Abstract
Post-exposure prophylaxis (PEP) for rabies is widely administered and highly effective. Nevertheless, sporadic breakthrough infections (ie, rabies in people who have started PEP) have been reported. We conducted a systematic review of articles published between Jan 1, 1980 and June 1, 2022 to characterise breakthrough infections. After reviewing 3380 articles from across all continents, we identified 52 articles, which included a total of 122 breakthrough infections. We classified breakthrough infections on the basis of adherence to core practices (ie, wound cleaning and vaccine administration). Of 86 breakthrough infections with data, median time from exposure to symptom onset was 20 days (IQR 16–24). Most (89 [77%] of 115) participants received PEP within 2 days of an exposure. Severe wounds (defined as those involving multiple wound sites or bites to the head, face, or neck) were common (80 [69%] of 116 [with data]). Deviations from core practices were reported in 68 (56%) of 122 cases. Other possible causes for breakthrough infections included errors in the administration of rabies immunoglobulin, delays in seeking health care, and comorbidities or immunosuppression. Cold-chain integrity assessments and potency testing of PEP biologics were only rarely assessed (8 [7%] of 122 cases), neither of which were found to be a cause of breakthrough infections. Timely and appropriate administration of PEP is crucial to prevent rabies, and although people with high-risk exposures or immunosuppression can develop rabies despite adherence to core practices, this occurrence remains exceedingly rare.
Introduction
Rabies causes approximately 59 000 human deaths annually, most of which occur in Asia and Africa, where dog rabies is enzootic.1 Humans become infected when Rabies lyssavirus in the saliva of an infected mammal breaches the skin barrier or mucous membrane through bites or scratches. Although rabies is nearly always fatal after symptom onset, advisory committees, including WHO and the US Advisory Committee on Immunization Practices (ACIP), assert that death can nearly always be prevented by timely post-exposure prophylaxis (PEP).2–4 PEP is especially recommended for severe exposures, like transdermal bites, the contamination of mucous membranes or broken skin with animal saliva, and direct contact with bats.2–4 PEP includes wound cleaning, intradermal or intramuscular rabies vaccines administered at specific time intervals, and infiltration of wounds with either human or equine rabies immunoglobulin when recommended for severe wounds. WHO defines wound cleaning as the thorough irrigation of wounds for 15 min, with either soap and water or with a virucidal or antiseptic agent, to remove and inactivate the virus in situ.3,4 Time from exposure to PEP is not explicitly described by WHO or ACIP recommendations, but it is implied that PEP should be administered as soon as possible.
Modern cell-culture vaccines against rabies were introduced in the 1980s. These vaccines are made from purified chick embryo, human diploid, or Vero cell lines, and have largely replaced previous rabies vaccines. Modern cell-culture vaccines are highly immunogenic, safe, and widely recognised for efficient induction of effective anti-rabies responses;5 however, rare breakthrough cases have been reported. This systematic review aimed to characterise rabies breakthrough infections after the administration of modern cell-culture vaccines, and to identify educational and clinical interventions that might prevent future cases.
Methods
Definitions
Vaccine schedules are established on the basis of scientific evidence.3,4 PEP guidelines by WHO3,4 and ACIP2 differ in some respects, but have the following recommendations in common: the need for appropriate wound cleaning, the injection of rabies vaccines intradermally or intramuscularly into an appropriate site,6,7 and the completion of the chosen vaccine series.3,4 As WHO recommendations are developed for use in low-income settings, we considered the previously stated three practices to be the minimum acceptable PEP core practices needed to prevent human rabies worldwide. We defined breakthrough infections with known or possible PEP deviations from core practices as those for which at least one of the core practices had been or might have been breached (eg, if wound cleaning was explicitly mentioned as not having been done or if there was no mention of whether it was done). Breakthrough infections without deviations from core practices were those for which the authors had reported wound cleaning (regardless of wound cleaning thoroughness), the authors had not indicated a concern about the injection site of the rabies vaccines (ie, incorrect administration into the gluteal muscle), and we could determine that the vaccine doses had been given according to a validated vaccine schedule (appendix p 5). We recognised that the infiltration of wounds with rabies immunoglobulin might be important for preventing rabies but did not include it in our core practices. Rabies immunoglobulin is not always available in some parts of Africa and Asia, yet there have been favourable outcomes in people exposed to rabies despite not having access to rabies immunoglobulin, suggesting that it might not always be essential to use it.4,8,9 Breakthrough infections were characterised as suspected, probable, or confirmed rabies cases on the basis of standard definitions developed by WHO, which classifies cases based on symptoms, history of contact with a rabid animal, and laboratory testing.4
Reasons for breakthrough infections, whether deviations from core practices or reasons stipulated by a study, were considered. Reasons were categorised into four groups: health-care provider contributions, patient behaviours, anatomical or health status attributes, and integrity of PEP biologics. Health-care provider contributions included inadequate wound cleaning, incorrect administration of intramuscular vaccine into the gluteal muscle, or no administration of rabies immunoglobulin when indicated. Patient behaviours included seeking medical attention rapidly and returning to receive follow-up doses of vaccine. Anatomical or health status attributes included exposures known to be high risk for rabies (ie, wounds to the head, neck, face, and the occurrence of multiple wounds) or to cause insufficient immunological response to vaccines (eg, comorbidities, immunocompromising conditions, and medication).2,4,10 Finally, we identified exposure pairs, consisting of one individual who had a breakthrough infection and another who did not develop rabies. We evaluated differences in clinical management and anatomical factors that might have contributed to only one of the two exposed people in the pair developing rabies, despite both receiving PEP.
Analysis
Descriptive analyses were performed, including stratifying cases with and without deviations from core practices by demographic and clinical variables, and by potential reasons for breakthrough infections. The same analyses were also performed on only patients with confirmed human rabies. No statistical comparisons were conducted because of low sample size and considerable missing data. Analyses were conducted using Stata (version 14.0).
Results
A total of 3380 articles met our search criteria. After screening, 52 publications, representing 122 breakthrough infections, were included in this Review (figure, appendix pp 6–29). All breakthrough infections were reported as case reports or case series.
Figure: Study selection.
