Abstract
Broadly directed hepatitis C virus (HCV)-specific cytotoxic T lymphocytes (CTL) have been identified from liver-infiltrating lymphocytes but have been more difficult to assess in peripheral blood of infected persons. To enhance the detection of CTL from peripheral blood mononuclear cells (PBMC), we cocultured PBMC with autologous Epstein-Barr virus-transformed B-lymphoblastoid cell lines that had been infected with recombinant vaccinia virus constructs so that they expressed the entire translated polyprotein of HCV-H, a type 1a strain. These stimulated cells from HCV-infected as well as exposed seronegative persons were then cloned at limiting dilution and tested for HCV-specific CTL activity using a standard 51Cr release assay. HCV-specific CTL were detected in PBMC from seven of nine persons with chronic hepatitis, including five of seven in whom CTL had previously been detected from liver biopsy specimens but not PBMC. In a single person with chronic HCV infection, CTL directed against as many as five different epitopes were detected in peripheral blood and were similar in specificity to those detected in liver tissue. This technique was used to evaluate eight subjects identified to be at high risk for HCV exposure due to continued injection drug abuse; no evidence of CTL in PBMC was found. We conclude that CTL can be detected in PBMC from the majority of persons with chronic HCV infection but are present at lower levels or absent in exposed but persistently seronegative persons. The high degree of concordance of HCV epitopes identified from liver and PBMC suggests that this strategy is a reasonable alternative to liver biopsy for characterizing the CTL response to HCV in chronically infected persons.
Cytotoxic T lymphocytes (CTL) potentially play a major role in the pathogenesis of chronic viral infections because they are capable of recognizing virus-infected cells (18, 29, 37) and responding either directly, by lysis of the infected cell, or indirectly, by secreting cytokines that inhibit viral replication and/or recruit other nonspecific inflammatory cells (9, 10, 40). In acute hepatitis C virus (HCV) infection, a strong virus-specific CTL response seems to be associated with spontaneous viral clearance (6, 8, 24), whereas in chronic infection, the role of CTL is still under debate. CTL studies of HCV-infected persons have been hampered by the relatively low frequency of specific cells in the peripheral blood and by the fact that the targeted epitopes vary depending on the HLA type of the individual. The majority of studies of HCV-specific CTL in peripheral blood have been based on testing predicted epitopes, and most have focused on HLA A2-positive persons (2, 4, 8, 23, 27, 28).
In subjects with chronic HCV infection, HCV-specific CTL responses presented by A2 and non-A2 alleles have been detected from liver-derived lymphocytes that have been polyclonally expanded using a CD3-specific monoclonal antibody (19–21, 26, 39). The CTL responses defined in this manner are presumed to reflect in vivo-determined specificities. However, liver-derived lymphocytes are not easily studied. The risks and discomforts of liver biopsy make it difficult to study subjects frequently in a longitudinal fashion or to study subjects with mild or acute disease for whom liver biopsies are not clinically indicated.
Peripheral blood-derived lymphocytes are easier to obtain than liver tissue, but previous attempts to detect HCV-specific CTL activity from peripheral blood mononuclear cells (PBMC) stimulated with anti-CD3 have been unsuccessful (21). This finding is consistent with the hypothesis that HCV-specific responses are compartmentalized to the liver and are present in PBMC in much lower frequencies. Other groups have detected CTL from PBMC that had been stimulated in an antigen-specific manner using synthetic HCV peptides, but these studies have been largely limited to detection of known or predicted class I-restricted epitopes (2, 4, 12, 15, 16, 23, 28, 32, 33). CTL responses identified in this manner do not allow detection of responses restricted by many class I alleles for which epitope predictions are not available or those that are missed by the prediction algorithms. Furthermore, some HCV peptides have been reported to induce CTL responses in HCV-seronegative subjects, raising the possibility that some of these responses may be due to primary in vitro sensitization (3, 22).
An alternative approach to antigen-specific stimulation has been described for detecting virus-specific CTL (14, 25). Recombinant vaccinia virus vectors can be used to introduce antigens into B-lymphoblastoid cell lines for processing through the class I pathway. After inactivation of the vaccinia virus by psoralen and long-wave UV irradiation, the cells are used to stimulate PBMC. This system has the advantage of having all potential CTL epitopes presented in the context of all the autologous HLA class I alleles.
In this study, we used a vaccinia virus system to express the entire translated polyprotein of HCV-H (a type 1a strain) in autologous B-lymphoblastoid cell lines (B-LCL) and then used these cells to stimulate the PBMC (13). Using this method, we have evaluated CTL responses from cryopreserved PBMC from subjects who had chronic HCV infection and who were previously assessed for the presence of liver-derived CTL (19–21, 39). In addition, we have used this technique to examine a cohort of injection drug users who remain HCV seronegative despite continued high-risk behavior (34, 35) to determine whether HCV-specific CTL may be detectable in PBMC. Our results indicate that HCV-specific CTL can be detected in PBMC in the majority of persons with chronic HCV infection but not, by this method, in persons who are repeatedly exposed but remain seronegative.
MATERIALS AND METHODS
Patient samples.
Cryopreserved PBMC from subjects previously studied for liver-derived HCV-specific CTL were examined (19–22). PBMC were also obtained from an additional 10 subjects selected from a cohort of injection drug users (34, 36). Eight of these individuals were chosen because they remained HCV seronegative for over 1 year of follow-up despite injection drug use patterns associated with the highest risks for HCV transmission (34). The other two subjects had developed chronic HCV infection, as determined by being repeatedly positive for anti-HCV antibodies and HCV RNA.
