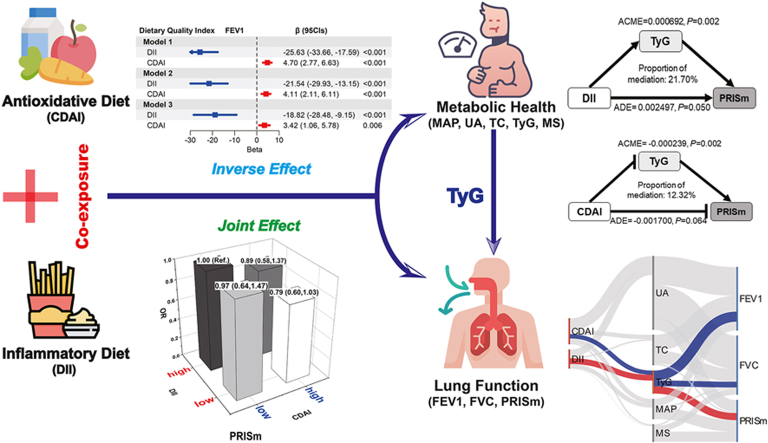
Abstract
Background
Previous studies have shown that inflammatory and antioxidant dietary patterns can modify the risk of COPD, yet few studies have examined the association of these diets with its early signs (PRISm), and the potential role of metabolic disorders remains to be elucidated.
Methods
Data from 9529 individuals who participated in the 2007–2012 National Health and Nutrition Examination Survey (NHANES) were analyzed. The Dietary Inflammation Index (DII) and the Dietary Antioxidant Composite Index (CDAI) were assessed using 24-h dietary recall, multiple metabolic indicators were calculated according to biochemical markers, and lung function parameters defined PRISm cases. Individual and joint effects of DII and CDAI were evaluated by generalized linear models and binary logistic regression models, and mediation effects of metabolic indicators were further explored by causal mediation analysis.
Results
Increased DII was associated with decreased lung function (FEV1: β = −18.82, FVC: β = −29.2; OR = 1.04) and increased metabolic indicators (β = 0.316, 0.036, 0.916, 0.033, and 0.145 on MAP, UA, TC, TyG, and MS, respectively). Contrary to this, CDAI were positively and negatively associated with lung function (FEV1: β = 3.42; FVC: β = 4.91; PRISm: OR = 0.99) and metabolic indicators (β < 0), respectively. Joint effects of DII and CDAI indicated the minimal hazard effects of DII on TyG (β = −0.11), FEV1 (β = 72.62), FVC (β = 122.27), and PRISm (OR = 0.79) in subjects with high CDAI when compared with those with low CDAI (low DII + high CDAI vs. high DII + low CDAI). Furthermore, TyG mediated 13.74 %, 8.29 %, and 21.70 % of DII- and 37.30 %, 20.90 %, and 12.32 % of CDAI-FEV1, -FVC, and -PRISm associations, respectively.
Conclusions
These findings indicated that CDAI can attenuate the adverse effects of DII on metabolic disorders and lung function decline, which provides new insight for diet modification in preventing early lung dysfunction.
Keywords: Dietary quality, Preserved ratio impaired spirometry, Triglyceride-glucose index, Mediation effects
Graphical abstract
Highlights
-
•
Whether CDAI attenuate DII induced metabolic disorders and subsequent PRISm remains unknown.
-
•
Both DII and CDAI are associated with metabolic indicators and lung function parameters but in a reversed direction.
-
•
DII and CDAI play joint effects on metabolic disorders, lung function parameters and PRISm.
-
•
TyG mediated the associations of DII and CDAI with FEV1, FVC, and PRISm.
Abbreviations
- NHANES
National Health and Nutrition Examination Survey
- DII
Dietary Inflammation Index
- CDAI
Composite Dietary Antioxidant Index
- PRISm
Preserved Ratio Impaired Spirometry
- TyG
Triglyceride-Glucose
- MAP
Mean Arterial Pressure
- UA
Uric Acid
- TC
Total Cholesterol
- MS
Metabolic Score
- IR
insulin resistance
- FEV1
Forced Expiratory Volume In 1 Second
- FVC
Forced Vital Capacity
- CRDs
Chronic Respiratory Diseases
- TAC
Total Antioxidant Capacity
- AGEs
Advanced Glycation End-products
- RAGE
The Receptor For Advanced Glycation End-products
- FFA
Free Fatty Acid
1. Introduction
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide, which has resulted in 3.23 million deaths in 2019, imposing a heavy burden on healthcare systems [1]. Given COPD is almost irreversible deterioration, early intervention is key to potentially preventing its onset or progression [2]. Preserved Ratio Impaired Spirometry (PRISm), is characterized by a reduction in both forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC), yet with a maintained FEV1/FVC ratio [3]. Recent studies have suggested that PRISm could be a precursor to COPD [4], and its reversibility is particularly important [5]. Therefore, prevention and early intervention of PRISm are of considerable significance in the management of COPD. However, most of the previous studies were mainly focused on the effects of traditional environmental factors (e.g., smoking and chemical exposure) [5,6], more research is needed to identify other potential risk factors, as well as the protective factors for PRISm.
Inflammation is a key mechanism in lung dysfunction [7], it has been shown that a variety of nutrients with anti- (e.g., vitamin D, omega-3 fatty acids) and pro-inflammatory properties (e.g., saturated fatty acids) have been linked to lung health [8]. The Dietary inflammatory index (DII), a scientifically validated index, assesses dietary inflammation by aggregating intakes of diverse nutrients with distinct anti- or pro-inflammatory properties [9]. Some studies observed that high levels of DII may hazard pulmonary health (e.g., increased risk of COPD and asthma) [[10], [11], [12]]. Regrettably, few studies have evaluated the relationship between DII and PRISm. Additionally, oxidative imbalance is another major mechanism causing lung injury [13]. Diet can change the levels of oxidative stress and consequently affect lung health, and a variety of dietary antioxidants (e.g., vitamins C, E, and plant active substances) exert positive effects on lung function [14]. The composite dietary antioxidant index (CDAI) is a validated composite score, derived from the cumulative intake of multiple dietary antioxidants, and effectively mirroring the dietary antioxidant intake level [15]. Although a negative association between CDAI and the prevalence of chronic respiratory disease including emphysema and chronic bronchitis was reported [16], whether CDAI plays protective impacts on PRISm remained to be investigated.
Diseased pulmonary microvasculature and lung stroma are shown in the subjects with metabolic disorders [17], since normal cellular function in the lung relies on metabolic activity and homeostasis. There was evidence indicating that metabolic diseases including hyperuricemia, hypercholesterolemia, and insulin resistance (IR) might affect pulmonary wellness [18]. For example, IR is a state of diminished response to the normal action of insulin that has a correlation with asthma, pulmonary fibrosis, and FEV1 [19]. Total cholesterol (TC) and uric acid (UA) are used as biomarkers for determining the presence of hyperlipidemia and hyperuricemia, respectively [20]. Mean arterial pressure (MAP) can be used as a biomarker of hypertension by integrating systolic and diastolic blood pressure data into a single measurement, thus overcoming the covariance challenges that can arise from using both blood pressures in a statistical model [21]. The construction of metabolic scores (MS) facilitates the uncovering of those with abnormalities in their overall metabolic levels [22]. Of particular interest is that triglyceride-glucose (TyG) index is a clinically useful surrogate that is better than glucose and triglyceride values alone in assessing metabolic dysfunction. And TyG index is more accurate than the homeostatic model assessment of insulin resistance (HOMA-IR) in the diagnosis of metabolic syndrome [23]. Given the advantages of these biochemical indicators and the combined parameters (e.g., MAP, UA, TC, TyG, and MS) in accessibility and availability for the general population, they are usually selected as metabolic dysfunction indicators [20,22]. Currently, the TyG index has been shown to be strongly associated with respiratory symptoms, chronic bronchitis, and restrictive spirometry pattern [24], but its relationship to the PRISm warrants further insights.
