Introduction
Direct oral anticoagulants have replaced vitamin K antagonists in the general population with nonvalvular atrial fibrillation due to their predictable anticoagulation profile, similar efficacy, and superior safety. However, their benefits remain unclear in patients on dialysis because studies involving apixaban were underpowered to determine their noninferiority to warfarin,1,2 and some concerns have been raised regarding their potential for accumulation and the risk of bleeding.3 These concerns are supported by a preliminary study in which a single 5 mg dose of apixaban, given immediately after a 4-hour session (off hemodialysis [HD]), resulted in a 36% increase in the area under the curve (AUC), indicating overexposure compared to healthy subjects.4 Although this study had a significant methodological flaw because it did not consider steady-state conditions, a second study conducted under steady-state conditions in patients on HD with 2.5 mg of apixaban twice daily (BID) resulted in significant accumulation over 8 days.3 Furthermore, a retrospective study involving 4313 patients with stage 4 or 5 CKD revealed that the use of apixaban at 5 mg BID was associated with a significantly higher risk of bleeding compared with the 2.5 mg BID (incidence rate of 4.9 vs. 2.9 events per 100 person-years), without any benefit concerning stroke or embolism (incidence rate of 3.3 vs. 3.0 events per 100 person-years) over a 2-year follow-up.5 Consequently, apixaban dosing in patients on HD requires careful consideration, and further research is needed on long-term accumulation to determine safe and effective dosing regimens for this population.
Hemodiafiltration (HDF), which takes advantage of convective forces, has demonstrated increased removal of uremic toxins, and reduced mortality compared with HD.6 As a result, HDF is currently considered for many groups as the technique of choice. While some studies indicate that HD can remove apixaban,3,4 there is a lack of information regarding the impact of HDF on the blood concentration of apixaban.
Therefore, the main objective of this clinical trial was to evaluate the pharmacokinetics, pharmacodynamics, and safety of apixaban 2.5 mg BID over a 4-week period in stable, steady-state patients on HDF in a Spanish population with nonvalvular atrial fibrillation. The secondary objectives included an assessment of the safety and tolerability as well as the impact of HDF on the plasma concentration of apixaban. The trial is registered as EudraCT: 2019-002353-29, AEMPS: 19-0502, and Clinical Trial: NCT04952792
Results
The study was conducted between May 2021 and October 2022. The study design is shown in Supplementary Figure S1, and a detailed protocol can be found in the supplementary material. Ten patients on dialysis (9 males and 1 female) with a mean (SD) age of 67.4 (6.6) years were enrolled. A CONSORT diagram describing the flow of participants through the study is shown in Supplementary Figure S2. Five patients had conserved residual kidney function defined by an interdialytic urine volume >100 ml/d. In Supplementary Table S1, we provide a summary of the patients’ demographics. No additional anticoagulation was administered during HDF and there were no interruptions due to clotting. Saline flushes were not routinely scheduled, and the catheter was locked with 30% citrate.
The methods used in this study are described in the supplementary material. In brief, apixaban concentrations were measured using a previously validated liquid chromatography with tandem mass spectrometry (LC-MS/MS) method.7 In addition, we estimated apixaban levels using an anti-FXa-based assay (AXA), which is considered an alternative in clinical practice when spectrometry is not available. The Bland-Altman analysis was used to assess the agreement between apixaban measurement with AXA and the gold standard LC-MS/MS.
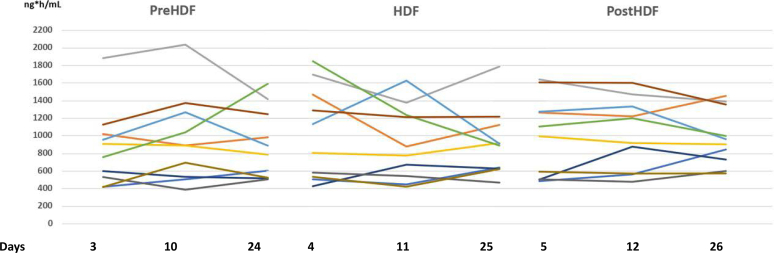
The main result was the absence of apixaban accumulation at a dose of 2.5 mg BID at any point between the first and fourth weeks (Table 1, Supplementary Tables S2 and S3). The mean and individual plasma concentration-time profiles are shown in Supplementary Figure S3. The AUC (mean [% coefficient of variation] AUC0–12) on day 4 (midweek dialysis) was 1030 (51%) ng·h/ml and on day 25 (midweek dialysis) was 921 (42%) ng·h/ml, reflecting a similar exposure and steady state (Figure 1). The AUC 0–12 reference levels for 2.5 mg apixaban BID reported was (median, 10th–90th percentile) 1269 ng·h/ml (615–1946). The accumulation index (mean [% coefficient of variation]), which provides a measure of the exposure to apixaban was 1.12 (34%) on day 3 (nondialysis), 0.98 (28%) on day 4 (midweek dialysis) and 1.07 (28%) on day 5 (nondialysis) (P = 0.6). This indicates that there was no significant accumulation. Furthermore, no bleeding events were observed, and no HDF had to be interrupted due to circuit clotting.