PBMC were isolated from venous blood by Ficoll-Hypaque density centrifugation. B-LCL were established through Epstein-Barr virus (EBV) transformation and maintained as previously described in RPMI 1640 medium (Sigma Chemical Co., St. Louis, Mo.) supplemented with 10 mM HEPES buffer, 2 mM l-glutamine plus 50 U of penicillin and 50 μg of streptomycin per ml along with 20% heat-inactivated fetal calf serum (R-20 medium). HLA typing was performed on additional samples of venous blood by the Massachusetts General Hospital Tissue Typing Laboratory, using standard serologic techniques. Sera and plasma, as well as the remaining PBMC, were stored at −80°C. Informed consent was obtained from all subjects, and the study was approved by the Massachusetts General Hospital Internal Review Board.
Stimulation of PBMC in bulk cultures.
Lymphocytes (106 cells) from PBMC were polyclonally expanded in an antigen-nonspecific manner with the CD3-specific monoclonal antibody 12F6 (0.1 μg/ml) and 20 × 106 irradiated (30 Gy) allogeneic PBMC feeder cells in 20 ml of R-10 medium. On day 3, recombinant interleukin-2 (rIL-2) was added to achieve a final concentration of 50 U/ml.
Lymphocytes were also expanded in an antigen-specific manner, using a protocol modified from Lubaki et al. (25). Stimulator cells that present all potential HCV antigens in the context of the autologous HLA alleles were prepared by infecting autologous B-LCL (8 × 106 cells) with the vaccinia virus-HCV (vv-HCV) recombinant viruses (see below) vv1-966(H1) and vv827-3011(H2) at a multiplicity of infection of 5 to 10 for 18 h. The vaccinia viruses were then inactivated by resuspending the stimulator cells in 5 ml of a 10-μg/ml psoralen solution (4′-aminomethyl-4′,5′-8-trimethylpsoralen hydrochloride; HRI Associates, Concord, Calif.) and then exposing the cells to long-wave UV light (8-W bulb, 350- to 400-nm light; Fisher Scientific) for 5 min. PBMC (4 × 106 to 6 × 106 cells) were stimulated with the stimulator B-LCL (4 × 106 cells) and allogeneic irradiated (30 Gy) PBMC feeder cells (20 × 106) in 20 ml of R-10 medium and incubated at 37°C with 5% CO2. rIL-2 (50 U/ml) was added on day 3. Bulk expanded cells were tested for CTL recognition of vv1-966(H1) and vv827-3011(H2) on day 14.
PBMC cloning.
Clones were derived following antigen-specific stimulation of PBMC by subculturing in 96-well plates at limiting dilution (25, 10, 5, and 3 cells per well) as described (39), using the anti-CD3 monoclonal antibody 12F6 as a stimulus for cellular proliferation. In designated instances, freshly isolated PBMC were directly cloned in this manner as well. Developing cells were restimulated in 24-well plates with irradiated (30 Gy) allogeneic feeder cells (105 cells/well), the CD3-specific monoclonal antibody 12F6 (0.1 μg/ml), and rIL-2 (100 U/ml) in R-10 medium and then tested for HCV-specific cytolytic recognition of HCV-H and HCV-1 antigens 7 days later. HCV-specific clones were maintained in long-term culture in T-25 flasks by restimulating 2 × 106 to 4 × 106 lymphocytes every 3 to 4 weeks with 20 × 106 irradiated (30 Gy) allogeneic PBMC feeders plus 0.1 μg of 12F6 and 50 U of rIL-2 per ml in 20 ml of R-10 medium.
vv-HCV constructs.
vv-HCV recombinant viruses were constructed to express the structural and nonstructural proteins of HCV as previously described. The following constructs expressed the entire translated polyprotein of an HCV type 1a strain, HCV-H (13): vv1-966(H1) expressed amino acids (aa) Met1 to Asp966, and vv827-3011(H2) expressed aa Met827 to Arg3011. In addition, the following constructs expressing the proteins of a second type 1a strain, HCV-1(5), were used: vv-core/E1 expressed aa Met1 to Ile340; vv-E2(NS1)/NS2 expressed aa Met347 to Leu906; vv-E2/NS2/NS3 expressed aa Met364 to His1619; vv-NS4 expressed Gln1590 to Arg2050; vv-NS5A expressed Gly2005 to Gly2396; and vv-NS5B expressed Gly2396 to Arg3011. All vv-HCV recombinant viruses were demonstrated to express the appropriate HCV proteins by radioimmunoprecipitation (7). These recombinant viruses have also been shown to sensitize B-LCL to lysis by HCV-specific CTL (data not shown). A construct expressing the Escherichia coli β-galactosidase gene (vv-Lac) was used as a negative control.
Synthetic peptides.
Peptides corresponding to the aa sequences of the HCV-1 strain were synthesized as free acids by Cambridge Research Biochemicals (Cambridge, Mass.) or Mimotopes (Chiron, Victoria, Australia) using the Fmoc (9-fluorenylmethoxy carbonyl) method. Peptides were 20 aa in length, overlapping adjacent peptides by 10 aa. Fine mapping was achieved using additional smaller peptides in free acid form that were synthesized on an automated peptide synthesizer (model 432A; Applied Biosystems, Inc., Foster City, Calif.). All peptides were reconstituted in sterile distilled water containing 10% dimethyl sulfoxide (Sigma Chemical Co.) and 1 mM dithiothreitol (Sigma Chemical Co.).
Cytotoxicity assay using vaccinia virus-infected target cells.
