Abstract
Neurofilaments (NFs) and GFAP are cytoskeletal intermediate filaments (IFs) that support cellular processes unfolding within the uniquely complex environments of neurons and astrocytes, respectively. This review highlights emerging concepts on the transitions between stable and destabilized IF networks in the nervous system. While self-association between transiently structured low-complexity IF domains promotes filament assembly, the opposing destabilizing actions of phosphorylation-mediated filament severing facilitate faster intracellular transport. Cellular proteases, including caspases and calpains, produce a variety of IF fragments, which may interact with N-degron and C-degron pathways of the protein degradation machinery. The rapid adoption of NF and GFAP-based clinical biomarker tests is contrasted with the lagging understanding of the dynamics between the native IF proteins and their fragments.
A low complexity view of complex IF assemblies.
Intermediate filament (IF) proteins form flexible and highly adaptable cytoskeletal networks that help various cell types meet physiological demands and manage stress. The activities of IFs are especially critical in cells with highly complex architecture and elongated cytoplasmic processes, such as astrocytes and neurons, to establish and maintain their characteristic morphology and cell-to-cell connections1, 2. Neurofilaments (NF) and glial fibrillary acidic proteins (GFAP) form the major IF networks in mature neurons and astrocytes, respectively. GFAP forms homo-polymeric assemblies that are highly sensitive to perturbations within the central alpha-helical rod domain of the molecule, as shown in recent mutagenesis studies3, 4. The three NF genes (NEFL, NEFM, and NEFH), encode the NF light, medium and heavy (NF-L, NF-M, and NF-H) proteins that associate to form the cytoskeletal networks of neurons. Recent seminal studies have highlighted the importance of the ‘low complexity’ N-terminal ‘head’ domain of NF-L in mediating homotypic interactions to assemble into mature networks5. Low complexity refers to the over-representation of specific amino acid in a given protein or protein segment6; and by that standard the head and tail domains of most IF proteins fit in this category. Previously thought to lack structural order, we now know that the N-termini transition between conformational disorder and labile β-strand polymers that promote self-associations and stabilize filament assembly5, 7.
Phosphorylation rules IF behavior.
Like other IFs, the head domains of NFs and GFAP are enriched in post-translational modification (PTMs) sites, particularly sites for phosphorylation. Nearly half of the NF-L head domain (92 residues) is represented by three residues: Ser, Tyr, or Thr, with 27, 9 and 4 residues respectively. Hence, phosphorylation plays a prominent role in NF dynamics, including the severing and re-annealing of mature filaments (Fig. 1) to continually facilitate adaptive responses in cells. This is independent of IF turnover, per se, via protein degradation and new protein synthesis8 and especially important in the nervous system, where NFs and GFAP are long-lived proteins with half-lives in vivo measured in weeks to months1,9, 10.
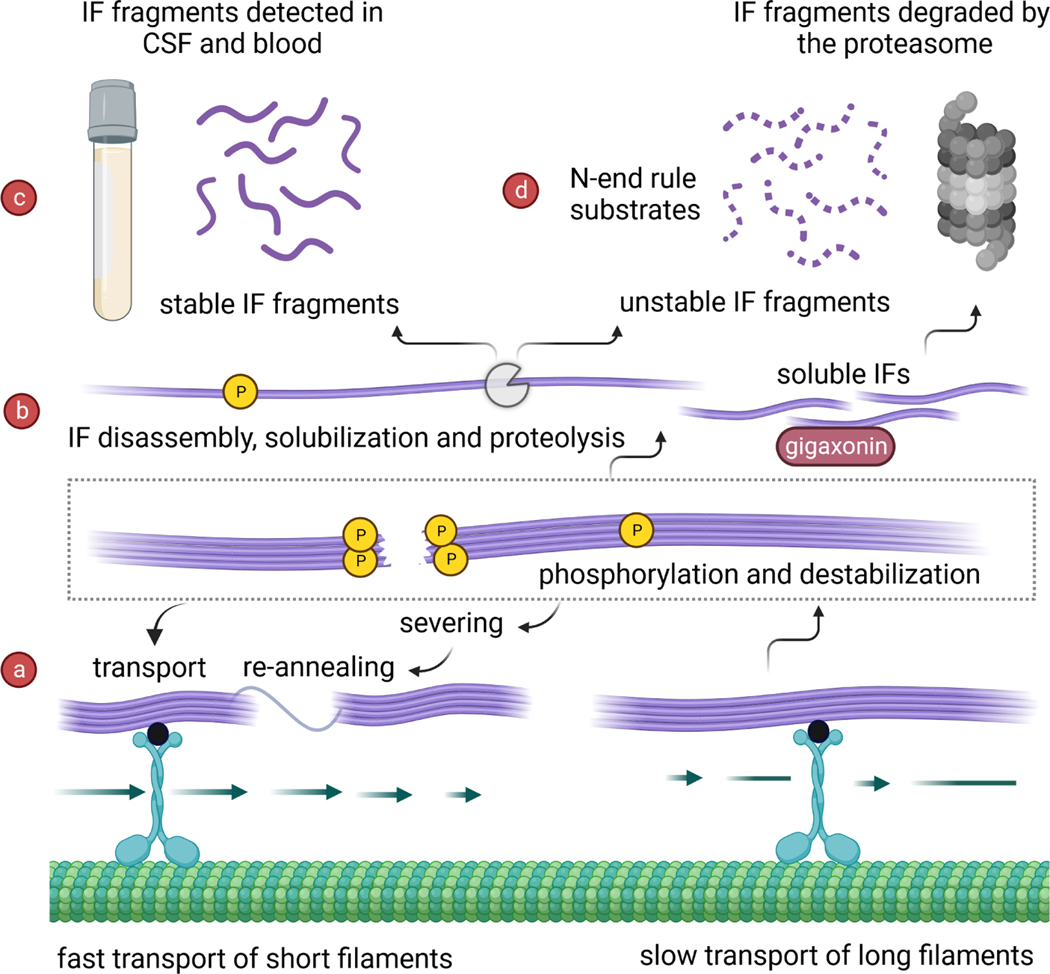
Figure 1: Stability and instability mechanisms of IFs in the nervous system.