Diet quality plays a critical role in the regulation and prevention of metabolic disorders. There were studies reported that a high level of DII was associated with an increased risk of metabolic syndrome [25,26], because chronic inflammation is usually accompanied by metabolic disorders [27]. In contrast, enhanced dietary antioxidant intake induced positive impacts on metabolic function [28]. Moreover, inflammation and oxidative stress are mutually reinforcing, with inflammation increasing ROS production and ROS exacerbating inflammation [29]. These studies evidence the necessity to reveal the joint effect of DII and CDAI on metabolic dysfunction. Moreover, considering the relations between metabolic disorders and lung health, a hypothesis is put forward that there are intercorrelations between DII, CDAI, and PRISm. Particularly, the underlying mediation effects of metabolic dysfunction are yet to be explored.
In the current study, we used the data from a representative sample of the US National Health and Nutrition Examination Survey (NHANES) to elucidate the associations between dietary quality (DII and CDAI co-exposure), metabolic dysfunction indicators (MAP, UA, TC, TyG, and MS), lung function parameters and PRISm; subsequently, to assess the joint effects of co-exposure to DII and CDAI; and further to elucidate the mediation roles of metabolic disorder on dietary quality-lung function associations.
2. Methods
2.1. Study population
NHANES is an ethically approved, large-scale, cross-sectional study in which a representative sample of the US population is selected through scientific sampling methods to complete a health examination and questionnaire. In this study, we used data collected from three consecutive cycles (2007–2008, 2009–2010, and 2011–2012) with available lung function information. A total of 30,442 participants were included, and our analyses were limited to 9529 participants. The exclusion criteria were the following: 1) age <20 years old (n = 12,729), or pregnant women (n = 180); 2) without spirometry examination or quality below graded B (n = 6037); 3) with airflow obstruction (FEV1/FVC <0.70, n = 1448); 4) no available or reliable dietary information (n = 465) or had significant abnormal energy intake (total energy intake >5000 kcal/d for women and >8000 kcal/d for men, or < 500 kcal/d, n = 28); 5) [30,31] had missing data on height (n = 26) (eFig. 1).
Fig. 1.
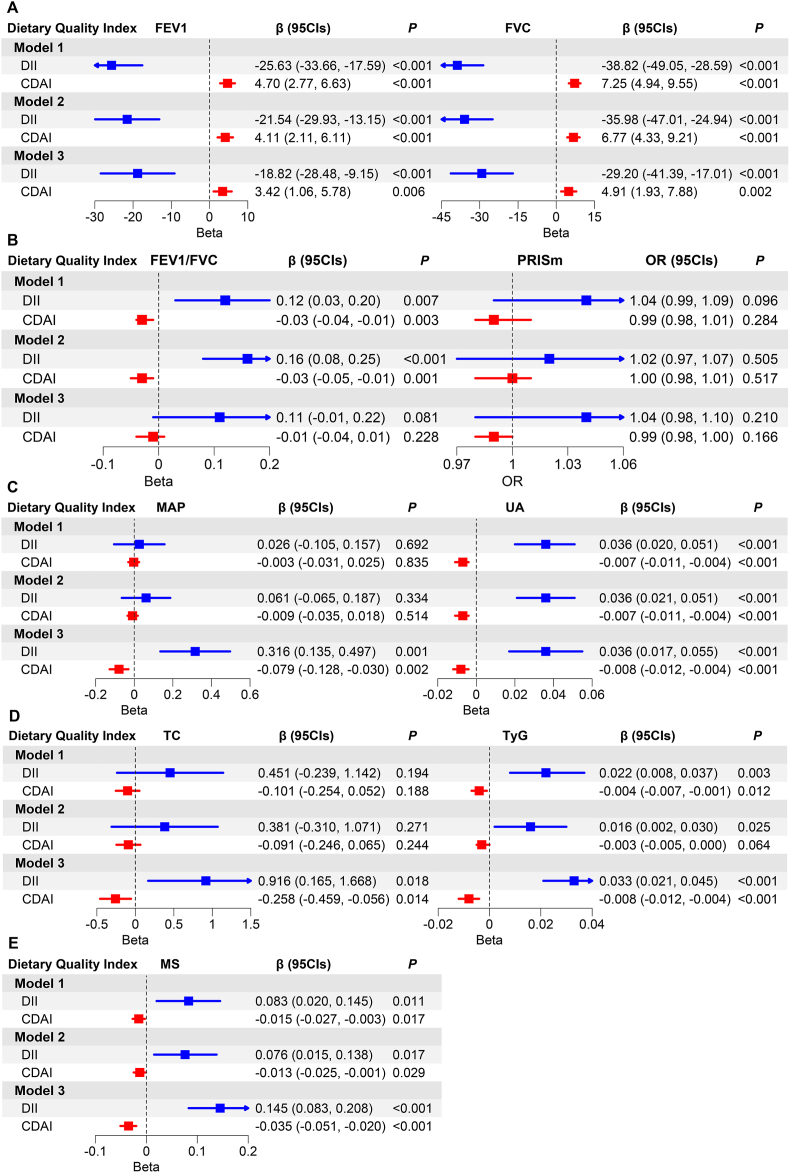
Associations of dietary quality index with lung function (A, B) and metabolic indicators (C, D, E).
Note: The blue and red dots and lines represent the point estimates (β or OR) and 95 % confidence intervals (CIs) of DII and CDAI on lung function and metabolic indicators. The arrows indicate that the 95%CIs exceed the plot limit. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.2. Dietary inflammatory index (DII)
NHANES collects dietary information at Mobile Examination Centers (MECs) through 24-h recall interviews. This study estimated the nutrient intake of participants, excluding supplements and meds, via the mean of two reliable 24-h recall intakes, or a single recall when only one was deemed reliable. DII is defined as a scoring system developed by Shivappa [9] that includes 45 dietary parameters, even if the number of nutrients used to calculate DII is < 30, the DII score is still available. Due to the limitation of the types of dietary components investigated in NHANES, 27 components were selected for the calculation of DII in this study, including carbohydrates, protein, total fat, fiber, cholesterol, saturated fatty acids, monounsaturated fatty acids, polyunsaturated fatty acids, n-3 fatty acids, n-6 fatty acids, vitamin C, vitamin A, carotene, vitamin D, vitamin E, niacin, thiamine, vitamin B2, vitamin B6, vitamin B12, folic acid, iron, magnesium, zinc, selenium, caffeine, and alcohol. The n-3 fatty acids contain linolenic acid (18:3), stearic acid (18:4), eicosapentaenoic acid (20:5), docosapentaenoic acid (22:5) and docosahexaenoic acid (22:6). The n-6 fatty acids contain linoleic acid (18:2) and arachidonic acid (20:4) [32]. DII was calculated using the following equation [9]:
2.3. Composite dietary antioxidant index (CDAI)
CDAI was assessed according to the method developed by Wright [15] with minor modification, which covered six minerals and vitamins including selenium, zinc, vitamins A, C, E, and carotenoids in food. CDAI was calculated using the following equations:
2.4. Measurements of lung function parameters and PRISm definition
Spirometry measurement was conducted at the MEC, and detailed methods have been described elsewhere [33]. Lung function parameters FEV1 and FVC were obtained from the NHANES SPX dataset. The predicted values for various spirometry parameters were calculated using the NHANES III equations [34]. PRISm cases are defined as FEV1/FVC ≥0.7 and FEV1% < 80 % [3]. Additionally, FEV1 and FVC were also considered as the important indicators of lung function in this study [35,36].
2.5. Metabolic dysfunction indicators
Blood pressure measurements were performed at the MEC. Fasting plasma glucose, triglyceride, TC, and UA levels were measured enzymatically by using an autoanalyzer. Detailed assay protocols are available on the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm/). MAP is diastolic BP + 1/3 × (systolic - diastolic) [20,21]. TyG index calculated as ln (fasting triglycerides [mg/dl] × fasting glucose [mg/dl]/2). In addition, we constructed a metabolic score (MS) as the sum of z-converted values of four factors including TC, UA, MAP, and TyG [21].
