Abstract
Baby corn, characterized by its high water activity and elevated respiration rate, poses a formidable obstacle to prolonged storage under standard ambient conditions and necessitates specialized treatments for transportation to distant locations. One of the primary postharvest challenges associated with baby corn is the occurrence of brown pigment formation because of enzymatic browning at the apex of its immature ovules, cut surfaces, and silk attached to the young ears. The present study was undertaken to investigate the effect of different blanching treatments on peroxidase inactivation, physicochemical properties, and functional properties of baby corn. The treatments applied were hot water blanching (HWB) at temperatures ranging from 70°C to 90 °C for 30–240 s, steam blanching (SB) for 30–240 s, and microwave blanching (MWB) at power levels of 360 W–900 W for 30–300 s. Results indicated that 90 % peroxidase enzyme inactivation occurred under different methods as 90 °C for 60 s for HWB, 100 °C for 60 s for SB, and 540 W for 30 s for MWB. These blanching methods have shown significant effects on the properties under investigation. MWB demonstrated the highest retention of ascorbic acid (94.15 %) and minimal color changes (ΔE = 5.72) in comparison to hot water and steam blanching. Similarly, the result for total flavonoid content for 540 W, 90 °C and 100 °C for 30, 60, and 60 s were found to be 3.01,1.99 and 2.10 mg QE/100g, phenols 48.98, 47.99 and 48.03 mg GAE/100g and DPPH (%) 42.55, 34.20 and 37.08 % respectively. The findings suggest that microwave blanching of baby corn at 540 W for 30 s holds promise to inactivate the peroxidase enzyme with better retention of physicochemical and functional properties.
Keywords: Baby corn, Blanching, Peroxidase, Antioxidant, Physical chemical properties
1. Introduction
Baby corn, scientifically known as Zea mays L. is a type of maize with distinguished and unique attributes harvested in its early, undeveloped stage, characterized by tender cobs adorned with silks measuring 2–3 cm in length [1]. Renowned for its delightful sweetness and succulence, baby corn can be savoured either in its raw form or when expertly prepared in various culinary dishes, it is not only tasty vegetable but is rich in carbohydrates, vitamins, amino acids, protein, minerals, fibre, β carotene, vitamin C and low in cholesterol and calories. As a result, this monoecious plant stands unique among vegetables, boasting a pristine absence of pesticide residues. This remarkable quality stems from its harvesting within a week of silk emergence, eliminating the necessity for pesticide application during this critical period [2].
Baby corn, characterized by its high water activity and elevated respiration rate, poses a formidable obstacle to prolonged storage under standard ambient conditions and necessitates specialized treatments for transportation to distant locations [3].One of the primary postharvest challenges associated with baby corn is the occurrence of brown pigment formation at the apex of its immature ovules, cut surfaces, and silk attached to the young ears [4]. Furthermore, browning is a common phenomenon in many fruits and vegetables and the underlying mechanisms for the same is either enzymatic or non-enzymatic [5]. The prominent factor influencing the acceptability by consumers is the cut surface browning of baby corn which is the consequence of intercellular enzymes like peroxidase and polyphenol oxidase (PPO) after harvest which affects the quality attributes like nutrients, color, texture, and flavor [6]. The principal cause of browning is the excess weight loss of young ears that causes the breakdown of vacuoles releasing the phenolic compounds that come in contact with PPO resulting in the development of brown pigments [4].
Blanching is a conventional technique for preventing enzymatic browning; Thermal treatment results in inactivating natural enzymes responsible for browning and destroys the microorganisms that might contaminate the crop during various stages of handling such as production, harvesting, transportation and storage. The treatment helps in preserving the vibrant color, flavor, and nutritional value of the crop. This process ensures that fruits and vegetables remain appealing, fresh-tasting, and have an extended shelf life. Traditionally hot water blanching is the most commonly technique followed by steam blanching. Advanced blanching technologies, including microwave [7], catalytic infrared [8], and high-pressure blanching [9], along with chemical methods utilizing antioxidants and chelating agents, further enhance its effectiveness [10]. Although extensive research has been conducted on blanching various vegetables, there is limited literature on its impact on baby corn, highlighting the need for further investigation into the best methods for preserving this high-value vegetable. Different time-temperature combinations influence the effectiveness of blanching. Therefore, the primary objective of this research was to examine the influence of various blanching methods on the characteristics of this high-value vegetable.
2. Materials and methods
The baby corn cobs of variety Syngenta 5417 were procured from Field Fresh Foods Pvt Ltd., Ladhowal, Ludhiana, India. Before blanching the cobs were dehusked and cleaned manually. The physicochemical and antioxidant parameters were analyzed before blanching.
2.1. Blanching treatment
Blanching was meticulously carried out with the primary objective of inactivating the peroxidase enzyme, a widely recognized marker enzyme associated with the blanching process. The effect of the treatments was studied on various physico-chemical properties of baby corn. Sample (100g) meticulously sliced in circular pieces, each having a diameter of precisely 15 ± 0.02 mm and a thickness within the range of 0.1 cm–0.2 cm, were subjected to various distinct blanching treatments viz. hot water blanching (HWB) at 70 °C, 80 °C, 90 °C for 30, 60, 120 180, and 240 s, steam blanching (SB) for 30, 60, 120 180 and 240 s, and microwave blanching (MWB) at 360 W–900 W for 30–300 s. The treatments were decided based on the preliminary trials carried out based on the work reported for different vegetables. Treated samples were analyzed in triplicates for shrinkage (%), mass of kernel (g), TSS, densities, color, enzyme inactivation and antioxidant assay.
