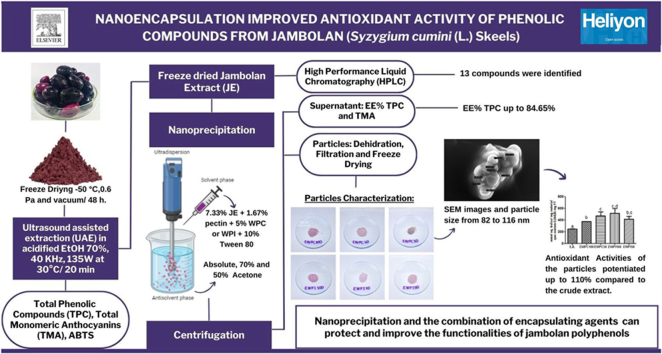
Abstract
Jambolan (Syzygium cumini L.) is an underutilized fruit rich in bioactive phenolic compounds, specially anthocyanins, but the low stability of these substances and interaction with other compounds in the food matrix limit their application as food additives; nanoencapsulation is the best strategy to overcome these limitations. This study aimed to nanoencapsulate a phenolic-rich jambolan extract using whey proteins and pectin by nanoprecipitation in different antisolvent compositions. Two formulations were synthesized (7.33 % extract, 1.67 % pectin, and 5 % concentrated or isolated whey protein) precipitated in different acetone concentrations (50, 70, and 100 % v/v). SEM showed particles with spherical shape and smooth surface. DLS pointed diameters between 82 nm and 116 nm. FTIR indicated chemical interactions between the materials. Encapsulation efficiency showed high phenolic compounds entrapment in all systems [73.81–84.65 %, p > 0.05]. However, particles precipitated in 50 and 100 % acetone (v/v) showed greater anthocyanins retention [56.89–35.24 %, p < 0.05]. Nanoencapsulation potentiated the antioxidant activity up to 110 % more than the crude extract (p < 0.05). These results show the potential of nanoprecipitation as an effective encapsulation process and the biopolymers combination to produce nanoparticles containing jambolan phenolic compounds to promote their application in foods and health products.
Keywords: Anthocyanins, Ultrasound assisted extraction, High-Performance liquid chromatography, Nanoprecipitation, Whey protein. pectin
Graphical abstract
Highlights
-
•
Jambolan extract by UAE yielded 3479.41 mg/100 g of TPC and 771.51 mg/100 g of TMA.
-
•
13 phenolic compounds were identified and quantified by HPLC on jambolan extract.
-
•
Biopolymer based nanoparticles with diameters of 82 nm–116 nm were produced.
-
•
Encapsulation Efficiency showed high phenolic compounds entrapment of 73.81–84.65 %.
-
•
Nanoencapsulation enhanced antioxidant activity up to 110 % more than the crude extract.
1. Introduction
Jambolan fruits (Syzygium cumini (L.) Skeels) have a rich chemical composition with high nutritional and biological values. Its edible parts contain significant phenolic compounds responsible for sensory attributes, such as color and flavor, and bioactive properties mainly associated with its antioxidant and anti-inflammatory activities [1]. Phenolic acids, flavones, and flavonoids such as gallic acid, myricetin, and anthocyanins are phenolic compounds found in these fruits [2]. Anthocyanins are an important subclass of flavonoids responsible for the purple shade of S. cumini fruit. Aside from presenting interesting biological activities, this class of compounds has been studied as alternatives to synthetic dyes due to their characteristic colors and high solubility in water [3].
The extraction of bioactive compounds is a strategy for characterizing and expanding the applications of phenolic-rich food matrices. Conventional methods for extracting phenolic compounds demand extended extraction periods, which can partially degrade the extracted compounds, particularly anthocyanins. Ultrasound-assisted extraction (UAE) is an emerging technique known to reduce extraction time and solvent usage while enhancing the yield and quality of extracts. These advantages result from a more effective mass transfer and solvent penetration due to the disruption of cell walls and vacuoles via acoustic cavitation [4].
Despite their potential, their molecular instability limits the use of phenolic compounds as food additives since they are sensitive to environmental conditions, such as pH, temperature, oxygen, light, and interaction with other food matrix components. Furthermore, they are also unstable to intestinal pH, the action of digestive enzymes, and biotransformation by the intestinal microbiota, which limits their biological use due to their relatively low bioavailability [5]. Aside from optimizing methods for the extraction of phenolic compounds, it is necessary to implement strategies that contribute to the preservation and stabilization of these compounds, such as nanoencapsulation techniques.
Nanotechnology is a field of science that deals with synthesizing particles ranging from 1 to 100 nm. A decrease in particle size, surface area, and volume increase leads to enhanced chemical, physical, and biological properties compared to larger particles [6]. In addition to protecting phenolic compounds by producing particles containing bioactive substances on the nanometer scale, nanoencapsulation presents other advantages over systems on the micro-scale, for example, greater thermodynamic stability, bioaccessibility, solubility and bioavailability of bioactives, acting in functionality and sensory characteristics. Besides, it allows the controlled release in target locations, producing suitable carriers for medicinal and nutritional purposes because of their high cellular uptake, enhancing the biological activity of the encapsulated compounds [7].
Many methods have been studied for the production of nanoparticles containing phenolic compounds, including the anthocyanins, or extracts rich in these substances, such as emulsification [8], nanoliposomes [9], ‘self-assembling’ or ‘self-assembly’ [5], coacervation [10], ultrasound [11], electrospinning [12] and antisolvent precipitation or nanoprecipitation [13].
The nanoprecipitation technique is a rapid and cost-effective encapsulation method for nanoparticle production. This method is particularly suitable for various classes of phenolic compounds, including anthocyanins [14]. Combining polysaccharides and proteins as wall materials for encapsulation has shown efficiency in stabilizing bioactive extracts. This combination achieves stability by increasing viscosity, promoting electrostatic repulsion, and raising the total solid content [11]. Although the cross-linking of biopolymers is thoroughly researched, adding plant extracts along with biopolymers to enhance functional properties is a new trend [15]. Complexes utilizing whey protein and pectin have been assessed as wall materials in encapsulation processes for bioactive compounds [16]. However, there is a lack of studies on obtaining nanoparticles based on whey protein and pectin containing phenolic compounds, mainly anthocyanins, using the nanoprecipitation method.
To describe a processing method for jambolan, an underutilized fruit, for the obtention of products with high added economic and technological values, by using simple, time and energy-efficient technologies, in addition to using solvents and safe ingredients for application in food for human consumption. This study aimed to produce and characterize nanoparticles derived from whey protein and pectin containing phenolic compounds from jambolan obtained by ultrasound-assisted extraction, and to evaluate the effect of the encapsulation process on antioxidant activity in vitro of the extract.
2. Materials and methods
2.1. Materials
The ripe fruits of Syzygium cumini (L.) Skeels were obtained from domestic crops in the city of Natal, Rio Grande do Norte/Brazil, between December/2022 and January/2023. A sample was deposited in the Herbarium of the Federal University of Rio Grande do Norte under registration number 27098. The research was registered in the National System for Management of Genetic Patrimony and Associated Traditional Knowledge (SISGEN) with the code ADAE032. Tween 80 was obtained from Sigma-Aldrich. Low methoxyl pectin (GRINDSTEDⓇ Pectin YSF357) was provided by International Flavors & Fragrances Inc. IFF.
2.2. Jambolan powder processing
The fruits were mixed and sanitized using a 200 ppm sodium hypochlorite solution. The edible parts (pulp and peel) were manually separated from the seeds, frozen, and freeze-dried (−50 °C/0.6 Pa and vacuum for 48 h). After drying, the samples were crushed in a blender, sieved (20 mesh) to obtain a fine powder, and stored (−20 °C) in dark packaging until testing. The edible parts of the fruits were characterized according to the proximal composition (water, ashes, lipid, proteins, and fiber content) [17]. The powder was characterized according to the granulometry by manually passing the powder through a set of sieves with different aperture sizes (10 mesh- 2.0 mm, 18 mesh- 1.0 mm, 35 mesh - 0.50 mm, 60 mesh- 0.250 mm, 115 mesh- 0.124 mm and 230 mesh- 0.061 mm).
2.3. Ultrasound-assisted extraction of phenolic compounds
Ultrasound-assisted extraction (UAE) was carried out as described by Sabino et al. [4] with modifications. Jambolan powder was suspended in solvent (70 % ethanol with 0.1 % trifluoroacetic acid) in a ratio of 1:25 (g of dried fruit/ml of solvent) and subjected to sonication using an ultrasound bath (Schuster L200) operating at 40 kHz, 135 W of nominal power, and an average temperature of 30 °C/20 min, followed by centrifugation (2540×g, 25 °C/10 min) to separate the supernatant.
2.3.1. Extract characterization
Total phenolic compounds (TPC) content was expressed in gallic acid equivalents (mg GAE/g jambolan powder) [18]. Diluted samples of the extracts (150 μl), distilled water (2400 μl), and Folin-Ciocalteu reagent (150 μl) were homogenized and left to rest for 3 min, followed by the addition of a saturated sodium carbonate solution (300 μl). The mixture was kept in the dark for 120 min. Absorbance was measured at 725 nm in a spectrophotometer (Bel Photonics 1105). The calibration curve was prepared from gallic acid solutions at 25–200 μg/ml.