2.6. Covariates
Based on the previous literature, factors that had been proven to be correlated with lung function and dietary quality were included. Including demographic characteristics including age, race, educational attainment, household income (expressed as the poverty-income ratio), Body Mass Index (BMI), serum cotinine levels, smoking status, and physical activity status. Participants were further categorized into three income groups based on their income-to-poverty ratio (PIR): low-income earners (with PIR <1), middle-income earners (with PIR ≥1 and <4), and high-income earners (with PIR ≥4). Other covariates included serum cotinine, smoking status (smoking ≥100 cigarettes in life or not), and exercise status (high level of physical activity is defined as > 600 MET-min/week, and light physical activity is defined as ≤ 600 MET-min/week) [37,38]. Since exposure to hazardous dust and fumes in the workplace is associated with reduced lung function, those who claimed to have been exposed to mineral dust, organic dust, exhaust fumes, or other fumes at work were defined as having occupational factors exposure or not in this study.
2.7. Statistical analysis
All analyses conducted in this study adhered to the standards outlined in the CDC guidelines, utilizing the recommended weighting scheme. The variance test was used for normally distributed data and the Kruskal-Wallis test was used for skewed data. Categorical variables were tested using the Chi-square test. Spearman correlation analyses were used in correlation analyses between dietary quality index, metabolic indicators, and lung function parameters.
Generalized linear models (GLM) were used for evaluating the relationships between dietary index (DII and CDAI), metabolism indicators (MAP, UA, TC, TyG, and MS), and lung function parameters (FEV1 and FVC). Binary logistic regression analyses were performed to calculate the odds ratios (OR) and 95 % confidence intervals (CIs) for dietary indices and metabolism indicators on PRISm. Three models were performed: Model 1 was adjusted for sociodemographic covariates including gender, age, BMI, ethnicity, and economic level; Model 2 was further adjusted for serum cotinine levels and exercise status; Model 3 additionally adjusted for mean energy consumption. Notably, alcohol intake was not adjusted because it was included in calculating DII.
To assess the joint effects between DII and CDAI on metabolic indicators and lung function, DII and CDAI were individually divided into two groups by a cut-off value of 0 based on an index calculation, where the scores of 0 means that the intake of each dietary component is exactly the same as the global per capita level. Subsequently, pairwise combinations were performed and induced four subgroups including high DII + low CDAI, high DII + high CDAI, low DII + high CDAI, and low DII + low CDAI. Joint effects of co-exposure to DII and CDAI in the latter three subgroups were assessed with the first subgroups as the reference, with adjustment for the covariates in model 3.
The cause mediation analysis was conducted to explore the mediation effects of metabolic indicators on the DII- and CDAI-lung function associations. Among selected metabolic indicators, only the TyG index was significantly associated with lung function parameters and PRISm. Therefore, the mediating effect of the TyG index on the relationships of DII and CDAI with lung function was evaluated. In mediation analysis, two models were constructed: a mediator model refers to the GLM for evaluating the relationships between DII and CDAI (the exposure variable) and TyG index (the mediator variable); an outcome model refers to the GLM and logistic regression model including both the exposure and mediator variable for assessing their effects on lung function parameters and PRISm (the outcome variable), respectively. The magnitude of the mediating effect was quantified by deriving a mediation percentage, which is calculated as the proportion of the indirect effect to the total effect. The significance of the mediating effect was tested using Bootstrap sampling (times = 1000) [39]. Given the interrelationship between dietary quality, metabolic dysfunction, and lung function, Sankey diagrams were further used to illustrate these relationships. In addition, considering the influence of important confounding factors from occupational exposures, the sensitivity analyses of additionally adjusted for occupational exposure (whether exposure to hazardous dusts and fumes in the workplace) were performed in GLM and binary logistic regression models.
Statistical analyses were performed using the R statistical package (version 4.3.2) with a significance threshold of 2-sided P < 0.05. GLM, mediation analysis, and Sankey diagram were operated with the “survey”, “mediation”, and “networkD3” packages, respectively.
3. Result
3.1. Characteristics of participants and distributions of dietary quality index, metabolic indicators, and lung function parameters by PRISm status
Among the 9529 study subjects, 1385 (14.5 %) were PRISm cases. The weighted prevalence of PRISm differed significantly with respect to age, BMI, ethnicity, PIR, serum cotinine, drinking status, average energy intake, dietary indices, metabolic indicators (except TC), and lung function (all P < 0.05). Higher DII [0.86 (−0.75, 2.08) vs. 0.37 (−1.21, 1.76)] and metabolic indicators (except TC) but lower CDAI [−2.64 (−6.38, 3.41) vs. −1.21 (−5.82, 4.84)] and lung function parameters (FEV1: 2432.5 vs. 3430.5; FVC: 3124.2 vs. 4270.6; FEV1/FVC: 78.1 % vs. 80.5 %) were shown in the PRISm cases than controls (Table 1).
Table 1.
Characteristics of participants and distributions of dietary quality index, metabolic indicators, and lung function parameters by PRISm status.
| Characteristics | Total (N = 9529) | PRISm status |
Pa | |
|---|---|---|---|---|
| Controls (N = 8144) | Cases (N = 1385) | |||
| General characteristics | ||||
| Gender, n (%) | 0.443 | |||
| Male | 4548 (47.7) | 3920 (48.1) | 628 (45.3) | |
| Female | 4981 (52.3) | 4224 (51.9) | 757 (54.7) | |
| Age (years) | 43.1 ± 14.7 | 42.7 ± 14.7 | 46.7 ± 14.6 | <0.001 |
| Height (cm) | 169.0 ± 9.9 | 169.1 ± 9.9 | 168.5 ± 10.1 | 0.180 |
| BMI (kg/m2) | 28.9 ± 6.7 | 28.6 ± 6.4 | 31.6 ± 8.5 | <0.001 |
| Ethnic, n (%) | <0.001 | |||
| Mexican American | 1638 (17.2) | 1555 (19.1) | 83 (6.0) | |
| Other Hispanic | 1069 (11.2) | 977 (12.0) | 92 (6.6) | |
| Non-Hispanic White | 4042 (42.4) | 3736 (45.9) | 306 (22.1) | |
| Non-Hispanic Black | 1976 (20.7) | 1232 (15.1) | 744 (53.7) | |
| Other Race-Including Multi-Racial | 804 (8.4) | 644 (7.9) | 160 (11.6) | |
| Income-to-poverty ratio, n (%) | <0.001 | |||
| Low income | 1820 (20.7) | 1507 (20.1) | 313 (24.5) | |
| Middle income | 4452 (50.7) | 3779 (50.4) | 673 (52.7) | |
| High income | 2507 (28.6) | 2216 (29.5) | 291 (22.8) | |
| Smoking status, n (%) | 0.300 | |||
| Non-smokers | 5541 (74.2) | 4787 (74.1) | 754 (74.4) | |
| Smokers | 1931 (25.8) | 1671 (25.9) | 260 (25.6) | |
| Serum cotinine, ng/mL | 0.04 (0.01, 4.09) | 0.04 (0.01, 2.34) | 0.10 (0.03, 98.25) | <0.001 |
| Drinking status, n (%) | <0.001 | |||
| Non-drinkers | 2262 (25.1) | 1837 (23.8) | 425 (32.6) | |
| Drinkers | 6758 (74.9) | 5879 (76.2) | 879 (67.4) | |
| Exercise status, n (%) | <0.001 | |||
| Light physical activity | 3498 (36.7) | 2848 (35.0) | 650 (46.9) | |
| High level of physical activity | 6031 (63.3) | 5296 (65.0) | 735 (53.1) | |
| Average energy intake (kJ) | 2143.1 ± 846.5 | 2150.3 ± 844.8 | 2078.7 ± 858.7 | 0.070 |
| Dietary quality index | ||||
| DII | 0.42 (−1.17, 1.79) | 0.37 (−1.21, 1.76) | 0.86 (−0.75, 2.08) | <0.001 |
| CDAI | −1.35 (−5.89, 4.59) | −1.21 (−5.82, 4.84) | −2.64 (−6.38, 3.41) | 0.001 |
| Metabolic indicators | ||||
| MAP, mm Hg | 87.48 ± 10.86 | 87.22 ± 10.58 | 89.76 ± 12.84 | <0.001 |
| Uric acid, mg/dL | 5.41 ± 1.37 | 5.38 ± 1.36 | 5.68 ± 1.47 | <0.001 |
| Total cholesterol, mg/dL | 197.49 ± 40.44 | 197.73 ± 40.23 | 195.24 ± 42.22 | 0.191 |
| TyG index | 8.63 ± 0.63 | 8.62 ± 0.62 | 8.79 ± 0.73 | <0.001 |
| MS | 0.68 ± 2.38 | 0.62 ± 2.35 | 1.24 ± 2.55 | <0.001 |
| Lung function parameters | ||||
| FEV1, mL | 3329.7 ± 866.5 | 3430.5 ± 829.4 | 2432.5 ± 644.5 | <0.001 |
| FVC, mL | 4154.8 ± 1062.3 | 4270.6 ± 1021.8 | 3124.2 ± 837.0 | <0.001 |
| FEV1/FVC, % | 80.2 ± 5.4 | 80.5 ± 5.3 | 78.1 ± 5.2 | <0.001 |
Note: PRISm, preserved ratio impaired spirometry; BMI, body mass index; DII, dietary inflammation index; CDAI, composite dietary antioxidant index; MAP, mean arterial pressure; TyG, triglyceride-glucose; MS, metabolic score; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s.
