Introduction
An estimated 2.5 million patients worldwide were being treated for end-stage kidney disease (ESKD) in 2016.1 The vast majority of patients with ESKD are treated with hemodialysis (HD, ∼89%) and peritoneal dialysis (PD); however, patterns of HD and PD vary internationally.2 Patients with ESKD who are on dialysis have been observed to be at increased risk of major bleeding likely due to multiple factors;3 however, the epidemiology of bleeding in different ESKD subgroups is not well-described. We aimed to determine rates of overall and cause-specific major bleeding events in patients with ESKD overall and by key characteristics, including HD versus PD, region, demographics, and comorbidities.
Data were obtained from the Dialysis Outcomes and Practice Patterns Study (DOPPS) and the Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS), which are both international prospective cohort studies of patients receiving chronic HD or PD, respectively.2,4 We assessed event rates for major bleed types (Supplementary Methods), including a composite of death or hospitalization due to any bleeding event, and the most frequently reported cause-specific bleeding (i.e., gastrointestinal [GI] or vascular access bleeding leading to hospitalization, and death due to hemorrhagic stroke).
Results
A total of 38,466 patients were included: 32,396 receiving HD (84%) and 6070 receiving PD (16%). On average, patients were aged 64 years (SD, 15 years), 14% were aged ≥80 years, and 61% were male (Supplementary Table S1). Although over half (58%) had a history of cardiovascular disease, only 9% were on an oral anticoagulant and 9% were on an antiplatelet (excluding aspirin) in patients with prescription data available (n = 32, 901).
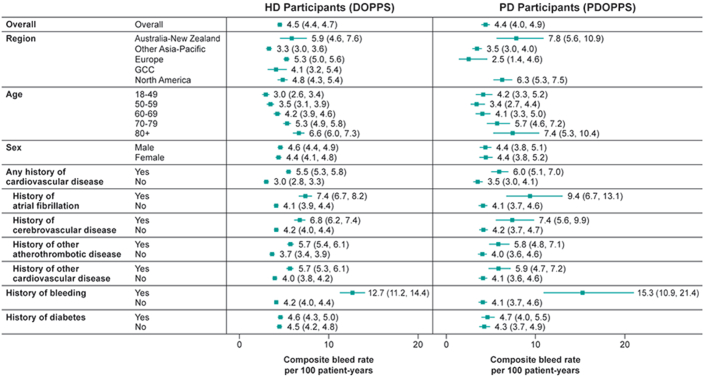
The overall rates for the composite outcome of hospitalization or death due to any bleeding event were similar in the HD (DOPPS) and PD (PDOPPS) cohorts: 4.5 (95% confidence interval [CI]: 4.4–4.7 and 4.4 (95% CI: 4.0–4.9) per 100 person-years, respectively (Figure 1). In both cohorts, bleeding rates were higher if patients had a prior history of bleeding; were aged ≥80 years; or had a history of any cardiovascular disease, including atrial fibrillation, cerebrovascular disease, atherothrombotic disease, or other cardiovascular disease. Minimal differences were observed by the history of diabetes.
Figure 1.
Composite major bleed rate in HD and PD. (a) The composite major bleed end point comprised any nonfatal bleeding event that resulted in hospitalization and any bleeding event that resulted in death. Nonfatal bleeding events that resulted in hospitalization included epistaxis, subdural hematoma, cerebral hemorrhage, evacuation of hematoma, abnormal bleeding, hemoptysis, hematuria, and vascular access bleeding. Any bleeding event that resulted in death included gastrointestinal hemorrhage, hemorrhage from vascular access, hemorrhage from ruptured vascular aneurysm, hemorrhage from surgery, and other hemorrhage. DOPPS, Dialysis Outcomes and Practice Patterns Study; HD, hemodialysis; PD, peritoneal dialysis; PDOPPS, Peritoneal Dialysis Outcomes and Practice Patterns Study.
The highest cause-specific event rate was for nonfatal GI bleeding, at 2.3 (95% CI: 2.1–2.4) and 2.0 (95% CI: 1.7–2.4) per 100 patient-years in the HD and PD cohorts, respectively (Table 1). In both HD and PD cohorts, subgroups with the highest event rates for nonfatal GI bleeding were similar to those for the composite bleeding endpoint. However, North America had the highest rates of hospitalizations due to GI bleeding at 3.1 (95% CI: 2.6–3.5) and 3.8 (95% CI: 3.0–4.7) per 100 patient-years for the HD and PD cohorts, respectively. Australia-New Zealand had the highest rates for the composite end point, at 5.9 (95% CI: 4.6–7.6) and 7.8 (95% CI: 5.6–10.9) per 100 patient-years for HD and PD cohorts, respectively. In the HD cohort, rates of hospitalization for vascular access bleeding were 3 to 4 times lower than for hospitalization caused by GI bleeding but followed a similar pattern across subgroups (Table 1).
