Abstract
T cell-redirecting therapies (TCRTs), such as chimeric antigen receptor (CAR) or T cell receptor (TCR) T cells and T cell engagers, have emerged as a highly effective treatment modality, particularly in the B and plasma cell-malignancy setting. However, many patients fail to achieve deep and durable responses; while the lack of truly unique tumor antigens, and concurrent on-target/off-tumor toxicities, have hindered the development of TCRTs for many other cancers. In this review, we discuss the recent developments in TCRT targets for hematological malignancies, as well as novel targeting strategies that aim to address these, and other, challenges.
Keywords: CAR T cell; T cell engager; T cell-redirecting therapies; immunotherapy; hematological malignancies; on-target, off-tumor toxicities
Graphical abstract
In this review, Chapman and Anderson summarize preclinical and early-stage clinical targets for T cell-redirecting therapies (TCRTs) in hematological malignancies and discuss novel targeting strategies that will improve the specificity of future TCRT therapies to enable safer and more effective treatment.
Introduction
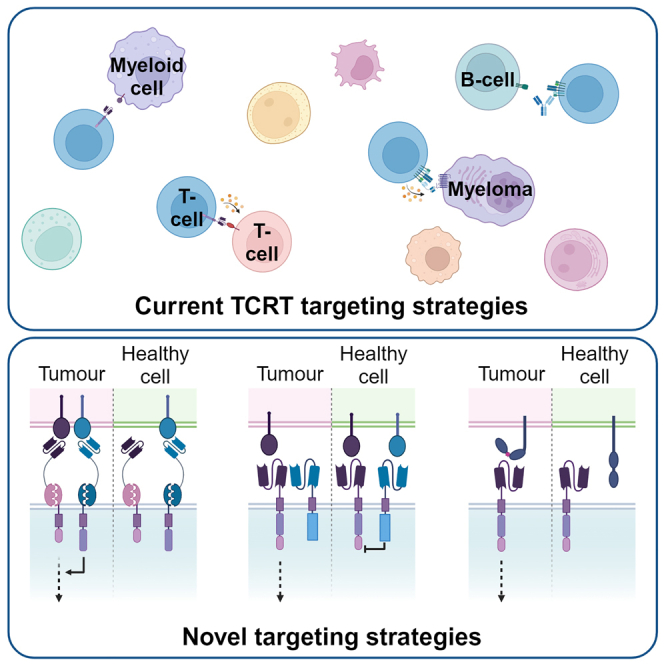
The introduction of immunotherapies has led to a paradigm shift in cancer treatment over the last two decades. The term immunotherapy encompasses a wide range of treatment modalities that use the immune system to help fight cancer. The initial focus was on antibodies. These can block or stimulate cell signaling pathways to induce cell death or reduce proliferation, recruit immune cells to induce cell death, reverse immunosuppression (checkpoint inhibition), or deliver toxins to malignant cells with antibody-drug conjugates (ADCs). T cell-redirecting therapies (TCRTs) have more recently moved into the spotlight. TCRT describes the use of T cell engagers (TCEs), bi- or tri-specific antibodies that can bring T cells and targets into close contact to initiate cell killing, and adoptive cell therapy using chimeric antigen receptor (CAR) or T cell receptor (TCR) T cells that have been genetically engineered to target a specific tumor antigen (Figure 1). CD19- and B cell maturation antigen (BCMA)-targeted CAR-T therapies have proved particularly successful in the treatment of B and plasma cell malignancies, respectively. Loss of lineage-restricted markers, such as CD19 for B cells, is reasonably well tolerated. However, other cancers have proved more challenging to target. In this review, we discuss emerging preclinical and early-phase targets in the TCRT field for hematological malignancies and novel targeting strategies to improve the specificity and efficacy of these therapies.
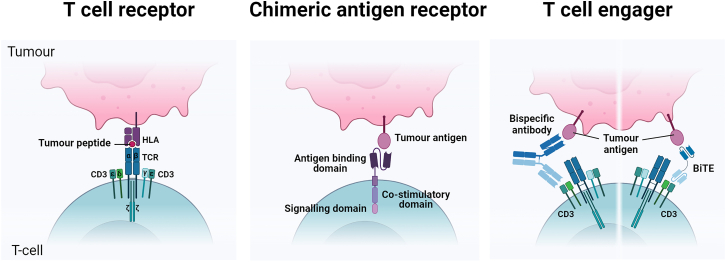
Figure 1.
Schematic view of T cell-redirecting therapies
The T cell receptor (TCR) complex, composed of two TCR chains (α/β or γ/δ) and six CD3 chains, recognizes peptides presented by the MHC on the target cell. A chimeric antigen receptor (CAR) contains an antigen-binding domain (typically a single-chain variable fragment [scFv]) fused to a co-stimulatory domain (such as CD28 or 4-1BB) and a CD3ζ signaling domain. TCEs: bispecific antibodies or bispecific TCEs (BiTEs) target a tumor antigen and CD3 simultaneously to activate and redirect T cells to tumor cells.
TCRTs in hematological malignancies
What makes an ideal TCRT target?
For a safe and effective TCRT, target selection is paramount. An ideal TCRT target should be consistently expressed across all tumor cells within a patient, including cancer stem cells, to achieve tumor clearance and prevent tumor recurrence. For CAR T cells and TCEs, targets must be localized at the cell surface and should be highly expressed to achieve full T cell activation.1 While not essential, expression of TCRT targets should not show significant inter-patient heterogeneity, to maximize the number of patients that can benefit from a given therapy. Off-tumor expression can be tolerated to some degree in non-vital cells, but absent expression in vital tissue is imperative to prevent severe on-target/off-tumor toxicities. To avoid fratricide, which can impair CAR-T cell manufacturing and efficacy,2 target antigens should not be expressed on T cells, and although not essential, an ideal target antigen would play a pro-tumorigenic role, such that loss of the antigen is unlikely, or would increase susceptibility to other therapies.
B cell malignancies
B cell malignancies encompass a large and highly heterogeneous group of cancers that can arise at various stages of the B cell differentiation pathway. Some B cell tumor targets are pan-B cell markers, such as CD19, CD20, and CD22, and the vast majority of TCRT clinical trials target one of these three antigens (clinicaltrials.gov). CD19-targeted therapies have shown unprecedented efficacy, achieving up to 90% complete response (CR) rates in trials for B cell acute lymphoblastic leukemia (B-ALL)3,4 and B cell lymphomas5,6,7 and there are now four US Food and Drug Administration (FDA)-approved CAR-T cell products targeting CD19. Despite these impressive responses, not all patients will respond, and many fail to achieve long-term remission.8 CD19-negative relapses can be treated with CD22 or CD20 TCRTs,9,10 but these antigens are also subject to antigen escape.11,12,13 Combination therapies against CD19, CD20, and CD22 may improve outcomes,14,15,16 but it is likely that additional targets will be needed. Possible preclinical and early-phase targets are summarized in Table 1.
Table 1.
Preclinical and early-phase TCRT targets for B cell malignancies
| Targeta | Reported on-tumor expression | Reported off-tumor expression | Advantages | Disadvantages | Additional notes |
|---|---|---|---|---|---|
| BAFF-R | high expression in mature B cell neoplasms (BL, MCL, FL, DLBCL, MZL, B-CLL) and aberrant expression in B-ALL18,19,20 | healthy mature B cells17 and low expression in hepatocytes280,281 | BAFF-R CAR T cells and mAbs are effective in vitro and in vivo against patient samples following CD19- and CD20-targeted immunotherapies20,21 pro-survival role in some healthy B cells, which may prevent antigen escape/loss17 not on normal pre-B cells, which may reduce the severity of B cell aplasia compared to CD19-targeted TCRTs18 |
low or varied expression in immature B cells could restrict application in some B cell neoplasms17 off-tumor expression poses a risk for hepatotoxicity and will cause B cell aplasia17,280,281 |
CAR T cells using BAFF as the antigen recognition domain can target all three BAFF receptors (BAFF-R, TACI and BCMA), which may mitigate the risk of antigen escape and broaden patient applicability, but would also cause plasma cell depletion17,281 BAFF-R targeting mAbs have been well tolerated, demonstrating potential safety for this target282 phase I trials for TCRTs are ongoing. Preliminary results from a phase I CAR T cell are encouraging with a 100% ORR in the three patients reported thus far22 |
| CD79ab | MALT, DLBCL, MCL, FL, BL, and MZL25,26,27 low expression in CLL25 |
healthy B cells25 and immature myeloid cells (CD79a)283 | highly restricted to the B cell lineage, limiting OTOT toxicities23 CD79a and b expression is retained in patient samples after CD19- and CD22-targeting TCRTs25,27,284 pro-survival role in some lymphomas may prevent antigen escape/loss23 |
off-tumor expression will cause B cell aplasia23 target of interest for mature B cell neoplasms only25,26,27 |
the FDA approval of a CD79b-ADC (polatuzumab vedotin) for the treatment of DLBCL, supports the safety and efficacy of CD79-TCRTs28 phase I/II trials for TCRTs ongoing with results pending |
| CD37 | DLBCL, BL, MCL, FL, and MZL30,285,286,287 | healthy B cells and minimal monocyte expression30,288 | high and homogeneous expression across B-NHL subtypes286,288 | target of interest for mature B cell lymphomas only287 off-tumor expression will result in B cell aplasia288 a case of CD37 antigen loss following CD37-CAR-T cell therapy has already been reported36 |
early-phase clinical trials of antibody-based therapies targeting CD37 had limited efficacy31,32,33 clinical trials are ongoing and suggest potential efficacy for CD37-CAR T cells (2 CR, 1 PR, and 1 PD) but two cases of prolonged pancytopenia with marrow aplasia is of concern36 CD37 is also aberrantly expressed in some T cell malignancies30 |
| CD72 | B-ALL and B-NHL289 particularly high expression in MLLr B-ALL38 |
healthy B cells38 | higher expression in DLBCL than CD22 which may improve efficacy9,38 expressed on all subtypes of B-ALL 38 CD72 loss may increase sensitivity to chemotherapy through decreased adhesion within the bone marrow niche38 low risk for OTOT toxicities38 CD72-CAR T cells were effective in a preclinical model of CD19low/neg relapse38 |
CD72low relapse has been reported in preclinical xenograft models290 | may also be a target of interest for AML289 not in clinical trials yet but Temple et al. suggest that their CD72-CAR T cell will be progressed to the clinic290 SHIP-1 inhibitors can increase CD72 expression, providing a rational combination strategy to improve efficacy38 |
| ROR1 | DLBCL, CLL, MCL, and a subset of B-ALL40,291,292 | absent in all B cells except a subset of normal B cell precursors40 adipose tissue, pancreas, gastrointestinal tract, parathyroid glands, and lung40,293,294 |
mature B cells would be spared, providing some short-term protection of humoral immunity40 side-population CLL cells, a chemo-resistant population, are sensitive to ROR-1 CAR T cells40 Increased expression in CD19-TCRT relapsed MCL patients292 |
risk for the long-term depletion of immature B cells40 non-lymphoid tissue expression poses a serious OTOT toxicity risk and lethal OTOT toxicity has been reported in preclinical ROR1-CAR-T cell xenograft models42,294 restricted to more mature B cell neoplasms40,291,292 |
clinical experience with the ROR1-ADC (zilovertamab vedotin [VLS-101]) in CLL and B-NHL, with no unexpected toxicities reported in a phase I trial295 preliminary results from ROR1 CAR T cells for solid malignancies and a ROR1 bispecific TCE in R/R MCL/CLL also suggest safety.296,297,298 However, a grade 5 AE (consistent with CRS and ICANS) in a separate ROR1-CAR-T cell trial warrants caution.299 Efficacy is promising thus far298,300 |
| TSPLR | TSLPR-overexpressing Philadelphia-like B-ALL43,44 | dendritic cells, subset of T cells and monocytes. Cytoplasmic staining in the kidney, colon, liver, and skin43,44 | highly expressed in a high-risk prognosis that has a high-rate of relapse and poor-response to chemotherapy301 pro-tumorigenic role in B-ALL may prevent antigen escape/loss302 |
the low expression on other immune cell subsets may pose an OTOT toxicity risk43 restricted expression to a small subset of B-ALL patients43 |
TSLPR-CAR T cells demonstrated comparable efficacy to CD19- and CD22-CAR T cells in in vivo xenograft models43 |
| Light chain (kappa or lambda) | late-stage immature/mature B cell neoplasms (B-NHL, CLL/SLL and MM)46,303 | late-stage immature/mature B cells expressing the target light chain (approximately half)46,303 | expressed in the majority of B-NHL subtypes46 a substantial proportion of healthy B cells would be spared, preserving humoral immunity and reducing the risk of severe infections compared to pan-B cell CAR T cells303 free immunoglobulins may provide low tonic-signaling that may promote CAR-T cell persistence303 pro-survival role for BCR signaling in some lymphomas may prevent antigen escape/loss23 |
possible loss of immune responses against particular epitopes (although this should be compensated for by reciprocal light chain Ig against different epitopes)303 expression restricted to mature B cell neoplasms46,303 |
kappa-light-chain phase I clinical trial safety results are encouraging, albeit with modest efficacy (2 CR, 1 PR, 1 SD, and 5 NR). This may be partly due to the lymphodepletion regime prior to infusion45 lambda-light-chain CAR T cells may be particularly beneficial for MCL, which is more commonly lambda-light-chain positive than kappa46 |
| IGHV4-34 | subset of late-stage immature/mature B cell neoplasms (B-NHL, CLL)47,304 | late-stage immature/mature B cells expressing IGHV4-34 (∼5%)47 | the majority (∼95%) of healthy B cells should be spared47 | although IGHV4-34 is commonly expressed in some subtypes (DLBCL, vitreoretinal lymphomas, HCL, and CLL), IGHV4-34 TCRTs would be highly restricted to a small subset of B cell neoplasms47,304 | IGHV-34 is also a target of interest for systemic lupus erythematosus305 still at the preclinical stage |
| CD70 | DLBCL, FL, HL, MM, and WM306 | subset of activated B and T cells and dendritic cells306 | CD70 CAR T cells have shown preclinical activity in vivo against CD19neg target cells307 low risk for OTOT toxicities: transient off-tumor expression that is restricted to a subset of activated immune cells306 |
elimination of CD70-positive T cells could impair T cell-mediated immunity, including anti-EBV responses308 | CD70-targeting ADCs have shown limited efficacy, and their clinical application is limited by frequency and severity of thrombocytopenia.309 However, this is likely due to treatment modality.309 B and T cell aplasias were not reported309 clinical trials are ongoing with results pending also a target of interest for AML, T-ALL, and MM306,310 |
| CD74 | B-NHL, HL, CLL, MM, and WM134,311,312,313 | healthy B cells, monocytes, dendritic cells, subset of myeloid cells, and subset of T cells134,312 | CD74-CAR T cells were effective in vitro against a post-CD19-TCRT relapse patient sample313 pro-survival role in B cells which may prevent antigen escape/loss313 |
expression on healthy B cells as well as other immune cells may be a risk for cytopenias313,314 | CD74-targeting mAbs and ADCs have shown safety but limited efficacy in clinical trials315,316,317 |
| CD32b | CLL/SLL, MCL, and SMZL318,319 | healthy B cells, subset of T- and dendritic- cells319,320 non-lymphoid tissue: airway smooth muscle cells, liver sinusoidal endothelial cells, Kupffer cells and placenta319 |
CD32b mediates resistance to rituximab by antibody internalization. Therefore, loss of target antigen could increase sensitivity to rituximab321 higher and more uniform than CD19 in CLL319 |
risk for OTOT toxicities toward non-lymphoid tissue and B cells319 T cell expression may lead to some CAR-T cell fratricide320 only approximately half of B-NHL are positive for CD32b, which would limit therapeutic applicability318 |
– |
AE, adverse event; ALL, acute lymphoblastic leukemia; BCR, B cell receptor; BL, Burkitt lymphoma; cHL, classical Hodgkin lymphoma; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; CR, complete response; CRS, cytokine release syndrome; DLBCL, diffuse large B cell lymphoma; FL, follicular lymphoma; HL, Hodgkin lymphoma; HSPC, hematopoietic stem and progenitor cell; ICANS, Immune effector cell-associated neurotoxicity syndrome; mAb, monoclonal antibody; MALT, mucosa-assisted lymphoid tissue lymphoma; MCL, mantle cell lymphoma; MM, multiple myeloma; MZL, marginal zone lymphoma; NHL, non-Hodgkin lymphoma; NR, no response; ORR, overall response rate; OTOT, on target, off tumor; PD, progressive disease; PR, partial response; R/R, relapsed/refractory; SD, stable disease; SMZL, splenic marginal zone lymphoma; TCE, T cell engager; TCRT, T cell-redirecting therapy; WM, Waldenstrom macroglobulinemia.
One of the most promising recent targets is the B cell-activating factor receptor (BAFF-R), which plays a key role in B cell viability, development, and survival.17 BAFF-R is also highly expressed in mature B cell neoplasms18,19 and expression is retained after relapse with CD19- and CD20-targeted therapies,20,21 making BAFF-R an attractive target in B cell disease. Even though BAFF-R is expressed at lower levels than CD19, anti-BAFF-R CAR T cells have shown preclinical efficacy against a wide range of lymphoma and chronic lymphocytic leukemia (CLL) cell lines.20 They are now in early-phase clinical trials, with promising initial results.22
The B cell receptor is a protein complex formed of surface immunoglobulin and its signaling component CD79, a heterodimer of CD79a and CD79b.23 These two proteins are restricted to the B cell lineage, are highly expressed in the majority of B cell lymphomas, and play a pro-survival role that can drive tumorigenesis.24,25,26,27 CD79b is the most clinically advanced target of the two and an anti-CD79b ADC (polatuzumab vedotin, CD79b-MMAE) has been FDA approved for the treatment of diffuse large B cell lymphoma (DLBCL), validating CD79 as a safe and effective target.28 Several CD79-targeting TCRT trials are currently ongoing with results pending (clinicaltrials.gov).
CD37 is another target of interest for mature B cell neoplasms.29,30 Although early-phase clinical trials of antibody-based therapies have proved disappointing, with several terminated early by their sponsor,31,32,33,34,35 it is possible that CAR T cells may fare better, with impressive preclinical30 and preliminary clinical data.36 One potential concern with targeting CD37 is off-tumor expression on monocytes,37 raising the prospect of on-target/off-tumor toxicity.
Excluding CD19 and CD22, CD72 is the only target known to be expressed across all B-ALL subtypes. Although currently only at the preclinical stage, Investigational New Drug approval for a nanobody-based CAR targeting CD72 is underway.38 CD72 is particularly highly expressed in the poor-prognosis MLLr B-ALL subtype, which is less responsive to more classic CAR-T cell targeting.38
Although these targets will expand the therapeutic repertoire in the post-CD19-relapse landscape, a particular challenge remains unaddressed (i.e., frequent B cell aplasia). That loss of healthy B cells can be clinically managed using immunoglobulin (Ig) infusions has facilitated the rapid advancements of TCRTs in this disease area. Although it is considered clinically tolerable, B cell aplasia can persist for several years post CAR-T cell infusion39—beyond the usual nonspecific cytopenias associated with CAR-T cell therapy—putting patients at an increased risk for severe infections. Identifying target antigens with minimal expression on healthy B cells would be highly desirable.
The oncofetal protein receptor tyrosine kinase-like orphan receptor 1 (ROR1) may enable more selective targeting. ROR1 is differentially expressed between normal and malignant B cells, and preclinical studies suggest that ROR1-CAR T cells selectively kill CLL cells while sparing resting and activated B cells.40 While this offers the potential of fewer infections compared to pan-B cell targets, the potential long-term loss of immature B cells and low-level expression in non-hematological cells may present a risk of other on-target/off-tumor toxicities.40,41,42
TSLPR (CRLF2) overexpression due to gene rearrangements is a frequent occurrence in the poor-prognosis Philadelphia chromosome-like (Ph-like) ALL subtype.43 In vivo studies have shown efficacy of both bispecific TCEs and CAR T cells,43,44 but results from an ongoing trial will be imperative to establish on-target/off-tumor toxicities. Further, TSLPR is highly restricted to a small subset of B-ALL, which will limit widespread utility of this target.43
Finally, the clonal nature of some B cell malignancies may offer a strategy to minimize B cell aplasia. Targeting the light chain (kappa or lambda) provides high specificity for the clonal malignant cells while sparing a proportion of the healthy B cells expressing the reciprocal light chain.45 Kappa-CAR T cells have shown safety and feasibility in mature B cell malignancies. Efficacy was limited but this may be in part due to the absence of a strong lymphodepleting regime.45 Preclinical evidence suggests lambda-light-chain CAR T cells would be similarly well tolerated.46 Similarly, the IGHV4-34 heavy-chain-variable gene is frequently expressed in a proportion of clonal mature B cell malignancies but only in ∼5% of the normal B cell repertoire.47 Other TCRT targets of interest but not discussed further herein include CD70, CD74, and CD32b (Table 1).
Multiple myeloma
Multiple myeloma (MM), a plasma cell malignancy, has seen dramatic improvements in survival over the last two decades with the advent of immunomodulatory agents, proteasome inhibitors, and anti-CD38 targeted monoclonal antibodies.48 Despite these advancements, responses are not typically durable, and patients will eventually relapse and become refractory to treatment. Like B cell malignancies, myeloma is well suited to immunotherapy as healthy plasma cell loss is reasonably well tolerated. BCMA (TNFRSF17) is the target that has paved the way in myeloma, achieving an impressive 81% overall response rate (ORR) in a first-in-human trial.49 Follow-up analyses have confirmed similar results across multiple trials50 and there are now four FDA-approved BCMA-targeted immunotherapies: two CAR T cells and two bispecific TCEs. Nonetheless, most patients still progress after BCMA-targeted therapy49,51,52,53 and additional targets for TCRTs will be required to maintain durable remissions, or even cure. Potential targets are summarized in Table 2.
Table 2.