Continuous variable with a normal or skewed distribution was presented as mean ± SD or median (25th-75th percentile), and categorical variables were presented as numbers (percentages). Variables between groups were compared by variance test, Kruskal-Wallis test, or Chi-square test.
3.2. Correlations between dietary quality index, metabolism indicators, and lung function parameters
For all studied subjects, analyses of the correlation between dietary indices, metabolism, and lung function parameters indicated negative correlations between DII and FEV1 (r = −0.23) and FVC (r = −0.25) (eFig. 2), whereas positive correlations between CDAI and both FEV1 (r = 0.27) and FVC (r = 0.28). Almost all metabolic indicators (except UA) showed negative correlations with FEV1 and FVC. Similar correlations were observed in PRISm cases and controls (eFig. 2).
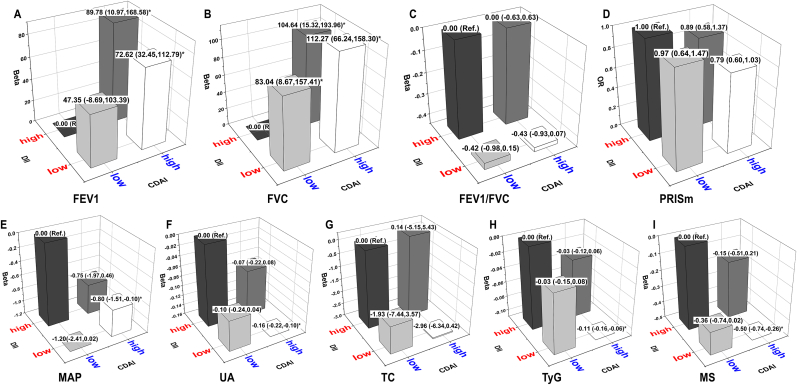
Fig. 2.
Joint effects of DII and CDAI on lung function (A–D) and metabolic indicators (E–I).
Note: Black, dark grey, light grey, and white prisms indicate 4 subgroups of DII and CDAI with different pairwise combinations, including high DII + low CDAI, high DII + high CDAI, low DII + low CDAI, and low DII + high CDAI, respectively. The height of the prism represents the effect value (β or OR and its 95 % confidence intervals) for each subgroup, and asterisks indicate the association is statistically significant.
3.3. Associations of DII, CDAI, and metabolic indicators with lung function
Both DII and CDAI were associated with lung function parameters (FEV1 and FVC) across Model 1 to Model 3 (both P < 0.05) (Fig. 1A), but in different directions. In Model 3, DII were negatively associated with FEV1 and FVC [β (95CIs) = −18.82 (−28.48, −9.15) and −29.20 (−41.39, −17.01), respectively]. CDAI had a positive association with both FEV1 and FVC [β (95CIs) = 3.42 (1.06, 5.78) and 4.91 (1.93, 7.88), respectively]. Meanwhile, DII and CDAI have nominally reversed effects on FEV1/FVC ratio and PRISm [β on FEV1/FVC = 0.11 vs. −0.01; OR on PRISm = 1.04 vs. 0.99, Fig. 1B).
All metabolic indicators including MAP, UA, TC, TyG, and MS had significant relationships with DII and CDAI (all P < 0.05) in Model 3 (Fig. 1C, D, and 1E). In brief, DII had positive associations with metabolic indicators (β on MAP, UA, TC, TyG, and MS is 0.316, 0.036, 0.916, 0.033, and 0.145, respectively). In contrast, CDAI had negative associations with them (β on MAP, UA, TC, TyG, and MS is −0.079, −0.008, −0.258, −0.008, and −0.035, respectively).
3.4. Relationships between metabolic dysfunction indicators and lung function
Of all the metabolic dysfunction indicators, only TyG index maintained significant relationships with lung function across three models [Model 3: FEV1, β (95CIs) = −89.85 (−129.30, −50.40); FVC, β (95CIs) = −109.23 (−163.28, −55.18); PRISm, OR (95CIs) = 1.44 (1.16, 1.78)]. In addition, MAP had a weak association with FEV1 and FVC [β (95CIs) = 1.68 (0.05, 3.32) and 2.52 (0.59, 4.44), respectively] in Model 3 (Table 2).
Table 2.
Associations of metabolic indicators with lung function parameters and PRISm.
