Abstract
Background
There is no clear consensus regarding the optimal risk stratification of high-degree atrioventricular block (HDAVB) after transcatheter aortic valve replacement (TAVR).
Methods
This prospective study sought to determine the utility of the pre- and post-TAVR His-ventricular (HV) interval in the risk stratification of post-TAVR HDAVB. One hundred twenty-one patients underwent an electrophysiology study before and after TAVR. The primary outcome was HDAVB requiring pacemaker implantation within 30 days post-TAVR. A separate retrospective cohort was analyzed to determine the postoperative interval at which the risk of HDAVB is reduced to <5%.
Results
HDAVB occurred in 12 (10%) patients. Baseline right bundle branch block (RBBB) (odds ratio [OR]: 13.6), implant depth >4 mm (OR: 3.9), use of mechanically- or self-expanding valves (OR: 6.3), and post-TAVR HV > 65 ms (OR: 4.9) were associated with increased risk of HDAVB, whereas PR intervals and pre-TAVR HV were not. In patients without baseline RBBB or new persistent left bundle branch block (LBBB), not one patient with post-TAVR HV < 65 ms developed HDAVB. In the separate retrospective cohort (N = 1049), the risk of HDAVB is reduced (<5%) on postoperative days 4 and 3 in patients with pre-TAVR RBBB and post-TAVR persistent LBBB, respectively.
Conclusions
Baseline RBBB, new persistent LBBB, implant depth >4 mm, and a post-TAVR HV ≥ 65 ms were associated with a higher risk of post-TAVR HDAVB, whereas an HV ≤ 65 ms was associated with a lower risk. The pre-TAVR HV was not associated with our outcome, and the delta HV did not have practical incremental prognostic value. Among those without pre-TAVR RBBB or post-TAVR persistent LBBB, no patients with post-TAVR HV < 65 ms developed HDAVB.
Keywords: Electrophysiology study, High degree AV block (HDAVB), H-V interval, Pacemaker, Transcatheter aortic valve replacement (TAVR)
Highlights
-
•
Baseline right bundle branch block (RBBB), new persistent left bundle branch block (LBBB), implant depth >4 mm, and a post-transcatheter aortic valve replacement (TAVR) His-ventricular (HV) interval ≥65 ms were associated with a higher risk of post-TAVR high-degree atrioventricular block (HDAVB).
-
•
In patients with a new LBBB, an HV interval ≤65 ms measured immediately post-TAVR was associated with a low risk of HDAVB and LBBB recovery.
-
•
Patients without a baseline pre-TAVR RBBB or new persistent post-TAVR LBBB are considered low-risk. An HV ≤65 ms completely ruled out the incidence of HDAVB.
-
•
The pre-TAVR HV interval was not associated with our outcome.
-
•
This prospective study, alongside our retrospective study, suggests that a longer postoperative monitoring period may be required for patients with baseline pre-TAVR RBBB (postoperative day 4) and new post-TAVR persistent LBBB (postoperative day 3).
-
•
Patients receiving valve-in-valve implants are at low risk. Moreover, patients with self-expanding valves are at a higher risk of HDAVB at the time of valve deployment. The risk was zero in the postoperative course.
Background
Transcatheter aortic valve replacement (TAVR) has become one of the most performed structural heart disease procedures.1,2 The number of TAVR procedures is only expected to grow with the expansion of its indications in treating low-risk and younger patients with severe aortic valve stenosis. Atrioventricular (AV) block is a common complication of TAVR1, 2, 3 due to the proximity of the His bundle to the valve deployment area.3, 4, 5 The reported incidence of high-degree atrioventricular block (HDAVB) after TAVR varies widely, from 7 to 28%,1,2,6, 7, 8, 9 and this complication is associated with worse outcomes.2,6 The timing of HDAVB development is neither uniform nor well-predicted,1,2,8,10 which makes postprocedure patient care challenging and may lead to morbidity and mortality for HDAVB on one side or unnecessary preemptive pacemaker implantations on the other. Pacemaker implantation after TAVR is associated with worse outcomes, including increased mortality and prolonged hospital stays.6
Currently, while several predictive algorithms have been proposed to assess the risk of HDAVB after TAVR,6,7,9 many of these prediction models were performed retrospectively and are not conclusive. Notably, the role of the electrophysiology (EP) study as part of HDAVB prediction algorithms remains unclear,3,11, 12, 13, 14, 15, 16, 17 and better predictive tools are needed to identify patients who may be at high risk of developing HDAVB after TAVR. Our study sought to prospectively evaluate the utility of preoperative and postoperative EP studies in risk stratification of postoperative HDAVB after TAVR.
Materials and Methods
This was a prospective single-center cohort study. We recruited adult patients with severe aortic stenosis who had elective TAVR procedures at Henry Ford Hospital in Detroit, Michigan, from January 2020 to January 2022. Patients were excluded from the study if they had an indication for pacemaker or defibrillator implantation or if both pre- and post-TAVR EP studies were not performed. Clinical data elements, including patient comorbid conditions, laboratory test results, echocardiography, cardiac computed tomography imaging, electrocardiography (ECG) results, and procedural data were collected. The study was approved by the Henry Ford Health Institutional Review Board. All patients provided written informed consent.
TAVR was performed via a transfemoral approach using a commercially available device (balloon expandable valve [Sapien], mechanically-expanding valve [Lotus], and self-expanding valve [Evolut]). The EP studies were performed in the TAVR catheterization labs. This was performed immediately before the commencement of and then after the TAVR procedure prior to extubation. An electrophysiologist performed the EP studies, made the measurements in real-time, and was thereafter blinded to the outcomes. The EP studies were performed using a portable GE Cardiolab 64-channel with GE Cardiolab amplifier and Micropace ORLab Cardiac Stimulator 320. A St. Jude Supreme 5-5-5 Crd-1 or Crd-2 were used His-ventricular (HV) interval measurement from the right ventricle (RV). The most proximal sharp His signal was identified using the largest A signal on the His bundle electrogram.18 The HV measurement was measured from the most proximal His signal to the onset of ventricular activation. An HV interval cut-off ≥65 ms was used as previously described.14,15
The pre- and post-TAVR EP studies refer to those tests performed immediately before and after every TAVR procedure. The His catheter was then used for RV pacing backup as well as rapid ventricular pacing for cardiac stabilization prior to valve deployment. A repeat EP study was offered to all patients 24-48 hours after TAVR for further risk stratification. All patients were given a 30-day monitor at discharge and had an in-person office visit with a 12-lead ECG at 30 days.