Preclinical and early-phase TCRT targets for myeloma
| Targeta | Reported on-tumor expression | Reported off-tumor expression | Advantages | Disadvantages | Additional notes |
|---|---|---|---|---|---|
| GPRC5D | MGUS, SMM, PCL, and MM59 | healthy PCs.59 hair follicles, nail beds, filiform papillae of the tongue and inferior olivary nucleus62,63 |
highly expressed in myeloma with no B cell expression and minimal PC expression. This may reduce the severity and/or frequency of infections as seen with BCMA-TCRTs.58,322 Clinical trial results support this, with lower rates of severe infections54,55,56,57,62 GPRC5D TCRTs have shown efficacy in patients with prior BCMA-targeting therapies exposure55,56,62 as a GPCR, exposed epitopes are likely to be membrane-proximal, which may enable the formation of more efficient immune synapses to enhance anti-tumor efficacy59 |
high frequency of nail- and skin-related AEs and dysgeusia in clinical trials due to off-target expression.55,56 OTOT expression may also be the reason for the cerebellar toxicities reported for two GPRC5D-CAR-T cell products54,62 GPRC5Dlow/neg progressive disease has reported in patients receiving CAR T cells and TCEs52,62 |
clinical trial results have shown promising efficacy, with both TCEs and CAR T cells achieving >70% ORRs across multiple trials54,55,56,57,62,323 talquetamab (GPRC5DXCD3 bispecific TCE) recently received accelerated approval and conditional marketing authorization in Europe for triple-class exposed R/R MM60,61 although cerebellar toxicities have been reported for two CAR-T cell products, no neural toxicities have been reported for a third CAR-T cell product or any bispecific TCEs54,55,56,57,62 |
| FCRL5/FCRH5 | MGUS, MM (and HCL, CLL, and MCL)64,67 | B lineage: pre-B cell to PC64,67 | low risk for OTOT with no know expression outside the B cell compartment67 lower expression on B cells and healthy PCs may limit cytotoxicity toward these cells64,66,67 higher and more uniform expression than BCMA, and expression is retained in patients post BCMA TCRT.66,67 FCRL5XCD3 TCEs have shown efficacy in patients who have previously received BCMA-targeted immunotherapies69 expression is associated with 1q21 gain, a poor prognostic marker in MM65,66,67 |
cleavage of FCRL5 could provide a means for antigen escape and may impair CAR-T cell cytolytic activity67 | phase I results for an FCRL5-ADC (DFRF4539A, NCT01432353) were disappointing with two (5%) PR, one (3%) MR, and 18 (46%) SD as best response.68 TCRTs may be a more effective means to target FCRH5 as suggested by early clinical results69,71 early results suggest lower response rates for FCRL5-TCEs than GPRC5D- and BCMA-TCEs (54.5% ORR at the 160-mg dose level, NCT03275103) but responses are durable69,71 may also be a target of interest for MCL, HCL, and CLL67 |
| SLAMF7 (CS1) | MGUS, SMM, MM324 | pro-B cells, plasma cells, NK cells, T cells, activated monocytes, dendritic cells324,325 | SLAMF7 is expressed in all MM patients and is retained in relapsed disease, including post BCMA-CAR-T cell therapy51,324,325 pro-tumorigenic326 |
SLAMF7 is expressed on nearly all CD8+ T cells, resulting in fratricide325 SLAMF7 can be cleaved, providing a mechanism for antigen escape, and CAR-T cell binding of soluble protein may limit efficacy327 Off-tumor expression on other immune cell subsets poses a serious risk for lymphopenia325 |
the FDA-approved SLAMF7-targeting mAb (elotuzumab) has shown anti-MM activity when used in combination for R/R MM, but activity in newly-diagnosed MM is limited328 SLAMF7-CAR-T cell trials are currently ongoing with results pending. Results from a bispecific BCMA-SLAMF7 CAR-T cell trial suggest that SLAMF7-targeting does not increase the rate of infections compared to BCMA CAR T cells alone, but these bispecific CAR T cells do have reduced cytolytic activity329 |
| Kappa-light chain | late-stage immature/mature B cell neoplasms (B-NHL, CLL/SLL) and kappa-restricted MM46,303 | late-stage immature/mature B cells expressing the kappa-light chain (approximately half)303 | CAR T cells selectively eliminate clonal malignant cells while sparing normal B cells with the reciprocal light chain (approximately half)45,303 although surface immunoglobulin is minimal on MM cells, surface immunoglobulin is expressed on myeloma-initiating cells330 |
kappa-light-chain immunoglobulins are secreted by myeloma cells, and so the low surface expression may limit efficacy. In addition, the high level of secreted immunoglobulins in MM might lead to excessive stimulation and exhaustion45 | in a phase I trial for kappa-CAR T cells for NHL/CLL and MM, modest anti-myeloma effects were observed (four of seven achieving SD as best response)45 KMA is a membrane-bound form of kappa-light chain found in kappa-restricted MM. KappaMab (MDX-1097), a KMA-targeting mAb, demonstrated efficacy in a phase IIb trial, which may support the targeting of KMA instead of surface kappa Ig for MM331 |
| CD229 | B-NHL, MM, and PCL72,73,74,332 | NK cells, mature B and T cells, and plasma cells72,74,332 | expression is highly restricted to the hematopoietic compartment72 pro-survival role may reduce the risk for antigen escape72 expressed on the chemo-resistant myeloma-initiating/propagating cells73,74 |
OTOT expression on other immune cells will likely result in cytopenias. CD229 is downregulated following CD3/CD28 stimulation, which may limit CAR-T cell fratricide during manufacturing, but it is currently unknown if this downregulation will be sustained post infusion74 soluble CD229 (sCD229) is increased in advanced disease.332 It is currently unknown if CAR T cells recognize sCD229 but it may abrogate activity |
affinity-tuned CD229 CAR T cells retain anti-myeloma activity but lack cytolytic activity toward healthy lymphocytes75 |
| CD1d | MGUS and MM333 | antigen-presenting cells, B cells, epithelial cells, thymocytes, activated T cells, and HSCs334,335,336,337 | CD1dXVδ2 bispecific Vγ9Vδ2-TCE can recruit both NKT- and Vγ9Vδ2 T cells, which preferentially target malignant cells over healthy cells (reducing the risk for OTOT toxicities) and have a lower risk for CRS334 | high risk for antigen escape as CD1d ligation induces B cell and PC death, and expression is lost with disease progression333,334 Expressed on in vitro activated T cells which may preclude CAR-T cell development335 |
low expression in advanced disease may limit efficacy in the R/R patient populations likely to constitute early-phase clinical trial cohorts334 CD1d is also a target of interest for (myelo)monocytic AML and CLL334 early clinical trial results for a CD1dXVδ2 TCE suggest limited efficacy in MM/CLL (disease stabilization in two of eight patients)338 |
| SEMA4A | MGUS, SMM, and MM79,80 | monocytes, granulocytes, healthy PCs, and a subset of T cells79 | pro-survival role in MM may reduce the risk for antigen escape79 SEMA4A is expressed in the majority (>90% of patients) and is retained in advanced R/R disease. Expression is higher than BCMA, FCRL5, and GPRC5D79,339 |
OTOT expression may pose a risk for cytopenias. However, expression is much lower than malignant cells and no cytopenias were seen in a murine toxicity model using a cross-reactive SEMA4A-ADC79 increased expression in T cells post activation poses a risk for fratricide during manufacturing but does not appear to affect cytolytic capabilities in vitro339 |
– |
| CD46 (MCP) | MGUS, SMM, MM78 | PC, monocytes, granulocytes, placenta, and prostate78 | expressed in myeloma-initiating cells76 higher expression in MM than healthy PCs may reduce the risk for hypoglobulinaemia78 expression is associated with 1q21 gain, a poor prognostic marker in MM78 |
moderate monocyte and granulocyte expression may pose a risk for cytopenias78 | phase I results of a CD46-ADC (FOR46) have shown modest efficacy (three PR in six patients) with severe cytopenia (3 Gr four neutropenia and one Gr 4 thrombocytopenia)77 |
| ILT3 (LILRB4) | MM80,82 | monocytes, macrophages, and dendritic cells80 | an ILT3XCD3 bispecific TCE has shown activity against samples from relapsed patients post BCMA-CAR-T cell therapy80 ILT3 is a negative immune receptor and can suppress T cell proliferation in MM and AML, providing a strong rationale for therapeutic targeting121 |
OTOT expression on other immune cells may pose a risk for cytopenias80 | also a target of interest for monocytic AML340 |
| CCR10 | MM82 | healthy PCs, T cells82 | low risk for OTOT toxicities as minimal expression on other hematopoietic cell subsets82 Expression is increased in R/R advanced disease82 |
CCR10 is upregulated on activated T cells, which resulted in fratricide and CAR-T cell manufacturing difficulties in a preclinical study of an anti-CCR10-CAR T cell82 | – |
AEs, adverse events; GPCR, G-protein-coupled receptor; HCL, hairy cell leukemia; KMA, kappa myeloma antigen; MGUS, monoclonal gammopathy of undetermined significance; MR, minimal response; PC, plasma cell; PCL, plasma cell leukemia; SMM, smoldering multiple myeloma. Other abbreviations as in Table 1.
Recently, GPRC5D has become one of the most prominently targeted surface proteins in myeloma.54,55,56,57 Hematopoietic expression is tightly restricted to plasma cells, and, unlike BCMA, expression is much greater in malignant cells compared to their healthy counterparts.58 As a G-protein-coupled receptor, it is postulated that GPRC5D is less likely to be shed, cutting off one means of antigen escape, and that the exposed epitopes will be closer to the membrane surface and enable the formation of more efficient immunological synapses between targets and T cells.59 Talquetamab, a GPRC5D bispecific TCE, has recently received accelerated FDA approval and conditional marketing authorization in Europe for relapsed/refractory myeloma60,61 on the back of impressive efficacy in a phase I/II trial (>70% ORR).56 Forimtamig, a second GPRC5D bispecific TCE, also has promising clinical efficacy,55 while GPRC5D-targeting CAR T cells have shown very encouraging results in early-phase trials.54,57,62 Unfortunately, expression of GPRC5D has been demonstrated outside the immune system, including in hair follicles, nail beds, filiform papillae of the tongue, and potentially the inferior olivary nucleus,54,55,56,62,63 and predictable on-target/off-tumor side effects have been observed in these clinical studies.54,55,56,62
FCRL5 (FCRH5) expression is also higher in malignant cells compared to healthy plasma cells and is minimally expressed on B cells.64,65,66 Expression is not correlated with BCMA and is generally higher and more consistent, making this another potential target in the post-BCMA landscape.66,67 Activity as an ADC target was underwhelming,68 but TCRTs often show greater clinical efficacy than ADCs, and FCRL5 has re-emerged as an effective TCE target. Response rates appear lower than GPRC5D- and BCMA-targeted TCEs, but responses were durable.56,69,70,71 Investigation into FCRL5 as a CAR-T cell target is currently at the preclinical stage but is showing promise, including in BCMA-negative disease.66,67 While FCRL5 and GPRC5D TCRTs have exhibited impressive anti-myeloma responses, especially in patients with prior BCMA therapy exposure, GPRC5Dlow/neg progressive disease has already been documented,52,62 and there is the potential for antigen escape through FCRL5 cleavage.67
With its anti-apoptotic role in MM and expression on potential MM-initiating/propagating cells, CD229 (LY9) may be a promising candidate target for inducing more durable remissions.72,73,74 However, expression on other hematopoietic cells may require affinity optimization to mitigate off-tumor toxicities.75 CD46 is another target present on myeloma-initiating cells,76 and a CD46-ADC has shown modest anti-myeloma activity in a phase I trial,77 but, again, moderate monocyte and granulocyte expression may necessitate additional engineering strategies to prevent longer-term cytopenias than are seen with other TCRTs.77,78 Although myeloma cells typically do not express surface immunoglobulin, it has been reported that kappa-restricted myeloma-initiating cells may do, providing a rationale for kappa-CAR T cells in myeloma. Clinically, kappa-CAR T cells have shown limited efficacy, only achieving stable disease in four patients as best response.45 This is likely because the low surface expression of the target and kappa-CAR T cells may fare better in combination therapy to enable the eradication of both malignant cells and initiating cells. We, and others, recently identified SEMA4A as a novel myeloma immunotherapeutic target using cell-surface proteomics.79,80 SEMA4A expression is essential for normal myeloma growth in vitro, suggesting a reduced risk for antigen escape.79 SEMA4A is expressed in other hematopoietic cells, but at a considerably lower level than in myeloma, and we did not see any cytopenias in a murine toxicity model.79 Other cell-surface proteomic studies have revealed ILT3 (LILRB4) and CCR10 as additional TCRT targets of interest for myeloma81,82 (Table 2).
Acute myeloid leukemia
Acute myeloid leukemia (AML), a malignancy of myeloid stem cells, is the most common form of adult acute leukemia. Standard care is chemotherapy, and, although most patients achieve complete remission, this response is often not durable, leading to relapse with chemo-resistant disease.83 Allogeneic hematopoietic stem cell (HSC) transplant (allo-HSCT), which exploits graft-versus-tumor cytotoxicity, was an early form of immunotherapy that is still utilized commonly in treatment. However, developing targeted immunotherapies for this disease has been challenging. This is partly a result of disease heterogeneity,84 but also because potential antigen targets are also expressed by hematopoietic stem and progenitor cells (HSPCs),84,85 whose long-term loss is less well tolerated than the B cell aplasia seen with CD19-targeted TCRTs. As a result, current TCRTs are predominantly being investigated as a bridge to transplant. Several such novel AML targets are summarized in Table 3.
Table 3.
Preclinical and early-phase TCRT targets for AML
| Targeta | Reported on-tumor expression | Reported off-tumor expression | Advantages | Disadvantages | Additional notes |
|---|---|---|---|---|---|
| CD33 | AML bulk cells and LSCs84 | myeloid progenitor cells, neutrophils, macrophages, T cells, dendritic cells, Kupffer cells, and hepatocytes84,86 | expressed in most AML patients, on both bulk cells and LSCs84 CD33 expression is retained in relapsed disease341 |
high risk for OTOT toxicities due to expression on hematopoietic cells, including CD34+CD38+ progenitor cells84 severe hepatotoxicity and fatal cytopenias have been reported using CD33-ADCs86,87,88 risk for CAR-T cell fratricide due to low T cell expression84 |
gemtuzumab ozogamicin, a CD33-ADC, received accelerated approval in 2000, but was withdrawn in 2010 due to serious adverse events. Gemtuzumab ozogamicin was approved again in 2017 KO of CD33 (discussed later) in CD34+ HSPCs prior to SCT may provide a means of safely targeting this antigen267 limited efficacy for CD33-targeting TCRTs in clinical trials89,90,91,94,342,343 |
| CD123 | AML bulk cells and LSCs84 | HPCs, monocytes, granulocytes, and endothelial cells106,344 | expressed in most AML patients, on both bulk cells and LSCs84 pro-survival role in AML95 lower expression on HPCs than AML blasts may provide protection97,98,195 |
expression on myeloid progenitors, monocytes, granulocytes pose a risk for severe myelotoxicity.96 OTOT expression on endothelial cells may cause serious adverse events (capillary leak syndrome and severe CRS)106 |
limited efficacy and severe CRS for CD123-targeting TCEs.99,100,101 CAR-T cell efficacy may be greater102,103 but severe adverse events (two grade 4 capillary leak syndromes and a grade 5 CRS) pose a serious concern.104,105 |
| CLL-1 (CLEC12A) | AML bulk cells and LSCs84,107 | myeloid progenitor cells, monocytes and granulocytes 107,108 | expressed in most AML patients, on both bulk cells and LSCs84,108 restricted to the myeloid-lineage, minimal expression on CD34+CD38− HSCs and lymphoid progenitor cells107,108 |
expression in myeloid-lineage cells pose a risk for severe myelotoxicity.107,108 CLEC12Aneg cells have been observed in some AML patients345 |
impressive efficacy for CLEC12A-CAR T cells in a phase I trial (70% ORR) but all patients developed severe pancytopenia and two died of severe infection due to chronic agranulocytosis112 |
| FLT3 | AML bulk cells and LSCs116 and B-ALL118 | HSPCs346 | expressed in most AML patients, on both bulk cells and LSCs116 activating mutations in AML are common and a marker of adverse prognosis347,348 low risk for OTOT toxicities: healthy tissue expression restricted to a subset of HSPCs116 pro-survival role in HSPCs suggests a reduced risk for antigen escape/loss346 |
expression on HSPCs could lead to profound myelosuppression346 cytoplasmic expression has been detected in the cerebellum346 |
FLT3 is overexpressed in KMT2Ar B-ALL. KMT2Ar B-ALL can lineage switch (ALL to AML) as a means of antigen escape to lymphoid-targeting therapies. FLT3 CAR T cells may provide therapeutic benefit in the lineage-switch setting118 clinical trials are ongoing with results pending |
| ILT3 (LILRB4) | monocytic AML121,340 | monocytes121,340 | expression in highly restricted to the monocyte lineage, with no expression on HSCs or on non-hematopoietic cells340 highly and homogenously expressed in monocytic AML, with variable partial expression in myelomonocytic AML121,340 expressed on immunosuppressive cells. Eliminating these cells may enhance anti-tumor efficacy121 immunoinhibitory and promotes AML migration and infiltration121 |
restricted to a subset of AML121,340 expressed on monocytic cells may result in monocytopenia121,340 |
a first-in-class myeloid checkpoint inhibitor ILT3-blocking antibody has been developed and a phase I study is underway (NCT04372433) |
| IL1RAP | AML bulk cells and LSCs126 | monocytes and epithelial cells349 | expressed on both bulk cells and LSCs126,350 pro-survival role in AML126,351 not expressed on HSPCs126 |
approximately one-third of patients do not express IL1RAP350,352 expression on monocytes and epithelial cells poses a risk for monocytopenia and endothelial cell damage (which can aggravate CRS)349 |
also a target of interest for CML353 |
| CD70 | AML bulk cells and LSCs125 DLBCL, FL, HL, WM, and MM 306 |
subset of activated B and T cells and dendritic cells 306 | pro-leukemic role, which may reduce the risk for antigen escape/loss354 not expressed on HSPCs and only transiently expressed on a subset of hematopoietic cells125 |
variable expression in AML125 | CD70 expression is upregulated by the hypomethylating agent azacitidine (already used clinically in AML), providing a rationale for combination therapy355 |
| CD44v6 | MM and AML (FLT3/DNMT3A mut)122 | keratinocytes, skin, and oral mucosa. Circulating monocytes and T cells122,190 | not expressed on HSCs122 pro-leukemic role, which may reduce the risk for antigen escape/loss122 |
variable expression in AML122 expression on monocytes poses a risk for modest monocytopenia122 transient expression on activated T cells may result in CAR-T cell fratricide190 |
a phase I/II trial (NCT04097301) for CD44v6 CAR T cells in AML or MM was terminated early due to lower-than-expected proportion of patients expressing CD44v6 (Clinicaltrials.gov, accessed 02.03.2024) |
| Folate receptor β | AML123 | myeloid-lineage cells123 | not detected on adult HSCs123 | expression on monocytes poses a risk for monocytopenia123 variable expression in AML123 |
– |
| GRP78 | AML124 | none under normal conditions | only expressed at the cell surface during ER stress, so should be absent on healthy cells124 | variable expression in AML and little to no expression LSCs124 | also a target of interest for myeloma202 |
Owing to its high and homogeneous expression in AML, including on the cancer-repopulating leukemic stem cells (LSCs),84,85 CD33 has long been established as a therapeutic target for AML. Although concerns over fatal cytopenias and hepatotoxicity, as seen with the CD33-ADCs, prompted caution,86,87,88 CD33 has been extensively investigated clinically as a TCRT target and initial results suggest that hepatic toxicity may be uncommon and cytopenias manageable.89,90,91 An initial CD33-CAR-T cell phase I trial illustrated the challenges of lymphopenia, a common occurrence in AML that can impede autologous CAR-T cell manufacturing and efficacy, with just three of the 10 enrolled patients in one study able to receive the CAR-T product.91 A more recent phase I/II trial, in the pediatric setting, showed manufacturing feasibility, successfully producing CAR-T therapies for 23 out of 24 enrolled patients.92 However, CD33-TCRTs have failed to recapitulate the impressive anti-tumor responses seen with CD19- and BCMA-TCRTs and efficacy has been limited.89,91,92,93,94
With its pro-survival role in AML, CD123 is an attractive target.95 Although monocyte and granulocyte expression pose a concern, low expression on hematopoietic progenitors may mitigate the risk of prolonged, severe myelosuppression.96,97,98 Indeed, this was confirmed in a phase I study, with arguably lower-than-expected levels of severe cytopenia.99 Unfortunately, the efficacy seen in this and other clinical studies of CD123 bispecific TCEs has not been impressive.99,100,101 Preliminary results from clinical trials suggest that CAR T cells may be a more effective modality for targeting CD123,102,103 but potentially at the cost of safety. Two patients in the Cellectis UCART123 trial developed grade 4 capillary leak syndrome, and one experienced grade 5 cytokine release syndrome (CRS).104,105 These serious adverse events may well be due to endothelial CD123-expression, which can be increased by interferon gamma (IFNγ) and tumor necrosis factor alpha (TNFα) during CRS, further exacerbating endothelial damage and CRS in a positive feedback loop.106
CLEC12A expression is mostly restricted to the myeloid compartment with minimal expression on CD34+CD38− HSCs and lymphoid progenitor cells,84,107,108 suggesting that CLEC12A targeting would not completely impair patients’ normal hematopoietic potential. Preclinical studies supported this, with retained progenitor cell function permitting count recovery after transient cytopenias.109,110,111 However, as is often the case with TCRT studies, the preclinical data were not predictive of clinical outcome. Chronic myelosuppression was a consistent feature in a phase I trial of a CLEC12A CAR T cell, and at least two deaths from infection in the setting of chronic agranulocytosis were reported.112 Nevertheless, severe myelosuppression is a general feature of all salvage therapies for AML, and response rates in this and a second phase I trial in pediatric AML were impressive, allowing a bridge to transplant.113 Early-phase trials with TCEs suggest more manageable myelosuppression, but perhaps at the expense of clinical response.114,115
Unlike CD33, CD123, and CLEC12A, FLT3 off-tumor expression is largely confined to a subset of HSPCs and is much lower than on malignant cells, which may provide a window for targeting AML without profound myeloablation.116 Preclinical studies attempting to gauge the degree of myelosuppression have been mixed, with some suggesting preserved stem cell numbers and function,117,118 and others suggesting that prolonged cytopenias are likely to be a feature.119,120 These differences almost certainly reflect differences in the models used, and it would seem prudent to assume that marked cytopenias are likely. Clinical trials of bispecific TCEs and CAR T cells are underway, but outcome data are not available at the time of writing. FLT3 is also highly expressed in KMT2Ar acute lymphoblastic leukemia (ALL), a disease that has been shown to undergo lymphoid-to-myeloid lineage switch following CD19-CAR-T cell therapy as a mechanism of antigen escape.118 Thus, FLT3 TCRTs may be beneficial to both treat and prevent lineage-switch relapses.118
Other targets of interest for AML that show minimal or no expression on HSPCs, and therefore may preclude the use of allo-HSCT, include ILT3 (LILRB4), CD44v6, folate receptor β, and GPR78 (Table 3).121,122,123,124 However, variable inter- and intra-patient target expression may limit therapeutic utility and increases the risk for antigen-negative/low relapse. Pro-leukemic proteins, such as CD70 and IL1RAP (Table 3), may also prove useful.125,126 However, given the challenges of targeting AML, it is likely that successful TCRTs will require some of the alternative engineering strategies discussed later on in this review.
T cell malignancies
T cell leukemias and lymphomas encompass a broad spectrum of phenotypically mature and immature neoplasms that can arise at any stage during T cell development. While prognosis can vary greatly between subtypes, even favorable subtypes adopt a very poor outlook in the relapsed-refractory setting.127,128 Contrary to B cell malignancies, the development of immunotherapies for T cell neoplasms has been slow. As with AML, a major challenge is the lack of tumor-unique antigens. B cell aplasia can be clinically managed using immunoglobulin infusions, but no equivalent therapy exists to replace T cell function. A further challenge, unique to T cells, is that target antigens are frequently shared by the effector CAR T cells, leading to CAR-T cell fratricide. This can impact both manufacturing and CAR-T cell persistence in vivo.2 A third challenge is that, in the autologous setting, the CAR-T infusion product could be contaminated with malignant cells. Nevertheless, these challenges are now being addressed, and there are several ongoing TCRT clinical trials. Most of these are against lineage-specific antigens and thus rely on the use of allo-HSCT or CAR-T cell suicide switches to reverse T cell aplasia. A summary of potential T cell targets is presented in Table 4.
Table 4.
