely, in the high content group, the ADC values were significantly lower in women than in men (p = 0.004). Details are shown in Table 5 and Fig. 4.
Table 5.
Sex differences in ADC values in various fat content groups.
| Fat content | Low content group | Middle content group | High content group |
|---|---|---|---|
| Men | 4.63 ± 1.08 | 4.40 ± 1.15 | 4.53 ± 0.86 |
| Women | 5.32 ± 0.79 | 4.76 ± 0.94 | 3.95 ± 0.74 |
| t | 3.010 | 1.377 | − 2.946 |
| P | 0.004 | 0.173 | 0.004 |
Note. ADC values are × 10–4 mm2/s.
Fig. 4.
The differences in apparent diffusion coefficient (ADC) values between sexes in three different fat content groups, as follows: (A) low, (B) middle, and (C) high content groups. Asterisks denote the significance level: * p < 0.05, ** p < 0.01, *** p < 0.001.
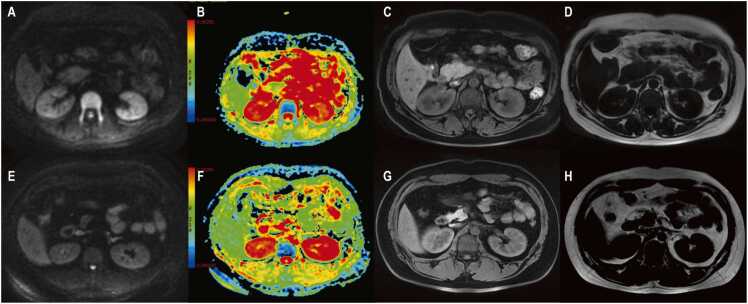
Fig. 5 illustrates the differences in the DWI signal and ADC values for the lumbar 1 vertebrae in the low fat content groups for men and women.
Fig. 5.
The differences in the diffusion-weighted imaging (DWI) performance between sexes for the lumbar 1 vertebrae in the low fat content groups. A 34-year-old woman exhibited a high DWI signal in lumbar 1 vertebrae (A). The apparent diffusion coefficient (ADC) value measured 4.87 × 10–4 mm2/s (B). The water phase map showed a signal intensity value of 211.81 (C), while the fat phase map showed 72.32 (D), indicating a fat fraction of 25.45 %. A 30-year-old man exhibited an iso DWI signal in lumbar 1 vertebrae (E). The ADC value measured 5.09 × 10–4 mm2/s (F). The water phase map showed a signal intensity value of 179.31 (G), while the fat phase map showed 49.00 (H), indicating a fat fraction of 21.46 %.
4. Discussion
The fat content significantly influences the DWI signal in lumbar spine, with the high DWI signal group having lower fat content, consistent with previous studies [9]. In the low and middle fat content groups, women exhibit a significantly higher proportion of a high DWI signal as compared to men in the lumbar spine. In general, fat content is utilized as an indicator of bone marrow composition, suggesting consistent DWI signals for identical fat fractions. However, this study reveals distinctive inconsistencies. This is an unexpected finding in our research and, to our knowledge, has not been reported elsewhere.
Besides fat content, some differences between the sexes have a significant effect on the bone marrow DWI signal. Firstly, the differences in intervertebral muscle signal on DWI may potentially have an influence on bone marrow signal intensity. A previous study found no significant difference in the fat content of the erector spinae muscles between men and women at the thoracic 12-lumbar 2 levels [19]. Other studies had reported that erector spinae perfusion and DWI signals remain unchanged with aging [10], [20]. Considering the relatively minimal changes in the erector spinae muscle, it was selected as a reference. Secondly, there are differences in lumbar spine bone mineral density between the sexes. It has been found that bone mineral density is negatively correlated with fat content in the vertebral body [20], [21], [22], [23].The study participants were divided into three groups based on their fat content, as a result, the variations in bone mineral density within the same group are minimal, and the impact is relatively insignificant. Finally, the most notable difference between the sexes was the functional status of the bone marrow. Previous studies have shown that a higher DWI signal corresponds to greater activity within the marrow [10], [13]. Specifically, it has been observed that young women exhibit higher marrow perfusion and more active bone marrow function compared to men [16], [23]. This increased perfusion leads to a higher DWI signal primarily [10]. Additionally, research has suggested that the high DWI signal in premenopausal women can be attributed to the considerably enriched cellular content of the bone marrow [9]. In conclusion, these findings demonstrate that bone marrow in younger women exhibits higher cell counts and perfusion, implying more active bone marrow function. In the group with a relatively low fat content, this is likely an explanation for the larger proportion of high DWI signals observed in the lumbar spine in women.
