Abstract
BACKGROUND:
The widespread use of recreational drugs has raised concerns regarding their effects on various organ systems. The use of cannabis and opioids in chronic pain management increases their prevalence among patients with musculoskeletal conditions whose bone health may already be compromised. This article aims to review the pathophysiology and toxic effects of recreational drug use on musculoskeletal health to establish appropriate pain regimens for patients with substance use.
METHODS:
Medical literature published from 1970 until 2022 was identified utilizing MEDLINE/PubMed and the Cochrane Library. In addition to the databases, references were obtained through the use of reference lists of published articles identified by the aforementioned databases. The initial search terms included opioids, inhalants, hallucinogens, cannabis, stimulants, and bone health. There were no methodological limitations in relation to the initial acquisition and analysis of data.
RESULTS:
A total of 55 research articles were included in this review. Cannabis, stimulants, opioids, and inhalants impact bone maintenance, specifically osteoblast and osteoclast activity, as well as impede hormone production. These substances inhibit bone remodeling and development, manifesting as lower bone mineral density and increased fracture risk in chronic users.
CONCLUSION:
Although the current literature suggests a deleterious effect of recreational drugs on bone health and musculoskeletal disease, further research is warranted to evaluate the clinical effects of long-term substance use. The evaluation of such effects will aid in establishing appropriate pain regimens, as well as appropriate screening and treatment plans for recreational drug users.
KEYWORDS: Musculoskeletal disorders, Bone health, Cannabis, Opioids, Stimulants
INTRODUCTION
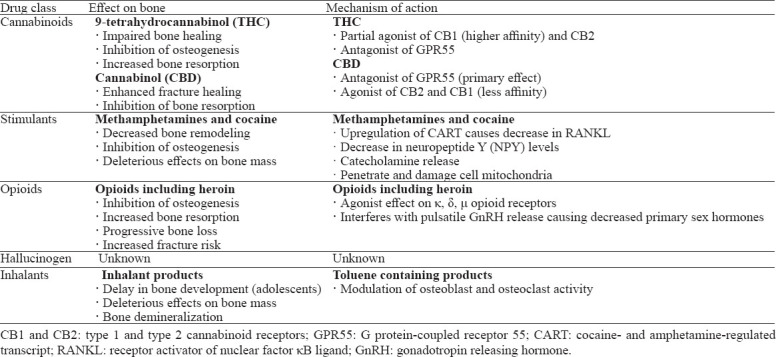
In the past decade, recreational drug use has become rampant within the USA. According to the National Center for Drug Abuse Statistics, 165 million people (60.2%) in the USA reported current substance use, while 31.9 million people (11.7%) reported illegal substance use. Cannabinoids, stimulants, opioids, hallucinogens, and inhalants are the most frequently used drugs in the USA, especially among the adolescent population (Table 1).[1-4] The use of cannabis, cocaine and opioids is particularly prevalent among individuals with chronic musculoskeletal ailments due to the analgesic effects of such substances.[5,6] Shmagel et al[7] noted that 46.5% of patients with chronic lower back pain used marijuana, 22% used cocaine, and 5% used heroin. Notably, 22.5% of patients reporting illicit substance use had an active prescription for opioid analgesics. Despite analgesic effects, current literature suggests that various recreational drugs may have deleterious effects on bone health, leading to increased fracture risk and growth impairment during musculoskeletal development in adolescents (Table 2).[8-11] With the exponential rise in recreational drug use within the USA, evaluating the effects of such substances on bone health and musculoskeletal disease has become crucial.
Table 1.
Overview of frequently used recreational drugs in the USA
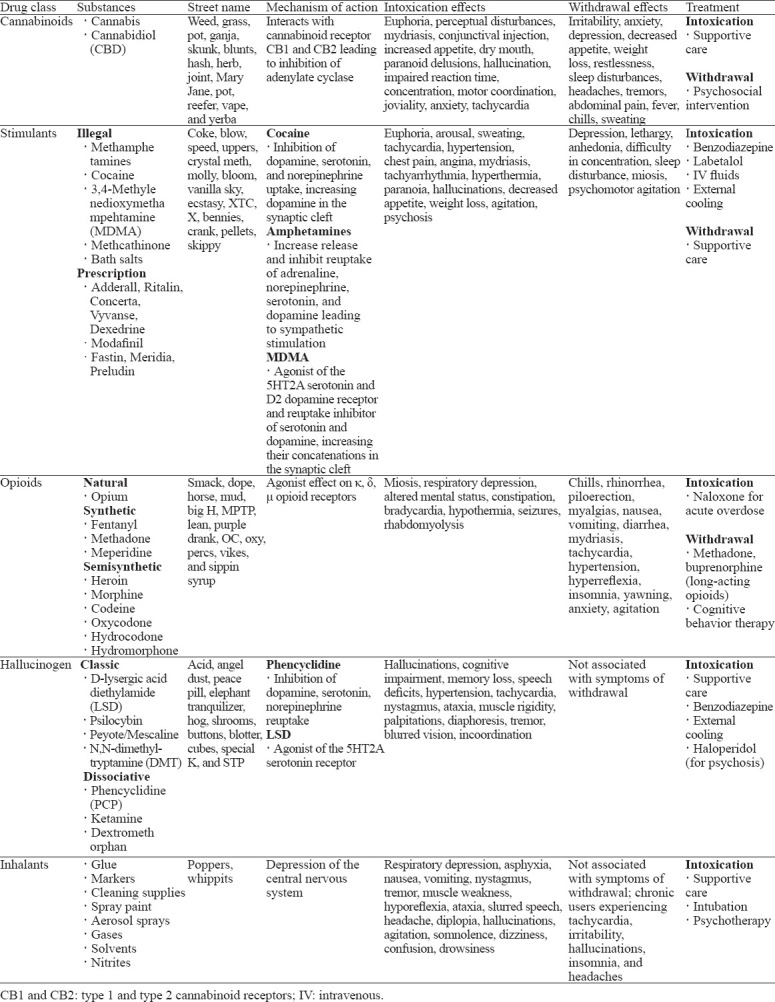
Table 2.