IF protein assembly and disassembly are dynamic processes facilitated by post-translational modifications (PTMs) and enzymatic proteolysis. (a) Phosphorylation (P) promotes destabilization of the IF network and severing of filaments. The speed of microtubule-dependent NF-L transport in neurons is controlled by cycles of filament severing and re-annealing: shorter IFs move faster compared to longer IFs, which stall frequently. A putative adapter molecule (represented by the black spheres) has been proposed to facilitate the interaction between IFs and kinesin motor proteins. (b) Both phosphorylation and citrullination (C), the enzymatic deimination of arginine to citrulline, destabilize IFs - including GFAP. This results in the formation of non-filamentous, soluble IFs. Soluble IFs become the substrates for the E3 ubiquitin ligase adaptor gigaxonin, which promotes normal IF protein turnover via the proteasome. IF clearance and transport are significantly impaired in the absence of functional gigaxonin. Further processing of non-filamentous IFs is carried out by various cellular proteases, such as caspases and calpains (represented by the pac-man shape). (c) Site-specific enzymatic proteolysis results in the formation of IF protein fragments with varying stability. Cerebrospinal fluid (CSF) and blood levels of NF-L and GFAP are currently utilized as biomarkers for neurological disorders and injuries. Recent studies show that NF-L biomarker assays detect only fragments, and that certain fragments are more strongly associated with disease activity. (d) Short-lived proteolytic IF fragments can become substrates for the N-end rule pathway, a ubiquitin-proteasome dependent system that targets protease-generated fragments for degradation. The residues exposed at the peptide cleavage site govern the stability of protein fragment products. Many predicted calpain cleavage sites on IFs expose highly stabilizing residues (such as serine and alanine), suggesting that IF protein fragments may be long-lived and may have important biological functions that are distinct from the fully assembled filaments. Created with BioRender.com
Citrullination destabilizes GFAP and marks reactive glia.
Another important, but incompletely understood PTM that has a strong destabilizing effect on IFs, is citrullination. Citrullination is the enzymatic deimination of the amino acid arginine to produce the non-essential amino acid citrulline. In the 1980s, work by Inagaki et al. demonstrated that head domain citrullination is highly destabilizing (Fig. 1) to vimentin, GFAP and desmin IFs, but the exact biological function of this PTM in various cells and physiological contexts has remained elusive. Recently, a series of studies by R. Mohan and colleagues have elegantly revealed a disease-associated role for citrullination of GFAP in reactive glial cells11–13. Müller glia (MG) exhibit robust compartmentalized GFAP citrullination in the their endfeet and processes in different mouse models of retinal degeneration, and this is also observed in human wet age-related macular degeneration tissues13. The enzyme peptidyl arginine deiminase-4 (PAD4), which facilitates citrullination, co-localizes with GFAP and GFAP hyper-citrullination is blunted in mice lacking PAD4 expression in glial cells. The authors have proposed that the MG endfeet serve as a “bunker” for citrullination throughout retinal degeneration, such that this highly localized stress response can still allow for phototransduction and visual processing to take place. This work also raises the possibility that citrullinated GFAP or cleavage of GFAP into citrullinated fragments may contribute to progressive disease pathology and highlights citrullinated GFAP as a potential biomarker for human degenerative retinal diseases. Recently developed methods to modulate protein citrullination in a site-specific manner14 should facilitate a better understanding of the cell biology behind this PTM on neuronal and glial IFs – especially in light of recent work linking citrullination more broadly with abnormal protein aggregation and neurodegeneration15.
L(IF)e in the fast lane: severed NFs move faster.
The regulated transport and degradation of NFs is essential for the maintenance of proper neuronal structure and cellular homeostasis16. A recent study using fluorescence photoactivation pulse-escape method found that the entire pool of neurofilaments is dynamic and moves (albeit slowly) within the myelinated axons of peripheral nerves in the adult mouse17, contrary to what was previously thought to be the case. The movement of NFs in axons is bidirectional with an anterograde bias, but the net velocity decreases during post-natal development, according to age and proximal-to-distal positioning along the nerve18. Increased cross-sectional area of myelinated axons is associated with increased influx and retention of NFs due to slower movement, which is partly related to decreased density of the microtubule network19. To accommodate microtubule-based transport, NFs undergo an active process of severing and re-annealing, similar to what has previously been established for vimentin20. Shorter segments move more quickly, while longer filaments (after annealing) move more slowly, change direction more frequently, or stall8. Phospho-mimetic substitutions at NF-L head domain serine residues 2, 55, 57 and 62 (PKA and CAMKII target sites) resulted in the formation of shorter and more rapidly moving NFs, while phospho-deficient mutations resulted in longer, slower moving NFs and wider axons8. Thus, these new studies suggest that head domain phosphorylation plays a destabilizing role within mature NF networks (Fig. 1).