Table 1.
Top nonfatal and fatal cause-specific major bleeding eventsa
| Patient group | Hemodialysis (DOPPS) n = 32,396 |
Peritoneal dialysis (PDOPPS) n = 6070 |
|||
|---|---|---|---|---|---|
| Hospitalization due to GI bleed | Hospitalization due to vascular accessb | Death due to hemorrhagic stroke | Hospitalization due to GI bleed | Death due to hemorrhagic stroke | |
| Overall | 2.26 (2.13–2.40) | 0.59 (0.52–0.67) | 0.42 (0.37–0.48) | 2.01 (1.72–2.35) | 0.32 (0.22–0.45) |
| Region | |||||
| Australia-New Zealand | 2.74 (1.90–3.94) | 1.21 (0.70–2.08) | 0.19 (0.06–0.60) | 3.29 (1.98–5.45) | 0.53 (0.17–1.65) |
| Other Asia-Pacific | 1.58 (1.39–1.79) | 0.29 (0.22–0.39) | 0.58 (0.48–0.70) | 1.24 (0.96–1.60) | 0.41 (0.28–0.61) |
| Europe | 2.53 (2.33–2.76) | 0.83 (0.72–0.96) | 0.39 (0.32–0.48) | 0.69 (0.22–2.14) | – |
| Gulf Cooperative Council (GCC) | 1.79 (1.21–2.64) | 0.28 (0.11–0.75) | 0.39 (0.17–0.87) | – | – |
| North America | 3.06 (2.64–3.53) | 0.46 (0.32–0.67) | 0.17 (0.09–0.31) | 3.76 (3.01–4.69) | 0.12 (0.04–0.36) |
| Age category (yr) | |||||
| 18–49 | 1.17 (0.95–1.45) | 0.44 (0.31–0.63) | 0.35 (0.24–0.50) | 1.43 (0.97–2.10) | 0.30 (0.14–0.63) |
| 50–59 | 1.66 (1.40–1.98) | 0.40 (0.28–0.57) | 0.43 (0.31–0.59) | 1.38 (0.94–2.03) | 0.34 (0.17–0.68) |
| 60–69 | 2.17 (1.92–2.46) | 0.59 (0.47–0.75) | 0.36 (0.27–0.47) | 1.89 (1.40–2.56) | 0.39 (0.22–0.70) |
| 70–79 | 2.77 (2.49–3.08) | 0.64 (0.51–0.80) | 0.47 (0.37–0.59) | 3.23 (2.39–4.35) | 0.24 (0.09–0.64) |
| ≥80 | 3.40 (2.98–3.88) | 0.88 (0.68–1.14) | 0.53 (0.39–0.72) | 3.88 (2.44–6.16) | 0.17 (0.02–1.23) |
| Sex | |||||
| Male | 2.28 (2.11–2.47) | 0.55 (0.47–0.65) | 0.45 (0.38–0.53) | 2.11 (1.72–2.58) | 0.32 (0.20–0.51) |
| Female | 2.23 (2.02–2.47) | 0.65 (0.54–0.78) | 0.37 (0.30–0.47) | 1.87 (1.46–2.41) | 0.32 (0.18–0.54) |
| Disease history | |||||
| Any cardiovascular | |||||
| Yes | 2.79 (2.60–3.00) | 0.69 (0.60–0.79) | 0.50 (0.43–0.59) | 2.96 (2.38–3.67) | 0.43 (0.26–0.72) |
| No | 1.44 (1.27–1.62) | 0.44 (0.35–0.55) | 0.29 (0.22–0.37) | 1.46 (1.16–1.84) | 0.26 (0.16–0.42) |
| Atrial fibrillation | |||||
| Yes | 3.94 (3.45–4.51) | 0.86 (0.65–1.14) | 0.61 (0.45–0.83) | 4.20 (2.57–6.85) | 0.63 (0.20–1.95) |
| No | 2.01 (1.87–2.16) | 0.55 (0.48–0.63) | 0.39 (0.34–0.46) | 1.85 (1.56–2.19) | 0.31 (0.21–0.45) |
| Cerebrovascular | |||||
| Yes | 3.08 (2.69–3.52) | 0.80 (0.61–1.04) | 0.75 (0.58–0.96) | 3.40 (2.24–5.16) | 0.64 (0.27–1.53) |
| No | 2.12 (1.98–2.27) | 0.56 (0.49–0.64) | 0.36 (0.31–0.42) | 1.88 (1.59–2.23) | 0.29 (0.20–0.43) |
| Other atherothrombotic | |||||
| Yes | 3.05 (2.81–3.30) | 0.71 (0.60–0.83) | 0.45 (0.37–0.55) | 3.49 (2.70–4.52) | 0.35 (0.16–0.73) |