The effects of recreational drugs on bone
METHODS
A narrative review was conducted by the authors of the study. Medical literature published from 1970 until 2022 was identified utilizing MEDLINE/PubMed and the Cochrane Library. In addition to the databases, references were obtained through use of reference lists of published articles identified by the aforementioned databases. The initial search terms included opioids, inhalants, hallucinogens, cannabis, stimulants, and bone health. There were no methodological limitations in relation to the initial acquisition and analysis of data. Articles were screened by abstract and then selected on the basis of full-text publications. The sample size and generalizability were considered in article selection. The primary author possessed a distinction in research and performed the initial data selection and extraction. The studies were then reviewed by the contributing authors for generalizability and relevance.
RESULTS
Overview of recreational drugs
Cannabis
A total of 55 research articles were included in the this review. Cannabis is used recreationally for its psychoactive properties. Cannabis is derived from the cannabis sativa plant and elicits its action through the active ingredient tetrahydrocannabinol (THC).[3] Along with euphoria, cannabis intoxication can result in dry mouth, bloodshot eyes, distorted perception, dizziness, impaired coordination, increased appetite, anxiety, paranoia, and short-term memory impairment.[12] The medicinal application of cannabis is growing in popularity and includes pain management, treatment of epilepsy and glaucoma.[5] The use of cannabis more than doubled (4.1% to 9.5%) from 2002 to 2013 and continues to increase with its recent legalization in many states.[13-15] The legalization status of cannabis in the USA differs from state to state and can be contingent on the context of use.[16]
Stimulants
Stimulants can be classified as legal (prescription) or illegal substances. Amphetamines are considered legal prescription stimulants used for the treatment of attention deficit hyperactive disorder (ADHD), narcolepsy, and obesity.[3] Despite their legalization, amphetamines frequently misused in the USA include those prescribed for ADHD (Adderall, Ritalin, Concerta, Vyvanse, and Dexedrine), those prescribed for narcolepsy (Modafinil), and those prescribed for weight loss (Fastin, Meridia, and Preludin).[3] In contrast to amphetamines, methamphetamines, cocaine, 3,4-methylenedioxymethampehtamine (MDMA, ecstasy), methcathinone, and bath salts (synthetic cathinones) are classified as illegal stimulants. In 2018, approximately 1.8 million (0.7%) Americans reported methamphetamine use, and 5.5 million (2.0%) individuals reported cocaine use.
Frequently used for the euphoric “high” and improved concentration associated with stimulant use, symptoms of intoxication include hyperactivity, mydriasis, anorexia, tachycardia, hypertension, diaphoresis, anxiety, mood swings, aggression, and elevated confidence.[17] Such sympathetic enhancement often leads to significant adverse cardiovascular effects. Cardiac arrests, arrhythmias, myocardial infarctions, aortic dissections, and cerebrovascular accidents occur more commonly in cocaine overdose than other forms of stimulant intoxication.[17,18] The mortality rate of cocaine overdose has increased from 1.4% in 2012 to 4.5% in 2018.[3] It is estimated that 1 in 5 deaths caused by drug overdose in the USA involve cocaine use.
Opioids
Opioids include naturally sourced opium, synthetic opioids (fentanyl, methadone, and meperidine), and semisynthetic opioids (morphine, codeine, oxycodone, hydrocodone, hydromorphone, and heroin).[3] All opioids are structurally similar analogs that act with varying affinities on κ, δ, and μ opioid receptors to elicit analgesic and euphoric effects. The pain relief associated with opioid use has fuelled the opioid epidemic, i.e., the development of addiction and often illegal substance use as a result of initial opioid analgesic prescriptions. According to the 2016 National Survey of Drug Use and Health, 11.8 million Americans self-reported opioid addiction, 92% of them reported pharmaceutical addiction and 8% of them reported heroin addiction.