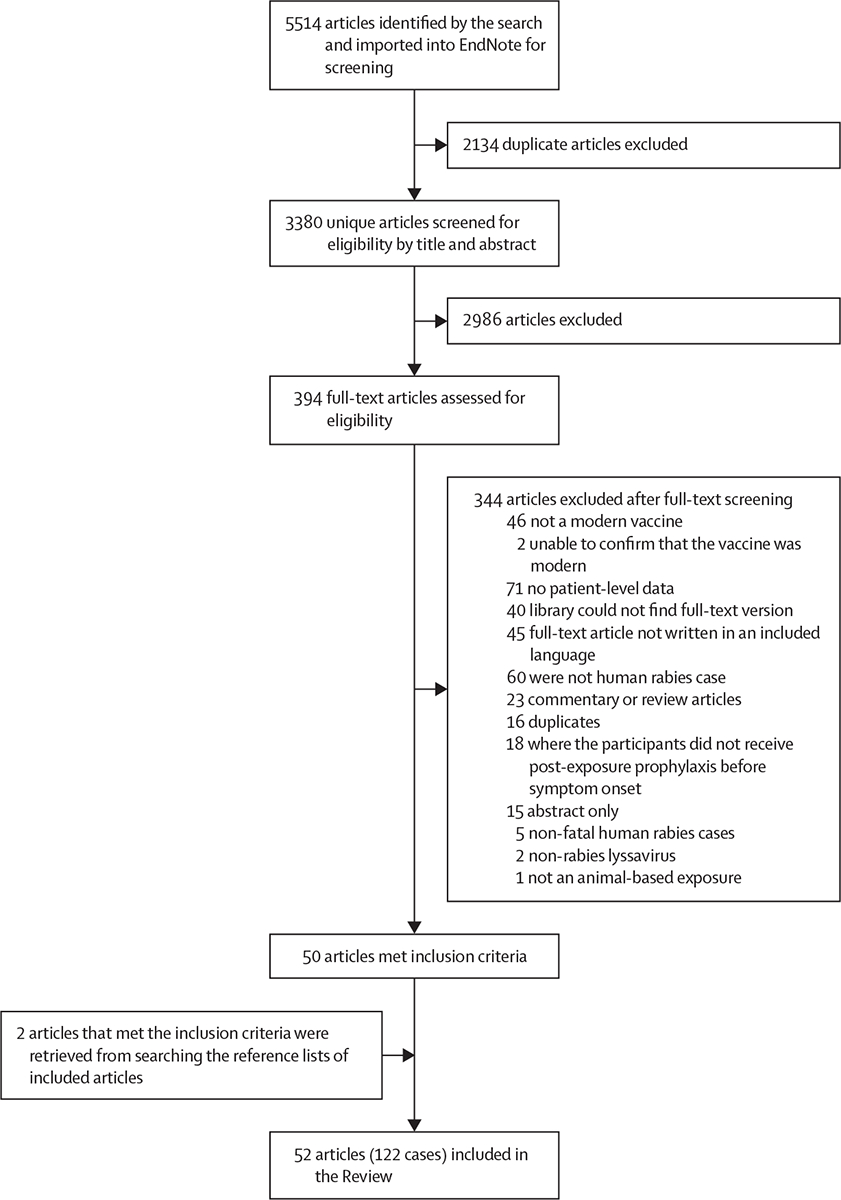
The literature review was done in accordance with the PRISMA guidelines.11
Cases were reported in Africa (n=30), Asia (n=64), and the Middle East (n=27). The country of exposure was missing for one case. Of the 122 breakthrough infections, 84 (69%) were among males and the median age of the patients was 14·5 years (IQR 7–50 years; range 11 months to 85 years; table 1). The exposure source was reported for 101 (83%) of 122 cases. Dogs were the most common source (88 [87%] of 101) and other implicated animals included foxes (n=4), wolves (n=3), mongooses (n=2), jackals (n=2), a giant pouched rat (Cricetomys emini), and a cat. Of the 120 cases associated with a known exposure type, all except two involved animal bites that broke the skin. The exposures that did not include a bite were a scratch from a dog on the head and neck of a male aged 6 years,9 and a deep cat scratch on the face of a female aged 15 years.12 More than half of the patients (62 [53%] of 116) had bites on their head, face, or neck. We were unable to evaluate the total number of bites or scratches sustained by each patient, which is a reasonable marker for exposure severity. However, for 35 (30%) of 116 patients, wounds were on at least two distinct anatomical sites (table 1). Breakthrough infections were either confirmed (56 [46%] of 122) or probable (66 [54%]) human rabies cases.