B-LCL were infected with recombinant vv-HCV vectors at a multiplicity of 5 to 10 PFU/cell, labeled with NA2[51Cr]O4 (New England Nuclear, Boston, Mass.), and incubated overnight at 37°C in 5% CO2. The following morning, the B-LCL target cells were washed three times with cold R-10 medium and incubated with effector cells at 37°C for 4 h. Cellular release of [51Cr]O4 into the supernatant was measured using a Top Count Microplate scintillation counter (Packard Instrument Company, Meriden, Conn.), and the percent specific cytotoxicity was calculated by the formula % lysis = [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100. Results are reported as the mean of triplicate values, with a standard deviation of <5%. Samples were scored positive if the specific lysis at an effector-to-target cell of 100:1 was greater than 20% and at least 15% higher than the percent lysis for the vv-Lac-infected B-LCL negative control (39).
Data analyses.
All statistical analyses were performed using the Statistics for Windows 5.1 software package (Statsoft, Tulsa, Okla.).
RESULTS
HCV-specific CTL are present in PBMC of persons with chronic HCV infection.
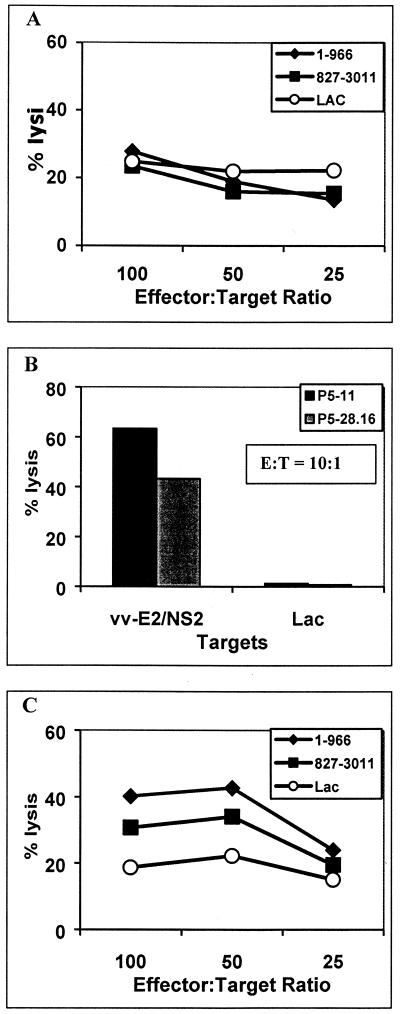
To determine if HCV-specific CTL can be detected in the peripheral blood of persons with chronic HCV infection, we initially examined a subject (P1) who had previously been shown to have a broadly directed response in the liver with five different CTL epitopes characterized (20). This subject had since completed a 5-month course of interferon therapy (she withdrew from the last month of treatment), resulting in a complete biochemical and virological response which has been sustained for over 1 year. A liver biopsy specimen, obtained prior to initiation of interferon therapy, and cryopreserved PBMC, obtained 3 months after starting interferon therapy, were available for study. Bulk stimulated cultures of PBMC expanded in an antigen-nonspecific manner with the anti-CD3 monoclonal antibody 12F6 had no evidence of HCV-specific CTL activity (Fig. 1A), which is consistent with our previous findings (19–21, 38). To increase the sensitivity of CTL detection, multiple clones were also established from PBMC by culturing at limiting dilution after stimulation with 12F6. Of the 105 clones tested, 2 lysed autologous B-LCL targets that expressed E2/NS2 (aa 339 to 906) of HCV-1 (Fig. 1B). CTL of similar specificity were obtained from intrahepatic lymphocytes with anti-CD3 stimulation (39). These data demonstrate that CTL of the same specificity as detected in liver can be detected at a clonal level in peripheral blood, but the frequencies are below the detection limit of bulk assays.
FIG. 1.
HCV-specific CTL are present in PBMC at low frequencies. Subject P1 was selected for study because she had chronic HCV infection and HCV-specific CTL previously identified from liver biopsy-derived lymphocytes. (A) PBMC were stimulated with 12F6, a CD3-specific monoclonal antibody, and tested after 2 weeks for CTL activity in a standard 4-h chromium release assay. HCV-H antigens were expressed on autologous B-LCL target cells using a recombinant HCV-vv infection. No HCV-specific CTL activity was detected compared to cells expressing the control LacZ protein. (B) Fresh (previously unstimulated) PBMC were cloned at limiting dilution and stimulated with 12F6 and allogeneic irradiated PBMC feeders. Of the 105 clones screened for CTL activity, 2 clones (P5-11 and P5-28.6) with CTL activity against target cells expressing the E2/NS2 proteins of HCV were identified. This suggested that HCV-specific CTL were present at low frequencies. (C) PBMC from subject P1 were also stimulated with antigens from the entire translated polyprotein of HCV-H expressed on autologous B-LCL. After 2 weeks in culture, the bulk stimulated cells were tested for CTL activity in a standard 4-h chromium release assay. HCV-specific CTL activity was detected against B-LCL expressing aa 1 to 966 and 827 to 3011 but not the negative control expressing the lacZ gene product.
Detection of HCV-specific CTL can be enhanced by antigen-specific stimulation.
To increase the sensitivity for detecting CTL, PBMC from the same individual were stimulated with autologous cells expressing HCV proteins. This was accomplished by infecting autologous B-LCL with recombinant vv-HCV constructs, leading to the expression of the entire translated polyprotein of HCV-H, a type 1a strain. Using this method, HCV-specific CTL activity was detected in bulk PBMC cultures (Fig. 1C). This bulk-stimulated PBMC culture was also cloned at limiting dilution using anti-CD3 monoclonal antibody so that the CTL response in peripheral blood could be characterized and compared to the CTL response in the liver. Of the 120 clones tested, 32 HCV-specific clones were identified. All five CTL epitopes recognized by the liver-derived clones were also recognized by at least one of the PBMC-derived clones (Fig. 2).
