Abstract
Introduction
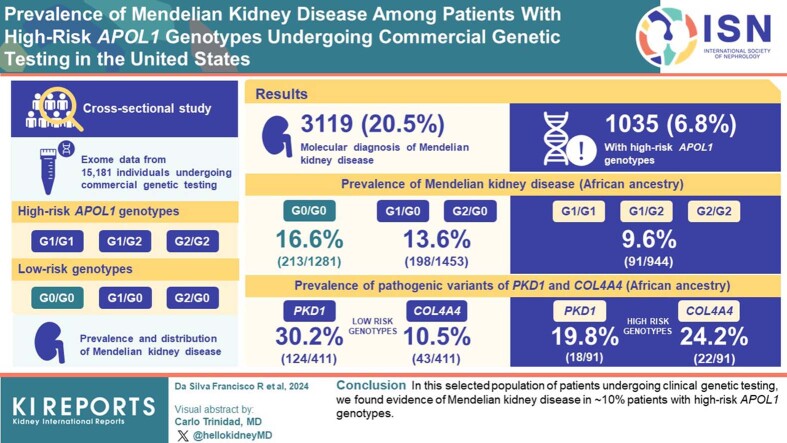
Among individuals with high-risk APOL1 genotypes, the lifetime risk of developing kidney failure is ∼15%, indicating that other genetic variants or nongenetic modifiers likely contribute substantially to an individual patient’s risk of progressive kidney disease. Here, we estimate the prevalence and distribution of Mendelian kidney diseases among patients with high-risk APOL1 genotypes undergoing commercial genetic testing in the United States.
Methods
We analyzed clinical exome sequencing data from 15,181 individuals undergoing commercial genetic testing for Mendelian kidney disease in the United States from 2020 to 2021. We identified patients with high-risk APOL1 genotypes by the presence of G1/G1, G1/G2, or G2/G2 alleles. Patients carrying single risk APOL1 alleles were identified as G1/G0, G2/G0; the remainder of patients were G0/G0. We estimated the prevalence and distribution of Mendelian kidney disease stratified by APOL1 genotype and genetically predicted ancestry.
Results
Of 15,181 patients, 3119 had genetic testing results consistent with a molecular diagnosis of Mendelian kidney disease (20.5%). Of 15,181 patients, 1035 (6.8%) had high-risk APOL1 genotypes. Among patients with recent genomic African ancestry, the prevalence of Mendelian kidney diseases was lower in those with high-risk APOL1 genotypes (9.6%; n = 91/944) compared with single risk APOL1 allele carriers (13.6%; n = 198/1453) and those with G0/G0 APOL1 genotypes (16.6%; n = 213/1281). Among patients with Mendelian kidney disease and recent genomic African ancestry, we observed differences in the prevalence of pathogenic/likely pathogenic variants in PKD1 (19.8% in high-risk vs. 30.2% in low-risk genotypes), and COL4A4 (24.2% in high-risk vs. 10.5% in low-risk genotypes).
Conclusion
In this selected population of patients undergoing clinical genetic testing, we found evidence of Mendelian kidney disease in ∼10% patients with high-risk APOL1 genotypes.
Keywords: African ancestry, APOL1, COL4A4, mendelian kidney disease, PKD1
Graphical abstract
Americans with recent African ancestry are at increased risk of kidney failure compared with Americans of European descent, and much of this excess risk has been attributed to APOL1-mediated kidney disease.1 Two risk alleles in APOL1, the gene encoding apolipoprotein-L1, have been identified as risk factors for the development and progression of chronic kidney disease.2 These coding variants, termed G1 and G2, are present relatively at high frequencies among people of recent African descent in part because of the positive natural selection of these alleles as they confer a protective advantage against the trypanosomes that cause African sleeping sickness.3,4 Approximately 13% of the Americans with recent African ancestry carry high-risk APOL1 genotypes, defined as having 2 high-risk alleles in APOL1 (G1/G1, G1/G2, or G2/G2).4 The risk of developing kidney disease is 3- to 30-fold higher among persons with APOL1 high-risk genotypes than those not carrying any APOL1 risk alleles.5 However, among persons with high-risk APOL1 genotypes, the lifetime risk of kidney failure is ∼15%, indicating that other genetic variants, or nongenetic modifiers, including environmental factors, likely contribute substantially to an individual patient’s risk of kidney disease.1,2
Recent studies have demonstrated that ∼10% of adult patients with chronic kidney disease have Mendelian forms of kidney disease,6 and as such, clinical genetic testing to identify monogenic kidney diseases has become increasingly common.7 The interaction between APOL1 risk alleles and other causes of kidney disease is an area of active research. For example, several studies have demonstrated that the presence of APOL1 high-risk genotypes is associated with worse disease trajectories than for patients with otherwise unrelated forms of kidney disease, and especially glomerular diseases (e.g., membranous nephropathy and systemic lupus erythematosus).8, 9, 10 The relationship between APOL1 risk alleles and Mendelian kidney diseases, in contrast, has been understudied.
Here, we analyze real-world data from a large, ethnically diverse cohort of over 15,000 patients undergoing commercial clinical genetic testing for Mendelian kidney disease in the United States. We characterize the estimated prevalence and distribution of Mendelian kidney diseases in this cohort, stratified by APOL1 genotype, with the hypothesis that Mendelian kidney diseases may contribute to kidney disease among persons with high-risk APOL1 genotypes.
Methods
This is a cross-sectional study of consented patients undergoing commercial genetic testing from April 1, 2020, to December 30, 2021. Patients were referred for genetic testing by their respective clinical providers. Demographic and clinical information collected at the time of testing includes age, race/ethnicity (either self-reported or designated by the clinical provider), sex (female or male), transplant status (yes/no), and International Classification of Diseases diagnostic codes. We received an exemption from institutional review board review (study ID 20099-03) from Ethical & Independent Review Services, Corte Madera, California. All data were deidentified to protect patient privacy. We excluded data from patients with missing information on age and/or sex. We also excluded persons undergoing clinical genetic testing in the setting of kidney donation.