Soluble IFs are gigaxonin substrates.
In addition to severing of filaments, phosphorylation can also trigger disassembly to form a soluble, non-filamentous pool of IFs that can be targeted for proteasomal degradation. Proteasomal turnover of NFs21 and GFAP22 is mediated by the ubiquitin ligase adaptor protein gigaxonin (Fig. 1), which is encoded by the gene KLHL16 (or GAN). Loss-of-function mutations in KLHL16 cause the rare pediatric neurodegenerative disease Giant Axonal Neuropathy (GAN)23. GAN is characterized by progressive axonal degeneration affecting the peripheral nervous system (PNS) and the central nervous system (CNS). The clinically debilitating effects of GAN are due to the preferential and severe involvement of axons, which are focally distended by densely packed NFs23. Recent work shows that astrocytes are significantly impacted in GAN, but their roles and the significance of GFAP accumulation are less clear24. Ectopic expression of high levels of gigaxonin in cells leads to the complete elimination of IFs, and this finding served as the basis of an ongoing clinical trial for GAN25. Despite recent progress in understanding the natural history of GAN23, the true function of gigaxonin and the specific reason behind the selective neuronal vulnerability, when many other cell types also contain prominent IF aggregates, have yet to be elucidated.
IF transport and degradation converge.
Although commonly assumed, it remains to be proven that gigaxonin facilitates the ubiquitination of IFs. It is possible that axons are more vulnerable to gigaxonin mutations because the focal NF accumulations ‘cement’ other organelles, such as mitochondria26 and block axonal traffic. In fact, recent work shows that gigaxonin itself appears to be important for the trafficking of NFs27. In the absence of gigaxonin, kinesin-1 dependent NF and mitochondria transport mechanisms are impaired, while other kinesin-1 cargo can move normally27. Interestingly, pharmacologic inhibition of HDAC6, which deacetylates and destabilizes microtubules28, improves IF morphology and mitochondria transport along axons in GAN mice, suggesting that tubulin acetylation may also play a role in this process29. Currently, it is not known if the IF transport-related defects in GAN cells are related to the function of gigaxonin as an E3 ligase adaptor, or another role. Moreover, aside from gigaxonin, the collective molecular machinery dedicated to ensuring the stability of the NFs remains to be defined. One possibility is that accumulation of a non-filamentous pool of NFs in GAN cells leads to the formation of large IF structures that are no longer effectively transported or degraded27.
Long-lived IF fragments.
Recent work in GAN patient fibroblasts reveals that in the absence of gigaxonin, IFs are more prone to destabilization via cleavage by calpains 30. Calpain-generated fragments may be short-lived or long-lived, and may have functions that differ from the native protein. Depending on the amino acids that are exposed during cleavage, the stability of protein fragments can vary from a few seconds to more than 20 hours31. Specifically, Arg, Lys, His, Leu, Trp, Phe, Tyr, and Ile are destabilizing residues when exposed following cleavage, while Ser, Ala, Thr, Met, Val, and Gly are highly stabilizing31. Recent work reveals the presence of multiple NF-L fragments in human brain tissue32, and at least three of these fragments are predicted to be highly stable: 117VLEAELLVLR126, 324GMNEALEK331 (from central the rod domain) and 530VEGAGEEQAAK540 (from the C-terminal tail domain). Moreover, recent evidence suggests that a calpain-generated tail domain fragment of NF-L translocates to the nucleus and interacts with DNA following oxidative injury in neurons33. Therefore, it is possible that IF fragments acquire new functions that are otherwise suppressed in the context of a mature filament. Similar to NFs, destabilizing effects on the filament network are observed with a Ser-13 phospho-mimic head domain mutation on GFAP. However, this particular phospho-mimic mutation promotes caspase-6 mediated cleavage to form a ~24kDa fragment34 resulting from cleavage at caspase recognition motif VELD22535. This cleavage product is detectable in tissue from patients with Alexander Disease, which is caused by GFAP mutations, suggesting it is highly stable. Whether this GFAP fragment occurs as part of a filament severing process or at the level of the soluble non-filamentous protein is not known, but the studies on NFs raise the importance of examining the active transport of GFAP in astrocytes and their processes. Although this has not been done to date, new and improved tools to study astrocyte morphology, physiology and molecular mechanisms should pave the way for a better understanding of GFAP dynamics in resting and reactive astrocytes36, 37. Currently the fate and function of GFAP fragments generated by calpains and caspases is not known, but their presence in patients with CNS injury well documented35, 38.
Short-lived IF fragments.