2.2. Physical properties
Dehusked baby corn cobs were cut in circular shapes and blanching treatments were given one by one including HWB, SB and MWB. Moreover, diameters of round pieces were taken before and after blanching by means of Vernier calliper to find out the effect of blanching on diameter or shrinkage (%) [11].
2.2.1. Determination of bulk density, true density and porosity
The determination of bulk density was accomplished by carefully transferring a known mass (g) of the sample into a measuring cylinder, and subsequently recording the volume occupied by roundels. The true density was precisely ascertained by toluene displacement method. Moreover, the specified quantity of toluene was put in a measuring cylinder followed by pouring the known mass of sample and the final volume was noted [12].Densities, both bulk and true, were computed employing Eq's. (1) and (2). Subsequently, the porosity (ԑ) was determined using Eq. (3).
| (1) |
| (2) |
| (3) |
where, ε represents the percentage of porosity, ρb denotes the bulk density in g/cc, and ρt signifies the true density in g/cc.
2.2.2. Determination of color values
The color values were recorded by Hunter lab colorimeter (Accuracy Micro sensors, Inc. Pittsford, New York) for fresh, blanched and dried sample with the aim to assess how the drying process influenced the color of the treated samples [13]. The transformation in color was quantified through the L*, a*, b* color system, where L* represents the degree of lightness, a* component ranges from green (-a) to red (+a), and b* component spans from blue (-b) to yellow (+b).The color difference (ΔE) and browning index were determined by using the formula as mentioned in Eq's. (4) and (5)
| (4) |
| (5) |
L₀*, a₀*, and b₀* correspond to the initial color values of L*, a*, and b*, respectively, value of X can be determined using the equation represented as Eq. (6).
| (6) |
2.3. Determination of chemical properties
2.3.1. Quantification of moisture content, water activity, TSS and ascorbic acid
The determination of moisture was done using hot air oven (Narang Scientific Works Pvt Ltd, Model: NSW-143, Ambala, India) method at 70 ± 5 °C until constant weight is obtained [14]. A water activity analyzer (Testo AG 400, Germany) was used to determine the water activity. Similarly, for the determination of Total Soluble Solids (TSS), a digital refractometer (model PT-32; ATAGO, Tokyo, Japan) with a measuring range spanning from 0 to 32 °Brix was employed [14] and the quantification of ascorbic acid content was conducted using the established standard procedure outlined by Ref. [15].
2.3.2. Determination of total phenol, flavonoid and free radical scavenging activity (DPPH)
The methodology described by Limmatvapirat et al. [16] was followed for determination of flavonoid and total phenol content. The method described by Kaur et al. [17] was employed for the purpose of determining free radical scavenging activity (FRSA or DPPH). An ethanolic solution of 2, 2-diphenyl-1-picrylhydrazyl (DPPH) at a concentration of 90 μmol/L was used in analysis. A 100 μl aliquot of the extract was carefully pipetted, and it was mixed with 1000 μl of the pre-prepared DPPH solution in a test tube. The resulting mixture was thoroughly shaken and then left in darkness for duration of 60 min. Subsequently, the absorbance of the solution was quantified at 517 nm (UV/Vis Spectrophotometer, Shimadzu Corporation, Japan). This measurement was conducted in comparison to a blank sample, as detailed in Eq. (7).
| Antioxidant activity (%) = A₀- A/ A₀ | (7) |
where, A₀ = Absorbance of DPPH solution as blank.
A = Absorbance of sample.
2.3.3. Peroxidase enzymatic activity
The treated samples (HWB, SB, and MWB) were analyzed for % residual enzymatic activity. Sample (1 g) was weighed and put in a test tube, 0.5 % guaiacol (10 ml) and 0.08 % hydrogen peroxide (10 ml) were introduced followed by vigorous shaking. The test tube was then allowed to stand undisturbed for 3 min, during which the color change was observed and recorded. The transition in color from a clear, colorless state to a vivid brick-red hue signifies a state of incomplete enzyme activation. For quantitative results, the absorbance was checked at 720 nm in a spectrophotometer [18].
2.4. Kinetics of variation in responses
The different responses during blanching methods at different time - temperature/power combinations of baby corn for hot water and microwave blanching were subjected to zero and first-order equations. In order to assess the fluctuation in various responses during the processing, below-mentioned equations (8), (9) were used.
| (8) |
| (9) |
Here, C represents the approximate quantity of diverse responses at specific processing times (θ) and temperatures (T) within various blanching treatments, C0 represents corresponding response in fresh sample, K0 and K1 represents kinetic rate constants.
2.5. Statistical analysis
Analytical determinations were conducted meticulously in triplicate to ensure data accuracy and reliability. The data collected from different treatments was meticulously recorded and subjected to rigorous statistical analysis. This entailed the application of one-way or two-way Analysis of Variance (ANOVA) and subsequent analysis using Duncan's Multiple Range Test (DMRT) with a significance level of p ≤ 0.05. Furthermore, the most appropriate models were selected based on the values of R2 (coefficient of determination), root mean square error (RMSE), and χ2 (chi-square statistic). These statistical assessments played a crucial role in identifying the best-fitted models for representing the observed data trends accurately.