In clinical practice, bone marrow function is primarily evaluated based on the fat content [24]. Several studies have suggested a link between changes in bone marrow fat content pre- and post-radiotherapy and the development of myelosuppression [25], [26]. Detecting active bone marrow regions with low fat content before radiotherapy can markedly decrease the severity and frequency of myelosuppression by confining radiation to these specific regions [27], [28]. The research shows significant differences in DWI signals between the sexes, despite identical fat content in the bone marrow. Hence, it is essential to consider gender differences when delineating active bone marrow regions based on fat content. In addition, DWI signals could provide valuable information.
This study has some limitations that should be considered. First, this is a retrospective study, and the study sample was obtained from a machine in our institution, resulting in a selection bias in the selection process of the population, and future research to explore the use of different machines and protocols to broaden the scope of our findings and improve the generalizability of the results. In addition, this study did not consider potential confounders such as altitude [14]. It is noteworthy that almost all patients at our institution come from a similar altitude, and this factor may influence the DWI performance of the bone marrow. Finally, fat content is measured using a two-point Dixon (LAVA-Flex), which is susceptible to magnetic field inhomogeneities, leading to less precise measurements.
In conclusion, the fat content significantly influences the DWI signal in the lumbar spine, with a slight sex difference observed. These sex differences suggest that DWI signals provide valuable information about the bone marrow beyond fat content, and highlight the need to consider sex when assessing bone marrow by fat content in future studies.
CRediT authorship contribution statement
Liang Hu: Writing – original draft, Visualization, Software, Project administration, Methodology, Funding acquisition. Jiang-Feng Pan: Writing – original draft, Resources, Project administration, Methodology. Zheng Han: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Funding acquisition. Xiu-Mei Xia: Supervision, Resources, Project administration, Methodology.
Ethical approval
The study was approved by the ethics review board of Jinhua Municipal Central Hospital (2023 - Ethics Review -65) in accordance with the Declaration of Helsinki. The data are anonymous, and the requirement for informed consent was therefore waived by the ethics committee review board of Jinhua Municipal Central Hospital.