The cleavage of a protein into two fragments results in the formation of a new C-terminus on one fragment and a new N-terminus on the other fragment (Fig. 2). The amino acids that define these newly exposed termini in short-lived fragments are called C-degrons or N-degrons, respectively39. A degron is a linear sequence motif that is the minimal segment required to facilitate an interaction between a protein target and the degradation machinery. N-degrons were described over three decades ago40, while C-degrons were discovered more recently41, 42. Many calpain-generated natural protein fragments are substrates for these degradation pathways43. It is assumed, though not proven explicitly, that these pathways always lead to the terminal destruction of a fragment via an ubiquitin-proteasome dependent system, previously known as the ‘N-end rule pathway’. Newly formed fragments containing N-degrons are recognized by specific E3 ubiquitin ligases. In mammalian cells, there are at least four such ligases (termed N-recognins): UBR1, UBR2, UBR4 and UBR5 (Fig. 2). Structural advances on UBR1 are providing new mechanistic insights into the process by which an N-degron is initially recognized by UBR1 to the mono- and poly-ubiquitination steps of this reaction44. Interestingly, HEK293 cells with a double UBR1 and UBR2 knockout have a near-complete loss of NF-L and NF-M proteins45, which are normally robustly expressed in the parental cell line, possibly due to their likely neuronal origin46. The changes at the NF protein level in the UBR1/2 knockout cells were independent of NEFL/NEFM mRNA expression45. Therefore, it appears that UBR1 and/or UBR2 ligases critically regulate NF-L and NF-M protein expression, but the mechanism remains to be defined – especially in neurons and in vivo. It is possible that absence of these ligases prevented regulated translation of NF mRNA, and/or stabilized a molecule that accelerated degradation of NFs. Curated data in the BioGrid repository47 from HEK293 cells reveal that human UBR1 and UBR2 have 201 and 153 unique interactors, respectively, but NFs were not among the interactors, which suggests a possible indirect mechanism.
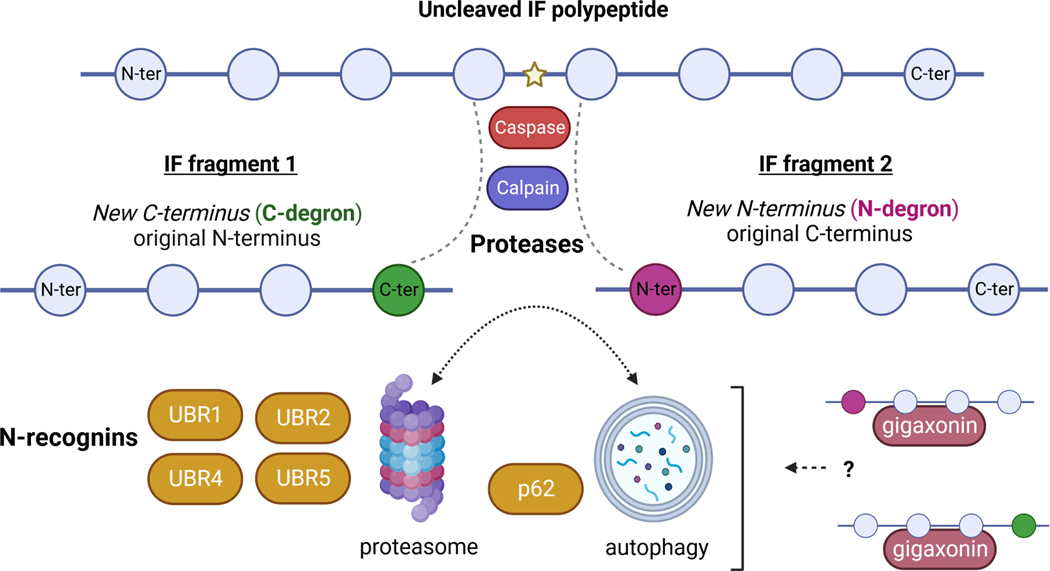
Figure 2: Intermediate filament (IF) fragments as potential substrates of the N-end rule pathway.
Schematic representation of proteolytic cleavage of an IF polypeptide (blue circles represent individual amino acids; blue line represents the peptide bond) by a cellular protease (e.g. caspase or calpain). This cleavage event (represented by the yellow star), results in the formation of two fragments: IF fragment 1, containing the original N-terminus (N-ter) and exposing a new C-terminal residue (green) and IF fragment 2, containing a newly exposed N-terminal residue (magenta) and the original C-terminus (C-ter). For short-lived fragments that are substrates for the N-end rule pathway, the new N-terminus and C-terminus are referred to as the N-degron and C-degron respectively. Degrons are short linear recognition motifs for specific E3 ubiquitin ligases, termed N-recognins, which can facilitate downstream fragment destruction via the proteasome or autophagy. Several proteasome-associated N-recognins have been identified in mammalian cells, including UBR1, UBR2, UBR4, and UBR5. Knockout of UBR1 and UBR2 leads to loss of NF-L and NF-M in cells, through unknown mechanisms. The protein p62, known to be associated with pathologic IF aggregates in diseased cells, acts as an N-recognin for autophagy. It is not presently known whether gigaxonin, which is considered to be an E3 ubiquitin ligase adaptor protein for IFs, may also be involved in the clearance of IF fragments through these pathways by associating with the various N-recognins in cells. Created with BioRender.com
N-degrons and links to developmental processes.
Interestingly, other IFs - including 17 keratin proteins, in addition to GFAP and vimentin– are interactors of UBR1 based on high throughput protein-protein interaction studies48. It is notable that mice with combined loss of UBR1 and UBR2 die in mid-gestation due to impaired neurogenesis marked by the reduced proliferation and migration of neuronal progenitors49. Studies to advance the role of these ligases on the proteostasis of different IFs (e.g. vimentin, nestin, peripherin,α-internexin) in developing neurons will shed insights into how the coordinated activities of the IF cytoskeleton support proper neuronal development50. In mature neurons, interactions between NFs and the N-degron pathway are likely to have functional consequences – perhaps beyond UBR ligases. For example, it was recently shown that NFs are degraded by autophagy in vivo51 and this may potentially involve p62, which is frequently associated with pathologic IF protein inclusions52 and was recently shown to function as an N-recognin regulating macroautophagy and autophagosome biogenesis53. Thus, interactions between IFs and the N-degron pathways are previously underappreciated mechanisms that could have significance in development, homeostasis and in disease.