Designated or Self-reported Race/Ethnicity
The following race and ethnicity categories were present on the intake form: African American, Ashkenazi Jewish, Caucasian, East Asian, French Canadian/Cajun, Hispanic, Mediterranean, Other, Sephardic Jewish, South Asian, and South-East Asian. To achieve a sufficiently large sample size for further analysis, certain race/ethnic categories, namely French Canadian/Cajun, Sephardic Jewish, and Ashkenazi Jewish, were combined with the category labeled as “Other” because of the limited number of individuals. We have opted to use “European American” in place of “Caucasian’ throughout this study to align with more accurate ethnic descriptors.11,12 The term “Multiple” refers to samples with >1 self-reported race and ethnicity category.
Sequencing
Genomic DNA was extracted from either the individual’s whole blood or saliva and processed for hybrid capture-based next-generation sequencing. Massively parallel sequencing was performed at 150 base-pairs, paired-end reads on a clinical exome backbone. The percentage of coverage was determined at a minimum of 20× per gene on the panel and Sanger sequencing was used to fill in regions of low coverage. The sequencing data were aligned to the GRCh37/hg19 genome assembly, and the variants were called and annotated using a bioinformatics pipeline that applied the Genome Analysis Toolkit framework.13 Single nucleotide variants, insertions and deletions, and copy number variants were detected using this assay. Orthogonal methods for confirmation of variants included Sanger sequencing for single nucleotide variants and insertions and deletions and quantitative polymerase chain reaction or multiplex-ligation dependent probe amplification for copy number variants.
Genetic Ancestry Determination
The population structure and admixture analyses were conducted using the continental ancestry groups from the combined, harmonized databases of the Human Genome Diversity Project and the 1000 Genomes Project 14 We included single nucleotide variants with a minor allele frequency >0.05 and missing in <5% of individuals within our dataset to determine ancestry. We retained samples for further analysis with an overall genotype rate >90% (i.e., missing <10% of selected single nucleotide variants). We performed principal component analysis using SNPW 8 version 210 and PLINK v1.9,15 and estimated global genomic ancestry proportions using Admixture v1.316 focusing on the following populations: African, admixed American, Central South Asia, East Asian, and European.
In additional analyses, we assigned a single ancestry group to each individual patient via a clustering method derived from the principal component analysis dimensions. We used the first 10 principal components and applied the Uniform Manifold Approximation and Projection algorithm, via the Umap package in R.17 The Uniform Manifold Approximation and Projection outputs were clustered using the Hierarchical Density-Based Spatial Clustering of Applications with Noise algorithm, via the dB scan R package.18 Five distinct clusters were identified, corresponding to inferred continental ancestries. These clusters aligned well with the predominant admixture proportions for their respective ancestry groups. We were also able to categorize samples with unknown self-designated or self-reported race/ethnicity into 1 of the 5 distinct ancestry clusters. Therefore, individuals with recent African ancestry were defined as those clustered within the African genetic cluster according to the first 10 principal components in the principal component analysis. This approach was similarly used to define the remaining ancestry groups.
Interpretation of Genetic Variants
Variants were analyzed in 343 genes associated with Mendelian forms of kidney disease (Supplementary Table S1), including the risk alleles associated with APOL1. Detected germline variants were classified using a 5-tier classification system (B, benign; LB, likely benign; VUS, variant of uncertain significance; LP, likely pathogenic; and P, pathogenic) in accordance with the American College of Medical Genetics and Genomics and the Association for Molecular Pathology guidelines at the time of testing.19 As part of routine clinical care, reports of identified variants and their relevant American College of Medical Genetics and Genomics and the Association for Molecular Pathology classifications were returned to the referring clinicians and patients at the time of testing.
Identifying Patients with Mendelian Kidney Diseases
Accurate diagnosis of Mendelian forms of kidney disease requires genotype-phenotype correlation based on variant classification information combined with the mode of inheritance for the disease. We classified individuals as having Mendelian kidney disease based on the presence of P/LP variants, meeting criteria informed by empirical data.19 We considered a test “positive” or consistent with a molecular diagnosis of Mendelian kidney disease if (i) the individual has a heterozygous or hemizygous P/LP variant in a gene associated with a dominant or X-linked inheritance pattern; or (ii) the patient has 2 P/LP variants (compound heterozygous or homozygous) in a gene with a recessive inheritance pattern. For genes with both dominant and recessive patterns of inheritance, we considered each variant’s specific phenotype, and the associated pattern of inheritance was considered. For HBB, only results consistent with autosomal recessive sickle cell anemia were included as “positive.” We considered the individuals harboring heterozygous P/LP variants in genes associated with an autosomal recessive inheritance as carriers. The remaining samples were considered negative.19
APOL1 Genotype Status Definition
We assessed 2 APOL1 high-risk alleles: (i) G1 (NM_003661.4:c.[1024A>G;1152T>G]) defined by the presence of 2 missense variants p.S342G (rs73885319) and p.I384M (rs60910145) that are nearly always in linkage disequilibrium; (ii) G2 (NM_003661.4:c.1164_1169del) defined by the presence of an in-frame deletion of 2 amino acid residues at codons 388 and 389, respectively (rs71785313). We defined APOL1 high-risk genotypes by the presence of 2 high-risk alleles (G1/G1, G1/G2, or G2/G2). We defined single risk APOL1 allele carriers by the presence of only 1 risk allele (G1/G0 or G2/G0). The lack of APOL1 risk alleles was denoted as (G0/G0). We deliberately considered single risk APOL1 allele carriers because, by definition, these patients have recent African ancestry.
Statistical Analysis
Continuous variables were summarized by means, medians, and interquartile ranges, where appropriate. Comparisons of continuous variables across groups were made via Wilcoxon rank sum test. We described categorical variables using proportions and compared groups using Fisher’s exact test. A P-value <0.05 was considered statistically significant. Analyses were conducted in R version 4.1.2.