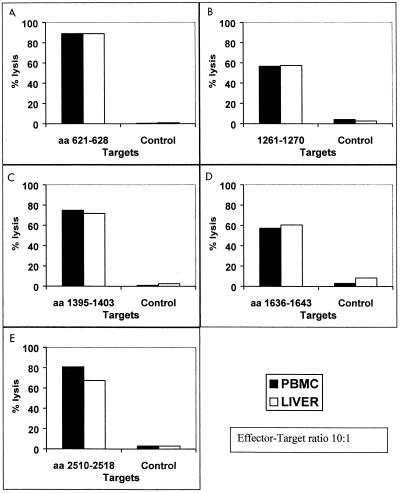
FIG. 2.
CTL identified from PBMC recognize the same epitopes as CTL identified from liver of subject P1. PBMC from subject P1 that had been stimulated with HCV-H antigens expressed by autologous B-LCL were subcloned and tested for recognition of five epitopes previously identified from this patient from liver CTL. Synthetic peptides corresponding to the identified CTL epitopes were incubated with 51Cr-labeled autologous B-LCL and used as target cells in a standard chromium release assay. CTL clones derived from PBMC (solid bars) and liver (clear bars) specifically recognized the epitopes (A) TINYTIFK, aa 621-628 in E2 envelope, (B) TLGFGAYMSK, aa 1261 to 1270, and (C) HSKKKCDEL, aa 1395 to 1403 in the NS3 protein, (D) TLGFGAYMSK, aa 1636 to 1643 in NS4, and (E) SLTPPHSAK, aa 2510 to 2518 in the NS5b protein.
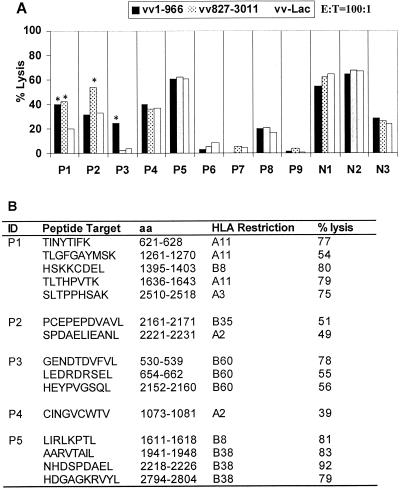
To assess the ability of this method to detect CTL in PBMC, we examined seven subjects with chronic HCV infection in whom CTL had been detected from liver-derived lymphocytes and for whom cryopreserved PBMC were available (Fig. 3A). PBMC stimulated with the anti-CD3 monoclonal antibody 12F6 had a 40- to 100-fold expansion in the total number of cells after 2 weeks, as assessed by absolute cell counts (Table 1). This indicates that the cryopreserved PBMC were viable and able to proliferate. Proliferation following stimulation with autologous B-LCL infected with recombinant vv-HCV, in which one would expect preferential expansion of HCV-specific CTL, had a 0.25- to 40-fold expansion over the same period of time. Nonspecific responses, possibly reflecting T-cell responses to vaccinia virus or EBV resulting in vv-Lac-specific lysis of >20%, were observed in 8 of the 12 subjects tested. However, three of the seven subjects with intrahepatic CTL also had detectable HCV-specific PBMC CTL using this antigen-specific stimulation method to establish polyclonal cell lines (P1, P2, and P3) (Fig. 3B). These results were confirmed by the establishment of PBMC clones from these three subjects (Fig. 3B). In addition, CTL were detected in PBMC from an additional two subjects (P4 and P5) after HCV-vv stimulated PBMC were cloned and screened for CTL responses (Fig. 3B). The specificities of the PBMC-derived CTL were identical to those that had been derived from liver. Another two subjects (P8 and P9) who did not have intrahepatic CTL were also studied, and neither of these had evidence of CTL in PBMC. Of the two subjects with discordant results between liver and PBMC CTL, P6 had a vigorous intrahepatic CTL response but no evidence of CTL from the PBMC sample obtained 1 year after the liver biopsy. Unfortunately, no PBMC samples were available from the time when the liver biopsy was obtained. P7 had a low frequency of intrahepatic CTL and no evidence of CTL from a PBMC sample from the same time point.
FIG. 3.
HCV-specific PBMC CTL detected in majority of subjects with intrahepatic CTL. (A) Seven subjects (P1 to P7) known to have intrahepatic CTL responses, two subjects with no detectable intrahepatic CTL responses (P8 and P9), and three HCV-seronegative controls (N1 to N3) were tested for PBMC CTL after stimulation with HCV-H antigens expressed on autologous B-LCL using a 4-h chromium release assay. Assays were scored as positive if the total specific lysis was greater than 20% and at least 15% greater than the background nonspecific lysis. HCV-specific CTL activity was detected in bulk stimulated cultures of subjects P1, P2, and P3 (indicated by asterisks). (B) Bulk-stimulated cells were subcloned and tested again for CTL activity. Where the bulk CTL assay had been scored as positive, HCV-specific CTL clones recognizing the appropriate regions identified using vaccinia virus vectors were detected in all three subjects (P1 to P3). Furthermore, PBMC CTL clones targeting epitopes in regions where the bulk CTL assay was scored as negative were found in subject P3 (aa 2152 to 2160) and in an additional two subjects (P4 and P5). All CTL clones recognized the optimal epitope in the context of a specific restricting HLA class I allele with high specificity. Nonspecific lysis to irrelevant peptides for all clones was <5%.
TABLE 1.