Small IF fragments, large gaps between biology and disease.
With advances of precision biomarker technologies, serum and cerebrospinal fluid (CSF) levels of NFs and GFAP are now widely used as biomarkers for many neurological diseases54, 55. The clinical biomarker assays are based on antibody-based capture and detection. However, despite their rapid adoption in the clinic, the precise species of protein captured by these assays are generally not known. Recent work on NFs showed that the most commonly used assays detect NF-L fragments — not the full-length protein32. In Alzheimer patients’ brain, a C-terminal fragment of NF-L (not known yet if this is the same fragment shown to translocate to the nucleus33) correlated most strongly with disease activity32. Calpain-generated GFAP truncated products can also be detected in patients biofluids35, 56. Specifically, a larger GFAP fragment is detected in patients within the first 24 hours following traumatic brain injury35. GFAP products formed after cleavage by caspase-6 are also detected in Alexander Disease (AxD)34. Knockdown of the Gfap gene in a rat model of AxD with a translationally relevant human-like phenotype can prevent disease progression and reverse disease that has already started to occur57. Whether the toxic effects of GFAP in AxD are related to the mRNA transcript, the full-length protein, a pathogenic cleaved fragment, or another mechanism (e.g. GFAP mRNA splicing58) remains to be determined, but studies addressing these gaps will have direct translational relevance in evaluating disease progression and therapeutic outcomes in patients.
Conclusions.
IF proteins in cells of the nervous system contribute to major processes throughout early development and beyond. Resilient IF networks are constantly being formed, remodeled and re-shaped via post-translational and proteolytic mechanisms to adapt to cellular and physiologic conditions. We also cannot rule out that non-enzymatic mechanisms contribute to filament breakage and fragmentation – as suggested by in vitro reconstitution studies and theoretical modeling on vimentin59. Enzymatic processing of NFs and GFAP by cellular proteases, including calpains and caspases, has long been recognized to occur. Still unknown are the dynamics and functions of the IF fragments that are generated after cleavage. Clinical advances show that these fragments are present outside of cells — yet, how the circulating fragments are formed and released from neurons and astrocytes, and how they are related to the pathogenesis and progression of disease is poorly understood. There are also challenges and limitations regarding the clinical utility of NF and GFAP biomarker assays that stem from the dynamic nature of these proteins, their tendency to undergo extensive PTM processing in stress and disease, the lack of a ‘normal’ range standard established across large cohorts of human subjects, and lack of knowledge about how various factors like age, stress, physical activity and other lifestyle factors could affect the levels of IFs in the blood of CSF60. The magnitude of elevation relative to normal subjects and patients affected with other conditions is an important consideration as such values could differ dramatically and these levels can change over time and according to the disease stage. Deployment of novel tools and methods to address these biological and clinical questions will contribute fundamental insights that will advance the disease-related roles of NFs and GFAP.
Funding acknowledgments:
The investigators’ research is funded by NIH grants GM122741 (NIGMS Molecular Medicine T32 to C.P.), R21NS121578 and Hannah’s Hope Fund.
Natasha Snider reports financial support was provided by National Institutes of Health. Cassandra Phillips reports financial support was provided by National Institutes of Health. Diane Armao reports financial support was provided by National Institutes of Health. Natasha Snider reports financial support was provided by Hannah’s Hope Fund. Diane Armao reports financial support was provided by Hannah’s Hope Fund.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Disclosure: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bomont P, The dazzling rise of neurofilaments: Physiological functions and roles as biomarkers. Current Opinion in Cell Biology, 2021. 68: p. 181–191. [DOI] [PubMed] [Google Scholar]
- 2.Jones JR, Kong L, Hanna MG, Hoffman B, Krencik R, Bradley R, Hagemann T, Choi J, Doers M, and Dubovis M, Mutations in GFAP disrupt the distribution and function of organelles in human astrocytes. Cell reports, 2018. 25(4): p. 947–958. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang A-W, Lin N-H, Yeh T-H, Snider N, and Perng M-D, Effects of Alexander disease–associated mutations on the assembly and organization of GFAP intermediate filaments. Molecular Biology of the Cell, 2022. 33(8): p. ar69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viedma-Poyatos Á., González-Jiménez P, Pajares MA, and Pérez-Sala D, Alexander disease GFAP R239C mutant shows increased susceptibility to lipoxidation and elicits mitochondrial dysfunction and oxidative stress. Redox Biology, 2022. 55: p. 102415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou X, Lin Y, Kato M, Mori E, Liszczak G, Sutherland L, Sysoev VO, Murray DT, Tycko R, and McKnight SL, Transiently structured head domains control intermediate filament assembly. Proceedings of the National Academy of Sciences, 2021. 118(8): p. e2022121118. **This study by Zhou et al. showed that the N-terminal ‘head’ domain of NF-L forms labile cross-β strands that promote self-association and facilitate filament assembly in vitro. This finding challenges the prevailing view that the N-terminal domains on IFs function in the absence of structural order.