Results
Prevalence of Mendelian Disease in the Overall Cohort
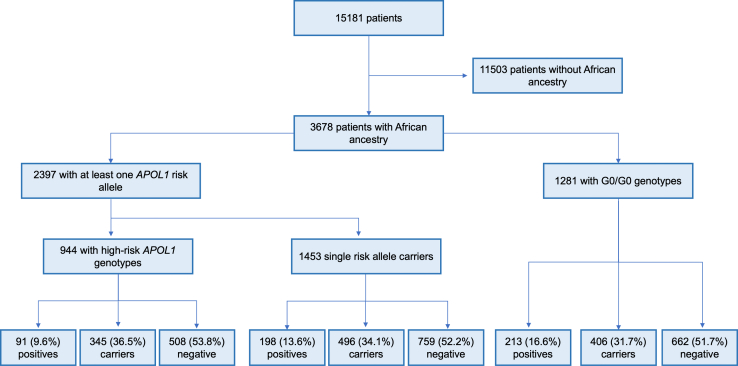
A total of 15,532 individuals underwent clinical genetic testing from April 1, 2020, to December 30, 2021, of whom 15,181 (97.7%) had sufficient data for further analysis (Figure 1). The median age at the time of testing was 47 years (25%, 75% range 30–62 years), with ∼50% of the patients identified as female (Table 1). Fewer than 3% (n = 492) had received a kidney transplant before the time of testing. Of 15,181 patients, 3119 had a molecular diagnosis of Mendelian kidney disease (20.5%; Table 1), defined by the presence of pathogenic or likely pathogenic variants (P/LP) consistent with the inheritance pattern of the specific disease. Of the total, 5049 (33.3%) patients were classified as carriers of Mendelian kidney disease, and 7013 (46.2%) were negative for P/LP variants in any of the 343 genes evaluated (Supplementary Table S1). Patients with Mendelian kidney disease were younger on average, at the time of testing, compared with carriers or patients with negative test results (40 vs. 48 years, P <0.0005; Table 1).
Figure 1.
Flowchart of patients included in this study. Positive samples refer to the number of patients with Mendelian kidney diseases. Carriers were defined as patients harboring heterozygous P/LP (pathogenic/likely pathogenic) variants in genes associated with a recessive inheritance pattern. The remaining samples were considered negative.
Table 1.
Clinical and demographic characteristics of patients, stratified by Mendelian kidney disease status in the overall cohort
| Category | Totala | Mendelian Diseaseb | Carriersb | Negativeb |
|---|---|---|---|---|
| N | 15181 (100) | 3119 (20.5) | 5049 (33.3) | 7013 (46.2) |
| Gender & Age | ||||
| Female | 7606 (50.1) | 1723 (22.7) | 2454 (32.3) | 3429 (45.1) |
| Male | 7575 (49.9) | 1396 (18.4) | 2595 (34.3) | 3584 (47.3) |
| Median age yr (IQR) | 47 (30–62) | 40 (23–56) | 49 (32–64) | 48 (31–63) |
| Self-reported race & ethnicity | ||||
| African American | 2960 (19.2) | 377 (12.7) | 1017 (34.4) | 1566 (52.9) |
| East Asian | 230 (1.5) | 62 (27) | 64 (27.8) | 104 (45.2) |
| European American | 5718 (37.1) | 1303 (22.8) | 2076 (36.3) | 2339 (40.9) |
| Hispanic | 1989 (12.9) | 437 (22) | 502 (25.2) | 1050 (52.8) |
| Mediterranean | 93 (0.6) | 16 (17.2) | 33 (35.5) | 44 (47.3) |
| Other | 570 (3.7) | 129 (22.6) | 185 (32.5) | 256 (44.9) |
| South-East Asian | 330 (2.1) | 76 (23) | 90 (27.3) | 164 (49.7) |
| Unknown | 3542 (23) | 775 (21.9) | 1171 (33.1) | 1596 (45.1) |
| Genetic cluster | ||||
| Admixed American | 2313 (15.2) | 501 (21.7) | 598 (25.9) | 1214 (52.5) |
| African | 3678 (24.2) | 502 (13.6) | 1247 (33.9) | 1929 (52.4) |
| Central-South Asian | 368 (2.4) | 79 (21.5) | 107 (29.1) | 182 (49.5) |
| East Asian | 769 (5.1) | 175 (22.8) | 208 (27) | 386 (50.2) |
| European | 7458 (49.1) | 1777 (23.8) | 2726 (36.6) | 2955 (39.6) |
| Unclustered samplesc | 595 (3.9) | 85 (14.3) | 163 (27.4) | 347 (58.3) |
| Clinical diagnosisd | ||||
| Chronic kidney disease | 8267 (49.1) | 1620 (19.6) | 2824 (34.2) | 3823 (46.2) |
| Cystic kidney disease | 1466 (8.7) | 658 (44.9) | 398 (27.1) | 410 (28) |
| End-stage kidney disease | 1616 (9.6) | 222 (13.7) | 524 (32.4) | 870 (53.8) |
| Hematuria | 584 (3.5) | 169 (28.9) | 165 (28.3) | 250 (42.8) |
| Hypertension | 467 (2.8) | 49 (10.5) | 147 (31.5) | 271 (58) |
| Kidney transplant | 432 (2.6) | 73 (16.9) | 138 (31.9) | 221 (51.2) |
| Proteinuria/nephrotic syndrome | 1024 (6.1) | 167 (16.3) | 354 (34.6) | 503 (49.1) |
| Others | 1554 (9.2) | 305 (19.6) | 518 (33.3) | 731 (47) |
| None available | 1431 (8.5) | 269 (18.8) | 506 (35.4) | 656 (45.8) |
IQR, interquartile range.
column-based proportions.
row-based proportions.