3. Results and discussion
3.1. Raw material analysis
The obtained raw material underwent a comprehensive evaluation, encompassing physicochemical and antioxidant assays, as illustrated in (Table 1).The physicochemical parameters moisture, ash, protein, fat, fiber, carbohydrates, pH, TSS, trititable acidity, ascorbic acid were found to be 86.2 ± 2.27 %, 0.40 ± 0.4 %, 3.54 ± 1.9 %, 0.94 ± 0.29 %, 3.72 ± 0.63 %, 5.20 ± 0.78 %, 5.21 ± 0.03, 9.80 ± 0.5 °Bx, 0.40 ± 0.04 % and 15 ± 2.25 mg/100 g respectively.
Table 1.
Raw material analysis prior to blanching.
| Moisture (%) | 86.2 ± 2.27 |
| Ash (%) | 0.40 ± 0.4 |
| Protein (%) | 3.54 ± 1.90 |
| Fat (%) | 0.94 ± 0.29 |
| Fiber (%) | 3.72 ± 0.63 |
| Carbohydrates (%) | 5.20 ± 0.78 |
| pH | 5.21 ± 0.03 |
| TSS (°Brix) | 9.80 ± 0.5 |
| Titratable Acidity (%) | 0.40 ± 0.04 |
| Ascorbic Acid(mg/100 g) | 15 ± 2.25 |
| DPPH (%) | 37.90 ± 0.42 |
| Flavonoid (mgQE/100 g) | 2.37 ± 0.05 |
| Total phenol content (mg GAE/100 g) | 49.02 ± 0.01 |
Values are expressed as Mean ± SD (n = 3) (p < 0.05).
The baby corn sample exhibited the total phenolic content of 49.02 ± 0.01 mgGAE/100 g, total flavonoid content of 2.37 ± 0.01 mgQE/100 g, and % radical scavenging activity in terms of DPPH was found to be 37.90 ± 0.42 %. Hitherto, no such data is available on the bioactive components present in baby corn. The values obtained for the DPPH assay are found to have weaker activity (37.90 %) than other vegetables like green chili (52.4 %), cabbage (58.05 %), carrot (69.04 %), and red chili (78.66 %) [19]. Moreover, various researchers have reported that maturation stages tremendously affect the concentration of polyphenols in fruits and vegetables. From the nutritional evaluation and bioactive components present in baby corn, study was focused to attain the maximum benefit of this high value vegetable that can be done by utilization of different blanching pre-treatments.
3.2. Effect of blanching treatments on shrinkage and mass of baby corn
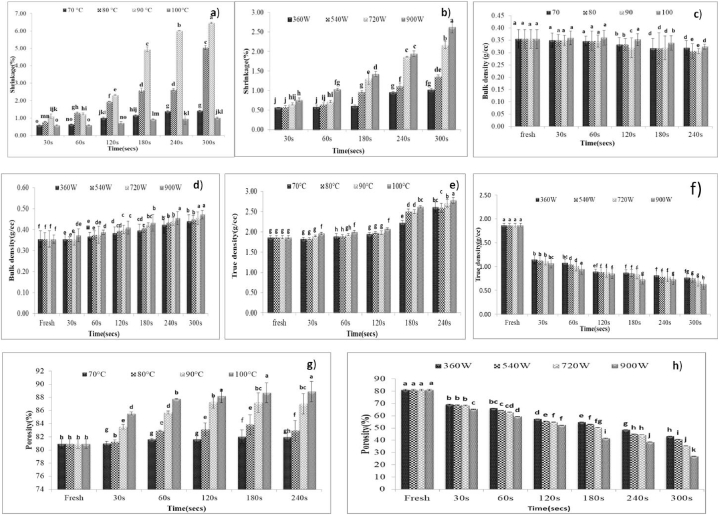
From the overall data, percent shrinkage as represented in Fig. 1a and b increased after being subjected to blanching treatments which may be due to several changes occurring due to thermal processing in the cellular structures (shape and size) and internal structure like causing the gaps, cracks, and voids. Consequently, the investigation conducted by Ref. [20] on freeze-dried red beetroots also revealed a similar effect of blanching on the cellular structure. The maximum increase of mass was observed in the case of the HWB roundels followed by SB roundels. The microwave blanching treatment causes moisture loss due to heating of roundels hence the mass was observed to decrease with increasing time and power.
Fig. 1.
a Effect of hot water and steam blanching on shrinkage %, b Effect of microwave blanching on shrinkage % c Effect of hot water and steam blanching on bulk density (g/cc) d Effect of microwave blanching on bulk density (g/cc) e Effect of hot water and steam blanching on true density (g/cc) f Effect of microwave blanching on true density (g/cc) g Effect of hot water and steam blanching on porosity (%) and h. Effect of microwave blanching on porosity (%).
