Abstract
Introduction
Peritoneal dialysis (PD) shows promise for urgent-start dialysis in end-stage renal disease (ESRD), with automated PD (APD) having advantages. However, there is limited multicenter randomized controlled trial (RCT) evidence comparing APD with temporary hemodialysis (HD) for this indication in China.
Methods
This multicenter RCT enrolled 116 patients with ESRD requiring urgent dialysis from 11 hospitals, randomized to APD or HD. Patients underwent a 2-week treatment with APD or HD via a temporary central venous catheter (CVC), followed by a maintenance PD. Outcomes were assessed over 12 months during 8 visits. The primary outcome was dialysis-related complications.
Results
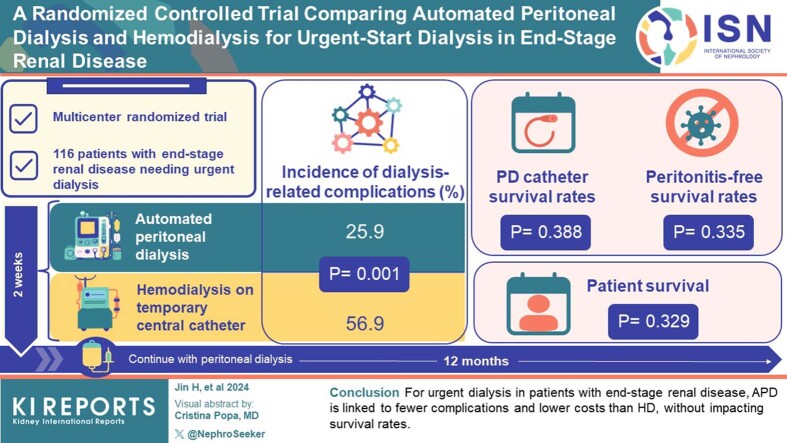
The 1-year incidence of dialysis-related complications was significantly lower in the APD group than in the HD group (25.9% vs. 56.9%, P = 0.001). No significant differences were found between the groups in terms of PD catheter survival rates (P = 0.388), peritonitis-free survival rates (P = 0.335), and patient survival rates (P = 0.329). In terms of health economics, the total direct medical cost of the initial hospitalization for patients with ESRD was significantly lower in the APD group (27,008.39 CNY) than in the HD group (42,597.54 CNY) (P = 0.001), whereas the duration of the first hospital stay showed no significant difference (P = 0.424).
Conclusion
For patients with ESRD needing urgent initiation of dialysis, APD was associated with a lower incidence of dialysis-related complications and lower initial hospitalization costs compared with HD, with no significant differences in PD catheter survival rate, peritonitis-free survival rates, or patient survival rates. These findings can guide clinical decision-making for the optimal dialysis modality for patients requiring urgent dialysis initiation.
Keywords: automated peritoneal dialysis, end-stage renal disease, hemodialysis, urgent-start peritoneal dialysis
Graphical abstract
See Commentary on Page 2588
ESRD, characterized by irreversible loss of renal function, is a global health challenge with rising incidence and prevalence.1 Dialysis serves as a life-saving treatment for ESRD, providing renal replacement therapy when renal function is insufficient. The choice of modality, whether PD or HD, is often influenced by a multitude of factors, including patient’s clinical condition, personal preferences, and availability of health care resources.2,3
In a subset of patients in whom ESRD is detected late or there is a rapid decline in renal function, an “urgent start” to dialysis becomes a necessity.4 Such situations pose significant clinical challenges due to the heightened risk of complications and a constrained timeframe for adequate preparation.5 In such contexts, HD via a CVC has traditionally been the go-to approach.6 Nevertheless, numerous studies have shown a range of complications associated with the placement and utilization of CVC, encompassing issues such as catheter-related infections, thrombosis, and hemodynamic instability, all of which negatively impact patient survival.7, 8, 9, 10
However, recent advancements in dialysis methods, particularly the advent of APD, have broadened the scope of available treatment options.11 Urgent-start PD can be defined as initiation of PD in patients with newly diagnosed ESRD who are not yet on dialysis and who require dialysis initiation less than 2 weeks after PD catheter placement, but who do not require emergency dialysis.12 Specifically, urgent-start PD is primarily designated for patients who have not prechosen a specific dialysis approach, but are considered appropriate candidates for PD.12, 13, 14 APD, a specific form of PD, is gaining traction as a viable modality for renal replacement therapy in urgent-start scenarios.15, 16, 17 The potential advantages of APD extend beyond mere physiological benefits such as fewer hemodynamic fluctuations and a continuous clearance of solutes;18,19 it also offers enhanced patient comfort, making it a favorable choice for patients and medical practitioners alike.20, 21, 22 Notably, compared to manual PD, APD provides the additional benefit of exerting less intraabdominal pressure, which is conducive to incision healing and prevents leakage, thus reducing related complications.19,23,24 Moreover, the dialysis adequacy can be boosted by increasing the frequency of exchanges, offering greater flexibility in managing patients' needs.17,19,23 Despite these potential advantages, the comparative efficacy and safety of APD and HD in urgent-start scenarios remain under researched, and the current body of evidence is largely rooted in retrospective or observational studies.
