Abstract
Introduction
Internationally, peritoneal dialysis (PD) is increasingly being commenced within 2 weeks of catheter insertion. Studies are warranted to evaluate outcomes of this strategy.
Methods
This study examines outcomes of early-start PD (ESPD) and conventional-start PD (CSPD), commencing at ≤14 days and >14 days after catheter insertion, respectively. All adults with kidney failure within a large metropolitan PD unit initiating PD through a new catheter, inserted using laparoscopic or modified Seldinger technique, between August 2019 and August 2022, were included in this retrospective observational study. Demographic data and episodes of infectious and mechanical complications were collected using electronic medical records. Analysis was conducted using analysis of variance and Chi-square testing. A P-value < 0.05 was significant with Bonferroni correction performed where relevant. Kaplan-Meier and competing risks analyses were performed for time to PD-related peritonitis and transfer to hemodialysis.
Results
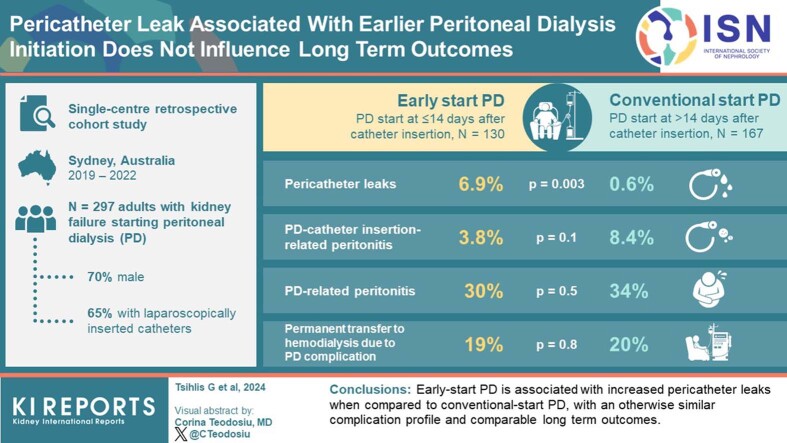
A total of 297 patients (70% male, mean age 58.7 years) were included, with 130 (43.8%) patients undertaking ESPD. Most patients had laparoscopically inserted catheters (65.3%) and 65 patients (22.0%) received prior hemodialysis. When compared to CSPD, ESPD was associated with a higher number of pericatheter leaks (6.9% vs. 0.6%, P = 0.003), with otherwise similar complication episodes and no significant difference with respect to time to PD-related peritonitis or transfer to hemodialysis. Catheter insertion technique or prior hemodialysis treatment did not significantly influence outcomes.
Conclusion
ESPD is associated with increased pericatheter leaks when compared to CSPD, with an otherwise similar complication profile.
Keywords: early-start peritoneal dialysis, pericatheter leak
Graphical abstract
See Commentary on Page 2588
PD carries numerous advantages when compared to hemodialysis, including higher patient satisfaction, greater preservation of residual kidney function and preservation of vascular access.1, 2, 3, 4 Despite this, PD only accounts for 20% of prevalent dialysis therapy in Australia5 and 10% in the United States.6 There are consistent efforts to improve the uptake of PD, and ESPD, which refers to the commencement of PD at ≤14 days of catheter insertion,7 may represent a strategy to achieve this objective.
Currently, existing guidelines recommend allowing a “break-in” period of at least 2 weeks after PD catheter insertion prior to elective start on PD, referred to as CSPD.3,8 However, there has been growing implementation of ESPD internationally in both developed and developing countries due to the many potential advantages this strategy offers.1,2,9,10 For the patient, ESPD facilitates a more streamlined introduction to dialysis, allowing commencement onto a home-based therapy with a single definitive access, bypassing bridging hemodialysis which may be a modality contrary to the patient’s choice.11 Moreover, this approach obviates the risks and complications associated with central venous catheter use, such as infection, thrombosis, and central vein stenosis, which may be needed for urgent hemodialysis prior to CSPD commencement, as well as hemodialysis-specific risks, including cardiovascular or hemodynamic compromise.2,8 From a program-centric perspective, early establishment of PD reduces the costs and infrastructure use associated with hemodialysis and can potentially increase the uptake of PD.7
However, numerous observational studies and 1 randomized controlled trial have demonstrated that ESPD may be associated with an increased risk of mechanical complications when compared to CSPD.1,8,12 The only available randomized controlled trial examining the optimal time for PD commencement demonstrated a higher risk of pericatheter leak at earlier commencement, particularly in diabetic patients.12 However, the small sample size and inclusion of patients with PD catheters inserted solely using an open surgical technique are limitations to consider when applying these results into clinical practice. A systematic review of available evidence found that ESPD may increase the risk of pericatheter leak when compared to CSPD but had uncertain effects on other mechanical and infectious complications, timing of transfer to hemodialysis, and patient survival.1 Based on the suboptimal quality of evidence available, the systematic review found that no strong recommendation could be made for or against ESPD.1
Considering that ESPD includes all patients starting PD within 14 days of catheter insertion, and the risk of mechanical complications can vary according to the time of PD commencement, a closer examination of the practice of “urgent-start” PD is warranted. This strategy, whereby patients commence PD within 3 days of catheter insertion, has seen increased uptake internationally.2,7,9,10 These patients typically have pressing indications for dialysis and would otherwise receive hemodialysis via a vascular catheter until definitive dialysis access is secured. Importantly, in some cases, such as life-threatening hyperkalemia or severe fluid overload, urgent-start PD is not appropriate, and hemodialysis is required. Current evidence suggests that patients commencing on urgent-start PD experience a higher frequency of mechanical complications, including pericatheter leak, than patients commencing PD after a longer break-in period; however, this data emerged from a limited number of small observational studies.2,9 Accordingly, further studies are required to examine the safety of both early and urgent PD initiation strategies. Moreover, with a paucity of studies centered on an Australian cohort and using modern PD catheter insertion techniques, investigation of outcomes within a large Australian PD program would better inform current practice.
This study’s primary objective was to compare outcomes of ESPD and CSPD for all patients initiating PD through a newly inserted PD catheter. Secondarily, this study assessed clinical outcomes for incident dialysis patients commencing PD within 72 hours of catheter insertion as compared to those commencing PD after 72 hours but before 14 days of catheter insertion. This study also examined outcomes for patients who have their PD catheter inserted laparoscopically as compared to the modified Seldinger technique, and patients who did and did not receive hemodialysis prior to commencing PD.
Methods
A single-center retrospective cohort study was performed within the guidelines stipulated by the Western Sydney Local Health District Human Research Ethics Committee (Approval: 2402-01). All adults with kidney failure initiating PD through a newly inserted PD catheter, whether inserted laparoscopically or using the modified Seldinger technique,13 between August 2019 and August 2022 within Western Renal Services, were included in the study. At the end of 2021, Western Renal Services was one of the largest dialysis service providers in Australia.14 With respect to PD, Western Renal Services provided care to 310 prevalent PD patients in 2021, representing 31% and 12% of all prevalent PD patients across New South Wales and Australia, respectively.14 In this study, we included incident dialysis patients, patients transferring to PD from hemodialysis, and patients resuming PD after catheter replacement.
To provide an assessment of “urgent” PD commencement, a subgroup of this cohort consisting solely of incident dialysis patients was evaluated separately. Incident dialysis patients commencing PD within 3 days of catheter insertion were classified as urgent ESPD (U-ESPD) patients,7 whereas the elective ESPD (E-ESPD) group consisted of those patients commencing PD between 3 and 14 days after catheter insertion.
All laparoscopic PD catheter insertions were performed by surgical teams. A double-cuffed Tenckhoff catheter was inserted, under direct vision, into the peritoneal cavity. Each catheter was then sutured to the bladder dome to enable the catheter pigtail to lie within the true pelvis. With the deep cuff located in a preperitoneal location, the catheter was then tunneled through the abdominal wall and secured to the skin with sutures. Intraoperative warm saline flushes were used to confirm appropriate catheter flow, with this fluid remaining in the peritoneum to reduce postprocedural peritoneal irritation.