Total monomeric anthocyanins (TMA) were determined by the pH differential method described by Lee et al. [19]. The samples were diluted in 0.025 M potassium chloride buffer, pH = 1.0, and a second dilution, in the same proportions, was carried out in 0.4 M sodium acetate buffer, pH 4.5. Absorbances were measured in a spectrophotometer (Bel Photonics 1105) at 510 and 700 nm. Differences between the absorptions of the dilution systems were evaluated using equations Eq. 1, Eq. 2 below. The results were then expressed in cyanidin 3-glucoside equivalents per gram of jambolan powder.
| A = [((A_520 nm-A_700 nm))_(pH = 1.0)]- [((A_520 nm-A_700 nm))_(pH = 4.5)] | Eq. 1 |
| AMT (cyd-3-glu eq.mg/L)=(AxM_w x DF x V x 1000)/(M_a x L x m) | Eq. 2 |
Where: Mw is the molecular weight of cyanidin-3-glucoside = 449.2 g/mol; FD: Sample dilution factor in the buffer; V: buffer volume in ml; Ma is extinction coefficient = 26,900 mol/Lxcm; L: optical length of the cuvette (1 cm); m: sample mass.
The ABTS+ (2,2′-Azinobis [3-ethylbenzothiazoline-6-sulfonic acid]) radical inhibition assay was based on the method proposed by Rufino et al. [20] and Morais et al. [21] with modifications. The ABTS+ solution was diluted to an absorbance of 0.80 ± 0.02 at 734 nm in a spectrophotometer (Bel Photonics 1105). Then, 40 μL of the diluted sample or Trolox standard (6-hydroxy-2,5,7,8-tetramethyl chroman-2- carboxylic acid) was added to 260 μL of ABTS+ solution, and absorbances were measured at 734 nm. An inhibition curve was prepared with concentrations of 0.03–0.3 mg/ml of the extract to determine the concentration that reduces the activity of the ABTS+ radical by 50 % (IC50). Ethanolic solutions containing 50–250 μmol of Trolox were used to prepare a calibration curve. Results were expressed in IC50 as in μmol equivalents of trolox/mL of extract and per gram of jambolan powder.
2.3.2. Identification of phenolic compounds by High-Performance Liquid Chromatography (HPLC)
To identify phenolic compounds by High-Performance Liquid Chromatography (HPLC), the extract was concentrated by rotary evaporation and freeze-dried. The results were expressed in mg/kg of freeze-dried extract. Phenolic compounds were determined on a WATERS chromatograph (model Aliance e2695) coupled with DAD and fluorescence detection (FD), according to the method described by Coelho et al. [22].
2.4. Encapsulation by nanoprecipitation
In the present study, the influence of the type of protein used as an encapsulating agent in combination with pectin and the composition of the antisolvent phase on the physical and chemical characteristics of the particles produced was evaluated. The other process variables were fixed based on preliminary tests and a study previously developed by Calliari et al. [13] with modifications.
For nanoprecipitation, the amount of freeze-dried extract used was defined according to the anthocyanin content, so 20 mg of pigment were added to the system. Two formulations were developed based on previous bench standardization tests to choose the technique and encapsulating agents, both containing approximately 7.33 % (2.2 g) of freeze-dried jambolan extract - JE (20 mg of anthocyanins and 97 mg of total phenolics), 1.67 % (0.5 g) of low methoxylation citrus pectin (PEC) and 5 % whey protein concentrate - EWPC (1.5 g) or whey protein isolate - EWPI (1.5 g).
Pectin was solubilized in 10 ml of distilled water at 70 °C. After cooling, the freeze-dried jambolan extract was dissolved in 5 ml of distilled water and solubilized on it, and the solution was refrigerated at 4 °C overnight. Separately, 1.5g of the proteins were dissolved in 10 ml of distilled water and left to hydrate overnight under refrigeration at 4 °C to ensure complete solvation. After this time, the PEC + JE mixture was added to each protein solution at a ratio of 1.5:1 and maintained under magnetic stirring at room temperature for 3 h. Tween 80 was added at a proportion of 10 % w/v. The volume of the solution was adjusted to 30 ml. The system remained under magnetic stirring at room temperature for an hour.
The compositions of the antisolvent phases (120 mL) evaluated in this study were absolute acetone (100 %), 70 %, and 50 % v/v. The formulations were precipitated into different acetone compositions under ultradispersion (IKA®) at 17,000 rpm for 10 min. Then, the dispersions were centrifuged (Fanem Excelsa® 4, model 280-R) at 800 rpm/10 min, and the precipitate was collected and subjected to dehydration in absolute acetone in a ratio of 1:10 (m/m) for approximately 16 h. After this time, the dehydrated material was vacuum filtered on qualitative filter paper, stored under freezing (−18 °C), and subsequently freeze-dried for the obtention of a powder. The final named formulations were: EWPC100 - encapsulated in pectin and concentrated whey protein precipitated in absolute acetone; EWPC70 - encapsulated in pectin and concentrated whey protein precipitated in 70 % v/v acetone; EWPC50 - encapsulated in pectin and concentrated whey protein precipitated in 50 % v/v acetone; EWPI100 - encapsulated in pectin and isolated whey protein precipitated in absolute acetone; EWPI70 - encapsulated in pectin and isolated whey protein precipitated in 70 % v/v acetone; and EWPI50 - encapsulated in isolated whey protein precipitated in 50 % v/v acetone.
2.5. Encapsulation efficiency (EE%)
After the centrifugation step of the encapsulation, the supernatant was collected, the recovered volume was registered, and the TPC and TMA contents were quantified according to the methods described above. Encapsulation efficiency (EE%) was used as a parameter to select the most suitable materials to proceed to the next stages of characterization. The EE% of phenolic compounds and anthocyanins was calculated using equations (Eq. 3), (Eq. 4)) described below.
| EE% TMA=((TMA added - TMA supernatant))/(TMA added)*100 | (Eq. 3) |
| EE% TPC=((TPC added - TPC supernatant))/(TPC added)*100 | (Eq. 4) |
2.6. Nanoparticles characterization
2.6.1. Scanning electron Microscopy (SEM)
The particles were suspended in acetone, and the dispersions were dripped onto silicon plates attached to stubs with carbon tape. The materials were analyzed at different magnifications in a high vacuum at 5.0 kV and without metallization using a FEG-SEM ZEISS microscope (AURIGA).
2.6.2. Particle size and polydispersity index (PDI) by DLS
Dynamic light scattering (DLS) technique was used to determine the hydrodynamic diameter of the particles in suspension and the polydispersity index values of the particles in solution. The average particle size was evaluated using the NANO-flex II 180° DLS Particle Size (Colloid Metrix) equipment, which has a monochromatic laser with a wavelength of 785 nm. The material was dispersed in phosphate buffer (pH 7.0) at 1 mg/ml and vortexed for approximately 1 min to measure particle diameter. This dispersion was used in the readings. The data was analyzed using the NANO-flex Control 0.9.7 software, and graphs with particle size distribution and polydispersity index were plotted.
2.6.3. Zeta potential (ζ)
The Zeta potential measurements of the formulations' different pH ranges were determined using the STABINO II Particle charge Titration (Colloid Metrix) equipment. For this, 10 mg of the sample was diluted in 10 ml of 10 mM KCl solution and then transferred to the cylindrical Teflon cell. The particles were titrated with solutions of 0.1 M HCl and 0.025 M NaOH to construct a Zeta potential curve as a function of pH at values from 1.5 to 9.0.
2.6.4. Fourier transform infrared spectroscopy (FTIR)
The encapsulating agents, the lyophilized crude extract, the surfactant, and the encapsulate powders were recorded in transmittance at the mid-infrared region in the range of 500–4000 cm−1, with a resolution of 4 cm−1, on a BRUKER spectrometer model FT-IR VERTEX 70.x.
2.6.5. X-ray diffraction (XRD)
This analysis was used to evaluate whether the dominant phase in the materials is crystalline or amorphous. The encapsulating agents and encapsulated formulations were inserted into the cylindrical sample holder of the BRUKER Model D2 PHASER Diffractometer, and the spectra were obtained with 2θ between 3° and 70° and angular step 0.02°.
2.7. Antioxidant activity of particles
Considering that total phenolic compounds are mainly responsible for the antioxidant activity of jambolan [23], the antioxidant capacity of the particles was compared to the freeze-dried jambolan extract (JE) based on the content of phenolic compound (TPC). In this sense, the amount in milligrams of each encapsulated was calculated in a way that, according to the encapsulation efficiency for TPC (EE% TPC), had the same content of phenolic compounds as JE. The antioxidant activity of JE and particles was evaluated by the ABTS+ radical scavenging assay, as described in topic 2.3.1 [21]. For JE, a stock solution was prepared at 40 mg/mL and then diluted at a 1:80 w/v. For the particles, a stock solution at a concentration of 8.2 mg/mL was prepared and diluted in buffer pH 7.0 in a ratio of 1:20 w/v. In the test, 40 μl of diluted sample was added to 260 μl of ABTS+ solution, and absorbances were measured at 734 nm. Ethanolic solutions containing 50–250 μmol of Trolox were used to construct the calibration curves. The results were expressed in μmol Trolox equivalents/mg of freeze-dried extract or encapsulate.
2.8. Statistical analysis
All tests were performed in triplicates, and the results were expressed as mean and standard deviation. GraphPad Prism 5.0 software (GraphPad Software, Inc.; La Jolla, CA, USA) was used to perform all statistical analyses. Data significance was determined using Analysis of Variance (ANOVA) and Tukey's post-test (p < 0.05).