Funding
This work was supported by young and middle-aged research promotion projects of Jinhua Municipal Central Hospital (No. JY2019-2-14) and public service projects grant from the Jinhua Municipal Science and Technology Bureau (Nos. 2021-4-033, 2023-4-092).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Filograna L., Magarelli N., Cellini F., et al. Diffusion weighted imaging (DWI) and apparent diffusion coefficient (ADC) values for detection of malignant vertebral bone marrow lesions. Eur. Rev. Med. Pharm. Sci. 2018;22(3):590–597. doi: 10.26355/eurrev_201802_14273. [DOI] [PubMed] [Google Scholar]
- 2.Schmeel F.C., Enkirch S.J., Luetkens J.A., et al. Diagnostic accuracy of quantitative imaging biomarkers in the differentiation of benign and malignant vertebral lesions: combination of diffusion-weighted and proton density fat fraction spine MRI. Clin. Neuroradiol. 2021;31(4):1059–1070. doi: 10.1007/s00062-021-01009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sartoretti E., Sartoretti-Schefer S., van Smoorenburg L., et al. Single shot zonal oblique multislice SE-EPI diffusion-weighted imaging with low to ultra-high b-values for the differentiation of benign and malignant vertebral spinal fractures. Eur. J. Radiol. Open. 2021;8 doi: 10.1016/j.ejro.2021.100377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhong X., Dong T., Tan Y., et al. Pelvic insufficiency fracture or bone metastasis after radiotherapy for cervical cancer? The added value of DWI for characterization. Eur. Radiol. 2020;30(4):1885–1895. doi: 10.1007/s00330-019-06520-1. [DOI] [PubMed] [Google Scholar]
- 5.Wang K., Lee E., Kenis S., et al. Application of diffusion-weighted whole-body MRI for response monitoring in multiple myeloma after chemotherapy: a systematic review and meta-analysis. Eur. Radiol. 2022;32(4):2135–2148. doi: 10.1007/s00330-021-08311-z. [DOI] [PubMed] [Google Scholar]
- 6.Zhang B., Bian B., Zhang Y., Zhang L., Zhang R., Wang J. The apparent diffusion coefficient of diffusion-weighted whole-body magnetic resonance imaging affects the survival of multiple myeloma independently. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.780078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui F.Z., Cui J.L., Wang S.L., et al. Signal characteristics of normal adult bone marrow in whole-body diffusion-weighted imaging. Acta Radiol. (Stockh. Swed.: 1987) 2016;57(10):1230–1237. doi: 10.1177/0284185115626477. [DOI] [PubMed] [Google Scholar]
- 8.Jie H., Hao F., Na L.X. Vertebral bone marrow diffusivity in healthy adults at 3T diffusion-weighted imaging. Acta Radiol. (Stockh. Swed.: 1987) 2016;57(10):1238–1243. doi: 10.1177/0284185116641346. [DOI] [PubMed] [Google Scholar]
- 9.Colombo A., Bombelli L., Summers P.E., et al. Effects of sex and age on fat fraction, diffusion-weighted image signal intensity and apparent diffusion coefficient in the bone marrow of asymptomatic individuals: a cross-sectional whole-body MRI study. Diagnostics. 2021;11(5):913. doi: 10.3390/diagnostics11050913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui F.Z., Yao Q.Q., Cui J.L., Wei W., Duan L.S., Yu H. The signal intensity characteristics of normal bone marrow in diffusion weighted imaging at various menstrual status women. Eur. J. Radiol. 2021;143 doi: 10.1016/j.ejrad.2021.109938. [DOI] [PubMed] [Google Scholar]
- 11.Munhoz L., Abdala Júnior R., Choi I., Arita E.S. Diffusion-weighted magnetic resonance imaging of mandibular bone marrow: do apparent diffusion coefficient values of the cervical vertebrae and mandible correlate with age. Oral. Radiol. 2022;38(1):72–79. doi: 10.1007/s11282-021-00528-4. [DOI] [PubMed] [Google Scholar]
- 12.Lavdas I., Rockall A.G., Castelli F., et al. Apparent diffusion coefficient of normal abdominal organs and bone marrow from whole-body DWI at 1.5 T: the effect of sex and age. AJR Am. J. Roentgenol. 2015;205(2):242–250. doi: 10.2214/AJR.14.13964. [DOI] [PubMed] [Google Scholar]