Left bundle branch block (LBBB) and right bundle branch block (RBBB) were defined according to standard criteria established by the American Heart Association, American College of Cardiology, and Heart Rhythm Society.19 LBBB was considered persistent if it persisted 24 hours after the TAVR implant. The primary outcome was HDAVB requiring pacemaker implantation within 30 days of TAVR surgery. HDAVB was defined as any of the following:
-
•
Complete or transient third-degree atrioventricular block (AV block).
-
•
Second-degree AV block type 2 (Mobitz II) in the presence of a QRS ≥120 ms.
-
•
A 2:1 AV block in the presence of a QRS 120 ms with ≥2 consecutive P waves at a constant physiologic rate did not conduct to the ventricles.
-
•
Transient third-degree AV block.
-
•
In the setting of atrial fibrillation, a prolonged pause (>3 s) with concomitant fixed slow (<50 beats/min) ventricular response rate.
-
•
A ≥5 second pause is not associated with increased vagal tone.
-
•
New symptoms associated with worsening post-TAVR conduction disease as assessed by an electrophysiologist.
Safety
Overall, the addition of these EP studies proved to be safe. There were no EP study-related deaths. There were four patients who developed atrial fibrillation during an EP study done during TAVR. These patients were all cardioverted to normal sinus rhythm without complications. One additional patient developed atrial fibrillation during an EP study done 24 hours after TAVR; this patient spontaneously converted to normal sinus rhythm without complication.
Separate Retrospective Cohort for Time to HDAVB
A separate retrospective cohort of patients who underwent TAVR at Henry Ford Hospital between January 2015 and December 2019 was analyzed to determine the post-TAVR time interval when the risk of HDAVB decreases, particularly for individuals with pre-TAVR RBBB and post-TAVR new persistent LBBB. Cumulative risk was compared among different groups during the postoperative period. A risk threshold of less than 5% was considered acceptable for discharge in the proposed risk stratification algorithm.
Statistical Analysis
Data were collected using REDCap (Research Electronic Data Capture) and analyzed using SPSS statistics software (IBM, Armonk, NY) and R (R Core Team, 2022). Descriptive statistics were obtained for all study variables. Continuous variables were described with a median and an interquartile range (IQR), given that the data were not normally distributed. Categorical data were described with frequency and percentage. Categorical variables were compared with chi-square or Fisher's exact tests based on cell count. Independent 2-group t tests or Wilcoxon rank sum tests based on normality were used for continuous variables. Univariate logistic regression models were used to obtain crude odds ratios (ORs) and 95% confidence intervals (CIs).
Results
Baseline and Post-TAVR Clinical Characteristics
A total of 124 patients were initially enrolled in the study; however, three patients were excluded because EP studies could not be performed before or after the TAVR procedure for logistical reasons. All included patients underwent both pre- and post-TAVR EP studies, except for two individuals who had either a pre-TAVR or a post-TAVR EP study performed.
Of the 121 patients, 56 (46%) were women, 65 (54%) were men, and the median age was 79 years (IQR 73-85). The patients had a significant burden of comorbidities and risk factors associated with cardiovascular disease, including hypertension (n = 101; 84%), diabetes mellitus (n = 42; 35%), atrial fibrillation (n = 42; 35%), coronary artery disease (n = 58; 48%), and cerebrovascular disease (n = 8; 7%). The median left ventricular ejection fraction was 60% (IQR 53-68), and the median annular area was 469 mm2 (IQR 398-540) (Table 1).
Table 1.
Comparison of baseline pre-TAVR and postprocedural characteristics of patients with and without HDAVB
| Variable | All patients N = 121 |
No HDAVB n = 109 |
HDAVB n = 12 |
p value |
|---|---|---|---|---|
| Age, y | 79 (73-85) | 79 (73-85) | 76 (70-81) | 0.33 |
| Male | 65 (54%) | 60 (55%) | 5 (42%) | 0.38 |
| BMI, kg/m2 | 29 (25-32) | 28 (29-32) | 30 (27-36) | 0.35 |
| Comorbidities | ||||
| Atrial fibrillation/flutter | 42 (35%) | 39 (36%) | 3 (25%) | 0.46 |
| Cerebrovascular disease | 8 (7%) | 7 (6%) | 1 (8%) | 0.80 |
| Coronary artery disease | 58 (48%) | 56 (51%) | 2 (17%) | 0.02 |
| Diabetes mellitus | 42 (35%) | 38 (35%) | 4 (33%) | 0.92 |
| Hypertension | 101 (8%) | 94 (86%) | 7 (58%) | 0.01 |
| STS score | 17 (12-25) | 17 (13-25) | 11 (9-20) | 0.23 |
| Imaging | ||||
| LVEF, % | 60 (53-68) | 60 (53-68) | 59 (55-66) | 0.98 |
| LVEF <50% | 26 (22%) | 24 (22%) | 2 (17%) | 0.67 |
| LVOT area, mm2 | 469 (368-559) | 447 (374-559) | 475 (362-559) | 0.84 |
| Annular area, mm2 | 469 (398-540) | 462 (398-540) | 469 (394-581) | 0.68 |
| EP Pre-OP ECG/EP studies | ||||
| Rhythm | ||||
| Sinus | 100 (83%) | 89 (82%) | 11 (92%) | 0.39 |
| Atrial fibrillation | 21 (17%) | 20 (18%) | 1 (8%) | |
| PR, ms | 189 (168-215) | 190 (168-213) | 185 (164-223) | 0.89 |
| PRE PRS ≥220 ms | 25 (25%) | 21 (23%) | 4 (36%) | 0.35 |
| QRS duration, ms | 106 (91-129) | 106 (91-120) | 136 (84-150) | 0.07 |
| Right bundle branch block | 22 (18%) | 14 (13%) | 8 (67%) | <0.01 |
| Left bundle branch block | 9 (7%) | 9 (8%) | 0 (0%) | 0.30 |
| AH interval, ms | 101 (80-123) | 100 (80-121) | 101 (87-160) | 0.53 |
| HV interval, ms | 52 (45-58) | 52 (45-59) | 50 (46-54) | 0.44 |
| Pre-TAVR EP studies HV ≥65 ms | 10 (8%) | 10 (9%) | 0 (0%) | 0.27 |
| TAVR | ||||
| Implant depth, mm | 4 (4-6) | 4 (4-6) | 6 (4-8) | 0.02 |
| Implant depth ≤4mm | 85 (70%) | 80 (73%) | 5 (42%) | 0.03 |
| Implant depth >4 mm | 36 (30%) | 29 (26%) | 7 (59%) | |
| Percent over/undersized | 5 (1-11) | 5 (1-10) | 8 (−1-20) | 0.09 |
| Type of valve | ||||
| Balloon expandable valve (Sapien) | 109 (90%) | 101 (93%) | 8 (67%) | <0.01∗ |
| Mechanically-expanding valve (Lotus) | 2 (2%) | 1 (1%) | 1 (8%) | |
| Self-expanding valve (Evolut) | 10 (8%) | 7 (6%) | 3 (25%) | |
| Valve-in-valve replacement | 9 (7%) | 9 (8%) | 0 (0%) | 0.30 |
Abbreviations: BMI, body mass index; ECG, electrocardiogram; EP, electrophysiology; HDAVB, high-degree atrioventricular block; HV, His-ventricular; LVEF, left ventricular ejection fraction; LVOT, left ventricular outflow tract; STS, Society of Thoracic Surgeons score; TAVR, transcatheter aortic valve replacement.