To address these gaps in knowledge, we conducted a prospective, multicenter, RCT to compare the outcomes of APD and HD for urgent-start dialysis in patients with ESRD. Our study focused on the incidence of dialysis-related complications, PD catheter survival rate, peritonitis-free survival rate, patient survival rate, and health economic indicators. This study aimed to provide evidence-based insights to guide the selection of dialysis modality in urgent-start scenarios, balancing both individual patient factors and health care system resources.
Methods
Study Design and Participants
We executed a prospective, multicenter, RCT across 11 hospitals involving a total of 116 patients with ESRD requiring urgent-start dialysis from March 2019 to December 2020, and the last follow-up occurred in December 2021. Patients were randomized in a 1:1 ratio into the APD group and the HD group, with each group comprising 58 patients.
Inclusion criteria included patients aged 18 to 80 years and having the necessity for urgent initiation of dialysis due to late presentation or rapid progression of renal disease without a preestablished functional dialysis access. Exclusion criteria included patients with contraindications to PD or HD; those with severe volume overload and pulmonary edema, severe hyperkalemia (serum potassium >6.5 mmol/l), or uremic encephalopathy; those with severe liver failure; those with uncorrectable shock, malignancy or mental disorders; those who were pregnant or lactating, and patients unable or unwilling to provide informed consent for the study. Patients were enrolled in the study if they met the eligibility criteria and none of the exclusion criteria.
This study received ethical approval from the ethics committees of 11 collaborating institutions. It was registered at ClinicalTrials.gov (identifier: NCT02946528) and conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization’s Guidelines for Good Clinical Practice, and applicable local legislation on non-interventional studies and/or observational studies. All patients provided written Informed Consent Forms pre-enrollment. The protocol and all its amendments were approved by the Shanghai Jiao Tong University School of Medicine, Ren Ji Hospital Ethics Committee (2018220) and the ethics committee of each participating center.
Randomization
In this study, we used SAS statistical software (SAS Institute Inc., Cary, NC) to generate a completely randomized sequence and randomly assigned participants in a 1:1 ratio to the APD group or the HD group. We adopted a block randomization method, with every 4 participants as a block, and 2 participants assigned to the APD group and 2 to the HD group in each block, to ensure balance between the treatment groups. The block randomization sequence was also generated using SAS. Participants who signed informed consent and met the inclusion criteria were assigned a random number according to the order of enrollment, and then allocated to the corresponding treatment group based on the random number. The allocation itself was done using opaque sealed envelopes. Allocation was not accessible to study personnel except to receive a treatment assignment for a specific participant. The entire randomization process was designed and supervised by a third-party statistical agency.
Interventions
In the APD group, patients initially underwent PD catheter insertion and were managed with APD as per an urgent-start PD protocol. In principle, tidal PD was used with a single dwell volume of 1.0 to 1.5 l and a total cyclic treatment time of 8 to 12 hours per day, resulting in a total dialysis dose of 5 to 10 l per day. The dwell volume was incrementally increased over time. After a period of 2 weeks, these patients transitioned to maintenance PD.
In contrast, patients in the HD group initially underwent temporary CVC insertion and initiated HD treatment. These patients underwent 2 to 3 HD sessions per week, employing intermittent HD, hemodiafiltration, or continuous renal replacement therapy, as per their individual clinical needs. Once these patients were stabilized, they underwent PD catheter insertion. Two weeks after PD catheter placement, they were switched to maintenance PD (Supplementary References). The trial protocol (Supplement 1) was approved by the Institutional Review Board at each participating center.
Follow-Up and Outcome Measures
Patients were followed-up with for 12 months, with 8 visits planned. The primary outcome was the incidence of dialysis-related complications. Dialysis-related complications were defined as a composite of noninfectious complications (PD catheter malposition, PD catheter obstruction, leakage, hernia, bleeding around the catheter, or thrombosis) and infectious complications (PD catheter-related infection, peritonitis, or CVC-related infection). Secondary outcomes included PD catheter survival rate (the percentage of PD catheters that remain functional throughout the study period without the need for surgical intervention), peritonitis-free survival rates, patient survival rate, total direct medical cost and duration of initial hospitalization.
Statistical Analysis
All statistical analyses were performed using SPSS 22.0 statistics software (SPSS Inc., Chicago, IL). Data were analyzed using appropriate statistical tests. Categorical variables were analyzed using chi-square or Fisher exact tests, and continuous variables were analyzed using Student’s t test or Mann-Whitney U test, as appropriate. Survival analyses were performed using the Kaplan-Meier method and compared using the log-rank test. Assuming 1-year composite complication rate after urgent-start PD and HD of 25% and 55%, respectively, according to our previous clinical experience, a 1:1 sampling ratio, a drop-out rate of 10%, and a 2-sided alpha of 0.05, a sample size of participants 58 per group was predicted to have 90% power of detecting a reduction in composite complications. A 2-tailed P-value < 0.05 was considered statistically significant. All analyses were performed using statistical software. The full analysis set, which was used for all analyses, included all enrolled patients. Missing data were not imputed unless otherwise specified.