According to the Addiction Center, 80% of individuals addicted to heroin were initially prescribed medicinal opioids.[19-21] Heroin is considered one of the most addictive illegal substances. Heroin is postulated to have a low binding affinity at the μ opioid receptors and therefore functions as a prodrug. Heroin is highly lipophilic and readily crosses the blood-brain barrier to deliver active metabolites, including morphine and 6-monoacetylmorphine (6-MAM). It is still contested which metabolite exerts the more potent and rapid effects on the central nervous system (CNS). Upon metabolite CNS penetration and binding of central opioid receptors, a rapid euphoric sensation (“rush”) is produced, along with skin flushing, dry mouth, and extremity “heaviness”. Following these initial effects, drowsiness, hypotension, bradycardia, hypothermia, miosis, head nodding, and respiratory depression may occur and persist for several hours.[20] Significant opioid ingestion can lead to coma or permanent brain damage through respiratory depression and hypoxia.[3]
Hallucinogens
Hallucinogens, often utilized in young adults for social purposes, distort an individual’s perception of reality and awareness.[22,23] According to the 2018 National Survey on Drug Use and Health, 15.8% of Americans admitted to hallucinogen use within their lifetime, while 2.0% admitted to hallucinogen use within the past year.[24] Two categories of hallucinogens exist: classic and dissociative.[23] Classic hallucinogens include D-lysergic acid diethylamide (LSD), psilocybin, peyote or mescaline, and N,N-dimethyltryptamine (DMT). Dissociative hallucinogens include phencyclidine (PCP), ketamine, and dextromethorphan.[23] Both categories of hallucinogens can intensify emotional or sensory perception, alter sense of time, and cause paranoia, panic, psychosis, or relaxation. Other side effects include tachycardia, tachypnea, hypertension, hyperthermia, diaphoresis, visual disturbances, anorexia, nausea, dry mouth, insomnia, and loss of coordination. Dissociative hallucinogens are known to cause seizures, amnesia, psychosis, drug-induced paralysis, and respiratory distress or apnea.[23] In addition, dissociative hallucinogens may result in a sense of self or environmental detachment and consequently result in a sense of loss of control.[23]
Inhalants
Inhalants consist of gases (nitrous oxide, refrigerants, propane tanks, whipped cream cans, chloroform), aerosols (spray paints, deodorant, hair sprays, fabric protectors), nitrites (food preservatives, leather cleaner, room deodorizer), or liquids (paint thinner, paint remover, markers, glues, gasoline, dry-cleaning fluids). Industrial or household use of such substances is common, often resulting in occupational exposure and involuntary intoxication. Inhalants also possess psychoactive effects and therefore are frequently used by children and adolescents through oral or nasal routes for relaxation, euphoria, and hunger cessation.[25,26] National surveys have suggested that the prevalence of inhalant use is greatest among 12- to 18-year-old children. In the 2018 National Survey on Drug Use and Health, 9.1% of individuals within the USA reported lifetime inhalant use, with 2.7% of users between the ages of 12 and 18 admitting to inhalant use within the past year.[27]
The pharmacologic mechanism by which inhalant intoxication occurs remains poorly understood. It has been theorized that once inhaled, rapid distribution to lipid-containing membranes within the CNS occurs, and inhalants exert their effects through non-specific CNS depression. Current literature now supports the theory that chemicals such as xylene, benzene, and trichloroethylene inhibit N-methyl-D-aspartate (NMDA) glutamate receptors. Toluene, an active solvent found in several commonly used inhalants, also inhibits NMDA receptors and activates the dopamine reward pathway in the nucleus accumbens, reinforcing its use.[26] The long-term use of inhalants can result in consequences, including organ damage, hearing loss, nerve damage, developmental and behavioral delays, seizure, coma, or death.[25]
Musculoskeletal effects of recreational drugs
Cannabis
The effects of cannabis on bone health in humans are relatively unknown.[8] Cannabinoid receptors and their ligands play a considerable role in bone turnover by regulating bone cell activity. Idris et al[28] reported that the binding of an agonist to type 1 cannabinoid (CB1) receptors in mice results in an increase in osteoclast activity and osteoblast differentiation. Increased osteoclast activity results in bone resorption while paradoxically maintaining bone mass through osteoblast differentiation.[8,15,28,29] Agonist binding to type 2 cannabinoid (CB2) receptors also stimulates osteoblast proliferation and activity. However, CB2 agonists inhibit osteoclast production by reducing the expression of receptor activator of nuclear factor κB ligand (RANKL) and consequently increasing bone formation.[30,31] In addition, antagonist activity on the G protein coupled receptor 55 (GPR55) inhibits osteoclast activity and reduces bone turnover.[32] With increasing age, CB1 and CB2 inactivation occurs and results in bone loss due to defective osteoblastic differentiation, adipocyte accumulation, and osteoporotic changes.[15,29] These findings suggest a protective role associated with endogenous cannabinoids in bone homeostasis and healing.[15]
THC and cannabinol (CBD) are active cannabinoids that interact with the human body’s endocannabinoid system. THC is the principal psychoactive constituent in cannabis, producing the “high” or euphoric sensation typically associated with the drug. THC is a partial agonist of CB1 and CB2 receptors and an antagonist of GPR55 receptors.[8,28] While endogenous cannabinoids play a protective role in bone health maintenance, THC is a partial agonist with considerably higher affinity for CB1 receptors. Such affinity results in increased bone resorption from CB1-induced osteoclastic activity. THC also signals mitochondrial apoptotic pathways in neurons and mesenchymal stem cells (MSCs). THC thus inhibits osteogenesis by inducing cell death and inhibiting stem cell differentiation.[15,33]
In contrast, CBD has significantly analgesic, anti-inflammatory, anti-convulsant, and anxiolytic effects without the psychoactive effects of THC. CBD acts predominantly as a GPR55 receptor antagonist, with weak agonistic effects on CB1 and CB2 receptors. CBD thus reduces osteoclast activity and inhibits bone resorption. CBD can also act as an allosteric modulator of CB1 receptors, reducing the efficacy and potency of THC.[15] Other effects of CBD include enhancement of MSC migration and differentiation during bone healing, as well as increased osteoblast expression of lysyl hydroxylase I to improve collagen cross-linking.[34] Unlike THC, CBD may enhance the rate and quality of fracture healing in rat models.[15,34] The diverse effects of various cannabinoid receptors on bone cell activity, as well as the influence of age and sex in pre-clinical animal studies, make it challenge to predict the effect of cannabis use on bone health.