Balloon expandable valve (Sapien) as a reference.
The median heart rate was 73 bpm (IQR: 60-80). The majority of patients (N = 100, 83%) were in sinus rhythm, while the remaining 21 (17%) were in atrial fibrillation. The baseline PR interval was 189 ms (IQR: 168-215). Of the total patient population, 22 (18%) had pre-existing RBBB, and 9 (7%) patients had pre-existing LBBB. The median pre-TAVR HV interval was 52 ms (IQR: 45-58), and of note, 10 (8%) patients had a baseline HV interval ≥65 ms.
The patients in this study received commercially available devices, including the Sapien balloon expandable (n = 109; 90%), the Evolut self-expanding (n = 10; 8%), and the Lotus mechanically expanding (n = 2; 2%) valves. The median annular area was 469 mm2 (IQR: 398-540), and the median percent oversized was more than +5% (IQR: +1 to 11%). The median implant depth was 4 mm (IQR: 4-6). Among the entire patient cohort, nine had a previous valve and underwent a valve-in-valve TAVR procedure.
Outcomes
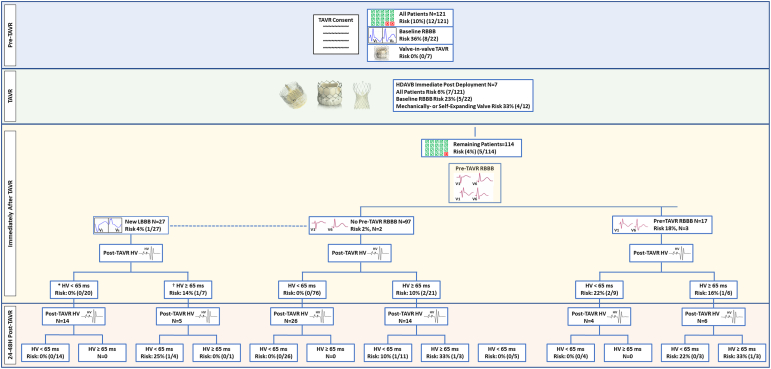
Figure 1 depicts the flow chart of our prospective study, illustrating risks at different time points and based on the risk stratification variables employed throughout our study.
Figure 1.
Study flow chart and risk stratification before and after TAVR. ∗18/20 had new transient LBBB, and 2/20 had new persistent LBBB after TAVR. † All 7/7 had new persistent LBBB after TAVR.
Abbreviations: HDAVB, high-degree atrioventricular block; HV, His-ventricular; LBBB, left bundle branch block; RBBB, right bundle branch block; TAVR, transcatheter aortic valve replacement.
A total of 12 (10%) patients developed HDAVB: seven occurring immediately after valve deployment, four in the delayed postoperative period, and 1 after discharge. The post-TAVR median PR interval was 198 ms (IQR: 178-226) and HV interval was 58 ms (IQR: 48-64). Additionally, 27 patients (22%) had new LBBB. All patients were offered and provided a 30-day ambulatory (amb) monitor; however, only 61 used the device for the follow-up period. All patients presented for a 30-day clinical follow-up examination and had a 12-lead ECG performed.
Analysis of Pre-TAVR Clinical Characteristics as Predictors of Post-TAVR HDAVB
Univariate analysis showed that pre-TAVR RBBB (OR: 13.57; 95% CI: 3.61-51.05; p < 0.001), implant depth >4 mm (OR: 3.86, 95% CI: 1.14-13.13, p = 0.023), and the use of mechanically- or self-expanding valves (compared to balloon expandable valves) (OR: 6.31; 95% CI: 1.56-25.59; p = 0.004) were associated with an increased risk of HDAVB. In contrast, hypertension (OR: 0.22; 95% CI: 0.06-0.80; p = 0.014) and coronary artery disease (OR: 0.19; 95% CI: 0.04-0.90; p = 0.022) were associated with a decreased risk of HDAVB. The pre-TAVR baseline HV interval, presence of baseline LBBB, and PR interval were not significantly associated with postprocedural HDAVB (Table 1).
Analysis of Post-TAVR Characteristics as Predictors of HDAVB
Patients who experienced persistent complete heart block immediately after valve deployment were excluded from this analysis because they did not undergo a post-TAVR EP study. Of the remaining patients, those who had HDAVB had a longer median post-TAVR HV interval than those without HDAVB (80 [IQR: 62-130] vs. 58 [IQR: 48-63] ms; p < 0.01). A post-TAVR HV interval ≥65 ms was associated with higher odds of HDAVB (OR: 4.9; 95% CI 0.79-31.49; p = 0.062). Assessment of the change in HV interval (delta HV = post-TAVR HV interval minus pre-TAVR HV interval) showed that patients with HDABV had a larger median delta HV (26 [IQR: 12-76] vs. 3 [IQR: 0-7]; p < 0.001), and a delta HV ≥ 15 ms was associated with higher odds of HDAVB (OR: 9.20; 95% CI: 1.42-59.73; p = 0.006) (Table 2).