- 6.Kato M, Zhou X, and McKnight SL, How do protein domains of low sequence complexity work? RNA, 2022. 28(1): p. 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou X, Sumrow L, Tashiro K, Sutherland L, Liu D, Qin T, Kato M, Liszczak G, and McKnight SL, Mutations linked to neurological disease enhance self-association of low-complexity protein sequences. Science, 2022. 377(6601): p. eabn5582. *This study by Zhou et al. showed that disease-associated mutations in neurofilaments enhance the stability of otherwise labile molecular self-associations of the head domain.
- 8. Uchida A, Peng J, and Brown A, Regulation of neurofilament length and transport by a dynamic cycle of phospho-dependent polymer severing and annealing. Molecular Biology of the Cell, 2023. 34(7): p. ar68. **This study by Uchida et al. demonstrated that head domain phosphorylation on NF-L is associated with focal destabilization and severing of filaments. This has consequences on the rate at which NFs are transported along axons and whether they form focal accumulations.
- 9.Messing A. and Brenner M, GFAP at 50. ASN neuro, 2020. 12: p. 1759091420949680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gafson AR, Barthélemy NR, Bomont P, Carare RO, Durham HD, Julien J-P, Kuhle J, Leppert D, Nixon RA, and Weller RO, Neurofilaments: neurobiological foundations for biomarker applications. Brain, 2020. 143(7): p. 1975–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wizeman JW, Nicholas AP, Ishigami A, and Mohan R, Citrullination of glial intermediate filaments is an early response in retinal injury. Molecular Vision, 2016. 22: p. 1137. [PMC free article] [PubMed] [Google Scholar]
- 12.Palko SI, Saba NJ, Bargagna-Mohan P, and Mohan R, Peptidyl arginine deiminase 4 deficiency protects against subretinal fibrosis by inhibiting Müller glial hypercitrullination. Journal of neuroscience research, 2023. 101(4): p. 464–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Palko SI, Saba NJ, Mullane E, Nicholas BD, Nagasaka Y, Ambati J, Gelfand BD, Ishigami A, Bargagna-Mohan P, and Mohan R, Compartmentalized citrullination in Muller glial endfeet during retinal degeneration. Proceedings of the National Academy of Sciences, 2022. 119(9): p. e2121875119. **This study by Palko et al. show that GFAP is heavily citrullinated by the enzyme PAD4 in a compartmentalized manner in Müller glia (MG) in vivo in mouse models and human diseases of retinal degeneration. The compartmentalization of hyper-citrullinated GFAP may enable the cells to carry out their main functions even in the presence of significant stress.
- 14.Mondal S, Wang S, Zheng Y, Sen S, Chatterjee A, and Thompson PR, Site-specific incorporation of citrulline into proteins in mammalian cells. Nature communications, 2021. 12(1): p. 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yusuf IO, Qiao T, Parsi S, Tilvawala R, Thompson PR, and Xu Z, Protein citrullination marks myelin protein aggregation and disease progression in mouse ALS models. Acta Neuropathologica Communications, 2022. 10(1): p. 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan A, Rao MV, and Nixon RA, Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harbor perspectives in biology, 2017. 9(4): p. a018309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nowier RM, Friedman A, Brown A, and Jung P, The role of neurofilament transport in the radial growth of myelinated axons. Molecular Biology of the Cell, 2023. 34(6): p. ar58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyer NP, Julien J-P, Jung P, and Brown A, Neurofilament Transport Is Bidirectional In Vivo. eneuro, 2022. 9(4): p. ENEURO.0138–22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenn JD, Li Y, Julien J-P, Jung P, and Brown A, The Mobility of Neurofilaments in Mature Myelinated Axons of Adult Mice. eneuro, 2023. 10(3): p. ENEURO.0029–23.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hookway C, Ding L, Davidson MW, Rappoport JZ, Danuser G, and Gelfand VI, Microtubule-dependent transport and dynamics of vimentin intermediate filaments. Molecular biology of the cell, 2015. 26(9): p. 1675–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahammad S, Murthy SP, Didonna A, Grin B, Israeli E, Perrot R, Bomont P, Julien J-P, Kuczmarski E, and Opal P, Giant axonal neuropathy–associated gigaxonin mutations impair intermediate filament protein degradation. The Journal of clinical investigation, 2013. 123(5): p. 1964–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin N-H, Huang Y-S, Opal P, Goldman RD, Messing A, and Perng M-D, The role of gigaxonin in the degradation of the glial-specific intermediate filament protein GFAP. Molecular biology of the cell, 2016. 27(25): p. 3980–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bharucha-Goebel DX, Norato G, Saade D, Paredes E, Biancavilla V, Donkervoort S, Kaur R, Lehky T, Fink M, and Armao D, Giant axonal neuropathy: cross sectional analysis of a large natural history cohort. Brain, 2021. *The study by Bharucha-Goebel is the first natural history study describing a large cohort of patients with Giant Axonal Neuropathy (GAN). Neurons and astrocytes in GAN patients exhibit abnormal accumulations of NF and GFAP due to loss of function in the E3 ubiquitin ligase adaptor gigaxonin, which associates with IF proteins.