HCV-specific CTL can be detected in the majority of subjects with chronic HCV infection in whom CTL had previously been detected in the livera
| Status | Subject | Stimulation | Expansion (fold) | No. of clones/no. tested |
|---|---|---|---|---|
| Chronic HCV infection with intrahepatic CTL | P1 (94F) | HCV-H | 36.8 | 32/120 |
| 12F6 | 52.9 | |||
| P2 (91E) | HCV-H | 0.3 | 3/165 | |
| 12F6 | 45.6 | |||
| P3 (94I) | HCV-H | 1.7 | 30/120 | |
| P4 (93K) | HCV-H | 1.2 | 7/147 | |
| P5 (95I) | HCV-H | 2.6 | 3/168 | |
| 12F6 | 52.6 | 0/84 | ||
| P6 (93I) | HCV-H | 1.2 | 0/120 | |
| 12F6 | 55.0 | |||
| P7 (95K) | HCV-H | 0.5 | 0/120 | |
| 12F6 | 53.0 | |||
| Chronic HCV infection without intrahepatic or PBMC CTL | P8 (95L) | HCV-H | 4.6 | 0/179 |
| 12F6 | 68.0 | |||
| P9 (97B) | HCV-H | 11.8 | 0/140 | |
| 12F6 | 86.0 | |||
| Seronegative controls | N1 (BW) | HCV-H | 6.7 | 0/126 |
| N2 (MK) | HCV-H | 21.8 | 0/97 | |
| 12F6 | 30.8 | |||
| N3 (CCW) | HCV-H | 0.2 | 0/120 | |
| 12F6 | 38.9 |
Cryopreserved PBMC were subjected to one round of stimulation with HCV-H antigens expressed on autologous B-LCL using a recombinant vaccinia virus infection system. On day 14, the cells were counted to assess the degree of expansion. The cells were also tested for HCV-specific CTL activity using a standard 4-h chromium release assay. Assays were scored as positive if the total percent specific lysis was greater than 20% and at least 15% greater than the background nonspecific lysis. The cells were then subcloned and tested for HCV-specific CTL activity. HCV-specific CTL activity was detected from five of seven subjects with chronic HCV in whom CTL had previously been detected from the liver. HCV-specific PBMC CTL were not detected in two subjects with chronic HCV in whom CTL had also not been detected in the liver or in three HCV-seronegative control subjects. These negative results were not due to poor cell viability, as cells expanded after stimulation with the anti-CD3 monoclonal antibody 12F6.
PBMC from three HCV-seronegative subjects were also examined, but no HCV-specific CTL were detected after antigen-specific stimulation with autologous B-LCL expressing HCV-H (Fig. 3B). Two of these three subjects were HLA B35 positive and were previously reported to have HLA B35-restricted CTL responses in PBMC after in vitro stimulation with the peptide P401 (aa 234 to 242, NASRCWVAM) (22). Once again, HLA B35-restricted CTL clones were obtained from both subjects following stimulation with peptide P401. However, similar to previous results, these CTL only recognized cells incubated with P401, not the endogenously processed antigen when the vaccinia virus expression system was used (22 and data not shown). Together, the data suggest that antigen-specific stimulation of PBMC using recombinant HCV-vv-driven expression of HCV antigens in autologous B-LCL can be used to detect CTL responses in the majority of subjects with intrahepatic CTL responses and that these responses reflect recognition of endogenously processed antigen.
No evidence of CTL in a cohort at high risk for HCV exposure.
A cohort of injection drug users in Baltimore has been followed for incidence and prevalence of several blood-borne pathogens. The prevalence of HCV was very high in this group, with frequent use and sharing of drug paraphernalia identified as factors for HCV seroconversion among those who were initially HCV seronegative (35). However, a subgroup remained HCV seronegative despite a history of continued high-risk behavior. This group was studied to determine if there was evidence of HCV-specific CTL that might account for their remaining HCV seronegative. Cryopreserved PBMC from 10 subjects were obtained from this cohort, 8 who remained HCV seronegative and 2 who had developed chronic HCV infection. Assays were performed in a blinded fashion. Two of the 10 subjects had detectable CTL responses in PBMC, and upon unblinding, both were shown to be HCV seropositive (Table 2). Subject A had a CTL response to one epitope within aa 347 to 906, and subject B had CTL responses to an epitope in the E1 (aa 285 to 293) and NS2 (aa 838 to 846) proteins (Table 2 and data not shown). The remaining eight subjects were HCV seronegative and had no evidence of HCV-specific CTL responses. These data are in conflict with the hypothesis that memory CTL generated from previous exposure were responsible for the continued HCV-seronegative status of these individuals. CTL may be present but below the limit of detection of this assay, in which case they are of lower magnitude than in persons with chronic progressive infection who do not control HCV replication.
TABLE 2.
HCV-specific CTL detected in subjects with chronic HCV infection but not in uninfected subjects despite the history of continued injection drug usea
| Subject | HCV ELISA result | Stimulation | Expansion (fold) | Bulk assay result | No. of clones/no. tested |
|---|---|---|---|---|---|
| A | + | HCV-H | 1.5 | ND | 5/105 |
| B | + | HCV-H | 30.0 | Positive | 8/78 |
| C | − | HCV-H | 3.0 | Negative | 0/120 |
| D | − | HCV-H | 5.0 | Negative | 0/120 |
| 12F6 | 92.0 | Negative | ND | ||
| E | − | HCV-H | 0.3 | ND | 0/105 |
| F | − | HCV-H | 28.0 | Negative | 0/120 |
| G | − | HCV-H | 0.2 | Negative | 0/120 |
| H | − | HCV-H | 3.2 | Negative | 0/58 |
| 12F6 | 34.0 | Negative | ND | ||
| 12970I | − | HCV-H | 22.2 | Negative | 0/120 |
| 12F6 | 46.6 | Negative | ND | ||
| 13173J | − | HCV-H | 2.8 | Negative | 0/44 |
Cryopreserved PBMC were stimulated and then tested for HCV-specific CTL activity using a standard 4-h chromium release assay. The cells were then subcloned and tested for HCV-specific CTL activity. HCV-specific CTL activity was detected in both subjects with chronic HCV infection but in none of the eight HCV-seronegative subjects who had been selected for high risk of viral exposure. ELISA, enzyme-linked immunosorbent assay. ND, not determined.