Table 2.
Post-TAVR electrocardiographic and electrophysiology study data for all patients, excluding those with complete heart block immediately after TAVR
| Variable | All patients N = 114 |
No HDAVB n = 109 |
HDAVB n = 5 |
p value |
|---|---|---|---|---|
| Post-TAVR PR, ms | 198 (178-226) | 198 (178-225) | 234 (170-312) | 0.13 |
| Post-TAVR QRS, ms | 123 (106-147) | 120 (104-148) | 138 (134-140) | 0.38 |
| Post-TAVR AH interval, ms | 103 (82-127) | 102 (82-127) | 140 (73-189) | 0.30 |
| Post-TAVR HV interval, ms | 58 (48-64) | 58 (48-63) | 80 (62-130) | <0.01 |
| Post-TAVR HV <65 ms | 85 (75%) | 83 (77%) | 2/5 (40%) | 0.06 |
| Post-TAVR HV ≥65 ms | 28 (25%) | 25 (23%) | 3/5 (60%) | |
| Post-TAVR new LBBB | 27/114 (24%) | 26/109 (24%) | 1/5 (20%) | 0.84 |
| Post-TAVR new persistent LBBB | 9/114 (8%) | 3 (7%) | 1 (20%) | 0.31 |
| Prolonged PR interval | 25/91 (28%) | 23/87 (26%) | 2/4 (50%) | 0.30 |
| Delta PR, ms | 7 (−4 to 22) | 7 (−4 to 21) | 29 (−5 to 79) | 0.02 |
| Delta PR ≥20 ms | 21 (25%) | 19 (23%) | 50 (65%) | 0.24 |
| Delta QRS, ms | −20 (26%) | −20 (26%) | −25 (44%) | 0.66 |
| Delta AH, ms | 1 (17%) | 2 (17%) | −2 (25%) | 0.71 |
| Delta HV, ms | 3 (0-7) | 3 (0-7) | 26 (12-76) | <0.01 |
| Delta HV ≥15 ms | 18/112 (16%) | 15/107 (14%) | 3/5 (60%) | <0.01 |
| ∗24-48 h post-TAVR HV ≥65 ms | 6/50 (12%) | 4/47 (9%) | 2/3 (67%) | <0.01 |
Abbreviations: EP, electrophysiology; HDAVB, high-degree atrioventricular block; HV, His-ventricular; LBBB, left bundle branch block; TAVR, transcatheter aortic valve replacement.
EP studies 24-48 h after TAVR were available for 50 patients. Values are median (interquartile range) or n (%).
Stratified Analysis
Patients With No Pre-TAVR RBBB
Of the 99 patients who did not have baseline RBBB, the median age was 78 (IQR: 72-84) years, and 4 (4%) developed HDAVB, with two occurring immediately after TAVR deployment, one occurring in the delayed postoperative period, and one after discharge (mean postoperative 19 hours, range 1-48 hours, Figure 2). The median baseline HV interval did not differ between patients with and without HDAVB. In this patient group, no patients with a post-TAVR HV interval <65 ms developed HDAVB, suggesting that this variable is a robust negative predictor of HDAVB.
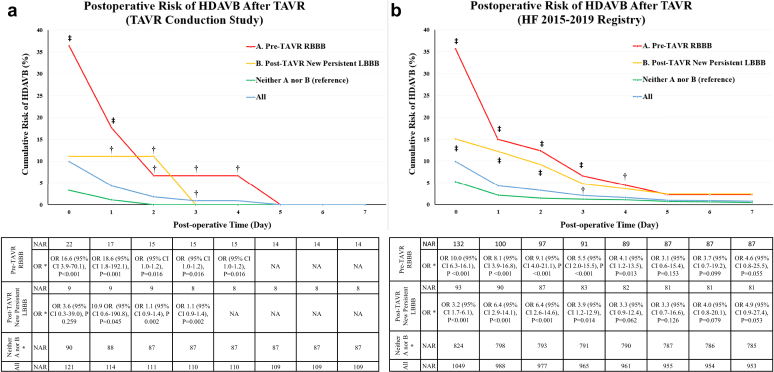
Figure 2.
The cumulative risk of HDAVB requiring pacemaker after TAVR in the (a) prospective TAVR conduction study and (b) a HF-separate retrospective cohort. Day 0 = Operative day. ∗As compared to the group with neither (a) (pre-TAVR RBBB) nor (b) (post-TAVR new persistent LBBB), †p < 0.05 ‡p < 0.001.
Abbreviations: HDAVB, high-degree atrioventricular block; HF, Henry Ford; LBBB, left bundle branch block; NAR, number at risk; OR, odds ratio; RBBB, right bundle branch block; TAVR, transcatheter aortic valve replacement.
Patients With New LBBB After TAVR
Among the 121 patients who underwent TAVR, 27 (24%) developed new LBBB. Of these, 18/27 (67%) were transient and resolved by the next day, while 9/27 (33%) were persistent. Only one patient developed HDAVB (1/27; 4%), and this patient had a persistent LBBB. All 18 patients with a transient LBBB had a post-TAVR HV <65 ms, and none (0/18) developed HDAVB. On the other hand, 7/9 of those with persistent LBBB had an HV interval ≥65 ms, and 1/9 developed HDAVB (HV ≥ 65 ms). None of the patients with persistent LBBB and an HV interval <65 ms (N = 2) developed HDAVB, whereas 1/7 (14%) with persistent LBBB and an HV ≥65 ms developed HDAVB. There was no significant difference in post-TAVR PR interval between those with the primary outcome and those without, and none of the patients with a new LBBB and a PR ≥220 ms or a delta PR of ≥20 ms developed HDAVB.