Preclinical and early-phase TCRT targets for T cell malignancies
| Targeta | Reported on-tumor expression | Reported off-tumor expression | Advantages | Disadvantages | Additional notes |
|---|---|---|---|---|---|
| CD7 | T-ALL/LBL, subset of PTCL2 | T, NK cells and B and myeloid-cell progenitors356 | highly expressed on the majority of T-ALL/LBL, and a subset of peripheral T cell lymphomas2 OTOT toxicities limited to the hematopoietic compartment356 |
healthy T cell expression results in severe CAR-T cell fratricide without modification. These modifications can complicate CAR-T cell manufacturing2 OTOT expression results in the short-term ablation of T- and NK cells, which may increase the risk of infections131 CD7 antigen escape post CD7-targeted TCRT has been reported131,132 |
clinical trial results suggest that CD7neg healthy T and NK cells can expand to reconstitute the immune system post CAR-T cell infusion129,132,357 CD7-CAR T cells have shown high CR rates across multiple trials but also high rates of severe infections129,130,131,132,133,308 also a target of interest for some AML 358 |
| CD5 | T-ALL, T-lymphoma, and some B cell malignancies 135 | thymocytes, peripheral T cells, and some B cells135 | expressed in the majority of T-ALL and T cell lymphomas135 CD5-CAR T cells have shown efficacy in CD7neg patients post CD7-CAR-T cell therapy139 CD5 negatively regulates T cell activation to prevent overactivation and activation-induced cell death. Thus, KO of CD5 in CAR T cells may minimize fratricide and also enhance anti-tumor efficacy359 OTOT toxicities limited to hematopoietic compartment135 |
CD5 is expressed on normal T cells, which poses a risk for CAR-T cell fratricide and increased exhaustion.359 Preclinical studies suggest expression is reduced during CAR-T cell manufacturing and that this isn’t a concern135,136 OTOT expression poses a risk T cell aplasia.139 Thus far, however, prolonged complete T cell aplasia have not been observed137,138,139 |
early results from CD5-CAR-T cell trials suggest efficacy139,360 |
| CD4 | mature T cell lymphomas and some T-ALL140,142 | most T cells (helper and regulatory T cells)361 | highly expressed in most mature T cell lymphomas (PTCL and CTCL) and some T-ALL140,142 OTOT toxicities limited to the hematopoietic compartment. CD4 is not expressed on HSCs so CD4+ T cell depletion could be reversed140 Targeting of Tregs may enhance anti-tumor efficacy142 |
prolonged CD4+ T cell aplasia can be fatal secondary to opportunistic infections141 CD4 expression on normal T cells leads to CD4+ CAR-T cell fratricide.140 CD4+ cells may be important for long-term responses362 CD4neg relapse was seen in a preclinical model363 |
clinical trials are ongoing with limited results, but early reports suggest efficacy142 |
| CD30 | HL, variable expression in NHL (both B and T cell), including DLBCL, ALCL and CTCL, and T-ALL 364,365,366 | activated HSPCs, T cells, B cells, and NK cells364,365,367 skin keratinocytes368 |
simultaneous elimination of CD30pos alloreactive T cells may minimize the risk of graft rejection when using allogenic CD30-CAR T cells369 CD30 plays an immunoregulatory role. Loss of CD30 on CAR T cells (such as by fratricide) may improve anti-tumor activity370 apart from a subset of activated immune cells, CD30 expression is highly restricted365 |
CD30 is transiently upregulated on activated B, T, NK cells, and HSPCs, which could pose a risk for OTOT toxicities. Preclinical studies suggest that the differential expression between these cells and tumor cells may provide protection.364,367 Cytopenias from CD30-CAR-T cell trials seem to be self-limiting and the risk of infections is low368,369,371 expression on skin keratinocytes may be the cause for reported transient skin rashes368,371 |
clinical experience with brentuximab-vedotin (CD30-ADC) in HL suggest that CD30 can be safely targeted.372 CD30-CAR T cells show similar safety and promising efficacy, although numbers of patients with T cell malignancies is low368,369,371,373,374 CD30-CAR T cells with transgenic CCR4 expression are currently being trialed in R/R CD30+ HL and CTCL, which may improve migration toward the tumor cells.374 Preliminary results support the use of CCR4 to improve tumor localization |
| TRBC1/2 | mature T cell malignancies (PTCL, AITL, T-PLL, ATLL, CTCL) and some T-ALL143,375 | approximately one-third to two-thirds (TBRC1/TRBC2) of healthy T cells143 | αβ TCR expressed in majority of PTCL-NOS and AITLs and approximately 30% of T-ALL143 clonal malignant T cells would be depleted, while sparing 35% or 65% (TRBC2 or TRBC1) of the normal T cell repertoire143 Contaminating malignant cells can be easily identified and removed376 |
CAR-T cell fratricide during manufacturing could limit persistence post-infusion376 targeting the TCR may result in bidirectional killing (target cell mediated killing of CAR T cells and other healthy T cells), limiting CAR-T cell persistence and efficacy377 |
a TRBC1-CAR-T cell trial is currently ongoing (NCT03590574) and preliminary results suggest durable responses144 |
| TRBV | mature T cell malignancies (PTCL, AITL, T-PLL, ATLL, CTCL, T-LGLL, and some T-ALL)143,147,375 | small subset(<10%) of healthy T cells146 | clonal malignant cells would be depleted while sparing most of the T cell repertoire375,378 minimal fratricide (depending on disease burden)146 the lower frequency of antigen-positive healthy cells may reduce the amount of bidirectional killing of healthy CARpos/neg T cells375,378 contaminating malignant cells can be easily identified and removed146 |
heterogeneity could limit the therapeutic applicability, although some variable segments appear to be more frequently used in some subtypes147 potential risk for cross-reactivity between similar variable genes375 |
TRBV9 mAbs are being currently trialed in axial spondyloarthritis, which will demonstrate the safety of this approach (NCT05445076 and NCT06333210) |
| CD1a | cortical T-ALL149 | cortical thymocytes, Langerhans cells, and a subset of myeloid DCs149 | not expressed on healthy mature T cells; therefore, little risk for CAR-T cell fratricide and T cell aplasia149 contaminating malignant T cells can be easily removed from the apheresis product by selecting for CD1a-negative cells149 |
target expression restricted to a subset of T cell malignancies (30%–40% of T-ALL)149 OTOT toxicities: Langerhans cell expression may pose a risk for skin-related AEs and loss of cortical thymocytes may compromise immunity149 |
Two CD1a-CAR-T cell trials are currently underway (NCT05745181 and NCT05679895) |
| CD37 | PTCL30 | healthy B cells and minimal monocyte expression30,288 | Is not expressed on T cells, so no risk for CAR-T cell fratricide or T cell aplasia30,288 | off-tumor expression will result in B cell aplasia288 variable expression in PTCLs30 |
CD37-CAR-T cell trials are ongoing. One patient with CTCL achieved a deep response36 |
| CCR9 | T-ALL 152 | small subset of healthy T cells and B cells (<5%), thymocytes, and gut-resident immune cells152 | expressed in the majority of T-ALL and only a subset of healthy T cells, minimizing risk of T cell aplasia152 contaminating malignant cells could be easily identified and removed |
CCR9 is unessential, which may pose a risk for antigen-negative/low clone escape152 expression on gut-resident immune cells and thymocytes may compromise immunity152 |
CCR9 small-molecule inhibitors have been trialled in Crohn’s disease, suggesting potential safety for this target152 |
| UMG1 (unique epitope of CD43) | cortical T-ALL and variable expression in other T-ALL subsets153 | small subset of healthy T cells (<5%) and cortical thymocytes153 | low risk for on-target toxicities and fratricide as minimal off-tumor expression (cortical thymocytes and a small subset of circulating T cells)153 low risk for fratricide153 contaminating malignant cells can be easily identified and removed |
target predominantly expressed by cortical T-ALL, with variable and mostly minimal expression in other subsets153 | may also be a target of interest for DLBCL153 |
AITL, angioimmunoblastic T cell lymphoma; ALL/LBL, acute lymphoblastic leukemia/lymphoblastic lymphoma; ALCL, anaplastic large-cell lymphoma; CTCL, cutaneous T cell lymphoma; PTCL, peripheral T cell lymphoma; PTCL-NOS, peripheral T cell lymphoma not otherwise specified; T-LGLL, T cell large granular lymphocytic leukemia; T-PLL, T cell-prolymphocytic leukemia. Other abbreviations as in Tables 1, 2, and 3.
CD7 is one of the most advanced targets for T cell malignancies. Attempts to mediate T cell fratricide include knockout of CD7 in the CAR T cells,2,129,130 sequestration of CD7 in the cytoplasm,131,132 and ignoring it altogether in a “survival-of-the-fittest” approach.133 CD7 allogeneic and autologous CAR T cells employing these approaches have achieved good clinical outcomes.129,130,131,132,133 Some, but not all, relapses were with CD7neg disease,130,131,132 suggesting that both limited CAR-T cell persistence and antigen escape are responsible for treatment failure. Loss of endogenous T cells was also a common feature of these trials and predictable infections, such as Epstein-Barr virus (EBV) and cytomegalovirus reactivation, were seen.129 In some cases, these infections proved fatal.129,130,131,134 Although some patients remained in remission and recovered their blood counts,130,131,132 it seems likely that CAR-T cell targeting of CD7 will be best employed as a bridge to transplant.
CD5 is another pan-T cell antigen, but, unlike CD7, expression is reportedly reduced at the cell surface on CAR T cells during manufacturing, limiting fratricide and permitting full expansion.135,136 Whether this is due to downregulation, masking, or sequestration is unclear, but it does not occur on target cells and there is no evidence of antigen escape preclinically.135,136 Clinical trials are somewhat in their infancy but suggest that CD5-CAR T cells are effective.137,138 Numbers are small, but there is a hint that efficacy and toxicity may be correlated; biepitopic targeting was associated with both deeper responses but also greater immune suppression and one grade 5 EBV infection.139 It is too early to comment on long-term toxicity, but again it is likely that CD5 CAR T cells will prove most useful as a bridge to transplant.
CD4 is a well-described TCR co-receptor expressed by helper and regulatory T cells and expressed in most mature T cell lymphomas and some T-ALL subsets.140 As CD4 is not expressed on HSCs, depletion may be reversible, reducing the risk for prolonged CD4 T cell aplasia, which can be fatal secondary to opportunistic infections.141 Preclinically, CD4+ CAR-T cell fratricide was prominent but preliminary results from phase I dose-escalation study suggest that CD4-CAR T cells are effective and that CD4+ T cell recovery is possible.142
While these target antigens have demonstrated promising efficacy, healthy T cell aplasia, even if transient, remains problematic. The TCR may offer a more specific way to target neoplastic T cells. Most T cells express an alpha and a beta TCR chain, with the constant region of the latter encoded by one of two genes: TRBC1 or TRBC2.143 As T cell malignancies are clonal, targeting one of these two proteins would deplete all the malignant cells but leave a substantial part of the normal T cell repertoire intact (∼35%–65%).143 Early results from a phase I/II dose-escalation study for TRBC1-CAR T cells support this theory, with modest, transient, and tolerable drops in T cell counts post infusion.144 Given the larger number of variable gene segments for the TCR beta chain, TRBV-TCRTs would similarly eliminate clonal tumor cells but spare a much larger proportion of the healthy T cells (>90%).145 Inter-patient heterogeneity could prove challenging, but some malignancies do demonstrate a degree of recurrent expression of certain segments, such as Vβ2, Vβ5, and Vβ8.146,147 Targeting the variable region for neoplasms is currently at the preclinical stage, but ongoing clinical trials with a TRBV9 monoclonal antibody (mAb) in axial spondyloarthritis will help validate the safety of this approach.148
More specific targeting may also be achieved using CD1a, CD37, CD30, CCR9, and UMG1 (Table 4), albeit with more limited therapeutic applicability. Cortical T-ALL, a major T-ALL subtype, comprising 30%–40% of disease, is characterized by CD1a expression.149 CD1a has minimal off-tumor expression, with no expression on mature healthy T cells. Consequently, neither CAR-T cell fratricide nor T cell aplasia are concerns, although targeting healthy cortical thymocytes may compromise immunity to some extent.150 CD37 is aberrantly expressed in some T cell lymphomas but not in resting or activated healthy T cells,30 and CD30 is similarly expressed in some T cell lymphomas with only transient expression in activated healthy T cells.151 Clinical trials for CD1a-, CD30-, and CD37-CAR-T cell trials are currently underway. CCR9 and UMG1 are still at the preclinical stage, but have both shown promise for the more specific targeting of T-ALL and cortical T-ALL respectively.152,153
Novel targeting strategies to improve TCRT tumor specificity
As mentioned above, CD19- and BCMA-targeting TCRTs are tolerated because loss of the healthy counterpart cells can be managed clinically. However, for other hematological malignancies, shared expression of target antigens with healthy cells poses a serious safety concern. Even prolonged B cell aplasia is not without consequence.39 In addition, even targets with acceptable off-tumor expression profiles may be less restricted than initially thought, leading to unexpected and severe on-target/off-tumor toxicities.57,62,104,105,154 In the second part of this review, we discuss alternative antigens and engineering strategies that aim to mitigate these toxicities and expand the clinical success of TCRTs.
Targeting neoantigens
Antibody and CAR-T cell targets are classically proteins expressed in their native conformation on the cell surface of a tumor. A neoantigen, on the other hand, is a peptide derived from a mutant protein (which may represent either a driver or passenger mutation) and presented by major histocompatibility complex (MHC) molecules to promote T cell engagement, expansion, and cytolysis. These tumor-unique mutations provide high specificity and, as they can arise from intra- and extracellular proteins, greatly expand the number of potential targets for TCRT therapy.155 The potential for neoantigens as immunotherapy targets arose from the realization that the clinical successes of tumor-infiltrating lymphocyte (TIL) therapy, in which patients’ TILs are isolated and expanded ex vivo before reinfusion, was in large part due to the presence of T cells reactive against somatic mutations present in the tumor.156,157 Although encouraging responses have been seen with TIL approaches, relapse, likely due to T cell exhaustion, appears to be the norm.158,159
TCR T cells and TCR-mimics
To improve clinical efficacy, neoantigen-reactive TCR sequences can be identified from patients and then cloned into healthy, naive T cells (TCR T cell), similar to CAR-T cell therapy. Neoantigens can also be targeted using antibodies specific for peptide-human leukocyte antigen (HLA) complexes, known as TCR-mimics. These TCR-mimics can be used as the antigen-recognition domain in CAR T cells or in bispecific TCEs,160,161,162 combining the specificity of TCR T cells with the simplicity of antibody manufacture.
The majority of neoantigens are unique to an individual’s cancer (i.e., private neoantigens). While offering the potential for individualized therapy, it can be prohibitively costly and labor intensive to develop TCRTs against these.163 Public neoantigens, which arise from mutational hotspots, are likely to be shared among multiple patients164,165,166 and present a more economically viable alternative. For example, Kim et al. recently reported the identification of 39 mutant p53-reactive TCRs.158 As p53 is such a common cancer mutation and because several of the identified TCRs paired with prevalent HLA molecules, the authors theorized that this library could be used to treat ∼7% of patients with solid cancers.158 Like p53, the RAS family of GTPases, especially KRAS, are frequently mutated in cancer.165 These mutations have been shown to be immunogenic167,168 and clinical trials for TCR T cells against KRAS neoantigens in solid tumors have now started recruiting (NCT03190941 and NCT03745326). As KRAS and p53 mutations are also found in hematological malignancies, albeit at a lower frequency, these therapies would likely be of benefit in these settings as well.169,170 Disease-specific recurrent mutations and fusions may provide more public neoantigens for hematological malignancies. TCR T cells reactive against FLT3D835, a mutation that occurs in approximately 7%–10% of AML patients, have demonstrated potent and highly selective anti-leukemic activity in vitro and in vivo.164 Other examples of frequent immunogenic neoantigens for hematological malignancies include the NPM1 mutations in AML171 and the BCR-ABL fusion protein for Ph-positive ALL.172
Phosphopeptides, which are immunogenic and immunologically distinct from parental un-phosphorylated peptides,173,174 further expand the repertoire of targetable neoantigens.175 Protein phosphorylation is typically dysregulated in cancer, with an increase in the global number of phosphopeptides presented by the MHC on malignant cells.175,176 Some phosphopeptides are both tumor specific and shared among patients, within and across cancer subtypes,175,176 and TCR T cells targeting these have shown promising preclinical results.173,175,176,177,178 Other post-translational modifications, such as methylation, acetylation, and glycosylation, have also been reported to provide a source of tumor-specific peptides.179,180
Although TCR and TCR-mimic T cells/TCEs enable truly specific cancer targeting, they present their own challenges. Firstly, although some neoantigens may be frequent, they are still not as prevalent as lineage-restricted antigens. Secondly, TCR T cell antigen recognition depends on the HLA allele presenting the peptide. This greatly reduces the number of patients that can benefit from any given therapy155 and creates HLA-subtype disparities: HLA-A2 is commonly targeted but this subtype is much more frequent in individuals of European descent compared to other ethnic groups, restricting access to novel therapies for these patients.181 Thirdly, although shared neoantigens are often essential for tumor survival, these targets are not immune to antigen escape and MHC loss is a frequent mechanism of resistance.158,167 Finally, while the on-target reactivities of TCRTs against conventional target antigens are relatively easy to predict, the off-target toxicities of TCRs and TCR-mimics, caused by cross-reactivities with other peptide-HLA complexes, are much more unpredictable.182,183,184 The severe consequences of this were demonstrated by the four patient deaths in two melanoma-associated antigen 3 (MAGE-A3) TCR T cell trials caused by the recognition of neurological MAGE-A12 and cardiomyocyte titin expression.183,184 Predicting these potential off-tumor toxicities is thus crucial but highly challenging.185
Isoforms and alternative splicing
Alternative splicing of pre-mRNA provides proteomic diversity from just one gene and is frequently co-opted by cancer cells, conferring drug resistance, increasing proliferation and/or survival, and inhibiting apoptosis.186 Alternative splicing in tumor cells is more prevalent than in normal cells.187 It can lead to isoform switching186—aberrant expression of normally tissue-restricted isoforms—and intron-retention and splice site neojunctions, which can generate cancer-specific neoantigens.187,188
CD44 is frequently upregulated in hematological and epithelial malignancies, promoting tumor survival and metastasis,189 but off-tumor expression (including on HSCs) precludes TCRT development.122 Alternative splicing of CD44 produces isoforms with much more restricted expression patterns, such as CD44v6, which is highly, but variably, expressed on AML and myeloma cells but not on HSCs.122 CD44v6 CAR T cells demonstrated effective anti-leukemic activity in vitro and in vivo, with only modest monocytopenia and no toxicity toward HSCs.122,190 Disappointingly, when CD44v6-CAR T cells progressed to the clinic, low patient recruitment rates resulted in an early termination of the trial (clinicaltrials.gov/study/NCT04097301, accessed 02.03.2024).
In addition to creating novel expressed proteins, alternative splicing can generate neoantigens that could represent targets of TCR T cells.187,191,192,193,194 For example, the D393-CD20 splice variant is expressed in some B cell lymphomas, but not resting healthy B cells, and has been shown to be immunogenic.193,194 Recently, circRNAs, back-spliced products of pre-mRNA, have been shown to encode proteins that are tumor specific and shared across patients and that generate immunogenic peptides,195,196,197,198 providing another potential source of targetable neoantigens.
Cancer-specific protein conformations
Altered protein conformations can provide a survival advantage for malignant cells, e.g., by maintaining a receptor in a constitutively active state or confining pro-apoptotic proteins to a non-functional state. However, this altered conformation can also expose unique epitopes that are not normally accessible.199,200 One example of this is ITGβ7 in myeloma. While ITGβ7 is not specific to myeloma, its constitutive activation exposes an epitope that can be recognized by a conformation-dependent CAR T cell that has demonstrated potent and selective killing of myeloma cells in vitro and in vivo.199 A second example is loss of function of the receptor, P2X7, which confers an anti-apoptotic phenotype in many cancers. This non-functional P2X7 has unique epitopes that can be targeted by CAR T cells200 and represents a potential pan-cancer target. Cancer-specific conformational changes represent promising targets, but unbiased identification is challenging. Recently, Mandal et al.201 reported a structural surfaceomic screen that combined cross-linking mass spectrometry with cell-surface proteomics as a method to identify proteins that are in an altered conformational state. They thus identified and validated ITGβ2 as a novel CAR-T cell target in AML.
Cancer-specific protein localization
Protein localization is dysregulated in cancer, and exposure of normally intracellular proteins to the cell surface represents another avenue for specific targeting. GRP78 is a regulator of the unfolded protein response that is normally retained in the endoplasmic reticulum (ER) by the binding of its C-terminal KDEL sequence to the KDEL-R1 receptor. Increased ER stress in cancer overwhelms this retention mechanism, leading to translocation of GRP78 to the cell surface in AML, where it can be targeted using CAR T cells.124 Cell-surface expression of GPR78 has also been reported for myeloma, and a GRP78-mAb was shown to be well tolerated in a phase I trial, although no objective responses were seen.202 Alternative splicing may also alter protein localization, as has been reported for some ESR1 (ERα) isoforms, thus creating further tumor-specific targets.203
While tumor-specific antigens may improve selectivity, innovative CAR designs can also be employed to overcome off-tumor toxicities, challenges of tumor heterogeneity, antigen escape, and limited CAR-T cell efficacy and/or persistence.
Logic gating
Boolean-logic gating describes the requirement for a CAR T cell to recognize and respond to multiple input signals, minimizing off-tumor toxicities by ensuring that CAR T cells are activated only in the presence of specific combinations of antigens found on the tumor.
OR gates (A OR B)
Antigenneg/low relapse following CAR-T cell therapy remains a major hurdle to durable remissions. Although tumors often respond to a second targeted therapy, targeting multiple-antigens simultaneous has been shown to be more efficacious and to reduce the risk of relapse.14,15,16,204,205,206,207 OR-gated logic enables CAR T cells to respond to one of several antigens (Figure 2). This not only addresses tumor heterogeneity but can enable the simultaneous targeting of immunosuppressive cells within the tumor microenvironment to improve efficacy.208,209 Due to their design simplicity, OR gates are the most clinically advanced logic gates. However, target antigens must still be highly tumor specific, and, as such, OR gates have been predominantly trialed in B cell malignancies14,16,210 and myeloma.211,212
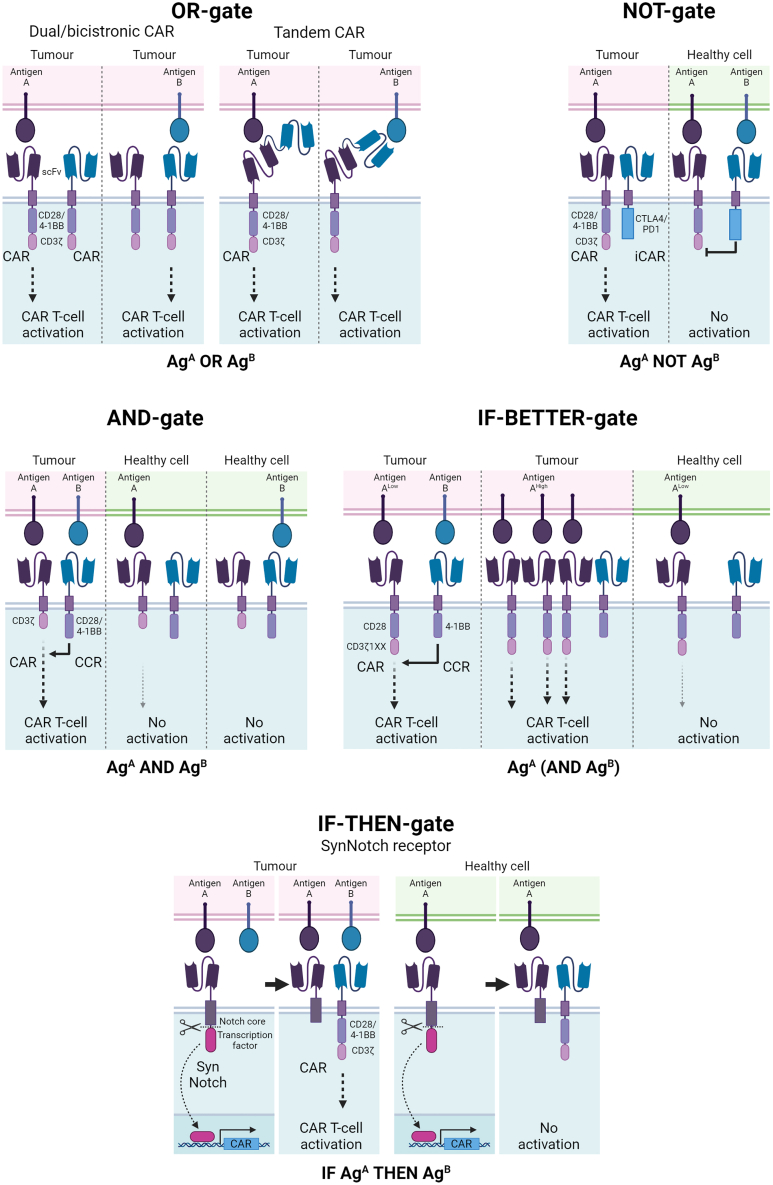
Figure 2.