Sophocleous et al[8] reported that the bone mineral density (BMD) of the spine, hip, and femoral neck was lower in patients who reported heavy cannabis use than in controls. Heavy cannabis users also had a lower body mass index (BMI) with higher incidence of fractures. Multiple regression analysis revealed that heavy cannabis use was an independent predictor of reduced spine and hip BMD. However, mediation analysis suggested that the effect on spine BMD was indirect and mediated through low BMI. In contrast, a cross-sectional study utilizing the National Health and Nutrition Examination Survey of 4,743 patients revealed that despite the association of cannabis use with lower BMI, no direct relationship could be derived between cannabis use and low BMD.[14]
Stimulants: methamphetamines and cocaine
Often used for its psychostimulatory effects and increased rates of attention deficit disorder diagnoses, methamphetamine use has become a systemic issue due to its highly addictive potential.[11,35,36] Methamphetamine not only results in multiple degenerative effects on the brain, heart, and liver but also has been implicated in decreased BMD and bone strength.[37-39] Due to the close relationship between CNS activity and bone metabolism, the current literature supports the notion that the stimulatory effects of methamphetamine adversely affect bone remodeling and turnover.[37,40]
Methamphetamine is commonly deposited in bone due to the high vascularity of marrow and the lipid content of the bone matrix.[37] As methamphetamine is lipophilic, it can penetrate cell membranes and various organelles, including mitochondria. Methamphetamine thus disrupts the Krebs cycle and electron transport chain, enhancing cellular susceptibility to oxidative stress, pro-apoptosis, and neuroinflammation.[37] Damage to MSC mitochondrial membranes hinders their differentiation into osteoblasts, impairing bone production. In an animal study by Shen et al,[37] MSCs treated with methamphetamine demonstrated a decrease in mitochondrial adenosine triphosphate (ATP) production and oxygen consumption rate with a down-regulation of mitochondrial DNA (mtDNA)-derived protein expression. Reductions in energy production and biogenesis support the role of methamphetamines in mitochondrial dysfunction and impaired differentiation during osteogenesis.[37]
The physiological process by which methamphetamine use negatively affects bone turnover and metabolism has been increasingly investigated in current literature. Recent literature has described the upregulation of the cocaine and amphetamine-regulated transcript (CART) gene that results from the hypothalamic stimulation of methamphetamine.[11,37] CART acts to decrease RANKL expression and inhibit osteoclast differentiation.[11,37,41] As a result of decreased osteoclast resorption, bone remodeling and metabolism are impaired. Similarly, low neuropeptide Y (NPY) levels have been attributed to methamphetamine use. NPY is essential for osteoblast activity, and therefore, its deficiency results in decreased bone formation.[37,42] Furthermore, the release of catecholamines stimulated by methamphetamine has deleterious effects on bone mass due to beta adrenergic receptor agonism.[11]
Tomita et al[11] studied the role of methamphetamine in the bone metabolism of mice. The mice were classified into three groups: (1) the control group, (2) the 5 mg/kg methamphetamine injection group, and (3) the 10 mg/kg methamphetamine injection group. Methamphetamine showed distinct dose-dependent effects on bone turnover, including a reduction in the serum protein concentration and increased formation of alkaline phosphatase and osteocalcin. A significant increase in osteoblasts was also observed in mice injected with 5 mg/kg methamphetamine. Bone remodeling was thought to be unbalanced, and decreased metabolic turnover was observed. The overall effects of methamphetamine are thus thought to lead to osteoporosis.
Kim et al[39] studied the frequency of osteoporosis in male methamphetamine users. Forty-six hospitalized male methamphetamine users and 100 reference controls were studied. BMD of the lumbar spine was measured by dual-energy X-ray absorptiometry (DXA). Considerable loss of BMD was observed in heavy methamphetamine users compared to controls, with an osteoporosis frequency of 22% and a lumbar spine osteopenia frequency of 76%. A clear correlation between drug use and the extent of bone mineral loss was observed.[39] Similarly, Bolognini and Ross[29] studied the speed of sound and broadband ultrasound attenuation at the calcaneus by Achilles ultrasound bone densitometer. The speed of sound and broadband ultrasound attenuation served as indicators of bone strength. A significant difference in bone strength was observed between patients with reported methamphetamine misuse and the control group.[38] Although current evidence suggests an adverse effect of methamphetamine on bone health, clinical studies with long-term follow-up are necessary to establish the consequences of chronic methamphetamine use on the musculoskeletal system and the resultant fracture risk.