3. Results and discussion
3.1. Jambolan fruits, powder, and extract characterization
The proximal composition on the wet basis of the jambolan fruits showed a moisture content of 88.14 % ± 0.52, ashes 0.30 % ± 0.01, proteins 0.86 % ± 0.05, lipids 0.19 % ± 0.01, carbohydrates 7.89 %, and total dietary fibers 2.36 % ± 0.27. The caloric value calculated was 37 Kcal/100 g. The granulometry analysis of the powder showed that most of the particles (87.70 %) were retained on sieves with openings of 35–115 mesh, which correspond to particles with sizes between 120 and 500 μm, with average particle size (calculated by geometric mean) of 249 μm.
Each step of the extraction process requires systematic control, from the pre-treatment of the material, which includes drying, crushing, and standardization of the sample by screening, to the final extraction. These steps are critical as they can significantly impact the overall process efficiency. Reducing particle size by grinding increases the diffusivity of compounds, as it increases the surface area, facilitating the transfer of active ingredients from the matrix to the solvent, which optimizes extraction time and kinetics [24].
The TPC and TMA in 100 g of jambolan powder were 3479.41 ± 6.6 mg GAE/100 g and 771.51 ± 11.6 mg eq. cyanidin 3-glucoside/100 g, respectively. Studies like Sabino et al. [4] have sought to optimize ultrasound-assisted extraction conditions to maximize the yield of phenolic compounds and anthocyanins. When evaluating the extraction of anthocyanins from jambolan using ultrasound technology (power of 5000 W/L/7.5 min, 79.6 % ethanol as solvent), the authors obtained values four times higher than those obtained by exhaustive serial extraction evaluated in this work.
The extract showed strong antioxidant activity, in which the concentration that reduced antioxidant activity by 50 % (IC50) of ABTS was 0.21 mg/mL. Lower values indicate the high antioxidant activity of the samples. Virgen-Carrillo et al. [25] investigated the antioxidant activity (IC50) of 8 varieties of 'berries' (raspberries, blueberries, and blackberries) grown in western Mexico. They found IC50 values for ABTS that ranged from 2.26 to 7.5 mg/mL in extracts prepared from freeze-dried fruits. Our result of antioxidant activity evaluated by ABTS in mmol eq. trolox/g dried fruit was 300.99 ± 25.66 mmol eq. Trolox/g. Huang et al. [26] evaluated the antioxidant activity by ABTS of blueberries, blackberries, and strawberries grown in Nanjing, China, and obtained results of 0.15, 0.11, and 0.04 mmol eq. Trolox/g dry weight, respectively. These results show the potential of the jambolan extracts evaluated in this study, even in comparison with other species of berries highly consumed and best known by the food industry.
3.2. Identification of phenolic compounds by High-Performance Liquid Chromatography (HPLC)
13 compounds were identified and quantified in the extract (mg/kg of dried extract), among the 30 standards compounds that were tested: caftaric acid (142.15 ± 11.25), gallic acid (76.13 ± 0.56), caffeic acid (16.76 ± 2.50) and chlorogenic acid (6.78 ± 5.50); flavan-3-ol monomers and condensed tannins catechin (23.63 ± 0.28), epicatechin (13.44 ± 0.13), procyanidins B1 (17.90 ± 0.37) and B2 (488.44 ± 35.64); flavonols myricetin (39.72), quercetin 3-glucoside (4.36), rutin (33.02), kaempferol 3-glucoside (5.23 ± 4.30); and anthocyanins malvidin 3,5-diglucoside (2438.60 ± 298.21), delphinidin 3-galactoside (514.88 ± 67.78), and cyanidin 3,5-diglucoside (281.49 ± 37.57), with a total of 4103 ± 455.2 mg/kg of phenolic compounds and 3235 ± 403.6 mg/kg of anthocyanins quantified by this method.
Anthocyanins were the major compounds found in the extract, corresponding to almost 80 % of the total phenolic compounds quantified by HPLC analysis. The identified profile presented the following anthocyanins: malvidin 3,5-diglucoside, delphinidin 3-galactoside, and cyanidin 3,5-diglucoside. The levels of malvidin 3,5-diglucoside and cyanidin 3,5-diglucoside found are consistent with the levels of these same compounds identified by Lestario et al. [27] [2683 ± 38 mg/kg and 356 ± 6.9 mg/kg, respectively] in jambolan fruits at the stage of complete ripening and by Brito et al. [28] (290 mg/kg), who also identified the presence of malvidin 3,5-diglucoside in the jambolan samples evaluated, but in smaller quantities than those found in this study (1660 mg/kg). Delphinidin 3-galactoside was not observed in the studies used as a reference. The discrepancy between these results may be due to varietal differences, variations in environmental growth conditions, or even differences in the protocols for obtaining the extracts.
The results obtained for the extracts at the wavelength of 520 nm showed significant peaks that could not be identified due to the lack of standards (Fig. S1 Supplementary Material). With the aid of literature data [[2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27]], it can be concluded that the peaks must correspond to delphinidin 3,5-diglucoside and petunidin 3,5-diglucoside. Therefore, there is a limitation regarding the conclusions for the qualitative and quantitative profile of anthocyanins since compounds possibly present in high concentrations were not analyzed.
In addition to anthocyanins, other compounds from the flavonoid group, such as myricetin, quercetin 3-glucoside, rutin (quercetin 3-rutinoside), and kaempferol 3-glucoside were found in the extract. Condensed tannins (procyanidin B1 and B2) were the compounds present in greater quantities in addition to anthocyanins. Flavan-3-ol monomers (catechin and epicatechin) were also identified. Procyanidin B2 [epicatechin-(4β-8)-epicatechin] (C30H26O12, PB2) is the majority isomer of procyanidins, consisting of two (−)-epicatechin molecules connected by a bond between the 4′ and 8′ positions in a β-configuration. PB2 has good solubility in water and organic solvents, including DMSO, methanol and ethanol, and has been associated with bioactive properties, including antioxidant action [29].
The main phenolic acids identified in the extracts were caftaric, gallic, caffeic, and chlorogenic acids. Phenolic acids constitute about one-third of the phenols consumed in the diet and are widely found in fruits and vegetables. Caffeic acid derivatives are a group of compounds derived from the esterification of caffeic acid with organic acids, such as quinic acid, which forms chlorogenic acid and more frequently with tartaric acid, caftaric acid [30]. Liu et al. [31] demonstrated in their studies that caftaric and caffeic acids presented high antioxidant activity with the ability to significantly inhibit DPPH, OH, and ABTS radicals in addition to a strong ability to reduce iron.
The results above highlighted the efficiency of ultrasound technology in producing extracts with good yields of bioactive compounds, good quality of anthocyanins and high antioxidant activity, without using high temperatures or long extraction periods, saving time and energy on the extractive processes. Despite the bioactive potential of phenolic compounds, their health benefits and application in industry may be affected by their instability. Thus, techniques to protect phytochemicals, such as nanoencapsulation, can help preserve and enhance their functionality.
3.3. Characterization of nanoparticles
3.3.1. Encapsulation efficiency (EE%)
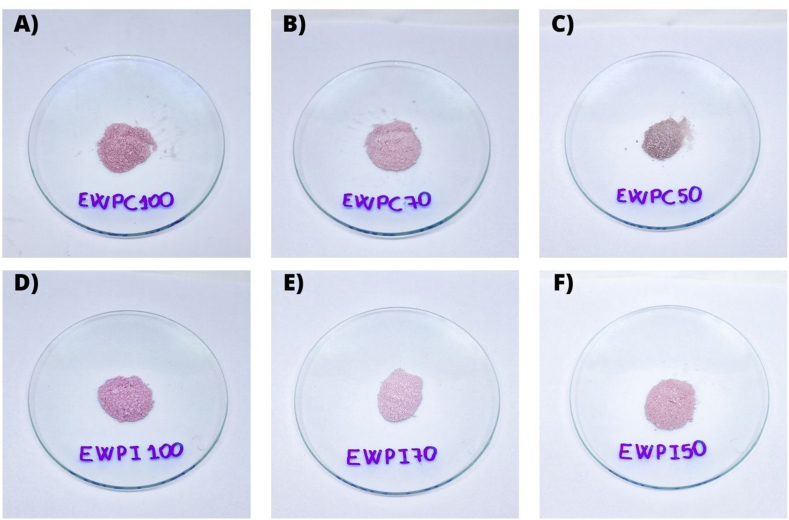
Table 1 presents the EE% results for TMA and TPC and Fig. 1(A–F) shows the freeze-dried powder resultant from the synthesized formulations. There was no significant difference between the EE% TPC for both encapsulating agents studied. However, for TMA, the EE% of the EWPC70 formulation was significantly lower than EWPC100 and EWPC50, a behavior observed for the same formulations based on WPI, showing the influence of the composition of the antisolvent phase on the EE% of anthocyanins in the systems.
Table 1.