All PD catheters inserted using the modified Seldinger technique were performed by either surgical or interventional radiology teams, using the following procedural approach.13 All catheters were placed under ultrasound and fluoroscopic guidance and using local anesthesia. Following a small paramedian superficial incision, a micropuncture set was used to establish a linear, sloping, cranio-caudal catheter tract through the rectus sheath, enabling low-entrance peritoneal access. Sequential tract dilatation then enabled the insertion of a double-cuffed Tenckhoff catheter within this tract, with the catheter pigtail orientated within the true pelvis. Completion peritoneography and a warm saline flush were then performed to confirm appropriate catheter position and flow, with fluid left within the peritoneum to reduce postprocedural peritoneal irritation. Bowel preparation was not administered as preparation for either catheter insertion procedure.
The timing of PD initiation was determined based on clinical need, as assessed by the nephrologist and PD nursing staff. According to the protocol, patients initiated on ESPD were started with low-volume dwells of 1 liter. Dwell volumes were gradually increased every 3 to 4 days until a 2-liter volume was reached, with frequent monitoring for pericatheter leak. Patients were advised to remain supine during dialysis to reduce intraperitoneal pressures and were instructed to use aperients as required to ensure daily bowel motions.
Data were collected from electronic medical records. Baseline demographic data assessed included patient age, sex, primary kidney disease, body mass index and presence of obesity, late referral to nephrologist (defined as first nephrologist review within 3 months of dialysis commencement), and presence of antiplatelet or anticoagulant therapy at time of PD catheter insertion. Further relevant PD-related characteristics sought included indication for dialysis commencement, PD catheter insertion technique, need for inpatient admission, hospital length of stay, time to PD commencement after catheter insertion, and kidney function at time of PD commencement.
The outcomes assessed were both infectious and mechanical complications. Infectious complications included PD-related peritonitis (defined as peritonitis occurring after PD commencement),15 PD catheter insertion–related peritonitis (defined as PD peritonitis within 1 month of catheter insertion),15 PD exit site infection, and PD catheter insertion–related exit site infection or tunnel infection (defined as PD exit site or tunnel infection within 1 month of catheter insertion).16 Mechanical complications assessed included immediate procedural complications (including failed catheter insertion, organ injury, hemoperitoneum, and postoperative surgical site bleeding), as well as pericatheter leak (defined by the leakage of fluid from the PD catheter exit site or surgical incision site and confirmed by positive glucose strip test),8 pleuroperitoneal leak (defined by a pleural effusion attributed to PD dialysate, confirmed through pleural fluid analysis, nuclear medicine imaging, and/or computed tomography), and catheter malposition (defined as catheter tip migration away from the pelvis).8 Furthermore, time to first PD-related peritonitis episode and time to permanent transfer to hemodialysis, defined as transfer to hemodialysis for ≥30 days,17,18 were also assessed. At the conclusion of follow-up, current modality of kidney replacement therapy and patient death events were recorded.
Statistical Analysis
Data was analyzed using SPSS software (v22.0; IBM Corp 2013) and R programming software (v4.1.2; R Core Team 2021). Analysis of variance was used to analyze continuous variables, and Chi-square test was applied to categorical variables. Kaplan-Meier estimate was used to examine the time to first episode of PD-related peritonitis and time to transfer to hemodialysis. A binomial logistic regression was performed to further evaluate factors contributing to pericatheter leak. A P-value < 0.05 was considered statistically significant, with Bonferroni correction performed where multiple comparisons were made.
Competing risks were analyzed with the subdistribution approach using finalfit and cmprsk using R programming software. To enable the assessment of the time to PD-related peritonitis, event time was adjusted to days from PD initiation to PD-related peritonitis, or transfer to hemodialysis, transplantation, and death, if these outcomes occurred before the PD-related peritonitis event. Explanatory variables included ESPD versus CSPD, catheter insertion technique, primary kidney disease, PD exit site infection, tunnel infection, catheter malposition and pericatheter leak. Similarly, for the assessment of time to transfer to hemodialysis, death and transplantation were treated as competing risks. The same explanatory variables were used as for time to PD-related peritonitis, with the addition of PD-related peritonitis and pleuroperitoneal leak.
Results were expressed as mean or median ± SD, total number of cases and proportion of cases (percentages), or odds ratio or hazard ratio (HR) with 95% confidence interval (CI) for upper and lower range, depending on the variable and analysis performed.
Results
Patient Characteristics
All patients meeting the eligibility criteria were suitable for inclusion into the study. A total of 297 patients were included in the study, which captured 225 incident dialysis patients, 65 patients who had received prior hemodialysis, and 7 patients who had their original PD catheter replaced (Table 1). This cohort had a mean age of 58.7 years, with male preponderance (70%). Diabetic nephropathy was the most common cause of kidney failure (43.1%). Almost two-thirds of patients had PD catheters inserted laparoscopically (65.3%), with the remainder inserted using the modified Seldinger technique. One hundred thirty patients (43.8%) commenced ESPD, with median time to PD commencement of 11 days after catheter insertion, and 167 patients commenced CSPD, with median time to PD commencement of 26 days after catheter insertion.
Table 1.
Demographic characteristics and clinical data: total cohort
| Variable | Total patients (N = 297) | Early-start peritoneal dialysis (n = 130) | Conventional-start peritoneal dialysis (n = 167) | P value |
|---|---|---|---|---|
| Age (yr, [SD]) | 58.7 ± 16.5 | 56.6 ± 17.3 | 60.3 ± 15.7 | 0.049 |
| Sex (male, n, %) | 208 (70.0%) | 90 (69.2%) | 118 (70.7%) | 0.8 |
| Primary kidney disease (n, %) | 0.5 | |||
| Diabetic nephropathy | 128 (43.1%) | 60 (46.2%) | 68 (40.7%) | |
| Hypertension | 41 (13.8%) | 15 (11.5%) | 26 (15.6%) | |
| Polycystic kidney disease | 12 (4.0%) | 3 (2.3%) | 9 (5.4%) | |
| Glomerulonephritis | 70 (23.6%) | 30 (23.1%) | 40 (24.0%) | |
| Other | 46 (15.5%) | 22 (16.9%) | 24 (14.4%) | |
| Obesity (n, %) | 85 (28.6%) | 35 (26.9%) | 50 (29.9%) | 0.3 |
| Body mass index (n, [SD]) | 27.4 ± 6.0 | 26.9 ± 5.8 | 27.8 ± 6.2 | 0.2 |
| Late referral to nephrologist (n, %) | 14 (4.7%) | 8 (6.2%) | 6 (3.6%) | 0.3 |
| Indication for dialysis commencement (n, %) | 0.06 | |||
| Mild uremia | 233 (78.5%) | 95 (73.1%) | 138 (82.6%) | 0.047a |
| Fluid overload | 34 (11.4%) | 15 (11.5%) | 19 (11.4%) | 0.97 |
| Hyperkalemia | 21 (7.1%) | 14 (10.8%) | 7 (4.2%) | 0.03a |
| Uremic encephalopathy or pericarditis | 3 (1.0%) | 3 (2.3%) | 0 (0.0%) | 0.048a |
| Metabolic acidosis | 6 (2.0%) | 3 (2.3%) | 3 (1.8%) | 0.8 |
| Antiplatelet or anticoagulant therapy (n, %) | 0.2 | |||
| No | 190 (64.0%) | 88 (67.7%) | 102 (61.1%) | |
| Single antiplatelet | 68 (22.9%) | 27 (20.8%) | 41 (24.6%) | |
| Dual antiplatelet | 11 (3.7%) | 7 (5.4%) | 4 (2.4%) | |
| Anticoagulation | 20 (6.7%) | 6 (4.6%) | 14 (8.4%) | |
| Anticoagulation and antiplatelet | 8 (2.7%) | 2 (1.5%) | 6 (3.6%) | |
| Technique for peritoneal dialysis (PD) catheter insertion (n, %) | <0.001 | |||
| Laparoscopic | 194 (65.3%) | 63 (48.5%) | 131 (78.4%) | <0.001 |
| Modified Seldinger | 103 (34.7%) | 67 (51.5%) | 36 (21.6%) | <0.001 |
| Estimated glomerular filtration rate at commencement (ml/min per 1.73 m2, [SD]) | 7.4 ± 2.9 | 6.8 ± 3.0 | 7.8 ± 2.9 | 0.002 |
| Serum creatinine at commencement (μmol/l, [SD]) | 696.3 ± 288.0 | 771.7 ± 323.2 | 636.9 ± 241.8 | <0.001 |
| Inpatient admission | 103 (34.7%) | 55 (42.3%) | 48 (28.7%) | 0.02 |
| Hospital length of stay (d [median], range) | 1.0 (0–52) | 1.0 (0–48) | 1.0 (0–52) | 0.7 |
| Time to PD commencement (d [median], [SD]) | 18.0 ± 15.3 | 11.0 ± 4.3 | 26.0 ± 14.2 | <0.001 |
| Bridging hemodialysis prior to PD (n, %) | 65 (22.0%) | 31 (23.8%) | 34 (20.5%) | 0.5 |
| Follow-up time (d [mean], [SD]) | 845.5 ± 305.3 | 821.9 ± 282.4 | 863.8 ± 321.6 | 0.2 |
PD, peritoneal dialysis.