| Metabolic indicators | FEV1 |
FVC |
FEV1/FVC |
PRISm |
||||
|---|---|---|---|---|---|---|---|---|
| β (95CIs) | P | β (95CIs) | P | β (95CIs) | P | OR (95CIs) | P | |
| Crude | ||||||||
| MAP | −1.51 (−4.26, 1.25) | 0.277 | 1.53 (−1.85, 4.90) | 0.368 | −0.07 (−0.08, −0.05) | <0.001 | 1.02 (1.01,1.03) | < 0.001 |
| Uric acid | 160.50 (142.86, 178.13) | <0.001 | 214.75 (192.76, 236.75) | <0.001 | −0.30 (−0.40, −0.20) | <0.001 | 1.17 (1.10,1.24) | < 0.001 |
| Cholesterol | −2.71 (−3.40, −2.01) | <0.001 | −2.49 (−3.38, −1.59) | <0.001 | −0.02 (−0.02, −0.01) | <0.001 | 1.00 (1.00,1.00) | 0.191 |
| TyG index | −110.24 (−178.20, −42.28) | 0.002 | −86.05 (−169.01, −3.09) | 0.042 | −1.07 (−1.38, −0.76) | <0.001 | 1.53 (1.27,1.84) | < 0.001 |
| MS | 5.90 (−15.76, 27.56) | 0.586 | 27.31 (0.84, 53.79) | 0.043 | −0.40 (−0.50, −0.30) | <0.001 | 1.11 (1.05,1.18) | < 0.001 |
| Model 1 | ||||||||
| MAP | 1.60 (0.00, 3.20) | 0.050 | 2.51 (0.61, 4.41) | 0.011 | −0.01 (−0.02, 0.00) | 0.210 | 1.00 (1.00,1.01) | 0.292 |
| Uric acid | −3.10 (−16.16, 9.97) | 0.634 | −7.28 (−24.88, 10.32) | 0.408 | 0.02 (−0.12, 0.15) | 0.798 | 1.10 (0.99,1.21) | 0.069 |
| Cholesterol | −0.08 (−0.46, 0.31) | 0.691 | 0.10 (−0.40, 0.60) | 0.683 | 0.00 (−0.01, 0.00) | 0.034 | 1.00 (1.00,1.00) | 0.192 |
| TyG index | −92.51 (−130.43, −54.58) | <0.001 | −108.28 (−160.63, −55.92) | <0.001 | −0.19 (−0.49, 0.12) | 0.223 | 1.50 (1.21,1.85) | < 0.001 |
| MS | −4.98 (−16.94, 6.98) | 0.405 | −0.45 (−15.25, 14.35) | 0.951 | −0.12 (−0.21, −0.02) | 0.018 | 1.05 (0.97,1.14) | 0.205 |
| Model 2 | ||||||||
| MAP | 1.81 (0.19, 3.43) | 0.029 | 2.76 (0.86, 4.65) | 0.006 | −0.01 (−0.02, 0.01) | 0.248 | 1.00 (1.00,1.01) | 0.268 |
| Uric acid | −2.83 (−16.09, 10.42) | 0.667 | −7.25 (−24.72, 10.23) | 0.406 | 0.02 (−0.11, 0.16) | 0.730 | 1.09 (0.99,1.21) | 0.078 |
| Cholesterol | −0.07 (−0.46, 0.32) | 0.713 | 0.07 (−0.42, 0.57) | 0.767 | 0.00 (−0.01, 0.00) | 0.083 | 1.00 (1.00,1.00) | 0.204 |
| TyG index | −88.02 (−127.02, −49.02) | <0.001 | −106.41 (−159.57, −53.26) | <0.001 | −0.11 (−0.42, 0.19) | 0.444 | 1.45 (1.17,1.79) | 0.001 |
| MS | −4.61 (−16.96, 7.73) | 0.454 | −0.79 (−15.99, 14.4) | 0.916 | −0.10 (−0.20, −0.01) | 0.039 | 1.05 (0.97,1.13) | 0.253 |
| Model 3 | ||||||||
| MAP | 1.68 (0.05, 3.32) | 0.044 | 2.52 (0.59, 4.44) | 0.012 | −0.01 (−0.02, 0.01) | 0.337 | 1.00 (1.00,1.01) | 0.274 |
| Uric acid | −1.95 (−15.03, 11.14) | 0.765 | −5.61 (−22.76, 11.53) | 0.511 | 0.01 (−0.12, 0.15) | 0.834 | 1.09 (0.99,1.21) | 0.079 |
| Cholesterol | −0.09 (−0.47, 0.30) | 0.657 | 0.05 (−0.45, 0.54) | 0.847 | 0.00 (−0.01, 0.00) | 0.104 | 1.00 (1.00,1.00) | 0.200 |
| TyG index | −89.85 (−129.30, −50.40) | <0.001 | −109.23 (−163.28, −55.18) | <0.001 | −0.11 (−0.41, 0.20) | 0.488 | 1.44 (1.16,1.78) | 0.001 |
| MS | −5.06 (−17.45, 7.33) | 0.413 | −1.48 (−16.87, 13.91) | 0.846 | −0.10 (−0.20, 0.00) | 0.045 | 1.05 (0.97,1.13) | 0.261 |
Note: Model 1: Adjusted for gender, age, BMI, ethnic, and economic level; Model 2: additionally adjusted for serum cotinine, and exercise status in Model 1; Model 3: average energy intake was further adjusted in Model 2.
3.5. Joint effects of DII and CDAI on metabolic indicators and lung function
Since both DII and CDAI had significant but opposite effects on metabolic and lung function, their joint effects were further assessed by pairwise combination (Fig. 2). Compared to the reference subgroup (high DII + low CDAI), significantly higher levels of FEV1 and FVC (Fig. 2A and B) but lower levels of MAP, UA, and MS (Fig. 2E, F, and 2I) were shown in other three subgroups, especially in the subgroup with low DII + high CDAI. Noteworthy, a significantly low risk of PRISm [OR (95CIs) = −0.80 (−1.51, −0.10)] (Fig. 2D), and low levels of TyG were also shown in the subjects exposed to low DII + high CDAI (Fig. 2H).
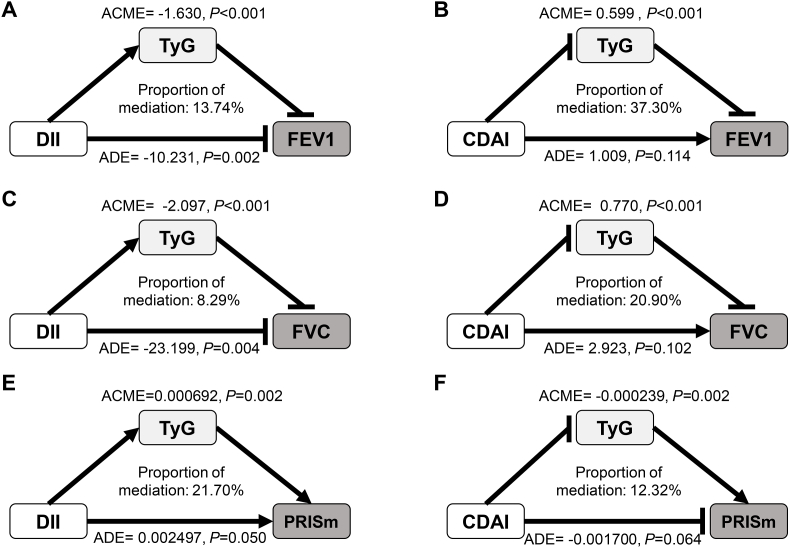
3.6. Mediation effects of TyG on DII- and CDAI-lung function associations
Given the intercorrelations between dietary quality (DII and CDAI), metabolic indicators (TyG), and lung function (FEV1, FVC, and PRISm status), mediation effects analyses were further performed (Fig. 3). TyG mediated 13.74 %, 8.29 %, and 21.70 % of the associations between DII and FEV1, FVC, and PRISm, respectively (Fig. 3A, C, 3E), and it also mediated 37.30 %, 20.90 %, and 12.32 % of the associations between CDAI and FEV1, FVC, and PRISm, respectively (Fig. 3B, D, 3F). Moreover, a Sankey diagram illustrated the relationships of dietary quality-metabolic status-lung function (eFig. 3).
Fig. 3.
Mediation effects of TyG on the associations of DII, CDAI with lung function parameters, and PRISm
Note: Arrows and rounded heads indicate promotion and inhibition, respectively. ACME, average causal mediation effects; ADE, average direct effects.
In addition, the sensitivity analyses with additional adjusted occupational exposure were further conducted in the GLM and binary logistic regression models. We found the impacts of DII and CDAI on TyG (eTable 1), and the associations between TyG and lung function decline (eTable 2) were not materially changed.
4. Discussion
This study innovatively explored the impacts of co-exposure to DII and CDAI on lung function, especially on PRISm, and further explored the underlying roles of metabolic disorders. We found both DII and CDAI were associated with lung function parameters (FEV1 and FVC) and metabolic indicators (MAP, UA, TC, TyG, MS), but in the opposite directions. Meanwhile, joint effects of co-exposure to DII and CDAI indicated the minimal hazard effects of DII on FEV1, FVC, and PRISm in subjects with high CDAI. Moreover, TyG mediated the associations of DII and CDAI with FEV1, FVC, and PRISm.
Two previous cross-sectional studies have shown that increased DII is strongly associated with the development of COPD [12,40]. PRISm is a prodromal symptom of COPD [4], this is the first study to explore the effects of co-exposure to DII and CDAI on PRISm. Although a nominal association between DII and PRISm was observed, increased DII was significantly and negatively associated with FEV1 and FVC, the characteristic indicators of PRISm. Consistent results were reported in previous studies of a cohort of adults aged 18–76 years, a cohort of adults aged ≥65 years in Italy, and asthmatics in Australia [11,41,42]. These findings indicated DII-induced lung function decline, which may subsequently cause PRISm that progresses over time. The mechanism of the DII-lung function association may be due to the pro-inflammatory properties of DII, since DII is positively correlated with inflammation factors (e.g., IL-1β, IL-6, TNF-α, and CRP) [9], and chronic inflammation leads to airway narrowing and destruction of the lung parenchyma [43]. Therefore, anti-DII intervention may be effective at reducing the decline of lung function.