Patients With Baseline Pre-TAVR RBBB
Among the study participants, 22 patients (18%) had RBBB at baseline, with a median age of 81 (IQR 75-86) years and a body mass index of 29 kg/m2 (IQR 26-32). Of these, eight patients (8/22; 36%) developed HDAVB: five occurring immediately after TAVR valve deployment and three in the postoperative period (mean postoperative 19 hours, range 1-96 hours, Figure 2a). The median baseline and postoperative HV interval did not significantly differ between those who developed HDAVB and those who did not, and there was no difference in the delta HV.
EP Study at 24-48 Hours
A repeat EP study at 24-48 hours after TAVR was offered to all patients for further risk stratification (Figure 1). A repeat EP study was performed for 50 patients only due to patient and/or physician preference.
In patients with no RBBB before TAVR, 26 out of 76 patients with an HV interval <65 ms immediately after TAVR had a repeat EP study, and all 26 patients continued to have an HV interval <65 ms with a 0% (0/26) risk for HDAVB. In those with an HV interval ≥65 ms immediately after TAVR (N = 14), the risk of HDAVB was reduced to 10% (1/11) if the repeat HV interval was <65 ms and 33% (1/3) if the HV interval was ≥65 ms.
In patients with a RBBB after TAVR, a repeat EP study was performed on 10 patients. If the implant depth was <4 mm (N = 7) or if the HV interval immediately after TAVR was <65 ms (N = 4), a repeat EP study with an HV interval <65 ms was associated with a 0% risk of HDAVB. If the implant depth was ≥4 mm or HV interval was ≥65 ms immediately after TAVR, a repeat EP study with an HV interval <65 ms was associated with a 0% risk for HDAVB.
Valve Type
Patients received implants with commercially available devices including balloon expandable valves (Sapien) in 109 patients (90%), mechanically expanding valves (Lotus) in 2 patients (2%), and self-expanding valves (Evolut) in 10 patients (8%). Patients who received mechanically expanding or self-expanding valves (n = 12) had higher odds of developing HDAVB than those who received balloon expandable valves (33 vs. 7%; OR: 6.31; 95% CI: 1.56-25.59; p = 0.004). In our cohort, receiving a mechanically expanding or self-expanding valve was associated with HDAVB immediately after TAVR (n = 4, 33%), but not in the postoperative period or during follow-up (0%). None of the nine patients who underwent valve-in-valve TAVR developed HDAVB after TAVR. The valve-in-valve TAVR group received balloon expandable valves in eight patients and a self-expanding valve in one patient.
Separate Retrospective Cohort for Time to HDAVB During the Postoperative Period
A total of 1253 patients had TAVR at Henry Ford Hospital between January 2015 and December 2019. Of these, 204 patients were excluded as they had a pre-TAVR permanent pacemaker, and 1049 patients were included in the retrospective analysis. Of these patients, 132 (13%) had pre-TAVR RBBB, and 93 (9%) had a new persistent LBBB after TAVR.
The incidence of post-TAVR HDAVB requiring pacemaker implantation was 10% (N = 104). The risk was significantly higher in patients with pre-TAVR RBBB (risk 36%, OR 10.0 [95% CI 6.3-16.1], p < 0.001) and new persistent LBBB (15%, OR 3.2 [95% CI 1.7-6.1], p < 0.001) than the remaining patients. None of the patients with transient LBBB (N = 56, 5%) developed HDAVB requiring pacemaker on follow-up. The median (IQR 25-75) time to the incidence of HDAVB was 0 (0-2) days. The cumulative risk of HDAVB was reduced to less than 5% on postoperative day 4 for patients with pre-TAVR RBBB and on day 3 for patients with new persistent LBBB, respectively. On those days, there were no longer any significant differences between both groups compared to the remaining patients (Figure 2b). The risk was reduced to less than 5% on postoperative day 1 for the remaining patients (Figure 2b). The majority (98/104, 94%) of incidences of post-TAVR HDAVB occurred by day 9 after TAVR with the remaining six occurring between postoperative days 161-275.
Discussion
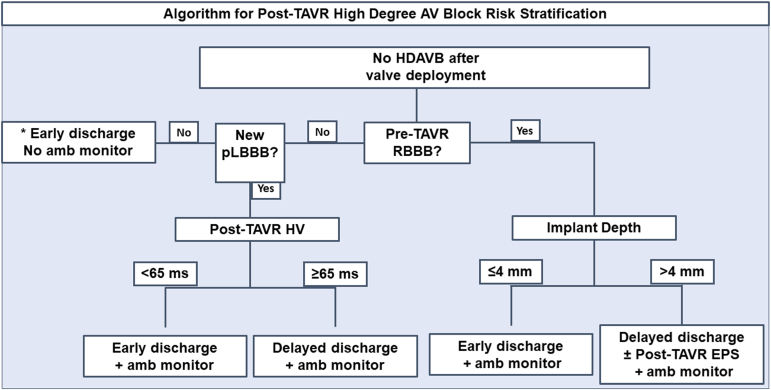
In this prospective cohort study, we assessed the incidence of HDAVB in patients who receive TAVR and identified potential predictors of post-TAVR HDAVB. Specifically, baseline RBBB, implant depth >4 mm, postoperative HV interval of ≥65 ms, and delta HV >15 ms were associated with postoperative development of HDAVB. Notably, for patients in our study with no baseline RBBB, inclusive of those with new LBBB, a postoperative HV interval <65 ms was associated with a 0% risk of HDAVB within 30 days of TAVR implantation. In patients with a baseline RBBB, the risk of HDAVB continues to be high even if HDAVB does not occur immediately after valve deployment. The risk is likewise high in patients with new persistent LBBB, but not if post-TAVR HV is <65 ms. We propose an algorithm utilizing pre-TAVR RBBB, post-TAVR new persistent LBBB, implant depth <4 mm, and post-TAVR HV <65 ms for risk stratification, along with time to event for a safe discharge strategy.
TAVR is one of the most commonly performed structural heart disease procedures,1 and its indication has now expanded to low-risk patients with severe aortic stenosis.14 Outcomes from TAVR, including mortality, stroke, and rehospitalizations, have improved ever since the Food and Drug Administration first approved this procedure in 2011.1 However, the rate of pacemaker implantation required for patients receiving this procedure remains high.1 Several studies have shown worse outcomes, including heart failure and mortality, in patients who require pacemaker implantation.