Demographic characteristics and clinical data of patients undertaking early-start and conventional-start PD. Early-start PD patients were younger, more likely to receive a catheter inserted using the modified Seldinger technique, had worse renal function at time of dialysis commencement, and were more likely to require a post-procedural inpatient admission.
Non-significant result after accounting for Bonferroni correction due to making multiple comparisons.
When compared to patients undergoing CSPD, ESPD patients were younger (56.6 vs. 60.3 years, P = 0.049); however, both groups had otherwise similar baseline characteristics. Compared to CSPD, patients undergoing ESPD were more likely to have a PD catheter inserted using the modified Seldinger technique (51.5% vs. 21.6%, P = 0.001), have worse renal function at time of dialysis commencement (estimated glomerular filtration rate, 6.8 ml/min per 1.73 m2 vs. 7.8 ml/min per 1.73 m2, P = 0.002), and require a postprocedural admission (42.3% vs. 28.7%, P = 0.02).
Ninety five of the 130 patients who underwent ESPD were incident dialysis patients, with 10 patients undergoing U-ESPD and the remaining 85 patients undergoing E-ESPD (Table 2). The median time to PD commencement was 1 day in the U-EPSD group and 12 days in the E-ESPD group. There were no significant between-group differences in baseline characteristics. However, patients undergoing U-ESPD were more likely to have received a PD catheter inserted using the modified Seldinger technique (90% vs. 41.2%, P = 0.004), require a postprocedural admission (80% vs. 37.6%, P = 0.01), and have a longer hospital length of stay (10.1 days vs. 2.5 days, P = 0.001).
Table 2.
Demographic characteristics and clinical data: incident dialysis patients undertaking early-start peritoneal dialysis (ESPD)
| Variable | Total patients (N = 95) | Urgent early-start peritoneal dialysis (n = 10) | Elective early-start peritoneal dialysis (n = 85) | P value |
|---|---|---|---|---|
| Age (yr, [SD]) | 57.5 ± 17.3 | 60.3 ± 17.7 | 57.1 ± 17.3 | 0.6 |
| Sex (Male, n, %) | 66 (69.5%) | 8 (80%) | 58 (68.2%) | 0.4 |
| Primary kidney disease (n, %) | 0.1 | |||
| Diabetic nephropathy | 43 (45.8% | 1 (10%) | 42 (49.4%) | |
| Hypertension | 14 (14.7%) | 2 (20%) | 12 (14.1%) | |
| Polycystic kidney disease | 2 (2.1%) | 0 (0%) | 2 (2.4%) | |
| Glomerulonephritis | 22 (23.2%) | 3 (30%) | 19 (22.4%) | |
| Other | 14 (14.7%) | 4 (40%) | 10 (11.8%) | |
| Obesity (n, %) | 24 (25.3%) | 1 (10%) | 23 (27.1%) | 0.2 |
| Body mass index (n, [SD]) | 26.7 +/− 5.9 | 24.6 +/− 5.5 | 27.0 +/− 6.0 | 0.2 |
| Late referral to nephrologist (n, %) | 8 (8.4%) | 0 (0%) | 8 (9.4%) | 0.4 |
| Indication for dialysis commencement (n, %) | 0.3 | |||
| Mild uremia | 74 (77.9%) | 6 (60%) | 68 (80%) | |
| Fluid overload | 12 (12.6%) | 3 (30%) | 9 (10.6%) | |
| Hyperkalemia | 7 (7.4%) | 1 (10%) | 6 (7.1%) | |
| Uremic encephalopathy or pericarditis | 0 (0%) | 0 (0%) | 0 (0%) | |
| Metabolic acidosis | 2 (2.1%) | 0 (0.0%) | 2 (2.4%) | |
| Antiplatelet or anticoagulant therapy (n, %) | 0.8 | |||
| No | 66 (69.5%) | 7 (70%) | 59 (69.4%) | |
| Single antiplatelet | 20 (21.1%) | 3 (30%) | 17 (20%) | |
| Dual antiplatelet | 4 (4.2%) | 0 (0%) | 4 (4.7%) | |
| Anticoagulation | 3 (3.2%) | 0 (0%) | 3 (3.5%) | |
| Anticoagulation and antiplatelet | 2 (2.1%) | 0 (0%) | 2 (2.4%) | |
| Technique for peritoneal dialysis (PD) catheter insertion (n, %) | 0.004 | |||
| Laparoscopic | 51 (53.7%) | 1 (10%) | 50 (58.8%) | 0.004 |
| Modified Seldinger | 44 (46.3%) | 9 (90%) | 35 (41.2%) | 0.004 |
| Estimated glomerular filtration rate at commencement (ml/min per 1.73 m2, [SD]) | 6.6 ± 2.6 | 6.7 ± 2.5 | 6.6 ± 2.7 | 0.9 |
| Serum creatinine at commencement (μmol/l, [SD]) | 788.2 ± 329.2 | 786.5 ± 349.2 | 781.7 ± 329.0 | 0.96 |
| Inpatient admission | 40 (42.1%) | 8 (80%) | 32 (37.6%) | 0.01 |
| Hospital length of stay (d [median], range) | 3.3 ± 7.2 | 10.1 ± 9.7 | 2.5 ± 6.4 | 0.001 |
| Time to PD commencement (d [median], [SD]) | 11.0 ± 4.1 | 1.0 ± 0.6 | 12.0 ± 2.8 | <0.001 |
| Follow-up time (d [mean], [SD]) | 807.7 ± 291.8 | 807.2 ± 321.0 | 807.7 ± 290.2 | 0.99 |
E-ESPD, elective ESPD; ESPD, early-start PD; PD, peritoneal dialysis; U-ESPD, urgent ESPD.
Demographic characteristics and clinical data of incident dialysis patients undertaking U-ESPD and E-ESPD. U-ESPD patients were more likely to receive a PD catheter inserted using the modified Seldinger technique, were more likely to require a post-procedural inpatient admission, and experienced a longer hospital length of stay, when compared to their E-ESPD counterparts.
Clinical Outcomes
During a median follow-up of 27.9 months (range 11.3–48.7 months), 58.6% of all patients experienced a PD-related complication (Table 3). Patients undertaking ESPD experienced a higher frequency of pericatheter leak episodes when compared to CSPD (ESPD 6.9% vs. CSPD 0.6%, P = 0.003), with 90% of pericatheter leak events occurring in the early-start group. All pericatheter leak episodes fit the definition of early pericatheter leak, occurring within 30 days of PD commencement.8 However, ESPD was not associated with an increased frequency of other early complications, with comparable episodes of PD catheter insertion–related peritonitis (ESPD 3.8% vs. CSPD 8.4%, P = 0.1), PD catheter insertion–related exit site infection (ESPD 3.8% vs. CSPD 9.6%, P = 0.1), and surgical site bleeding (ESPD 3.8% vs. CSPD 1.2%, P = 0.1).