There are beneficial effects of antioxidant diets on lung function [44]. As an indicator reflecting the overall antioxidative potential of dietary nutrients [15], CDAI was associated with a decreased risk of COPD [45] and a low prevalence of chronic respiratory diseases (CRDs) [16]. However, none of the previous studies had investigated the role of CDAI on pre-clinical lung disease. Our results indicated that increased CDAI had a nominal association with decreased risk of PRISm, and was significantly positively associated with both FEV1 and FVC. Similar results were detected in previous studies. For example, a UK cross-sectional study showed that dietary total antioxidant capacity (TAC) was positively associated with FEV1 and FVC [46], and a Swedish birth cohort suggests that dietary TAC affects FEV1 in asthmatic children up to adolescence [47]. Given the fact that oxidative stress and DNA damage often accompany the transformation of a healthy lung into COPD [48,49], dietary antioxidants may slow the decline in lung function by ameliorating oxidative lung damage [13,50,51]. Thus, CDAI may be an important modifiable factor in the protection of lung function decline and prevention of subsequent PRISm.
Additionally, we observed the associations of DII and CDAI with metabolic indicators including MAP, UA, TC, TyG, and MS. An elevated DII appeared to exacerbate the unhealthy metabolic state, which was comparable to the findings in previous studies [25,26,28]. Although limited studies had directly explored the impacts of dietary quality on TyG, the associations between dietary quality and TyG components (calculated from fasting triglycerides and fasting glucose) were reported. High DII was associated with increased numbers of lipoproteins including VLDL, LDL, and HDL particles, as well as glucose homeostasis imbalance [52]. In contrast, micronutrients (e.g., zinc and vitamins A, C, and E) included in CDAI are associated with lipids, glucose, inflammation, and IR [53]. Usually, metabolic abnormalities are associated with decreased lung function [54]. Of the metabolic indicators mentioned above, we found that only the TyG index was negatively associated with FEV1 and FVC, but positively associated with increased risk of PRISm. Similarly, a highly restrictive spirometry pattern (FVC <80 % of predicted and FEV1/FVC ≥0.70) was presented in subjects with high levels of TyG [24]. As a recognized marker of metabolic disorders and systemic inflammation, increased TyG implies enhanced IR, which is closely related to blood glucose [23] and adversely affects lung function [55].
The harmful effects of DII were lower among those with high levels of CDAI. One of the critical mechanisms is due to the fact that high levels of DII drive the production of inflammation and oxidative stress, whereas high CDAI may antagonize these effects through antioxidants [9,15,29]. It suggests that increasing the level of dietary antioxidants seems to be beneficial for lung health when the level of dietary inflammation is high.
We further observed that TyG played a mediating role in the link between dietary quality and lung health. Although the mechanism is not clear, a number of possible explanations remain. For example, saturated fatty acids are pro-inflammatory components in the DII score [9], and consuming foods with high levels of saturated fatty acids leads to fatty acid infiltration of macrophages, which activates cytotoxic T-cells and perpetuates the inflammatory response [56]. On the contrary, vitamin A in the CDAI scoring system acts as an antioxidant component that weakens inflammation in the body [57]. It is worth mentioning that both DII and CDAI were noted to show some association with inflammatory factors IL-6 and TNF-α [9,58]. IL-6 is mainly involved in the activation of the JAK/STAT pathway, and TNF-α alters the adhesion properties of pancreatic islet B-cells, thereby promoting jeopardization of the pancreas and increasing IR [59,60]. TyG is the available marker for IR [23], and metabolic imbalances produced by IR may affect lung function through a number of pathways. For example, advanced glycation end-products (AGEs) are formed in response to hyperglycemia, and binding of AGEs to the receptor for advanced glycation end-products (RAGE), which is highly expressed in the lungs, leads to an increased inflammatory response that is associated with the development of COPD [61,62]. In addition, IR inhibits adipocyte lipoprotein lipase activity, elevates free fatty acid (FFA) release, promotes the production of inflammatory factors such as IL-6 and TNF-α [63], and further leads to lung injury [[64], [65], [66]]. Another possible cause is a change in gut flora. Some studies have shown that high-inflammatory diets may lead to dysbiosis of the gut microflora in the body, while antioxidant-rich diets may relatively increase the abundance of beneficial bacteria within the gut flora [67,68]. This alteration in microbiota composition and function may stimulate the development of metabolic disorders through a variety of mechanisms [69]. The gut microbiome is associated with IR (or TyG) and impaired insulin secretion, possibly due to the pro-inflammatory properties of lipopolysaccharides from gram-negative bacteria (harmful flora) in the gut [70]. These findings suggested changes in TyG and subsequent inflammation are the common mechanisms (play mediating roles) of the joint effects of DII and CDAI on lung function including PRISm.
The novelty of this study is that we focused on early lung dysfunction including PRISm and simultaneously explored its risk and protective factors. The joint effects of these factors were also assessed and further revealed the mediating role of TyG on dietary quality-lung function association. Our study provides novel insight into the modifiable factors and mechanisms for targeted intervention and prevention of early lung function decline. However, it must be recognized that there were some limitations. First, a cross-sectional study was unable to verify a causal relationship between diet quality, metabolic disorders, and lung function. However, our findings indicate that dietary pattern/quality is an important influencing factor of changes in lung function, which may play a role in affecting metabolism. This provides us with clues and foundations for further causal inference. Second, the intakes of various dietary nutrients used in calculations of DII and CDAI were derived from the average of 24-h dietary recalls, which may not fully represent the daily dietary intake with long-term exposure. Despite this, there is evidence that 24-h dietary recall is in good agreement with the food frequency questionnaire, a primary method to assess long-term dietary consumption, in evaluating dietary habits [71]. Besides, we did not include plant actives with antioxidant properties in the calculation of DII due to the limitation of food composition in NHANES, our findings may therefore have some bias. Other limitations include unmeasured confounding (co-exposure to environmental and genetic factors, etc.). Therefore, our findings need to be further explored and confirmed in cohort studies as well as in ex vivo experiments.
5. Conclusion
Our findings suggest that DII and CDAI were associated with decreased and increased lung function, respectively. Their joint effects indicate that CDAI may weaken the hazard effects of DII on metabolic disorders, lung function parameters, and PRISm. As a metabolic indicator, TyG mediated the relationships of DII and CDAI with FEV1, FVC, and PRISm. This study provides new evidence and operationalization for preventing lung function decline through early dietary intervention, as well as revealing metabolic mechanisms involved in the process. More cohort studies and mechanistic research are warranted.
Financial support
This work was supported by the National Natural Scientific Foundation of China (grant no. 82203998), the 2022 Annual Student Innovation Capability Enhancement Program of Guangzhou Medical University, the Scientific Research Project of Guangzhou Education Bureau (grant no. 202235417), and the Guangzhou Science and Technology Project (grant no. 2023A04J0560).
CRediT authorship contribution statement
Yuyu Zheng: Writing – review & editing, Writing – original draft, Visualization, Validation, Formal analysis, Data curation, Conceptualization. Wanlu Liu: Writing – review & editing, Writing – original draft, Conceptualization. Xinyu Zhu: Writing – review & editing, Formal analysis. Mengya Xu: Writing – review & editing, Formal analysis. Baihao Lin: Writing – review & editing, Formal analysis. Yansen Bai: Writing – review & editing, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank the other researchers, staff and participants in the NHANES study for their valuable contributions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2024.103334.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Chronic obstructive pulmonary disease (COPD) 2024. https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd 2024-5-12, 2024.