Various studies have investigated the utility of EP studies for predicting HDAVB after TAVR.11, 12, 13, 14, 15, 16 However, the data are inconclusive. The His-bundle traverses from the inferior edge of the membranous septum to the ventricular septal crest in proximity to the junction of the right and noncoronary cusps.4,5 It is, therefore, vulnerable to injury during the TAVR procedure. The utility of the HV interval as a predictor of outcomes, either before or after TAVR, has been explored in several studies.12, 13, 14, 15, 16 In our study, the pre-TAVR HV interval alone was not associated with HDAVB, and this is consistent with previous studies.12,14,15 However, our study and others14,15 have shown that the postoperative HV interval is associated with HDAVB. There is no consensus on the cutoffs associated with post-TAVR HDAVB, but multiple studies have shown an association between an HV interval of ≥65 ms and HDAVB.14,15 Only one study by Badenco et al12 showed no correlation between the postoperative HV interval and HDAVB. Additionally, the change in the HV interval (the delta HV) before and after the TAVR procedure has also been assessed.12,14,15 The delta HV interval may theoretically account for the dynamic effect of inflammation due to mechanical compression affecting the underlying conduction system. The delta HV interval cutoff associated with HDAVB is in the range of 13-15 ms.14,15
In our study, a postoperative HV interval >65 ms and a delta HV >15 ms were independently associated with postoperative HDAVB, even after controlling for other traditional risk factors, including a baseline pre-TAVR RBBB. Both variables and the cutoff showed no risk of HDAVB in the 30-day postoperative period in patients without pre-TAVR RBBB. While a delta HV ≥15 ms yielded a larger OR than a post-TAVR HV ≥65 ms, obtaining a delta HV requires performing EP studies before and after the TAVR procedure, which is more logistically challenging, as in our experience. In patients with no pre-TAVR RBBB, including those with a new LBBB, an EP study immediately after TAVR with an HV <65 ms identified patients who are at low risk of developing HDAVB.
The post-TAVR PR interval as a predictor of HDAVB has had its share of focus with conflicting evidence.7,9,12,14,15 In our study, the PR interval was not significantly associated with the incidence of HDAVB, but the delta PR was. However, we could not establish a meaningful clinical cutoff for this parameter. The PR interval is measured by ECG as the interval from the beginning of the P wave to the beginning of the QRS complex. Thus, the PR interval is the time it takes for the impulse to travel through the atria, the AV node (AH interval), and the His-Purkinje system (HV interval). Assuming that the intra-atrial conduction is not affected by the TAVR procedure, the PR prolongation should be accounted for by either a prolongation in the AH or HV interval. Interestingly, in our study, the delta PR was comparable to the delta HV, and there was no significant difference in the delta AH between patients who did and did not develop HDAVB (Table 2). The PR interval prolongation may represent HV interval prolongation, that is, infranodal rather than AV nodal disease. This was also described in a study by Akin et al,11 which showed that the new first-degree AV block was driven by the increase in the HV interval. In retrospect and considering other studies, we recommend identifying the His signal and HV interval even if complete heart block occurs immediately after TAVR deployment to elucidate whether the conduction block is occurring at the level of the AV node or below either intra- or infrahisian block.
A new LBBB after TAVR is one of the complications associated with worse outcomes, and how to address this occurrence is unclear.12,14,15 In our study, a new transient LBBB was associated with a zero risk of HDAVB, and none of these patients had an HV < 65 ms. However, these results should be interpreted with caution since only 27 patients developed new LBBB, of which 18 were transient. On the other hand, a new persistent LBBB was associated with a higher risk of post-TAVR HDAVB in our prospective (11%) and retrospective (15%) cohorts. In the prospective cohort, patients with new persistent LBBB and a post-TAVR HV < 65 ms did not develop HDAVB. Additionally, the PR interval did not have an incremental predictive value in patients with new LBBB. Patients with new LBBB and a long (>220 ms) or prolonged PR interval (delta 20 ms) did not exhibit a higher risk of HDAVB. This is again to be cautiously interpreted given our limited cohort of new persistent LBBB. Rivard et al15 reported a higher mean PR value (mean 217 ± 43 ms) in patients with LBBB and HDAVB, although this range falls within the normal range, making this value not clinically practical. Lastly, a multicenter trial by Massoullie et al20 specifically looked at the prognosis assessment of patients with persistent post-TAVR LBBB via an electrophysiological and remote monitoring risk-adapted algorithm; however, the results are yet to be published. The risk of postoperative HDAVB was decreased to less than 5% on postoperative day 3. Therefore, we propose that patients with a new persistent LBBB have a post-TAVR EP study performed to utilize the HV interval for further risk stratification and consider a delayed discharge strategy, whereas those with a transient LBBB or an HV < 65 ms may be safely discharged. Further research with a larger cohort with persistent LBBB is needed to identify the predictors of HDAVB in this higher-risk patient population.
The treatment and risk stratification of patients with baseline RBBB who undergo TAVR remain uncertain since our study had a small number of patients. RBBB has been uniformly associated with a high incidence of HDAVB requiring pacemaker implantation,7, 8, 9,12,14,15,21 as with our prospective and retrospective cohorts, and this risk should be thoroughly discussed with patients as part of the consent process. We analyzed data from 27 patients with baseline RBBB who underwent TAVR and found that EP studies by themselves did not effectively risk stratify these patients as they did for the other groups. This could be because, in patients with baseline RBBB, an injury thickness deep enough to affect only the left bundle may be sufficient to cause AV block. In contrast, those without RBBB would require a larger injury thickness to sever both right and left bundles, or the His bundle, which is protected by thick fibrous tissue. We observed that implant depth >4 mm was associated with HDAVB in this patient population. This is an important variable to consider because it is one of the modifiable variables, and every attempt should be made to optimize this variable. In these patients, if the implant depth is > 4 mm, we suggest considering a conservative approach with a delayed discharge strategy (postoperative day 4 when risk is <5%) and a post-TAVR EP study for further risk stratification (Figure 3). Further research is needed to identify the predictors of HDAVB in this high-risk patient population.
Figure 3.