Table 1.
PK parameters
| Apixaban 2.5 mg twice daily | D3 | HDF_D4 | D5 | D10 | HDF_D11 | D12 | D24 | HDF_D25 | D26 | P |
|---|---|---|---|---|---|---|---|---|---|---|
| AUC 0–12; ng·h/ml (CV) | 862 (51%) | 1030 (51%) | 998 (46%) | 962 (52%) | 920 (46%) | 1025 (39%) | 907 (44%) | 921 (42%) | 982 (33%) | 0.9 |
| Cmax;ng/ml (CV) | 102 (43%) | 103 (51%) | 111 (46%) | 115 (51%) | 103 (43%) | 110 (32%) | 100 (33%) | 97 (34%) | 114 (29%) | 0.9 |
| Tmax;h (CV) | 2.5 (51%) | 2.4 (28%) | 2.5 (54%) | 2.2 (60%) | 2.9 (64%) | 2.6 (32%) | 2.5 (57%) | 2.4 (60%) | 2 (41%) | 0.5 |
| Cmin;ng/ml (CV) | 57 (71%) | 74 (60%) | 66 (49%) | 82 (77%) | 63 (58%) | 68 (48%) | 67 (59%) | 69 (45%) | 71 (45%) | 0.7 |
| t1/2; h (CV) | 15 (47%) | 20 (54%) | 22 (53%) | 23 (54%) | 21 (63%) | 24 (41%) | 22 (69%) | 26 (68%) | 22 (44%) | 0.14 |
| Creatinine clearance; ml/min (CV) | 0.35 (52%) | 0.15 (41%) | 0.25 (51%) | 0.2 (30%) | 0.05 (28%) | 0.17 (95%) | 0.17 (64%) | 0.1 (76%) | 0.11 (33%) | 0.048 |
| AI from 1st week (CV) | N/A | N/A | N/A | 1.13 (23%) | 0.95 (31%) | 1.07 (22%) | 1.12 (34%) | 0.98 (28%) | 1.07 (28%) | 0.6 |
| AI from 2nd week (CV) | N/A | N/A | N/A | N/A | N/A | N/A | 0.99 (26%) | 1.07 (27%) | 1.01 (22%) | 0.8 |
AI, accumulation index; AUC0–12, area under plasma concentration-time from 0 to 12 h after morning dose; Cmax, maximum observed plasma concentration; Cmin, minimum observed plasma concentration; CV, coefficient of variation expressed as a percentage; HDF, hemodiafiltration, N/A: not applicable; t½, terminal half-life; Tmax, time to peak apixaban concentration.
P was calculated using a paired t-test between days 4 and day 25.
The P-value for creatinine clearance and AI were calculated using analysis of variance.
Results are expressed as mean (% CV).
Figure 1.
Progression of the area under the concentration-time curve (AUC0–12) for apixaban at 2.5 mg twice-daily dose across the first, second, and fourth weeks. The figure illustrates the evolution of the AUC0–12 for each patient throughout the study. We present the data in 3 separate panels representing the evolution of AUC0–12 the nondialysis day (preHDF), midweek dialysis (HDF), and nondialysis day after HDF (postHDF). The x-axis denotes the specific days from the initiation of the study after the steady state of apixaban was reached, with the first day representing the first week, the second day representing the second week, and the third day representing the fourth week. Each color corresponds to the same patient. Notably, no accumulation of apixaban was observed.
The variability in apixaban levels among subjects ranged from 33% to 52%, whereas intrasubject variability was comparatively lower, ranging from 8% to 29% (Table 1, Supplementary Tables S2 and S3). In addition, the impact of renal clearance of apixaban (apixaban clearance = 0.19 [0.07] ml/min) was limited because the 5 patients with preserved residual kidney function did not show a significant difference in apixaban levels (1029 [50%] ng·h/ml vs. 695 [45%] ng·h/ml in patients without residual kidney function, P = 0.25).