Table 1:
Demographic and exposure characteristics of 122 human rabies breakthrough infections with or without deviations from core practices
| All cases (n=122) | Breakthrough infections with reported or possible deviations from core practices (n=68)* | Breakthrough infections without deviations from core practices (n=54)* | |
|---|---|---|---|
| WHO classification of human rabies case 4 | |||
| Confirmed | 56/122 (46%) | 24/68 (35%) | 32/54 (59%) |
| Probable | 66/122 (54%) | 44/68 (65%) | 22/54 (41%) |
| Age, years | |||
| 0–9 | 42/122 (34%) | 17/68 (25%) | 25/54 (46%) |
| 10–19 | 27/122 (22%) | 20/68 (29%) | 7/54 (13%) |
| 20–29 | 9/122 (7%) | 7/68 (10%) | 2/54 (4%) |
| 30–39 | 8/122 (7%) | 5/68 (7%) | 3/54 (6%) |
| 40–49 | 5/122 (4%) | 2/68 (3%) | 3/54 (6%) |
| 50–59 | 17/122 (14%) | 8/68 (12%) | 9/54 (17%) |
| 60–69 | 8/122 (7%) | 5/68 (7%) | 3/54 (6%) |
| 70–79 | 4/122 (3%) | 3/68 (4%) | 1/54 (2%) |
| ≥80 | 2/122 (2%) | 1/68 (1%) | 1/54 (2%) |
| Sex | |||
| Female | 37/121 (31%) | 20/67 (30%) | 17/54 (31%) |
| Male | 84/121 (69%) | 47/67 (70%) | 37/54 (69%) |
| Exposure | |||
| Dog | 88/101 (87%) | 41/47 (87%) | 47/54 (87%) |
| Fox | 4/101 (4%) | 1/47 (2%) | 3/54 (6%) |
| Wolf | 3/101 (3%) | 1/47 (2%) | 2/54 (4%) |
| Mongoose | 2/101 (2%) | 1/47 (2%) | 1/54 (2%) |
| Jackal | 2/101 (2%) | 1/47 (2%) | 1/54 (2%) |
| Emin’s pouched rat (Cricetomys emini) | 1/101 (1%) | 1/47 (2%) | 0/54 (0%) |
| Cat | 1/101 (1%) | 1/47 (2%) | 0/54 (0%) |
| Exposure type | |||
| Bite | 118/122 (97%) | 66/68 (97%) | 52/54 (96%) |
| Scratch | 2/122 (2%) | 2/68 (3%) | 0/54 (0%) |
| Not specified | 2/122 (2%) | 0/68 (0%) | 2/54 (4%) |
| Wound location | |||
| Face or neck | 62/116 (53%) | 28/63 (44%) | 34/53 (64%) |
| Arms or hands | 55/116 (47%) | 30/63 (48%) | 25/53 (47%) |
| Trunk or back | 16/116 (14%) | 8/63 (13%) | 8/53 (15%) |
| Legs or feet | 25/116 (22%) | 15/63 (24%) | 10/53 (19%) |
| Number of anatomical wound locations | |||
| 1 | 81/116 (70%) | 47/63 (75%) | 34/53 (64%) |
| 2 | 29/116 (25%) | 14/63 (22%) | 15/53 (28%) |
| 3 | 5/116 (4%) | 2/63 (3%) | 3/53 (6%) |
| 4 | 1/116 (1%) | 0/63 (0%) | 1/53 (2%) |
Data are n/N (%).
Breakthrough infections without deviations from core practices were defined as infections for which the study reported wound cleaning (regardless of the thoroughness of wound cleaning), the study did not indicate a concern with the injection site of rabies vaccines (ie, about incorrect administration into the gluteal muscle), and the current authors could determine that vaccine doses had been given according to a validated vaccine schedule. Breakthrough infections with known or possible post-exposure prophylaxis deviations from core practices included those with deviations or possible deviations from at least one of the core practices.
The median time from exposure to onset of rabies symptoms was 20 days (n=86; IQR 16–24 days; range 9–61 days) and median time from exposure to death was 27 days (n=85; IQR 20–37 days; range 9–81 days; table 2). The median time from exposure to vaccine administration was 0 days (n=115; IQR 0–2 days; range 0–65 days) and exposure to rabies immunoglobulin administration was 0 days (n=64; IQR 0–2 days; range: 0–40 days). Rabies immunoglobulin (either human or equine) was administered to 67 (57%) of 117 patients, but was sometimes only given intramuscularly (14 [21%] of 67). None of the affected patients had previously received pre-exposure or post-exposure rabies prophylaxis. Two patients with rabies received suckling-mouse-brain vaccine at 21 h and 7 days, respectively, before receiving cell-culture vaccine.13,14 Five patients received PEP after substantial delays (22, 40, 55, 61, and 65 days) and developed symptoms (two people) or died (three people) within 2 days of receiving their first vaccine dose (appendix pp 6–29).9,15 No studies reported known breaches in the vaccine cold chain and only one study mentioned this as a suspected reason for breakthrough infection. Potency testing of rabies immunoglobulin (n=3) and vaccine (n=5) were completed in five patients, all of which confirmed vaccine or rabies immunoglobulin integrity. For three additional patients, the studies reported that the vaccines and rabies immunoglobulin were found to be potent by manufacturer testing or were administered to other patients who survived following a bite from a confirmed rabid dog (table 3; appendix pp 6–29).16
Table 2:
Clinical characteristics of 122 reported cases of human rabies breakthrough infections with and without deviations from core practices
| All cases (n=122) | Breakthrough infection with reported or possible deviations from core practices (n=68)* | Breakthrough infection without deviations from core practices (n=54)* | |
|---|---|---|---|
| Time from exposure, days | |||
| Wound care | 33; 0 (0–0); 0–40 | 8; 0 (0–1); 0–1 | 25; 0 (0–0); 0–40 |
| Administration of rabies immunoglobulin | 64; 0 (0–2); 0–40 | 25; 0 (0–2); 0–29 | 39; 0 (0–2); 0–40 |
| Administration of vaccine | 115; 0 (0–2); 0–65 | 61; 1 (0–2); 0–65 | 54; 0 (0–2); 0–40 |
| Onset of rabies symptoms | 86; 20 (16–24); 9–61 | 35; 21 (18–27); 9–60 | 51; 20 (15–23); 10–61 |
| Death | 85; 27 (20–37); 9–81 | 36; 30·5 (21·5-37·5); 9–70 | 49; 25 (20–33); 12–81 |
| Rabies immunoglobulin administration | |||
| Administered | 67/117 (57%) | 25/63 (40%) | 42/54 (78%) |
| Human type | 32/67 (48%) | 12/25 (48%) | 20/42 (48%) |
| Equine type | 32/67 (48%) | 11/25 (44%) | 21/42 (50%) |
| Not specified | 3/67 (4%) | 2/25 (8%) | 1/42 (2%) |
Data are N; median (IQR); range, or n/N (%).
Breakthrough infections without deviations in core practices were defined as those for which studies reported wound cleaning (regardless of wound cleaning thoroughness), studies did not indicate a concern with the injection site of rabies vaccines (ie, about incorrect administration into the gluteal muscle), and the current authors could determine that vaccine doses had been given according to a validated vaccine schedule. Breakthrough infections with known or possible post-exposure prophylaxis deviations included those with deviations or possible deviations from at least one of the core practices.