No. of Samples excluded during PCA filtering steps due to missing the minimum number of single-nucleotide variants after quality control.
Clinical diagnoses are based on ICD codes, summarized into the categories presented. The total count exceeds the number of unique patients due to the presence of multiple ICD codes in some patients.
Self-reported or Designated Race/Ethnicity and Genomic Ancestry in the Overall Cohort
Based on the self-reported or designated race/ethnicity of each patient, the majority of samples were collected from European Americans (37.1%, n = 5718), followed by African Americans (19.2%, n = 2960), and Hispanic persons (12.9%, n = 1989), whereas the remaining samples were collected from persons from other race/ethnicities (29.7%, n = 4514; Table 1). Two hundred forty-one (1.6%) individuals had a self-reported multiracial/multiethnic designation and 23% of patients in the overall cohort had unknown race/ethnicity information. We used a panel of ancestry informative markers from the clinical exome sequencing data to predict each patient’s genetic ancestry. We found that 49.1% (n = 7458) of patients were assigned to the European genetic cluster, 24.2% (n = 3678) to the African genetic cluster, 15.2% (n = 2313) to the Admixed American cluster, and 7.5% (n = 1137) to the Asian cluster. A total of 595 samples (3.9%) were excluded from this analysis because of quality control steps (Table 1). Admixture analysis revealed that the patients in the African cluster had a median inferred continental African ancestry of 70% (interquartile range: 63%–76%) (Supplementary Table S2). Approximately 74% (n = 2718) of individuals in the African ancestry cluster had a self-reported or designated race/ethnicity of (Supplementary Table S3). Overall, self-reported or designated race/ethnicity was highly concordant with genetic ancestry (Supplementary Figure S1 and Supplementary Tables S2–S4).
Prevalence of APOL1 Risk Alleles in the Overall Cohort
Of 15,181 individuals, 1035 (6.8%) had high-risk APOL1 genotypes (Figure 1). Homozygous haplotypes G1/G1 and G2/G2 accounted for 43.7% (n = 452) and 12.9% (n = 134) of patients, respectively; 449 patients with high-risk APOL1 genotypes had 1 G1 allele and 1 G2 allele (43.4%; G1/G2). Of the total, 1687 (11.1%) patients were single-risk APOL1 allele carriers, of which 1064 were with G1 (63.1%), and 623 with G2 (36.9%); 12459 patients were G0/G0 (82.1%).
The majority of patients with high-risk APOL1 genotypes had self-reported or designated African American race (815/1035; 78.9%; Supplementary Table S5). Single-risk allele carriers had a high proportion of self-reported African American race as well (1158/1687; 68.6%; Supplementary Table S5). As expected, individuals carrying APOL1 variants predominantly overlapped with the African ancestry cluster from reference continental population samples (Supplementary Figure S2). However, individuals with G0/G0 genotypes had a much lower proportion of self-reported African American race (987/12459; 7.9%), because this group also included samples from non-African clusters. When considering genetically predicted ancestry, 91.2% of patients with high-risk APOL1 genotypes (n = 944/1035), 86.1% of patients with single-risk alleles (n = 1453/1687), and 10.3% of patient with G0/G0 were assigned to the African genetic cluster (n = 1281/12459; Supplementary Figure S3 and Supplementary Table S5). Given that high-risk APOL1 genotypes only occur in a background of recent African ancestry, and non-African individuals are unlikely to carry APOL1 risk variants, we focused our analysis exclusively on the samples within the African ancestry cluster.
Prevalence of Mendelian Kidney Disease Stratified by APOL1 Genotype Among Patients with Recent African Ancestry
We subset patients from the overall cohort who belonged to the African genetic cluster for further analysis (n = 3678/15181; 24.2%, Table 1). Of 3678 patients with recent African ancestry, 502 had a diagnosis of Mendelian kidney disease (Table 2), a prevalence lower than in those with recent European ancestry (13.6% vs. 23.8%, P <0.00001; Supplementary Table S5). Of the total, 1247 (33.9%) of the patients with recent African ancestry were classified as carriers of Mendelian kidney disease, and 1929 (52.4%) were negative (Table 2).
Table 2.
Clinical and demographic characteristics among patients with recent African ancestry, stratified by Mendelian kidney disease status
| Categories | All |
High risk genotype |
Single risk allele carriers |
G0/G0 |
|---|---|---|---|---|
| 3678 | 944 | 1453 | 1281 | |
| Gender & age | ||||
| Female | 1776 (48.3) | 435 (46.1) | 691 (47.6) | 650 (50.7) |
| Male | 1902 (51.7) | 509 (53.9) | 762 (52.4) | 631 (49.3) |
| Age yr (IQR) | 46 (31–60) | 45 (32–57) | 47 (31–61) | 47 (27–61) |
| Mendelian status | ||||
| Affected | 502 (13.6) | 91 (9.6) | 198 (13.6) | 213 (16.6) |
| Carrier | 1247 (33.9) | 345 (36.5) | 496 (34.1) | 406 (31.7) |
| Negative | 1929 (52.4) | 508 (53.8) | 759 (52.2) | 662 (51.7) |
| Self-reported race & ethnicity | ||||
| African American | 2718 (73.9) | 746 (79) | 1093 (75.2) | 879 (68.6) |
| East Asian | 1 (0) | 0 (0) | 0 (0) | 1 (0.1) |
| European American | 51 (1.4) | 6 (0.6) | 13 (0.9) | 32 (2.5) |
| Hispanic | 115 (3.1) | 14 (1.5) | 27 (1.9) | 74 (5.8) |
| Mediterranean | 1 (0) | 0 (0) | 0 (0) | 1 (0.1) |
| Other | 107 (2.9) | 27 (2.9) | 34 (2.3) | 46 (3.6) |
| Unknown | 685 (18.6) | 151 (16) | 286 (19.7) | 248 (19.4) |
| Genetic admixture proportion | ||||
| AFR | 0.7 (0.63–0.76) | 0.72 (0.66–0.77) | 0.7 (0.63–0.76) | 0.69 (0.59–0.76) |
| AMR | 0.01 (0–0.06) | 0.02 (0–0.06) | 0.01 (0–0.05) | 0.02 (0–0.06) |
| CSA | 0 (0–0.04) | 0 (0–0.04) | 0 (0–0.04) | 0 (0–0.05) |
| EAS | 0.01 (0–0.05) | 0 (0–0.05) | 0.01 (0–0.05) | 0.01 (0–0.05) |
| EUR | 0.22 (0.15–0.29) | 0.2 (0.15–0.27) | 0.22 (0.15–0.29) | 0.23 (0.16–0.31) |
| Clinical diagnosisa | ||||
| Chronic kidney disease | 2136 | 528 (24.7) | 866 (40.5) | 742 (34.7) |
| End-stage kidney disease | 572 | 221 (38.6) | 203 (35.5) | 148 (25.9) |
| Proteinuria/nephrotic syndrome | 279 | 73 (26.2) | 111 (39.8) | 95 (34.1) |
| Cystic kidney disease | 233 | 33 (14.2) | 97 (41.6) | 103 (44.2) |
| Hypertension | 145 | 27 (18.6) | 57 (39.3) | 61 (42.1) |
| Hematuria | 85 | 16 (18.8) | 36 (42.4) | 33 (38.8) |
| Kidney transplant | 61 | 22 (36.1) | 20 (32.8) | 19 (31.1) |
| Others | 272 | 54 (19.9) | 95 (34.9) | 123 (45.2) |
| None available | 286 | 66 (23.1) | 119 (41.6) | 101 (35.3) |
AFR, African; AMR, Admixed American; CSA, Central/South Asian; EAS, East Asian; EUR, European.