In addition, our study aimed to evaluate the impact of convection-driven forces in HDF on apixaban clearance. To achieve this, we conducted a consecutive pharmacokinetics analysis on the nondialysis day, the subsequent mid-week HDF session, and the following day, over 3 different weeks. Apixaban was not detected in the dialysis effluent, and we did not observe any impact of HDF on apixaban AUCs as shown in Table 1.
Finally, we observed a dose-dependent linear correlation between the apixaban levels estimated from the AXA and measured with LC-MS/MS (Supplementary Figure S3). In addition, the Bland-Altman analysis showed a mean bias of 52.8 ± 31.5 ng/ml (95% CI: −9 to 114.6 ng/ml) in the AXA estimation.
Discussion
Our study was conducted to determine whether apixaban 2.5 mg BID could accumulate further after day 8 in stable patients on HD, as previously suggested.3 In a cohort of patients on HD using 2.5 mg BID, the AUC0–12, represented as mean (% coefficient of variation), was 298.6 (38%) ng·h/ml on day 1 and increased to 1009.8 (30.7%) ng·h/ml on day 8.3 This result did not differ from the AUC0–12 of 1269 ng·h/ml (with 10th to 90th percentiles ranging from to 615–1946 ng·h/ml) in 20 patients who received 2.5 mg BID in the RENAL-AF study.1 Accordingly, in our study the AUC0–12 on day 4 (midweek dialysis) was 1030 (51%) ng·h/ml, but on day 25 (midweek dialysis) was 921 (42%) ng·h/ml, demonstrating the absence of apixaban accumulation at any point between the first and fourth weeks. In addition, apixaban 2.5 mg BID in patients on HD resulted in drug exposure comparable to that of 5 mg BID in patients with preserved renal function.3 Thus, our study contributes to the understanding of the safety of 2.5 mg BID apixaban by demonstrating that it does not accumulate beyond day 8.
Our study also aimed to investigate whether HDF affects apixaban plasma concentration, because HD has been suggested to have the capacity to remove apixaban from blood. A previous cohort study involving 8 patients with end-stage renal disease treated with a single dose 5 mg of apixaban reported a 14% reduction in the AUC associated with HD.4 These authors recovered approximately 0.33 mg apixaban in the dialysis effluent, representing 6.7% of the apixaban dose, with a calculated HD extraction ratio of −1.1%. It is important to note that that study has a methodologic flaw because patients were not in a steady state, which might not accurately reflect the typical behavior of apixaban. In addition, a separate study conducted in steady-state conditions observed a 4% removal of apixaban by dialysis, though the dialysis effluent was not analyzed.3 In our study, also conducted with patients in a steady state, apixaban was not detected in the dialysate effluent, and we did not observe any effect of HDF on the apixaban plasma concentration. This result is not surprising considering that apixaban is highly (87%) bound to proteins and has a large volume of distribution (21l),8 although its relatively small molecular weight (459.5 g/mol) might suggest a potential removal by dialysis. Consequently, in patients on HDF, the apixaban 2.5 BID dose can be used without any dose increase, because the medication is not removed by HDF.
In healthy subjects, total plasma clearance of apixaban is approximately 55 ml/min and renal clearance of apixaban is approximately 15 ml/min (approximately 27% of total clearance).8 Consequently, renal impairment may contribute to higher apixaban exposure because regression analysis of AUC0–∞ versus creatinine clearance showed that in subjects with mild (creatinine clearance of 65 ml/min), moderate (40 ml/min), or severe (15 ml/min) renal impairment, apixaban AUC 0–∞ increased by 16%, 29%, and 44%, respectively, compared to healthy subjects when a single oral dose of 10 mg was administered.9 However, the AUC0–12 for the 2.5 mg dose in the RENAL-AF trial did not differ from the AUC for patients with estimated creatinine clearance ≥15 and <90 ml/min from the ARISTOTLE trial and the use of apixaban at 5 mg BID in patients on CKD stage 4 or 5 was associated with a significantly higher risk of bleeding compared with the 2.5 mg BID (incidence rate of 4.9 vs. 2.9 events per 100 person-years), without any benefit with respect to stroke or embolism (incidence rate of 3.3 vs. 3.0 events per 100 person-years)5 supporting the apixaban dosing recommendation of 2.5 mg BID for patients with kidney disfunction. In our study, the renal clearance of apixaban in the 5 patients with HD with residual kidney function was poor (0.19 ± 0.07 ml/min) and had no significant effect on apixaban blood levels, suggesting that no further dose adjustment is required for residual renal function in dialysis patients and that the dose of 2.5 mg BID can also be used in those patients.