- 24.Battaglia R, Faridounnia M, Beltran A, Robinson J, Kinghorn K, Ezzell JA, Bharucha-Goebel D, Bonnemann C, Hooper JE, Opal P, Bouldin TW, Armao D, and Snider N, Intermediate filament dysregulation in astrocytes in the human disease model of KLHL16 mutation in giant axonal neuropathy (GAN). Mol Biol Cell, 2023: p. mbcE23030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bailey RM, Armao D, Kalburgi SN, and Gray SJ, Development of intrathecal AAV9 gene therapy for giant axonal neuropathy. Molecular Therapy-Methods & Clinical Development, 2018. 9: p. 160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Israeli E, Dryanovski DI, Schumacker PT, Chandel NS, Singer JD, Julien JP, Goldman RD, and Opal P, Intermediate filament aggregates cause mitochondrial dysmotility and increase energy demands in giant axonal neuropathy. Human molecular genetics, 2016. 25(11): p. 2143–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Renganathan B, Zewe JP, Cheng Y, Paumier JM, Kittisopikul M, Ridge KM, Opal P, and Gelfand VI, Gigaxonin is required for intermediate filament transport. The FASEB Journal, 2023. 37(5). *The study by Renganathan et al. demonstrated that gigaxonin is important in NF transport. This new mechanism sheds light on how gigaxonin mutations may lead to ‘giant’ focal axonal swellings with NF accumulations in patients with GAN.
- 28.Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D, Osada H, Komatsu Y, Nishino N, and Khochbin S, In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. The EMBO journal, 2002. 21(24): p. 6820–6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nath B, Phaneuf D, and Julien J-P, Axonal Transport Defect in Gigaxonin Deficiency Rescued by Tubastatin A. Neurotherapeutics, 2023: p. 1–14. *Nath et al. provide an extensive preclinical study showing that an HDAC6 inhibitor can rescue axonal transport and reduce NF accumulation in GAN, potentially by promoting tubulin acetylation.
- 30.Phillips CL, Fu D, Herring LE, Armao D, and Snider NT, Calpain-mediated proteolysis of vimentin filaments is augmented in giant axonal neuropathy fibroblasts exposed to hypotonic stress. Front Cell Dev Biol, 2022. 10: p. 1008542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bachmair A, Finley D, and Varshavsky A, In vivo half-life of a protein is a function of its amino-terminal residue. science, 1986. 234(4773): p. 179–186. [DOI] [PubMed] [Google Scholar]
- 32. Budelier MM, He Y, Barthelemy NR, Jiang H, Li Y, Park E, Henson RL, Schindler SE, Holtzman DM, and Bateman RJ, A map of neurofilament light chain species in brain and cerebrospinal fluid and alterations in Alzheimer’s disease. Brain communications, 2022. 4(2): p. fcac045. **The Budelier et al. study provides a comprehensive characterization of NF-L in brain tissue and CSF from Alzheimer Disease patients and healthy controls. The findings reveal that widely used clinical biomarker tests detect NF-L fragments (not the full-length protein) in the CSF, highlighting the need to understand the source and dynamics of such fragments in healthy and diseased brains.
- 33.Arsić A. and Nikić-Spiegel I, The tail domain of neurofilament light chain accumulates in neuronal nuclei during oxidative injury. bioRxiv, 2022: p. 2022.03. 03.481279. [Google Scholar]
- 34.Battaglia RA, Beltran AS, Delic S, Dumitru R, Robinson JA, Kabiraj P, Herring LE, Madden VJ, Ravinder N, and Willems E, Site-specific phosphorylation and caspase cleavage of GFAP are new markers of Alexander disease severity. Elife, 2019. 8: p. e47789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Z, Arja RD, Zhu T, Sarkis GA, Patterson RL, Romo P, Rathore DS, Moghieb A, Abbatiello S, and Robertson CS, Characterization of calpain and caspase-6-generated glial fibrillary acidic protein breakdown products following traumatic brain injury and astroglial cell injury. International Journal of Molecular Sciences, 2022. 23(16): p. 8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu X, Nagai J, and Khakh BS, Improved tools to study astrocytes. Nature Reviews Neuroscience, 2020. 21(3): p. 121–138. [DOI] [PubMed] [Google Scholar]
- 37.Escartin C, Galea E, Lakatos A, O’Callaghan JP, Petzold GC, Serrano-Pozo A, Steinhäuser C, Volterra A, Carmignoto G, and Agarwal A, Reactive astrocyte nomenclature, definitions, and future directions. Nature neuroscience, 2021. 24(3): p. 312–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jonesco DS., Hassager C, Frydland M, Kjærgaard J, Karsdal M, and Henriksen K, A caspase-6-cleaved fragment of glial fibrillary acidic protein as a potential serological biomarker of CNS injury after cardiac arrest. Plos one, 2019. 14(11): p. e0224633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Varshavsky A, N-degron and C-degron pathways of protein degradation. Proceedings of the National Academy of Sciences, 2019. 116(2): p. 358–366. *A. Varshavsky provides a comprehensive overview of the N-degron and C-degron pathways, including a historic perspective and proposed nomenclature changes to reflect novel discoveries.