Table 3.
Complications analyzed according to timing of peritoneal dialysis commencement: total cohort
| Complication | Total patients (N = 297) | Early-start PD (n = 130) | Conventional-start PD (n = 167) | P value |
|---|---|---|---|---|
| PD catheter complication (n, %) | 174 (58.6%) | 73 (56.2%) | 101 (60.5%) | 0.5 |
| Infectious complications | ||||
| PD catheter insertion–related peritonitisa (n, %) | 19 (6.4%) | 5 (3.8%) | 14 (8.4%) | 0.1 |
| PD-related peritonitisb (n, %) | 96 (32.3%) | 39 (30%) | 57 (34.1%) | 0.5 |
| Time to PD-related peritonitis (d, [SD]) | 241.7 ± 233.6 | 248.5 ± 238.0 | 237.1 ± 232.6 | 0.8 |
| PD catheter insertion–related exit site infectionc (n, %) | 21 (7.1%) | 5 (3.8%) | 16 (9.6%) | 0.1 |
| PD exit site infection (n, %) | 80 (26.9%) | 34 (26.2%) | 46 (27.5%) | 0.8 |
| PD catheter insertion–related tunnel infectiond (n, %) | 1 (0.3%) | 0 (0%) | 1 (0.6%) | 0.6 |
| Tunnel infection (n, %) | 15 (5.1%) | 6 (4.6%) | 9 (5.4%) | 0.8 |
| Mechanical Complications | ||||
| Failed insertion (n, %) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Organ Injury (n, %) | 1 (0.3%) | 1 (0.8%) | 0 (0.0%) | 0.3 |
| Hemoperitoneum (n, %) | 1 (0.3%) | 1 (0.8%) | 0 (0.0%) | 0.3 |
| Surgical site bleeding (n, %) | 7 (2.4%) | 5 (3.8%) | 2 (1.2%) | 0.1 |
| Pericatheter leak (n, %) | 10 (3.4%) | 9 (6.9%) | 1 (0.6%) | 0.003 |
| Malposition (n, %) | 18 (6.1%) | 6 (4.6%) | 12 (7.2%) | 0.4 |
| Pleuroperitoneal leak (n, %) | 6 (2.0%) | 1 (0.8%) | 5 (3.0%) | 0.2 |
| Other complication (n, %) | 6 (2.1%) | 2 (1.6%) | 4 (2.5%) | 0.6 |
| Bridging hemodialysis due to PD complication (n, %) | 11 (3.7%) | 5 (3.8%) | 6 (3.6%) | 0.9 |
| Need for PD catheter removal (n, %) | 64 (21.5%) | 24 (18.5%) | 40 (24.0%) | 0.3 |
| Permanent transfer to hemodialysis due to PD complicatione (n, %) | 57 (19.4%) | 24 (18.6%) | 33 (20.0%) | 0.8 |
PD, peritoneal dialysis.
Complication episodes according to timing of PD commencement. Patients undertaking early-start PD experienced an increased frequency of pericatheter leak episodes when compared to conventional-start PD patients, with an otherwise comparable safety profile.
PD catheter insertion–related peritonitis defined as peritonitis occurring within 30 days of PD catheter insertion.15
PD-related peritonitis defined as peritonitis occurring after PD commencement.15
PD catheter insertion–related exit site infection defined as exit site infection occurring within 30 days of PD catheter insertion.16
PD catheter insertion–related tunnel infection defined as tunnel infection occurring within 30 days of PD catheter insertion.16
ESPD was associated with a similar frequency of longer-term complications when compared with CSPD. Episodes of mechanical complications during the lifetime of the PD catheter, including malposition (ESPD 4.6% vs. CSPD 7.2%, P = 0.4) and pleuroperitoneal leak (ESPD 0.8% vs. CSPD 3.0%, P = 0.2), were comparable between groups. Furthermore, ESPD was associated with comparable infectious complications, including PD-related peritonitis (ESPD 30% vs. CSPD 34.1%, P = 0.5), PD exit site infection (ESPD 26.2% vs. CSPD 27.5%, P = 0.8) and tunnel infection (ESPD 4.6% vs. CSPD 5.4%, P = 0.8). There were no peritonitis events that did not meet the definition for either PD catheter insertion-related peritonitis or PD-related peritonitis.
Subgroup analysis of incident dialysis patients enabled an assessment of U-ESPD when compared to E-ESPD (Table 4). Although pericatheter leak occurred more frequently in the E-ESPD group, this result was not statistically significant (U-ESPD 0% vs. E-ESPD 7.1%, P = 0.5). U-ESPD patients experienced a similar frequency of mechanical and infectious complications when compared to E-ESPD.
Table 4.
Complications analyzed according to timing of peritoneal dialysis (PD) commencement: incident dialysis patients undertaking early-start peritoneal dialysis (ESPD)
| Complication | Total patients (N = 95) | Urgent early-start PD (n = 10) | Elective early-start PD (n = 85) | P value |
|---|---|---|---|---|
| PD catheter complication (n, %) | 53 (55.8%) | 6 (60%) | 47 (55.3%) | 0.8 |
| Infectious complications | ||||
| PD catheter insertion-related peritonitisa (n, %) | 2 (2.1%) | 1 (10%) | 1 (1.2%) | 0.1 |
| PD-related peritonitisb (n, %) | 27 (28.4%) | 4 (40%) | 23 (27.1%) | 0.4 |
| Time to PD-related peritonitis (d, [SD]) | 282.9 ± 231.8 | 381.3 ± 394.5 | 265.8 ± 200.5 | 0.4 |
| PD catheter insertion–related exit site infectionc (n, %) | 2 (2.1%) | 0 (0%) | 2 (2.4%) | 0.6 |
| PD exit site infection (n, %) | 24 (25.3%) | 3 (30%) | 21 (24.7%) | 0.7 |
| PD catheter insertion–related tunnel infectiond (n, %) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Tunnel infection (n, %) | 6 (6.3%) | 0 (0.0%) | 6 (7.1%) | 0.5 |
| Mechanical complications | ||||
| Failed insertion (n, %) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Organ Injury (n, %) | 1 (1.1%) | 1 (10%) | 0 (0%) | 0.1 |
| Hemoperitoneum (n, %) | 1 (1.1%) | 1 (10%) | 0 (0%) | 0.1 |
| Surgical site bleeding (n, %) | 3 (3.2%) | 0 (0%) | 3 (3.5%) | 0.7 |
| Pericatheter leak (n, %) | 6 (6.3%) | 0 (0%) | 6 (7.1%) | 0.5 |
| Malposition (n, %) | 3 (3.2%) | 1 (10%) | 2 (2.4%) | 0.3 |
| Pleuroperitoneal leak (n, %) | 1 (1.1%) | 0 (0%) | 1 (1.2%) | 0.9 |
| Other complications (n, %) | 2 (2.1%) | 0 (0%) | 2 (2.4%) | 0.8 |
| Bridging hemodialysis due to PD complication (n, %) | 4 (4.2%) | 0 (0%) | 4 (4.7%) | 0.6 |
| Need for PD catheter removal (n, %) | 18 (18.9%) | 2 (20%) | 16 (18.8%) | 0.6 |
| Permanent transfer to hemodialysis due to PD complicatione (n, %) | 19 (20%) | 1 (10%) | 18 (21.2%) | 0.4 |
E-ESPD, elective ESPD; ESPD, early-start PD; PD, peritoneal dialysis; U-ESPD, urgent ESPD.
Complication episodes according to timing of PD commencement. Patients undertaking U-ESPD had comparable complications when compared to E-ESPD.