Our study revealed elevated intersubject variability in AUC0–12, ranging from 33% to 52%. This finding is consistent with another cohort study involving patients on HD, which reported variability between 30.7% and 38%.3 In contrast, this observed range differs from the previously reported AUC0–24 variability of 20% to 24% in healthy subjects.8 These differences may involve patient characteristics that contribute to variations in apixaban absorption and metabolism.8 However, a standard range for routine monitoring of apixaban in the general population has not been established, largely due to its more predictable pharmacokinetics/pharmacodynamics profile In patients on HD, monitoring plasma levels could be necessary to identify an optimal therapeutic range, helping to ensure appropriated dosage, and improve patient safety.
Finally, the Bland-Altman analysis was used to assess the agreement between apixaban measurement with AXA and the gold standard LC-MS/MS method. This analysis showed that the AXA measurements were, on average, 52.8 ng/ml higher than the LC-MS/MS (95% CI: −9.0 to 114.6 ng/ml). In addition, we presented a regression analysis that can be used to calibrate AXA (Supplementary Figure S4). These results suggest that the uremic milieu slightly alters the anticoagulant properties of apixaban and that slightly higher anti Xa activity may be observed at the same drug levels in patients on HD.
Conclusion
Our study revealed no significant accumulation of apixaban between the first and fourth weeks, with a dosage of 2.5 mg BID. In addition, HDF and residual renal function did not affect the plasma concentrations of apixaban. Despite high interpatient variability, intrasubject variability remained low. Our results have significant clinical implications, providing pharmacokinetic and pharmacodynamic insights that support the use of 2.5 mg BID in patients with nonvalvular atrial fibrillation undergoing dialysis.
Disclosure
All the authors declared no conflicting interests.
Acknowledgments
Thanks to the patients on dialysis who trusted us and accepted participation in this study. We thank the CERCA program/Generalitat de Catalunya for institutional support. This study was presented in Late Clinical trials during Kidney Week 2023. This study was funded by the Sociedad Española de Nefrología (SEN) and Baxter SL through the grant Fondo para el Fomento y Ayuda a la Investigación en Diálisis, Baxter 2018 to MH, and by RICORS (RD21/0005/0001).
Data Availability Statement
Data were collected using the research electronic data capture system (REDCap) and can be accessed via the following link: (https://redcap.idibell.cat/redcap_v13.4.12/index.php?pid=347).
The individual-level data collected during the study are not publicly available due to protection of participants’ privacy and confidentiality. However, these data are available for sharing and reuse upon request from the corresponding author.
Author Contributions
Conceptualization was by AO, SC, SV, and MH. Methodology was by AO, ERP, JP, NLL, RRB, SV, and MH. Validation was by AO, YM, ERP, NLL, NS, JP, and RRB. Formal analysis was by AO, ERP, JP, NLL, RRB, SV, and MH. Investigation was by AO, ERP, SC, YM, G, CP, CG, SV, and MH. Resources were provided by AO, ERP, RRB, SV, and MH. Data curation was done by AO, ERP, NLL, NS, JP, RRB, SV, and MH. Writing of the original draft was by AO, ERP, NLL, RRB, SV, and MH. Visualization was done by AO, ERP, NLL, NS, JP, RRB, SV, and MH. Supervision was done by AO, SV, and MH. Project administration was done by AO, SV, and MH. Funding was acquired by MH.
Footnotes
Supplementary Methods.
Detailed Protocol.
Figure S1. Study Design.
Figure S2. CONSORT diagram describing the flow of participants through the study.
Figure S3. Arithmetic means apixaban plasma concentration over time during the first, second, and fourth week.
Figure S4. Linear regression model showing apixaban levels measured using AXA and LC-MS/MS.
Table S1. Population demographics.
Table S2. Unadjusted marginal means and 90% confidence interval for the log-transformed PK parameters.
Table S3. Adjusted by age marginal means and 90% confidence interval for the log-transformed PK parameters.
STROBE Checklist.
Contributor Information
Sebastià Videla, Email: svidelaces@gmail.com.