Encapsulation Efficiency (EE%) results for total monomeric anthocyanins (TMA) and total phenolic compounds (TPC) of encapsulates based on pectin and whey protein concentrate (EWPC) or whey protein isolate (EWPI) precipitated in different antisolvent phase compositions.
| Sample Code | EE% TMA | EE% TPC | Sample Code | EE% TMA | EE% TPC |
|---|---|---|---|---|---|
| EWPC100 | 56.89 ± 1.29aA | 82.39 ± 1.59aA | EWPI100 | 35.24 ± 1.24aB | 84.61 ± 1.64aA |
| EWPC70 | 24.33 ± 5.35bA | 82.44 ± 1.22aA | EWPI70 | 26.79 ± 1.06bA | 84.65 ± 1.26aA |
| EWPC50 | 48.65 ± 1.74aA | 73.81 ± 6.72aA | EWPI50 | 36.33 ± 2.76aB | 75.80 ± 6.91aA |
Mean ± SD. Equal lowercase letters in the same column indicate means that did not differ from each other (Tukey, p ≤ 0.05). Equal capital letters on the same line (comparison between systems containing concentrated and isolated whey protein) indicate means that did not differ from each other in the same analysis (Tukey, p ≤ 0.05). Legend: EWPC100- encapsulate in pectin and concentrated whey protein precipitated in absolute acetone; EWPC70- encapsulate in pectin and concentrated whey protein precipitated in 70 % v/v acetone; EWPC50- encapsulate in pectin and concentrated whey protein precipitated in 50 % v/v acetone; EWPI100- encapsulate in pectin and isolated whey protein precipitated in absolute acetone; EWPI70- encapsulate in pectin and isolated whey protein precipitated in 70 % v/v acetone; and EWPI50- encapsulate in isolated whey protein precipitated in 50 % v/v acetone.
Fig. 1.
Powdered encapsulated materials. A) EWPC100; B) EWPC70; C) EWPC50; D) EWPI100 E) EWPI70; and F) EWPI150.
Legend: EWPC100- encapsulate in pectin and concentrated whey protein precipitated in absolute acetone; EWPC70- encapsulate in pectin and concentrated whey protein precipitated in 70 % v/v acetone; EWPC50- encapsulate in pectin and concentrated whey protein precipitated in 50 % v/v acetone; EWPI100- encapsulate in pectin and isolated whey protein precipitated in absolute acetone; EWPI70- encapsulate in pectin and isolated whey protein precipitated in 70 % v/v acetone; and EWPI50- encapsulate in isolated whey protein precipitated in 50 % v/v acetone.
Similarly, Calliari et al. [13] encapsulated an extract of Hibiscus sabdariffa rich in polyphenols, using zein as an encapsulating agent and nanoprecipitation method, achieving an EE% of 89 %. Gali et al. [32] also used the nanoprecipitation method to produce nanoparticles based on zein and gum arabic containing an extract of Ruta chalepensis L. rich in rutin and obtained an EE% of 69.9 %. From an efficiency point of view, the nanoencapsulation of anthocyanins still requires optimization. Escobar-Puentes et al. [33] used a nanoprecipitation technique to produce corn starch-based nanoparticles containing anthocyanins from Ardisia compressa, achieving EE% of 52 % for particles produced with traditional corn starch and 49 % for particles produced with amylopectin. Fierri et al. [34] encapsulated anthocyanins extracted from red cabbage (Brassica oleracea L. var. Capitata f. rubra, cultivar Cairo) in whey protein isolate and pectin nanoparticles through coacervation and found EE% of 29.3 %. According to the authors, a complex interplay of different factors, including the degree of glycosylation and/or acylation in the anthocyanin structure, appears to affect the affinity of each ACN to the polymers and, in turn, the overall encapsulation efficiency and stability of the molecules.
Since the type of encapsulating agent and the composition of the antisolvent phase had no influence on the EE% TPC and with the future application of the particles as a natural food colorant in mind, we prioritized working with the encapsulated systems that demonstrated the highest anthocyanin encapsulation efficiencies. In this context, the remaining particle characterizations were conducted for EWPC100, EWPC50, EWPI100, and EWPI50.
3.3.2. Scanning electron Microscopy (SEM)
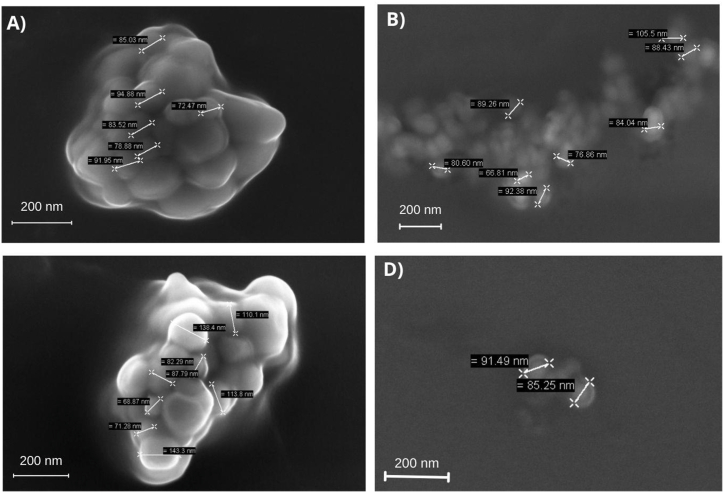
Fig. 2(A–D) shows the SEM images of the studied formulations, indicating that the particles generally exhibited a circular shape and a smooth surface without cracks or depressions.
Fig. 2.
Scanning Electron Microscopy Images obtained for each formulation studied: A) EWPC100 at a magnitude of 80,000×; B) EWPC50 at a magnitude of 50,000X; C) EWPI100 at a magnitude of 80,000X; D) EWPI50 at a magnitude of 50,000X.
Legend: EWPC100- encapsulate in pectin and concentrated whey protein precipitated in absolute acetone; EWPC50- encapsulate in pectin and concentrated whey protein precipitated in 50 % v/v acetone; EWPI100- encapsulate in pectin and isolated whey protein precipitated in absolute acetone; EWPI50- encapsulate in isolated whey protein precipitated in 50 % v/v acetone.
The encapsulate EWPC100 (Fig. 2A) and EWPI100 (Fig. 2C) are found in small clusters, with physical sizes ranging from 72 to 95 nm for systems synthesized with whey protein concentrate and from 69 to 143 nm for systems based on whey protein isolate. EWPC50 (Fig. 2B) presents large agglomerates of particles with sizes around 100 nm, and this tendency to agglomerate is probably due to the increase in attraction forces and reduction in repulsion forces between the molecules due to the nanometric size [35]. EWPI50 particles (Fig. 2D) appear as small clusters of particles with sizes below 100 nm. The sizes observed in the SEM analysis were consistent with the results obtained through the DLS analysis. A homogeneous morphology is essential for the stability of nanoparticles. It can influence storage time when applied in food formulations and affect cellular absorption due to the interaction between the nanoparticle surface and the epithelium, influencing biological effects [5].
3.3.3. Particle size and polydispersity index (PDI) by DLS
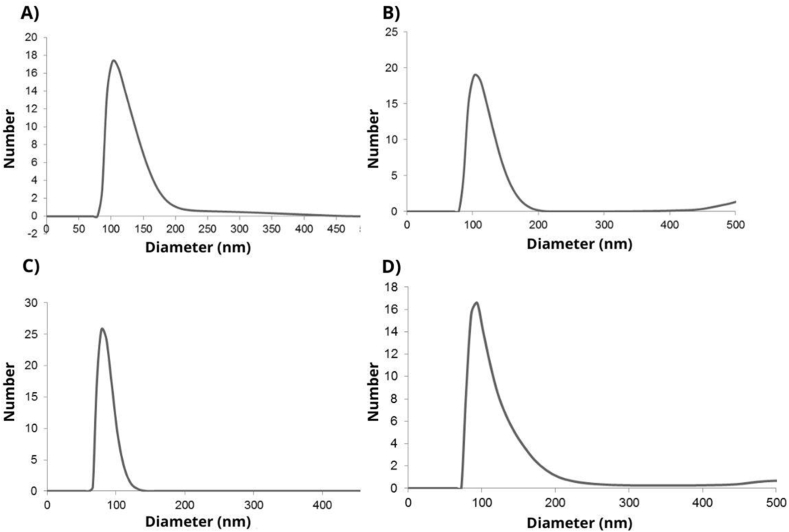
The results of particle size (nm) and PDI analyses of the particles (EWPC100: 116 ± 29.50 nm and 0.003 ± 0.004; EWPC50: 111 ± 23.40 nm and 0.005 ± 0.008; EWPI100: 82 ± 10.80 nm and 0; EWPI50: 107 ± 33.90 nm and 0.001 ± 0.001) are consistent with the physical sizes observed by SEM images. Furthermore, it is possible to note that the low PDI values indicate that the evaluated systems are highly monodisperse, with a very homogeneous size distribution, and can be seen by the size distribution graphs (Fig. 3A–D).
Fig. 3.
Particle size distribution for EWPC100 (A), EWPC50 (B), EWPI100 (C) and EWPI50 (D)
Legends: EWPC100- encapsulate in pectin and concentrated whey protein precipitated in absolute acetone; EWPC50- encapsulate in pectin and concentrated whey protein precipitated in 50 % v/v acetone; EWPI100- encapsulate in pectin and isolated whey protein precipitated in absolute acetone; EWPI50- encapsulate in isolated whey protein precipitated in 50 % v/v acetone.
Recent studies using similar wall materials to produce nanoparticles containing phenolic compounds have reported comparable particle size values to those in this study. Rosales et al. [5] encapsulated blackberry anthocyanins in nanoparticles produced by self-assembling pectin and lysozyme, obtaining sizes of 198.5 nm and PDI of 0.18. Rashid et al. [11] encapsulated a pomegranate peel extract rich in phenolic compounds in particles of whey protein isolate with maltodextrin and Tween 80 as surfactant, with ultrasonication to reduce particle size, obtaining sizes of 218.8 nm and PDI of 0.15.
It is also important to highlight the effect of nanoprecipitation as an efficient encapsulation method to obtain particles containing phenolic compounds on the nanoscale. Calliari et al. [13] produced nanoparticles containing Hibiscus sabdariffa extract rich in phenolic compounds with zein as wall material, obtaining particles 176 nm in size and a PDI of 0.18. Gali et al. [31] produced nanoparticles based on zein and gum arabic containing an extract of Ruta chalepensis L. rich in rutin, a flavonoid, and obtained particle sizes from 80 to 170 nm.