- 2.Christenson S.A., Smith B.M., Bafadhel M., Putcha N. Chronic obstructive pulmonary disease. Lancet. 2022;399(10342):2227–2242. doi: 10.1016/S0140-6736(22)00470-6. [DOI] [PubMed] [Google Scholar]
- 3.Wan E.S., Balte P., Schwartz J.E., et al. Association between preserved ratio impaired spirometry and clinical outcomes in US adults. JAMA, J. Am. Med. Assoc. 2021;326(22):2287–2298. doi: 10.1001/jama.2021.20939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higbee D.H., Granell R., Davey S.G., Dodd J.W. Prevalence, risk factors, and clinical implications of preserved ratio impaired spirometry: a UK Biobank cohort analysis. Lancet Respir. Med. 2022;10(2):149–157. doi: 10.1016/S2213-2600(21)00369-6. [DOI] [PubMed] [Google Scholar]
- 5.Wan E.S., Hokanson J.E., Regan E.A., et al. Significant spirometric transitions and preserved ratio impaired spirometry among ever smokers. Chest. 2022;161(3):651–661. doi: 10.1016/j.chest.2021.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C., Zhang S., Yang T., Wang C., Han G. Associations between environmental heavy metals exposure and preserved ratio impaired spirometry in the U.S. adults. Environ. Sci. Pollut. Res. Int. 2023;30(49):108274–108287. doi: 10.1007/s11356-023-29688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie C., Ya L.M., Luo Q.L., Dong J.C. Role of cellular senescence in inflammatory lung diseases. Cytokine Growth Factor Rev. 2023;70:26–40. doi: 10.1016/j.cytogfr.2023.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Wood L.G. Diet, obesity, and asthma. Ann Am Thorac Soc. 2017;14(Supplement_5):S332–S338. doi: 10.1513/AnnalsATS.201702-124AW. [DOI] [PubMed] [Google Scholar]
- 9.Shivappa N., Steck S.E., Hurley T.G., Hussey J.R., Hebert J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Publ. Health Nutr. 2014;17(8):1689–1696. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Visser E., de Jong K., van Zutphen T., Kerstjens H., Ten B.A. Dietary inflammatory index and clinical outcome measures in adults with moderate-to-severe asthma. J. Allergy Clin. Immunol. Pract. 2023;11(12):3680–3689.e7. doi: 10.1016/j.jaip.2023.08.032. [DOI] [PubMed] [Google Scholar]
- 11.Han Y.Y., Jerschow E., Forno E., et al. Dietary patterns, asthma, and lung function in the hispanic community health study/study of latinos. Ann Am Thorac Soc. 2020;17(3):293–301. doi: 10.1513/AnnalsATS.201908-629OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C., Yang T., Wang C. The dietary inflammatory index and early COPD: results from the national health and nutrition examination survey. Nutrients. 2022;14(14):2841. doi: 10.3390/nu14142841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnes P.J. Oxidative stress-based therapeutics in COPD. Redox Biol. 2020;33 doi: 10.1016/j.redox.2020.101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Larsen V., Amigo H., Bustos P., Bakolis I., Rona R.J. Ventilatory function in young adults and dietary antioxidant intake. Nutrients. 2015;7(4):2879–2896. doi: 10.3390/nu7042879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright M.E., Mayne S.T., Stolzenberg-Solomon R.Z., et al. Development of a comprehensive dietary antioxidant index and application to lung cancer risk in a cohort of male smokers. Am. J. Epidemiol. 2004;160(1):68–76. doi: 10.1093/aje/kwh173. [DOI] [PubMed] [Google Scholar]
- 16.Wang S., Teng H., Zhang L., Wu L. Association between dietary antioxidant intakes and chronic respiratory diseases in adults. World Allergy Organ J. 2024;17(1) doi: 10.1016/j.waojou.2023.100851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lecube A., Simo R., Pallayova M., et al. Pulmonary function and sleep breathing: two new targets for type 2 diabetes care. Endocr. Rev. 2017;38(6):550–573. doi: 10.1210/er.2017-00173. [DOI] [PubMed] [Google Scholar]
- 18.Baffi C.W., Wood L., Winnica D., et al. Metabolic syndrome and the lung. Chest. 2016;149(6):1525–1534. doi: 10.1016/j.chest.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allinson J.P., Patel P.H., Donaldson G.C. Obesity, insulin resistance, and asthma. Am. J. Respir. Crit. Care Med. 2022;206(9):1057–1058. doi: 10.1164/rccm.202207-1271ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyai N., Shiozaki M., Yabu M., et al. Increased mean arterial pressure response to dynamic exercise in normotensive subjects with multiple metabolic risk factors. Hypertens. Res. 2013;36(6):534–539. doi: 10.1038/hr.2012.215. [DOI] [PubMed] [Google Scholar]
- 21.Fritz J., Brozek W., Concin H., et al. The association of excess body weight with risk of ESKD is mediated through insulin resistance, hypertension, and hyperuricemia. J. Am. Soc. Nephrol. 2022;33(7):1377–1389. doi: 10.1681/ASN.2021091263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haggstrom C., Stocks T., Nagel G., et al. Prostate cancer, prostate cancer death, and death from other causes, among men with metabolic aberrations. Epidemiology. 2014;25(6):823–828. doi: 10.1097/EDE.0000000000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin J.L., Yang J., Song X.J., et al. Triglyceride-glucose index and health outcomes: an umbrella review of systematic reviews with meta-analyses of observational studies. Cardiovasc. Diabetol. 2024;23(1):177. doi: 10.1186/s12933-024-02241-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu T.D., Fawzy A., Brigham E., et al. Association of triglyceride-glucose index and lung health: a population-based study. Chest. 2021;160(3):1026–1034. doi: 10.1016/j.chest.2021.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canto-Osorio F., Denova-Gutierrez E., Sanchez-Romero L.M., Salmeron J., Barrientos-Gutierrez T. Dietary Inflammatory Index and metabolic syndrome in Mexican adult population. Am. J. Clin. Nutr. 2020;112(2):373–380. doi: 10.1093/ajcn/nqaa135. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Q., Tan X., Su Z., et al. The relationship between the dietary inflammatory index (DII) and metabolic syndrome (MetS) in middle-aged and elderly individuals in the United States. Nutrients. 2023;15(8):1857. doi: 10.3390/nu15081857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saltiel A.R., Olefsky J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Invest. 2017;127(1):1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gantenbein K.V., Kanaka-Gantenbein C. Mediterranean diet as an antioxidant: the impact on metabolic health and overall wellbeing. Nutrients. 2021;13(6):1951. doi: 10.3390/nu13061951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mittal M., Siddiqui M.R., Tran K., Reddy S.P., Malik A.B. Reactive oxygen species in inflammation and tissue injury. Antioxidants Redox Signal. 2014;20(7):1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gibson R., Lau C.E., Loo R.L., et al. The association of fish consumption and its urinary metabolites with cardiovascular risk factors: the International Study of Macro-/Micronutrients and Blood Pressure (INTERMAP) Am. J. Clin. Nutr. 2020;111(2):280–290. doi: 10.1093/ajcn/nqz293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abstracts of the 21th European Congress on Obesity (ECO2014) May 28-31, 2014, sofia, Bulgaria. Obes. Facts. 2014;7:1–188. doi: 10.1159/000363668. Suppl 1(Suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J., Sun B., Zhang D. Association of dietary n3 and n6 fatty acids intake with hypertension: NHANES 2007-2014. Nutrients. 2019;11(6):1232. doi: 10.3390/nu11061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nhanes 2011-2012: spirometry - pre and post-bronchodilator data documentation, codebook, and frequencies. 2024. https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/SPX_G.htm 2024-7-14, 2024.
- 34.Bowerman C., Bhakta N.R., Brazzale D., et al. A race-neutral approach to the interpretation of lung function measurements. Am. J. Respir. Crit. Care Med. 2023;207(6):768–774. doi: 10.1164/rccm.202205-0963OC. [DOI] [PubMed] [Google Scholar]
- 35.Wan E.S., Fortis S., Regan E.A., et al. Longitudinal phenotypes and mortality in preserved ratio impaired spirometry in the COPDGene study. Am. J. Respir. Crit. Care Med. 2018;198(11):1397–1405. doi: 10.1164/rccm.201804-0663OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miura S., Iwamoto H., Omori K., et al. Preserved ratio impaired spirometry with or without restrictive spirometric abnormality. Sci. Rep. 2023;13(1):2988. doi: 10.1038/s41598-023-29922-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention National health and nutrition examination survey 2011–2012 data documentation, codebook, and frequencies: physical acitivty (PAQ_G) 2024. https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/PAQ_G.htm 2024-6-11, 2024.