3.3. Effect on densities and porosity of blanched baby corn
The initial bulk density of fresh baby corn roundels was found to be 0.36 g/cc. Subsequently, various blanching treatments, including hot water, steam, and microwave blanching, were employed. It was observed that the bulk density exhibited a decreasing trend with an increase in temperature and time in case of hot water blanching and SB from 0.36 g/cc to 0.31 g/cc and 0.32 g/cc (Fig. 1c). The decrease in the bulk density after HWB and SB can be attributed to the absorption of moisture by a roundel which leads to expansion [21]. Similar reduction was reported by Ref. [18] for sweet corn kernels whereas it increased in case of microwave blanching with increasing time and power, increased from 0.36 to 0.47 g/cc (Fig. 1d). The rise in bulk density could be attributed to the decrease in dimensions due to microwave treatment which has led to the more compact structure of treated roundels [22]. The true density of fresh baby corn roundels was found to be 1.86g/cc which increased in the case of HWB and SB to 2.71 g/cc and 2.78 g/cc respectively (Fig. 1e) whereas it was found to decrease with increasing time and power in case of MWB to 0.64 g/cc (Fig. 1f). The less effect on the overall dimensions of roundels resulted in decreasing true densities. Initial porosity of baby corn roundel was about 80.95 % which increased with increasing processing time and temperature from 80.95 % to 88.72 % and 88.89 % (Fig. 1g). The more the void spaces, the more will be the porosity of roundels in the case of HWB and SB roundels. The same was found to decrease with increasing time and power to 26.82 % (Fig. 1h) which is due to compactness of roundels to microwave blanching treatment. This can be co-related with the data obtained from shrinkage (%).
3.4. Influence of blanching treatments on moisture content
The determination of moisture content in baby corn was conducted for untreated and blanched samples at different time-temperature/power combinations. The moisture content of unblanched samples was assessed using a classification method and it was found to be approximately 86.76 ± 1.06 % whereas the moisture content of hot water and steam blanched samples were found to increase from 86.76 ± 1.06 % to 92 ± 0.43 % and 89.5 ± 0.0 % respectively. However, the same decreased to 84 ± 0.04 % in case of microwave blanching. This significant increase in moisture content in during conventional blanching methods, such as hot water and steam blanching can be attributed to moisture absorption. In the context of microwave-blanching, it was observed that the moisture content decreased as the microwave power increased, owing to the heat treatment applied to the samples. Notably, the minimal alteration in moisture content during microwave blanching was consistent with findings reported by Ref. [18].
3.5. Influence of blanching treatments on TSS and water activity
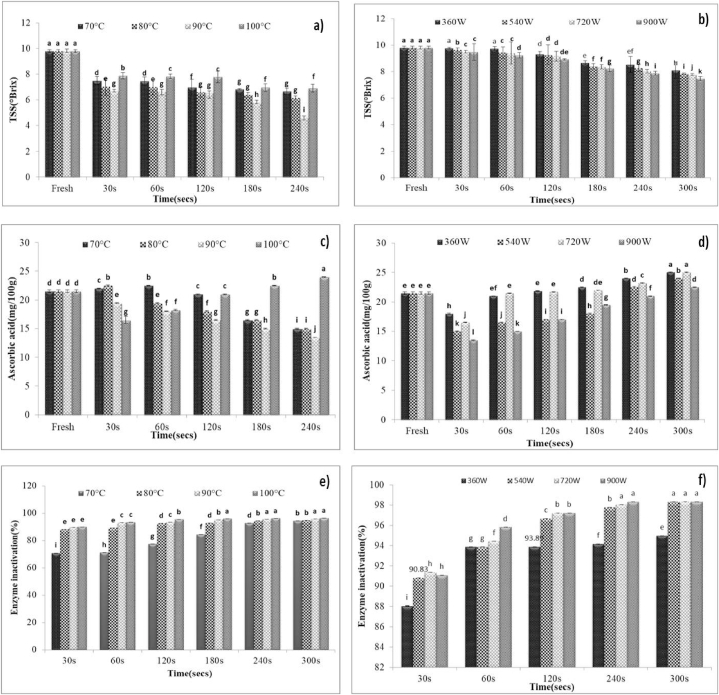
The TSS was determined for fresh and treated samples. It was observed that TSS decreased from 9.80 to 4.60, 6.90, and 7.47 °Brix respectively in the case of HWB, SB, and MWB (Fig. 2a and 2b). The decrease in TSS content is due to the leaching of water-soluble components during HWB and SB [23].The reduction in Total Soluble Solids (TSS) observed in the case of microwave-treated samples is linked to the chemical reaction between reducing sugars and amino groups, a phenomenon supported by previous studies conducted by Ref. [18].The minimum loss of TSS happened in MWB followed by SB and HWB respectively. The water activity of fresh sample was 0.979 that increased with increasing processing time and temperature to 0.999, 0.993 in case of HWB and SB treated roundels respectively. It decreased with increasing processing power and time to 0.970.
Fig. 2.
a Effect of hot water and steam blanching on the TSS (°Brix), b Effect of microwave blanching on TSS (°Brix), c Effect of hot water and steam blanching on ascorbic acid (mg/100 g) d Effect of microwave blanching on ascorbic acid (mg/100 g) e Effect of hot water and steam blanching on enzyme inactivation(%) and f. Effect of microwave blanching on enzyme inactivation(%).