Interactions between phenolic compounds and pectin have been studied to preserve the properties and stabilize the molecular structure of these compounds, protecting them from degradation and preserving their antioxidant action [3]. Furthermore, the choice of using whey proteins in combination with pectin for the synthesis of nanoparticles was due to the functionality of the proteins to act as surfactants. When interacting with different materials, proteins undergo conformational changes to allow more favorable bonds, stabilizing the complex through electrostatic interactions and reducing system surface tension. Therefore, when opting for the combination of biopolymers, whey proteins acted as wall material and surfactant, forming spherical nanoparticles with a smooth surface [35], as observed in the images obtained by SEM.
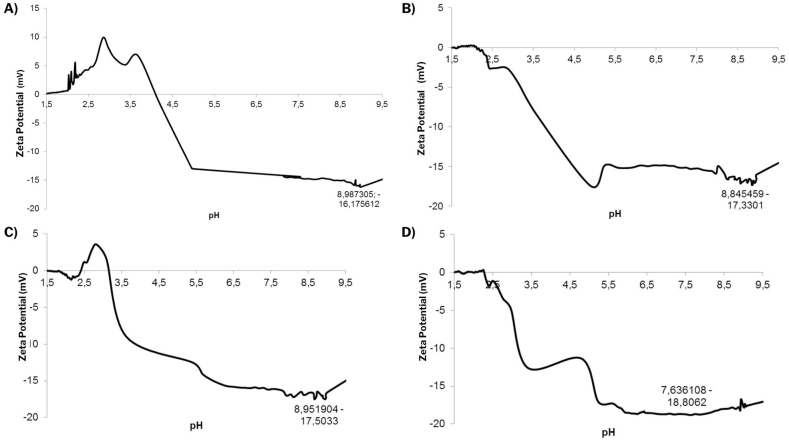
3.3.4. Zeta potential (ζ)
The pH is the parameter that most influences Zeta potential measurements, especially in aqueous dispersions, which makes it relevant when considering the application of particles in food and pharmaceutical formulations. The Zeta potential varies with pH and becomes more positive and negative in magnitude with acidic and basic pH, respectively [36].
In this study, the systems presented higher instability at acidic pH, where the Zeta potentials tended to zero in the pH ranges between 2.0 and 4.0. An inflection point at pH close to 5.0–5.5 was identified, corresponding to the pH range of the isoelectric point of whey proteins. From the inflection point onwards, the Zeta potential assumed higher values, indicating the pH range where the systems presented greater stability, that is, in the pH range outside the pI of the proteins used. For EWPC100 (Fig. 4 A), the highest value of Zeta potential was −16.18 mV at pH ~ 9, EWPC50 (Fig. 4 B) was −17.33 mV at pH = 8.85, EWPI100 (Fig. 4C) was −17.5 mV at pH = 8.95, and EWPI50 (Fig. 4 D) was −18.80 mV at pH = 7.64, values that categorize the evaluated systems as relatively stable. Although the best Zeta potential values occur at higher pH, the curves remain almost constant from pH ~ 6.0, where the systems enter a zone of relative stability, indicating the most appropriate pH range for future applications.
Fig. 4.
Zeta potential curves as a function of pH for each formulation studied: A) EWPC100; B) EWPC50; C) EWPI100; D) EWPI50.
Legends: EWPC100- encapsulate in pectin and concentrated whey protein precipitated in absolute acetone; EWPC50- encapsulate in pectin and concentrated whey protein precipitated in 50 % v/v acetone; EWPI100- encapsulate in pectin and isolated whey protein precipitated in absolute acetone; EWPI50- encapsulate in isolated whey protein precipitated in 50 % v/v acetone.
Similarly, Ghasemi et al. [16] evaluated the effect of pH on the Zeta potential of nanocomplexes of whey protein concentrate and pectin containing orange peel oil produced by ultrasonication and using Tween 80 as an emulsifying agent and observed that the Zeta potential at pH values of 3.0, 6.0 and 9.0 were +1.7, −19.0 and −30.3 mV, respectively. This result followed a very similar trend to what was observed in the present study. The authors argue that at pH = 3.0, below the isoelectric point (pI) of proteins, the biopolymers neutralize themselves, and the resulting charge is very close to zero (+1.7 mV).
As the pH increases, an increase in Zeta potential values is observed. This may occur due to the excess of unbound carboxylic acids of pectin in the nanoparticles, in which the deprotonation caused by the increase in pH (especially at pH 4 to 10) overcomes the protonation of the proteins, leading to negative values of the Zeta potential. Consequently, electrostatic repulsion occurs between the nanoparticles, thus preventing sedimentation and flocculation [5].
During the experiments, it was observed that the pH of the encapsulated dispersed directly in distilled water was around 5.0. This value is in the range of particle instability, which generated flocculation and aggregation of systems in solution. The slightly acidic characteristic of the particles, when dispersed directly in distilled water, probably comes from the particle encapsulation process. Considering that the freeze-dried jambolan extract and pectin, when in solution, presented pH values around 3.0, and when added to the solution containing whey proteins, they reached pH close to 4.0, a pH range in the encapsulation process occurred. The stability results obtained through the Zeta potential analysis were decisive for the other characterization analyses to be carried out, justifying the use of the phosphate buffer (pH = 7.0) to determine particle size by DLS and antioxidant activity.
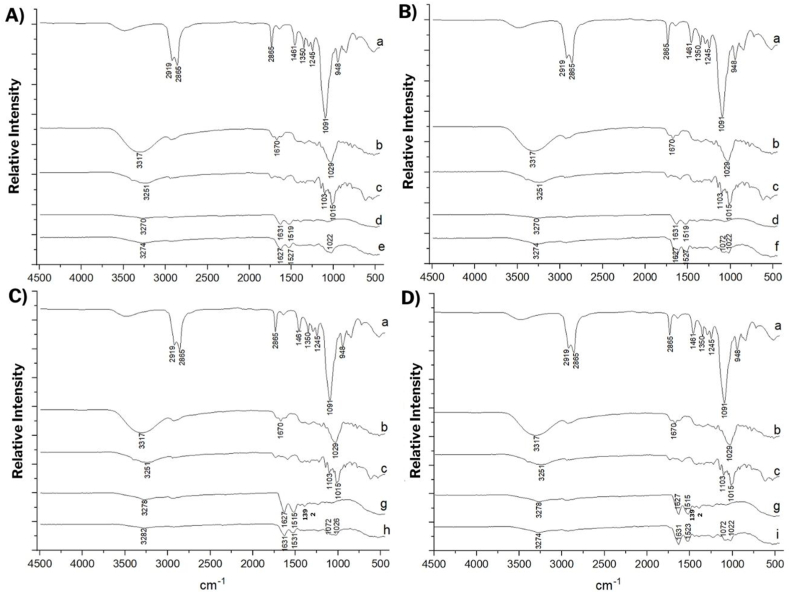
3.3.5. Fourier transform infrared spectroscopy (FTIR)
The FTIR spectra (Fig. 5A–D) show the results for Tween 80, encapsulating agents, crude jambolan extract, and the encapsulates obtained (EWPC100, EWPC50, EWPI100, and EWPI50).
Fig. 5.
Fourier transform infrared spectra of the different encapsulated powders obtained by the nanoprecipitation technique. A) EWPC100, B) EWPC50, C) EWPI100 and D) EWPI50.
Legends: a) Tween 80, b) Crude extract, c) Pectin, d) whey protein concentrate; e) EWPC100- encapsulate in pectin and whey protein concentrate precipitated in absolute acetone; f) EWPC50 - encapsulate in pectin and whey protein concentrate precipitated in 50 % v/v acetone; g) whey protein isolate, h) EWPI100 - encapsulate in pectin and whey protein isolate precipitated in absolute acetone, i) EWPI50 - encapsulate in pectin and whey protein isolate precipitated in acetone 50 % v/v.
For the surfactant Tween 80, bands around 2919 and 2865 cm−1 were observed, indicating the stretching of C⼀H bonds from the methylene group, and at 1461 cm−1, which corresponds to the folding of C⼀H bonds from this same group. Furthermore, the band at 1097 cm-1 is associated with stretching C⼀O⼀C bonds [37].
In the pectin spectrum, it is possible to observe the band 3251 cm−1, which represents the interactions between groups O⼀H of carboxylic acids, and the bands 1015 and 1103 cm−1 that correspond to the stretching of the C⼀O bond of the galacturonic acids from pectin chains [38].
For whey protein isolate, the presence of the characteristic 3278 cm-1 band represents the absorption of N⼀H groups amines and amides of proteins; in WPC, this band appears at 3270 cm-1. The band 1392 cm−1 corresponds to the stretching of the C⼀O bond, 1627 cm-1 to amide I, and 1515 cm-1 to N⼀H folding and C⼀ N stretching of amide II. For whey protein concentrate, the absorption bands for amides I and II, respectively, were observed at 1631 cm−1 and 1519 cm−1 [39].
In the jambolan extract-rich polyphenols spectrum, bands associated with interactions between O⼀H groups of carboxylic acids (3317 cm−1) can be identified. The softness and width of these bands are associated with materials rich in anthocyanins. At 1029 cm−1, the band that may be related to the stretching of the C⼀O bond of the functional group of phenols and the folding of C⼀H in the carbon chains of anthocyanins and other phenolic compounds [38] can also be observed.