| No | 1.68 (1.53–1.84) | 0.51 (0.43–0.60) | 0.40 (0.33–0.47) | 1.60 (1.31–1.95) | 0.31 (0.21–0.47) |
| Other cardiovascular | |||||
| Yes | 2.81 (2.55–3.09) | 0.73 (0.61–0.88) | 0.50 (0.41–0.62) | 2.65 (1.93–3.62) | 0.44 (0.22–0.87) |
| No | 1.99 (1.83–2.15) | 0.52 (0.45–0.61) | 0.38 (0.32–0.45) | 1.86 (1.55–2.23) | 0.29 (0.19–0.44) |
| Bleedingc | |||||
| Yes | 8.74 (7.54–10.1) | 0.87 (0.56–1.37) | 0.55 (0.33–0.93) | 8.13 (5.19–12.8) | 0.65 (0.16–2.61) |
| No | 1.96 (1.83–2.10) | 0.58 (0.51–0.66) | 0.41 (0.36–0.48) | 1.82 (1.53–2.15) | 0.31 (0.21–0.44) |
| Diabetes | |||||
| Yes | 2.50 (2.28–2.74) | 0.49 (0.40–0.60) | 0.44 (0.36–0.54) | 2.04 (1.61–2.59) | 0.41 (0.25–0.65) |
| No | 2.09 (1.93–2.28) | 0.67 (0.57–0.77) | 0.41 (0.34–0.48) | 1.98 (1.61–2.45) | 0.25 (0.15–0.43) |
CI, confidence interval; DOPPS, Dialysis Outcomes and Practice Patterns Study; GCC, Gulf Cooperation Council; GI, gastrointestinal; PDOPPS, Peritoneal Dialysis Outcomes and Practice Patterns Study.
Data is reported as event rates per 100 patients-years (95% CI).
Vascular access for hemodialysis via catheter, fistula, or graft.
History of hospitalization due to any bleeding.
Rates of death due to hemorrhagic stroke were similar in the HD and PD cohorts, respectively: 0.4 (95% CI: 0.4–0.5) and 0.3 (95% CI: 0.2–0.5) per 100 patient-years. Regional heterogeneity in rates of death due to hemorrhagic stroke, showed highest rate among patients living in the Other Asia-Pacific region in the HD cohort and in Australia-New Zealand in the PD cohort.
Discussion
In this large, observational dataset of over 38,000 patients receiving dialysis, rates of hospitalization or death due to bleeding were approximately 4% to 5% per year, most frequently caused by GI bleeding, and were similar in patients receiving HD or PD. Subgroups with the highest bleeding rates had a prior history of bleeding, older age, and comorbid cardiovascular disease. There were geographic differences in bleeding rates as well as the use of HD versus PD.
In this population of patients, bleeding rates varied minimally by dialysis modality, which contrasts with results of other recent studies.3,5 A Taiwanese study found higher risk of subdural hematoma in the HD cohort than in the PD cohort (adjusted hazard ratio: 1.6; 95% CI: 1.2–2.3).5 Another study in the Netherlands showed that bleeding risk for patients on HD compared with PD increased 1.5-fold (95% CI: 1.0–2.2) based on adjusted results.3 However, these studies were conducted in a single population, and while we assessed bleeding rates by region, rates could vary substantially by practice patterns and country, neither of which were included in DOPPS or PDOPPS.