DISCUSSION
The role of HCV-specific CTL in the pathogenesis of HCV infection is still unclear. Most studies have been conducted on chronically infected subjects, which have been the easiest to identify. HCV-specific CTL have been identified in the majority of these subjects from lymphocytes isolated from liver (26, 39). CTL have also been detected by in vitro stimulation with viral peptides. These methods are largely limited to detection of predicted epitopes and therefore do not provide a comprehensive analysis of CTL to all possible presented epitopes (2, 4, 12, 15, 17, 23, 28, 32, 33). In addition, peptide-specific approaches limit the analysis to known particular peptides and alleles. The ability to study CTL responses in HLA-diverse populations and to study responses to the entire translated polyprotein will allow a more comprehensive characterization of this response. This would be particularly advantageous for situations when liver biopsy is not feasible, such as for subjects with acute HCV infection, uninfected subjects, and studies for which frequent samples are required.
In this study, we have used vaccinia virus infection to express HCV-H antigens (type 1a strain) in autologous B-lymphoblastoid cells. These cells were then used to stimulate PBMC. The advantage of this system is that the PBMC are exposed to antigens from the entire HCV-H genome and expressed in the context of all autologous HLA alleles. Furthermore, over 75% of subjects in North America are infected with genotype 1 viruses, which would have a relatively high degree of homology with HCV-H (1). Our results indicate that the majority of persons with chronic HCV infection have circulating CTL and that these target epitopes are similar to those targeted by the intrahepatic CTL response.
A potential problem with this system is that the PBMC are also exposed to vaccinia virus antigens and EBV antigens (EBV was used to establish the B-LCL). However, our results show that HCV-specific CTL clones can be established in the majority of subjects after one round of in vitro stimulation. Use of cold targets might be expected to lower background levels of lysis for the bulk expanded cells even further, as has been shown using this system for other viral infections (25). The high degree of concordance of HCV epitopes identified from liver and PBMC CTL suggests that this strategy is a reasonable alternative to liver biopsy studies for identifying CTL responses and epitopes. However, whether the T-cell receptor repertoire in the liver is fully represented in the peripheral blood and whether the frequency of CTL in blood will reflect frequencies in the liver are important issues that still need to be addressed.
Virus-specific CTL have been implicated as a possible protective mechanism in subjects with high-risk viral exposure, such as those exposed to human immunodeficiency virus (30, 31). To see if this was also true for HCV, we studied a cohort of injection drug users who reported prolonged and persistent high-risk behavior. We found no evidence of HCV-specific CTL that might be responsible for the persistent lack of HCV infection in these eight individuals. This did not appear to be caused by technical reasons, such as poor cell viability, as CTL were identified in the two chronically infected subjects, whose PBMC were analyzed identically. However, this is a difficult hypothesis to reject, as there is no guarantee of HCV exposure despite the history of high-risk behavior. In addition, it is also possible that CTL are present but below the detection limit. However, one can say that the levels of CTL are at least well below those seen in some persons with chronic uncontrolled HCV infection.
Estimating the magnitude of the CTL response using methods that require in vitro stimulation is potentially problematic since the measurements reflect the antigen-specific cells that have survived the incubation period rather than the magnitude of the immune response at the time the sample of PBMC was obtained. Newer technologies, such as Elispot detection of cytokine secretion in response to specific antigen (24) and the use of tetrameric complexes of HLA class I and viral peptide (11), have an advantage in that they do not require in vitro stimulation and can give an estimate of the frequency of antigen-specific CD8 cells within the population of PBMC. However, both techniques require initial identification of the relevant CTL epitopes and do not test the ability of CD8 cells to mediate cell lysis. To date, we have identified 39 different CTL epitopes restricted by 17 different HLA class I alleles in the HCV genome (19–21, 39). The technique reported here provides a means of identifying CTL epitopes without the need for preselecting epitopes for screening or for a liver biopsy. It also allows assessment of the lytic potential of CTL responses. Together, these techniques provide the means to investigate the role that CTL play in subjects for whom liver biopsies cannot be obtained routinely.
In summary, we report the frequent detection of HCV-specific CTL in PBMC in persons with chronic HCV infection. In these subjects, the identified epitopes have a high degree of correlation with the epitopes identified from liver-derived CTL. These data indicate that CTL circulate in peripheral blood in persons with chronic HCV infection, and this method provides a means to assess CTL without the need for liver biopsy.