The spectra of the encapsulates showed that all synthesized materials retained the characteristic profile of the proteins used as wall material, suggesting that the proteins cover the exterior of the particles but with certain displacements caused by interactions with other materials present in the systems. The absorption bands for amides I and II, respectively, were observed in EWPC100 and EWPC50 at 1627 and 1527 cm−1, in EWPI100 at 1631 and 1531 cm−1, and in EWPI50 at 1631 and 1523 cm−1.
The formation of new bands was identified in the spectra of the synthesized materials in the regions from 1072 to 1022 cm−1 in EWPC100, EWPC50, and EWPI50, and 1072 and 1026 cm−1 for EWPI100. The surging of these bands indicates the presence of new chemical interactions between the proteins, the extract, and the pectin, showing that the nanoprecipitation method was efficient in encapsulating the jambolan extract rich in phenolic compounds in whey protein and pectin nanoparticles.
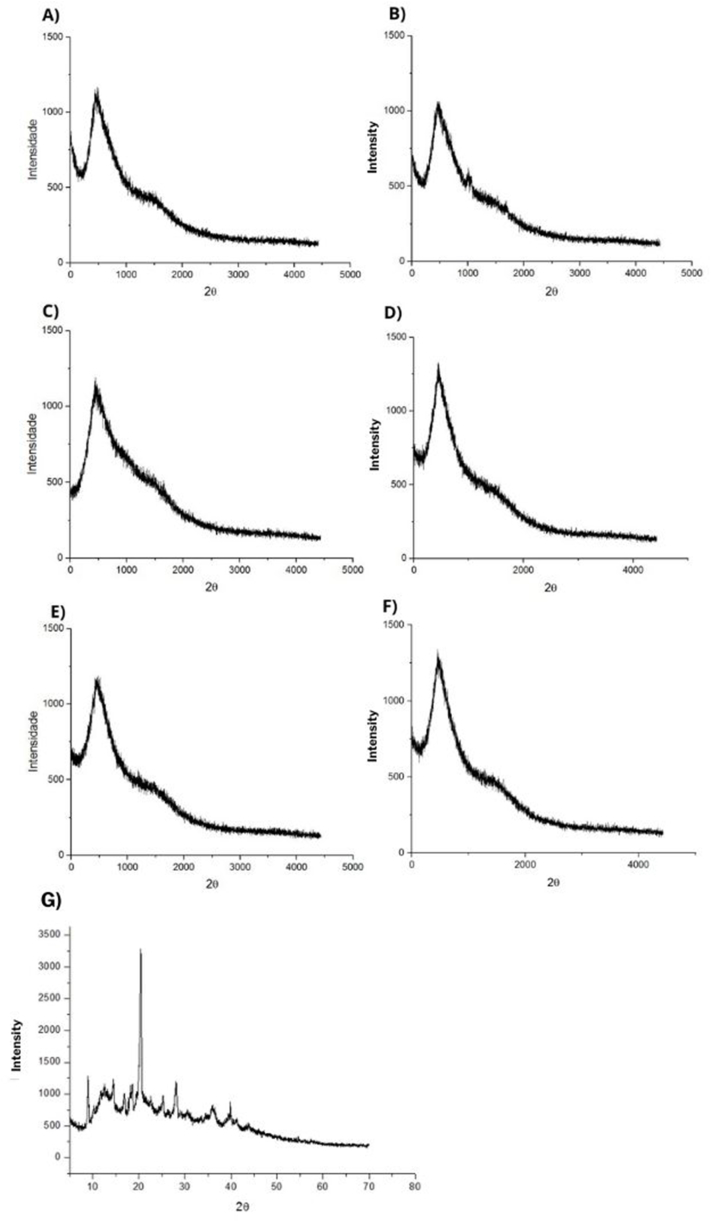
3.3.6. X-ray diffraction
Fig. 6(A–G) shows the diffractograms for the proteins used as wall materials and the nanoparticles obtained. It is possible to observe that the particles preserved the same pattern and amorphous structure observed in the diffractograms of whey proteins, which shows the predominance of structures with disorganized molecular chains.
Fig. 6.
X-ray diffraction patterns of encapsulating agents and encapsulates A) WPC, B) WPI, C) EWPC100, D) EWPI100, E) EWPC50, F) EWPI50 and G) PEC.
Legends: A) WPC- whey protein concentrate; B) WPI- whey protein isolate; C) EWPC100- encapsulate in pectin and whey protein concentrate precipitated in absolute acetone; D) EWPI100- encapsulate in pectin and whey protein isolate precipitated in acetone to Absolute; E) EWPC50- encapsulate in pectin and whey protein concentrate precipitated in 50 % v/v acetone; F) EWPI50- encapsulate in pectin and whey protein isolate precipitated in 50 % v/v acetone; G) PEC- Pectin.
By evaluating the diffractograms of pectin (Fig. 6G), it can be concluded that it has a semi-crystalline structure due to the presence of defined and more intense peaks in signals at 2θ of ∼10°, 20°, and 30°. However, such behavior was not observed in the encapsulated materials Fig. 6(A–F), which corroborates the FTIR findings that suggest that the particles are covered by proteins, presenting an almost identical diffractogram profile.
3.4. Antioxidant activity of particles
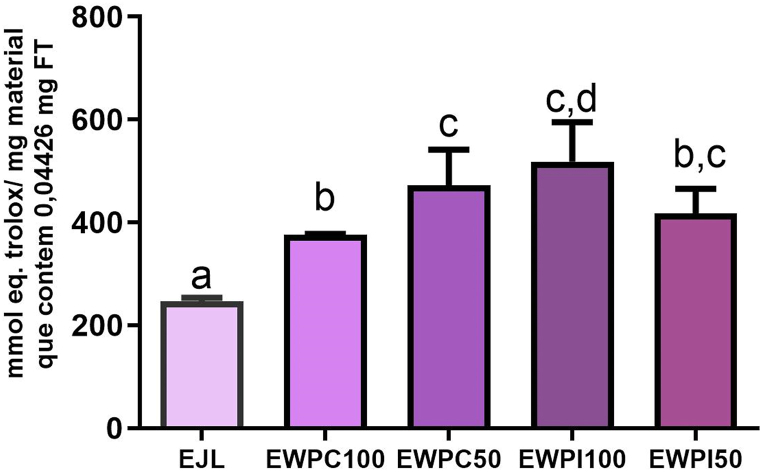
Based on the results of total phenolic compounds in the jambolan extract (JE) (0.04426 mg TPC/mg JE) and considering that 2.2 g of this extract were used in each encapsulation system, it can be inferred that 97.38 mg TPC were initially added to each system. Subsequently, the encapsulation efficiency was evaluated for each system based on the TPC determination (EE% TPC). In this sense, it was possible to establish the amount in milligrams of powdered encapsulate that would present the TPC content of 0.04426 mg, based on JE (Table 1 Supplementary Material). This quantity of each powdered particle system was then used to determine the antioxidant activity.
Initially, the antioxidant activity of the freeze-dried jambolan extract (JE) was determined, obtaining a value of 246.81 ± 2.76 μmol Trolox Eq./mg of JE. The particle antioxidant activity results for EWPC100 (375.36 ± 0.89 μmol eq. of Trolox/0.82 mg), EWPC50 (472.62 ± 27.68 μmol eq. of Trolox/0.94 mg), EWPI100 (518.31 ± 30.96 μmol eq. of Trolox/1.19 mg) and EWPI50 (417.14 ± 19.62 μmol eq. of Trolox/0.89 mg) show that the antioxidant activity of all particle systems was significantly higher than the antioxidant activity of EJL for the same CFT content (0.04426 mg FT). The antioxidant activity of these phenolic compounds was enhanced by 52.08 % in EWPC100, 91.49 % in EWPC50, 110 % in EWPI100, and 69.01 % in EWPI50 (Fig. 7).
Fig. 7.
Antioxidant activity of freeze-dried jambolan extract (JE) compared to the synthesized particle systems.
Legends: Equal lowercase letters in the same column indicate means that did not differ from each other (Tukey, p ≤ 0.05). Legends: EWPC100- encapsulate in pectin and concentrated whey protein precipitated in absolute acetone; EWPC50- encapsulate in pectin and concentrated whey protein precipitated in 50 % v/v acetone; EWPI100- encapsulate in pectin and isolated whey protein precipitated in absolute acetone; and EWPI50- encapsulate in isolated whey protein precipitated in 50 % v/v acetone.
Among the systems, EWPI100 exhibited no significant difference in antioxidant activity compared to EWPC50. However, it showed a significantly higher antioxidant activity than that observed in EWPC100 and EWPI50, with no significant difference between the latter two. EWPC50 also did not differ from EWPC100 and EWPI50. The results highlight that EWPI100 and EWPC50 were the most effective systems in enhancing the antioxidant activity of jambolan phenolic compounds. These results prove that the encapsulation process of phenolic compounds, especially anthocyanins, from jambolan using pectin and whey proteins concentrate and isolate by nanoprecipitation not only preserved the antioxidant activity of the encapsulated compounds but also enhanced this activity. It is important to emphasize that the antioxidant activity is related to bioactive properties attributed to the phenolic compounds in jambolan and their benefits for human health [1].
Nanoencapsulation of bioactive compounds has been studied for protection, maintenance, or enhancement of the antioxidant activity of bioactive compounds. Kenari & Razavi [40] produced nanoemulsions (particle sizes from 76.5 to 99.3 nm) containing extract from Bouganvillea spetabilis, a source of phenolic compounds and anthocyanins, using Urtica dioica seed gum as wall materials and Tween 80 as surfactant. They observed that the encapsulated extract presented higher antioxidant activity values than the crude extract, which was evaluated by DPPH and FRAP tests. Similar results were presented by Soleymanfallah et al. [41] by encapsulating an aqueous extract of grapes in chitosan and tripolyphosphate nanoparticles.