Overview of logic-gated CAR-T cell designs
OR gate: CAR T cells may recognize one of two (or more) antigens. Dual or bicistronic CAR designs contain two separate CAR molecules, while tandem CARs contain two scFvs fused to a single stalk. NOT gate: an activating CAR is paired with an inhibitory CAR (iCAR) that contains an inhibitory domain, such as PD-1 or CTLA-4, against a healthy-cell-exclusive target antigen. CAR-T cell activation is inhibited by the iCAR when encountering a healthy cell, while cytolytic activity is maintained against single-target-positive tumor cells. AND gate: a first-generation CAR (does not contain a co-stimulatory domain) is paired with a chimeric co-stimulatory receptor (CCR) that lacks an intracellular signaling domain. Both receptors must be engaged for full target cell activation and effector cell function; therefore, healthy cells expressing only one target antigen are spared. IF-BETTER gate: an attenuated CAR is paired with a CCR. The CCR amplifies CAR signaling to enable T cell effector functions when encountering antigen Alow tumor cells. Antigen Alow healthy cells that do not express antigen B are spared.233 IF-THEN gate: the SynNotch receptor consists of an scFv fused to part of the Notch receptor and a transcription factor. Engagement of the cognate antigen results in the release of the transcription factor, which drives expression of a conventional CAR against a second protein.234
Despite good response rates, the emergence of single-target-positive cells at relapse suggest that single-antigen targeting may be compromised in OR gates, particularly in tandem CARs. In a CD19/CD22 tandem-CAR trial in large B cell lymphoma and B-ALL (NCT03233854), CD19neg/low relapse was common, consistent with a selection pressure against CD19, whereas CD22 loss or decrease was not seen.213 Follow-up in vitro studies revealed reduced reactivity against CD22 in the bispecific compared to the monospecific CAR T cells. Other preclinical studies have similarly reported that tandem CARs are superior in eliminating dual-target-positive cells but can have reduced efficacy against single-target-positive cells, likely due to reduced antigen sensitivity.26,207 Thus, optimal OR-gate CAR-T cell effector function requires rational design, with consideration for the length and orientation of each scFv relative to its target antigen.205,207,214 In some situations, compromised antigen recognition may actually be beneficial: in a BCMA/CD38 tandem-CAR trial for myeloma, the low-affinity anti-CD38 scFv was placed in a sub-optimal orientation to minimize on-target/off-tumor toxicities (ChiCTR1900026286).212
Despite initial concerns about increased CRS severity and on-target/off-tumor toxicities from multi-antigen targeting, bispecific CAR T cells for MM and B cell malignancies have been well tolerated with manageable toxicity.215
NOT gates (A NOT B)
NOT-gated CAR T cells improve tumor specificity by pairing an activating receptor against a tumor-associated antigen with an inhibitory receptor (iCAR) specific to a healthy-cell-exclusive antigen (Figure 2). This system was first demonstrated by Fedorov, who used a PSMA-targeting scFv linked to cytotoxic T lymphocyte-associated protein 4 (CTLA-4) or programmed cell death protein 1 (PD-1) inhibitory domains to selectively inhibit CD19 CAR T cells against PSMAposCD19pos target cells, while maintaining efficacy against PSMAnegCD19pos cells.216 This reversible inhibition offers advantages over suicide switches by preserving long-term CAR-T cell immunity and is preventive rather than reactive. In addition, NOT-gated CAR T cells may be subject to reduced antigen-dependent exhaustion and CRS.217,218 However, Fedorov also identified a key limitation: the efficiency of the system hinges on the expression level of the inhibitory receptor target and low-density iCAR targets may not sufficiently limit activity.216,219 In some situations, NOT gates can even inadvertently increase cytotoxicity through enhanced avidity and/or target cell engagement,219 posing challenges for clinical application. Design adjustments, such as receptor length, stronger or dual inhibitory domains, and increasing the iCAR:CAR ratio, as well as careful target selection to avoid large, bulky, or low-density antigens, could help mitigate these issues.220,221
Probably owing to these challenges, only one NOT-gate CAR T cell has progressed into clinical trials. A2B530, an autologous carcinoembryonic antigen (CEA)-targeting CAR T cell with an HLA-A∗02 inhibitory receptor (Tmod), is in phase I/II trials (NCT05736731) for CEA+ solid tumors with HLA-A∗02 loss of heterozygosity (LOH).222,223 Given that HLA LOH is a common occurrence in solid cancers, this design provides a universal iCAR receptor when targeting multiple tumors.222,223 However, HLA LOH is much rarer in hematological malignancies, which may limit translation in these conditions.224 Furthermore, subjecting CAR-T cell therapy to HLA type greatly limits patient eligibility, negating one of the major benefits of CAR over TCR T cell therapy.
In the absence of unique tumor antigens, inhibitory receptors could greatly expand the number of available targets for CAR-T cell therapy and enable the targeting of essential genes to preclude antigen escape.218,225 Although beyond the scope of this review, it should be noted that more progress for NOT-gate CAR NK cells has been made in hematological malignancies and a trial for CD33/FLT3-NOT-EMCN CAR NK cells (SENTI-202) in AML is currently recruiting (NCT06325748).
AND gates (A AND B)
AND gates offer another strategy to enhance CAR-T cell precision by requiring dual antigen recognition. A sub-optimal first-generation CAR against one antigen is paired with a chimeric co-stimulatory receptor (CCR), which lacks an intracellular signaling domain, specific to a second antigen (Figure 2). As the simultaneous engagement of both receptors is required to pass the activation threshold for full effector function, healthy cells that share only one antigen with the tumor are protected.226
When targeting highly expressed antigens, CCRs can enhance CAR-T cell reactivity toward low-density antigens targeted by the other, first-generation CARs. Hence, AND-gated CAR T cells have been shown to eliminate targetlow tumor cells that were resistant to standard second-generation CAR-T cell targeting in vitro.227,228 Thus, in addition to improving specificity, AND gates can enhance sensitivity. This may reduce the risk for cancer recurrence from antigenlow clones and may widen the CAR-T cell therapeutic repertoire by enabling the targeting of low-density antigens that were previously considered unamenable to CAR targeting.
Applying AND gates to more heterogeneous diseases such as AML may be more challenging due to the scarcity of paired tumor-associated antigens homogeneously expressed across the disease.84 Nonetheless, provided these antigens are expressed at sufficiently low levels on HSCs, AND gating has the potential to reduce toxicity, even if it is not completely eliminated.229 In the absence of shared tumor antigens, Sukumaran et al. proposed targeting tumor-specific patterns. By combining a first-generation anti-PSCA CAR with two hybrid cytokine receptors to convert immunosuppressive transforming growth factor beta (TGFβ) and interleukin 4 (IL4) cytokine signaling into co-stimulatory signaling,230 maximal CAR-T cell activity was restricted to the tumor site. This AND-gate design has the added benefit of rendering CAR T cells resistant to these immunosuppressive signals, improving anti-tumor potency and persistence.
Despite their potential, AND gates must be carefully designed to avoid unintended activation and toxicity through the first-generation CAR.226 Tousley et al. recently proposed an alternative design to reduce the “leakiness” of the CAR. By replacing the CD3ζ and co-stimulatory domains with the proximal signaling molecules LAT and SLP-76, the authors demonstrated complete abrogation of single-target signaling without compromising efficacy.231
IF-BETTER
IF-BETTER gates, whereby antigen B enhances the targeting of antigen A but is not obligate for CAR-T cell function, aim to strike a balance between anti-tumor efficacy and selectivity (Figure 2). Using a second-generation CD19 CAR paired with a CD38 CCR with dual-co-stimulatory domains, Katsarou demonstrated the improved targeting of ultra-low-density CD19 tumor cells (∼20 molecules/cell) that were resistant to second-generation CD19-CAR T cells.232 While healthy cells expressing CD19 may still be affected, a sufficient difference in antigen density between healthy and tumor cells could offer some protection. This concept was validated by Haubner et al., who employed a similar strategy to target ADGRE2 in AML, an antigen whose expression in HSPCs prevents conventional CAR-T cell targeting. The researchers used an ADGRE2-targeting CAR with limited sensitivity (ADGRE2-CD28ζ1XX) to minimize reactivity against ADGRE2low HSPCs while maintaining strong anti-tumor activity against ADGRE2high AML cells. To counteract the increased risk of escape by antigenlow tumor cells—a potential drawback of affinity-tuning strategies—a CLEC12A-CCR was introduced to enhance the elimination of ADGRE2lowCLEC12Amed/high AML cells. CLEC12Aneg HSPCs were spared.233
IF-THEN
IF-THEN gates describe the sequential recognition of two antigens for target cell elimination. The SynNotch receptor design, developed by the Lim lab, is central to most IF-THEN strategies234 and is composed of an antigen-recognition domain, the Notch receptor’s core regulatory domain, and a transcription factor (Figure 2). Upon antigen engagement, the receptor is cleaved, releasing the transcription factor to drive expression of a CAR molecule. This theoretically confines CAR toxicity to dual-target-positive cells, and it also enhances efficacy and persistence by limiting antigen stimulation to the tumor site.235,236 However, because of the temporal delay in CAR induction and decay, neighboring single-target cells are not protected.42 Thus, antigen selection for IF-THEN pairing requires careful consideration, especially for metastatic or circulating tumors.
This reduced stringency, however, may benefit heterogeneous tumors. Highly specific, but heterogeneous, tumor antigens can “prime” CAR T cells and drive the localized expression of CARs against more homogeneous, but less tumor-specific, antigens. This enables the use of CARs against targets with otherwise unacceptable off-tumor toxicity.235 Based on promising preclinical findings, a phase I trial for a epidermal growth factor receptor variant III (EGFRvIII) SynNotch receptor induced EphA2/IL13Rα2 tan-CAR (E-SYNC) in glioblastoma is currently recruiting (NCT06186401) with results pending.
The modular nature of these synthetic receptors means they are highly versatile and can be used to drive multiple outputs, such as the production of immunostimulatory cytokines or therapeutic antibodies to enhance anti-tumor potency, or to initiate apoptotic signals, akin to a NOT gate.234,237 Recently, the Lim lab investigated the potential for the SynNotch system to integrate multiple recognition events to achieve highly specific tumor-targeting using one, two, or even three antigen input AND/OR/NOT gates. The high precision of this approach was demonstrated by the selective killing of CD19posGFPposHER2neg target cells, while sparing CD19posGFPnegHER2pos and CD19posGFPposHER2pos cells.237 Improved specificity may also be achieved by enhanced antigen-density discrimination. By placing a high-affinity HER2-CAR under the control of a low-affinity HER2-SynNotch receptor, Hernandez-Lopez et al. created “ultra-sensitive” CARs that spared HER2low cells but were more potent against HER2high cells than affinity-modulated CARs.238
Although most IF-THEN gates feature the SnyNotch design, alternative systems that use environmental cues to confine CAR expression to the tumor microenvironment exist. This includes the use of a hypoxia-sensing domain to promote CAR degradation under normoxic conditions,239 scFvs with pH-restricted target binding,240 and masked CARs that are only exposed by proteases exclusive to the tumor microenvironment.241
Pharmacological modulation
For some targets currently undergoing clinical investigation, on-target/off-tumor expression necessitates a suicide switch to rapidly ablate CAR T cells in the event of severe toxicities and/or to restore normal hematopoiesis.242 Protein tags, such as CD20 and truncated EGFR, enable antibody-mediated destruction,242,243 although there are potential risks for on-target toxicities from the antibodies themselves.242,244 The inducible caspase 9 (iCasp9) system offers a much faster alternative to the slow kinetics of antibody-mediated killing. The pro-apoptotic caspase9 and drug-binding domain fusion protein triggers rapid apoptosis following dimerization by the small molecule rimiducid. The safety and speed of this approach was first demonstrated in a graft-versus-host-disease setting, in which >90% of the administered modified T cells were eliminated within 30 min, resulting in resolution of symptoms within 24–48 h, and no recurrence.245 In CAR-T cell therapy, iCas9 switches have been reported to effectively resolve severe, persistent immune effector cell-associated neurotoxicity syndrome (ICANS).246 While effective, the irreversible nature of suicide switches greatly limits CAR-T cell efficacy. Given the high cost, long production time, and limited manufacturing capacity of CAR T cells, this raises significant concerns regarding the long-term practicality of this approach.
Pharmacological modulation provides a means to reversibly switch CARs on or off, repeatedly, without affecting effector functions. One way to achieve this is to target the CAR for degradation. The incorporation of a zinc-finger degron tag into the CAR construct allows lenalidomide-mediated ubiquitination and proteasomal degradation, effectively creating an “OFF switch” (Figure 3).247 Conversely, an “ON switch” can be achieved by inserting hepatitis C virus NS3 proteases into the CAR construct, causing in cis proteolysis and CAR fragmentation. Cleavage is inhibited by NS3 protease inhibitors, enabling CAR activation.248 Pharmacological modulation can also be achieved by splitting the antigen-recognition and signaling domains and by using small molecules to regulate dimerization.247,248,249,250 The extracellular positioning of the drug-binding domain in the dimerizing agent-related immunoreceptor complex (DARIC) ON switch allows the use of exogenous rapamycin-prebound scFvs against additional targets to provide target flexibility.250 Synthetic zinc-finger gene regulators fused to drug-binding domains (synZiFTRs) can be used to regulate CAR expression at the transcriptional level. The number of synZiFTRs and small-molecule combinations enables multiplexing, providing temporal control over multiple cellular programs to prime and then activate CAR T cells and optimize clinical efficacy.251
Figure 3.
Overview of pharmacologically modulated CAR T cells
Lenalidomide-modulated OFF-switch degradable CAR: administration of lenalidomide (len) targets the CAR construct for CRL4CRBN-mediated ubiquitination and proteasomal degradation, turning the CAR off.247 ON VIPER CAR: versatile protease regulatable CARs contain the hepatitis C virus NS3 protease, which triggers in cis proteolysis and CAR fragmentation. NS3 inhibitors (i.e., grazoprevir [GZV]) prevent CAR proteolysis and enable the formation of full-length, signaling-competent CARs.248 DARIC CAR: dimerizing agent–regulated immunoreceptor complex CARs are composed of two receptors, one containing the antigen-recognition domain and the other the signaling domain. The small molecule rapamycin induces dimerization of the CAR to form a signaling competent receptor. Rapamycin-prebound scFvs (DARIC Plug-Ins) can redirect the CAR against a second antigen.250 synZiFTR: synthetic zinc-finger transcription regulators are DNA-binding zinc fingers fused to drug-binding domains. synZiFTRs, regulated by small molecules, drive the expression of a conventional CAR.251
An ongoing trial in AML for CD33-CAR T cells employing a DARIC ON switch will help determine the safety and feasibility of incorporating ON/OFF switches clinically (NCT05105152). It is important to note that, although these designs (and the adaptor CARs [AdCARs] described below) can improve CAR safety, they will not improve tumor specificity and cannot completely prevent on-tumor/off-target toxicity.
AdCARs
AdCARs utilize adaptor molecules to bridge target antigens and CAR T cells (Figure 4). Similar to pharmacologically modulated CAR T cells, they enable considerable precision over the activation, expansion, and persistence of CAR T cells to reduce on-tumor/off-target toxicity, reduce CRS/ICANS severity, optimize therapeutic efficacy, and limit exhaustion.252,253,254,255,256 They also provide greater flexibility for changing targets without additional manufacturing. AdCARs are particularly promising for hematological malignancies to avoid prolonged hematotoxicity. Provided HSCs are not eliminated by the CAR T cells, normal hematopoiesis can be restored following cessation of adaptor treatment.257 This enables the use of CAR T cells against targets such as CD33 and CLEC12A, which would otherwise be restricted to the bridge-to-transplant setting.254
Figure 4.
Overview of adaptor CARs
The universal CAR (UniCAR) is composed of two components: a universal CAR construct, which recognizes the peptide motif included in the adaptor, and an adaptor containing an scFv against the target antigen. Administration of the adaptor is required for CAR-T cell activation and recognition of the target cell.256 RevCAR: bispecific scFvs are used to link the CAR, containing a peptide motif as the extracellular domain, and the target antigen.262 The SUPRA CAR design features a zipCAR containing a leucine zipper instead of an antigen-recognition domain, and the cognate leucine zipper fused to an scFv against the target antigen (zipFv). An AND-gate system is created by separating the co-stimulatory and signaling domains and a NOT gate is achieved through competition: when zipFvs against antigen A and B are both engaged, their complementary zippers are engaged, preventing CAR binding.263 Co-LOCKR: The colocalization-dependent latching orthogonal cage/key protein system is composed of a “cage” protein that uses a latch domain to sequester a peptide (i.e., Bim) in an inactive conformation. The binding of a key protein triggers a conformational change enabling the peptide to bind to an effector protein (i.e., Bcl-2 fused to a CAR). A second key with a different targeting domain provides an OR gate, while a decoy that sequesters the key can be used to create a NOT gate.264
Phase I trials of UniCAR T cells with a CD123 adaptor in AML (UniCAR-T-CD123, NCT04230265) provide validation for this hypothesis. Neutrophil counts rapidly recovered following withdrawal of the adaptor with no lasting treatment-induced myelosuppression, and none of the 19 patients required a stem cell transplant for white blood cell reconstitution.256,258 Three grade 3 CRS events were reported, but were all resolved within 24 h of adaptor withdrawal. Importantly, UniCAR T cells could be re-expanded with adaptor re-administration.256,258 A trial for a second AdCAR with a CD123 adaptor is currently recruiting (Allo-RevCAR01-T-CD123, NCT05949125), and a CD19-adaptor CAR for B cell malignancies is also under investigation (CLBR001+SWI019, NCT04450069).255
Boolean logic has been applied to AdCARs to further enhance tumor specificity. Split-CAR approaches using dual adaptors against distinct antigens have yielded AND-gated AdCARs for glioblastoma (EGFR/GD2), colorectal cancer (CEA/EpCAM), and AML (CD33/CD123).259,260,261,262 The split, universal, and programmable (SUPRA) design provides a NOT-gated AdCAR,263 and the colocalization-dependent latching orthogonal cage/key proteins (Co-LOCKR) design impressively recognizes three different inputs using AND, NOT, and OR logic.264 These preclinical studies highlight the versatility of logic-gated AdCARs. However, ensuring sufficient bioavailability of the adaptor molecules to balance activating and/or inhibitory signals, already a major hurdle for conventional logic-gated CARs, could preclude clinical translation.
Generation of tumor-specific antigens
An innovative solution to the lack of truly unique tumor antigens is to create them. Gene editing has already been demonstrated as an effective tool to circumvent CAR-T cell fratricide in T cell malignancies,265 but it can also be applied to remove the target antigen or epitope from healthy HSCs ex vivo. This renders them immune to CAR- or antibody-mediated destruction and enables the specific targeting of residual cells post HSCT. This approach has been explored in AML to enable the targeting of CD33, an attractive target otherwise limited by the risk of severe myelotoxicity. As demonstrated by Kim and others, CD33 is non-essential, and gene-edited CD33null HSCs are capable of restoring hematopoiesis, while resisting CD33-targeted therapies post transplantation.266,267,268 Tremtelectogene empogeditemcel (trem-cel, VOR33), a CD33null HSPC product, is currently being trialed in AML in combination with post-transplant gemtuzumab ozogamicin (CD33-ADC) administration, and the results are eagerly anticipated (NCT04849910).
Preclinical trem-cel studies confirmed no significant off-target effects from CD33-gRNA, and many other trials have demonstrated the safety of gene editing.269 However, Cas9-induced double-strand breaks can cause chromosomal rearrangements and deletions, risking genetic instability.270,271 Base editing offers a safer alternative and allows for precise epitope engineering of proteins on HSCs to escape CAR-T cell targeting without compromising normal protein function, a requisite for essential genes.272 Multiplex epitope engineering enables heterogeneous antigen targeting, further minimizing the risk for antigenneg/low relapse,273 and CD123, KIT, and FLT3 have all been proved amenable to this approach.273,274 Epitope engineering also enables the targeting of pan-hematologic antigens, such as CD45, offering a universal therapeutic strategy for hematological malignancies. As CD45 is essential for T cell function, base editing provides a means to disrupt the CD45 epitope without impairing CAR-T cell and HSC function.275
Alternatively, introducing unique antigens onto tumor cells is possible with oncolytic viruses. These viruses can specifically infect tumor cells and induce surface expression of CAR-T cell targets with acceptable off-tumor expression profiles, such as CD19 or even GFP.276,277 Oncolytic viruses have innate anti-tumor activity and the release of antigens from lysed cells can contribute to the priming of endogenous T cells against additional tumor antigens in a process known as epitope spreading.278 While this tumor-decorating approach is currently at the preclinical stage, oncolytic viruses are under clinical investigation for their role in boosting other TCRT approaches such as CAR T cells and TILs (NCT03740256 and NCT05057715).
Conclusions
It is likely that, at least with proteins in their natural conformation and location, there is no such thing as a genuinely specific immunotherapy target. Indeed, we have recently reported that this is the case for myeloma.225 It is thus probably not a coincidence that TCRTs, and CAR T cells in particular, are most advanced in B cell malignancies and myeloma, where targeting of healthy cellular counterparts is perhaps best tolerated. Targeting of AML and T cell malignancies, where potential on-target, off-tumor toxicity is more pronounced, lags somewhat behind. However, innovative CAR designs are showing considerable promise to achieve more selective targeting in the absence of targets with acceptable safety profiles and it is hoped they will advance TCRT development for these diseases. It is remarkable that many of the successful TCRT targets, such as CD19 and BCMA, were identified on the basis of RNA expression profiles, even though we know that RNA and protein expression are virtually un-correlated at the cell surface.79 Cell-surface proteomics is now achieving unprecedented depth and specificity,79,80,82,84 but its use for target identification is limited to cancers where relatively homogeneous cell populations can be isolated. Another major challenge, by no means unique to TCRT but particularly pertinent to the field, is the disconnect between preclinical models, especially of toxicity, and clinical outcomes, a point we have argued elsewhere.279 Furthermore, the time and expense of conducting clinical trials, means that we need to find more efficient approaches for prioritizing and advancing immunotherapeutic targets. Nevertheless, we believe that this review highlights the considerable drive and ingenuity in this field, and that there are good grounds for optimism in the use of the immune system to target cancer in the future.
Acknowledgments
This work is supported by a Medical Research Council program grant, MC_UU_00025/10. Figures were created with BioRender.com.