PD catheter insertion–related peritonitis defined as peritonitis occurring within 30 days of PD catheter insertion.15
PD-related peritonitis defined as peritonitis occurring after PD commencement.15
PD catheter insertion–related exit site infection defined as exit site infection occurring within 30 days of PD catheter insertion.16
PD catheter insertion–related tunnel infection defined as tunnel infection occurring within 30 days of PD catheter insertion.16
Binomial logistic regression was performed to ascertain the effects of potential confounding factors on pericatheter leak events, namely diabetes, body mass index, and PD catheter insertion technique. The timing of PD commencement was also examined. The logistic regression model was statistically significant (x2 [4] = 15.5, P = 0.004). The Nagelkerke R2 was 0.2 and the model correctly classified 96.6% of cases. Patients undertaking ESPD were 16.3 times more likely to experience pericatheter leak than CSPD patients (95% CI: 1.9–137.9, P = 0.01). However other factors such as the presence of diabetes (odds ratio 0.2, 95% CI: 0.04–1.1, P = 0.1), higher body mass index (odds ratio 1.1, 95% CI: 0.98–1.2, P = 0.1), or laparoscopically inserted PD catheter (odds ratio 1.3, 95% CI: 0.3–5.1, P = 0.7) were not associated with an increased likelihood of pericatheter leak.
There was heterogeneity in the management of pericatheter leak. For half of the patients experiencing pericatheter leak, PD was continued, with 4 patients remaining on continuous ambulatory PD with lower dialysate dwell volumes, and 1 patient changing to automated PD. For the remaining 5 patients, PD was temporarily discontinued. Four of these patients were managed medically with diuresis, diet modification, and pharmacological treatment of electrolyte disturbances, and were successfully resumed on PD within 2 weeks of PD cessation. The remaining patient in this group required hemodialysis via a central venous catheter due to diuresis-refractory fluid overload and resumed PD after 6 weeks. No patients required catheter removal due to persistent pericatheter leak.
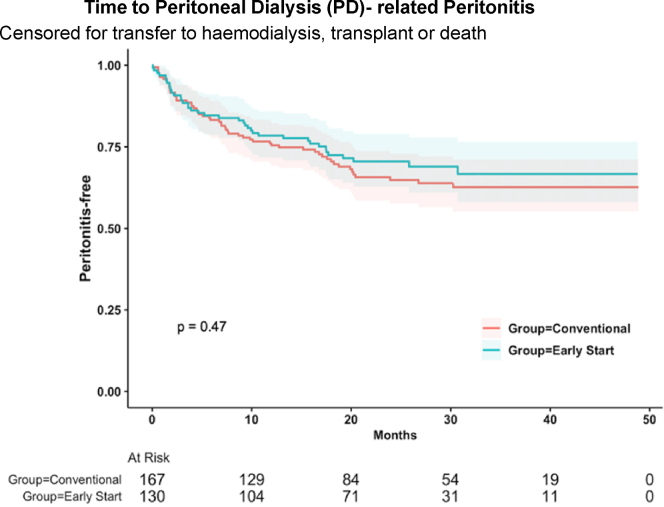
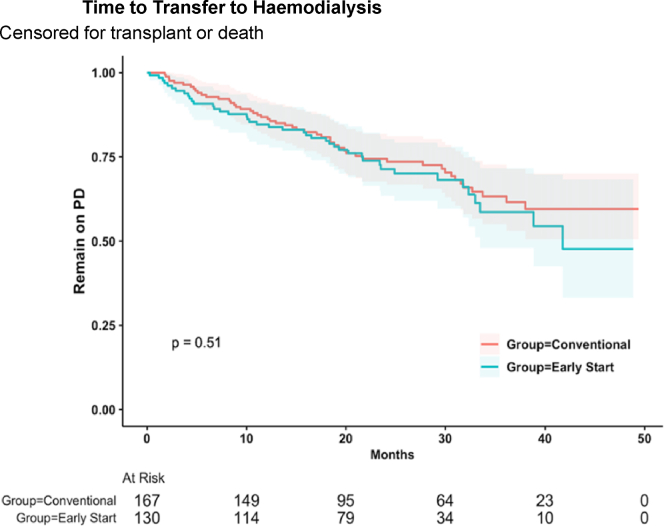
At the conclusion of follow-up, 204 patients (68.7%) remained on PD, 53 patients (17.8%) transferred to hemodialysis, 17 patients (5.7%) received a kidney transplant, and 23 patients (7.7%) died. There were no patients who experienced recovery of kidney function to enable cessation of kidney replacement therapy. Kaplan Meier analysis showed no significant difference between ESPD and CSPD with respect to time to first PD-related peritonitis (P = 0.47), censored for transfer to hemodialysis, transplantation, and death (Figure 1); or time to transfer to hemodialysis (P = 0.51), censored for transplantation and death (Figure 2). These results were confirmed with competing risk analysis, which demonstrated no significant difference between ESPD and CSPD with respect to time to PD-related peritonitis (HR: 1.0, P = 0.99) when death, transplantation, or transfer to hemodialysis were considered as competing risks (Table 5). Similarly, timing of PD commencement did not significantly impact transfer to hemodialysis (HR: 0.8, P = 0.5) when death or transplantation were considered as competing risks (Table 6).
Figure 1.
Kaplan-Meier analysis of time to first episode of PD-related peritonitis, censored for transfer to hemodialysis, transplant or death, showed no significant difference between early-start and conventional-start PD. PD, peritoneal dialysis.
Figure 2.
Kaplan-Meier analysis of time to transfer to hemodialysis, censored for transplant or death, showed no significant difference between early-start and conventional-start PD. PD, peritoneal dialysis.
Table 5.
Risk of peritoneal dialysis (PD)-related peritonitis - competing risks analysis
| Variable | Hazard ratio (competing risks multivariable) | P value |
|---|---|---|
| Primary kidney disease | ||
| Diabetic nephropathy | ||
| Hypertension | 0.9 (95% CI: 0.6–1.2) | 0.4 |
| Polycystic kidney disease | 1.1 (95% CI: 0.6–2.1) | 0.9 |
| Glomerulonephritis | 0.9 (95% CI: 0.6–1.3) | 0.6 |
| Other | 0.6 (95% CI: 0.4–1.1) | 0.1 |
| Early-start peritoneal dialysis (ESPD) vs. conventional-start peritoneal dialysis (CSPD) | 1.0 (95% CI: 0.7–1.4) | 0.99 |
| Modified Seldinger vs. laparoscopic PD catheter insertion technique | 1.3 (95% CI: 0.9–1.9) | 0.1 |
| Infectious complications | ||
| PD exit site infection | 0.8 (95% CI: 0.6–1.0) | 0.1 |
| Tunnel infection | 0.5 (95% CI: 0.2–1.2) | 0.1 |
| Mechanical complications | ||
| Pericatheter leak | 0.8 (95% CI: 0.4–1.5) | 0.4 |
| Malposition | 0.6 (95% CI: 0.3–1.1) | 0.1 |
CI, confidence interval.
A multivariate competing risk analysis was performed to examine variables contributing to time to PD-related peritonitis. The timing of PD commencement or catheter insertion technique did not significantly influence this outcome.
Table 6.
Risk of transfer to hemodialysis-competing risks analysis
| Variable | Hazard ratio (competing risks multivariable) | P value |
|---|---|---|
| Primary kidney disease | ||
| Diabetic nephropathy | ||
| Hypertension | 0.3 (95% CI: 0.1–1.3) | 0.1 |
| Polycystic kidney disease | 0.7 (95% CI: 0.2–2.5 | 0.6 |
| Glomerulonephritis | 1.2 (95% CI: 0.6–2.4) | 0.6 |
| Other | 1.0 (95% CI: 0.4–2.2) | 0.97 |
| Early-start peritoneal dialysis (ESPD) vs. conventional-start peritoneal dialysis (CSPD) | 0.8 (95% CI: 0.4–1.5) | 0.5 |
| Modified Seldinger vs. laparoscopic peritoneal dialysis (PD) catheter insertion technique | 1.0 (95% CI: 0.5–1.9) | 0.98 |
| Infectious complications | ||
| PD-related peritonitisa | 2.7 (95% CI: 1.5–4.8) | 0.001 |
| PD exit site infection | 0.9 (95% CI: 0.5–1.6) | 0.7 |
| Tunnel infection | 1.9 (95% CI: 0.7–5.2) | 0.2 |
| Mechanical Complications | ||
| Pericatheter leak | 1.4 (95% CI: 0.4–4.5) | 0.6 |
| Malposition | 2.8 (95% CI: 1.3–6.0) | 0.01 |
| Pleuroperitoneal leak | 12.7 (95% C.I 5.7–28.4) | <0.001 |
A multivariate competing risk analysis was performed to examine variables contributing to time transfer to hemodialysis. Although timing of PD commencement and catheter insertion technique did not influence this outcome, the presence of PD-related peritonitis, catheter malposition, or pleuroperitoneal leak significantly shortened the time to transfer to hemodialysis.