The enhancement of antioxidant activity observed in the present study and in the literature cited can be associated with the increase in the surface area provided by the small sizes obtained through nanoencapsulation. This allows for a greater number of chemical interactions, stabilizes the active compounds, and increases the dispersibility, bioaccessibility, and bioavailability of the encapsulated compounds [21]. Future studies may clarify the mechanisms involved in the findings of the present work.
The biopolymers used as wall materials can also affect the antioxidant properties observed in the particles. By encapsulating naringenin, a phenolic compound from the flavonoid group with high antioxidant activity, in nanoparticles (140 nm) of isolated whey protein, Yin et al. [42] observed a synergistic antioxidant effect between protein and flavonoid activities. The authors reported that whey proteins, composed of β-lactoglobulin, α-lactalbumin, and bovine serum albumin, may have antioxidant activity. β-lactoglobulin contains five cysteines, which are converted into two intramolecular disulfide bridges and a free thiol group (-SH), and the free radical scavenging capacity of these proteins is mainly related to the thiol group.
The antioxidant activity of phenolic compounds is attributed to the hydroxyl groups linked to the phenol rings of their structures. In the present study, the particle characterization results (FTIR and XRD) demonstrated that the particles mainly preserved the structural profile of the proteins used as encapsulating agents, which may contribute to the increase observed in the antioxidant potential. Moreover, the increase in surface area resulting from nanoencapsulation, along with the stabilization and subsequent reduction in nanoparticle aggregation at pH 7.0, likely enhances the exposure of functional groups, thereby facilitating interactions and enhancing antioxidant activity.
Fierri et al. [34] compared the antioxidant activity by ABTS assay of extracted and encapsulated anthocyanins from red cabbage in whey protein isolate and pectin nanoparticles (368.2 nm) through coacervation. They reported that the antioxidant activity of the nanoparticles (16.07 mM) was lower than the extract (22.05 mM) at the same anthocyanin concentration of 1042 mg/L, which the authors attribute to the interaction between the biopolymers and the ACNs that limited the scavenging capacity of these compounds. The results of the antioxidant activity of the particles support the choice of nanoprecipitation for the encapsulation of anthocyanins and other phenolic compounds from jambolan. This method has proved to be an excellent option for the production of nanocarrier systems, with emphasis on the EWPC50 and EWPI100 systems. These systems exhibited outstanding physicochemical and structural characteristics, in addition to high antioxidant activity, enhanced by 91.49 and 110 % over the raw extract, respectively. This property is directly related to several benefits to human health.
Future analysis will investigate which of these systems has the best characteristics for application in foods and nutraceuticals, in addition to evaluation in pre-clinical studies. Whey proteins and pectin used as wall materials are natural biopolymers of high biological value and have diverse technological applications, especially in the food industry. The nanoparticle systems produced and characterized by the present study have great potential for developing future studies that evaluate the particle's stability, bioavailability, and safety, aiming to expand their application possibilities.
4. Conclusions
Ultrasound-assisted extraction proved an effective method for obtaining phenolic compounds from jambolan, including anthocyanins. Notably, the nanoparticles produced through the nanoprecipitation technique, using a combination of different encapsulating agents and antisolvent phases, have demonstrated efficiency in encapsulating the target compounds. This process yields nanoscale particles with satisfactory physicochemical properties and enhanced antioxidant potential. Such advancements can ensure the protection and improvement of the application of these compounds in food products.
Funding
This study was funded by the Foundation for Support and Promotion of Science, Technology and Innovation of Rio Grande do Norte (FAPERN) – Process No.: SEI no. 10910019.000264/2021-98, with the proposal title of Nutritional and Functional Bioprospecting of the Socio Biodiversity of the Semiarid Region for products.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Jessica Anarellis Barbosa dos Santos: Writing – original draft, Validation, Methodology, Investigation, Formal analysis, Conceptualization. Cristiane Fernandes Assis: Resources, Methodology. Cicero Flavio Soares Aragao: Supervision. Marcos dos Santos Lima: Resources, Investigation. Thais Souza Passos: Writing – review & editing, Validation, Resources, Methodology, Conceptualization. Juliana Kelly da Silva-Maia: Writing – review & editing, Validation, Project administration, Methodology, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was carried out with the support of the Coordination for the Improvement of Higher Education Personnel – Brazil (CAPES) – Financing Code 001.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e36973.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Qamar M., Akhtar S., Ismail T., Wahid M., Abbas M.W., Mubarak M.S., Yuan Y., Barnard R.T., Ziora Z.M., Esatbeyoglu T. Phytochemical profile, biological properties, and food applications of the medicinal plant Syzygium cumini. Foods. 2022;11:378. doi: 10.3390/foods11030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tavares I.M. de C., Lago-Vanzela E.S., Rebello L.P.G., Ramos A.M., Gómez-Alonso S., García-Romero E., Da-Silva R., Hermosín-Gutiérrez I. Comprehensive study of the phenolic composition of the edible parts of jambolan fruit (Syzygium cumini (L.) Skeels) Food Res. Int. 2016;82:1–13. doi: 10.1016/j.foodres.2016.01.014. [DOI] [Google Scholar]
- 3.Rosales O. João Paulo Fabi, Valorization of polyphenolic compounds from food industry by-products for application in polysaccharide-based nanoparticles. Front. Nutr. 2023;10 doi: 10.3389/fnut.2023.1144677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabino L.B. de S., Filho E.G.A., Fernandes F.A.N., de Brito E.S., Júnior I.J. da S. Optimization of pressurized liquid extraction and ultrasound methods for recovery of anthocyanins present in jambolan fruit (Syzygium cumini L.) Food Bioprod. Process. 2021;127:77–89. doi: 10.1016/j.fbp.2021.02.012. [DOI] [Google Scholar]
- 5.Rosales O., Silva, Rebello Lourenço Felipe, Aymoto M. João Paulo Fabi, Nanoencapsulation of anthocyanins from blackberry (Rubus spp.) through pectin and lysozyme self-assembling. Food Hydrocolloids. 2021;114:106563. doi: 10.1016/j.foodhyd.2020.106563. [DOI] [Google Scholar]
- 6.Ahmad A., Mushtaq Z., Saeed F. Muhammad Afzaal, Entessar Al Jbawi, Ultrasonic-assisted green synthesis of silver nanoparticles through cinnamon extract: biochemical, structural, and antimicrobial properties. Int. J. Food Prop. 2023;26:1984–1994. doi: 10.1080/10942912.2023.2238920. [DOI] [Google Scholar]
- 7.Andishmand Hashem, Azadmard-Damirchi Sodeif, Hamishekar Hamed, Torbati M., Rafi Kharazmi Mohammad, Savage G.P., Tan C. Seid Mahdi Jafari, Nano-delivery systems for encapsulation of phenolic compounds from pomegranate peel. Adv. Colloid Interface Sci. 2023;311:102833. doi: 10.1016/j.cis.2022.102833. [DOI] [PubMed] [Google Scholar]
- 8.Homayouni Rad A., Ebrahimi B., Gharehbeglou P. Influence of polymeric complexes on the stability and releasing behavior of phenol-loaded nano-emulsions: modeling and optimization. J. Mol. Liq. 2022;349 doi: 10.1016/j.molliq.2021.118089. [DOI] [Google Scholar]
- 9.Sun Y., Chi J., Ye X., Wang S., Liang J., Yue P., Julian McClements David, Gao X. Nanoliposomes as delivery system for anthocyanins: physicochemical characterization, cellular uptake, and antioxidant properties. LWT (Lebensm.-Wiss. & Technol.) 2021;139:110554. doi: 10.1016/j.lwt.2020.110554. [DOI] [Google Scholar]
- 10.Salah M., Mansour M., Zogona D., Xu X. Nanoencapsulation of anthocyanins-loaded β-lactoglobulin nanoparticles: characterization, stability, and bioavailability in vitro. Food Res. Int. 2020;137 doi: 10.1016/j.foodres.2020.109635. [DOI] [PubMed] [Google Scholar]
- 11.Rashid R., Ahmad Wani Sajad, Manzoor S., Masoodi F.A., Altaf A. Nanoencapsulation of pomegranate peel extract using maltodextrin and whey protein isolate. Characterisation, release behaviour and antioxidant potential during simulated invitro digestion. Food Biosci. 2022;50:102135. doi: 10.1016/j.fbio.2022.102135. [DOI] [Google Scholar]