- 38.Piercy K.L., Troiano R.P., Ballard R.M., et al. The physical activity guidelines for Americans. JAMA, J. Am. Med. Assoc. 2018;320(19):2020–2028. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valente M.J., Rijnhart J., Smyth H.L., Muniz F.B., Mackinnon D.P. Causal mediation programs in R, mplus, SAS, SPSS, and stata. Struct. Equ. Model. 2020;27(6):975–984. doi: 10.1080/10705511.2020.1777133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu H., Tan X., Liu Z., et al. Association between diet-related inflammation and COPD: findings from NHANES III. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.732099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alessi A., Trevisan C., Citron A., et al. Dietary inflammatory index is associated with lung function in healthy older adults. Nutrition. 2022;99–100 doi: 10.1016/j.nut.2022.111653. [DOI] [PubMed] [Google Scholar]
- 42.Wood L.G., Shivappa N., Berthon B.S., Gibson P.G., Hebert J.R. Dietary inflammatory index is related to asthma risk, lung function and systemic inflammation in asthma. Clin. Exp. Allergy. 2015;45(1):177–183. doi: 10.1111/cea.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan W., Shen H.M., Wong W. Dysregulated autophagy in COPD: a pathogenic process to be deciphered. Pharmacol. Res. 2019;144:1–7. doi: 10.1016/j.phrs.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Garcia-Larsen V., Potts J.F., Omenaas E., et al. Dietary antioxidants and 10-year lung function decline in adults from the ECRHS survey. Eur. Respir. J. 2017;50(6) doi: 10.1183/13993003.02286-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Z., Li J., Chen T., et al. Association between dietary antioxidant levels and chronic obstructive pulmonary disease: a mediation analysis of inflammatory factors. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1310399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okubo H., Shaheen S.O., Ntani G., et al. Processed meat consumption and lung function: modification by antioxidants and smoking. Eur. Respir. J. 2014;43(4):972–982. doi: 10.1183/09031936.00109513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sdona E., Hallberg J., Andersson N., et al. Dietary antioxidant intake in school age and lung function development up to adolescence. Eur. Respir. J. 2020;55(2) doi: 10.1183/13993003.00990-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dauchet L. Propylene oxide, lung function, and oxidative stress: what are the public health implications? Chest. 2023;163(6):1344–1345. doi: 10.1016/j.chest.2023.02.046. [DOI] [PubMed] [Google Scholar]
- 49.Makena P., Kikalova T., Prasad G.L., Baxter S.A. Oxidative stress and lung fibrosis: towards an adverse outcome pathway. Int. J. Mol. Sci. 2023;24(15) doi: 10.3390/ijms241512490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ilari S., Vitiello L., Russo P., et al. Daily vegetables intake and response to COPD rehabilitation. The role of oxidative stress, inflammation and DNA damage. Nutrients. 2021;13(8):2787. doi: 10.3390/nu13082787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carotenuto F., Albertini M.C., Coletti D., et al. How diet intervention via modulation of DNA damage response through MicroRNAs may have an effect on cancer prevention and aging, an in silico study. Int. J. Mol. Sci. 2016;17(5):752. doi: 10.3390/ijms17050752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phillips C.M., Shivappa N., Hebert J.R., Perry I.J. Dietary inflammatory index and biomarkers of lipoprotein metabolism, inflammation and glucose homeostasis in adults. Nutrients. 2018;10(8) doi: 10.3390/nu10081033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia O.P., Ronquillo D., Del C.C.M., et al. Zinc, iron and vitamins A, C and e are associated with obesity, inflammation, lipid profile and insulin resistance in Mexican school-aged children. Nutrients. 2013;5(12):5012–5030. doi: 10.3390/nu5125012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wielscher M., Amaral A., van der Plaat D., et al. Genetic correlation and causal relationships between cardio-metabolic traits and lung function impairment. Genome Med. 2021;13(1):104. doi: 10.1186/s13073-021-00914-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reay W.R., El S.S., Geaghan M.P., et al. Genetic association and causal inference converge on hyperglycaemia as a modifiable factor to improve lung function. Elife. 2021;10 doi: 10.7554/eLife.63115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fritsche K.L. The science of fatty acids and inflammation. Adv. Nutr. 2015;6(3):293S–301S. doi: 10.3945/an.114.006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blaner W.S., Shmarakov I.O., Traber M.G. Vitamin A and vitamin E: will the real antioxidant please stand up? Annu. Rev. Nutr. 2021;41:105–131. doi: 10.1146/annurev-nutr-082018-124228. [DOI] [PubMed] [Google Scholar]
- 58.Luu H.N., Wen W., Li H., et al. Are dietary antioxidant intake indices correlated to oxidative stress and inflammatory marker levels? Antioxidants Redox Signal. 2015;22(11):951–959. doi: 10.1089/ars.2014.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lesina M., Wormann S.M., Neuhofer P., Song L., Algul H. Interleukin-6 in inflammatory and malignant diseases of the pancreas. Semin. Immunol. 2014;26(1):80–87. doi: 10.1016/j.smim.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 60.Cirulli V., Halban P.A., Rouiller D.G. Tumor necrosis factor-alpha modifies adhesion properties of rat islet B cells. J. Clin. Invest. 1993;91(5):1868–1876. doi: 10.1172/JCI116403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Twarda-Clapa A., Olczak A., Bialkowska A.M., Koziolkiewicz M. Advanced glycation end-products (AGEs): formation, chemistry, classification, receptors, and diseases related to AGEs. Cells. 2022;11(8):1312. doi: 10.3390/cells11081312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu L., Ma L., Nicholson L.F., Black P.N. Advanced glycation end products and its receptor (RAGE) are increased in patients with COPD. Respir. Med. 2011;105(3):329–336. doi: 10.1016/j.rmed.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 63.Ormazabal V., Nair S., Elfeky O., Aguayo C., Salomon C., Zuniga F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018;17(1):122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu H., Zhang H., Lu K., et al. Chlorinated organophosphate flame retardants impair the lung function via the IL-6/JAK/STAT signaling pathway. Environ. Sci. Technol. 2022;56(24):17858–17869. doi: 10.1021/acs.est.2c05357. [DOI] [PubMed] [Google Scholar]
- 65.Chen Y.C., Sung H.C., Chuang T.Y., et al. Vitamin D(3) decreases TNF-alpha-induced inflammation in lung epithelial cells through a reduction in mitochondrial fission and mitophagy. Cell Biol. Toxicol. 2022;38(3):427–450. doi: 10.1007/s10565-021-09629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo Z., Yang H., Zhang J.R., Zeng W., Hu X. Leptin receptor signaling sustains metabolic fitness of alveolar macrophages to attenuate pulmonary inflammation. Sci. Adv. 2022;8(28) doi: 10.1126/sciadv.abo3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beam A., Clinger E., Hao L. Effect of diet and dietary components on the composition of the gut microbiota. Nutrients. 2021;13(8) doi: 10.3390/nu13082795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Riaz Rajoka M.S., Shi J., Mehwish H.M., et al. Interaction between diet composition and gut microbiota and its impact on gastrointestinal tract health. Food Sci. Hum. Wellness. 2017;6(3):121–130. doi: 10.1016/j.fshw.2017.07.003. [DOI] [Google Scholar]
- 69.Moszak M., Szulinska M., Bogdanski P. You are what you eat-the relationship between diet, microbiota, and metabolic disorders-A review. Nutrients. 2020;12(4) doi: 10.3390/nu12041096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gurung M., Li Z., You H., et al. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020;51 doi: 10.1016/j.ebiom.2019.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cui Q., Xia Y., Wu Q., Chang Q., Niu K., Zhao Y. Validity of the food frequency questionnaire for adults in nutritional epidemiological studies: a systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2023;63(12):1670–1688. doi: 10.1080/10408398.2021.1966737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.