Clinical diagnoses are based on ICD codes.
Considering APOL1 genotypes, 25.6% of patients in the genetic African ancestry cluster had high-risk APOL1 genotypes (n = 944), 39.5% were single risk allele carriers (n = 1453), and 34.8% had G0/G0 (n = 1281; Table 2 and Supplementary Figure S3). The prevalence of Mendelian kidney disease varied across APOL1 genotypes among patients in the African genetic cluster: 9.6% of patients with high-risk APOL1 genotypes had a concomitant diagnosis of Mendelian kidney disease compared with 13.6% of single risk allele carriers and 16.6% of patients with G0/G0 (Table 2; P <0.01 for single risk allele vs. high-risk, P <0.00001 for G0/G0 vs. high-risk). We observed a higher prevalence of high-risk APOL1 genotypes (n = 853/3176, 26.9%) among patients without Mendelian kidney disease when compared with patients with Mendelian kidney disease (n = 91/502; 18.1%, P <0.0012).
Patients with Mendelian kidney disease had similar ages at the time of testing across APOL1 genotypes (36–41 years) and tended to be younger than patients without Mendelian kidney disease (Table 3). We did not find evidence that patients with high-risk APOL1 genotypes and Mendelian kidney disease present for clinical genetic testing at younger ages than patients without high-risk APOL1 genotypes and Mendelian kidney disease (Table 3, P = 0.357 for single risk allele vs. high-risk; P = 0.409 for G0/G0 vs. high risk).
Table 3.
Demographic data of patients included, stratified by presence of APOL1 risk alleles among patients with recent genomic African ancestry
| Category | All patients | High-risk APOL1 genotype |
Single risk allele carriers |
G0/G0 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Positive | Carrier | Negative | All | Positive | Carrier | Negative | All | Positive | Carrier | Negative | ||
| N | 3678 | 944 (25.7) | 91 (9.6) | 345 (36.5) | 508 (53.8) | 1453 (39.5) | 198 (13.6) | 496 (34.1) | 759 (52.2) | 1281 (34.8) | 213 (16.6) | 406 (31.7) | 662 (51.7) |
| Gender & Age | |||||||||||||
| Female | 1776 | 435 (24.5) | 53 (12.2) | 145 (33.3) | 237 (54.5) | 691 (38.9) | 112 (16.2) | 223 (32.3) | 356 (51.5) | 650 (36.6) | 122 (18.8) | 194 (29.8) | 334 (51.4) |
| Male | 1902 | 509 (26.8) | 38 (7.5) | 200 (39.3) | 271 (53.2) | 762 (40.1) | 86 (11.3) | 273 (35.8) | 403 (52.9) | 631 (33.2) | 91 (14.4) | 212 (33.6) | 328 (52) |
| Median Age yr (IQR) | 46 (31–60) | 45 (32–57) | 37 (20–52) | 46 (35–57) | 46 (33–59) | 47 (31–61) | 41 (23–54) | 47 (31–63) | 48 (34–61) | 47 (27–61) | 36 (14–50) | 49 (34–64) | 49 (29–63) |
| Self-reported race & ethnicity | |||||||||||||
| African American | 2768 | 758 (27.4) | 71 (9.4) | 281 (37.1) | 406 (53.6) | 1112 (40.2) | 148 (13.3) | 386 (34.7) | 578 (52) | 898 (32.4) | 139 (15.5) | 291 (32.4) | 468 (52.1) |
| East Asian | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (100) | 0 | 0 | 1 (100) |
| European American | 69 | 9 (13) | 1 (11.1) | 3 (33.3) | 5 (55.6) | 21 (30.4) | 4 (19) | 6 (28.6) | 11 (52.4) | 39 (56.5) | 5 (12.8) | 8 (20.5) | 26 (66.7) |
| Hispanic | 137 | 18 (13.1) | 0 | 10 (55.6) | 8 (44.4) | 34 (24.8) | 9 (26.5) | 12 (35.3) | 13 (38.2) | 85 (62) | 19 (22.4) | 23 (27.1) | 43 (50.6) |
| Mediterranean | 2 | 0 | 0 | 0 | 0 | 1 (50) | 0 | 0 | 1 (100) | 1 (50) | 0 | 1 (100) | 0 |
| Other | 70 | 21 (30) | 2 (9.5) | 10 (47.6) | 9 (42.9) | 17 (24.3) | 2 (11.8) | 8 (47.1) | 7 (41.2) | 32 (45.7) | 4 (12.5) | 10 (31.2) | 18 (56.2) |
| South-East Asian | 2 | 0 | 0 | 0 | 0 | 1 (50) | 0 | 0 | 1 (100) | 1 (50) | 1 (100) | 0 | 0 |
| Unknown | 685 | 151 (22) | 18 (11.9) | 49 (32.5) | 84 (55.6) | 286 (41.8) | 38 (13.3) | 94 (32.9) | 154 (53.8) | 248 (36.2) | 47 (19) | 82 (33.1) | 119 (48) |
Distribution of Common Mendelian Kidney Diseases by APOL1 Genotype Among Patients with Recent African Ancestry
We grouped Mendelian forms of kidney disease into 5 nonexclusive categories: (i) cystic and tubulointerstitial diseases, (ii) glomerular diseases, (iii) tubulopathies and other tubular diseases, (iv) congenital anomalies of the kidney and urinary tract and other structural diseases, and (v) complement-related kidney diseases (Supplementary Table S1). Among patients with recent African ancestry and Mendelian kidney disease, 40.7% had cystic and tubulointerstitial diseases, 33.4% had glomerular diseases, 15.7% tubulopathies and other tubular diseases, 8.8% had congenital anomalies of the kidney and urinary tract and other structural diseases and 1.4% had complement-related diseases.