Table 3:
Potential causes of 122 breakthrough rabies infections with and without deviations from core practices
| Breakthrough infection with reported or possible deviation from core practices (n=68)* | Breakthrough infection without deviation from core practices (n=54)* | |
|---|---|---|
| Health-care provider contributions | ||
| Wound care | ||
| No appropriate wound care | 10/68 (15%) | 0/54 (0%) |
| No information on wound care | 50/68 (73%) | 0/54 (0%) |
| Vaccine administration | ||
| Vaccine administration in the gluteal muscle | 7/68 (10%) | 0/54 (0%) |
| Did not complete vaccine series (reason unknown) | 17/68 (25%) | 0/54 (0%) |
| Received incorrect vaccine regimen† | 2/68 (3%) | 0/54 (0%) |
| Vaccine regimen and series completion unknown | 5/68 (7%) | 0/54 (0%) |
| Developed symptoms before completion of vaccine series‡ | 32/68 (47%) | 30/54 (56%) |
| Rabies immunoglobulin administration | ||
| Given intramuscularly only | 9/25 (36%) | 5/42 (12%) |
| Wound sutured beforehand | 2/25 (8%) | 5/42 (12%) |
| Not all wounds infiltrated | 1/25 (4%) | 3/42 (7%) |
| Not administered | 38/63 (60%) | 12/54 (22%) |
| Anatomic and health status attributes | ||
| Wounds to the head, neck, or face | 28/63 (44%) | 34/53 (64%) |
| Exposed at two or more anatomical locations | 16/63 (25%) | 19/53 (36%) |
| Immunosuppression | 5§/68 (7%) | 3¶/54 (6%) |
| Integrity of post-exposure prophylaxis biologics | ||
| Rabies immunoglobulin potency testing doneǁ | 0**/68 (0%) | 3/54 (6%) |
| Vaccine potency testing doneǁ | 2**/68 (3%) | 2/54 (4%) |
Data are n/N (%).
Breakthrough infections without deviations from core practices were defined as infections for which the study reported wound cleaning (regardless of the thoroughness of wound cleaning), the study did not indicate a concern with the injection site of rabies vaccines (ie, about incorrect administration into the gluteal muscle), and the current authors could determine that vaccine doses had been given according to a validated vaccine schedule. Breakthrough infections with known or possible post-exposure prophylaxis deviations from core practices included those with deviations or possible deviations from at least one of the core practices.
One patient incorrectly received four doses on day 0; a second patient incorrectly received three doses on day 0.
Includes one person who either developed symptoms before they completed their fifth dose of vaccine or received only a four-dose series so would have completed their vaccination (n=1 with deviations).
Immunosuppressive conditions were specified as liver cirrhosis secondary to alcoholism (n=2), age-related immunosuppression (n=1), chronic lymphoproliferative leukaemia (n=1), and unspecified advanced immunodeficiency (n=1).
Immunosuppressive conditions were specified as uncontrolled diabetes (n=1) and liver cirrhosis secondary to alcoholism (n=2).
All rabies immunoglobulins and vaccines that were tested were found to be potent.
There were three patients for whom the study reported that the vaccine and rabies immunoglobulin batches used were found by the manufacturer to be effective or not associated with death in other confirmed recipients of post-exposure prophylaxis (not included in this table) following a bite by a dog with confirmed rabies.16
A total of 68 (56%) of 122 cases were classified as breakthrough infections with reported or possible deviations from core practices, and 54 (44%) were classified as breakthrough infections without deviations from core practices (table 3, 4).
Table 4:
Patient behaviours of 122 breakthrough rabies infections with and without deviations from core practices
| Breakthrough infection with reported or possible deviation from core practices (n=68)* | Breakthrough infection without deviation from core practices (n=54)* | |||
|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | |
| Time from exposure to wound care, days | 8 | 0 (0–1) | 39 | 0 (0–0) |
| Time from exposure to vaccine administration, days | 61 | 1 (0–2) | 54 | 0 (0–2) |
| Time from exposure to rabies immunoglobulin administration, days | 25 | 0 (0–2) | 39 | 0 (0–2) |
| Patient did not return for additional doses of vaccine | 68 | 2 (3%)† | 54 | 0 (0%)† |
Data are N, median (IQR) range, or n (%).
Breakthrough infections without deviations in core practices were defined as those for which studies reported wound cleaning (regardless of wound cleaning thoroughness), studies did not indicate a concern with the injection site of rabies vaccines (ie, about incorrect administration into the gluteal muscle), and the current authors could determine that vaccine doses had been given according to a validated vaccine schedule. Breakthrough infections with known or possible post-exposure prophylaxis deviations included those with deviations or possible deviations from at least one of the core practices.
n (%).
Most of the errors in health-care provision involved deviations from core practices (table 3; appendix pp 6–29). Other errors not related to core practices involved rabies immunoglobulin and occurred in breakthrough infections with and without deviations from core practices. These errors included: no rabies immunoglobulin being administered (n=50); rabies immunoglobulin only administered intramuscularly (n=14); some but not all wounds infiltrated with rabies immunoglobulin (n=4); wound closure before rabies immunoglobulin administration (n=7); and administration of vaccine and rabies immunoglobulin in the same anatomical location (n=1). For two cases, studies reported that the infiltration of rabies immunoglobulin into all wounds was unachievable because of the specific anatomical locations of the wounds (ie, eyelid or lips). Although WHO does not define an acceptable timeframe in which to seek PEP, 89 (77%) of 115 patients received the first dose of the rabies vaccine within 2 days of the exposure. Among breakthrough infections with possible or known deviations from core practices, only two patients did not return for additional doses after the first dose of vaccine (table 4).17,18
Anatomical or health status attributes included wounds to the head or neck (28 [44%] of 63 with deviations from core practices vs 34 [64%] of 53 breakthrough infections without deviations), multiple wounds (16 [25%] of 63 breakthrough infections with deviations vs 19 [36%] of 53 without deviations), and immunocompromising conditions reported by the studies as uncontrolled diabetes (n=1), liver cirrhosis secondary to alcoholism (n=4), age-related immunosuppression (n=1), chronic lymphoproliferative leukaemia (n=1), and unspecified advanced immunodeficiency (n=1; table 3; appendix pp 6–29). Additional reasons suggested by the studies for breakthrough infections included direct inoculation into highly innervated tissue or nerves (eg, wounds with exposed nerves or highly innervated areas like the face or fingers; 22 [18%] of 122), severity of the bites (4 [3%] of 122), concurrent administration of anti-malarial medication (1 [<1%] of 122) or ketamine (1 [<1%] of 122), concerns about failure in correct PEP administration practices because two fatal cases occurred in rapid succession in the same clinic (2 [2%] of 122), and hypotheses that the rabies virus strain could have been more virulent (4 [3%] of 122; appendix pp 6–29). The authors did not identify a reason for breakthrough infection in 13 (24%) of 54 patients without deviations from core practices and one (1%) of 68 patients with a possible deviation (information about wound care was missing).