3.6. Influence of blanching treatments on the ascorbic acid content
The blanched samples were air dried and analyzed for ascorbic acid content, enzyme assay and antioxidant activity. The ascorbic acid content of the treated samples is visually represented in Fig. 2c. An analysis of Fig. 2c suggests a notable decline in ascorbic acid content as the intensity of the blanching treatment, characterized by varying time-temperature combinations, increases. Furthermore, it is observed that an extended blanching time is associated with a greater loss in ascorbic acid content, primarily attributed to the leaching of water-soluble ascorbic acid into the blanching water. These observations align with research findings presented by Ref. [24] in their study on broccoli. Consequently, high-temperature thermal degradation during HWB pre-treatment was not found to be favourable for the withholding ascorbic acid content because of its sensitive nature towards heat during blanching process [25]. Moreover, these findings are closely linked to the leaching of soluble solids into the water, a significant drawback associated with conventional blanching methods such as hot water blanching (HWB) [26]. However, it's worth noting that samples treated with steam blanching exhibited a notable upward trend in ascorbic acid content with prolonged treatment durations, as illustrated in Fig. 2c. These findings align with research conducted by Ref. [27] which indicated that steam blanching, when applied before the drying process, resulted in an increase in ascorbic acid content in dry flakes and raw potatoes. This phenomenon may be attributed to the structural openings caused by blanching, which potentially facilitate the rise in ascorbic acid content.
From Fig. 2d, it is evident that microwave blanching effectively preserves ascorbic acid content, showcasing minimal leaching losses. This outcome is consistent with findings reported by Ref. [6], which also observed a substantial increase in ascorbic acid content due to the low leaching losses associated with this blanching method. The notable increase in ascorbic acid content can be attributed to the capability of thermal blanching to disrupt the cell wall, facilitating the release of antioxidants. The results depicted above are in accordance with the results revealed by several studies done by Ref. [28].The outcome of their studies revealed a significant increase in the extractability of chemicals from plant tissue after heat treatment, leading to a rise in the ascorbic acid content of the treated samples. In comparison to conventional methods, microwave blanching was found to yield higher retention of ascorbic acid. The percentage of retained ascorbic acid was found to be 83.5, 94 and 94.15 % for HWB, SB and MWB samples at 90 °C, 100 °C and 540 W and for 60, 60 and 30 s, respectively. Furthermore, prolonged treatment can lead to texture losses.
3.7. Impact of blanching treatments on the color characteristics of baby corn
The L*, a*, and b* values for fresh baby corn samples were measured at 75.17 ± 0.37, 4.53 ± 0.21, and 30.27 ± 0.22, respectively. Notably, the lightness (L*) value decreased, while a* (redness) and b* (yellowness) values increased. The reduction in lightness was attributed to non-enzymatic browning [29] the yellowness (b*) value was found to increase due to the presence of the yellow pigments which increases ones the samples are blanched [18,21]. ΔL values were found to be 161.03 ± 2.29, 187.41 ± 5.90 and 3.20 ± 1.24 respectively for HWB (90 °C, 60 s), SB (100 °C, 60 s) and MWB (540 W, 30 s) respectively. Similarly Δa, Δb for HWB (90 °C, 60 s), SB (100 °C, 60 s) and MWB (540 W, 30 s) were found to be (0.11 ± 0.01, 0.004 ± 0.01, 0.02 ± 0.02) and (11.56 ± 1.20, 67.73 ± 1.22, 29.48 ± 1.10) respectively. The ΔE was found to be 13.14, 15.97 and 5.72 for HWB, SB and MWB samples. The variations in color L∗, a∗, b∗ and ΔE were due to the different blanching treatments and temperature/power. The maximum retention of the color was found to be more in case of samples treated with microwave blanching (Table S1).
3.8. Influence of blanching treatments on the antioxidant activity of baby corn
The treated/blanched samples were analyzed for antioxidant activity which shows a declining trend during hot water blanching with increasing time and temperature combinations. The time temperature has a negative effect on antioxidant activity, with temperature showing more effect than time (p ≤ 0.05) (Table S1). The highest level of antioxidant activity was observed in the samples treated at 70 °C for 30 s. As the blanching time and temperatures increased, a decrease in antioxidant activity was noted. This trend aligns with findings reported by Ref. [25], for blanched bell pepper where the decline was attributed to the leaching of these antioxidant components.
In the case of steam blanching time has a positive effect on antioxidant activity (p ≤ 0.05). A slight increase in the flavonoid, TPC, and DPPH was witnessed with the increase in time as visible in the results of DPPH (%), flavonoids (mgQE/100 g), TPC (mg GAE/100 g) reported in Table S1. Moreover, the study carried out by Ref. [30] on frozen blueberry purees shows the same declining trend of steam-blanched samples. The disadvantage with steam blanching is that prolonged steaming time can lead to softness hence degrading the texture of treated samples [31].
Microwave blanching, an efficient blanching method was employed for blanching baby corn samples, and the effect it was observed on the antioxidant activity viz., flavonoids (mgQE/100 g), total phenols (mg GAE/100 g), DPPH (%). Numerous studies have investigated microwave blanching and its impact on antioxidant activity. These studies have consistently reported an increase in antioxidant activity with longer exposure times and higher power levels. The power and temperature have a positive effect on antioxidants, with power showing more effect than temperature (p ≤ 0.05) (Table S1). Antioxidants in vegetables exist in both free and bound forms. Heat treatment has the potential to release antioxidants from bound sites, leading to an increase in antioxidant activity, as noted in the study by Ref. [32].