Results
Study Population
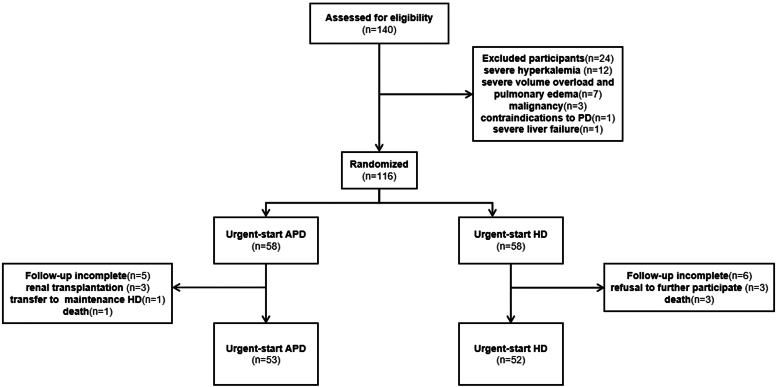
The study initially screened 140 patients. Among these, 24 participants were excluded due to various reasons. A total of 116 patients were included in the study, 58 in each of the APD and HD groups (Figure 1). Seven patients (4 in the APD group and 3 in the HD group) were withdrawn from the study early because of kidney transplantation (n = 3), transfer to maintenance HD (n = 1), or refusal to further participate in the study (n = 3). No loss to follow-up occurred; however, 4 participants died during the maintenance dialysis period (1 in the APD group and 3 in the HD group). Therefore, 53 participants in the APD group and 52 participants in the HD group completed the follow-up. The mean age of the participants was 52.2 ± 14.2 years, with 66.4% being male. The median time from catheter insertion to the initiation of dialysis was 4 days in the APD group, as the protocol specified a transition to maintenance PD 14 days after catheter insertion. Baseline patient characteristics are reported in Table 1.
Figure 1.
Patient flowchart. APD, automated peritoneal dialysis; HD, hemodialysis.
Table 1.
Baseline characteristics of study patients
| Characteristics | APD (n = 58) | HD (n = 58) | P-value |
|---|---|---|---|
| Gender (n [%] men) | 40 (69.0) | 37 (63.8) | 0.555 |
| Age (yr) | 52.4 ± 14.6 | 51.9 ± 13.8 | 0.862 |
| Weight (kg) | 68 (59, 80) | 65 (58, 75) | 0.413 |
| BMI (kg/m2) | 24.95 ± 4.52 | 23.77 ± 3.82 | 0.149 |
| Primary disease (n [%] men) | |||
| Primary glomerulonephritis | 25 (43.1) | 19 (32.8) | 0.251 |
| Diabetic kidney disease | 10 (17.2) | 15 (25.9) | 0.259 |
| Hypertensive nephrosclerosis | 5 (8.6) | 2 (3.4) | 0.242 |
| Polycystic kidney disease | 1 (1.7) | 1 (1.7) | 1.000 |
| Lupus nephritis | 1 (1.7) | 0 (0.0) | 0.315 |
| Comorbidities (n [%]) | |||
| Hypertension | 56 (96.6) | 52 (89.7) | 0.143 |
| Diabetes | 28 (48.3) | 25 (43.1) | 0.576 |
| Infection | 11 (19.0) | 9 (15.5) | 0.623 |
| Cerebrovascular disease | 5 (8.6) | 7 (12.1) | 0.542 |
| Coronary atherosclerotic heart disease | 6 (10.3) | 4 (6.9) | 0.508 |
| Heart failure | 2 (3.4) | 7 (12.1) | 0.083 |
| Abdominal surgical history | 2 (3.4) | 5 (8.6) | 0.242 |
| Scr (umol/l) | 875.7 ± 254.0 | 880.3 ± 271.2 | 0.928 |
| BUN (mmol/l) | 30.4 (24.3, 35.9) | 27.3 (22.7, 32.9) | 0.135 |
| eGFR (ml/min per 1.73 m2) | 5.6 (4.0, 7.0) | 5.8 (4.0, 7.5) | 0.535 |
| K (mmol/l) | 4.47 ± 0.60 | 4.41 ± 0.67 | 0.600 |
| Na (mmol/l) | 141.0 ± 3.6 | 140.6 ± 3.1 | 0.541 |
| Cl (mmol/l) | 102.7 ± 4.8 | 102.9 ± 4.6 | 0.793 |
| pH | 7.34 ± 0.06 | 7.35 ± 0.06 | 0.773 |
| HCO3 | 21.6±3.6 | 21.2±3.9 | 0.608 |
| Hb (g/l) | 85.4 ± 20.2 | 83.7 ± 18.6 | 0.635 |
| Alb (g/l) | 34.5 ± 4.7 | 33.6 ± 6.4 | 0.369 |
| Pre-albumin (g/l) | 302.6 ± 74.6 | 289.3 ± 86.8 | 0.402 |
| Ca (mmol/l) | 1.96 ± 0.24 | 1.94 ± 0.26 | 0.668 |
| Corrected Ca (mmol/l) | 2.09 (1.92, 2.25) | 2.14 (1.92, 2.25) | 0.855 |
| P (mmol/l) | 2.09 (1.78, 2.57) | 2.00 (1.72, 2.45) | 0.475 |
| PTH (ng/l) | 313.0 (218.6, 413.1) | 424.3 (248.0, 610.0) | 0.094 |
| TC (mmol/l) | 4.40 ± 1.03 | 4.37 ± 1.22 | 0.864 |
| LDL (mmol/l) | 2.82 (1.98, 3.46) | 2.26 (1.82, 3.24) | 0.285 |
Alb, serum albumin; APD, automated peritoneal dialysis; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; Ca, serum calcium; Corrected Ca, serum corrected calcium; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; HCO3, serum bicarbonate; HD, hemodialysis; K, serum potassium; LDL, low-density lipoprotein; Na, serum sodium; P, serum phosphate; PTH, parathyroid hormone; Scr, serum creatinine; TC, total cholesterol.