In general, patients aged ≥80 years had the highest fatal and nonfatal bleed rates compared to other age groups with the exception that patients on PD who were aged ≥80 years had the lowest rate of death due to hemorrhagic stroke compared to other age groups. Consistent with other studies,6,7 in general, patients with and without diabetes had similar rates of hospitalizations due to GI bleeds and deaths due to hemorrhagic stroke in both dialysis cohorts, though patients on HD without comorbid diabetes had higher rates of hospitalizations due to vascular access bleeds versus those with diabetes. These observations among elderly patients receiving PD and patients without diabetes in the HD cohort are unexpected and highlight the need for further research.
A prior history of bleeding was also associated with higher rates of subsequent bleeding. This observation has been made in other cardiovascular populations where bleeding history is included in risk scores for treatment with more potent agents.8 The observation that such history is also associated with higher rates of bleeding in patients receiving dialysis suggests that such risk scores may extend to the dialysis population to assist in identifying patients who would benefit from strategies that lower bleeding risk.
In this context, the observation that bleeding rates were increased approximately 2-fold among those with a history of atrial fibrillation, as well as any cardiovascular disease, underscores the complexity and unmet need for patients receiving dialysis who have atrial fibrillation or other comorbid cardiovascular conditions associated with high thrombotic risk, where the benefit-risk of currently available antithrombotic therapies is unclear. Novel approaches to reducing thrombotic risk with a more favorable safety profile are needed. In this study, utilization of anticoagulant or antiplatelet (excluding aspirin) therapies was low overall. Aspirin use was not included because the drug utilization data for this study were based on prescription records, and most aspirin use is over the counter, thus aspirin use would be significantly underestimated in prescription records. However, further investigation is required to examine the utilization pattern of antithrombotic therapies in the subgroups of the study sample with a history of atrial fibrillation or any cardiovascular disease; and is the subject of a forthcoming manuscript.
Event rates described and compared between groups are crude, often in limited sample size, and do not reflect associations independent of other patient characteristics (or each other) and should be interpreted with caution. We can speculate that some of the observed differences by geographic setting might be related to differences in the source patient populations and/or in local clinical practice. For example, oral anticoagulants might be prescribed with varying doses or international normalized ratio measurement targets, which impact the subsequent risk of bleeding.6 Finally, DOPPS and PDOPPS include different countries over differing time periods, which should be considered when drawing comparisons.
Overall, the observations of this large dataset of patients receiving dialysis highlights that impactful bleeding events leading to hospitalization or death are frequent regardless of HD or PD and are associated with regional variation, age, prior history of bleeding, and comorbid cardiovascular disease. The use of antithrombotic agents overall in patients receiving dialysis was low. The majority of bleeds were GI in etiology and not related to dialysis access. Because the factors associated with the highest rates of bleeding are also associated with the risk of severe thrombotic events, these data highlight the unmet need for strategies to reduce thrombotic risk with a more favorable safety profile than currently available agents.
Disclosure
AK, MG, and CA are employees of Arbor Research Collaborative for Health, which administers the DOPPS and PDOPPS studies. CRC, LDB, DRR, GBA, and IB are employees of Merck & Co., Inc., Rahway, NJ, USA.