Of 54 breakthrough infections without deviation from core practices, the vaccine series was completed in 24 (44%) of 54 patients. For 26 (48%) of 54 cases, at least three vaccine doses or sessions (if multiple doses per day were involved) were given before the onset of rabies symptoms with most (16 [62%] of 26) patients treated with at least four vaccine doses or sessions. A single dose of vaccine was received at 22 days after an exposure by one patient and at 40 days after an exposure by another, and both developed symptoms the next day.15
We repeated the analysis for 56 cases with confirmed human rabies to see if there were major differences in demographics, exposure, clinical characteristics, and potential reasons for breakthrough infection between these individuals and the entire cohort (appendix pp 30–32). No major differences were observed compared with the entire cohort. For example, 31 (56%) of 55 individuals (one with missing data) reported head, face, or neck wounds and 16 (29%) people reported multiple wound locations. Health-care provider errors were also similar in type and frequency to the entire cohort, including three (13%) individuals with administration of vaccine intramuscularly in the gluteal muscle and eight (33%) individuals with no wound care among breakthrough infections with deviations, and 15 (27%) individuals with errors in rabies immunoglobulin administration among all cases.
Three articles reported pairs of people bitten by the same animal who received PEP, but with different clinical outcomes.19–21 Although there were differences in clinical management (including timeliness of PEP administration and lack of rabies immunoglobulin administration and wound cleaning), fatal cases were also associated with more severe wounds (eg, wounds to the head and neck, wounds close to cranial nerves, or wounds requiring surgical intervention; table 5).
Table 5:
Pairs of individuals who were bitten by the same animal but who had different outcomes
| Details of exposed patients who died of rabies | Details of exposed patients who survived | Potential explanation for the breakthrough infection in the fatal case reported by study | |
|---|---|---|---|
| Gadekar, et al19 | Male aged 30 years with left middle finger dog bite; no wound care done; received four doses of-cell culture vaccine per ESSEN* regimen; no reported delay in seeking care; died 27 days after dog bite; probable rabies case | Male aged 5 years with two dog bites in right gluteal region; wound cleaning and irrigation with povidone iodine; vaccine and rabies immunoglobulin administered per five-dose ESSEN* regimen; no reported delay in seeking care | No immediate wound cleaning; no rabies immunoglobulin administration; bite in a high-risk site (ie, finger compared with gluteal region) |
| Fescharek, et al20 | Male aged 6 years with dog bites on upper lip, left calf, upper arm, and scalp; wound care with disinfection; vaccine given on the day of the bite and rabies immunoglobulin administered the next day; received tetanus prophylaxis and antibiotics; patient received purified chick embryo cell-culture vaccine by intramuscular injection on days 0, 3, 7, and 14; had additional surgery for facial wound with ketamine 3 days post-bite; died 3 days after the fourth vaccine dose; probable rabies case | Male aged 8 years with dog bite on left check, left ear, and scalp; wound care with disinfection; vaccine given on the day of the bite and rabies immunoglobulin administrated the next day; received tetanus prophylaxis and antibiotics | Suppression of immune system by ketamine after high-risk bite; potential for more severe wound because the patient required additional surgery |
| Tabbara and Al-Omar21 | Female aged 7 years with eyelid bite from fox; had a delay of 48 h in seeking care; wound cleaning information missing; received vaccination, rabies immunoglobulin, tetanus prophylaxis, and antibiotics; did not report regimen or how many vaccine doses received; died weeks after bites; probable rabies case | Female aged 18 months with bite from fox on eyelid and abrasion on left check and nose; no reported delay in seeking care or receiving post-exposure prophylaxis; wound cleaning information missing; received vaccine, rabies immunoglobulin, and antibiotics | Delay in rabies immunoglobulin administration; amount of rabies virus inoculated could have been larger; laceration was proximate to the cranial nerves |
Discussion
We identified 122 breakthrough infections that occurred between 1980 and 2022, despite an estimated 29 million people receiving rabies PEP each year, largely from dog bite exposures (87% of all breakthrough infections with known exposure source).8 The patients who were cases in our Review cohort resembled patients with rabies worldwide in terms of age, gender, and exposure source (ie, predominantly dogs).8,10,22,23 However, many of our patients had features indicative of particularly severe and high-risk exposures; wounds were frequently to the head, neck, or face, and the time intervals from exposure to rabies illness onset were remarkably short (median 27 days compared with 1–3 months for typical rabies infections).8,10,24,25 Most (77%) patients in our Review cohort sought medical attention within 2 days, which is uncommonly prompt (other publications24,26–29 report that only 57–87% of exposed people seek care so quickly), supporting our suspicion that the wounds that these patients received were probably considerable. Indeed, among the 54 patients for whom breaches in fundamental PEP recommendations did not occur, anatomical or health status contributions (eg, bites to head, neck, or face) were most often implicated.
Head, neck, or face bites (54%), or multiple bites (30%) were more common in our Review than in published reports of people who have been bitten by dogs with studies reporting 2–6% with head, neck, or face bites and 3–18% with multiple bites in Africa (Kenya,24 Chad,25 and Nigeria),28,29 Asia (India,30 China,31 and Thailand),32 and the Middle East (Iran).33 For 62 patients in our cohort, rabies illness onset occurred so quickly that the full course of rabies PEP could not be completed. The studies hypothesised that these short incubations could be due to the rapid transportation of the rabies virus to the central nervous system from highly innervated surfaces (eg, the tips of the fingers and the face). Four studies commented on the potential effect of virulent or unusual rabies strains (appendix pp 6–29); however, there is no clear evidence to suggest a difference in virulence among different rabies virus variants.