Different time and power combinations resulted in the increase in total flavonoid from 2.99 ± 0.002 to 3.41 ± 0.00 (mgQE/100 g), total phenols from 48.94 ± 0.02 to 49.17 ± 0.03 (mg GAE/100 g) and DPPH 40.05 ± 0.01 to 47.01 ± 0.01 %. The enhanced antioxidant activity observed in the treated samples is a result of the cell wall's breakdown caused by thermal blanching, which leads to the release of antioxidants, as reported by Ref. [6]. Additionally, blanching has been found to create openings in the cell matrix, leading to an increase in polyphenols, which, in turn, contributes to the overall increase in antioxidants. Microwave power, time as well interactive effect contributed significantly to the increase in total flavonoid, total phenols, and DPPH activity at p ≤ 0.01 as shown in Table S1.
3.9. Influence of blanching treatments on the peroxidase activity of baby corn
The treated samples were analyzed for peroxidase activity to assess the efficacy of different blanching methods, including hot water (70–90 °C for 30–240 s), steam (100 °C for 60–240 s), and microwave blanching (360–900 W for 30–300 s). From the quantitative POD assay, it was found that the blanched samples (hot water, steam and microwave) showed decreasing enzyme activity with increasing time, temperature/power, the 90 % inactivation of peroxidase enzyme was achieved at 540 W, 90 °C and 100 °C for 30, 60 and 60 s respectively (Fig. 2e 2f). Similar results for different blanching methods have been reported by Ref. [18]. Furthermore, a study conducted by Ref. [33] on steam blanching treatments revealed a similar pattern of decreasing enzyme activity with increasing blanching time.
3.10. Process kinetics during different blanching treatments of baby corn
Zero and first order kinetic models was used for evaluating different responses for different treatments. The best fitted model was determined by values of R2, RMSE and ꭓ2. The model with higher R2 values and lower RMSE and ꭓ2 values is considered to be the best-fitted model. From Table 2 it can be observed that first order model fitted well for shrinkage (%) and ascorbic acid content (mg/100 g) whereas the zero-order models proved to be a good fit for antioxidant and peroxidase activity, but higher values of ꭓ2 and RMSE limit the applicability of the model for estimating POD activity. Among the three blanching treatments given, the steam-blanched samples showed higher R2 and lower RMSE and ꭓ2 values.
Table 2.
Coefficients and statistical parameters on various responses during different blanching treatments of baby corn kernels.
| Response | Coefficients |
Statistical Parameters |
||||
|---|---|---|---|---|---|---|
| C0 | K1 | K2 | R2 | χ2 | RMSE | |
| Shrinkage (%)(**) | ||||||
| HWB | −4.60 | 0.056 | 0.005 | 0.89 | 2.21 | 0.60 |
| MWB | −1.33 | 0.001 | 0.003 | 0.92 | 0.36 | 0.15 |
| Ascorbic acid (mg/100g)(**) | ||||||
| HWB | 3.74 | −0.007 | −0.001 | 0.94 | 0.40 | 0.72 |
| MWB | 2.95 | −0.01 | 0.002 | 0.75 | 3.45 | 1.77 |
| Moisture (%)(*) | ||||||
| HWB | 86.01 | 0.046 | 0.007 | 0.92 | 0.006 | 0.20 |
| MWB | 87.80 | −0.001 | −0.006 | 0.81 | 0.024 | 0.32 |
| Flavanoid (mg QE/100)(*) | ||||||
| HWB | 2.98 | −0.002 | −9.28E-05 | 0.33 | 0.0043 | 0.03 |
| MWB | 2.85 | 0.0002 | 0.001 | 0.96 | 0.004 | 0.02 |
| Phenol (mgGAE/100g)(*) | ||||||
| HWB | 48.07 | −0.001 | −0.001 | 0.97 | 0.00003 | 0.003 |
| MWB | 48.92 | 0.0002 | 0.00049 | 0.94 | 6.55232E-05 | 0.01 |
| DPPH (%)(*) | ||||||
| HWB | 49.01 | −0.09 | −0.01 | 0.93 | 0.05 | 0.37 |
| MWB | 40.57 | 0.001 | 0.019 | 0.89 | 0.24 | 0.67 |
| Peroxidase activity (%)(*) | ||||||
| HWB | 34.29 | 0.59 | 0.048 | 0.72 | 3.55 | 4.11 |
| MWB | 88.55 | 0.005 | 0.02 | 0.69 | 0.58 | 1.65 |
(*)- Zero order model; (**)-First order model; HWB: Hot water blanching; SB: Steam blanching; MWB: Microwave blanching.
4. Conclusion
In conclusion, the study highlights the significant impact of various blanching treatments on the physicochemical and antioxidant properties of baby corn. The comprehensive analysis of raw materials revealed valuable insights into moisture, nutrient content, and bioactive components. Different blanching methods, including hot water, steam, and microwave, were shown to affect the physical structure, density, porosity, moisture content, total soluble solids, and antioxidant activity of baby corn. Hot water and steam blanching generally increased moisture content but resulted in greater nutrient loss, whereas microwave blanching effectively preserved nutrients, including ascorbic acid and antioxidants, with minimal leaching. All the methods showed inactivation of peroxidase enzyme but for microwave, hot water, and steam-blanched samples, a 90 % inactivation of peroxidase enzyme was achieved at 540 W, 90 °C, and 100 °C for 30, 60, and 60 s, respectively. The study also demonstrated that microwave blanching provided the best retention of color and the highest increase in antioxidant activity due to the disruption of the cell wall and release of bound antioxidants. The retention % of ascorbic acid was found to be 94.15 %, 83.5 %, and 94 % for microwave, hot water, and steam-blanched samples, respectively. The findings suggest that microwave blanching of baby corn at 540 W for 30 s holds promise to inactivate the peroxidase enzyme with better retention of physicochemical and functional properties.Higher time temperature/power combinations can lead to physical-chemical and texture losses. Furthermore, while microwave blanching showed promising results, the scalability and economic feasibility of this method for large-scale industrial applications require further investigation. These limitations highlight the need for continued research to optimize blanching processes for baby corn and other high-value vegetables.