REFERENCES
- 1.Alter M J, Kruszon-Moran D, Nainan O V, McQuillan G M, Gao F, Moyer L A, Kaslow R A, Margolis H S. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 2.Battegay M, Fikes J, Di Bisceglie A M, Wentworth P A, Sette A, Celis E, Ching W M, Grakoui A, Rice C M, Kurokohchi K, et al. Patients with chronic hepatitis C have circulating cytotoxic T cells which recognize hepatitis C virus-encoded peptides binding to HLA-A2.1 molecules. J Virol. 1995;69:2462–2470. doi: 10.1128/jvi.69.4.2462-2470.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cerny A, Fowler P, Brothers M A, Houghton M, Schlicht H J, Chisari F V. Induction in vitro of a primary human antiviral cytotoxic T cell response. Eur J Immunol. 1995;25:627–630. doi: 10.1002/eji.1830250248. [DOI] [PubMed] [Google Scholar]
- 4.Cerny A, McHutchison J G, Pasquinelli C, Brown M E, Brothers M A, Grabscheid B, Fowler P, Houghton M, Chisari F V. Cytotoxic T lymphocyte response to hepatitis C virus-derived peptides containing the HLA A2.1 binding motif. J Clin Investig. 1995;95:521–530. doi: 10.1172/JCI117694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choo Q L, Kuo G, Weiner A J, Overby L R, Bradley D W, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:362–364. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 6.Cooper S, Erickson A L, Adams E J, Kansopon J, Weiner A J, Chien D Y, Houghton M, Parham P, Walker C M. Analysis of a successful immune response against hepatitis C virus. Immunity. 1999;10:439–449. doi: 10.1016/s1074-7613(00)80044-8. [DOI] [PubMed] [Google Scholar]
- 7.Grakoui A, Wychowski C, Lin C, Feinstone S M, Rice C M. Expression and identification of hepatitis C virus polyprotein cleavage products. J Virol. 1993;67:1385–1395. doi: 10.1128/jvi.67.3.1385-1395.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gruner N H, Gerlach T J, Jung M C, Diepolder H M, Schirren C A, Schraut W W, Hoffmann R, Zachoval R, Santantonio T, Cucchiarini M, Cerny A, Pape G R. Association of hepatitis C virus-specific CD8+ T cells with viral clearance in acute hepatitis C. J Infect Dis. 2000;181:1528–1536. doi: 10.1086/315450. [DOI] [PubMed] [Google Scholar]
- 9.Guidotti L G, Ando K, Hobbs M V, Ishikawa T, Runkel L, Schreiber R D, Chisari F V. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc Natl Acad Sci USA. 1994;91:3764–3768. doi: 10.1073/pnas.91.9.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guidotti L G, Ishikawa T, Hobbs M V, Matzke B, Schreiber R, Chisari F V. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 11.He X S, Rehermann B, Lopez-Labrador F X, Boisvert J, Cheung R, Mumm J, Wedemeyer H, Berenguer M, Wright T L, Davis M M, Greenberg H B. Quantitative analysis of hepatitis C virus-specific CD8(+) T cells in peripheral blood and liver using peptide-MHC tetramers. Proc Natl Acad Sci USA. 1999;96:5692–5697. doi: 10.1073/pnas.96.10.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibe M, Sakaguchi T, Tanaka K, Saito S, Yokota S, Tanaka T, Shimotohno K, Chujoh Y, Shiratori Y, Omata M, Miwa K, Takiguchi M. Identification and characterization of a cytotoxic T cell epitope of hepatitis C virus presented by HLA-B*3501 in acute hepatitis. J Gen Virol. 1998;79:1735–1744. doi: 10.1099/0022-1317-79-7-1735. [DOI] [PubMed] [Google Scholar]
- 13.Inchauspe G, Zebedee S, Lee D H, Sugitani M, Nasoff M, Prince A M. Genomic structure of the human prototype strain H of hepatitis C virus: comparison with American and Japanese isolates. Proc Natl Acad Sci USA. 1991;88:10292–10296. doi: 10.1073/pnas.88.22.10292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson R P, Glickman R L, Yang J Q, Kaur A, Dion J T, Mulligan M J, Desrosiers R C. Induction of vigorous cytotoxic T-lymphocyte responses by live attenuated simian immunodeficiency virus. J Virol. 1997;71:7711–7718. doi: 10.1128/jvi.71.10.7711-7718.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaneko T, Nakamura I, Kita H, Hiroishi K, Moriyama T, Imawari M. Three new cytotoxic T cell epitopes identified within the hepatitis C virus nucleoprotein. J Gen Virol. 1996;77:1305–1309. doi: 10.1099/0022-1317-77-6-1305. [DOI] [PubMed] [Google Scholar]
- 16.Kita H, Hiroishi K, Moriyama T, Okamoto H, Kaneko T, Ohnishi S, Yazaki Y, Imawari M. A minimal and optimal cytotoxic T cell epitope within hepatitis C virus nucleoprotein. J Gen Virol. 1995;76:3189–3193. doi: 10.1099/0022-1317-76-12-3189. [DOI] [PubMed] [Google Scholar]
- 17.Kita H, Moriyama T, Kaneko T, Okamoto H, Hiroishi K, Ohnishi S, Imawari M. HLA B44-restricted cytotoxic T lymphocyte responses to the peptides of HCV nucleoprotein residues 81–100 in patients with chronic hepatitis C. J Gastroenterol. 1995;30:809–812. doi: 10.1007/BF02349654. [DOI] [PubMed] [Google Scholar]
- 18.Koelle D M, Posavad C M, Barnum G R, Johnson M L, Frank J M, Corey L. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Investig. 1998;101:1500–1508. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koziel M J, Dudley D, Afdhal N, Choo Q L, Houghton M, Ralston R, Walker B D. Hepatitis C virus (HCV)-specific cytotoxic T lymphocytes recognize epitopes in the core and envelope proteins of HCV. J Virol. 1993;67:7522–7532. doi: 10.1128/jvi.67.12.7522-7532.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koziel M J, Dudley D, Afdhal N, Grakoui A, Rice C M, Choo Q L, Houghton M, Walker B D. HLA class I-restricted cytotoxic T lymphocytes specific for hepatitis C virus: identification of multiple epitopes and characterization of patterns of cytokine release. J Clin Investig. 1995;96:2311–2321. doi: 10.1172/JCI118287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koziel M J, Dudley D, Wong J T, Dienstag J, Houghton M, Ralston R, Walker B D. Intrahepatic cytotoxic T lymphocytes specific for hepatitis C virus in persons with chronic hepatitis. J Immunol. 1992;149:3339–3344. . (Erratum, 150:2563.) [PubMed] [Google Scholar]