Baseline characteristics are expressed as mean ± SD for normally distributed data, median (25th percentile, 75th percentile) for nonnormally distributed data, and frequencies and percentages for categorical data.
Dialysis-Related Complications
The incidence of dialysis-related complications at 1-year follow-up was considerably lower in the APD group in comparison to the HD group (15 [25.9%] vs. 33 [56.9%], P = 0.001). Specifically, there are statistically significant differences in noninfectious complications (9 [15.5%] vs. 20 [34.5%], P = 0.032). Moreover, the incidence of infection-related complications was higher in the HD group, although this difference was not statistically significant (6 [10.3%] vs. 13 [22.4%], P = 0.132). Notably, the APD group faced complications such as PD-catheter malposition (3.4%), PD-catheter obstruction (6.9%), leakage (3.4%), hernia (1.7%), PD catheter-related infection (5.2%), and peritonitis (5.2%). Conversely, in the HD group, CVC-related or anticoagulation-related complications accounted for 24.1% of cases, with bleeding around the catheter (15.5%), CVC-related infection (5.2%), and thrombosis (3.4%). Meanwhile, complications were more frequent and varied, with a remarkable 32.7% of patients experiencing PD-related complications. These included PD-catheter malposition (5.2%), PD-catheter obstruction (3.4%), leakage (3.4%), hernia (3.4%), PD catheter-related infection (5.2%), and peritonitis (12.1%) (Table 2).
Table 2.
One-year dialysis-related complications distribution (APD and HD Groups)
| Complications distribution | APD (n = 58) | HD (n = 58) | P-value |
|---|---|---|---|
| Total | 15 (25.9) | 33 (56.9) | 0.001 |
| Noninfectious complications | 9 (15.5) | 20 (34.5) | 0.032 |
| PD-catheter malposition | 2 (3.4) | 3 (5.2) | 1.000 |
| PD-catheter obstruction | 4 (6.9) | 2 (3.4) | 0.675 |
| Leakage | 2 (3.4) | 2 (3.4) | 1.000 |
| Hernia | 1 (1.7) | 2 (3.4) | 1.000 |
| Bleeding around the catheter | 0 (0.0) | 9 (15.5) | 0.006 |
| Thrombosis | 0 (0.0) | 2 (3.4) | 0.476 |
| Infectious complications | 6 (10.3) | 13 (22.4) | 0.132 |
| PD-catheter-related infection | 3 (5.2) | 3 (5.2) | 1.000 |
| Peritonitis | 3 (5.2) | 7 (12.1) | 0.321 |
| CVC-related infection | 0 (0.0) | 3 (5.2) | 0.242 |
APD, automated peritoneal dialysis; CVC, central venous catheter; HD, hemodialysis; PD, peritoneal dialysis.
Complication distributions for APD and HD are expressed as n (%).
A significant difference in the complication rates within 6 weeks was observed, with 15.5% (9/58) in the APD group and 36.2% (21/58) in the HD group (P = 0.020). Specifically, the HD group showed significantly higher instances of bleeding around the catheter (P = 0.006). In addition, the HD group had a higher prevalence of CVC-related infections (5.2%) and thrombosis (3.4%); whereas the APD group exhibited a marginally higher incidence of PD catheter obstruction (5.2%) and leakage (3.4%), although these differences were not statistically significant. PD-related infectious complications were comparably distributed between the 2 groups (Supplementary Table S1).
PD Catheter Survival Rate and Peritonitis-Free Survival Rate
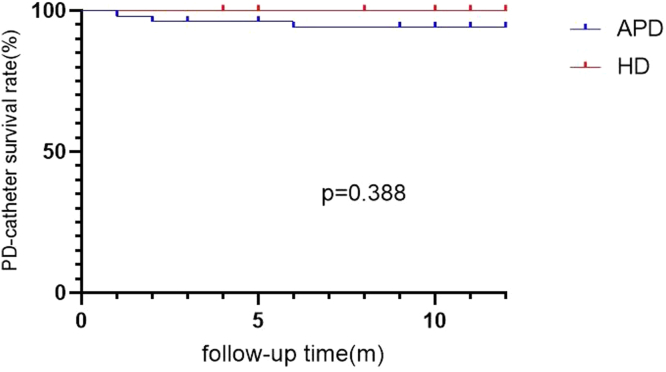
There was no significant difference in the PD catheter survival rate between the 2 groups (log-rank = 0.744, P = 0.388) (Figure 2).