Author contributions
All authors wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
References
- 1.Turatti F., Figini M., Balladore E., Alberti P., Casalini P., Marks J.D., Canevari S., Mezzanzanica D. Redirected activity of human antitumor chimeric immune receptors is governed by antigen and receptor expression levels and affinity of interaction. J. Immunother. 2007;30:684–693. doi: 10.1097/CJI.0b013e3180de5d90. [DOI] [PubMed] [Google Scholar]
- 2.Gomes-Silva D., Srinivasan M., Sharma S., Lee C.M., Wagner D.L., Davis T.H., Rouce R.H., Bao G., Brenner M.K., Mamonkin M. CD7-edited T cells expressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood. 2017;130:285–296. doi: 10.1182/blood-2017-01-761320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J., Chew A., Gonzalez V.E., Zheng Z., Lacey S.F., et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davila M.L., Riviere I., Wang X., Bartido S., Park J., Curran K., Chung S.S., Stefanski J., Borquez-Ojeda O., Olszewska M., et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kochenderfer J.N., Dudley M.E., Kassim S.H., Somerville R.P.T., Carpenter R.O., Stetler-Stevenson M., Yang J.C., Phan G.Q., Hughes M.S., Sherry R.M., et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuster S.J., Bishop M.R., Tam C.S., Waller E.K., Borchmann P., McGuirk J.P., Jäger U., Jaglowski S., Andreadis C., Westin J.R., et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 7.Locke F.L., Ghobadi A., Jacobson C.A., Miklos D.B., Lekakis L.J., Oluwole O.O., Lin Y., Braunschweig I., Hill B.T., Timmerman J.M., et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20:31–42. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruella M., Maus M.V. Catch me if you can: Leukemia Escape after CD19-Directed T Cell Immunotherapies. Comput. Struct. Biotechnol. J. 2016;14:357–362. doi: 10.1016/j.csbj.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fry T.J., Shah N.N., Orentas R.J., Stetler-Stevenson M., Yuan C.M., Ramakrishna S., Wolters P., Martin S., Delbrook C., Yates B., et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 2018;24:20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang A., Ye S., Li P., Huang J., Zhu S., Yao X., Zhou L., Xu Y., Zhu J., Zheng C., et al. Safety and efficacy of a novel anti-CD20 chimeric antigen receptor (CAR)-T cell therapy in relapsed/refractory (r/r) B-cell non-Hodgkin lymphoma (B-NHL) patients after failing CD19 CAR-T therapy. J. Clin. Oncol. 2021;39:2508. [Google Scholar]
- 11.Shah N.N., Highfill S.L., Shalabi H., Yates B., Jin J., Wolters P.L., Ombrello A., Steinberg S.M., Martin S., Delbrook C., et al. CD4/CD8 T-Cell Selection Affects Chimeric Antigen Receptor (CAR) T-Cell Potency and Toxicity: Updated Results From a Phase I Anti-CD22 CAR T-Cell Trial. J. Clin. Oncol. 2020;38:1938–1950. doi: 10.1200/JCO.19.03279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duell J., Leipold A.M., Appenzeller S., Fuhr V., Rauert-Wunderlich H., Da Via M., Dietrich O., Toussaint C., Imdahl F., Eisele F., et al. Sequential antigen loss and branching evolution in lymphoma after CD19- and CD20-targeted T-cell-redirecting therapy. Blood. 2024;143:685–696. doi: 10.1182/blood.2023021672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aminov S., Giricz O., Melnekoff D.T., Sica R.A., Polishchuk V., Papazoglu C., Yates B., Wang H.W., Sahu S., Wang Y., et al. Immunotherapy-resistant acute lymphoblastic leukemia cells exhibit reduced CD19 and CD22 expression and BTK pathway dependency. J. Clin. Invest. 2024;134 doi: 10.1172/JCI175199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah N.N., Johnson B.D., Schneider D., Zhu F., Szabo A., Keever-Taylor C.A., Krueger W., Worden A.A., Kadan M.J., Yim S., et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat. Med. 2020;26:1569–1575. doi: 10.1038/s41591-020-1081-3. [DOI] [PubMed] [Google Scholar]
- 15.Cui W., Zhang X., Dai H., Cui Q., Song B., Wu D., Tang X., Yin J., Li Z. Tandem CD19/CD22 Dual Targets CAR-T Cells Therapy Acquires Superior CR Rate Than CD19 CAR-T Cells: A Case Controlled Study. Blood. 2020;136:44. [Google Scholar]
- 16.Cordoba S., Onuoha S., Thomas S., Pignataro D.S., Hough R., Ghorashian S., Vora A., Bonney D., Veys P., Rao K., et al. CAR T cells with dual targeting of CD19 and CD22 in pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia: a phase 1 trial. Nat. Med. 2021;27:1797–1805. doi: 10.1038/s41591-021-01497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smulski C.R., Eibel H. BAFF and BAFF-Receptor in B Cell Selection and Survival. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.02285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parameswaran R., Müschen M., Kim Y.M., Groffen J., Heisterkamp N. A functional receptor for B-cell-activating factor is expressed on human acute lymphoblastic leukemias. Cancer Res. 2010;70:4346–4356. doi: 10.1158/0008-5472.CAN-10-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novak A.J., Grote D.M., Stenson M., Ziesmer S.C., Witzig T.E., Habermann T.M., Harder B., Ristow K.M., Bram R.J., Jelinek D.F., et al. Expression of BLyS and its receptors in B-cell non-Hodgkin lymphoma: correlation with disease activity and patient outcome. Blood. 2004;104:2247–2253. doi: 10.1182/blood-2004-02-0762. [DOI] [PubMed] [Google Scholar]
- 20.Qin H., Dong Z., Wang X., Cheng W.A., Wen F., Xue W., Sun H., Walter M., Wei G., Smith D.L., et al. CAR T cells targeting BAFF-R can overcome CD19 antigen loss in B cell malignancies. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aaw9414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin H., Wei G., Sakamaki I., Dong Z., Cheng W.A., Smith D.L., Wen F., Sun H., Kim K., Cha S., et al. Novel BAFF-Receptor Antibody to Natively Folded Recombinant Protein Eliminates Drug-Resistant Human B-cell Malignancies In Vivo. Clin. Cancer Res. 2018;24:1114–1123. doi: 10.1158/1078-0432.CCR-17-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budde L.E., Del Real M.M., Baird J.H., Chen L., Song J.Y., Kambhampati S., Macias A., Kim T., Dulan S., Barva B., et al. Promising Safety and Anti-Lymphoma Efficacy of Autologous Pmb-CT01 (BAFFRCAR T Cell) Therapy in a First-in-Human Phase 1 Study. Blood. 2023;142:221. [Google Scholar]
- 23.Tkachenko A., Kupcova K., Havranek O. B-Cell Receptor Signaling and Beyond: The Role of Igalpha (CD79a)/Igbeta (CD79b) in Normal and Malignant B Cells. Int. J. Mol. Sci. 2023;25 doi: 10.3390/ijms25010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seda V., Mraz M. B-cell receptor signalling and its crosstalk with other pathways in normal and malignant cells. Eur. J. Haematol. 2015;94:193–205. doi: 10.1111/ejh.12427. [DOI] [PubMed] [Google Scholar]
- 25.Ding S., Mao X., Cao Y., Wang N., Xu H., Zhou J. Targeting CD79b for Chimeric Antigen Receptor T-Cell Therapy of B-Cell Lymphomas. Target. Oncol. 2020;15:365–375. doi: 10.1007/s11523-020-00729-7. [DOI] [PubMed] [Google Scholar]
- 26.Leung I., Templeton M.L., Lo Y., Rajan A., Stull S.M., Garrison S.M., Salter A.I., Smythe K.S., Correnti C.E., Srivastava S., et al. Compromised antigen binding and signaling interfere with bispecific CD19 and CD79a chimeric antigen receptor function. Blood Adv. 2023;7:2718–2730. doi: 10.1182/bloodadvances.2022008559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu F., Cao J., Liu J., Yang H., Davis T.J., Kuang S.Q., Cheng X., Zhang Z., Karri S., Vien L.T., et al. Chimeric antigen receptor T cells to target CD79b in B-cell lymphomas. J. Immunother. Cancer. 2023;11 doi: 10.1136/jitc-2023-007515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palanca-Wessels M.C.A., Czuczman M., Salles G., Assouline S., Sehn L.H., Flinn I., Patel M.R., Sangha R., Hagenbeek A., Advani R., et al. Safety and activity of the anti-CD79B antibody-drug conjugate polatuzumab vedotin in relapsed or refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukaemia: a phase 1 study. Lancet Oncol. 2015;16:704–715. doi: 10.1016/S1470-2045(15)70128-2. [DOI] [PubMed] [Google Scholar]
- 29.Xu-Monette Z.Y., Li L., Byrd J.C., Jabbar K.J., Manyam G.C., Maria de Winde C., van den Brand M., Tzankov A., Visco C., Wang J., et al. Assessment of CD37 B-cell antigen and cell of origin significantly improves risk prediction in diffuse large B-cell lymphoma. Blood. 2016;128:3083–3100. doi: 10.1182/blood-2016-05-715094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scarfò I., Ormhøj M., Frigault M.J., Castano A.P., Lorrey S., Bouffard A.A., van Scoyk A., Rodig S.J., Shay A.J., Aster J.C., et al. Anti-CD37 chimeric antigen receptor T cells are active against B- and T-cell lymphomas. Blood. 2018;132:1495–1506. doi: 10.1182/blood-2018-04-842708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moshe Y.L., Deepa J., Zhanet G.-P., Marek T., Wojciech J., Halyna P., Marc A., Sunita D.N., Dalit R.-R., Sara T., et al. Safety and Efficacy of CD37-Targeting Naratuximab Emtansine PLUS Rituximab in Diffuse Large B-Cell Lymphoma and Other NON-Hodgkin'S B-Cell Lymphomas - a Phase 2 Study. Blood. 2021;138:526. [Google Scholar]
- 32.Sawas A., Savage K.J., Perez R.P., Advani R.H., Zaine J.M., Lackey J.M., Trave F., Anand B., Chu R., Reyno L.M., O'Connor O. A PHASE 1 STUDY OF THE ANTI-CD37 ANTIBODY-DRUG CONJUGATE AGS67E IN ADVANCED LYMPHOID MALIGNANCIES. INTERIM RESULTS. Hematological Oncol. 2017;35:49. [Google Scholar]
- 33.Balzarotti M., Magagnoli M., Canales M.Á., Corradini P., Grande C., Sancho J.M., Zaja F., Quinson A.M., Belsack V., Maier D., Carlo-Stella C. A phase Ib, open-label, dose-escalation trial of the anti-CD37 monoclonal antibody, BI 836826, in combination with gemcitabine and oxaliplatin in patients with relapsed/refractory diffuse large B-cell lymphoma. Invest. New Drugs. 2021;39:1028–1035. doi: 10.1007/s10637-020-01054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.ClinicalTrials.gov. Identifier NCT02610062, A Study to Evaluate Safety, Tolerability, and Pharmacokinetics of Escalating Doses of AGS67E Given as Monotherapy in Subjects With Acute Myeloid Leukemia (AML). [Internet] [cited 2024 March 1]; Available from: https://www.clinicaltrials.gov/study/NCT02610062.
- 35.ClinicalTrials.gov. Identifier NCT04358458, First-in-Human (FIH) Trial of GEN3009 in Subjects With Relapsed or Refractory B-Cell Non-Hodgkin Lymphomas. [Internet] [cited 2024 March 1]; Available from: https://clinicaltrials.gov/study/NCT04358458.
- 36.Frigault M.J., Chen Y.-B., Gallagher K.M., Horick N.K., El-Jawahri A., Scarfò I., Wehrli M., Huang L., Casey K., Cook D., et al. Phase 1 Study of CD37-Directed CAR T Cells in Patients with Relapsed or Refractory CD37+ Hematologic Malignancies. Blood. 2021;138:653. [Google Scholar]
- 37.de Winde C.M., Zuidscherwoude M., Vasaturo A., van der Schaaf A., Figdor C.G., van Spriel A.B. Multispectral imaging reveals the tissue distribution of tetraspanins in human lymphoid organs. Histochem. Cell Biol. 2015;144:133–146. doi: 10.1007/s00418-015-1326-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nix M.A., Mandal K., Geng H., Paranjape N., Lin Y.H.T., Rivera J.M., Marcoulis M., White K.L., Whitman J.D., Bapat S.P., et al. Surface Proteomics Reveals CD72 as a Target for In Vitro-Evolved Nanobody-Based CAR-T Cells in KMT2A/MLL1-Rearranged B-ALL. Cancer Discov. 2021;11:2032–2049. doi: 10.1158/2159-8290.CD-20-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cappell K.M., Sherry R.M., Yang J.C., Goff S.L., Vanasse D.A., McIntyre L., Rosenberg S.A., Kochenderfer J.N. Long-Term Follow-Up of Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy. J. Clin. Oncol. 2020;38:3805–3815. doi: 10.1200/JCO.20.01467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hudecek M., Schmitt T.M., Baskar S., Lupo-Stanghellini M.T., Nishida T., Yamamoto T.N., Bleakley M., Turtle C.J., Chang W.C., Greisman H.A., et al. The B-cell tumor-associated antigen ROR1 can be targeted with T cells modified to express a ROR1-specific chimeric antigen receptor. Blood. 2010;116:4532–4541. doi: 10.1182/blood-2010-05-283309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berger C., Sommermeyer D., Hudecek M., Berger M., Balakrishnan A., Paszkiewicz P.J., Kosasih P.L., Rader C., Riddell S.R. Safety of targeting ROR1 in primates with chimeric antigen receptor-modified T cells. Cancer Immunol. Res. 2015;3:206–216. doi: 10.1158/2326-6066.CIR-14-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Srivastava S., Salter A.I., Liggitt D., Yechan-Gunja S., Sarvothama M., Cooper K., Smythe K.S., Dudakov J.A., Pierce R.H., Rader C., Riddell S.R. Logic-Gated ROR1 Chimeric Antigen Receptor Expression Rescues T Cell-Mediated Toxicity to Normal Tissues and Enables Selective Tumor Targeting. Cancer Cell. 2019;35:489–503.e8. doi: 10.1016/j.ccell.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qin H., Cho M., Haso W., Zhang L., Tasian S.K., Oo H.Z., Negri G.L., Lin Y., Zou J., Mallon B.S., et al. Eradication of B-ALL using chimeric antigen receptor–expressing T cells targeting the TSLPR oncoprotein. Blood. 2015;126:629–639. doi: 10.1182/blood-2014-11-612903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian Z., Shi C., Yang G., Allen J.K., Shi Q., Al-Shami A., Olson J.W., Smith M.G., Chang Q., Kaur J., et al. Preclinical development of 1B7/CD3, a novel anti-TSLPR bispecific antibody that targets CRLF2-rearranged Ph-like B-ALL. Leukemia. 2023;37:2006–2016. doi: 10.1038/s41375-023-02010-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramos C.A., Savoldo B., Torrano V., Ballard B., Zhang H., Dakhova O., Liu E., Carrum G., Kamble R.T., Gee A.P., et al. Clinical responses with T lymphocytes targeting malignancy-associated κ light chains. J. Clin. Invest. 2016;126:2588–2596. doi: 10.1172/JCI86000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ranganathan R., Shou P., Ahn S., Sun C., West J., Savoldo B., Dotti G. CAR T cells Targeting Human Immunoglobulin Light Chains Eradicate Mature B-cell Malignancies While Sparing a Subset of Normal B Cells. Clin. Cancer Res. 2021;27:5951–5960. doi: 10.1158/1078-0432.CCR-20-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen I.J., Bochi-Layec A.C., Kim K.H., Jenks S., Ugwuanyi O., Ghilardi G., Gabrielli G., Harris J., Wang L.-P., Zhang Y., et al. Precision Targeting of the Malignant Clone in Diffuse Large B Cell Lymphoma Using Chimeric Antigen Receptor T Cells Against the Clonotypic IGHV4-34 B Cell Receptor. Blood. 2023;142:1020. [Google Scholar]
- 48.Stalker M.E., Mark T.M. Clinical Management of Triple-Class Refractory Multiple Myeloma: A Review of Current Strategies and Emerging Therapies. Curr. Oncol. 2022;29:4464–4477. doi: 10.3390/curroncol29070355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brudno J.N., Maric I., Hartman S.D., Rose J.J., Wang M., Lam N., Stetler-Stevenson M., Salem D., Yuan C., Pavletic S., et al. T Cells Genetically Modified to Express an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J. Clin. Oncol. 2018;36:2267–2280. doi: 10.1200/JCO.2018.77.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang L., Shen X., Yu W., Li J., Zhang J., Zhang R., Li J., Chen L. Comprehensive meta-analysis of anti-BCMA chimeric antigen receptor T-cell therapy in relapsed or refractory multiple myeloma. Ann. Med. 2021;53:1547–1559. doi: 10.1080/07853890.2021.1970218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Da Via M.C., Dietrich O., Truger M., Arampatzi P., Duell J., Heidemeier A., Zhou X., Danhof S., Kraus S., Chatterjee M., et al. Homozygous BCMA gene deletion in response to anti-BCMA CAR T cells in a patient with multiple myeloma. Nat. Med. 2021;27:616–619. doi: 10.1038/s41591-021-01245-5. [DOI] [PubMed] [Google Scholar]
- 52.Lee H., Ahn S., Maity R., Leblay N., Ziccheddu B., Truger M., Chojnacka M., Cirrincione A., Durante M., Tilmont R., et al. Mechanisms of antigen escape from BCMA- or GPRC5D-targeted immunotherapies in multiple myeloma. Nat. Med. 2023;29:2295–2306. doi: 10.1038/s41591-023-02491-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Samur M.K., Fulciniti M., Aktas Samur A., Bazarbachi A.H., Tai Y.-T., Prabhala R., Alonso A., Sperling A.S., Campbell T., Petrocca F., et al. Biallelic loss of BCMA as a resistance mechanism to CAR T cell therapy in a patient with multiple myeloma. Nat. Commun. 2021;12:868. doi: 10.1038/s41467-021-21177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bal S., Htut M., Nadeem O., Anderson L.D., Jr., Koçoğlu H., Gregory T., Rossi A.C., Martin T., Egan D.N., Costa L., et al. BMS-986393 (CC-95266), a G Protein-Coupled Receptor Class C Group 5 Member D (GPRC5D)-Targeted Chimeric Antigen Receptor (CAR) T-Cell Therapy for Relapsed/Refractory Multiple Myeloma (RRMM): Updated Results from a Phase 1 Study. Blood. 2023;142:219. [Google Scholar]
- 55.Carlo-Stella C., Mazza R., Manier S., Facon T., Yoon S.-S., Koh Y., Harrison S.J., Er J., Pinto A., Volzone F., et al. RG6234, a GPRC5DxCD3 T-Cell Engaging Bispecific Antibody, Is Highly Active in Patients (pts) with Relapsed/Refractory Multiple Myeloma (RRMM): Updated Intravenous (IV) and First Subcutaneous (SC) Results from a Phase I Dose-Escalation Study. Blood. 2022;140:397–399. [Google Scholar]
- 56.Schinke C.D., Touzeau C., Minnema M.C., van de Donk N.W., Rodríguez-Otero P., Mateos M.-V., Rasche L., Ye J.C., Vishwamitra D., Ma X., et al. Pivotal phase 2 MonumenTAL-1 results of talquetamab (tal), a GPRC5DxCD3 bispecific antibody (BsAb), for relapsed/refractory multiple myeloma (RRMM) J. Clin. Oncol. 2023;41:8036. [Google Scholar]
- 57.Zhang M., Wei G., Zhou L., Zhou J., Chen S., Zhang W., Wang D., Luo X., Cui J., Huang S., et al. GPRC5D CAR T cells (OriCAR-017) in patients with relapsed or refractory multiple myeloma (POLARIS): a first-in-human, single-centre, single-arm, phase 1 trial. Lancet Haematol. 2023;10:e107–e116. doi: 10.1016/S2352-3026(22)00372-6. [DOI] [PubMed] [Google Scholar]
- 58.Verkleij C.P.M., Broekmans M.E.C., van Duin M., Frerichs K.A., Kuiper R., de Jonge A.V., Kaiser M., Morgan G., Axel A., Boominathan R., et al. Preclinical activity and determinants of response of the GPRC5DxCD3 bispecific antibody talquetamab in multiple myeloma. Blood Adv. 2021;5:2196–2215. doi: 10.1182/bloodadvances.2020003805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pillarisetti K., Edavettal S., Mendonça M., Li Y., Tornetta M., Babich A., Majewski N., Husovsky M., Reeves D., Walsh E., et al. A T-cell-redirecting bispecific G-protein-coupled receptor class 5 member D x CD3 antibody to treat multiple myeloma. Blood. 2020;135:1232–1243. doi: 10.1182/blood.2019003342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson & Johnson. (2023). U.S. FDA Approves TALVEY™ (talquetamab-tgvs), a First-in-Class Bispecific Therapy for the Treatment of Patients with Heavily Pretreated Multiple Myeloma. [Press Release] [cited 2024 February 26]; Available from: https://www.jnj.com/media-center/press-releases/u-s-fda-approves-talvey-talquetamab-tgvs-a-first-in-class-bispecific-therapy-for-the-treatment-of-patients-with-heavily-pretreated-multiple-myeloma.
- 61.Johnson & Johnson. (2023). European Commission Approves TALVEY®▼ (talquetamab), Janssen’s Novel Bispecific Therapy for the Treatment of Patients with Relapsed and Refractory Multiple Myeloma. [Press Release] [cited 2024 Feburary 26]; Available from: https://www.jnj.com/media-center/press-releases/european-commission-approves-talvey-talquetamab-janssens-novel-bispecific-therapy-for-the-treatment-of-patients-with-relapsed-and-refractory-multiple-myeloma.