PD-related peritonitis defined as peritonitis occurring after PD commencement.15
Overall, complications were similar for patients who had their PD catheter inserted laparoscopically as compared to the modified Seldinger technique (laparoscopic 61.3% vs. modified Seldinger 53.4%, P = 0.1) (Table 7). There were no significant between-group differences in the assessed infectious or mechanical complications, including early complications such as PD catheter insertion–related peritonitis (laparoscopic 6.2% vs. modified Seldinger 6.8%, P = 0.8) and surgical site bleeding (laparoscopic 1.5% vs. modified Seldinger 3.9%, P = 0.2). Furthermore, patients who had received hemodialysis prior to PD commencement had comparable overall complications to patients without prior hemodialysis exposure (prior hemodialysis 60.0% vs. dialysis-naïve 58.4%, P = 0.5) (Table 8). Complications were also similar between patients requiring inpatient and outpatient care following catheter insertion (inpatient 56.3% vs. outpatient 59.8%, P = 0.3) (Supplementary Table S1).
Table 7.
Complications analyzed according to peritoneal dialysis (PD) catheter insertion technique: total cohort
| Outcomes | Total patients (N = 297) | Laparoscopic (n = 194) | Modified Seldinger (n = 103) | P value |
|---|---|---|---|---|
| PD catheter complication (n, %) | 174 (58.6%) | 119 (61.3%) | 55 (53.4%) | 0.1 |
| Infectious complications | ||||
| PD catheter insertion–related peritonitisa (n, %) | 19 (6.4%) | 12 (6.2%) | 7 (6.8%) | 0.8 |
| PD-related peritonitisb (n, %) | 96 (32.3%) | 62 (32.0%) | 34 (33.0%) | 0.9 |
| Time to PD-related peritonitis (d, [SD]) | 241.7 ± 233.6 | 244.5 ± 234.4 | 236.7 ± 235.6 | 0.9 |
| PD catheter insertion–related PD exit site infectionc (n, %) | 21 (7.1%) | 17 (8.8%) | 4 (3.9%) | 0.1 |
| PD exit site infection (n, %) | 80 (26.9%) | 60 (30.9%) | 20 (19.4%) | 0.03d |
| PD catheter insertion–related tunnel infectione (n, %) | 1 (0.3%) | 1 (0.5%) | 0 (0.0%) | 0.7 |
| Tunnel infection (n, %) | 15 (5.1%) | 10 (5.2%) | 5 (4.9%) | 0.6 |
| Mechanical complications | ||||
| Failed insertion (n, %) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Organ injury (n, %) | 1 (0.3%) | 0 (0.0%) | 1 (1.0%) | 0.3 |
| Hemoperitoneum (n, %) | 1 (0.3%) | 0 (0.0%) | 1 (1.0%) | 0.3 |
| Surgical site bleeding (n, %) | 7 (2.4%) | 3 (1.5%) | 4 (3.9%) | 0.2 |
| Pericatheter leak (n, %) | 10 (3.4%) | 6 (3.1%) | 4 (3.9%) | 0.5 |
| Malposition (n, %) | 18 (6.1%) | 12 (6.2%) | 6 (5.8%) | 0.6 |
| Pleuroperitoneal leak (n, %) | 6 (2.0%) | 5 (2.6%) | 1 (1.0%) | 0.3 |
| Other complication (n, %) | 6 (2.1%) | 4 (2.1%) | 2 (2.0%) | 0.6 |
| Bridging hemodialysis due to PD complication (n, %) | 11 (3.7%) | 9 (4.7%) | 2 (1.9%) | 0.2 |
| Need for PD catheter removal (n, %) | 64 (21.5%) | 46 (23.7%) | 18 (17.5%) | 0.1 |
| Permanent transfer to hemodialysis due to PD complicationf (n, %) | 57 (19.4%) | 37 (19.3%) | 20 (19.6%) | 0.5 |
PD, peritoneal dialysis.
Complication episodes according to PD catheter insertion technique. There was no significant between-group difference in complications when comparing laparoscopic and modified Seldinger insertion techniques.
PD catheter insertion-related peritonitis defined as peritonitis occurring within 30 days of PD catheter insertion.15
PD-related peritonitis defined as peritonitis occurring after PD commencement.15
PD catheter insertion–related exit site infection defined as exit site infection occurring within 30 days of PD catheter insertion.16
Non-significant result after accounting for Bonferroni correction due to making multiple comparisons.
PD catheter insertion–related tunnel infection defined as tunnel infection occurring within 30 days of PD catheter insertion.16
Table 8.
Complications analyzed according to prior hemodialysis treatment: total cohort
| Outcomes | Total patients (N = 296) | Bridging hemodialysis prior to peritoneal dialysis (PD) (n = 65) | No hemodialysis prior to PD (n = 231) | P value |
|---|---|---|---|---|
| PD catheter complication (n, %) | 174 (58.8%) | 39 (60.0%) | 135 (58.4%) | 0.5 |
| Infectious complications | ||||
| PD catheter insertion–related peritonitisa (n, %) | 19 (6.4%) | 6 (9.2%) | 13 (5.6%) | 0.3 |
| PD-related peritonitisb (n, %) | 96 (32.4%) | 25 (38.5%) | 71 (30.7%) | 0.2 |
| Time to PD-related peritonitis (d, [SD]) | 241.7 ± 233.6 | 219.1 ± 238.1 | 249.7 ± 233.3 | 0.6 |
| PD catheter insertion-related exit site infectionc (n, %) | 21 (7.1%) | 5 (7.7%) | 16 (6.9%) | 0.8 |
| PD exit site infection (n, %) | 80 (27.0%) | 22 (33.8%) | 58 (25.1%) | 0.2 |
| PD catheter insertion-related tunnel infectiond (n, %) | 1 (0.3%) | 0 (0.0%) | 1 (0.4%) | 0.8 |
| Tunnel infection (n, %) | 15 (5.1%) | 3 (4.6%) | 12 (5.2%) | 0.6 |
| Mechanical complications | ||||
| Failed insertion (n, %) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Organ injury (n, %) | 1 (0.03%) | 0 (0.0%) | 1 (0.4%) | 0.8 |
| Hemoperitoneum (n, %) | 1 (0.03%) | 0 (0.0%) | 1 (0.4%) | 0.8 |
| Surgical site bleeding (n, %) | 7 (2.4%) | 1 (1.5%) | 6 (2.6%) | 0.5 |
| Pericatheter leak (n, %) | 10 (3.4%) | 3 (4.6%) | 7 (3.0%) | 0.4 |
| Malposition (n, %) | 18 (6.1%) | 5 (7.7%) | 13 (5.6%) | 0.4 |
| Pleuroperitoneal leak (n, %) | 6 (2.0%) | 1 (1.5%) | 5 (2.2%) | 0.6 |
| Other complication (n, %) | 6 (2.1%) | 1 (1.6%) | 5 (2.2%) | 0.6 |
| Bridging hemodialysis due to PD complication (n, %) | 11 (3.7%) | 1 (1.5%) | 10 (4.3%) | 0.3 |
| Need for PD catheter removal (n, %) | 64 (21.6%) | 15 (23.1%) | 49 (21.2%) | 0.4 |
| Permanent transfer to hemodialysis due to PD complicatione (n, %) | 57 (19.4%) | 10 (15.6%) | 47 (20.4%) | 0.3 |
PD, peritoneal dialysis.