Figure 2.
PD-catheter survival rate between APD and HD group.
APD, automated peritoneal dialysis; HD, hemodialysis; PD, peritoneal dialysis.
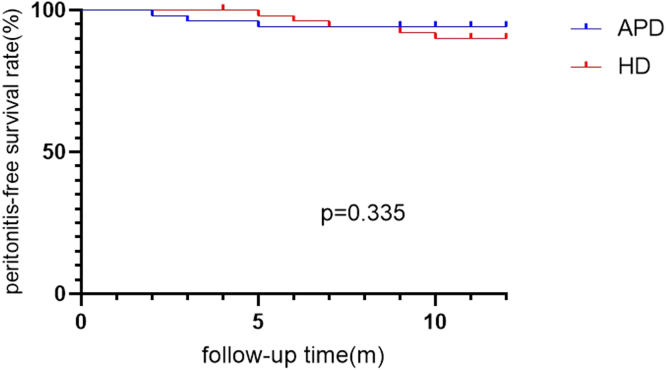
During the follow-up period, 3 cases of peritonitis occurred in the APD group, and 7 in the HD group. No significant difference in peritonitis-free survival was observed between the 2 groups (log-rank = 0.931, P = 0.335) (Figure 3).
Figure 3.
Peritonitis-free survival rate between APD and HD group.
APD, automated peritoneal dialysis; HD, hemodialysis.
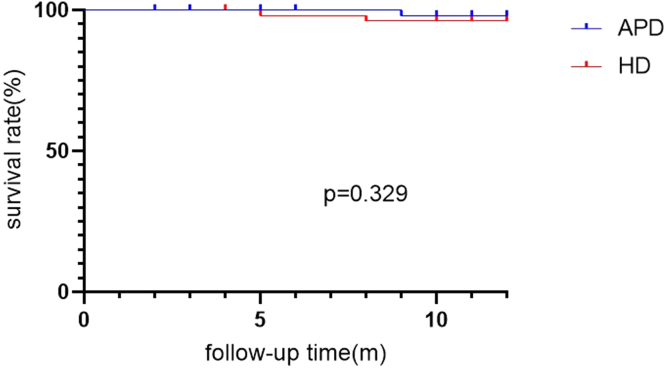
Patient Survival Rate
The 1-year patient survival rate was 97.9% in the APD group and 94.3% in the HD group. There was no significant difference between the 2 groups (log-rank = 0.953, P = 0.329) (Figure 4).
Figure 4.
Patient survival rate between APD and HD group. APD, automated peritoneal dialysis; HD, hemodialysis.
Health Economic Indicators
The total direct medical cost of the first hospitalization for patients with ESRD in the APD group was significantly lower than that in the HD group (27,008.39 [17,676.54– 37748.30] vs. 42,597.54 [17,764.57–56,312.28] CNY, P = 0.001). The duration of the initial hospitalization was comparable between the 2 groups, with the APD group averaging 23 days and the HD group averaging 22.5 days, resulting in a statistically insignificant difference (P = 0.424).
Discussion
To our knowledge, our research stands as the first multicenter RCT in our country, rigorously comparing the outcomes and complications of APD and HD as primary dialysis methods for patients with ESRD in need of urgent-start dialysis with long-term follow-up. This study marks a significant advancement in the field because it not only introduces a novel approach to utilizing APD as the urgent initiation PD method but also follows a standardized APD prescription. The prescription has been honed through rigorous clinical practice at our center.15,16,25, 26, 27, 28 Furthermore, our research sets itself apart from previous work by offering a longer follow-up period, because past studies have predominantly focused on short-term complications.29, 30, 31, 32 Importantly, our study also factors in economic considerations, expanding the scope beyond clinical outcomes alone. This comprehensive approach sheds new light on the utility of PD as a first-line treatment for patients with ESRD requiring urgent-start dialysis, especially in settings where HD resources may be limited.
Significantly, this study’s findings align with previous research, demonstrating a notably lower incidence of dialysis-related complications in the APD group compared to the HD group at 1-year follow-up. The lower incidence can be attributed to several factors. The APD group avoided the use of temporary CVC for HD, thereby minimizing the risk of complications such as thrombosis, CVC-related infections, and HD-associated risks such as increased bleeding due to anticoagulation. In addition, early initiation of APD did not significantly elevate the risk of short-term PD-related complications such as peritonitis, hernia, and leakage. Consequently, the incidence of dialysis-related complications was lower in the APD group. Our results echo previous shorter-term studies,25, 26, 27,29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 while providing a more comprehensive picture due to our multicenter RCT study design and extended follow-up period. This emphasizes that, even in the context of an urgent need for dialysis, PD may present a safer therapeutic choice for managing patients with ESRD. Moreover, the diminished complication rate underscores that APD might be better tolerated by patients over the long-term, potentially enhancing treatment adherence and improving overall outcomes. This study further extends the understanding of ESRD management, highlighting the enduring benefits of PD and APD treatment in the context of longer-term patient care.