- 12.Lima F., de D., Barbosa J., Buchveitz Pires Juliani, Jéssica Siebeneichler Tatiane, Hüttner Kringel Dianini, Fajardo A.R., Valmor Rombaldi Cesar, Renato A., Martins F.T. Encapsulation of anthocyanic extract of jambolan (Syzygium cumini (L.)) in zein sub-micron fibers produced by electrospinning. Food Biophys. 2022;18:133–147. doi: 10.1007/s11483-022-09758-3. [DOI] [Google Scholar]
- 13.Calliari C.M., Campardelli R., Pettinato M., Perego P. Encapsulation of Hibiscus sabdariffa extract into zein nanoparticles. Chem. Eng. Technol. 2020;43:2062–2072. doi: 10.1002/ceat.202000194. [DOI] [Google Scholar]
- 14.Daniel W., Verruck Silvani, Rodrigues Monteiro Alcilene, Valencia Germán Ayala. The mechanism, biopolymers and active compounds for the production of nanoparticles by anti-solvent precipitation: a review. Food Res. Int. 2023;168:112728. doi: 10.1016/j.foodres.2023.112728. [DOI] [PubMed] [Google Scholar]
- 15.Raza M.A., Ahmad A., Saeed F., Hussain M., Afzaal Muhammad, Rasheed A. Maize bran arabinoxylans mediated green synthesis of silver nanoparticles and their incorporation in gelatin-based packaging film. Food Packag. Shelf Life. 2024;43:101301. doi: 10.1016/j.fpsl.2024.101301. [DOI] [Google Scholar]
- 16.Ghasemi S., Jafari S.M., Assadpour E., Khomeiri M. Production of pectin-whey protein nano-complexes as carriers of orange peel oil. Carbohydr. Polym. 2017;177:369–377. doi: 10.1016/j.carbpol.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Association of Official Analytical Chemists . Association of Official Analytical Chemists; 1995. Official Methods of Analysis of the. [Google Scholar]
- 18.Swain T., Hillis W.E. The phenolic constituents ofPrunus domestica. I.—the quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959;10:63–68. doi: 10.1002/jsfa.2740100110. [DOI] [Google Scholar]
- 19.Lee J., Durst R.W., Wrolstad R.E., Eisele T., Giusti M.M., Hach J., Hofsommer H., Koswig S., Krueger D.A., Kupina S., Martin S.K., Martinsen B.K., Miller T.C., Paquette F., Ryabkova A., Skrede G., Trenn U., Wightman J.D. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J. AOAC Int. 2005;88:1269–1278. doi: 10.1093/jaoac/88.5.1269. [DOI] [PubMed] [Google Scholar]
- 20.Rufino M. do S.M., Alves R.E., de Brito E.S., Pérez-Jiménez J., Saura-Calixto F., Mancini-Filho J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010;121:996–1002. doi: 10.1016/j.foodchem.2010.01.037. [DOI] [Google Scholar]
- 21.Morais N. de S., Passos T.S., Ramos G.R., Ferreira V.A.F., Moreira S.M.G., Chaves Filho G.P., Barreto A.P.G., Leite P.I.P., de Almeida R.S., Paulo C.L.R., Fernandes R., da Silva S.Â.D., Nascimento S.S. da C., de Sousa Júnior F.C., de Assis C.F. Nanoencapsulation of buriti oil (Mauritia flexuosa L.f.) in porcine gelatin enhances the antioxidant potential and improves the effect on the antibiotic activity modulation. PLoS One. 2022;17 doi: 10.1371/journal.pone.0265649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coelho E.M., de Azevêdo L.C., Corrêa L.C., Bordignon-Luiz M.T., Lima M. dos S. Phenolic profile, organic acids and antioxidant activity of frozen pulp and juice of the jambolan (Syzygium cumini) J. Food Biochem. 2015;40:211–219. doi: 10.1111/jfbc.12209. [DOI] [Google Scholar]
- 23.Singh B., Singh J.P., Kaur A., Singh N. Insights into the phenolic compounds present in jambolan (Syzygium cumini) along with their health-promoting effects. Int. J. Food Sci. Technol. 2018;53:2431–2447. doi: 10.1111/ijfs.13841. [DOI] [Google Scholar]
- 24.Alsaud N., Farid M. Insight into the influence of grinding on the extraction efficiency of selected bioactive compounds from various plant leaves. Appl. Sci. 2020;10:6362. doi: 10.3390/app10186362. [DOI] [Google Scholar]
- 25.Virgen-Carrillo C.A., Valdés Miramontes E.H., Fonseca Hernández D., Luna-Vital D.A., Mojica L. West Mexico berries modulate α-amylase, α-glucosidase and pancreatic lipase using in vitro and in silico approaches. Pharmaceuticals. 2022;15:1081. doi: 10.3390/ph15091081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang W., Zhang H., Liu W., Li C. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. - Sci. B. 2012;13:94–102. doi: 10.1631/jzus.b1100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lestario L.N., Howard L.R., Brownmiller C., Stebbins N.B., Liyanage R., Lay J.O. Changes in polyphenolics during maturation of Java plum (Syzygium cumini Lam.) Food Res. Int. 2017;100:385–391. doi: 10.1016/j.foodres.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 28.de Brito E.S., de Araújo M.C.P., Alves R.E., Carkeet C., Clevidence B.A., Novotny J.A. Anthocyanins present in selected tropical fruits: acerola, jambolão, jussara, and guajiru. J. Agric. Food Chem. 2007;55:9389–9394. doi: 10.1021/jf0715020. [DOI] [PubMed] [Google Scholar]
- 29.Chen J., Zhong K., Jing Y., Liu S., Qin S., Peng F., Li D., Peng C. Procyanidin B2: a promising multi-functional food-derived pigment for human diseases. Food Chem. 2023;420:136101. doi: 10.1016/j.foodchem.2023.136101. [DOI] [PubMed] [Google Scholar]
- 30.Vendramin V., Viel A., Vincenzi S. Caftaric acid isolation from unripe grape: a “green” alternative for hydroxycinnamic acids recovery. Molecules. 2021;26:1148. doi: 10.3390/molecules26041148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Q., Liu F., Zhang L., Niu Y., Liu Z., Liu X. Comparison of chicoric acid, and its metabolites caffeic acid and caftaric acid: in vitro protection of biological macromolecules and inflammatory responses in BV2 microglial cells. Food Sci. Hum. Wellness. 2017;6:155–166. doi: 10.1016/j.fshw.2017.09.001. [DOI] [Google Scholar]
- 32.Gali L., Bedjou F., Ferrari G., Donsì F. Formulation and characterization of zein/gum Arabic nanoparticles for the encapsulation of a rutin-rich extract from Ruta chalepensis L. Food Chem. 2022;367 doi: 10.1016/j.foodchem.2021.129982. [DOI] [PubMed] [Google Scholar]
- 33.Escobar-Puentes A.A., García-Gurrola A., Rincón S., Zepeda A., Martínez-Bustos F. Effect of amylose/amylopectin content and succinylation on properties of corn starch nanoparticles as encapsulants of anthocyanins. Carbohydr. Polym. 2020;250:116972. doi: 10.1016/j.carbpol.2020.116972. [DOI] [PubMed] [Google Scholar]
- 34.Fierri I., De Marchi L., Chignola R., Rossin G., Bellumori M., Perbellini A., Mancini I., Romeo A., Ischia G., Saorin A. Federica mainente, gianni zoccatelli, nanoencapsulation of anthocyanins from red cabbage (Brassica oleracea L. Var. Capitata f. rubra) through coacervation of whey protein isolate and apple high methoxyl pectin. Antioxidants. 2023;12:1757. doi: 10.3390/antiox12091757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Queiroz J.L.C., De Araújo Costa R.O., Rodrigues Matias L.L., De Medeiros A.F., Teixeira Gomes A.F., Santos Pais T.D., Passos T.S., Maciel B.L.L., Dos Santos E.A., De Araújo Morais A.H. Chitosan-whey protein nanoparticles improve encapsulation efficiency and stability of a trypsin inhibitor isolated from Tamarindus indica L. Food Hydrocolloids. 2018;84:247–256. doi: 10.1016/j.foodhyd.2018.06.010. [DOI] [Google Scholar]
- 36.Bhattacharjee S. DLS and zeta potential – what they are and what they are not? J. Contr. Release. 2016;235:337–351. doi: 10.1016/j.jconrel.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 37.Roy Choudhury S., Mandal A., Chakravorty D., Gopal M., Goswami A. Evaluation of physicochemical properties, and antimicrobial efficacy of monoclinic sulfur-nanocolloid. J. Nanoparticle Res. 2013;15 doi: 10.1007/s11051-013-1491-y. [DOI] [Google Scholar]
- 38.Salleh Nurhazwani, Goh Kelvin K.T., Waterland M.R., Huffman L.M., Weeks M., Matia-Merino L. The influence of anthocyanins in pectin-whey protein complexation using a natural pigmented blackcurrant pectin. Food Hydrocolloids. 2023;140:108672. doi: 10.1016/j.foodhyd.2023.108672. [DOI] [Google Scholar]
- 39.de Medeiros A.K., Gomes C., Louize M., Freitas L., Medeiros I., Lopes Porto Dayanne, Soares F., Leal B., Heloneida A., Souza Passos Thaís. Nanoencapsulation improved water solubility and color stability of carotenoids extracted from Cantaloupe melon (Cucumis melo L.) Food Chem. 2019;270:562–572. doi: 10.1016/j.foodchem.2018.07.099. [DOI] [PubMed] [Google Scholar]
- 40.Kenari R.E., Razavi R. Encapsulation of bougainvillea (Bougainvillea spectabilis) flower extract in Urtica dioica L. seed gum: characterization, antioxidant/antimicrobial properties, and in vitro digestion. Food Sci. Nutr. 2022 doi: 10.1002/fsn3.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soleymanfallah S., Khoshkhoo Z., Hosseini S.E., Azizi M.H. Preparation, physical properties, and evaluation of antioxidant capacity of aqueous grape extract loaded in chitosan‐TPP nanoparticles. Food Sci. Nutr. 2022;10:3272–3281. doi: 10.1002/fsn3.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yin X., Fu Xiaojun, Cheng H., Wusigale L. Liang. α-Tocopherol and naringenin in whey protein isolate particles: Partition, antioxidant activity, stability and bioaccessibility. 2020;106:105895. doi: 10.1016/j.foodhyd.2020.105895. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.