Although there are no specific recommendations about deviations from core practices in the vaccine schedule, seven (13%) of the 54 patients without a deviation from core practices had schedule deviations of up to 5 days (appendix pp 6–29). It is possible that even minor deviations of a few days early in the schedule could lead to a delayed and insufficient immune response to prevent rabies in patients with severe wounds. Additional studies are needed to explore the effect of deviations on immune response and risk for breakthrough infections in people with severe exposures. Immunocompromising conditions were rightly considered as possible reasons for breakthrough infections, but several conditions listed by studies (eg, diabetes and liver cirrhosis) were, in isolation, unlikely to have caused altered immunity. A shortage of preventive medicine in developing countries probably resulted in underdiagnosis of these conditions (eg, HIV and malignancy) and an inability to adequately assess how frequently breakthrough infections were associated with immunocompromising conditions. Nevertheless, WHO and ACIP guidelines indicate that patients with immunocompromising conditions might need additional doses of PEP, and verification of adequate immune response by antibody titre if feasible.2,4 None of the patients in our cohort received additional doses, nor did they have their antibody titres checked when vaccine effectiveness was questioned because of potential immunocompromise, indicating a need for provider education about this issue.
Although host and virus contributions to breakthrough infections might be difficult to circumvent, we identified preventable reasons for such events. Overall, health-care provider contributions, including deviations from core practices, were the most common (95 [78%] of 122) observations in our review, a finding consistent with previous publications.28,34,35 Although incorrect practices might not always be enough to cause breakthrough infections, patients with severe exposures, such as those described in this Review, might benefit from clinician adherence to established, feasible, and effective best practices. For one patient, four doses of rabies vaccine were administered on the same day and for another, suckling-mouse-brain vaccine was administered even though modern cell-culture vaccine was available. The fact that the thoroughness of wound cleaning, or the occurrence of wound cleaning at all, was often not stated by studies suggests that the crucial contribution that wound cleaning makes to preventing rabies is not fully appreciated by treatment givers.19,36,37 WHO guidelines4 underscore the importance of wound cleaning by explicitly stating that thorough wound cleaning and timely vaccinations alone might lead to survival in more than 99% of rabies cases. However, these same guidelines recommend that rabies immunoglobulin should be administered for severe exposures, which most of our patients unequivocally had received.4 A substantial number of patients in our cohort received rabies immunoglobulin, indicating that it was rightly prioritised for patients with severe exposures. Whether or not it was available for the other patients is not known; however, the administration of rabies immunoglobulin only intramuscularly and the infiltration of only some of the wounds indicate the need for continuing education to address easily avoidable errors.
Patients in our Review sought care quickly and adhered to the recommended PEP (only two patients did not return for all doses). Nevertheless, continual outreach should emphasise the importance of immediate wound flushing and cleansing, especially when health-care facilities are scarce or difficult to access.25,30,38 In addition, communities might also rely on traditional and herbal medicine.39 There were several patients who had received wound care with herbs before accessing a health-care facility, highlighting the importance of engaging with community-based healers for the treatment of animal bites. In our cohort, two patients did not receive rabies immunoglobulin, or had a delay in vaccine administration, because of financial constraints. Medical and government entities should consider how to remove barriers to accessing PEP, particularly for individuals with the most severe bites.
There are several limitations to our analysis. We relied on the studies’ accounts of exposures, wound locations, and treatment, many of which were incomplete. For example, we could not evaluate the number of wounds or the presence of underlying immunosuppressive conditions, if unassessed by the study. Approximately half of reported breakthrough infections were laboratory-confirmed rabies cases; some deaths might have been from other causes, such as bacterial infection at the wound site. However, when looking at the subset of laboratory-confirmed human rabies cases (appendix pp 30–32), we did not establish any considerably different conclusions. In five cases, we were unable to discern the PEP series used and whether it was completed, raising the possibility (albeit unlikely) that an incomplete PEP series was an unrecognised reason for a breakthrough infection. In five cases, patients had considerable delays in accessing PEP and had symptom onset or died within 2 days of receiving such treatment (appendix pp 6–29), and only two of those cases received rabies immunoglobulin. Given the time required to mount an antibody response after vaccination, it is unlikely that these cases would be considered PEP failures, even though they met our inclusion criteria. None of the studies identified a breach in the cold-chain integrity, an event that could affect vaccine potency. Cold-chain problems are more likely in remote regions and in clinics with an unreliable electrical supply. Several studies (eight cases) reported that vaccine or rabies immunoglobulin potency had been investigated (appendix pp 6–29) and although no abnormalities were found, it is possible that additional cases of breakthrough infections could have been due to defective vaccine or rabies immunoglobulin. This Review is based on observational case study data, thus causality of breakthrough infections cannot be inferred. Finally, this analysis included only published cases with sufficient clinical details. Aggregated human rabies publications that include epidemiological studies of bite victims or human rabies cases that might include reports of breakthrough infections, could not be evaluated.23,40,41
Our Review suggests that breakthrough infections are rare but that, in rare cases, breakthrough infections might not be preventable because of anatomical and health status factors. We identified common educational gaps among health-care providers that could be addressed to improve adherence to recommended PEP practices. Even though we know that many patients globally do not receive PEP as recommended (eg, owing to inadequate wound care and errors in vaccine administration), most patients do not develop rabies after PEP.34 We identified 24 cases of breakthrough infections in people who had received complete post-exposure vaccination with no reported deviations from core practices, and 13 cases for which the study authors could not identify any discernible reason for breakthrough infections. Although this represents a small number of cases when compared with the millions of people that receive PEP each year, further research is needed to elucidate true breakthrough infections and their underlying causes.8,42 This research includes increased understanding the role of wound closure or possible interactions between PEP and other medications, such as ketamine8 and antimalarials, although antimalarials are probably not a concern.43 Perhaps most importantly, improved surveillance for breakthrough infections is essential to identify and describe their occurrence. In the past 5 years, WHO shortened the PEP series to three rabies vaccine doses; the cited study16 explicitly calls for the improved detection of breakthrough infections where shortened regimens are introduced. Enhanced surveillance of breakthrough infections, including improved reporting using standardised data collection tools, is therefore crucial.