- 22.Koziel M J, Wong D K, Dudley D, Houghton M, Walker B D. Hepatitis C virus-specific cytolytic T lymphocyte and T helper cell responses in seronegative persons. J Infect Dis. 1997;176:859–866. doi: 10.1086/516546. [DOI] [PubMed] [Google Scholar]
- 23.Kurokohchi K, Akatsuka T, Pendleton C D, Takamizawa A, Nishioka M, Battegay M, Feinstone S M, Berzofsky J A. Use of recombinant protein to identify a motif-negative human cytotoxic T-cell epitope presented by HLA-A2 in the hepatitis C virus NS3 region. J Virol. 1996;70:232–240. doi: 10.1128/jvi.70.1.232-240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lechner F, Wong D K, Dunbar P R, Chapman R, Chung R T, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker B D. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lubaki M N, Egan M A, Siliciano R F, Weinhold K J, Bollinger R C. A novel method for detection and ex vivo expansion of HIV type 1-specific cytolytic T lymphocytes. AIDS Res Hum Retrovirol. 1995;10:1427–1431. doi: 10.1089/aid.1994.10.1427. [DOI] [PubMed] [Google Scholar]
- 26.Nelson D R, Marousis C G, Davis G L, Rice C M, Wong J, Houghton M, Lau J Y. The role of hepatitis C virus-specific cytotoxic T lymphocytes in chronic hepatitis C. J Immunol. 1997;158:1473–1481. [PubMed] [Google Scholar]
- 27.Rehermann B, Chang K M, McHutchinson J, Kokka R, Houghton M, Rice C M, Chisari F V. Differential cytotoxic T-lymphocyte responsiveness to the hepatitis B and C viruses in chronically infected patients. J Virol. 1996;70:7092–7102. doi: 10.1128/jvi.70.10.7092-7102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rehermann B, Chang K M, McHutchison J G, Kokka R, Houghton M, Chisari F V. Quantitative analysis of the peripheral blood cytotoxic T lymphocyte response in patients with chronic hepatitis C virus infection. J Clin Investig. 1996;98:1432–1440. doi: 10.1172/JCI118931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rooney C M, Smith C A, Ng C Y, Loftin S K, Sixbey J W, Gan Y, Srivastava D K, Bowman L C, Krance R A, Brenner M K, Heslop H E. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- 30.Rowland-Jones S L, Dong T, Fowke K R, Kimani J, Krausa P, Newell H, Blanchard T, Ariyoshi K, Oyugi J, Ngugi E, Bwayo J, MacDonald K S, McMichael A J, Plummer F A. Cytotoxic T cell responses to multiple conserved HIV epitopes in HIV-resistant prostitutes in Nairobi. J Clin Investig. 1998;102:1758–1765. doi: 10.1172/JCI4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowland-Jones S L, Nixon D F, Aldhous M C, Gotch F, Ariyoshi K, Hallam N, Kroll J S, Froebel K, McMichael A. HIV-specific cytotoxic T-cell activity in an HIV-exposed but uninfected infant. Eur J Immunol. 1993;23:447–453. doi: 10.1016/0140-6736(93)93063-7. [DOI] [PubMed] [Google Scholar]
- 32.Shirai M, Okada H, Nishioka M, Akatsuka T, Wychowski C, Houghten R, Pendleton C D, Feinstone S M, Berzofsky J A. An epitope in hepatitis C virus core region recognized by cytotoxic T cells in mice and humans. J Virol. 1994;68:3334–3342. doi: 10.1128/jvi.68.5.3334-3342.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takaki A, Wiese M, Maertens G, Depla E, Seifert U, Liebetrau A, Miller J L, Manns M P, Rehermann B. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med. 2000;6:578–582. doi: 10.1038/75063. [DOI] [PubMed] [Google Scholar]
- 34.Thomas D L, Vlahov D, Solomon L, Cohn S, Taylor E, Garfein R, Nelson K E. Correlates of hepatitis C virus infections among injection drug users. Medicine (Baltimore) 1995;74:212–220. doi: 10.1097/00005792-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Villano S A, Vlahov D, Nelson K E, Lyles C M, Cohn S, Thomas D L. Incidence and risk factors for hepatitis C among injection drug users in Baltimore, Maryland. J Clin Microbiol. 1997;35:3274–3277. doi: 10.1128/jcm.35.12.3274-3277.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vlahov D, Anthony J C, Munoz A, Margolick J, Nelson K E, Celentano D D, Solomon L, Polk B F. The ALIVE study, a longitudinal study of HIV-1 infection in intravenous drug users: description of methods and characteristics of participants. NIDA Res Monogr. 1991;109:75–100. [PubMed] [Google Scholar]
- 37.Walter E A, Greenberg P D, Gilbert M J, Finch R J, Watanabe K S, Thomas E D, Riddell S R. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 38.Wong D A, Tong L K, Lim W. High prevalence of hepatitis C virus genotype 6 among certain risk groups in Hong Kong. Eur J Epidemiol. 1998;14:421–426. doi: 10.1023/a:1007400304726. [DOI] [PubMed] [Google Scholar]
- 39.Wong D K, Dudley D D, Afdhal N H, Dienstag J, Rice C M, Wang L, Houghton M, Walker B D, Koziel M J. Liver-derived CTL in hepatitis C virus infection: breadth and specificity of responses in a cohort of persons with chronic infection. J Immunol. 1998;160:1479–1488. [PubMed] [Google Scholar]
- 40.Yang O O, Walker B D. CD8+ cells in human immunodeficiency virus type 1 pathogenesis: cytolytic and noncytolytic inhibition of viral replication. Adv Immunol. 1997;66:273–311. doi: 10.1016/s0065-2776(08)60600-8. [DOI] [PubMed] [Google Scholar]