Opioids
Traditionally used for analgesic effects in moderate to severe pain management, opioid use has also increasingly been associated with osteoporosis and low BMD.[9,43] Three types of opioid receptors have been identified on human osteoblast cells: μ, δ, and κ.[44] Upon activation of these receptors by either endogenous opioids or an exogenous drug, osteoblast activity decreases, as evidenced by reduced osteocalcin production. Low osteocalcin levels have been observed in chronic heroin users, suggesting an inhibitory effect on osteoblast activity and bone production with opioid misuse.[44,45]
Furthermore, activation of opioid receptors on the hypothalamus interferes with the pulsatile release of gonadotropin releasing hormone (GnRH). A lack of pulsatile GnRH release results in a reduction of luteinizing hormone and follicle stimulating hormone.[10] Significant disruption of the hypothalamic-pituitary-gonadal axis results in hypogonadism and a decrease in testosterone and estradiol hormone production by the testes and ovaries, respectively.[10] In men, testosterone and other androgens are essential for stimulating periosteal apposition during bone development and maintenance, while estrogens increase endosteal apposition in women.[46] As primary sex hormones are necessary for bone health and maintenance, increased osteoporosis risk and compression fractures have been reported in long-term male and female opioid users with associated hypogonadism.[10,47] Women are particularly at risk for fractures due to estrogen withdrawal at the onset of menopause. Estrogen deficiencies prolong the lifespan of osteoclasts and reduce osteoblast duration.[46] The increase in bone resorption with decreased osteoblastic production leads to poor remodeling and skeletal weakening. Opioid-induced hypogonadism has the potential to hasten the progression of bone loss in women prior to menopause and exacerbate fracture risk following the onset of menopause.[46]
Gotthardt et al[43] reported a 74.3% prevalence rate of low bone mass in patients on opioid maintenance therapy. Similarly, Kim et al[48] reported low BMD in 83% of 92 male and female opioid users receiving methadone maintenance therapy. Thirty-five percent of the low BMD group had a dual-energy X-ray absorptiometry (DEXA) scan T-score in the osteoporosis range (< -2.5). The direct alterations in bone metabolism caused by opioids, along with the indirect effects of opioids on hypothalamic hormones, provide potential mechanisms by which long-term opioid use can result in deterioration of bone quality.[10, 44, 46]
Other adverse effects of opioid use include the development of infections, including osteomyelitis. Osteomyelitis is frequently associated with intravenous heroin use.[49] Disease occurs through the hematogenous spread of bacteria from the injection site to various skeletal locations during intravenous use. This is potentiated with the use of unclean needles and syringes.[49] Preferred sites of infection include the lumbar spine, cervical spine, and clavicles. The most common causative organisms include Staphylococcus species, gram-negative rods, and yeast.[49,50] Patients initially complain of severe localized pain with erythema and edema at the site of the infected bone, with systemic symptoms of fever and fatigue.[51] If left untreated, osteomyelitis can lead to osteonecrosis, septic arthritis in nearby joints, and impaired growth in children if the infection extends to the growth plate.[51] Depending on the severity, osteonecrosis is generally managed by surgical resection of the infected or dead portion of bone with post-operative intravenous (IV) antibiotics in the inpatient setting.[52]
Hallucinogens
The existing literature lacks evidence regarding the biochemical and clinical effects of hallucinogenic substances on bone metabolism and musculoskeletal disease. As such substances are becoming increasingly approved by the federal drug administration, particularly for depression and pain control, further research is warranted to identify the effects of short- and long-term use of hallucinogens on bone health. The unexplored consequences of more widespread therapeutic use, especially in the adolescent population, must be considered.
Inhalants
Inhalant use in adolescents has been associated with growth impairment and reduced bone density.[53] A cross-sectional study comparing BMD in 25 adolescent glue inhalant users to that of 30 age-matched controls revealed that DEXA scan measurements were considerably lower in the inhalant group. Such results suggest either a delay in development and/or decreased bone mass with frequent inhalant use in children and adolescents.[54] Although the exact substance eliciting the deleterious effects is not evident, toluene in particular has been linked to bone demineralization. An animal study conducted by Atay et al[55] reported lower BMD measurements in mice chronically exposed to toluene than in control mice. Although the mechanism by which toluene elicits its effects on bone metabolism remains uncertain, it is hypothesized that toluene influences osteoblast and osteoclast activity.[53,55] Nevertheless, lower peak bone mass produced by the use of inhalants during development raises concern over the future development of osteoporosis and increased fracture risk.[54]
DISCUSSION
The widespread use of recreational drugs in the USA raises significant concerns regarding their effects on various organ systems. Furthermore, the use of cannabis and opioids in chronic pain management increases the prevalence of these substances among patients with musculoskeletal conditions whose bone health may already be compromised. Cannabis, stimulants, opioids, and inhalants impact bone maintenance, specifically osteoblast and osteoclast activity, and impede hormone production. Such substances therefore lead to the inhibition of bone remodeling and development, manifesting as lower BMD and increased fracture risk in chronic users. Although the current literature suggests a deleterious effect of recreational drugs on bone health and musculoskeletal disease, further research is warranted to evaluate the clinical effects of long-term substance use. The evaluation of such effects will aid in establishing appropriate pain management regimens, as well as appropriate screening and treatment plans for recreational drug users.