- 62.Mailankody S., Devlin S.M., Landa J., Nath K., Diamonte C., Carstens E.J., Russo D., Auclair R., Fitzgerald L., Cadzin B., et al. GPRC5D-Targeted CAR T Cells for Myeloma. N. Engl. J. Med. 2022;387:1196–1206. doi: 10.1056/NEJMoa2209900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shinichi I., Tadahiro N., Toshiyasu S. The RAIG Family Member, GPRC5D, Is Associated with Hard-Keratinized Structures. J. Invest. Dermatol. 2004;122:565–573. doi: 10.1046/j.0022-202X.2004.12628.x. [DOI] [PubMed] [Google Scholar]
- 64.Elkins K., Zheng B., Go M., Slaga D., Du C., Scales S.J., Yu S.-F., McBride J., de Tute R., Rawstron A., et al. FcRL5 as a Target of Antibody–Drug Conjugates for the Treatment of Multiple Myeloma. Mol. Cancer Ther. 2012;11:2222–2232. doi: 10.1158/1535-7163.MCT-12-0087. [DOI] [PubMed] [Google Scholar]
- 65.Li J., Stagg N.J., Johnston J., Harris M.J., Menzies S.A., DiCara D., Clark V., Hristopoulos M., Cook R., Slaga D., et al. Membrane-Proximal Epitope Facilitates Efficient T Cell Synapse Formation by Anti-FcRH5/CD3 and Is a Requirement for Myeloma Cell Killing. Cancer Cell. 2017;31:383–395. doi: 10.1016/j.ccell.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu Z., Li H., Lu Q., Zhang Z., Tong A., Niu T. Fc receptor-like 5 (FCRL5)-directed CAR-T cells exhibit antitumor activity against multiple myeloma. Signal Transduct. Target. Ther. 2024;9:16. doi: 10.1038/s41392-023-01702-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang D., Huang H., Qin H., Tang K., Shi X., Zhu T., Gao Y., Zhang Y., Tian X., Fu J., et al. Chimeric antigen receptor T cells targeting FcRH5 provide robust tumour-specific responses in murine xenograft models of multiple myeloma. Nat. Commun. 2023;14:3642. doi: 10.1038/s41467-023-39395-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stewart A.K., Krishnan A.Y., Singhal S., Boccia R.V., Patel M.R., Niesvizky R., Chanan-Khan A.A., Ailawadhi S., Brumm J., Mundt K.E., et al. Phase I study of the anti-FcRH5 antibody-drug conjugate DFRF4539A in relapsed or refractory multiple myeloma. Blood Cancer J. 2019;9:17. doi: 10.1038/s41408-019-0178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trudel S., Cohen A.D., Krishnan A.Y., Fonseca R., Spencer A., Berdeja J.G., Lesokhin A., Forsberg P.A., Laubach J.P., Costa L.J., et al. Cevostamab Monotherapy Continues to Show Clinically Meaningful Activity and Manageable Safety in Patients with Heavily Pre-Treated Relapsed/Refractory Multiple Myeloma (RRMM): Updated Results from an Ongoing Phase I Study. Blood. 2021;138:157. [Google Scholar]
- 70.Moreau P., Garfall A.L., van de Donk N.W.C.J., Nahi H., San-Miguel J.F., Oriol A., Nooka A.K., Martin T., Rosinol L., Chari A., et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2022;387:495–505. doi: 10.1056/NEJMoa2203478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lesokhin A.M., Richter J., Trudel S., Cohen A.D., Spencer A., Forsberg P.A., Laubach J.P., Thomas S.K., Bahlis N.J., Costa L.J., et al. Enduring Responses after 1-Year, Fixed-Duration Cevostamab Therapy in Patients with Relapsed/Refractory Multiple Myeloma: Early Experience from a Phase I Study. Blood. 2022;140:4415–4417. [Google Scholar]
- 72.Djordje A., Jens P., York H., Adam J., Sebastian K., Yanran C., Julia T., Sabrina M., Henrike R., Katrin B., et al. Surface molecule CD229 as a novel target for the diagnosis and treatment of multiple myeloma. Haematologica. 2011;96:1512–1520. doi: 10.3324/haematol.2010.036814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yousef S., Kovacsovics-Bankowski M., Salama M.E., Bhardwaj N., Steinbach M., Langemo A., Kovacsovics T., Marvin J., Binder M., Panse J., et al. CD229 is expressed on the surface of plasma cells carrying an aberrant phenotype and chemotherapy-resistant precursor cells in multiple myeloma. Hum. Vaccin. Immunother. 2015;11:1606–1611. doi: 10.1080/21645515.2015.1046658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Radhakrishnan S.V., Luetkens T., Scherer S.D., Davis P., Vander Mause E.R., Olson M.L., Yousef S., Panse J., Abdiche Y., Li K.D., et al. CD229 CAR T cells eliminate multiple myeloma and tumor propagating cells without fratricide. Nat. Commun. 2020;11:798. doi: 10.1038/s41467-020-14619-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vander Mause E.R., Baker J.M., Dietze K.A., Radhakrishnan S.V., Iraguha T., Omili D., Davis P., Chidester S.L., Modzelewska K., Panse J., et al. Systematic single amino acid affinity tuning of CD229 CAR T cells retains efficacy against multiple myeloma and eliminates on-target off-tumor toxicity. Sci. Transl. Med. 2023;15:eadd7900. doi: 10.1126/scitranslmed.add7900. [DOI] [PubMed] [Google Scholar]
- 76.VanWyngarden M.J., Walker Z.J., Su Y., Perez de Acha O., Stevens B.M., Forsberg P.A., Mark T.M., Matsui W., Liu B., Sherbenou D.W. CD46-ADC Reduces the Engraftment of Multiple Myeloma Patient-Derived Xenografts. Cancers (Basel) 2023;15 doi: 10.3390/cancers15225335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sandy W., Philip I., Tomer M., Jonathan K., Ajai I., Jeffrey A.Z., Zachary W., Daniel S., Mark S., Jill A., et al. P-225: A first-in-human study of FOR46 in patients with triple refractory Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2021;21:S164. [Google Scholar]
- 78.Sherbenou D.W., Aftab B.T., Su Y., Behrens C.R., Wiita A., Logan A.C., Acosta-Alvear D., Hann B.C., Walter P., Shuman M.A., et al. Antibody-drug conjugate targeting CD46 eliminates multiple myeloma cells. J. Clin. Invest. 2016;126:4640–4653. doi: 10.1172/JCI85856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Anderson G.S.F., Ballester-Beltran J., Giotopoulos G., Guerrero J.A., Surget S., Williamson J.C., So T., Bloxham D., Aubareda A., Asby R., et al. Unbiased cell surface proteomics identifies SEMA4A as an effective immunotherapy target for myeloma. Blood. 2022;139:2471–2482. doi: 10.1182/blood.2021015161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Di Meo F., Iyer A., Akama K., Cheng R., Yu C., Cesarano A., Kurihara N., Tenshin H., Aljoufi A., Marino S., et al. A target discovery pipeline identified ILT3 as a target for immunotherapy of multiple myeloma. Cell Rep. Med. 2023;4 doi: 10.1016/j.xcrm.2023.101110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Di Meo F., John S., Zhang C., Freeman C.L.L., Perna F. Chimeric Antigen Receptor T Cells Targeting LILRB4, an Immunoreceptor Mediating T-Cell Suppression, Are Potently Effective in Multiple Myeloma. Blood. 2023;142:4804. [Google Scholar]
- 82.Ferguson I.D., Patiño-Escobar B., Tuomivaara S.T., Lin Y.-H.T., Nix M.A., Leung K.K., Kasap C., Ramos E., Nieves Vasquez W., Talbot A., et al. The surfaceome of multiple myeloma cells suggests potential immunotherapeutic strategies and protein markers of drug resistance. Nat. Commun. 2022;13:4121. doi: 10.1038/s41467-022-31810-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dohner H., Weisdorf D.J., Bloomfield C.D. Acute Myeloid Leukemia. N. Engl. J. Med. 2015;373:1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 84.Perna F., Berman S.H., Soni R.K., Mansilla-Soto J., Eyquem J., Hamieh M., Hendrickson R.C., Brennan C.W., Sadelain M. Integrating Proteomics and Transcriptomics for Systematic Combinatorial Chimeric Antigen Receptor Therapy of AML. Cancer Cell. 2017;32:506–519.e5. doi: 10.1016/j.ccell.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ehninger A., Kramer M., Röllig C., Thiede C., Bornhäuser M., von Bonin M., Wermke M., Feldmann A., Bachmann M., Ehninger G., Oelschlägel U. Distribution and levels of cell surface expression of CD33 and CD123 in acute myeloid leukemia. Blood Cancer J. 2014;4:e218. doi: 10.1038/bcj.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maniecki M.B., Hasle H., Bendix K., Møller H.J. Is hepatotoxicity in patients treated with gemtuzumabozogamicin due to specific targeting of hepatocytes? Leuk. Res. 2011;35:e84–e86. doi: 10.1016/j.leukres.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 87.Larson R.A., Sievers E.L., Stadtmauer E.A., Löwenberg B., Estey E.H., Dombret H., Theobald M., Voliotis D., Bennett J.M., Richie M., et al. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer. 2005;104:1442–1452. doi: 10.1002/cncr.21326. [DOI] [PubMed] [Google Scholar]
- 88.BusinessWire Seattle Genetics Discontinues Phase 3 CASCADE Trial of Vadastuximab Talirine (SGN-CD33A) in Frontline Acute Myeloid Leukemia. 2017. https://www.businesswire.com/news/home/20170619005466/en/Seattle-Genetics-Discontinues-Phase-3-CASCADE-Trial-of-Vadastuximab-Talirine-SGN-CD33A-in-Frontline-Acute-Myeloid-Leukemia
- 89.Narayan R., Piérola A.A., Donnellan W.B., Yordi A.M., Abdul-Hay M., Platzbecker U., Subklewe M., Kadia T.M., Alonso-Domínguez J.M., McCloskey J., et al. First-in-human study of JNJ-67571244, a CD33 × CD3 bispecific antibody, in relapsed/refractory acute myeloid leukemia and myelodysplastic syndrome. Clin. Transl. Sci. 2024;17 doi: 10.1111/cts.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sallman D.A., Elmariah H., Sweet K., Mishra A., Cox C.A., Chakaith M., Semnani R., Shehzad S., Anderson A., Sabzevari H., et al. Phase 1/1b Safety Study of Prgn-3006 Ultracar-T in Patients with Relapsed or Refractory CD33-Positive Acute Myeloid Leukemia and Higher Risk Myelodysplastic Syndromes. Blood. 2022;140:10313–10315. [Google Scholar]
- 91.Tambaro F.P., Singh H., Jones E., Rytting M., Mahadeo K.M., Thompson P., Daver N., DiNardo C., Kadia T., Garcia-Manero G., et al. Autologous CD33-CAR-T cells for treatment of relapsed/refractory acute myelogenous leukemia. Leukemia. 2021;35:3282–3286. doi: 10.1038/s41375-021-01232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bowser B., Yang X., Roach J., Blake J., Donnelly K., Paskey B., VanBockel H., Yates B., Hoang C., Tasian S.K., et al. Success of Centralized Manufacturing of CD33 CAR T-Cells (CD33CART) for Children and Young Adults with Relapsed/Refractory AML. Transplant. Cell Ther. 2024;30:S153–S154. [Google Scholar]
- 93.Ravandi F., Stein A.S., Kantarjian H.M., Walter R.B., Paschka P., Jongen-Lavrencic M., Ossenkoppele G.J., Yang Z., Mehta B., Subklewe M. A Phase 1 First-in-Human Study of AMG 330, an Anti-CD33 Bispecific T-Cell Engager (BiTE®) Antibody Construct, in Relapsed/Refractory Acute Myeloid Leukemia (R/R AML) Blood. 2018;132:25. [Google Scholar]
- 94.Subklewe M., Stein A., Walter R.B., Bhatia R., Wei A.H., Ritchie D., Bücklein V., Vachhani P., Dai T., Hindoyan A., et al. Preliminary Results from a Phase 1 First-in-Human Study of AMG 673, a Novel Half-Life Extended (HLE) Anti-CD33/CD3 BiTE® (Bispecific T-Cell Engager) in Patients with Relapsed/Refractory (R/R) Acute Myeloid Leukemia (AML) Blood. 2019;134:833. [Google Scholar]
- 95.Testa U., Riccioni R., Militi S., Coccia E., Stellacci E., Samoggia P., Latagliata R., Mariani G., Rossini A., Battistini A., et al. Elevated expression of IL-3Ralpha in acute myelogenous leukemia is associated with enhanced blast proliferation, increased cellularity, and poor prognosis. Blood. 2002;100:2980–2988. doi: 10.1182/blood-2002-03-0852. [DOI] [PubMed] [Google Scholar]
- 96.Gill S., Tasian S.K., Ruella M., Shestova O., Li Y., Porter D.L., Carroll M., Danet-Desnoyers G., Scholler J., Grupp S.A., et al. Anti-CD123 Chimeric Antigen Receptor T Cells (CART-123) Provide A Novel Myeloablative Conditioning Regimen That Eradicates Human Acute Myeloid Leukemia In Preclinical Models. Blood. 2013;122:143. [Google Scholar]
- 97.Al-Hussaini M., Rettig M.P., Ritchey J.K., Karpova D., Uy G.L., Eissenberg L.G., Gao F., Eades W.C., Bonvini E., Chichili G.R., et al. Targeting CD123 in acute myeloid leukemia using a T-cell–directed dual-affinity retargeting platform. Blood. 2016;127:122–131. doi: 10.1182/blood-2014-05-575704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mardiros A., Dos Santos C., McDonald T., Brown C.E., Wang X., Budde L.E., Hoffman L., Aguilar B., Chang W.-C., Bretzlaff W., et al. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood. 2013;122:3138–3148. doi: 10.1182/blood-2012-12-474056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Uy G.L., Aldoss I., Foster M.C., Sayre P.H., Wieduwilt M.J., Advani A.S., Godwin J.E., Arellano M.L., Sweet K.L., Emadi A., et al. Flotetuzumab as salvage immunotherapy for refractory acute myeloid leukemia. Blood. 2021;137:751–762. doi: 10.1182/blood.2020007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boyiadzis M., Desai P., Daskalakis N., Donnellan W., Ferrante L., Goldberg J.D., Grunwald M.R., Guttke C., Li X., Perez-Simon J.A., et al. First-in-human study of JNJ-63709178, a CD123/CD3 targeting antibody, in relapsed/refractory acute myeloid leukemia. Clin. Transl. Sci. 2023;16:429–435. doi: 10.1111/cts.13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ravandi F., Bashey A., Foran J., Stock W., Mawad R., Short N., Yilmaz M., Kantarjian H., Odenike O., Patel A., et al. Phase 1 study of vibecotamab identifies an optimized dose for treatment of relapsed/refractory acute myeloid leukemia. Blood Adv. 2023;7:6492–6505. doi: 10.1182/bloodadvances.2023010956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Naik S., Madden R.M., Lipsitt A., Lockey T., Bran J., Rubnitz J.E., Klco J., Shulkin B., Patil S.L., Schell S., et al. Safety and Anti-Leukemic Activity of CD123-CAR T Cells in Pediatric Patients with AML: Preliminary Results from a Phase 1 Trial. Blood. 2022;140:4584–4585. [Google Scholar]
- 103.Sallman D.A., DeAngelo D.J., Pemmaraju N., Dinner S., Gill S., Olin R.L., Wang E.S., Konopleva M., Stark E., Korngold A., et al. Ameli-01: A Phase I Trial of UCART123v1.2, an Anti-CD123 Allogeneic CAR-T Cell Product, in Adult Patients with Relapsed or Refractory (R/R) CD123+ Acute Myeloid Leukemia (AML) Blood. 2022;140:2371–2373. [Google Scholar]
- 104.Cellectis Cellectis Reports Clinical Hold of UCART123 Studies. 2017. https://www.cellectis.com/en/press/cellectis-reports-clinical-hold-of-ucart123-studies/
- 105.Cellectis Cellectis Presents Clinical Data on AMELI-01 and Preclinical Data on Multiplex Engineering for Superior Generation of CAR T-cells at ASGCT 2023. 2023. https://www.cellectis.com/en/press/cellectis-presents-clinical-data-on-ameli-01-and-preclinical-data-on-multiplex-engineering-for-superior-generation-of-car-t-cells-at-asgct-2023
- 106.Sun Y., Wang S., Zhao L., Zhang B., Chen H. IFN-γ and TNF-α aggravate endothelial damage caused by CD123-targeted CAR T cell. Onco. Targets Ther. 2019;12:4907–4925. doi: 10.2147/OTT.S205678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zheng B., Yu S.-F., del Rosario G., Leong S.R., Lee G.Y., Vij R., Chiu C., Liang W.-C., Wu Y., Chalouni C., et al. An Anti–CLL-1 Antibody–Drug Conjugate for the Treatment of Acute Myeloid Leukemia. Clin. Cancer Res. 2019;25:1358–1368. doi: 10.1158/1078-0432.CCR-18-0333. [DOI] [PubMed] [Google Scholar]
- 108.Wang J., Chen S., Xiao W., Li W., Wang L., Yang S., Wang W., Xu L., Liao S., Liu W., et al. CAR-T cells targeting CLL-1 as an approach to treat acute myeloid leukemia. J. Hematol. Oncol. 2018;11:7. doi: 10.1186/s13045-017-0553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Leong S.R., Sukumaran S., Hristopoulos M., Totpal K., Stainton S., Lu E., Wong A., Tam L., Newman R., Vuillemenot B.R., et al. An anti-CD3/anti-CLL-1 bispecific antibody for the treatment of acute myeloid leukemia. Blood. 2017;129:609–618. doi: 10.1182/blood-2016-08-735365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tashiro H., Sauer T., Shum T., Parikh K., Mamonkin M., Omer B., Rouce R.H., Lulla P., Rooney C.M., Gottschalk S., Brenner M.K. Treatment of Acute Myeloid Leukemia with T Cells Expressing Chimeric Antigen Receptors Directed to C-type Lectin-like Molecule 1. Mol. Ther. 2017;25:2202–2213. doi: 10.1016/j.ymthe.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jiang Y.P., Liu B.Y., Zheng Q., Panuganti S., Chen R., Zhu J., Mishra M., Huang J., Dao-Pick T., Roy S., et al. CLT030, a leukemic stem cell-targeting CLL1 antibody-drug conjugate for treatment of acute myeloid leukemia. Blood Adv. 2018;2:1738–1749. doi: 10.1182/bloodadvances.2018020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jin X., Zhang M., Sun R., Lyu H., Xiao X., Zhang X., Li F., Xie D., Xiong X., Wang J., et al. First-in-human phase I study of CLL-1 CAR-T cells in adults with relapsed/refractory acute myeloid leukemia. J. Hematol. Oncol. 2022;15:88. doi: 10.1186/s13045-022-01308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang H., Bu C., Peng Z., Li G., Zhou Z., Ding W., Zheng Y., He Y., Hu Z., Pei K., et al. Characteristics of anti-CLL1 based CAR-T therapy for children with relapsed or refractory acute myeloid leukemia: the multi-center efficacy and safety interim analysis. Leukemia. 2022;36:2596–2604. doi: 10.1038/s41375-022-01703-0. [DOI] [PubMed] [Google Scholar]
- 114.Mascarenhas J., Cortes J., Huls G., Venditti A., Breems D., De Botton S., DeAngelo D.J., van de Loosdrecht A., Jongen-Lavrencic M., Borthakur G., et al. European Hematology Association; 2020. Update from the Ongoing Phase I Multinational Study of MCLA-117, a Bispecific CLEC12A X CD3 T-Cell Engager, in Patients (Pts) with Acute Myelogenous Leukemia. [Google Scholar]
- 115.Lee E., Lee S., Park S., Son Y.-G., Yoo J., Koh Y., Shin D.-Y., Lim Y., Won J. Asymmetric anti-CLL-1×CD3 bispecific antibody, ABL602 2+1, with attenuated CD3 affinity endows potent antitumor activity but limited cytokine release. J. Immunother. Cancer. 2023;11:e007494. doi: 10.1136/jitc-2023-007494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brauchle B., Goldstein R.L., Karbowski C.M., Henn A., Li C.-M., Bücklein V.L., Krupka C., Boyle M.C., Koppikar P., Haubner S., et al. Characterization of a Novel FLT3 BiTE Molecule for the Treatment of Acute Myeloid Leukemia. Mol. Cancer Ther. 2020;19:1875–1888. doi: 10.1158/1535-7163.MCT-19-1093. [DOI] [PubMed] [Google Scholar]
- 117.Chen L., Mao H., Zhang J., Chu J., Devine S., Caligiuri M.A., Yu J. Targeting FLT3 by chimeric antigen receptor T cells for the treatment of acute myeloid leukemia. Leukemia. 2017;31:1830–1834. doi: 10.1038/leu.2017.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Niswander L.M., Graff Z.T., Chien C.D., Chukinas J.A., Meadows C.A., Leach L.C., Loftus J.P., Kohler M.E., Tasian S.K., Fry T.J. Potent preclinical activity of FLT3-directed chimeric antigen receptor T-cell immunotherapy against FLT3 - mutant acute myeloid leukemia and KMT2A -rearranged acute lymphoblastic leukemia. Haematologica. 2023;108:457–471. doi: 10.3324/haematol.2022.281456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Karbowski C., Goldstein R., Frank B., Kim K., Li C.M., Homann O., Hensley K., Brooks B., Wang X., Yan Q., et al. Nonclinical Safety Assessment of AMG 553, an Investigational Chimeric Antigen Receptor T-Cell Therapy for the Treatment of Acute Myeloid Leukemia. Toxicol. Sci. 2020;177:94–107. doi: 10.1093/toxsci/kfaa098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sommer C., Cheng H.Y., Nguyen D., Dettling D., Yeung Y.A., Sutton J., Hamze M., Valton J., Smith J., Djuretic I., et al. Allogeneic FLT3 CAR T Cells with an Off-Switch Exhibit Potent Activity against AML and Can Be Depleted to Expedite Bone Marrow Recovery. Mol. Ther. 2020;28:2237–2251. doi: 10.1016/j.ymthe.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Deng M., Gui X., Kim J., Xie L., Chen W., Li Z., He L., Chen Y., Chen H., Luo W., et al. LILRB4 signalling in leukaemia cells mediates T cell suppression and tumour infiltration. Nature. 2018;562:605–609. doi: 10.1038/s41586-018-0615-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Casucci M., Nicolis di Robilant B., Falcone L., Camisa B., Norelli M., Genovese P., Gentner B., Gullotta F., Ponzoni M., Bernardi M., et al. CD44v6-targeted T cells mediate potent antitumor effects against acute myeloid leukemia and multiple myeloma. Blood. 2013;122:3461–3472. doi: 10.1182/blood-2013-04-493361. [DOI] [PubMed] [Google Scholar]
- 123.Lynn R.C., Poussin M., Kalota A., Feng Y., Low P.S., Dimitrov D.S., Powell D.J., Jr. Targeting of folate receptor β on acute myeloid leukemia blasts with chimeric antigen receptor-expressing T cells. Blood. 2015;125:3466–3476. doi: 10.1182/blood-2014-11-612721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hebbar N., Epperly R., Vaidya A., Thanekar U., Moore S.E., Umeda M., Ma J., Patil S.L., Langfitt D., Huang S., et al. CAR T cells redirected to cell surface GRP78 display robust anti-acute myeloid leukemia activity and do not target hematopoietic progenitor cells. Nat. Commun. 2022;13:587. doi: 10.1038/s41467-022-28243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu G., Guo S., Luo Q., Wang X., Deng W., Ouyang G., Pu J.J., Lei W., Qian W. Preclinical evaluation of CD70-specific CAR T cells targeting acute myeloid leukemia. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1093750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Barreyro L., Will B., Bartholdy B., Zhou L., Todorova T.I., Stanley R.F., Ben-Neriah S., Montagna C., Parekh S., Pellagatti A., et al. Overexpression of IL-1 receptor accessory protein in stem and progenitor cells and outcome correlation in AML and MDS. Blood. 2012;120:1290–1298. doi: 10.1182/blood-2012-01-404699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bellei M., Foss F.M., Shustov A.R., Horwitz S.M., Marcheselli L., Kim W.S., Cabrera M.E., Dlouhy I., Nagler A., Advani R.H., et al. The outcome of peripheral T-cell lymphoma patients failing first-line therapy: a report from the prospective, International T-Cell Project. Haematologica. 2018;103:1191–1197. doi: 10.3324/haematol.2017.186577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Raetz E.A., Teachey D.T. T-cell acute lymphoblastic leukemia. Hematol. Am. Soc. Hematol. Educ. Program. 2016;2016:580–588. doi: 10.1182/asheducation-2016.1.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hu Y., Zhou Y., Zhang M., Zhao H., Wei G., Ge W., Cui Q., Mu Q., Chen G., Han L., et al. Genetically modified CD7-targeting allogeneic CAR-T cell therapy with enhanced efficacy for relapsed/refractory CD7-positive hematological malignancies: a phase I clinical study. Cell Res. 2022;32:995–1007. doi: 10.1038/s41422-022-00721-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang X., Zhou Y., Yang J., Li J., Qiu L., Ge W., Pei B., Chen J., Han L., Ren J., Lu P. A Novel Universal CD7-Targeted CAR-T Cell Therapy for Relapsed or Refractory T-Cell Acute Lymphoblastic Leukemia and T-Cell Lymphoblastic Lymphoma. Blood. 2022;140:4566–4567. [Google Scholar]
- 131.Tan Y., Shan L., Zhao L., Deng B., Ling Z., Zhang Y., Peng S., Xu J., Duan J., Wang Z., et al. Long-term follow-up of donor-derived CD7 CAR T-cell therapy in patients with T-cell acute lymphoblastic leukemia. J. Hematol. Oncol. 2023;16:34. doi: 10.1186/s13045-023-01427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang M., Chen D., Fu X., Meng H., Nan F., Sun Z., Yu H., Zhang L., Li L., Li X., et al. Autologous Nanobody-Derived Fratricide-Resistant CD7-CAR T-cell Therapy for Patients with Relapsed and Refractory T-cell Acute Lymphoblastic Leukemia/Lymphoma. Clin. Cancer Res. 2022;28:2830–2843. doi: 10.1158/1078-0432.CCR-21-4097. [DOI] [PubMed] [Google Scholar]
- 133.Lu P., Liu Y., Yang J., Zhang X., Yang X., Wang H., Wang L., Wang Q., Jin D., Li J., Huang X. Naturally selected CD7 CAR-T therapy without genetic manipulations for T-ALL/LBL: first-in-human phase 1 clinical trial. Blood. 2022;140:321–334. doi: 10.1182/blood.2021014498. [DOI] [PubMed] [Google Scholar]