Moreover, our results showed no significant difference between the APD and HD groups in terms of PD catheter survival rate, peritonitis-free survival rate, and patient survival rate. These findings further underscore the feasibility and safety of APD as an acceptable treatment modality alternative to HD in terms of an urgent need for dialysis. Importantly, despite the high prevalence of comorbid conditions such as diabetes and hypertension in our study population, the survival rates in both groups were encouraging, indicating that both methods can be effectively implemented for ESRD treatment.
In terms of health economics, we found that the total direct medical cost of the first hospitalization for patients with ESRD in the APD group was significantly lower than that in the HD group. This suggests that APD could potentially offer a cost-effective alternative to HD, particularly in resource-limited settings. However, the length of the first hospital stay did not differ significantly between the 2 groups, indicating that the overall burden on health care facilities might be similar with both dialysis methods.
Despite these promising findings, several limitations must be acknowledged and necessitate careful interpretation. The open-label design could introduce observer and performance biases, and the lack of blinding among outcome assessors and absence of an adjudication committee may permit detection bias. In addition, our findings may not extend to different ethnicities or regions, given the potential for more acceptance of small volume PD in Asian populations with generally smaller body sizes. This may limit the generalizability to populations with higher body mass indexes or where PD catheter placement is not typically performed by nephrologists. Lastly, the lack of patient-reported outcomes such as quality of life in this study underscores the need for their inclusion in future investigations. As such, though our findings are promising, future research should account for these limitations for a more comprehensive understanding of PD.
In conclusion, our study suggests that APD is a viable, safe, and potentially cost-effective option for the management of urgent-start dialysis in ESRD, with a lower incidence of dialysis-related complications and comparable survival rates to HD.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We would like to express our sincere gratitude to all the participating patients and their families, without whom this study would not have been possible. We are profoundly grateful to the medical staff of the 11 hospitals involved in this study for their unwavering commitment and dedication to patient care and data collection. We would like to thank our research team for their tireless efforts in study design, data analysis, and manuscript preparation. Special thanks to all the data collectors, statisticians, and administrative staff who made significant contributions to this research. We are grateful for the guidance and support provided by our mentors and colleagues. Their insightful feedback was invaluable in conducting this study and preparing the manuscript. Finally, we wish to express our appreciation to all those who directly or indirectly contributed to the success of this study.
This study was funded by the Multicenter Clinical Research Project of Shanghai Jiao Tong University School of Medicine (DLY201805), Clinical Research Plan of SHDC (SHDC2020CR3029B), and the Sailing Program of Shanghai Municipal Science and Technology Commission (21YF1425400).
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
HJ wrote the original manuscript. WF and LW performed data curation and supervision. XZ, YD, GW, YL, XC, NW, GJ, ZG, XW, and YQ were subprincipal investigators. SL conducted the statistical analysis. ZN conceived and designed the study, revised the manuscript and was the principal investigator. All the authors critically reviewed the manuscript and approved the final version for submission.
Footnotes
Supplement 1. Research Protocol.
Supplementary References.
Table S1. Six-week-dialysis-related complications distribution (automated peritoneal dialysis and hemodialysis groups).
CONSORT 2010 checklist.
Supplementary Material
Supplement 1. Research Protocol. Supplementary References. Table S1. Six-week-dialysis-related complications distribution (automated peritoneal dialysis and hemodialysis groups). CONSORT 2010 checklist.