Supplementary Material
Key messages.
A systematic literature review of articles published between 1980 and 2022 identified 122 cases of breakthrough rabies infections (ie, rabies in people who had started post-exposure prophylaxis)
Confirmed or suspected breaches in post-exposure prophylaxis practices (eg, inadequate wound cleaning or inappropriate vaccine administration methods) were found in more than half of the patients with breakthrough infections
Multiple wound locations and bites to the head, neck, and face were much more common among our cohort when compared with epidemiological studies of animal bites in similar geographical regions
We identified educational opportunities for communities and health-care providers to improve post-exposure prophylaxis practices, including improved wound cleansing and appropriate administration of rabies immunoglobulin
Enhanced surveillance for breakthrough infections is crucial to better understand their cause and to adapt recommendations for providers
Search strategy and selection criteria.
On Dec 13, 2018, July 8, 2020, and July 11, 2022, we searched MEDLINE (OVID), Embase (OVID), Global Health (OVID), CINAHL (EBSCO), Cochrane Library, and Scopus, for articles published between Jan 1, 1980, and June 1, 2022. We used an intentionally broad search strategy that included subject headings for “rabies” and “post-exposure”, to capture published reports of breakthrough rabies infections in humans. The search strategy for each database was made in accordance with the PRISMA guidelines, which was developed in MEDLINE and modified appropriately for subsequent databases. All 5514 publications that met the search criteria were imported into EndNote (version 20) and exported to Covidence (Cochrane, VIC, Australia). We also searched bibliographies of included publications for additional relevant references.
We included articles written in English, French, German, or Spanish. We included any journal publications with patient-level data that described an author-reported fatal human case of rabies that occurred after a bite or a scratch from a domestic or wild mammal (including bats), for which at least one dose of cell-culture vaccine had been given before the onset of rabies symptoms (ie, a breakthrough infection). We excluded articles on infections with antigenic typing or sequencing-confirmed non-rabies Lyssaviruses (eg, European bat 1 lyssavirus), as this Review assessed breakthrough infections from rabies virus infections only. We also excluded cases for which full-text articles were unavailable.
We screened all publication titles and abstracts to identify potentially relevant articles. Discordances about whether to include an article resulted in the inclusion of full-text reviews. Data from full-text articles were extracted in duplicate using a standardised data extraction tool designed in Google Forms. Extracted data comprised patient-level information about demographics, exposure history, laboratory test results, clinical course, clinical management, and vaccine integrity (eg, maintenance of vaccine cold chain and potency testing of vaccines and rabies immunoglobulin). We also reported the reasons for breakthrough infections as hypothesised by the study authors. If articles were unclear about whether a case had been treated with a modern cell-culture vaccine, we contacted the study authors for clarification. We included 52 articles, which included a total of 122 breakthrough infections. Cases reported in multiple articles were consolidated into one extraction. We resolved discordances between duplicated data extractions through adjudication by a third Review author.
Acknowledgments
We thank Ryan Wallace and Andrea McCollum of the Poxvirus and Rabies Branch, Division of High Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, GA, USA. The findings and conclusions in this Review are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Declaration of interests
We declare no competing interests.
See Online for appendix
References
- 1.Hampson K, Coudeville L, Lembo T, et al. Estimating the global burden of endemic canine rabies. PLoS Negl Trop Dis 2015; 9: e0003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manning SE, Rupprecht CE, Fishbein D, et al. Human rabies prevention—United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2008; 57: 1–28. [PubMed] [Google Scholar]
- 3.WHO. Rabies vaccines: WHO position paper—April 2018. Wkly Epidemiol Rec 2018; 16: 201–20. [Google Scholar]
- 4.WHO. WHO expert consultation on rabies: third report. 2018. https://apps.who.int/iris/handle/10665/272364 (accessed July 11, 2022).
- 5.WHO. The immunological basis for immunization series: module 17: rabies. Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 6.Fishbein DB, Sawyer LA, Reid-Sanden FL, Weir EH. Administration of human diploid-cell rabies vaccine in the gluteal area. N Engl J Med 1982; 318: 124–25. [DOI] [PubMed] [Google Scholar]
- 7.Reid-Sanden FL, Fishbein DB, Stevens CA, Briggs DJ. Administration of rabies vaccine in the gluteal area: a continuing problem. Arch Intern Med 1991; 151: 821. [PubMed] [Google Scholar]
- 8.WHO. Rabies. Fact sheet. May 17, 2021. https://www.who.int/news-room/fact-sheets/detail/rabies (accessed July 11, 2022).