In patients presenting to the emergency department or outpatient clinics with acute or chronic pain, it is critical to ascertain their relationship with drug use. Patients with chronic substance use are more likely to suffer from lower BMD and increased fracture risk. Opioids, steroids, and other pain regimens that further lower bone density should be avoided in such patients, with alternative agents being prescribed when feasible. Patients should be educated on their increased fracture risk. Education and resources regarding the cessation of substance use should also be provided. Additionally, patients can be given addiction medicine, pain medicine, and physical medicine and rehabilitation referrals for further care if needed.
CONCLUSION
In patients presenting to the emergency department or outpatient clinics with acute or chronic pain, it is critical to ascertain their relation with drug use. Patients with chronic substance use likely suffer from lower bone mineral density and increased fracture risk. Opioids, steroids, and other pain regimens that further lower bone density should be avoided in such patients, with alternative agents being prescribed when feasible. Patients should be educated on their increased fracture risk and provided resources regarding cessation of substance use if amenable.
ACKNOWLEDGEMENT
We would like to extend our appreciation to Sophia Seik-Ismail for her assistance with the illustrations included in this review.
Footnotes
Funding: None.
Ethical approval: Noe needed.
Conflicts of interest: All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Contributors: All contributing authors participated in conceptualization, data curation, formal analysis, investigation, methodology, resources, software, supervision, validation, visualization, writing, reviewing and editing the draft, and final approval of the draft for publication.
REFERENCES
- 1.National Institute on Drug Abuse. What drugs are most frequently used by adolescents? Available at:https://www.drugabuse.gov/publications/principles-adolescent-substance-use-disorder-treatment-research-based-guide/frequently-asked-questions/what-drugs-are-most-frequently-used-by-adolescents .
- 2.Schulden JD, Thomas YF, Compton WM. Substance abuse in the United States:findings from recent epidemiologic studies. Curr Psychiatry Rep. 2009;11(5):353–9. doi: 10.1007/s11920-009-0053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Center for Drug Abuse Statistics. Drug Abuse Statistics. Available at:https://drugabusestatistics.org/
- 4.AMBOSS. Substance-related and addictive disorders. Available at:https://www.amboss.com/us/knowledge/substance-related-and-addictive-disorders .
- 5.Ehrenkranz J, Levine MA. Bones and joints:the effects of cannabinoids on the skeleton. J Clin Endocrinol Metab. 2019;104(10):4683–94. doi: 10.1210/jc.2019-00665. [DOI] [PubMed] [Google Scholar]
- 6.Richards CJ, Graf KW, Jr, Mashru RP. The effect of opioids, alcohol, and nonsteroidal anti-inflammatory drugs on fracture union. Orthop Clin North Am. 2017;48(4):433–43. doi: 10.1016/j.ocl.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Shmagel A, Krebs E, Ensrud K, Foley R. Illicit substance use in US adults with chronic low back pain. Spine. 2016;41(17):1372–7. doi: 10.1097/BRS.0000000000001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sophocleous A, Robertson R, Ferreira NB, McKenzie J, Fraser WD, Ralston SH. Heavy cannabis use is associated with low bone mineral density and an increased risk of fractures. Am J Med. 2017;130(2):214–21. doi: 10.1016/j.amjmed.2016.07.034. [DOI] [PubMed] [Google Scholar]
- 9.Ding ZH, Chen YY, Wang X, Zhou X, Xu Y, Ma ZC, et al. A comparison of bone quality and its determinants in young opioid-dependent women with healthy control group. Drug Alcohol Depend. 2017;175:232–6. doi: 10.1016/j.drugalcdep.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Katz N, Mazer NA. The impact of opioids on the endocrine system. Clin J Pain. 2009;25(2):170–5. doi: 10.1097/AJP.0b013e3181850df6. [DOI] [PubMed] [Google Scholar]
- 11.Soo JEJ, Ng M, Chong TKL, Tan BKK, Ponampalam R. A case of persistent refractory hypoglycemia from polysubstance recreational drug use. World J Emerg Med. 2023;14(1):75–7. doi: 10.5847/wjem.j.1920-8642.2022.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Addiction Center. Marijuana symptoms and warning signs. Available at:https://www.addictioncenter.com/drugs/marijuana/symptoms-signs/
- 13.Grucza RA, Agrawal A, Krauss MJ, Cavazos-Rehg PA, Bierut LJ. Recent trends in the prevalence of marijuana use and associated disorders in the United States. JAMA Psychiatry. 2016;73(3):300–1. doi: 10.1001/jamapsychiatry.2015.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bourne D, Plinke W, Hooker ER, Nielson CM. Cannabis use and bone mineral density:NHANES 2007-2010. Arch Osteoporos. 2017;12(1):29. doi: 10.1007/s11657-017-0320-9. [DOI] [PubMed] [Google Scholar]
- 15.O'Connor CM, Anoushiravani AA, Adams C, Young JR, Richardson K, Rosenbaum AJ. Cannabinoid use in musculoskeletal illness:a review of the current evidence. Curr Rev Musculoskelet Med. 2020;13(4):379–84. doi: 10.1007/s12178-020-09635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DISA. Marijuana legality by state. Available at:https://disa.com/map-of-marijuana-legality-by-state .