References
- 1.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torreggiani M., Piccoli G.B., Moio M.R., et al. Choice of the dialysis modality: practical considerations. J Clin Med. 2023;12:3328. doi: 10.3390/jcm12093328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan C.T., Blankestijn P.J., Dember L.M., et al. Dialysis initiation, modality choice, access, and prescription: conclusions from a Kidney Disease: improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019;96:37–47. doi: 10.1016/j.kint.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Lok C.E. Urgent peritoneal dialysis or hemodialysis catheter dialysis. J Vasc Access. 2016;17:S56–S59. doi: 10.5301/jva.5000535. [DOI] [PubMed] [Google Scholar]
- 5.Hassan R., Akbari A., Brown P.A., Hiremath S., Brimble K.S., Molnar A.O. Risk factors for unplanned dialysis initiation: a systematic review of the literature. Can J Kidney Health Dis. 2019;6 doi: 10.1177/2054358119831684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansen K.L., Chertow G.M., Gilbertson D.T., et al. US renal data system 2022 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2023;81:A8–A11. doi: 10.1053/j.ajkd.2022.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xue H., Ix J.H., Wang W., et al. Hemodialysis access usage patterns in the incident dialysis year and associated catheter-related complications. Am J Kidney Dis. 2013;61:123–130. doi: 10.1053/j.ajkd.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vachharajani T.J., Taliercio J.J., Anvari E. New devices and technologies for hemodialysis vascular access: a review. Am J Kidney Dis. 2021;78:116–124. doi: 10.1053/j.ajkd.2020.11.027. [DOI] [PubMed] [Google Scholar]
- 9.Arhuidese I.J., Orandi B.J., Nejim B., Malas M. Utilization, patency, and complications associated with vascular access for hemodialysis in the United States. J Vasc Surg. 2018;68:1166–1174. doi: 10.1016/j.jvs.2018.01.049. [DOI] [PubMed] [Google Scholar]
- 10.Ravani P., Palmer S.C., Oliver M.J., et al. Associations between hemodialysis access type and clinical outcomes: a systematic review. J Am Soc Nephrol. 2013;24:465–473. doi: 10.1681/ASN.2012070643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domenici A., Giuliani A. Automated peritoneal dialysis: patient perspectives and outcomes. Int J Nephrol Renovasc Dis. 2021;14:385–392. doi: 10.2147/IJNRD.S236553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blake P.G., Jain A.K. Urgent start peritoneal dialysis: defining what it is and why it matters. Clin J Am Soc Nephrol. 2018;13:1278–1279. doi: 10.2215/CJN.02820318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dias D.B., Mendes M.L., Caramori J.T., Falbo Dos Reis P., Ponce D. Urgent-start dialysis: comparison of complications and outcomes between peritoneal dialysis and haemodialysis. Perit Dial Int. 2021;41:244–252. doi: 10.1177/0896860820915021. [DOI] [PubMed] [Google Scholar]
- 14.Crabtree J.H., Shrestha B.M., Chow K.M., et al. Creating and maintaining optimal peritoneal dialysis access in the adult patient: 2019 update. Perit Dial Int. 2019;39:414–436. doi: 10.3747/pdi.2018.00232. [DOI] [PubMed] [Google Scholar]
- 15.Jin H., Lv S., Wang L., et al. Automated peritoneal dialysis in urgent-start dialysis ESRD patients: safety and dialysis adequacy. Ther Apher Dial. 2023;27:464–470. doi: 10.1111/1744-9987.13943. [DOI] [PubMed] [Google Scholar]
- 16.Jin H., Lu R., Lv S., et al. Automated peritoneal dialysis as a cost-effective urgent-start dialysis option for ESRD patients: a prospective cohort study. Int J Artif Organs. 2022;45:672–679. doi: 10.1177/03913988221105903. [DOI] [PubMed] [Google Scholar]
- 17.Xie J., Wang H., Li S., et al. Low-volume tidal peritoneal dialysis is a preferable mode in patients initiating urgent-start automated peritoneal dialysis: a randomized, open-label, prospective control study. Ther Apher Dial. 2019;23:409–417. doi: 10.1111/1744-9987.12791. [DOI] [PubMed] [Google Scholar]
- 18.Giuliani A., Crepaldi C., Milan Manani S., et al. Evolution of automated peritoneal dialysis machines. Contrib Nephrol. 2019;197:9–16. doi: 10.1159/000496302. [DOI] [PubMed] [Google Scholar]
- 19.Roumeliotis A., Roumeliotis S., Leivaditis K., Salmas M., Eleftheriadis T., Liakopoulos V. APD or CAPD: one glove does not fit all. Int Urol Nephrol. 2021;53:1149–1160. doi: 10.1007/s11255-020-02678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duncanson E., Chur-Hansen A., Jesudason S. Patient perspectives of coping with automated peritoneal dialysis. Perit Dial Int. 2022;42:344–352. doi: 10.1177/08968608211043411. [DOI] [PubMed] [Google Scholar]
- 21.Luo P.T., Li W., Li X.Y., Zhang Y., Du B., Cui W.P. Impact of peritoneal dialysis modality on patient and PD survival: a systematic review. Perit Dial Int. 2023;43:128–138. doi: 10.1177/08968608221140788. [DOI] [PubMed] [Google Scholar]
- 22.Sun H., Zhuang Y., Gao L., et al. Impact of dialysis modality conversion on the health-related quality of life of peritoneal dialysis patients: a retrospective cohort study in China. PeerJ. 2022;10 doi: 10.7717/peerj.12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Javaid M.M., Khan B.A., Subramanian S. The modality of choice, manual or automated, for urgent start peritoneal dialysis. Clin Kidney J. 2019;12:443–446. doi: 10.1093/ckj/sfz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang I.K., Yu T.M., Yen T.H., et al. Comparison of patient survival and technique survival between continuous ambulatory peritoneal dialysis and automated peritoneal dialysis. Perit Dial Int. 2020;40:563–572. doi: 10.1177/0896860820942987. [DOI] [PubMed] [Google Scholar]