Complication episodes according to prior hemodialysis treatment. There was no significant between-group difference in complications when comparing patients who received and did not receive prior hemodialysis.
PD catheter insertion–related peritonitis defined as peritonitis occurring within 30 days of PD catheter insertion.15
PD-related peritonitis defined as peritonitis occurring after PD commencement.15
PD catheter insertion–related exit site infection defined as exit site infection occurring within 30 days of PD catheter insertion.16
PD catheter insertion–related tunnel infection defined as tunnel infection occurring within 30 days of PD catheter insertion.16
Discussion
This retrospective cohort study compared clinical outcomes of patients commencing ESPD and CSPD at a large PD unit in Australia. It found that ESPD was associated with a higher number of pericatheter leaks than CSPD but had an otherwise comparable safety profile and did not impact the longer-term outcomes of time to PD-related peritonitis or transfer to hemodialysis. We also found that patients commencing PD within 72 hours of PD catheter insertion had a comparable safety profile as compared to those who commenced PD between 3 to 14 days after catheter insertion. Furthermore, this study found that prior hemodialysis treatment, or differences in PD catheter insertion technique, did not significantly influence clinical outcomes.
The higher frequency of pericatheter leak episodes encountered in the ESPD cohort in our study, when compared to CSPD, is consistent with existing evidence.1,8,12 This complication can carry significant clinical implications, necessitating temporary cessation of PD in most cases and requiring some patients to undergo bridging hemodialysis until PD can be safely restarted.4,8,12,19 Patients with persisting pericatheter leak typically require surgical catheter repair or replacement.4,8,12,19 In our cohort, the consequence of pericatheter leak was significant for only 1 patient (10%), who required bridging hemodialysis via a vascular catheter. No patients experienced persisting pericatheter leak requiring surgical intervention. Moreover, competing risks analysis demonstrated that pericatheter leak did not significantly impact the time to PD-related peritonitis (HR: 0.8, P = 0.4) or time to transfer to hemodialysis (HR: 1.4, P = 0.6). The incidence of pericatheter leaks in our ESPD cohort (6.9%) was comparable to that experienced by ESPD patients in a multicenter study in the United States (5.0%)20 and single-center cohort in Korea (5.8%),21 and lower than that seen in another Australian-based ESPD group (12%); a complication rate considered by the investigators to be acceptably low when compared to the benefits of an ESPD strategy.22
There are numerous recognized risk factors of pericatheter leaks. Dialysis-related and catheter-related factors include midline catheter implantation, ESPD, and large-volume dialysate dwells or administration of PD in the erect position, both of which are associated with higher intraperitoneal pressures.8 Patient-related factors include obesity, corticosteroid use, diabetes mellitus, and physical exertion during PD.8,12 Being a retrospective cohort study, our study is limited by its inability to completely control for these variables when examining episodes of pericatheter leak.
To examine the effect of potential confounding factors affecting pericatheter leaks, a binomial logistic regression was performed on measured variables. The small number of pericatheter leak events in our cohort limits the strength of this analysis. The significant association identified between ESPD and pericatheter leak aligns with existing evidence; however, the presence of diabetes mellitus was not associated with an increased risk of pericatheter leaks, contrasting with the findings of the Timely PD study.12 Moreover, though pericatheter leaks occurred more frequently in obese patients compared to nonobese patients (5.9% vs. 2.4%), this association was not statistically significant, and binomial logistic regression found that higher body mass index did not correlate with a significantly increased risk of pericatheter leak.
Importantly, the variables interrogated in our model were found to have an overall weak relationship with pericatheter leak, indicating that additional factors may be contributing to this complication. Although our center follows an ESPD protocol, which specifies the stepwise uptitration of dialysate dwell volumes and encourages supine dialysis, the precise initial PD prescription for each patient, including dialysate dwell volume and patient positioning during dialysis, were inconsistently recorded. This information would have augmented our examination of factors contributing to pericatheter leak events. Moreover, with physical strain recognized as a cause for pericatheter leaks,8 constipation may be an important factor to consider and suggests that bowel care strategies surrounding PD commencement may be an important variable to assess in further studies.
Our examination of outcomes between patients who did and did not receive prior hemodialysis stemmed from our hypothesis that correction of uremia, which is associated with platelet dysfunction and impaired wound healing,23, 24, 25 would reduce the incidence of surgical site bleeding and pericatheter leaks. To our understanding, this hypothesis has not been previously interrogated. In our study, we found that despite patients transferring from hemodialysis having a significantly lower preoperative urea level than that for incident dialysis patients (22.1 mmol/l vs. 31.4 mmol/l, P < 0.001), the frequency of surgical site bleeding and pericatheter leak were comparable between both groups, suggesting against a protective role of hemodialysis in preventing these complications.
The comparable incidence of infectious and mechanical complications between U-ESPD and E-ESPD patients, particularly pericatheter leak, may be explained by the high proportion of U-ESPD patients who commenced PD within an inpatient setting. Nurse-led administration of PD, and close observation and implementation of bedrest and bowel care, may have contributed to improved outcomes for the U-ESPD cohort. Interestingly, 90% of patients commencing U-ESPD had their PD catheters inserted via the modified Seldinger technique, compared with 41.2% in the E-ESPD cohort, due to the timely availability of this method relative to laparoscopic insertion, particularly during the COVID-19 pandemic. This could also have contributed to the low number of pericatheter leaks in the U-ESPD group, because this technique as opposed to the laparoscopic technique requires a smaller incision and the skin and soft tissues tightly approximate the PD catheter, potentially reducing the risk of pericatheter leaks.
There are limitations recognized in this study. First, as an observational study, unmeasured confounding factors influencing the incidence for pericatheter leaks are present, and recognized risk factors thought to contribute to pericatheter leaks accounted for a low proportion of risk for the occurrence of this complication. Second, as a single-center cohort study, selection bias is inherently likely to impact the outcomes, with patients selected into either an ESPD or CSPD initiation, or to receive a PD catheter inserted laparoscopically or using the modified Seldinger technique. Third, the small sample size for U-ESPD patients limits the evaluation of clinical outcomes pertaining to this dialysis strategy. Furthermore, there was considerable divergence in proceduralist expertise, ranging from proceduralists with decades of experience to training doctors. Although this diversity of proceduralist experience is reflective of practice within a tertiary teaching hospital, it may have influenced clinical outcomes.
However, our study is strengthened by its overall large sample size relative to existing observational studies in this area.1,9,21,22,26,27 Furthermore, patients in our cohort had PD catheters inserted laparoscopically or using the modified Seldinger technique, which contrasts with previous studies in this field where patients had PD catheters inserted using the comparably outdated open surgical approach,12,28,29 thereby enhancing the generalizability of these results to modern clinical practice.
Conclusion
In our cohort study, ESPD was associated with more episodes of pericatheter leaks when compared to CSPD; however, the 2 modalities had an otherwise comparable safety profile and similar long-term outcomes. These results align with existing evidence surrounding ESPD and suggest that ESPD can be considered a safe approach to dialysis initiation in patients with kidney failure. Furthermore, U-ESPD was associated with a similar safety profile to E-ESPD, indicating that this can be considered a safe approach for patients needing urgent dialysis commencement, particularly after catheter insertion by modified Seldinger technique. Future studies should further investigate potential factors which may contribute to pericatheter leaks, as this would help facilitate the implementation of strategies to reduce the occurrence of this complication and enhance the safety of ESPD.
Disclosure
KS is on the Fresenius Medical Advisory Board for Australia and New Zealand; and has received speaker’s honoraria from Baxter Healthcare, Roche, and Fresenius Medical Care. KC has received a speaker’s honoraria from Baxter Healthcare. All the other authors declared no competing interests.
Acknowledgments
We would like to thank KC for her guidance and supervision throughout this study.
Footnotes
Table S1. Complications analyzed according to post-procedural care setting: total cohort.