- 17.Addiction Center. stimulant symptoms and warning signs. Available at:https://www.addictioncenter.com/stimulants/symptoms-signs/
- 18.American Heart Association. Cardiovascular effects of cocaine. Available at:https://www.ahajournals.org/doi/full/10.1161/circulationaha.110.940569 .
- 19.Addiction Center. The opioid epidemic. Available at:https://www.addictioncenter.com/opiates/opioid-epidemic/
- 20.Center for Substance Abuse Treatment. Physical detoxification services for withdrawal from specific substances. Available at:https://www.ncbi.nlm.nih.gov/books/NBK64116/
- 21.Lyden J, Binswanger IA. The United States opioid epidemic. Semin Perinatol. 2019;43(3):123–31. doi: 10.1053/j.semperi.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Institute on Drug Abuse. Hallucinogens. Available at:https://www.drugabuse.gov/drug-topics/hallucinogens.
- 23.National Institute on Drug Abuse. Hallucinogens drug facts. Available at:https://www.drugabuse.gov/publications/drugfacts/hallucinogens .
- 24.National Institute on Drug Abuse. Hallucinogens trends and statistics. Available at:https://www.drugabuse.gov/drug-topics/hallucinogens/hallucinogens-trends-statstics .
- 25.National Institute on Drug Abuse. Inhalants drug facts. Available at:https://www.drugabuse.gov/publications/drugfacts/inhalants .
- 26.National Institute on Drug Abuse. Inhalants research report. How do inhalants produce their effects? Available at:https://www.drugabuse.gov/publications/research-reports/inhalants/how-do-inhalants-produce-their-effects .
- 27.National Institute on Drug Abuse. Inhalants trends and statistics. Available at:https://www.drugabuse.gov/drug-topics/inhalants/inhalants-trends-statistics .
- 28.Idris AI, Sophocleous A, Landao-Bassonga E, van't Hof RJ, Ralston SH. Regulation of bone mass, osteoclast function, and ovariectomy-induced bone loss by the type 2 cannabinoid receptor. Endocrinology. 2008;149(11):5619–26. doi: 10.1210/en.2008-0150. [DOI] [PubMed] [Google Scholar]
- 29.Bolognini D, Ross RA. Medical cannabis vs. synthetic cannabinoids:what does the future hold? Clin Pharmacol Ther. 2015;97(6):568–70. doi: 10.1002/cpt.107. [DOI] [PubMed] [Google Scholar]
- 30.Sophocleous A, Landao-Bassonga E, van't Hof RJ, Idris AI, Ralston SH. The type 2 cannabinoid receptor regulates bone mass and ovariectomy-induced bone loss by affecting osteoblast differentiation and bone formation. Endocrinology. 2011;152(6):2141–9. doi: 10.1210/en.2010-0930. [DOI] [PubMed] [Google Scholar]
- 31.Ofek O, Attar-Namdar M, Kram V, Dvir-Ginzberg M, Mechoulam R, Zimmer A, et al. CB2 cannabinoid receptor targets mitogenic Gi protein-cyclin D1 axis in osteoblasts. J Bone Miner Res. 2011;26(2):308–16. doi: 10.1002/jbmr.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whyte LS, Ryberg E, Sims NA, Ridge SA, Mackie K, Greasley PJ, et al. The putative cannabinoid receptor GPR55 affects osteoclast function in vitro and bone mass in vivo . Proc Natl Acad Sci USA. 2009;106(38):16511–6. doi: 10.1073/pnas.0902743106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gowran A, McKayed K, Campbell VA. The cannabinoid receptor type 1 is essential for mesenchymal stem cell survival and differentiation:implications for bone health. Stem Cells Int 2013. 2013:796715. doi: 10.1155/2013/796715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kogan NM, Melamed E, Wasserman E, Raphael B, Breuer A, Stok KS, et al. Cannabidiol, a major non-psychotropic cannabis constituent enhances fracture healing and stimulates lysyl hydroxylase activity in osteoblasts. J Bone Miner Res. 2015;30(10):1905–13. doi: 10.1002/jbmr.2513. [DOI] [PubMed] [Google Scholar]
- 35.Nordahl TE, Salo R, Leamon M. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition:a review. J Neuropsychiatry Clin Neurosci. 2003;15(3):317–25. doi: 10.1176/jnp.15.3.317. [DOI] [PubMed] [Google Scholar]
- 36.Wijetunga M, Seto T, Lindsay J, Schatz I. Crystal methamphetamine-associated cardiomyopathy:tip of the iceberg? J Toxicol Clin Toxicol. 2003;41(7):981–6. doi: 10.1081/clt-120026521. [DOI] [PubMed] [Google Scholar]