MB is the Executive Director of CPC, a nonprofit academic research organization affiliated with the University of Colorado, that receives or has received research grant/consulting funding between February 2021 and present from the following: Abbott Laboratories; Adamis Pharmaceuticals Corporation; Agios Pharmaceuticals, Inc.; Alexion Pharma; Alnylam Pharmaceuticals, Inc.; Amgen Inc.; Angionetics, Inc.; ARCA Biopharma, Inc.; Array BioPharma, Inc.; AstraZeneca and Affiliates; Atentiv LLC; Audentes Therapeutics, Inc.; Bayer and Affiliates; Beth Israel Deaconess Medical Center; Better Therapeutics, Inc.; BIDMC; Boston Clinical Research Institute; Bristol-Meyers Squibb Company; Cambrian Biopharma, Inc.; Cardiol Therapeutics Inc.; CellResearch Corp.; Cook Medical Incorporated; Covance; CSL Behring LLC; Eidos Therapeutics, Inc.; EP Trading Co. Ltd.; EPG Communication Holdings Ltd.; Epizon Pharma, Inc.; Esperion Therapeutics, Inc.; Everly Well, Inc.; Exicon Consulting Pvt. Ltd.; Faraday Pharmaceuticals, Inc.; Foresee Pharmaceuticals Co. Ltd.; Fortress Biotech, Inc.; HDL Therapeutics Inc.; HeartFlow Inc.; Hummingbird Bioscience; Insmed Inc.; Ionis Pharmaceuticals; IQVIA Inc.; JanOne Biotech Holdings Inc.; Janssen and Affiliates; Kaneka; Kowa Research Institute, Inc.; Kyushu University; Lexicon Pharmaceuticals, Inc.; LSG Kyushu University; Medimmune Ltd.; Medpace; Merck & Co., Inc., Rahway, NJ, USA; Novartis Pharmaceuticals Corp.; Novate Medical, Ltd.; Novo Nordisk, Inc.; Pan Industry Group; Pfizer Inc.; PhaseBio Pharmaceuticals, Inc.; PPD Development, LP; Prairie Education and Research Cooperative; Prothena Biosciences Limited; Regeneron Pharmaceuticals, Inc.; Regio Biosciences, Inc.; Rexgenero, Sanifit Therapeutics S.A.; Sanofi-Aventis Groupe; Silence Therapeutics PLC; Smith & Nephew PLC; Stealth BioTherapeutics Inc.; State of Colorado CCPD Grant; The Brigham & Women's Hospital, Inc.; The Feinstein Institutes for Medical Research; Thrombosis Research Institute; University of Colorado; University of Pittsburgh; VarmX; Virta Health Corporation; WCT Atlas; Worldwide Clinical Trials Inc.; WraSer, LLC; and Yale Cardiovascular Research Group. MB also receives support from the AHA SFRN under award numbers 18SFRN3390085 (BWH-DH SFRN Center) and 18SFRN33960262 (BWH-DH Clinical Project). RP-F received honoraria (paid to employer) from Astra Zeneca, Boehringer-Lilly, Akebia, Bayer, GSK, and Novo Nordisk for participation in Advisory Boards and educational activities; consulting fees scientific leadership in clinical trials from the George Clinical; research grants from Fresenius Medical Care, National Council for Scientific and Technological Development. RP-F is also employed by Arbor Research Collaborative for Health, who runs the DOPPS studies.
Acknowledgments
This research was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. Medical writing assistance was provided by Gail Pyne-Geithman, MS, D.Phil. in collaboration with ScribCo. Global support for the ongoing DOPPS Programs is provided without restriction on publications by a variety of funders. For details see https://www.dopps.org/AboutUs/Support.aspx. All funds were made to Arbor Research Collaborative for Health and not directly to the authors.
Footnotes
Supplementary Methods.
Supplementary References.
Table S1. Baseline patient characteristics.
Supplementary Material
Supplementary Methods. Supplementary References. Table S1. Baseline patient characteristics.
References
- 1.Thurlow J.S., Joshi M., Yan G., et al. Global epidemiology of end-stage kidney disease and disparities in kidney replacement therapy. Am J Nephrol. 2021;52:98–107. doi: 10.1159/000514550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perl J., Davies S.J., Lambie M., et al. The peritoneal dialysis outcomes and practice patterns study (PDOPPS): unifying efforts to inform practice and improve global outcomes in peritoneal dialysis. Perit Dial Int. 2016;36:297–307. doi: 10.3747/pdi.2014.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Eck van der Sluijs A., Abrahams A.C., Rookmaaker M.B., et al. Bleeding risk of haemodialysis and peritoneal dialysis patients. Nephrol Dial Transplant. 2021;36:170–175. doi: 10.1093/ndt/gfaa216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DOPPS Dialysis outcomes and practice patterns study. https://www.dopps.org/AboutUs.aspx
- 5.Wang I.K., Cheng Y.K., Lin C.L., et al. Comparison of subdural hematoma risk between hemodialysis and peritoneal dialysis patients with ESRD. Clin J Am Soc Nephrol. 2015;10:994–1001. doi: 10.2215/CJN.08140814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sood M.M., Larkina M., Thumma J.R., et al. Major bleeding events and risk stratification of antithrombotic agents in hemodialysis: results from the DOPPS. Kidney Int. 2013;84:600–608. doi: 10.1038/ki.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox C.S., Matsushita K., Woodward M., et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380:1662–1673. doi: 10.1016/S0140-6736(12)61350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magnani G., Ardissino D., Im K., et al. Predictors, type, and impact of bleeding on the net clinical benefit of long-term ticagrelor in stable patients with prior myocardial infarction. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.017008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods. Supplementary References. Table S1. Baseline patient characteristics.