- 25.Jin H., Fang W., Zhu M., et al. Urgent-start peritoneal dialysis and hemodialysis in ESRD patients: complications and outcomes. PLoS One. 2016;11 doi: 10.1371/journal.pone.0166181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin H., Ni Z., Mou S., et al. Feasibility of urgent-start peritoneal dialysis in older patients with end-stage renal disease: a single-center experience. Perit Dial Int. 2018;38:125–130. doi: 10.3747/pdi.2017.00002. [DOI] [PubMed] [Google Scholar]
- 27.Jin H., Ni Z., Che X., et al. Peritoneal dialysis as an option for unplanned dialysis initiation in patients with end-stage renal disease and diabetes mellitus. Blood Purif. 2019;47:52–57. doi: 10.1159/000493176. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y., Zhang L., Lin A., Ni Z., Qian J., Fang W. Impact of break-in period on the short-term outcomes of patients started on peritoneal dialysis. Perit Dial Int. 2014;34:49–56. doi: 10.3747/pdi.2012.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Htay H., Johnson D.W., Craig J.C., Teixeira-Pinto A., Hawley C.M., Cho Y. Urgent-start peritoneal dialysis versus haemodialysis for people with chronic kidney disease. Cochrane Database Syst Rev. 2021;1 doi: 10.1002/14651858.CD012899.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lobbedez T., Lecouf A., Ficheux M., Henri P., Hurault de Ligny B., Ryckelynck J.P. Is rapid initiation of peritoneal dialysis feasible in unplanned dialysis patients? a single-centre experience. Nephrol Dial Transplant. 2008;23:3290–3294. doi: 10.1093/ndt/gfn213. [DOI] [PubMed] [Google Scholar]
- 31.Koch M., Kohnle M., Trapp R., Haastert B., Rump L.C., Aker S. Comparable outcome of acute unplanned peritoneal dialysis and haemodialysis. Nephrol Dial Transplant. 2012;27:375–380. doi: 10.1093/ndt/gfr262. [DOI] [PubMed] [Google Scholar]
- 32.Parapiboon W., Sangsuk J., Nopsopon T., et al. Randomized study of urgent-start peritoneal dialysis versus urgent-start temporary hemodialysis in patients transitioning to kidney failure. Kidney Int Rep. 2022;7:1866–1877. doi: 10.1016/j.ekir.2022.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang D., Calabro-Kailukaitis N., Mowafy M., et al. Urgent-start peritoneal dialysis results in fewer procedures than hemodialysis. Clin Kidney J. 2019;13:166–171. doi: 10.1093/ckj/sfz053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karpinski S., Sibbel S., Cohen D.E., et al. Urgent-start peritoneal dialysis: association with outcomes. Perit Dial Int. 2023;43:186–189. doi: 10.1177/08968608221083781. [DOI] [PubMed] [Google Scholar]
- 35.Zang X., Du X., Li L., Mei C. Complications and outcomes of urgent-start peritoneal dialysis in elderly patients with end-stage renal disease in China: a retrospective cohort study. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-032849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding X., Gao W., Guo Y., Cai Q., Bai Y. Comparison of mortality and complications between urgent-start peritoneal dialysis and urgent-start hemodialysis: a systematic review and meta-analysis. Semin Dial. 2022;35:207–214. doi: 10.1111/sdi.13001. [DOI] [PubMed] [Google Scholar]
- 37.Liu S., Zhuang X., Zhang M., et al. Application of automated peritoneal dialysis in urgent-start peritoneal dialysis patients during the break-in period. Int Urol Nephrol. 2018;50:541–549. doi: 10.1007/s11255-018-1785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alkatheeri A.M., Blake P.G., Gray D., Jain A.K. Success of urgent-start peritoneal dialysis in a Large Canadian renal program. Perit Dial Int. 2016;36:171–176. doi: 10.3747/pdi.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghaffari A. Urgent-start peritoneal dialysis: a quality improvement report. Am J Kidney Dis. 2012;59:400–408. doi: 10.1053/j.ajkd.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 40.Wong L.P., Li N.C., Kansal S., et al. Urgent peritoneal dialysis starts for ESRD: initial multicenter experiences in the United States. Am J Kidney Dis. 2016;68:500–502. doi: 10.1053/j.ajkd.2016.03.426. [DOI] [PubMed] [Google Scholar]
- 41.See E.J., Cho Y., Hawley C.M., Jaffrey L.R., Johnson D.W. Early and late patient outcomes in urgent-start peritoneal dialysis. Perit Dial Int. 2017;37:414–419. doi: 10.3747/pdi.2016.00158. [DOI] [PubMed] [Google Scholar]
- 42.Xu D., Liu T., Dong J. Urgent-start peritoneal dialysis complications: prevalence and risk factors. Am J Kidney Dis. 2017;70:102–110. doi: 10.1053/j.ajkd.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 43.Yan H., Fang W., Lin A., Cao L., Ni Z., Qian J. Three versus 4 daily exchanges and residual kidney function decline in incident CAPD patients: a randomized controlled trial. Am J Kidney Dis. 2017;69:506–513. doi: 10.1053/j.ajkd.2016.08.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement 1. Research Protocol. Supplementary References. Table S1. Six-week-dialysis-related complications distribution (automated peritoneal dialysis and hemodialysis groups). CONSORT 2010 checklist.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.