- 9.Gerber F, Tetchi M, Kallo V, Léchenne M, Hattendorf J, Bonfoh B, et al. Rabies immunoglobulin: brief history and recent experiences in Côte d’Ivoire. Acta Trop 2020; 211: 105629. [DOI] [PubMed] [Google Scholar]
- 10.Cleaveland S, Fèvre EM, Kaare M, Coleman PG. Estimating human rabies mortality in the United Republic of Tanzania from dog bite injuries. Bull World Health Organ 2002; 80: 304–10. [PMC free article] [PubMed] [Google Scholar]
- 11.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gowda VK, Basavaraja GV, Reddy H, Ramaswamy P. Paralytic rabies following cat scratch and intra-dermal anti-rabies vaccination. J Pediatr Neurosci 2014; 9: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thongcharoen P, Wasi C. Possible factors influencing unsuccessful protection of post-exposure prophylaxis for rabies by human diploid cell vaccine. J Med Assoc Thai 1985; 68: 386–87. [PubMed] [Google Scholar]
- 14.Centers for Disease Control. Human rabies despite treatment with rabies immune globulin and human diploid cell rabies vaccine—Thailand. MMWR Morb Mortal Wkly Rep 1988; 259: 25. [PubMed] [Google Scholar]
- 15.Farahtaj F, Fayaz A, Howaizi N, Biglari P, Gholami A. Human rabies in Iran. Trop Doct 2014; 44: 226–29. [DOI] [PubMed] [Google Scholar]
- 16.Tarantola A, Ly S, Chan M, et al. Intradermal rabies post-exposure prophylaxis can be abridged with no measurable impact on clinical outcome in Cambodia, 2003–2014. Vaccine 2019; 37 (suppl): A118–27. [DOI] [PubMed] [Google Scholar]
- 17.Bennasrallah C, Ben Fredj M, Mhamdi M, et al. Animal bites and post-exposure prophylaxis in Central-West Tunisia: a 15-year surveillance data. BMC Infect Dis 2021; 21: 1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monson MH. Practical management of rabies. Trop Doct 1985; 15: 50–54. [DOI] [PubMed] [Google Scholar]
- 19.Gadekar RD, Domple VK, Inamdar IF, Aswar NR, Doibale MK. Same dog bite and different outcome in two cases—case report. J Clin Diagn Res 2014; 8: 11–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fescharek R, Franke V, Samuel M. Do anaesthetics and surgical stress increase the risk of post-exposure rabies treatment failure? Vaccine 1994; 12: 12–13. [DOI] [PubMed] [Google Scholar]
- 21.Tabbara KF, Al-Omar O. Eyelid laceration sustained in an attack by a rabid desert fox. Am J Ophthalmol 1995; 119: 651–52. [DOI] [PubMed] [Google Scholar]
- 22.Gadre G, Satishchandra P, Mahadevan A, et al. Rabies viral encephalitis: clinical determinants in diagnosis with special reference to paralytic form. J Neurol Neurosurg Psychiatry 2010; 81: 812–20. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh S, Rana MS, Islam MK, et al. Trends and clinico-epidemiological features of human rabies cases in Bangladesh 2006–2018. Sci Rep 2020; 10: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ngugi JN, Maza AK, Omolo OJ, Obonyo M. Epidemiology and surveillance of human animal-bite injuries and rabies post-exposure prophylaxis, in selected counties in Kenya, 2011–2016. BMC Public Health 2018; 18: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madjadinan A, Hattendorf J, Mindekem R, et al. Identification of risk factors for rabies exposure and access to post-exposure prophylaxis in Chad. Acta Trop 2020; 209: 105484. [DOI] [PubMed] [Google Scholar]
- 26.Liu Q, Wang X, Liu B, et al. Improper wound treatment and delay of rabies post-exposure prophylaxis of animal bite victims in China: prevalence and determinants. PLoS Negl Trop Dis 2017; 11: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Addai JA, Nuertey BD. Pattern of animal bites and delays in initiating rabies postexposure prophylaxis among clients receiving care in Korle-Bu Teaching Hospital. J Trop Med 2020; 2020: 6419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abubakar SA, Bakari AG. Incidence of dog bite injuries and clinical rabies in a tertiary health care institution: a 10-year retrospective study. Ann Afr Med 2012; 11: 108–11. [DOI] [PubMed] [Google Scholar]
- 29.Iyalomhe GBS, Iyalomhe SI. Dog bite and clinical rabies in a surburban hospital in Nigeria: a 20-year retrospective study of the prevalence and treatment with anti-rabies vaccine. World J Pharm Res 2015; 4: 113–21. [Google Scholar]
- 30.Ichhpujani RL, Mala C, Veena M, et al. Epidemiology of animal bites and rabies cases in India. A multicentric study. J Commun Dis 2008; 40: 27–36. [PubMed] [Google Scholar]
- 31.Gong Z, He F, Chen Z. Risk factors for human rabies in China. Zoonoses Public Health 2012; 59: 39–43. [DOI] [PubMed] [Google Scholar]
- 32.Yurachai O, Hinjoy S, Wallace RM. An epidemiological study of suspected rabies exposures and adherence to rabies post-exposure prophylaxis in eastern Thailand, 2015. PLoS Negl Trop Dis 2020; 14: e0007248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poorolajal J, Babaee I, Yoosefi R, Farnoosh F. Animal bite and deficiencies in rabies post-exposure prophylaxis in Tehran, Iran. Arch Iran Med 2015; 18: 822–26. [PubMed] [Google Scholar]
- 34.Benavides JA, Megid J, Campos A, Rocha S, Vigilato MAN, Hampson K. An evaluation of Brazil’s surveillance and prophylaxis of canine rabies between 2008 and 2017. PLoS Negl Trop Dis 2019; 13: e0007564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilde H, Lumlertdacha B, Meslin FX, Ghai S, Hemachudha T. Worldwide rabies deaths prevention—a focus on the current inadequacies in postexposure prophylaxis of animal bite victims. Vaccine 2016; 34: 187–89. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan MM, Cohen D, Koprowski H, Dean D, Ferrigan L. Studies on the local treatment of wounds for the prevention of rabies. Bull World Health Organ 1962; 26: 765–75. [PMC free article] [PubMed] [Google Scholar]
- 37.Dean DJ, Baer GM, Thompson WR. Studies on the local treatment of rabies-infected wounds. Bull World Health Organ 1963; 28: 477–86. [PMC free article] [PubMed] [Google Scholar]
- 38.Bonaparte SC, Adams L, Bakamutumaho B, et al. Rabies post-exposure healthcare-seeking behaviors and perceptions: results from a knowledge, attitudes, and practices survey, Uganda, 2013. PLoS One 2021; 16: e0251702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mshelbwala PP, Weese JS, Sanni-Adeniyi OA, et al. Rabies epidemiology, prevention and control in Nigeria: scoping progress towards elimination. PLoS Negl Trop Dis 2021; 15: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo C, Li Y, Huai Y, et al. Exposure history, post-exposure prophylaxis use, and clinical characteristics of human rabies cases in China, 2006–2012. Sci Rep 2018; 8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montgomery JP, Zhang Y, Wells EV, et al. Human rabies in Tianjin, China. J Public Health 2012; 34: 505–11. [DOI] [PubMed] [Google Scholar]
- 42.Pieracci EG, Pearson CM, Wallace RM, et al. Vital signs: trends in human rabies deaths and exposures—United States, 1938–2018. MMWR Morb Mortal Wkly Rep 2019; 68: 524–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Endy TP, Keiser PB, Cibula D, et al. Effect of antimalarial drugs on the immune response to intramuscular rabies vaccination using a postexposure prophylaxis regimen. J Infect Dis 2020; 221: 927–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