Funding statement
The study is supported by the Researchers Supporting Project number (RSP2024R45) at King Saud University Riyadh Saudi Arabia.
Data availability statement
Data will be made available on request.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Ubaida Akbar: Writing – original draft, Investigation. Jyoti Singh: Writing – review & editing. Prasad Rasane: Writing – review & editing. Vikas Nanda: Writing – review & editing, Supervision, Conceptualization. Achyuta Basak: Formal analysis. Amine Assouguem: Writing – review & editing. Riaz Ullah: Writing – review & editing. Essam A. Ali: Writing – review & editing. Sawinder Kaur: Writing – review & editing, Supervision, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors acknowledge the support and central instrumentation facilities provided by Lovely Professional University for carrying out the work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e36964.
Contributor Information
Amine Assouguem, Email: assougam@gmail.com.
Riaz Ullah, Email: rullah@ksu.edu.sa.
Essam A. Ali, Email: esali@ksu.edu.sa.
Sawinder Kaur, Email: sawi_raman@yahoo.co.in.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Singh M., Kumar A., Kaur P. Respiratory dynamics of fresh baby corn (Zea mays L.) under modified atmospheres based on enzyme kinetics. J. Food Sci. Technol. 2014 doi: 10.1007/s13197-012-0735-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh V., Kaur K. Development, formulation and shelf life evaluation of baby corn soup mix from industrial by-products. J. Food Sci. Technol. 2020 doi: 10.1007/s13197-019-04227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehan S., Kaur P., Singh M. Studies on effect of storage on quality of minimally processed babycorn. J. Food Process. Technol. 2014 doi: 10.4172/2157-7110.1000388. [DOI] [Google Scholar]
- 4.Attia M.M., Saleh S.M.M., El-Shabrawy E.M. Effect of anti-browning agents and wrapping films on browning inhibition and maintaining quality of baby corn during storage. J. Plant Prod. 2011 doi: 10.21608/JPP.2011.85768. [DOI] [Google Scholar]
- 5.Moon K.M., Kwon E.B., Lee B., Kim C.Y. Recent trends in controlling the enzymatic browning of fruit and vegetable products. Molecules. 2020 doi: 10.3390/molecules25122754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J., Yang X.H., Mujumdar A.S., Wang D., Zhao J.H., Fang X.M., Xiao H.W. Effects of various blanching methods on weight loss, enzymes inactivation, phytochemical contents, antioxidant capacity, ultrastructure and drying kinetics of red bell pepper (Capsicum annuum L.) Lebensm. Wiss. Technol. 2017 doi: 10.1016/j.lwt.2016.11.070. [DOI] [Google Scholar]
- 7.Zhang C., Lyu X., Aadil R.M., Tong Y., Zhao W., Yang R. Microwave heating instead of blanching to produce low-fat French fries. Innovat. Food Sci. Emerg. Technol. 2023;84 doi: 10.1016/j.ifset.2023.103298. [DOI] [Google Scholar]
- 8.Wang Y., Zhang L., Yu X., Zhou C., Yagoub A.E.A., Li D. A catalytic infrared system as a hot water replacement strategy: a future approach for blanching fruits and vegetables to save energy and water. Food Rev. Int. 2024;40(2):641–657. doi: 10.1080/87559129.2023.2187060. [DOI] [Google Scholar]
- 9.Ranjan S., Dasgupta N., Walia N., Thara Chand C., Ramalingam C. Microwave blanching: an emerging trend in food engineering and its effects on Capsicum annuum L. J. Food Process. Eng. 2017 doi: 10.1111/jfpe.12411. [DOI] [Google Scholar]
- 10.Ruiz-Ojeda L.M., Penas F.J. Comparison study of conventional hot-water and microwave blanching on quality of green beans. Innovat. Food Sci. Emerg. Technol. 2013 doi: 10.1016/j.ifset.2013.09.009. [DOI] [Google Scholar]
- 11.Al-Mitewty M.I., Yahya A., Razif M., Mat N. Physical and mechanical properties of sweet corn plant. Agric. Eng. Int. CIGR J. 2019;21(4):152–160. [Google Scholar]
- 12.Deshpande H.W., Poshadri A. Physical and sensory characteristics of extruded snacks prepared from Foxtail millet based composite flours. Int. Food Res. J. 2011;18:751–756. [Google Scholar]
- 13.Bhat S., Saini C.S., Kumar M., Sharma H.K. Peroxidase as indicator enzyme of blanching in bottle gourd (Lagenaria siceraria): changes in enzyme activity, color, and morphological properties during blanching. J. Food Process. Preserv. 2019 doi: 10.1111/jfpp.14017. [DOI] [Google Scholar]
- 14.AOAC . Association of Official Analytical Chemists; Washington, USA: 2012. Official Methods of Analysis. 17thed. [Google Scholar]