Algorithm for post-TAVR high-degree AV block discharge risk stratification. Delayed discharge strategy: discharge on POD 3-4 for patients with new persistent LBBB and POD 4 for patients with pre-TAVR RBBB. The duration of ambulatory monitoring should be at least 14 days. ∗ In this low-risk population, post-TAVR EPS with HV <65 ms had a 100% sensitivity in ruling out HDAVB.
Abbreviations: amb, ambulatory; AV, atrioventricular; EPS, electrophysiology study; HDAVB, high-degree atrioventricular block; HV, His-ventricular; LBBB, left bundle branch block; pLBBB, Persistent left bundle branch block; POD, postoperative day; RBBB, right bundle branch block; TAVR, transcatheter aortic valve replacement.
Type of valve is also associated with the risk of HDAVB, with self-expanding and mechanically expanding valves being associated with the highest risk. What we observed regarding valve type in our study was similar to what has already been seen.6,12, 13, 14, 15, 16,22 Interestingly, patients who received self- and mechanically-expanding valves in our study developed HDAVB immediately after TAVR but not in the delayed or postdischarge period. However, this was limited to a small number of patients. A study by Rubin et al16 looked at the utility of the CoreValve, and the rate of HDAVB with this valve was 4 of 18 patients, with three occurring immediately after valve deployment and 1 after 10 days. In our study, patients who had a valve-in-valve TAVR had no incidence of HDAVB, and a lower rate in this situation has been reported.21
Overall, we propose a simple HDAVB risk stratification algorithm for patients receiving TAVR (Figure 3). Multiple studies, including ours, have shown that the pre-TAVR EPS12,14,15 in addition to the post-TAVR atrial pacing (atrioventricular Wenckebach and effective refractory periods) do not provide any additional benefit to post-TAVR HDAVB risk stratification.14, 15, 16 Instead, we recommend a simple HV measurement immediately after TAVR. Traditionally, in TAVRs, a femoral or jugular venous sheath is inserted, and a temporary transvenous pacer is advanced into the RV apex to provide ventricular pacing into ventricular standstill during valve deployment. Using the same venous access, instead of the temporary transvenous pacer, a quadripolar catheter can be advanced into the RV apex for the same purpose and then withdrawn to the His region for HV interval measurement. For patients without pre-TAVR RBBB, if the patient does not develop immediate post-TAVR HDAVB or a new persistent LBBB, an early discharge strategy without a monitor can be employed. For high-risk patients, including those with pre-TAVR RBBB and post-TAVR new persistent LBBB, a more conservative strategy should be employed, and more research is required to develop more nuanced risk stratification strategies. In patients with new persistent LBBB, a post-TAVR HV interval of ≤65 should allow for early discharge in addition to amb rhythm monitoring. Conversely, a postoperative HV interval of ≥65 ms may necessitate a delayed discharge strategy (consider postoperative day 3) in addition to an amb monitor. For patients with a baseline pre-TAVR RBBB, if the implant depth is >4 mm, we suggest a postoperative EP study and a delayed discharge strategy (consider postoperative day 4) with an amb monitor. In our study, HDAVB occurred within 5 days in the prospective study and within 9 days in the larger separate retrospective cohort. Therefore, the duration of amb monitoring should be at least 14 days.
Limitations
It is important to note that our study had several limitations. Although it is the largest prospective study on the use of EP studies for predicting HDAVB after TAVR to date, it was a single-center study, which limits generalizability. The findings from the EP studies, such as AH and HV intervals, were obtained during the TAVR procedures, which may introduce nonphysiologic autonomic tone due to general anesthesia. Nonetheless, both pre- and post-TAVR EP studies were performed under the same conditions. The study was designed to offer a repeat EP study at 24-48 hours after TAVR; however, only 50 patients had this performed because of patient preference to avoid another procedure and also because of the implanting team’s and/or electrophysiologist’s decision that the study was not clinically necessary prior to discharge. Consequently, this limits the generalizability of the study findings, especially for patients who have immediate post-TAVR EP studies. This in part drives our recommendation of a single catheter EP study with a straightforward HV measurement immediately after TAVR. Additionally, a complete EP study should also include atrioventricular Wenckebach assessment in addition to drug provocation such as with atropine and procainamide to enhance the specificity of the AV nodal and His-Purkinje system assessments. This was not performed to allow for a single catheter procedure and TAVR procedural time restraints, as the EP study was performed immediately post-TAVR in the TAVR labs. While all patients had a 30-day follow-up examination and a 12-lead ECG, not all wore the 30-day monitor device. The monitoring period offered to patients covered only 30 days, but delayed HDAVB beyond that period has been reported. This limitation is in part addressed by our separate retrospective cohort. Finally, the patients at highest risk for HAD atrioventricular block—pre-TAVR RBBB and post-TAVR persistent LBBB patients—represent a minority cohort in this study; large studies primarily in these patients are warranted.
Conclusions
Our prospective cohort study showed that the HV interval measured immediately after TAVR valve deployment has a predictive value for identifying patients who are more likely to develop HDAVB after TAVR. Importantly, the post-TAVR HV interval <65 ms showed a high negative predictive value for HDAVB in patients with no baseline RBBB, including those who developed a new LBBB after TAVR. More research is needed to confirm our findings and identify the risk of post-TAVR HDAVB and management strategies of high-risk patients with pre-TAVR RBBB or post-TAVR new persistent LBBB.
Ethics Statement
The study was conducted in adherence to research ethical guidelines and received approval from the Henry Ford Health Institutional Review Board.
Funding
No relevant funding sources exist to disclose.
Disclosure Statement
P. Villablanca is a consultant for Edwards LifeSciences and Teleflex. D. D. Wang is a consultant for Abbott, Boston Scientific, and Edwards LifeSciences. D. D. Wang receives research grant support from Boston Scientific assigned to her employer, Henry Ford Health. B. O'Neill is a consultant to Abbott, Edwards LifeSciences, and Medtronic and receives research support from Edwards LifeSciences assigned to his employer, Henry Ford Health. T. Frisoli is a clinical proctor for Edwards Lifesciences, Abbott, Boston Scientific, and Medtronic. W. O'Neill is a consultant to Abiomed, Medtronic, and Boston Scientific. The other authors had no conflicts to declare.