- 37.Shen YL, Wu L, Wang J, Wu X, Zhang XM. The role of mitochondria in methamphetamine-induced inhibitory effects on osteogenesis of mesenchymal stem cells. Eur J Pharmacol. 2018;826:56–65. doi: 10.1016/j.ejphar.2018.02.049. [DOI] [PubMed] [Google Scholar]
- 38.Katsuragawa Y. Effect of methamphetamine abuse on the bone quality of the calcaneus. Forensic Sci Int. 1999;101(1):43–8. doi: 10.1016/s0379-0738(99)00010-9. [DOI] [PubMed] [Google Scholar]
- 39.Kim EY, Kwon DH, Lee BD, Kim YT, Ahn YB, Yoon KY, et al. Frequency of osteoporosis in 46 men with methamphetamine abuse hospitalized in a national hospital. Forensic Sci Int. 2009;188(1-3):75–80. doi: 10.1016/j.forsciint.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 40.Takeda S. Osteoporosis:a neuroskeletal disease? Int J Biochem Cell Biol. 2009;41(3):455–9. doi: 10.1016/j.biocel.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang XL, Liu XY, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434(7032):514–20. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 42.Kobeissy FH, Jeung JA, Warren MW, Geier JE, Gold MS. Changes in leptin, ghrelin, growth hormone and neuropeptide-Y after an acute model of MDMA and methamphetamine exposure in rats. Addict Biol. 2008;13(1):15–25. doi: 10.1111/j.1369-1600.2007.00083.x. [DOI] [PubMed] [Google Scholar]
- 43.Gotthardt F, Huber C, Thierfelder C, Grize L, Kraenzlin M, Scheidegger C, et al. Bone mineral density and its determinants in men with opioid dependence. J Bone Miner Metab. 2017;35(1):99–107. doi: 10.1007/s00774-015-0732-9. [DOI] [PubMed] [Google Scholar]
- 44.Pérez-Castrillón JL, Olmos JM, Gómez JJ, Barrallo A, Riancho JA, Perera L, et al. Expression of opioid receptors in osteoblast-like MG-63 cells, and effects of different opioid agonists on alkaline phosphatase and osteocalcin secretion by these cells. Neuroendocrinology. 2000;72(3):187–94. doi: 10.1159/000054586. [DOI] [PubMed] [Google Scholar]
- 45.Rico H, Costales C, Cabranes JA, Escudero M. Lower serum osteocalcin levels in pregnant drug users and their newborns at the time of delivery. Obstet Gynecol. 1990;75(6):998–1000. [PubMed] [Google Scholar]
- 46.Seeman E. Pathogenesis of bone fragility in women and men. Lancet. 2002;359(9320):1841–50. doi: 10.1016/S0140-6736(02)08706-8. [DOI] [PubMed] [Google Scholar]
- 47.Anderson FH, Francis RM, Selby PL, Cooper C. Sex hormones and osteoporosis in men. Calcif Tissue Int. 1998;62(3):185–8. doi: 10.1007/s002239900414. [DOI] [PubMed] [Google Scholar]
- 48.Kim TW, Alford DP, Malabanan A, Holick MF, Samet JH. Low bone density in patients receiving methadone maintenance treatment. Drug Alcohol Depend. 2006;85(3):258–62. doi: 10.1016/j.drugalcdep.2006.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holzman RS, Bishko F. Osteomyelitis in heroin addicts. Ann Intern Med. 1971;75(5):693–6. doi: 10.7326/0003-4819-75-5-693. [DOI] [PubMed] [Google Scholar]
- 50.Endress C, Guyot DR, Fata J, Salciccioli G. Cervical osteomyelitis due to i.v. heroin use:radiologic findings in 14 patients. AJR Am J Roentgenol. 1990;155(2):333–5. doi: 10.2214/ajr.155.2.2115262. [DOI] [PubMed] [Google Scholar]
- 51.Mayo Clinic. Osteomyelitis:symptoms and causes. Available at:https://www.mayoclinic.org/diseases-conditions/osteomyelitis/symptoms-causes/syc-20375913 .
- 52.Mayo Clinic. Osteomyelitis:diagnosis &treatment. Available at:https://www.mayoclinic.org/diseases-conditions/osteomyelitis/diagnosis-treatment/drc-20375917 .
- 53.Crossin R, Qama A, Andrews ZB, Lawrence AJ, Duncan JR. The effect of adolescent inhalant abuse on energy balance and growth. Pharmacol Res Perspect. 2019;7(4):e00498. doi: 10.1002/prp2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dündaröz MR, Sarici SU, Türkbay T, Baykal B, Kocaoğlu M, Aydin HI, et al. Evaluation of bone mineral density in chronic glue sniffers. Turk J Pediatr. 2002;44(4):326–9. [PubMed] [Google Scholar]
- 55.Atay AA, Kismet E, Turkbay T, Kocaoglu M, Demirkaya E, Sarici SU, et al. Bone mass toxicity associated with inhalation exposure to toluene. Biol Trace Elem Res. 2005;105(1-3):197–203. doi: 10.1385/BTER:105:1-3:197. [DOI] [PubMed] [Google Scholar]