- 15.Ranganna S. In: Handbook of Analysis and Quality Control for Fruit and Vegetable Products. Ranganna S., editor. Tata McGraw-Hill Publications; New Delhi: 2001. pp. 719–724. [Google Scholar]
- 16.Limmatvapirat C., Nateesathittarn C., Dechasathian K., Moohummad T., Chinajitphan P., Limmatvapirat S. Phytochemical analysis of baby corn silk extracts. J. Ayurveda Integr. Med. 2020 doi: 10.1016/j.jaim.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaur R., Kaur K., Ahluwalia P. Effect of drying temperatures and storage on chemical and bioactive attributes of dried tomato and sweet pepper. Lebensm. Wiss. Technol. 2020 doi: 10.1016/j.lwt.2019.108604. [DOI] [Google Scholar]
- 18.Kachhadiya S., Kumar N., Seth N. Process kinetics on physico-chemical and peroxidase activity for different blanching methods of sweet corn. J. Food Sci. Technol. 2018 doi: 10.1007/s13197-018-3416-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghasemzadeh A., Azarifar M., Soroodi O., Jaafar H.Z. Flavonoid compounds and their antioxidant activity in extract of some tropical plants. J. Med. Plants Res. 2012 doi: 10.5897/JMPR11.1531. [DOI] [Google Scholar]
- 20.Ciurzynska A., Falacinska J., Kowalska H., Kowalska J., Galus S., Marzec A., Domian E. The effect of pre-treatment (blanching, ultrasound and freezing) on quality of freeze-dried red beets. Foods. 2021 doi: 10.3390/foods10010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popalia C., Kumar N. Effect of temperature and processing time on physico-chemical characteristics in hot water blanching of sweet corn kernels. J. Inst. Eng. India Ser. 2021;A doi: 10.1007/s40030-021-00508-1. [DOI] [Google Scholar]
- 22.Popaliya C., Kumar N. Physicochemical characteristics of sweet corn kernels during microwave blanching. J. Food Process. Preserv. 2022 doi: 10.1111/jfpp.16413. [DOI] [Google Scholar]
- 23.Adetoro A.O., Opara U.L., Fawole O.A. Effect of blanching on enzyme inactivation, physicochemical attributes and antioxidant capacity of hot-air dried pomegranate (Punica gratum L.) arils (cv. wonderful) Processes. 2020 doi: 10.3390/pr9010025. [DOI] [Google Scholar]
- 24.Severini C., Giuliani R., De Filippis A., Derossi A., De Pilli T. Influence of different blanching methods on colour, ascorbic acid and phenolics content of broccoli. J. Food Sci. Technol. 2016 doi: 10.1007/s13197-015-1878-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang C., Zhang B., Song L., Li P., Hao Y., Zhang J. Assessment of different blanching strategies on quality characteristics and bioactive constituents of Toona sinensis. Lebensm. Wiss. Technol. 2020 doi: 10.1016/j.lwt.2020.109549. [DOI] [Google Scholar]
- 26.Latorre M.E., de Escalada Pla M.F., Rojas A.M., Gerschenson L.N. Blanching of red beet (Beta vulgaris L. var. conditiva) root. Effect of hot water or microwave radiation on cell wall characteristics. LWT LWT-Food Sci. Technol. 2013 doi: 10.1016/j.lwt.2012.06.004. [DOI] [Google Scholar]
- 27.Nayak B., Berrios J.D.J., Powers J.R., Tang J., Ji Y. Colored potatoes (Solanum tuberosum L.) dried for antioxidant‐rich value‐added foods. J. Food Process. Preserv. 2011 doi: 10.1111/j.1745-4549.2010.00502.x. [DOI] [Google Scholar]
- 28.Oerlemans K., Barrett D.M., Suades C.B., Verkerk R., Dekker M. Thermal degradation of glucosinolates in red cabbage. Food Chem. 2006 doi: 10.1016/j.foodchem.2004.12.013. [DOI] [Google Scholar]
- 29.Chikpah S.K., Korese J.K., Sturm B., Hensel O. Colour change kinetics of pumpkin (Cucurbita moschata) slices during convective air drying and bioactive compounds of the dried products. J. Agric. Food Res. 2022 doi: 10.1016/j.jafr.2022.100409. [DOI] [Google Scholar]
- 30.Brambilla A., Maffi D., Rizzolo A. Study of the influence of berry-blanching on syneresis in blueberry purees. Procedia Food Science. 2011;1:1502–1508. doi: 10.1016/j.profoo.2011.09.222. [DOI] [Google Scholar]
- 31.Xiao H.W., Pan Z., Deng L.Z., El-Mashad H.M., Yang X.H., Mujumdar A.S., Zhang Q. Recent developments and trends in thermal blanching–A comprehensive review. Inf. Process. Agric. 2017 doi: 10.1016/j.inpa.2017.02.001. [DOI] [Google Scholar]
- 32.Dibanda R.F., Akdowa E.P., Tongwa Q.M. Effect of microwave blanching on antioxidant activity, phenolic compounds and browning behaviour of some fruit peelings. Food Chem. 2020 doi: 10.1016/j.foodchem.2019.125308. [DOI] [PubMed] [Google Scholar]
- 33.Ndiaye C., Xu S.Y., Wang Z. Steam blanching effect on polyphenol oxidase, peroxidase and colour of mango (Mangifera indica L.) slices. Food Chem. 2009 doi: 10.1080/10408398.2011.654142. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.