The prevalence of cystic and tubulointerstitial diseases was significantly different between patients with high-risk APOL1 genotypes (n = 29/944, 3.07%) and low-risk genotypes (n = 206/2734, 7.53%, including both single risk allele carriers and G0/G0, P <0.00001; Supplementary Table S6). In contrast, the prevalence of Mendelian glomerular diseases was higher in those with high-risk APOL1 genotypes (53.85%, n = 49/91) than those with low-risk genotypes (35.04%, n = 144/411, P <0.004; Supplementary Table S6).
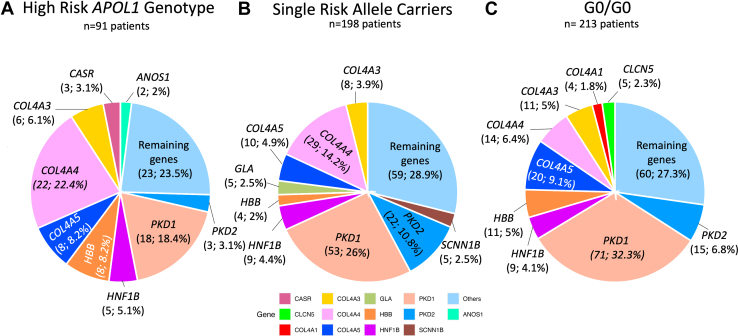
The differences in the prevalence of glomerular and cystic/tubulointerstitial diseases are predominantly driven by variants identified in COL4A4 and PKD1, respectively. We found that the prevalence of Alport syndrome/thin basement membrane disease because of P/LP variants in COL4A4 varied across APOL1 genotypes, comprising 24.2% of patients with Mendelian kidney and high-risk APOL1 genotypes and 10.5% of patients with low risk APOL1 genotypes (including both G0/G0 and single-risk allele carriers, P = 0.0009). Similarly, the prevalence of autosomal dominant polycystic kidney disease because of P/LP variants in PKD1 was 30.2% among patients with Mendelian kidney disease and low risk APOL1 genotypes, and 19.8% among patients with Mendelian kidney disease and high-risk APOL1 genotypes (P < 0.05). The most common forms of Mendelian kidney disease across the APOL1 genotypes are presented in Figure 2 and Supplementary Figure S4. The full list of Mendelian kidney diseases is presented in Supplementary Table S7.
Figure 2.
Most common genes affected among patients with recent African ancestry and Mendelian kidney diseases stratified by APOL1 genotype. Proportional representation (n, %) of most common genes in patients with recent African ancestry and (a) high-risk APOL1 genotypes, (b) single-risk allele carriers and (c) G0/G0 patients.
Discussion
Because the lifetime risk of developing kidney failure among persons with high-risk APOL1 genotypes is ∼15%, there is a need to understand how other genetic and nongenetic modifiers contribute to the risk of kidney disease and kidney disease progression.20 Here, we studied Mendelian kidney disease in individuals with high-risk APOL1 genotypes using genomic data derived from a multiethnic cohort of >15,000 patients who underwent commercial clinical genetic testing for Mendelian kidney disease in the United States.
We found that 9.6% of patients with high-risk APOL1 genotypes who underwent genetic testing in this cohort had concurrent Mendelian kidney diseases. To our knowledge, only 1 single-center study (n = 239 patients with high-risk APOL1 genotypes) has explored the prevalence of Mendelian kidney disease among patients with chronic kidney disease and APOL1 high-risk genotypes (excluding patients with congenital anomalies of the kidney and urinary tract and polycystic kidney disease),20 demonstrating that 2.5% of these patients had concurrent Mendelian kidney diseases. In contrast, our cohort of patients undergoing commercial clinical genetic testing had a much higher prevalence, likely explained by different patient selection in our highly selected cohort of patients undergoing clinical genetic testing. Nonetheless, our data highlights the potential contribution of Mendelian forms of kidney disease in patients with high-risk APOL1 genotypes. The majority of patients with high-risk APOL1 genotypes and concurrent Mendelian kidney diseases in our cohort had glomerular diseases (53.85%), dominated by variants in Alport syndrome genes: COL4A3, COL4A4 and COL4A5. Because patients with APOL1-mediated kidney disease and Alport syndrome can present with overlapping clinical (e.g., hypertension and proteinuria) and histologic findings on kidney biopsy (e.g., focal segmental glomerulosclerosis), this finding may support consideration of broader diagnostic genetic testing in a subset of patients with high-risk APOL1 genotypes.