- 134.Li X., Abrahams C., Yu A., Embry M., Henningsen R., DeAlmeida V., Matheny S., Kline T., Yam A., Stafford R., et al. Targeting CD74 in B-cell non-Hodgkin lymphoma with the antibody-drug conjugate STRO-001. Oncotarget. 2023;14:1–13. doi: 10.18632/oncotarget.28341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mamonkin M., Rouce R.H., Tashiro H., Brenner M.K. A T-cell-directed chimeric antigen receptor for the selective treatment of T-cell malignancies. Blood. 2015;126:983–992. doi: 10.1182/blood-2015-02-629527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wada M., Zhang H., Fang L., Feng J., Tse C.O., Zhang W., Chen Q., Sha S., Cao Y., Chen K.H., et al. Characterization of an Anti-CD5 Directed CAR T-Cell against T-Cell Malignancies. Stem Cell Rev. Rep. 2020;16:369–384. doi: 10.1007/s12015-019-09937-9. [DOI] [PubMed] [Google Scholar]
- 137.Feng J., Xu H., Cinquina A., Wu Z., Chen Q., Zhang P., Wang X., Shan H., Xu L., Zhang Q., et al. Treatment of Aggressive T Cell Lymphoblastic Lymphoma/leukemia Using Anti-CD5 CAR T Cells. Stem Cell Rev. Rep. 2021;17:652–661. doi: 10.1007/s12015-020-10092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hill L.C., Rouce R.H., Wu M.J., Wang T., Ma R., Zhang H., Mehta B., Lapteva N., Mei Z., Smith T.S., et al. Antitumor efficacy and safety of unedited autologous CD5.CAR T cells in relapsed/refractory mature T-cell lymphomas. Blood. 2024;143:1231–1241. doi: 10.1182/blood.2023022204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pan J., Tan Y., Shan L., Deng B., Ling Z., Song W., Feng X., Hu G. Phase I study of donor-derived CD5 CAR T cells in patients with relapsed or refractory T-cell acute lymphoblastic leukemia. J. Clin. Oncol. 2022;40:7028. [Google Scholar]
- 140.Pinz K., Liu H., Golightly M., Jares A., Lan F., Zieve G.W., Hagag N., Schuster M., Firor A.E., Jiang X., Ma Y. Preclinical targeting of human T-cell malignancies using CD4-specific chimeric antigen receptor (CAR)-engineered T cells. Leukemia. 2016;30:701–707. doi: 10.1038/leu.2015.311. [DOI] [PubMed] [Google Scholar]
- 141.Anglaret X., Minga A., Gabillard D., Ouassa T., Messou E., Morris B., Traore M., Coulibaly A., Freedberg K.A., Lewden C., et al. AIDS and non-AIDS morbidity and mortality across the spectrum of CD4 cell counts in HIV-infected adults before starting antiretroviral therapy in Cote d'Ivoire. Clin. Infect. Dis. 2012;54:714–723. doi: 10.1093/cid/cir898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Feng J., Xu H., Cinquina A., Wu Z., Zhang W., Sun L., Chen Q., Tian L., Song L., Pinz K.G., et al. Treatment of aggressive T-cell lymphoma/leukemia with anti-CD4 CAR T cells. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.997482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maciocia P.M., Wawrzyniecka P.A., Philip B., Ricciardelli I., Akarca A.U., Onuoha S.C., Legut M., Cole D.K., Sewell A.K., Gritti G., et al. Targeting the T cell receptor β-chain constant region for immunotherapy of T cell malignancies. Nat. Med. 2017;23:1416–1423. doi: 10.1038/nm.4444. [DOI] [PubMed] [Google Scholar]
- 144.Cwynarski K., Iacoboni G., Tholouili E., Menne T., Irvine D., Balasubramaniam N., Wood L., Stephens C., Shang J., Xue E., et al. FIRST IN HUMAN STUDY OF AUTO4, A TRBC1-TRAGETTING CART T CELL THERAPY IN RELAPSED/REFRACTORY TRBC1-POSITIVE PERIPHERAL T-CELL LYMPHOMA. Hematological Oncol. 2023;41:80–81. [Google Scholar]
- 145.van der Geest K.S.M., Abdulahad W.H., Horst G., Lorencetti P.G., Bijzet J., Arends S., van der Heiden M., Buisman A.M., Kroesen B.J., Brouwer E., Boots A.M.H. Quantifying Distribution of Flow Cytometric TCR-Vβ Usage with Economic Statistics. PLoS One. 2015;10:e0125373. doi: 10.1371/journal.pone.0125373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li F., Zhang H., Wang W., Yang P., Huang Y., Zhang J., Yan Y., Wang Y., Ding X., Liang J., et al. T cell receptor β-chain-targeting chimeric antigen receptor T cells against T cell malignancies. Nat. Commun. 2022;13:4334. doi: 10.1038/s41467-022-32092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ren J., Liao X., Lewis J.M., Chang J., Qu R., Carlson K.R., Foss F., Girardi M. Generation and optimization of off-the-shelf immunotherapeutics targeting TCR-Vβ2+ T cell malignancy. Nat. Commun. 2024;15:519. doi: 10.1038/s41467-024-44786-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Britanova O.V., Lupyr K.R., Staroverov D.B., Shagina I.A., Aleksandrov A.A., Ustyugov Y.Y., Somov D.V., Klimenko A., Shostak N.A., Zvyagin I.V., et al. Targeted depletion of TRBV9+ T cells as immunotherapy in a patient with ankylosing spondylitis. Nat. Med. 2023;29:2731–2736. doi: 10.1038/s41591-023-02613-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sanchez-Martinez D., Baroni M.L., Gutierrez-Aguera F., Roca-Ho H., Blanch-Lombarte O., Gonzalez-Garcia S., Torrebadell M., Junca J., Ramirez-Orellana M., Velasco-Hernandez T., et al. Fratricide-resistant CD1a-specific CAR T cells for the treatment of cortical T-cell acute lymphoblastic leukemia. Blood. 2019;133:2291–2304. doi: 10.1182/blood-2018-10-882944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Roosen J., Oosterlinck W., Meyns B. Routine thymectomy in congenital cardiac surgery changes adaptive immunity without clinical relevance. Interact. Cardiovasc. Thorac. Surg. 2015;20:101–106. doi: 10.1093/icvts/ivu343. [DOI] [PubMed] [Google Scholar]
- 151.Muta H., Podack E.R. CD30: from basic research to cancer therapy. Immunol. Res. 2013;57:151–158. doi: 10.1007/s12026-013-8464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Maciocia P.M., Wawrzyniecka P.A., Maciocia N.C., Burley A., Karpanasamy T., Devereaux S., Hoekx M., O’Connor D., Leon T., Rapoz-D’Silva T., et al. Anti-CCR9 chimeric antigen receptor T cells for T-cell acute lymphoblastic leukemia. Blood. 2022;140:25–37. doi: 10.1182/blood.2021013648. [DOI] [PubMed] [Google Scholar]
- 153.Caracciolo D., Polerà N., Belmonte B., Conforti F., Signorelli S., Gulino A., Staropoli N., Tuccillo F.M., Bonelli P., Juli G., et al. UMG1/CD3ε-bispecific T-cell engager redirects T-cell cytotoxicity against diffuse large B-cell lymphoma. Br. J. Haematol. 2024;204:555–560. doi: 10.1111/bjh.19183. [DOI] [PubMed] [Google Scholar]
- 154.Van Oekelen O., Aleman A., Upadhyaya B., Schnakenberg S., Madduri D., Gavane S., Teruya-Feldstein J., Crary J.F., Fowkes M.E., Stacy C.B., et al. Neurocognitive and hypokinetic movement disorder with features of parkinsonism after BCMA-targeting CAR-T cell therapy. Nat. Med. 2021;27:2099–2103. doi: 10.1038/s41591-021-01564-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schumacher T.N., Scheper W., Kvistborg P. Cancer neoantigens. Annu. Rev. Immunol. 2019;37:173–200. doi: 10.1146/annurev-immunol-042617-053402. [DOI] [PubMed] [Google Scholar]
- 156.Tran E., Ahmadzadeh M., Lu Y.C., Gros A., Turcotte S., Robbins P.F., Gartner J.J., Zheng Z., Li Y.F., Ray S., et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science. 2015;350:1387–1390. doi: 10.1126/science.aad1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tran E., Turcotte S., Gros A., Robbins P.F., Lu Y.C., Dudley M.E., Wunderlich J.R., Somerville R.P., Hogan K., Hinrichs C.S., et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim S.P., Vale N.R., Zacharakis N., Krishna S., Yu Z., Gasmi B., Gartner J.J., Sindiri S., Malekzadeh P., Deniger D.C., et al. Adoptive Cellular Therapy with Autologous Tumor-Infiltrating Lymphocytes and T-cell Receptor-Engineered T Cells Targeting Common p53 Neoantigens in Human Solid Tumors. Cancer Immunol. Res. 2022;10:932–946. doi: 10.1158/2326-6066.CIR-22-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lo W., Parkhurst M., Robbins P.F., Tran E., Lu Y.C., Jia L., Gartner J.J., Pasetto A., Deniger D., Malekzadeh P., et al. Immunologic Recognition of a Shared p53 Mutated Neoantigen in a Patient with Metastatic Colorectal Cancer. Cancer Immunol. Res. 2019;7:534–543. doi: 10.1158/2326-6066.CIR-18-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chandran S.S., Ma J., Klatt M.G., Dündar F., Bandlamudi C., Razavi P., Wen H.Y., Weigelt B., Zumbo P., Fu S.N., et al. Immunogenicity and therapeutic targeting of a public neoantigen derived from mutated PIK3CA. Nat. Med. 2022;28:946–957. doi: 10.1038/s41591-022-01786-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Douglass J., Hsiue E.H.C., Mog B.J., Hwang M.S., DiNapoli S.R., Pearlman A.H., Miller M.S., Wright K.M., Azurmendi P.A., Wang Q., et al. Bispecific antibodies targeting mutant RAS neoantigens. Sci. Immunol. 2021;6 doi: 10.1126/sciimmunol.abd5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yarmarkovich M., Marshall Q.F., Warrington J.M., Premaratne R., Farrel A., Groff D., Li W., di Marco M., Runbeck E., Truong H., et al. Targeting of intracellular oncoproteins with peptide-centric CARs. Nature. 2023;623:820–827. doi: 10.1038/s41586-023-06706-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Foy S.P., Jacoby K., Bota D.A., Hunter T., Pan Z., Stawiski E., Ma Y., Lu W., Peng S., Wang C.L., et al. Non-viral precision T cell receptor replacement for personalized cell therapy. Nature. 2023;615:687–696. doi: 10.1038/s41586-022-05531-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Giannakopoulou E., Lehander M., Virding Culleton S., Yang W., Li Y., Karpanen T., Yoshizato T., Rustad E.H., Nielsen M.M., Bollineni R.C., et al. A T cell receptor targeting a recurrent driver mutation in FLT3 mediates elimination of primary human acute myeloid leukemia in vivo. Nat. Cancer. 2023;4:1474–1490. doi: 10.1038/s43018-023-00642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hobbs G.A., Der C.J., Rossman K.L. RAS isoforms and mutations in cancer at a glance. J. Cell Sci. 2016;129:1287–1292. doi: 10.1242/jcs.182873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Baugh E.H., Ke H., Levine A.J., Bonneau R.A., Chan C.S. Why are there hotspot mutations in the TP53 gene in human cancers? Cell Death Differ. 2018;25:154–160. doi: 10.1038/cdd.2017.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tran E., Robbins P.F., Lu Y.C., Prickett T.D., Gartner J.J., Jia L., Pasetto A., Zheng Z., Ray S., Groh E.M., et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N. Engl. J. Med. 2016;375:2255–2262. doi: 10.1056/NEJMoa1609279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Peace D.J., Chen W., Nelson H., Cheever M.A. T cell recognition of transforming proteins encoded by mutated ras proto-oncogenes. J. Immunol. 1991;146:2059–2065. [PubMed] [Google Scholar]
- 169.Ward A.F., Braun B.S., Shannon K.M. Targeting oncogenic Ras signaling in hematologic malignancies. Blood. 2012;120:3397–3406. doi: 10.1182/blood-2012-05-378596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xu-Monette Z.Y., Medeiros L.J., Li Y., Orlowski R.Z., Andreeff M., Bueso-Ramos C.E., Greiner T.C., McDonnell T.J., Young K.H. Dysfunction of the TP53 tumor suppressor gene in lymphoid malignancies. Blood. 2012;119:3668–3683. doi: 10.1182/blood-2011-11-366062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.van der Lee D.I., Reijmers R.M., Honders M.W., Hagedoorn R.S., de Jong R.C., Kester M.G., van der Steen D.M., de Ru A.H., Kweekel C., Bijen H.M., et al. Mutated nucleophosmin 1 as immunotherapy target in acute myeloid leukemia. J. Clin. Invest. 2019;129:774–785. doi: 10.1172/JCI97482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Comoli P., Basso S., Riva G., Barozzi P., Guido I., Gurrado A., Quartuccio G., Rubert L., Lagreca I., Vallerini D., et al. BCR-ABL-specific T-cell therapy in Ph+ ALL patients on tyrosine-kinase inhibitors. Blood. 2017;129:582–586. doi: 10.1182/blood-2016-07-731091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dao T., Mun S.S., Molvi Z., Korontsvit T., Klatt M.G., Khan A.G., Nyakatura E.K., Pohl M.A., White T.E., Balderes P.J., et al. A TCR mimic monoclonal antibody reactive with the "public" phospho-neoantigen pIRS2/HLA-A∗02:01 complex. JCI Insight. 2022;7 doi: 10.1172/jci.insight.151624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Patskovsky Y., Natarajan A., Patskovska L., Nyovanie S., Joshi B., Morin B., Brittsan C., Huber O., Gordon S., Michelet X., et al. Molecular mechanism of phosphopeptide neoantigen immunogenicity. Nat. Commun. 2023;14:3763. doi: 10.1038/s41467-023-39425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Molvi Z., Klatt M.G., Dao T., Urraca J., Scheinberg D.A., O'Reilly R.J. The landscape of MHC-presented phosphopeptides yields actionable shared tumor antigens for cancer immunotherapy across multiple HLA alleles. J. Immunother. Cancer. 2023;11 doi: 10.1136/jitc-2023-006889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cobbold M., De La Peña H., Norris A., Polefrone J.M., Qian J., English A.M., Cummings K.L., Penny S., Turner J.E., Cottine J., et al. MHC class I-associated phosphopeptides are the targets of memory-like immunity in leukemia. Sci. Transl. Med. 2013;5:203ra125. doi: 10.1126/scitranslmed.3006061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Engelhard V.H., Obeng R.C., Cummings K.L., Petroni G.R., Ambakhutwala A.L., Chianese-Bullock K.A., Smith K.T., Lulu A., Varhegyi N., Smolkin M.E., et al. MHC-restricted phosphopeptide antigens: preclinical validation and first-in-humans clinical trial in participants with high-risk melanoma. J. Immunother. Cancer. 2020;8 doi: 10.1136/jitc-2019-000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zarling A.L., Obeng R.C., Desch A.N., Pinczewski J., Cummings K.L., Deacon D.H., Conaway M., Slingluff C.L., Jr., Engelhard V.H. MHC-restricted phosphopeptides from insulin receptor substrate-2 and CDC25b offer broad-based immunotherapeutic agents for cancer. Cancer Res. 2014;74:6784–6795. doi: 10.1158/0008-5472.CAN-14-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sun M., Liu J., Qi J., Tefsen B., Shi Y., Yan J., Gao G.F. Nα-terminal acetylation for T cell recognition: molecular basis of MHC class I-restricted nα-acetylpeptide presentation. J. Immunol. 2014;192:5509–5519. doi: 10.4049/jimmunol.1400199. [DOI] [PubMed] [Google Scholar]
- 180.Malaker S.A., Penny S.A., Steadman L.G., Myers P.T., Loke J.C., Raghavan M., Bai D.L., Shabanowitz J., Hunt D.F., Cobbold M. Identification of Glycopeptides as Posttranslationally Modified Neoantigens in Leukemia. Cancer Immunol. Res. 2017;5:376–384. doi: 10.1158/2326-6066.CIR-16-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Olivier T., Haslam A., Tuia J., Prasad V. Eligibility for Human Leukocyte Antigen–Based Therapeutics by Race and Ethnicity. JAMA Netw. Open. 2023;6:e2338612. doi: 10.1001/jamanetworkopen.2023.38612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kurosawa N., Midorikawa A., Ida K., Fudaba Y.W., Isobe M. Development of a T-cell receptor mimic antibody targeting a novel Wilms tumor 1-derived peptide and analysis of its specificity. Cancer Sci. 2020;111:3516–3526. doi: 10.1111/cas.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Linette G.P., Stadtmauer E.A., Maus M.V., Rapoport A.P., Levine B.L., Emery L., Litzky L., Bagg A., Carreno B.M., Cimino P.J., et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122:863–871. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Morgan R.A., Chinnasamy N., Abate-Daga D., Gros A., Robbins P.F., Zheng Z., Dudley M.E., Feldman S.A., Yang J.C., Sherry R.M., et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 2013;36:133–151. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lee C.H., Salio M., Napolitani G., Ogg G., Simmons A., Koohy H. Predicting Cross-Reactivity and Antigen Specificity of T Cell Receptors. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.565096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Climente-González H., Porta-Pardo E., Godzik A., Eyras E. The Functional Impact of Alternative Splicing in Cancer. Cell Rep. 2017;20:2215–2226. doi: 10.1016/j.celrep.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 187.Kahles A., Lehmann K.V., Toussaint N.C., Hüser M., Stark S.G., Sachsenberg T., Stegle O., Kohlbacher O., Sander C., Cancer Genome Atlas Research Network. Rätsch G. Comprehensive Analysis of Alternative Splicing Across Tumors from 8,705 Patients. Cancer Cell. 2018;34:211–224.e6. doi: 10.1016/j.ccell.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Smart A.C., Margolis C.A., Pimentel H., He M.X., Miao D., Adeegbe D., Fugmann T., Wong K.-K., Van Allen E.M. Intron retention is a source of neoepitopes in cancer. Nat. Biotechnol. 2018;36:1056–1058. doi: 10.1038/nbt.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chen C., Zhao S., Karnad A., Freeman J.W. The biology and role of CD44 in cancer progression: therapeutic implications. J. Hematol. Oncol. 2018;11:64. doi: 10.1186/s13045-018-0605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tang L., Huang H., Tang Y., Li Q., Wang J., Li D., Zhong Z., Zou P., You Y., Cao Y., et al. CD44v6 chimeric antigen receptor T cell specificity towards AML with FLT3 or DNMT3A mutations. Clin. Transl. Med. 2022;12:e1043. doi: 10.1002/ctm2.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Li G., Mahajan S., Ma S., Jeffery E.D., Zhang X., Bhattacharjee A., Venkatasubramanian M., Weirauch M.T., Miraldi E.R., Grimes H.L., et al. Splicing neoantigen discovery with SNAF reveals shared targets for cancer immunotherapy. Sci. Transl. Med. 2024;16:eade2886. doi: 10.1126/scitranslmed.ade2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Oka M., Xu L., Suzuki T., Yoshikawa T., Sakamoto H., Uemura H., Yoshizawa A.C., Suzuki Y., Nakatsura T., Ishihama Y., et al. Aberrant splicing isoforms detected by full-length transcriptome sequencing as transcripts of potential neoantigens in non-small cell lung cancer. Genome Biol. 2021;22:9. doi: 10.1186/s13059-020-02240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gamonet C., Bole-Richard E., Delherme A., Aubin F., Toussirot E., Garnache-Ottou F., Godet Y., Ysebaert L., Tournilhac O., Caroline D., et al. New CD20 alternative splice variants: molecular identification and differential expression within hematological B cell malignancies. Exp. Hematol. Oncol. 2015;5:7. doi: 10.1186/s40164-016-0036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Vauchy C., Gamonet C., Ferrand C., Daguindau E., Galaine J., Beziaud L., Chauchet A., Henry Dunand C.J., Deschamps M., Rohrlich P.S., et al. CD20 alternative splicing isoform generates immunogenic CD4 helper T epitopes. Int. J. Cancer. 2015;137:116–126. doi: 10.1002/ijc.29366. [DOI] [PubMed] [Google Scholar]
- 195.Chichili G.R., Huang L., Li H., Burke S., He L., Tang Q., Jin L., Gorlatov S., Ciccarone V., Chen F., et al. A CD3xCD123 bispecific DART for redirecting host T cells to myelogenous leukemia: Preclinical activity and safety in nonhuman primates. Sci. Transl. Med. 2015;7:289ra82. doi: 10.1126/scitranslmed.aaa5693. [DOI] [PubMed] [Google Scholar]
- 196.Song R., Guo P., Ren X., Zhou L., Li P., Rahman N.A., Wołczyński S., Li X., Zhang Y., Liu M., et al. A novel polypeptide CAPG-171aa encoded by circCAPG plays a critical role in triple-negative breast cancer. Mol. Cancer. 2023;22:104. doi: 10.1186/s12943-023-01806-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tang X., Deng Z., Ding P., Qiang W., Lu Y., Gao S., Hu Y., Yang Y., Du J., Gu C. A novel protein encoded by circHNRNPU promotes multiple myeloma progression by regulating the bone marrow microenvironment and alternative splicing. J. Exp. Clin. Cancer Res. 2022;41:85. doi: 10.1186/s13046-022-02276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wenwen W., Lili M., Zheng X., Tinggan Y., Jinxia B., Yanjing Z., Xiaofang Z., Yan Z., Yali Z., Yani Z., et al. Tumor-Specific CircRNA-Derived Antigen Peptide Identification for Hepatobiliary Tumors. Engineering. 2023;22:159–170. [Google Scholar]
- 199.Hosen N., Matsunaga Y., Hasegawa K., Matsuno H., Nakamura Y., Makita M., Watanabe K., Yoshida M., Satoh K., Morimoto S., et al. The activated conformation of integrin β7 is a novel multiple myeloma–specific target for CAR T cell therapy. Nat. Med. 2017;23:1436–1443. doi: 10.1038/nm.4431. [DOI] [PubMed] [Google Scholar]
- 200.Bandara V., Foeng J., Gundsambuu B., Norton T.S., Napoli S., McPeake D.J., Tyllis T.S., Rohani-Rad E., Abbott C., Mills S.J., et al. Pre-clinical validation of a pan-cancer CAR-T cell immunotherapy targeting nfP2X7. Nat. Commun. 2023;14:5546. doi: 10.1038/s41467-023-41338-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mandal K., Wicaksono G., Yu C., Adams J.J., Hoopmann M.R., Temple W.C., Izgutdina A., Escobar B.P., Gorelik M., Ihling C.H., et al. Structural surfaceomics reveals an AML-specific conformation of integrin β2 as a CAR T cellular therapy target. Nat. Cancer. 2023;4:1592–1609. doi: 10.1038/s43018-023-00652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rasche L., Duell J., Castro I.C., Dubljevic V., Chatterjee M., Knop S., Hensel F., Rosenwald A., Einsele H., Topp M.S., Brändlein S. GRP78-directed immunotherapy in relapsed or refractory multiple myeloma - results from a phase 1 trial with the monoclonal immunoglobulin M antibody PAT-SM6. Haematologica. 2015;100:377–384. doi: 10.3324/haematol.2014.117945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Barone I., Brusco L., Fuqua S.A.W. Estrogen receptor mutations and changes in downstream gene expression and signaling. Clin. Cancer Res. 2010;16:2702–2708. doi: 10.1158/1078-0432.CCR-09-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ruella M., Barrett D.M., Kenderian S.S., Shestova O., Hofmann T.J., Perazzelli J., Klichinsky M., Aikawa V., Nazimuddin F., Kozlowski M., et al. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J. Clin. Invest. 2016;126:3814–3826. doi: 10.1172/JCI87366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zah E., Nam E., Bhuvan V., Tran U., Ji B.Y., Gosliner S.B., Wang X., Brown C.E., Chen Y.Y. Systematically optimized BCMA/CS1 bispecific CAR-T cells robustly control heterogeneous multiple myeloma. Nat. Commun. 2020;11:2283. doi: 10.1038/s41467-020-16160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hamieh M., Dobrin A., Cabriolu A., van der Stegen S.J.C., Giavridis T., Mansilla-Soto J., Eyquem J., Zhao Z., Whitlock B.M., Miele M.M., et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature. 2019;568:112–116. doi: 10.1038/s41586-019-1054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Fernández de Larrea C., Staehr M., Lopez A.V., Ng K.Y., Chen Y., Godfrey W.D., Purdon T.J., Ponomarev V., Wendel H.-G., Brentjens R.J., Smith E.L. Defining an Optimal Dual-Targeted CAR T-cell Therapy Approach Simultaneously Targeting BCMA and GPRC5D to Prevent BCMA Escape–Driven Relapse in Multiple Myeloma. Blood Cancer Discov. 2020;1:146–154. doi: 10.1158/2643-3230.BCD-20-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sakemura R., Hefazi M., Siegler E.L., Cox M.J., Larson D.P., Hansen M.J., Manriquez Roman C., Schick K.J., Can I., Tapper E.E., et al. Targeting cancer-associated fibroblasts in the bone marrow prevents resistance to CART-cell therapy in multiple myeloma. Blood. 2022;139:3708–3721. doi: 10.1182/blood.2021012811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Alberti G., Arsuffi C., Pievani A., Salerno D., Mantegazza F., Dazzi F., Biondi A., Tettamanti S., Serafini M. Engineering tandem CD33xCD146 CAR CIK (cytokine-induced killer) cells to target the acute myeloid leukemia niche. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1192333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Locke F.L., Miklos D.B., Tees M., Bees T., Li A., Truppel-Hartmann A., Flinn I.W. CRC-403: A Phase 1/2 Study of bbT369, a Dual CD79a and CD20 Targeting CAR T Cell Drug Product with a Gene Edit, in Relapsed and/or Refractory B Cell Non-Hodgkin's Lymphoma (NHL) Blood. 2022;140:12716–12717. [Google Scholar]