Miguel Hueso, Email: mhueso@idibell.cat.
Supplementary Material
Supplementary Methods. Detailed Protocol. Figure S1. Study Design. Figure S2. CONSORT diagram describing the flow of participants through the study. Figure S3. Arithmetic means apixaban plasma concentration over time during the first, second, and fourth week. Figure S4. Linear regression model showing apixaban levels measured using AXA and LC-MS/MS. Table S1. Population demographics. Table S2. Unadjusted marginal means and 90% confidence interval for the log-transformed PK parameters. Table S3. Adjusted by age marginal means and 90% confidence interval for the log-transformed PK parameters. STROBE Checklist.
References
- 1.Pokorney S.D., Chertow G.M., Al-Khalidi H.R. Apixaban for patients with atrial fibrillation on hemodialysis: a multicenter randomized controlled trial. Circulation. 2022;146:1735–1745. doi: 10.1161/CIRCULATIONAHA.121.054990. and others. [DOI] [PubMed] [Google Scholar]
- 2.Reinecke H., Engelbertz C., Bauersachs R. A randomized controlled trial comparing Apixaban with the vitamin K antagonist phenprocoumon in patients on chronic hemodialysis: the AXADIA-AFNET 8 study. Circulation. 2023;147:296–309. doi: 10.1161/CIRCULATIONAHA.122.062779. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mavrakanas T.A., Samer C.F., Nessim S.J., Frisch G., Lipman M.L. Apixaban pharmacokinetics at steady state in hemodialysis patients. J Am Soc Nephrol. 2017;28:2241–2248. doi: 10.1681/ASN.2016090980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X., Tirucherai G., Marbury T.C., et al. Pharmacokinetics, pharmacodynamics, and safety of apixaban in subjects with end-stage renal disease on hemodialysis. J Clin Pharmacol. 2016;56:628–636. doi: 10.1002/jcph.628. [DOI] [PubMed] [Google Scholar]
- 5.Xu Y., Chang A.R., Inker L.A., McAdams-DeMarco M., Grams M.E., Shin J.I. Associations of Apixaban dose with safety and effectiveness outcomes in patients with atrial fibrillation and severe chronic kidney disease. Circulation. 2023;148:1445–1454. doi: 10.1161/CIRCULATIONAHA.123.065614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blankestijn P.J., Vernooij R.W.M., Hockham C., et al. Effect of Hemodiafiltration or Hemodialysis on Mortality in Kidney Failure. N Engl J Med. 2023;389(8):700–709. doi: 10.1056/NEJMoa2304820. [DOI] [PubMed] [Google Scholar]
- 7.Rigo-Bonnin R., Rosselló-Palmer E., Sánchez-García A., et al. Measurement of apixaban concentrations in different human biological fluids by UHPLC-MS/MS. Clinical pharmacokinetic application in a subject with chronic kidney disease and nonvalvular atrial fibrillation on haemodialysis. Clin Chim Acta. 2023;549 doi: 10.1016/j.cca.2023.117554. [DOI] [PubMed] [Google Scholar]
- 8.Byon W., Garonzik S., Boyd R.A., Frost C.E. Apixaban: a clinical pharmacokinetic and pharmacodynamic review. Clin Pharmacokinet. 2019;58:1265–1279. doi: 10.1007/s40262-019-00775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang M., Yu Z., Shenker A., et al. Effect of renal impairment on the pharmacokinetics, pharmacodynamics, and safety of apixaban. J Clin Pharmacol. 2016;56:637–645. doi: 10.1002/jcph.633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods. Detailed Protocol. Figure S1. Study Design. Figure S2. CONSORT diagram describing the flow of participants through the study. Figure S3. Arithmetic means apixaban plasma concentration over time during the first, second, and fourth week. Figure S4. Linear regression model showing apixaban levels measured using AXA and LC-MS/MS. Table S1. Population demographics. Table S2. Unadjusted marginal means and 90% confidence interval for the log-transformed PK parameters. Table S3. Adjusted by age marginal means and 90% confidence interval for the log-transformed PK parameters. STROBE Checklist.
Data Availability Statement
Data were collected using the research electronic data capture system (REDCap) and can be accessed via the following link: (https://redcap.idibell.cat/redcap_v13.4.12/index.php?pid=347).
The individual-level data collected during the study are not publicly available due to protection of participants’ privacy and confidentiality. However, these data are available for sharing and reuse upon request from the corresponding author.