Our analysis also demonstrated that patients with recent African ancestry had lower prevalence of Mendelian kidney disease than patients with more remote African Ancestry or patients without African ancestry. This finding aligns with existing research indicating a generally lower diagnostic yield for genetic diseases in African American patients with kidney disease.21, 22, 23 Such discrepancies might be attributed to the underrepresentation of African Americans in genomic sequencing studies.23, 24, 25 This work highlights the potential need for more equitable genetic disease identification across racial and ethnic groups, including African Americans, a critical area for future research.
We found differences in the distribution of Mendelian kidney diseases among patients with recent African ancestry and different APOL1 genotypes. Specifically, we found that P/LP variants in PKD1 were less frequent, and P/LP variants in COL4A4 were more frequent, among patients with high-risk APOL1 genotypes compared to single risk allele carriers or those with G0/G0. These findings are interpreted with caution due to the relatively small number of patients evaluated (e.g., 91 individuals with Mendelian kidney disease and high-risk APOL1 genotypes), and the highly selected nature of the cohort. Future studies are warranted to evaluate how the type of Mendelian kidney disease may vary with APOL1 genotype status.
Several limitations in this study should be acknowledged. First, our cohort is composed of a highly selected population of patients undergoing clinical genetic testing for Mendelian kidney disease; we had limited clinical information, and we can only infer (but not document) that these patients had kidney disease and that a genetic cause was contemplated. As such, we should be cautious in extrapolating these findings to unrestricted populations of patients with chronic kidney disease. The lack of clinical information prevents genotype-phenotype correlation because we have no information on any patient’s phenotype. Despite this limitation, we note that variants classified as P/LP meet stringent criteria, and in a patient population selected for clinical genetic testing, the likelihood that such variants contribute causally to a patient’s kidney disease would be high. Second, our analysis focused on a set of 343 genes known to be associated with kidney disease, which, although extensive, may not encompass all possible genetic variants contributing to kidney disease. We also cannot rule out Mendelian kidney disease in negative cases because there may be clinically significant variants within the 343 genes that have either not been captured by the assay or not classified as P/LP based on the variant classification guidelines, which are subject to updates over time.
Overall, our data suggest that Mendelian kidney disease may be an important contributor in a fraction of patients with high-risk APOL1 genotypes. Future research to confirm these findings in diverse, unselected cohorts, and to further elucidate the interaction between Mendelian kidney disease and APOL1 risk alleles is needed.
Disclosure
SP, LV, NS, and DKK are full time employees of Natera, Inc. GMC serves on the Steering Committee of the AMPLITUDE trial, sponsored by Vertex. All the authors declared no competing interests.
Acknowledgments
VC is supported by KL2 TR003143. VC takes full responsibility for the work, including the study design, access to data, and the decision to submit and publish the manuscript.
Authors Contributions
RSFJ developed the research idea and study design, did the data analysis and interpretation, as well as statistical analysis. SP developed theresearch idea and study design, data analysis and interpretation. LV developed the research idea and study design, data analysis and interpretation. NS developed research idea and study design, data analysis and interpretation. VB worked on data interpretation. GMC worked on data interpretation. DKK developed the research idea and study design, data analysis and interpretation. VC developed the research idea and study design, data analysis and interpretation, statistical analysis. Each author contributed important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual’s own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even one in which the author was not directly involved, are appropriately investigated and resolved, including with documentation in the literature if appropriate.
Footnotes
Figure S1. Comparison of self-reported or designated race/ethnicity with genomic ancestry derived from HGDP and 1KG.
Figure S2. Population Stratification of individuals harboring APOL1 risk alleles.
Figure S3. Flowchart of patients with recent African ancestry included in this study.
Figure S4. Most common genes among all patients with Mendelian kidney diseases stratified by APOL1 genotype included in this study.
Table S1. Comprehensive list of 343 genes implicated in Mendelian kidney diseases.
Table S2. Distribution of global ancestry proportions across five continental populations in genetic clusters from principal component analysis.
Table S3. Comparative analysis of self-reported or designated race/ethnicity and genetic clusters derived from principal component analysis.
Table S4. Global ancestry proportions in five continental populations based on self-reported or designated race/ethnicity.
Table S5. Demographic data of patients stratified by presence of APOL1 risk alleles in the overall cohort.
Table S6. Prevalence and association between APOL1 high-risk genotype and APOL1 low-risk genotypes among the Mendelian kidney disease categories in patients with recent African ancestry.
Table S7. Frequency of Mendelian kidney disease stratified by APOL1 genotype in the overall cohort.
Supplementary Material
Figure S1. Comparison of self-reported or designated race/ethnicity with genomic ancestry derived from HGDP and 1KG. Figure S2. Population Stratification of individuals harboring APOL1 risk alleles. Figure S3. Flowchart of patients with recent African ancestry included in this study. Figure S4. Most common genes among all patients with Mendelian kidney diseases stratified by APOL1 genotype included in this study. Table S1. Comprehensive list of 343 genes implicated in Mendelian kidney diseases. Table S2. Distribution of global ancestry proportions across five continental populations in genetic clusters from principal component analysis. Table S3. Comparative analysis of self-reported or designated race/ethnicity and genetic clusters derived from principal component analysis. Table S4. Global ancestry proportions in five continental populations based on self-reported or designated race/ethnicity. Table S5. Demographic data of patients stratified by presence of APOL1 risk alleles in the overall cohort. Table S6. Prevalence and association between APOL1 high-risk genotype and APOL1 low-risk genotypes among the Mendelian kidney disease categories in patients with recent African ancestry. Table S7. Frequency of Mendelian kidney disease stratified by APOL1 genotype in the overall cohort.