Supplementary Material
Table S1. Complications analyzed according to post-procedural care setting: total cohort.
References
- 1.Htay H., Johnson D.W., Craig J.C., Teixeira-Pinto A., Hawley C.M., Cho Y. Urgent-start peritoneal dialysis versus conventional-start peritoneal dialysis for people with chronic kidney disease. Cochrane Database Syst Rev. 2020;12:1–70. doi: 10.1002/14651858.CD012913.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Povlsen J.V., Ivarsen P. How to start the late referred ESRD patient urgently on chronic APD. Nephrol Dial Transplant. 2006;21:56–59. doi: 10.1093/ndt/gfl192. [DOI] [PubMed] [Google Scholar]
- 3.Stanley M. CARI. The CARI guidelines. Peritoneal dialysis versus haemodialysis (adult) Nephrology (Carlton) 2010;15:S24–S31. doi: 10.1111/j.1440-1797.2010.01228.x. [DOI] [PubMed] [Google Scholar]
- 4.Peppelenbosch A., Kuijk W.H.M., Bouvy N.D., et al. Peritoneal dialysis catheter placement technique and complications. NDT Plus. 2008;1:23–28. doi: 10.1093/ndtplus/sfn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Australia and New Zealand dialysis and transplant registry (ANZDATA) CHAPTER 5 - PERITONEAL DIALYSIS. https://www.anzdata.org.au/wp-content/uploads/2023/05/Chapter-5-Peritoneal-Dialysis-ANZDATA-Annual-Report-2022.pdf
- 6.Hansson J.H., Finkelstein F.O. Peritoneal dialysis in the United States: lessons for the future. Kidney Med. 2020;2:529–531. doi: 10.1016/j.xkme.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blake P.G., Jain A.K. Urgent start peritoneal dialysis: defining what it is and why it matters. Clin J Am Soc Nephrol. 2018;13:1278–1279. doi: 10.2215/CJN.02820318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crabtree J.H., Shrestha B.M., Chow K.M., et al. ISPD Guidelines/Recommendations: creating and maintaining optimal peritoneal dialysis access in the adult patient: 2019 update. Perit Dial Int. 2019;39:414–436. doi: 10.3747/pdi.2018.00232. [DOI] [PubMed] [Google Scholar]
- 9.Nayak K.S., Subhramanyam S.V., Pavankumar N., Antony S., Sarfaraz Khan M.A. Emergent start peritoneal dialysis for end-stage renal disease: outcomes and Advantages. Blood Purif. 2018;45:313–319. doi: 10.1159/000486543. [DOI] [PubMed] [Google Scholar]
- 10.Dias D.B., Mendes M.L., Banin V.B., Barretti P., Ponce D. Urgent-start peritoneal dialysis: the first year of Brazilian experience. Blood Purif. 2017;44:283–287. doi: 10.1159/000478970. [DOI] [PubMed] [Google Scholar]
- 11.Rubin H.R., Fink N.E., Plantinga L.C., Sadler J.H., Kliger A.S., Powe N.R. Patient ratings of dialysis care with peritoneal dialysis vs haemodialysis. JAMA. 2004;291:697–703. doi: 10.1001/jama.291.6.697. [DOI] [PubMed] [Google Scholar]
- 12.Ranganathan D., John G.T., Yeoh E., et al. A randomised controlled trial to determine the appropriate time to initiate peritoneal dialysis after insertion of catheter (Timely PD Study) Perit Dial Int. 2017;37:420–428. doi: 10.3747/pdi.2016.00066. [DOI] [PubMed] [Google Scholar]
- 13.Swinnen J., Davidson I., Baker L. Modified seldinger peritoneal dialysis catheter insertion: a game changer in renal replacement therapy. Endovasc Today. 2022;21:61. https://evtoday.com/articles/2022-june/modified-seldinger-peritoneal-dialysis-catheter-insertion-a-game-changer-in-renal-replacement-therapy 54. [Google Scholar]
- 14.Australia and New Zealand dialysis and transplant registry (ANZDATA) Quality Indicators Annual Report. https://www.anzdata.org.au/wp-content/uploads/2023/10/20240130_QI_annual_locked_2022.pdf
- 15.Li P.K.T., Chow K.M., Cho Y., et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit Dial Int. 2022;42:110–153. doi: 10.1177/08968608221080586. [DOI] [PubMed] [Google Scholar]
- 16.Chow K.M., Li P.K.T., Cho Y., et al. ISPD catheter-related infection recommendations: 2023 Update. Perit Dial Int. 2023;43:1–19. doi: 10.1177/08968608231172740. [DOI] [PubMed] [Google Scholar]
- 17.Bonenkamp A.A., Sluijs A.E., Dekker F.W., et al. Technique failure in peritoneal dialysis: modifiable causes and patient-specific risk factors. Perit Dial Int. 2023;43:73–83. doi: 10.1177/08968608221077461. [DOI] [PubMed] [Google Scholar]
- 18.Lan P.G., Clayton P.A., Johnson D.W., et al. Duration of haemodialysis following peritoneal dialysis cessation in Australia and New Zealand: proposal for a standardised definition of technique failure. Perit Dial Int. 2016;36:623–630. doi: 10.3747/pdi.2015.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leblanc M., Denis O., Vincent P. Dialysate leaks in peritoneal dialysis. Semin Dial. 2001;14:50–54. doi: 10.1046/j.1525-139x.2001.00014.x. [DOI] [PubMed] [Google Scholar]
- 20.Wong L.P., Li N.C., Kansal S., et al. Urgent peritoneal dialysis starts for ESRD: initial Multicentre Experiences in the United States. Am J Kidney Dis. 2016;68:499–502. doi: 10.1053/j.ajkd.2016.03.426. [DOI] [PubMed] [Google Scholar]
- 21.Kim K., Son Y.K., Lee S.M., Kim S.E., An W.S. Early technical complications and long-term survival of urgent peritoneal dialysis according to break-in periods. PLoS One. 2018;13:1–9. doi: 10.1371/journal.pone.0206426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.See Hawley C.M., Jaffrey L.R., Hawley C.M., Jaffrey L.R., Johnson D.W. Early and late patient outcomes in urgent-start peritoneal dialysis. Perit Dial Int. 2017;37:414–419. doi: 10.3747/pdi.2016.00158. [DOI] [PubMed] [Google Scholar]
- 23.Boccardo P., Remuzzi G., Galbusera M. Platelet dysfunction in renal failure. Semin Thromb Hemost. 2004;30:579–589. doi: 10.1055/s-2004-835678. [DOI] [PubMed] [Google Scholar]
- 24.Kaw D., Malhotra D. Platelet dysfunction and end-stage renal disease. Semin Dial. 2006;19:317–322. doi: 10.1111/j.1525-139X.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 25.Maroz N., Simman R. Wound healing in patients with impaired kidney function. J Am Coll Clin Wound Spec. 2013;5:2–7. doi: 10.1016/j.jccw.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pai M.F., Yang J.Y., Chen H.Y., et al. Comparing long-term outcomes between early and delayed initiation of peritoneal dialysis following catheter implantation. Ren Fail. 2016;38:875–881. doi: 10.3109/0886022X.2016.1165069. [DOI] [PubMed] [Google Scholar]
- 27.Javaid M.M., Lee E., Khan B.A., Subramanian S. Description of an urgent-start peritoneal dialysis program in Singapore. Perit Dial Int. 2017;37:500–502. doi: 10.3747/pdi.2016.00308. [DOI] [PubMed] [Google Scholar]
- 28.Ye H., Yang X., Yi C., et al. Urgent-start peritoneal dialysis for patients with end stage renal disease: a 10-year retrospective study. BMC Nephrol. 2019;20:1–10. doi: 10.1186/s12882-019-1408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y., Xhang L., Lin A., Ni Z., Qian J., Fang W. Impact of break-in period on the short-term outcomes of patients started on peritoneal dialysis. Perit Dial Int. 2014;34:49–56. doi: 10.3747/pdi.2012.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Complications analyzed according to post-procedural care setting: total cohort.