- 13.Tsujikawa T., Oikawa H., Tasaki T., et al. Whole-body bone marrow DWI correlates with age, anemia, and hematopoietic activity. Eur. J. Radiol. 2019;118:223–230. doi: 10.1016/j.ejrad.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 14.Bao H., He X., Li X., Cao Y., Zhang N. Magnetic resonance imaging study of normal cranial bone marrow conversion at high altitude. Quant. Imaging Med. Surg. 2022;12(6):3126–3137. doi: 10.21037/qims-21-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bani Hassan E., Ghasem-Zadeh A., Imani M., et al. Bone marrow adipose tissue quantification by imaging. Curr. Osteoporos. Rep. 2019;17(6):416–428. doi: 10.1007/s11914-019-00539-5. [DOI] [PubMed] [Google Scholar]
- 16.James F., Griffith Age-related changes in the bone marrow. Curr. Radiol. Rep. 2017;5(6):24. [Google Scholar]
- 17.Ording Müller L.S., Avenarius D., Olsen O.E. High signal in bone marrow at diffusion-weighted imaging with body background suppression (DWIBS) in healthy children. Pediatr. Radiol. 2011;41(2):221–226. doi: 10.1007/s00247-010-1774-8. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y.Y., Wu C.L., Shen S.H. High signal in bone marrow on diffusion-weighted imaging of female pelvis: correlation with anemia and fibroid-associated symptoms. J. Magn. Reson. Imaging. 2018;48(4):1024–1033. doi: 10.1002/jmri.26002. [DOI] [PubMed] [Google Scholar]
- 19.Sollmann N., Zoffl A., Franz D., et al. Regional variation in paraspinal muscle composition using chemical shift encoding-based water-fat MRI. Quant. Imaging Med. Surg. 2020;10(2):496–507. doi: 10.21037/qims.2020.01.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffith J.F., Yeung D.K., Antonio G.E., et al. Vertebral marrow fat content and diffusion and perfusion indexes in women with varying bone density: MR evaluation. Radiology. 2006;241(3):831–838. doi: 10.1148/radiol.2413051858. [DOI] [PubMed] [Google Scholar]
- 21.Chang F.X., Fan D.H., Huang G., He J.H. Lumbar spine bone mineral density measurement: comparison of dual-energy X-ray absorptiometry and fat content evaluation by dixon chemical shift MRI. Int. J. Gen. Med. 2022;15:6415–6424. doi: 10.2147/IJGM.S370814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim D., Kim S.K., Lee S.J., Choo H.J., Park J.W., Kim K.Y. Simultaneous estimation of the fat fraction and R₂* via T₂*-corrected 6-echo dixon volumetric interpolated breath-hold examination imaging for osteopenia and osteoporosis detection: correlations with sex, age, and menopause. Korean J. Radiol. 2019;20(6):916–930. doi: 10.3348/kjr.2018.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu W., Gong T., Niu J., et al. Study of bone marrow microstructure in healthy young adults using intravoxel incoherent motion diffusion-weighted MRI. Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.958151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawless M., Byrns K., Bednarz B.P., et al. Feasibility of identifying proliferative active bone marrow with fat fraction MRI and multi-energy CT. Phys. Med. Biol. 2024;69(13) doi: 10.1088/1361-6560/ad58a0. [DOI] [PubMed] [Google Scholar]
- 25.Gassert F.G., Kranz J., Gassert F.T., et al. Longitudinal MR-based proton-density fat fraction (PDFF) and T2* for the assessment of associations between bone marrow changes and myelotoxic chemotherapy. Eur. Radiol. 2024;34(4):2437–2444. doi: 10.1007/s00330-023-10189-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C., Qin X., Gong G., Wang L., Su Y., Yin Y. Correlation between changes of pelvic bone marrow fat content and hematological toxicity in concurrent chemoradiotherapy for cervical cancer. Radiat. Oncol. 2022;17(1):70. doi: 10.1186/s13014-022-02029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miszczyk M., Wu T., Kuna K., et al. Clinical outcomes of pelvic bone marrow sparing radiotherapy for cervical cancer: a systematic review and meta-analysis of randomised controlled trials. Clin. Transl. Radiat. Oncol. 2024;47 doi: 10.1016/j.ctro.2024.100801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou P., Zhang Y., Luo S., Zhang S. Pelvic bone marrow sparing radiotherapy for cervical cancer: a systematic review and meta-analysis. Radiol. Oncol. 2021;165:103–118. doi: 10.1016/j.radonc.2021.10.015. [DOI] [PubMed] [Google Scholar]