References
- 1.Dummer P.D., Limou S., Rosenberg A.Z., et al. APOL1 kidney disease risk variants: an evolving landscape. Semin Nephrol. 2015;35:222–236. doi: 10.1016/j.semnephrol.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foster M.C., Coresh J., Fornage M., et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24:1484–1491. doi: 10.1681/ASN.2013010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Genovese G., Friedman D.J., Ross M.D., et al. Association of trypanolytic ApoL1 variants with kidney disease in African-Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman D.J. A brief history of APOL1: a gene evolving. Semin Nephrol. 2017;37:508–513. doi: 10.1016/j.semnephrol.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Friedman D.J., Pollak M.R. APOL1 and kidney disease: from genetics to biology. Annu Rev Physiol. 2020;82:323–342. doi: 10.1146/annurev-physiol-021119-034345. [DOI] [PubMed] [Google Scholar]
- 6.Groopman E.E., Marasa M., Cameron-Christie S., et al. Diagnostic utility of exome sequencing for kidney disease. N Engl J Med. 2019;380:142–151. doi: 10.1056/NEJMoa1806891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cocchi E., Nestor J.G., Gharavi A.G. Clinical genetic screening in adult patients with kidney disease. Clin J Am Soc Nephrol. 2020;15:1497–1510. doi: 10.2215/CJN.15141219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsen C.P., Beggs M.L., Saeed M., Walker P.D. Apolipoprotein L1 risk variants associate with systemic lupus erythematosus-associated collapsing glomerulopathy. J Am Soc Nephrol. 2013;24:722–725. doi: 10.1681/ASN.2012121180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blazer A., Dey I.D., Nwaukoni J., et al. Apolipoprotein L1 risk genotypes in Ghanaian patients with systemic lupus erythematosus: a prospective cohort study. Lupus Sci Med. 2021;8 doi: 10.1136/lupus-2020-000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C.Y., Pollack S., Hunter D.J., Hirschhorn J.N., Kraft P., Price A.L. Improved ancestry inference using weights from external reference panels. Bioinformatics. 2013;29:1399–1406. doi: 10.1093/bioinformatics/btt144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flanagin A., Frey T., Christiansen S.L., Bauchner H. The reporting of race and ethnicity in medical and science journals: comments invited. JAMA. 2021;325:1049–1052. doi: 10.1001/jama.2021.2104. [DOI] [PubMed] [Google Scholar]
- 12.Bhopal R., Donaldson L. White, European, Western, Caucasian, or what? Inappropriate labeling in research on race, ethnicity, and health. Am J Public Health. 1998;88:1303–1307. doi: 10.2105/AJPH.88.9.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKenna A., Hanna M., Banks E., et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koenig Z., Yohannes M.T., Lethukuthula L., et al. A harmonized public resource of deeply sequenced diverse human genomes. https://doi.org/10.1101/2023.01.23.525248 bioRxiv. 2023.01.23.525248. [DOI] [PMC free article] [PubMed]
- 15.Purcell S., Neale B., Todd-Brown K., et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexander D.H., Novembre J., Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19:1655–1664. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McInnes L., Healy J., Melville J. Published online September 17, 2020. UMAP: Uniform manifold approximation and projection for dimension reduction. [DOI] [Google Scholar]
- 18.Hahsler M., Piekenbrock M., Doran D. dbscan: Fast density-based clustering with R. Journal of Statistical Software. 2019;91:1–30. doi: 10.18637/jss.v091.i01. [DOI] [Google Scholar]
- 19.Richards S., Aziz N., Bale S., et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elliott M.D., Marasa M., Cocchi E., et al. Clinical and genetic characteristics of CKD patients with high-risk APOL1 genotypes. J Am Soc Nephrol. 2023;34:909–919. doi: 10.1681/ASN.0000000000000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abul-Husn N.S., Marathe P.N., Kelly N.R., et al. Molecular diagnostic yield of genome sequencing versus targeted gene panel testing in racially and ethnically diverse pediatric patients. Genetics in Medicine. 2023 doi: 10.1101/2023.03.18.23286992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrovski S., Goldstein D.B. Unequal representation of genetic variation across ancestry groups creates healthcare inequality in the application of precision medicine. Genome Biol. 2016;17:157. doi: 10.1186/s13059-016-1016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gudmundsson S., Singer-Berk M., Watts N.A., et al. Variant interpretation using population databases: lessons from gnomAD. Hum Mutat. 2022;43:1012–1030. doi: 10.1002/humu.24309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mavura Y., Sahin-Hodoglugil N., Hodoglugil U., et al. Genetic ancestry and diagnostic yield of exome sequencing in a diverse population. NPJ Genom Med. 2024;9:1. doi: 10.1038/s41525-023-00385-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Florentine M.M., Rouse S.L., Stephans J., et al. Racial and ethnic disparities in diagnostic efficacy of comprehensive genetic testing for sensorineural hearing loss. Hum Genet. 2022;141:495–504. doi: 10.1007/s00439-021-02338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Comparison of self-reported or designated race/ethnicity with genomic ancestry derived from HGDP and 1KG. Figure S2. Population Stratification of individuals harboring APOL1 risk alleles. Figure S3. Flowchart of patients with recent African ancestry included in this study. Figure S4. Most common genes among all patients with Mendelian kidney diseases stratified by APOL1 genotype included in this study. Table S1. Comprehensive list of 343 genes implicated in Mendelian kidney diseases. Table S2. Distribution of global ancestry proportions across five continental populations in genetic clusters from principal component analysis. Table S3. Comparative analysis of self-reported or designated race/ethnicity and genetic clusters derived from principal component analysis. Table S4. Global ancestry proportions in five continental populations based on self-reported or designated race/ethnicity. Table S5. Demographic data of patients stratified by presence of APOL1 risk alleles in the overall cohort. Table S6. Prevalence and association between APOL1 high-risk genotype and APOL1 low-risk genotypes among the Mendelian kidney disease categories in patients with recent African ancestry. Table S7. Frequency of Mendelian kidney disease stratified by APOL1 genotype in the overall cohort.