- 40.Johnson ES, Gonda DK, and Varshavsky A, Cis-trans recognition and subunit-specific degradation of short-lived proteins. Nature, 1990. 346(6281): p. 287–291. [DOI] [PubMed] [Google Scholar]
- 41.Koren I, Timms RT, Kula T, Xu Q, Li MZ, and Elledge SJ, The eukaryotic proteome is shaped by E3 ubiquitin ligases targeting C-terminal degrons. Cell, 2018. 173(7): p. 1622–1635. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin H-C, Yeh C-W, Chen Y-F, Lee T-T, Hsieh P-Y, Rusnac DV, Lin S-Y, Elledge SJ, Zheng N, and Yen H-CS, C-terminal end-directed protein elimination by CRL2 ubiquitin ligases. Molecular cell, 2018. 70(4): p. 602–613. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piatkov KI, Oh J-H, Liu Y, and Varshavsky A, Calpain-generated natural protein fragments as short-lived substrates of the N-end rule pathway. Proceedings of the National Academy of Sciences, 2014. 111(9): p. E817–E826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pan M, Zheng Q, Wang T, Liang L, Mao J, Zuo C, Ding R, Ai H, Xie Y, and Si D, Structural insights into Ubr1-mediated N-degron polyubiquitination. Nature, 2021. 600(7888): p. 334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vu TT, Mitchell DC, Gygi SP, and Varshavsky A, The Arg/N-degron pathway targets transcription factors and regulates specific genes. Proceedings of the National Academy of Sciences, 2020. 117(49): p. 31094–31104. **Vu et al. conducted a proteomic profiling experiment to examine the substrates for the N-recognins UBR1 and UBR2. A striking finding is the complete disappearance of neurofilaments when these two enzymes are knocked out. The mechanism for this is presently not known.
- 46.Shaw G, Morse S, Ararat M, and Graham FL, Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. The FASEB journal, 2002. 16(8): p. 869–871. [DOI] [PubMed] [Google Scholar]
- 47.Oughtred R, Rust J, Chang C, Breitkreutz BJ, Stark C, Willems A, Boucher L, Leung G, Kolas N, and Zhang F, The BioGRID database: A comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Science, 2021. 30(1): p. 187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huttlin EL, Bruckner RJ, Navarrete-Perea J, Cannon JR, Baltier K, Gebreab F, Gygi MP, Thornock A, Zarraga G, and Tam S, Dual proteome-scale networks reveal cell-specific remodeling of the human interactome. Cell, 2021. 184(11): p. 3022–3040. e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.An JY, Seo JW, Tasaki T, Lee MJ, Varshavsky A, and Kwon YT, Impaired neurogenesis and cardiovascular development in mice lacking the E3 ubiquitin ligases UBR1 and UBR2 of the N-end rule pathway. Proceedings of the National Academy of Sciences, 2006. 103(16): p. 6212–6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bott CJ and Winckler B, Intermediate filaments in developing neurons: Beyond structure. Cytoskeleton, 2020. 77(3–4): p. 110–128. [DOI] [PubMed] [Google Scholar]
- 51.Rao MV, Darji S, Stavrides PH, Goulbourne CN, Kumar A, Yang D-S, Yoo L, Peddy J, Lee J-H, and Yuan A, Autophagy is a novel pathway for neurofilament protein degradation in vivo. Autophagy, 2022: p. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zatloukal K, Stumptner C, Fuchsbichler A, Heid H, Schnoelzer M, Kenner L, Kleinert R, Prinz M, Aguzzi A, and Denk H, p62 Is a common component of cytoplasmic inclusions in protein aggregation diseases. The American journal of pathology, 2002. 160(1): p. 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cha-Molstad H, Yu JE, Feng Z, Lee SH, Kim JG, Yang P, Han B, Sung KW, Yoo YD, and Hwang J, p62/SQSTM1/Sequestosome-1 is an N-recognin of the N-end rule pathway which modulates autophagosome biogenesis. Nature communications, 2017. 8(1): p. 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, Barro C, Kappos L, Comabella M, and Fazekas F, Neurofilaments as biomarkers in neurological disorders. Nature Reviews Neurology, 2018. 14(10): p. 577–589. [DOI] [PubMed] [Google Scholar]
- 55.Abdelhak A, Foschi M, Abu-Rumeileh S, Yue JK, D’Anna L, Huss A, Oeckl P, Ludolph AC, Kuhle J, and Petzold A, Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nature Reviews Neurology, 2022. 18(3): p. 158–172. [DOI] [PubMed] [Google Scholar]
- 56.Yang Z. and Wang KK, Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker. Trends in neurosciences, 2015. 38(6): p. 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hagemann TL, Powers B, Lin N-H, Mohamed AF, Dague KL, Hannah SC, Bachmann G, Mazur C, Rigo F, and Olsen AL, Antisense therapy in a rat model of Alexander disease reverses GFAP pathology, white matter deficits, and motor impairment. Science translational medicine, 2021. 13(620): p. eabg4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Helman G, Takanohashi A, Hagemann TL, Perng MD, Walkiewicz M, Woidill S, Sase S, Cross Z, Du Y, and Zhao L, Type II Alexander disease caused by splicing errors and aberrant overexpression of an uncharacterized GFAP isoform. Human mutation, 2020. 41(6): p. 1131–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tran QD, Sorichetti V, Pehau-Arnaudet G, Lenz M, and Leduc C, Fragmentation and Entanglement Limit Vimentin Intermediate Filament Assembly. Physical Review X, 2023. 13(1): p. 011014. [Google Scholar]
- 60.Yuan A. and Nixon RA, Neurofilament proteins as biomarkers to monitor neurological diseases and the efficacy of therapies. Frontiers in neuroscience, 2021. 15: p. 689938. [DOI] [PMC free article] [PubMed] [Google Scholar]