References
- 1.Carroll J.D., Mack M.J., Vemulapalli S., et al. STS-ACC TVT registry of transcatheter aortic valve replacement. J Am Coll Cardiol. 2020;76(21):2492–2516. doi: 10.1016/j.jacc.2020.09.595. [DOI] [PubMed] [Google Scholar]
- 2.Grover F.L., Vemulapalli S., Carroll J.D., et al. 2016 annual report of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. J Am Coll Cardiol. 2017;69(10):1215–1230. doi: 10.1016/j.jacc.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 3.Lilly S.M., Deshmukh A.J., Epstein A.E., et al. 2020 ACC Expert consensus decision pathway on management of conduction disturbances in patients undergoing transcatheter aortic valve replacement: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(20):2391–2411. doi: 10.1016/j.jacc.2020.08.050. [DOI] [PubMed] [Google Scholar]
- 4.Massing G.K., James T.N. Anatomical configuration of the his bundle and bundle branches in the human heart. Circulation. 1976;53(4):609–621. doi: 10.1161/01.cir.53.4.609. [DOI] [PubMed] [Google Scholar]
- 5.Shumpei Mori K.S. Cardiotext; 2022. Atlas of Cardiac Anatomy. [Google Scholar]
- 6.Fadahunsi O.O., Olowoyeye A., Ukaigwe A., et al. Incidence, predictors, and outcomes of permanent pacemaker implantation following transcatheter aortic valve replacement: analysis from the U.S. Society of Thoracic Surgeons/American College of Cardiology TVT Registry. JACC Cardiovasc Interv. 2016;9(21):2189–2199. doi: 10.1016/j.jcin.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 7.Maan A., Refaat M.M., Heist E.K., et al. Incidence and predictors of pacemaker implantation in patients undergoing transcatheter aortic valve replacement. Pacing Clin Electrophysiol. 2015;38(7):878–886. doi: 10.1111/pace.12653. [DOI] [PubMed] [Google Scholar]
- 8.Ream K., Sandhu A., Valle J., et al. Ambulatory rhythm monitoring to detect late high-grade atrioventricular block following transcatheter aortic valve replacement. J Am Coll Cardiol. 2019;73(20):2538–2547. doi: 10.1016/j.jacc.2019.02.068. [DOI] [PubMed] [Google Scholar]
- 9.Siontis G.C., Jüni P., Pilgrim T., et al. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: a meta-analysis. J Am Coll Cardiol. 2014;64(2):129–140. doi: 10.1016/j.jacc.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 10.Reiter C., Lambert T., Kellermair J., et al. Delayed total atrioventricular block after transcatheter aortic valve replacement assessed by implantable loop recorders. JACC Cardiovasc Interv. 2021;14(24):2723–2732. doi: 10.1016/j.jcin.2021.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Akin I., Kische S., Schneider H., et al. Surface and intracardiac ECG for discriminating conduction disorders after CoreValve implantation. Clin Res Cardiol. 2012;101(5):357–364. doi: 10.1007/s00392-011-0400-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Badenco N., Chong-Nguyen C., Maupain C., et al. Respective role of surface electrocardiogram and his bundle recordings to assess the risk of atrioventricular BLOCK after transcatheter aortic valve replacement. Int J Cardiol. 2017;236:216–220. doi: 10.1016/j.ijcard.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 13.Eksik A., Yildirim A., Gul M., et al. Comparison of Edwards sapien XT versus Lotus valve devices in terms of electrophysiological study parameters in patients undergoing TAVI. Pacing Clin Electrophysiol. 2016;39(10):1132–1140. doi: 10.1111/pace.12917. [DOI] [PubMed] [Google Scholar]
- 14.Ferreira T., Da Costa A., Cerisier A., et al. Predictors of high-degree conduction disturbances and pacemaker implantation after transcatheter aortic valve replacement: prognostic role of the electrophysiological study. Pacing Clin Electrophysiol. 2021;44(5):843–855. doi: 10.1111/pace.14225. [DOI] [PubMed] [Google Scholar]
- 15.Rivard L., Schram G., Asgar A., et al. Electrocardiographic and electrophysiological predictors of atrioventricular block after transcatheter aortic valve replacement. Heart Rhythm. 2015;12(2):321–329. doi: 10.1016/j.hrthm.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 16.Rubin J.M., Avanzas P., del Valle R., et al. Atrioventricular conduction disturbance characterization in transcatheter aortic valve implantation with the CoreValve prosthesis. Circ Cardiovasc Interv. 2011;4(3):280–286. doi: 10.1161/CIRCINTERVENTIONS.111.961649. [DOI] [PubMed] [Google Scholar]
- 17.Rodes-Cabau J., Ellenbogen K.A., Krahn A.D., et al. Management of conduction disturbances associated with transcatheter aortic valve replacement: JACC scientific expert panel. J Am Coll Cardiol. 2019;74(8):1086–1106. doi: 10.1016/j.jacc.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Callans D.J. Lippincott Williams & Wilkins (LWW); 2020. Josephson's Clinical Cardiac Electrophysiology: Techniques and Interpretations. [Google Scholar]
- 19.Surawicz B., Childers R., Deal B.J., et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm SOCIETY: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119(10):e235–e240. doi: 10.1161/CIRCULATIONAHA.108.191095. [DOI] [PubMed] [Google Scholar]
- 20.Massoullie G., Bordachar P., Irles D., et al. Prognosis assessment of persistent left bundle branch block after TAVI by an electrophysiological and remote monitoring risk-adapted algorithm: rationale and design of the multicentre LBBB-TAVI Study. BMJ Open. 2016;6(10) doi: 10.1136/bmjopen-2015-010485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuzcu E.M., Kapadia S.R., Vemulapalli S., et al. Transcatheter aortic valve replacement of failed surgically implanted bioprostheses: the STS/ACC registry. J Am Coll Cardiol. 2018;72(4):370–382. doi: 10.1016/j.jacc.2018.04.074. [DOI] [PubMed] [Google Scholar]
- 22.Rogers T., Devraj M., Thomaides A., et al. Utility of Invasive electrophysiology studies in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation. Am J Cardiol. 2018;121(11):1351–1357. doi: 10.1016/j.amjcard.2018.02.015. [DOI] [PubMed] [Google Scholar]