- 211.Shi M., Wang J., Huang H., Liu D., Cheng H., Wang X., Chen W., Yan Z., Sang W., Qi K., et al. Bispecific CAR T cell therapy targeting BCMA and CD19 in relapsed/refractory multiple myeloma: a phase I/II trial. Nat. Commun. 2024;15:3371. doi: 10.1038/s41467-024-47801-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Tang Y., Yin H., Zhao X., Jin D., Liang Y., Xiong T., Li L., Tang W., Zhang J., Liu M., et al. High efficacy and safety of CD38 and BCMA bispecific CAR-T in relapsed or refractory multiple myeloma. J. Exp. Clin. Cancer Res. 2022;41:2. doi: 10.1186/s13046-021-02214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Spiegel J.Y., Patel S., Muffly L., Hossain N.M., Oak J., Baird J.H., Frank M.J., Shiraz P., Sahaf B., Craig J., et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat. Med. 2021;27:1419–1431. doi: 10.1038/s41591-021-01436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zah E., Lin M.Y., Silva-Benedict A., Jensen M.C., Chen Y.Y. T Cells Expressing CD19/CD20 Bispecific Chimeric Antigen Receptors Prevent Antigen Escape by Malignant B Cells. Cancer Immunol. Res. 2016;4:498–508. doi: 10.1158/2326-6066.CIR-15-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Xie B., Li Z., Zhou J., Wang W. Current Status and Perspectives of Dual-Targeting Chimeric Antigen Receptor T-Cell Therapy for the Treatment of Hematological Malignancies. Cancers (Basel) 2022;14 doi: 10.3390/cancers14133230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Fedorov V.D., Themeli M., Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci. Transl. Med. 2013;5:215ra172. doi: 10.1126/scitranslmed.3006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tao L., Farooq M.A., Gao Y., Zhang L., Niu C., Ajmal I., Zhou Y., He C., Zhao G., Yao J., et al. CD19-CAR-T Cells Bearing a KIR/PD-1-Based Inhibitory CAR Eradicate CD19+HLA-C1− Malignant B Cells While Sparing CD19+HLA-C1+ Healthy B Cells. Cancers. 2020;12:2612. doi: 10.3390/cancers12092612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Richards R.M., Zhao F., Freitas K.A., Parker K.R., Xu P., Fan A., Sotillo E., Daugaard M., Oo H.Z., Liu J., et al. NOT-Gated CD93 CAR T Cells Effectively Target AML with Minimized Endothelial Cross-Reactivity. Blood Cancer Discov. 2021;2:648–665. doi: 10.1158/2643-3230.BCD-20-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Funk M.A., Heller G., Waidhofer-Söllner P., Leitner J., Steinberger P. Inhibitory CARs fail to protect from immediate T cell cytotoxicity. Mol. Ther. 2024;32:982–999. doi: 10.1016/j.ymthe.2024.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Partin A.C., Bruno R., Shafaattalab S., Vander Mause E., Winters A., Daris M., Gahrs C., Jette C.A., DiAndreth B., Sandberg M.L., et al. Geometric parameters that affect the behavior of logic-gated CAR T cells. Front. Immunol. 2024;15 doi: 10.3389/fimmu.2024.1304765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Bangayan N.J., Wang L., Burton Sojo G., Noguchi M., Cheng D., Ta L., Gunn D., Mao Z., Liu S., Yin Q., et al. Dual-inhibitory domain iCARs improve the efficiency of the AND-NOT gate CAR T strategy. Proc. Natl. Acad. Sci. USA. 2023;120 doi: 10.1073/pnas.2312374120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Punekar S.R., Welling T., Randolph Hecht J., Molina J., Smith C., Garon E., Pia Morelli M., Fakih M., Kirtane K., Grierson P.M., et al. 634 EVEREST-1: A seamless phase 1/2 study of CEA logic-gated Tmod CAR T-cell therapy (A2B530) in patients with solid tumors associated with CEA expression also exhibiting HLA loss of heterozygosity (LOH) J. ImmunoTherapy Cancer. 2023;11:A724–A725. [Google Scholar]
- 223.Sandberg M.L., Wang X., Martin A.D., Nampe D.P., Gabrelow G.B., Li C.Z., McElvain M.E., Lee W.H., Shafaattalab S., Martire S., et al. A carcinoembryonic antigen-specific cell therapy selectively targets tumor cells with HLA loss of heterozygosity in vitro and in vivo. Sci. Transl. Med. 2022;14 doi: 10.1126/scitranslmed.abm0306. [DOI] [PubMed] [Google Scholar]
- 224.Arnold P.Y. Review: HLA loss and detection in the setting of relapse from HLA-mismatched hematopoietic cell transplant. Hum. Immunol. 2022;83:712–720. doi: 10.1016/j.humimm.2022.03.001. [DOI] [PubMed] [Google Scholar]
- 225.Walker I.G., Roy J., Anderson G., Lopez J.G., Chapman M.A. Targeting Myeloma Essential Genes using NOT Gated CAR- T Cells, a computational approach. Leukemia. 2024;38:1848–1852. doi: 10.1038/s41375-024-02247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kloss C.C., Condomines M., Cartellieri M., Bachmann M., Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat. Biotechnol. 2013;31:71–75. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Halliwell E., Vitali A., Muller H., Alonso-Ferrero M., Barisa M., Gavriil A., Piapi A., Leboreiro-Babe C., Gileadi T., Yeung J., et al. Targeting of low ALK antigen density neuroblastoma using AND logic-gate engineered CAR-T cells. Cytotherapy. 2023;25:46–58. doi: 10.1016/j.jcyt.2022.10.007. [DOI] [PubMed] [Google Scholar]
- 228.Anderson G.S.F., Walker I.G., Roy J.P., Chapman M.A. Cell surface proteomics reveal a strategy for targeting myeloma through combinatorial targeting of TNFRSF8 and TMPRSS11E. bioRxiv. 2023 doi: 10.1101/2023.04.04.535580. Preprint at. [DOI] [Google Scholar]
- 229.Perriello V.M., Rotiroti M.C., Pisani I., Galimberti S., Alberti G., Pianigiani G., Ciaurro V., Marra A., Sabino M., Tini V., et al. IL-3-zetakine combined with a CD33 costimulatory receptor as a dual CAR approach for safer and selective targeting of AML. Blood Adv. 2023;7:2855–2871. doi: 10.1182/bloodadvances.2022008762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sukumaran S., Watanabe N., Bajgain P., Raja K., Mohammed S., Fisher W.E., Brenner M.K., Leen A.M., Vera J.F. Enhancing the Potency and Specificity of Engineered T Cells for Cancer Treatment. Cancer Discov. 2018;8:972–987. doi: 10.1158/2159-8290.CD-17-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Tousley A.M., Rotiroti M.C., Labanieh L., Rysavy L.W., Kim W.J., Lareau C., Sotillo E., Weber E.W., Rietberg S.P., Dalton G.N., et al. Co-opting signalling molecules enables logic-gated control of CAR T cells. Nature. 2023;615:507–516. doi: 10.1038/s41586-023-05778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Katsarou A., Sjöstrand M., Naik J., Mansilla-Soto J., Kefala D., Kladis G., Nianias A., Ruiter R., Poels R., Sarkar I., et al. Combining a CAR and a chimeric costimulatory receptor enhances T cell sensitivity to low antigen density and promotes persistence. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abh1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Haubner S., Mansilla-Soto J., Nataraj S., Kogel F., Chang Q., de Stanchina E., Lopez M., Ng M.R., Fraser K., Subklewe M., et al. Cooperative CAR targeting to selectively eliminate AML and minimize escape. Cancer Cell. 2023;41:1871–1891.e6. doi: 10.1016/j.ccell.2023.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Roybal K.T., Williams J.Z., Morsut L., Rupp L.J., Kolinko I., Choe J.H., Walker W.J., McNally K.A., Lim W.A. Engineering T Cells with Customized Therapeutic Response Programs Using Synthetic Notch Receptors. Cell. 2016;167:419–432.e16. doi: 10.1016/j.cell.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Choe J.H., Watchmaker P.B., Simic M.S., Gilbert R.D., Li A.W., Krasnow N.A., Downey K.M., Yu W., Carrera D.A., Celli A., et al. SynNotch-CAR T cells overcome challenges of specificity, heterogeneity, and persistence in treating glioblastoma. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abe7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Hyrenius-Wittsten A., Su Y., Park M., Garcia J.M., Alavi J., Perry N., Montgomery G., Liu B., Roybal K.T. SynNotch CAR circuits enhance solid tumor recognition and promote persistent antitumor activity in mouse models. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abd8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Williams J.Z., Allen G.M., Shah D., Sterin I.S., Kim K.H., Garcia V.P., Shavey G.E., Yu W., Puig-Saus C., Tsoi J., et al. Precise T cell recognition programs designed by transcriptionally linking multiple receptors. Science. 2020;370:1099–1104. doi: 10.1126/science.abc6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Hernandez-Lopez R.A., Yu W., Cabral K.A., Creasey O.A., Lopez Pazmino M.d.P., Tonai Y., De Guzman A., Mäkelä A., Saksela K., Gartner Z.J., Lim W.A. T cell circuits that sense antigen density with an ultrasensitive threshold. Science. 2021;371:1166–1171. doi: 10.1126/science.abc1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Juillerat A., Marechal A., Filhol J.M., Valogne Y., Valton J., Duclert A., Duchateau P., Poirot L. An oxygen sensitive self-decision making engineered CAR T-cell. Sci. Rep. 2017;7 doi: 10.1038/srep39833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhang W., He Q., Lopez B., Hu J., Kundu A., Andraza M.C., Kerner A.R., Schreiber G.H., Shepard H.M., Frost G.I. Abstract PO074: Logic-gating HER2 CAR-T to the tumor microenvironment mitigates on-target, off-tumor toxicity without compromising cytotoxicity against HER2-over-expressing tumors. Cancer Immunol. Res. 2021;9 [Google Scholar]
- 241.Han X., Bryson P.D., Zhao Y., Cinay G.E., Li S., Guo Y., Siriwon N., Wang P. Masked Chimeric Antigen Receptor for Tumor-Specific Activation. Mol. Ther. 2017;25:274–284. doi: 10.1016/j.ymthe.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Paszkiewicz P.J., Fräßle S.P., Srivastava S., Sommermeyer D., Hudecek M., Drexler I., Sadelain M., Liu L., Jensen M.C., Riddell S.R., Busch D.H. Targeted antibody-mediated depletion of murine CD19 CAR T cells permanently reverses B cell aplasia. J. Clin. Invest. 2016;126:4262–4272. doi: 10.1172/JCI84813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Introna M., Barbui A.M., Bambacioni F., Casati C., Gaipa G., Borleri G., Bernasconi S., Barbui T., Golay J., Biondi A., Rambaldi A. Genetic modification of human T cells with CD20: a strategy to purify and lyse transduced cells with anti-CD20 antibodies. Hum. Gene Ther. 2000;11:611–620. doi: 10.1089/10430340050015798. [DOI] [PubMed] [Google Scholar]
- 244.Kouhi A., Pachipulusu V., Kapenstein T., Hu P., Epstein A.L., Khawli L.A. Brain Disposition of Antibody-Based Therapeutics: Dogma, Approaches and Perspectives. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22126442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Di Stasi A., Tey S.K., Dotti G., Fujita Y., Kennedy-Nasser A., Martinez C., Straathof K., Liu E., Durett A.G., Grilley B., et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Foster M.C., Savoldo B., Lau W., Rubinos C., Grover N., Armistead P., Coghill J., Hagan R.S., Morrison K., Buchanan F.B., et al. Utility of a safety switch to abrogate CD19.CAR T-cell-associated neurotoxicity. Blood. 2021;137:3306–3309. doi: 10.1182/blood.2021010784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Jan M., Scarfo I., Larson R.C., Walker A., Schmidts A., Guirguis A.A., Gasser J.A., Slabicki M., Bouffard A.A., Castano A.P., et al. Reversible ON- and OFF-switch chimeric antigen receptors controlled by lenalidomide. Sci. Transl Med. 2021;13 doi: 10.1126/scitranslmed.abb6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Li H.S., Wong N.M., Tague E., Ngo J.T., Khalil A.S., Wong W.W. High-performance multiplex drug-gated CAR circuits. Cancer Cell. 2022;40:1294–1305.e4. doi: 10.1016/j.ccell.2022.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zajc C.U., Dobersberger M., Schaffner I., Mlynek G., Pühringer D., Salzer B., Djinović-Carugo K., Steinberger P., De Sousa Linhares A., Yang N.J., et al. A conformation-specific ON-switch for controlling CAR T cells with an orally available drug. Proc. Natl. Acad. Sci. USA. 2020;117:14926–14935. doi: 10.1073/pnas.1911154117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Leung W.H., Gay J., Martin U., Garrett T.E., Horton H.M., Certo M.T., Blazar B.R., Morgan R.A., Gregory P.D., Jarjour J., Astrakhan A. Sensitive and adaptable pharmacological control of CAR T cells through extracellular receptor dimerization. JCI Insight. 2019;5 doi: 10.1172/jci.insight.124430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Li H.S., Israni D.V., Gagnon K.A., Gan K.A., Raymond M.H., Sander J.D., Roybal K.T., Joung J.K., Wong W.W., Khalil A.S. Multidimensional control of therapeutic human cell function with synthetic gene circuits. Science. 2022;378:1227–1234. doi: 10.1126/science.ade0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Tamada K., Geng D., Sakoda Y., Bansal N., Srivastava R., Li Z., Davila E. Redirecting Gene-Modified T Cells toward Various Cancer Types Using Tagged Antibodies. Clin. Cancer Res. 2012;18:6436–6445. doi: 10.1158/1078-0432.CCR-12-1449. [DOI] [PubMed] [Google Scholar]
- 253.Rodgers D.T., Mazagova M., Hampton E.N., Cao Y., Ramadoss N.S., Hardy I.R., Schulman A., Du J., Wang F., Singer O., et al. Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proc. Natl. Acad. Sci. USA. 2016;113:E459–E468. doi: 10.1073/pnas.1524155113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Nixdorf D., Sponheimer M., Berghammer D., Engert F., Bader U., Philipp N., Kazerani M., Straub T., Rohrbacher L., Wange L., et al. Adapter CAR T cells to counteract T-cell exhaustion and enable flexible targeting in AML. Leukemia. 2023;37:1298–1310. doi: 10.1038/s41375-023-01905-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Nikolaenko L., Mulroney C., Riedell P.A., Flinn I.W., Cruz J.C., Vaidya R., van Besien K., Emde M., Klyushnichenko V., Strauss J., et al. First in Human Study of an on/Off Switchable CAR-T Cell Platform Targeting CD19 for B Cell Malignancies (CLBR001 + SWI019) Blood. 2021;138:2822. [Google Scholar]
- 256.Wermke M., Kraus S., Ehninger A., Bargou R.C., Goebeler M.E., Middeke J.M., Kreissig C., von Bonin M., Koedam J., Pehl M., et al. Proof of concept for a rapidly switchable universal CAR-T platform with UniCAR-T-CD123 in relapsed/refractory AML. Blood. 2021;137:3145–3148. doi: 10.1182/blood.2020009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Loff S., Dietrich J., Meyer J.E., Riewaldt J., Spehr J., von Bonin M., Gründer C., Swayampakula M., Franke K., Feldmann A., et al. Rapidly Switchable Universal CAR-T Cells for Treatment of CD123-Positive Leukemia. Mol. Ther. Oncolytics. 2020;17:408–420. doi: 10.1016/j.omto.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Martin W., Stephan M., Sabrina K., Elisa S., Vladan V., Walter F., Katrin W., Jonas S., Maria-Elisabeth G., Jan K., et al. Updated Results from a Phase I Dose Escalation Study of the Rapidly-Switchable Universal CAR-T Therapy UniCAR-T-CD123 in Relapsed/Refractory AML. Blood. 2023;142:3465. [Google Scholar]
- 259.Feldmann A., Hoffmann A., Bergmann R., Koristka S., Berndt N., Arndt C., Rodrigues Loureiro L., Kittel-Boselli E., Mitwasi N., Kegler A., et al. Versatile chimeric antigen receptor platform for controllable and combinatorial T cell therapy. Oncoimmunology. 2020;9 doi: 10.1080/2162402X.2020.1785608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Saleh H.A., Mitwasi N., Ullrich M., Kubeil M., Toussaint M., Deuther-Conrad W., Neuber C., Arndt C., R Loureiro L., Kegler A., et al. Specific and safe targeting of glioblastoma using switchable and logic-gated RevCAR T cells. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1166169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Soto K.E.G., Loureiro L.R., Bartsch T., Arndt C., Kegler A., Mitwasi N., Drewitz L., Hoffmann L., Saleh H.A., Crespo E., et al. Targeting colorectal cancer cells using AND-gated adaptor RevCAR T-cells. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1302354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kittel-Boselli E., Soto K.E.G., Loureiro L.R., Hoffmann A., Bergmann R., Arndt C., Koristka S., Mitwasi N., Kegler A., Bartsch T., et al. Targeting Acute Myeloid Leukemia Using the RevCAR Platform: A Programmable, Switchable and Combinatorial Strategy. Cancers (Basel) 2021;13 doi: 10.3390/cancers13194785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Cho J.H., Collins J.J., Wong W.W. Universal Chimeric Antigen Receptors for Multiplexed and Logical Control of T Cell Responses. Cell. 2018;173:1426–1438.e11. doi: 10.1016/j.cell.2018.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Lajoie M.J., Boyken S.E., Salter A.I., Bruffey J., Rajan A., Langan R.A., Olshefsky A., Muhunthan V., Bick M.J., Gewe M., et al. Designed protein logic to target cells with precise combinations of surface antigens. Science. 2020;369:1637–1643. doi: 10.1126/science.aba6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Cooper M.L., Choi J., Staser K., Ritchey J.K., Devenport J.M., Eckardt K., Rettig M.P., Wang B., Eissenberg L.G., Ghobadi A., et al. An "off-the-shelf" fratricide-resistant CAR-T for the treatment of T cell hematologic malignancies. Leukemia. 2018;32:1970–1983. doi: 10.1038/s41375-018-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Humbert O., Laszlo G.S., Sichel S., Ironside C., Haworth K.G., Bates O.M., Beddoe M.E., Carrillo R.R., Kiem H.P., Walter R.B. Engineering resistance to CD33-targeted immunotherapy in normal hematopoiesis by CRISPR/Cas9-deletion of CD33 exon 2. Leukemia. 2019;33:762–808. doi: 10.1038/s41375-018-0277-8. [DOI] [PubMed] [Google Scholar]
- 267.Kim M.Y., Yu K.R., Kenderian S.S., Ruella M., Chen S., Shin T.H., Aljanahi A.A., Schreeder D., Klichinsky M., Shestova O., et al. Genetic Inactivation of CD33 in Hematopoietic Stem Cells to Enable CAR T Cell Immunotherapy for Acute Myeloid Leukemia. Cell. 2018;173:1439–1453.e19. doi: 10.1016/j.cell.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Lydeard J.R., Lin M.I., Ge H.G., Halfond A., Wang S., Jones M.B., Etchin J., Angelini G., Xavier-Ferrucio J., Lisle J., et al. Development of a gene edited next-generation hematopoietic cell transplant to enable acute myeloid leukemia treatment by solving off-tumor toxicity. Mol. Ther. Methods Clin. Dev. 2023;31 doi: 10.1016/j.omtm.2023.101135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Tao R., Han X., Bai X., Yu J., Ma Y., Chen W., Zhang D., Li Z. Revolutionizing cancer treatment: enhancing CAR-T cell therapy with CRISPR/Cas9 gene editing technology. Front. Immunol. 2024;15 doi: 10.3389/fimmu.2024.1354825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Fu Y., Foden J.A., Khayter C., Maeder M.L., Reyon D., Joung J.K., Sander J.D. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol. 2013;31:822–826. doi: 10.1038/nbt.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Tsuchida C.A., Brandes N., Bueno R., Trinidad M., Mazumder T., Yu B., Hwang B., Chang C., Liu J., Sun Y., et al. Mitigation of chromosome loss in clinical CRISPR-Cas9-engineered T cells. Cell. 2023;186:4567–4582.e20. doi: 10.1016/j.cell.2023.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Webber B.R., Lonetree C.L., Kluesner M.G., Johnson M.J., Pomeroy E.J., Diers M.D., Lahr W.S., Draper G.M., Slipek N.J., Smeester B.A., et al. Highly efficient multiplex human T cell engineering without double-strand breaks using Cas9 base editors. Nat. Commun. 2019;10:5222. doi: 10.1038/s41467-019-13007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Casirati G., Cosentino A., Mucci A., Salah Mahmoud M., Ugarte Zabala I., Zeng J., Ficarro S.B., Klatt D., Brendel C., Rambaldi A., et al. Epitope editing enables targeted immunotherapy of acute myeloid leukaemia. Nature. 2023;621:404–414. doi: 10.1038/s41586-023-06496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Marone R., Landmann E., Devaux A., Lepore R., Seyres D., Zuin J., Burgold T., Engdahl C., Capoferri G., Dell'Aglio A., et al. Epitope-engineered human hematopoietic stem cells are shielded from CD123-targeted immunotherapy. J. Exp. Med. 2023;220 doi: 10.1084/jem.20231235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Wellhausen N., O'Connell R.P., Lesch S., Engel N.W., Rennels A.K., Gonzales D., Herbst F., Young R.M., Garcia K.C., Weiner D., et al. Epitope base editing CD45 in hematopoietic cells enables universal blood cancer immune therapy. Sci. Transl. Med. 2023;15 doi: 10.1126/scitranslmed.adi1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Park A.K., Fong Y., Kim S.I., Yang J., Murad J.P., Lu J., Jeang B., Chang W.C., Chen N.G., Thomas S.H., et al. Effective combination immunotherapy using oncolytic viruses to deliver CAR targets to solid tumors. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.aaz1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Aalipour A., Le Boeuf F., Tang M., Murty S., Simonetta F., Lozano A.X., Shaffer T.M., Bell J.C., Gambhir S.S. Viral Delivery of CAR Targets to Solid Tumors Enables Effective Cell Therapy. Mol. Ther. Oncolytics. 2020;17:232–240. doi: 10.1016/j.omto.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Tang X.Y., Ding Y.S., Zhou T., Wang X., Yang Y. Tumor-tagging by oncolytic viruses: A novel strategy for CAR-T therapy against solid tumors. Cancer Lett. 2021;503:69–74. doi: 10.1016/j.canlet.2021.01.014. [DOI] [PubMed] [Google Scholar]
- 279.Parol W., Anderson G.S.F., Chapman M.A. CAR-T cell therapies for cancer: what novel technologies are being developed for toxicity data? Expert Opin. Drug Metab. Toxicol. 2022;18:241–244. doi: 10.1080/17425255.2022.2085551. [DOI] [PubMed] [Google Scholar]
- 280.Chou C.H., Ho C.M., Lai S.L., Chen C.N., Wu Y.M., Shun C.T., Wen W.F., Lai H.S. B-Cell Activating Factor Enhances Hepatocyte-Driven Angiogenesis via B-Cell CLL/Lymphoma 10/Nuclear Factor-KappaB Signaling during Liver Regeneration. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20205022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Wong D.P., Roy N.K., Zhang K., Anukanth A., Asthana A., Shirkey-Son N.J., Dunmire S., Jones B.J., Lahr W.S., Webber B.R., et al. A BAFF ligand-based CAR-T cell targeting three receptors and multiple B cell cancers. Nat. Commun. 2022;13:217. doi: 10.1038/s41467-021-27853-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Kerry A.R., Ian W.F., Carolyn M., Liangke Connie G., Nadia H., Janghee W., John C.B. Phase Ib Study of Ianalumab (VAY736) and Ibrutinib in Patients with Chronic Lymphocytic Leukemia (CLL) on Ibrutinib Therapy. Blood. 2020;136:13–14. [Google Scholar]
- 283.Luger D., Yang Y.A., Raviv A., Weinberg D., Banerjee S., Lee M.J., Trepel J., Yang L., Wakefield L.M. Expression of the B-cell receptor component CD79a on immature myeloid cells contributes to their tumor promoting effects. PLoS One. 2013;8 doi: 10.1371/journal.pone.0076115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Sattva S.N., John M.R., Caron A.J., Frederick L.L., David B.M., Patrick M.R., Scott J.R., Lazaros J.L., Ian W.F., Lianqing Z., et al. CD19-Loss with Preservation of Other B Cell Lineage Features in Patients with Large B Cell Lymphoma Who Relapsed Post-Axi-Cel. Blood. 2019;134:203. [Google Scholar]
- 285.Deckert J., Park P.U., Chicklas S., Yi Y., Li M., Lai K.C., Mayo M.F., Carrigan C.N., Erickson H.K., Pinkas J., et al. A novel anti-CD37 antibody-drug conjugate with multiple anti-tumor mechanisms for the treatment of B-cell malignancies. Blood. 2013;122:3500–3510. doi: 10.1182/blood-2013-05-505685. [DOI] [PubMed] [Google Scholar]
- 286.van der Horst H.J., Oostindie S.C., Cillessen S.A.G.M., Gelderloos A.T., Overdijk M.B., Nijhof I.S., Zweegman S., Chamuleau M.E.D., Breij E.C.W., Mutis T. Potent Preclinical Efficacy of DuoHexaBody-CD37 in B-Cell Malignancies. Hemasphere. 2021;5:e504. doi: 10.1097/HS9.0000000000000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Barrena S., Almeida J., Yunta M., López A., Fernández-Mosteirín N., Giralt M., Romero M., Perdiguer L., Delgado M., Orfao A., Lazo P.A. Aberrant expression of tetraspanin molecules in B-cell chronic lymphoproliferative disorders and its correlation with normal B-cell maturation. Leukemia. 2005;19:1376–1383. doi: 10.1038/sj.leu.2403822. [DOI] [PubMed] [Google Scholar]
- 288.Köksal H., Dillard P., Josefsson S.E., Maggadottir S.M., Pollmann S., Fåne A., Blaker Y.N., Beiske K., Huse K., Kolstad A., et al. Preclinical development of CD37CAR T-cell therapy for treatment of B-cell lymphoma. Blood Adv. 2019;3:1230–1243. doi: 10.1182/bloodadvances.2018029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Buldini B., Faggin G., Porcù E., Scarparo P., Polato K., Tregnago C., Varotto E., Rizzardi P., Rizzari C., Locatelli F., et al. CD72 is a pan-tumor antigen associated to pediatric acute leukemia. Cytometry. A. 2023;103:1004–1009. doi: 10.1002/cyto.a.24790. [DOI] [PubMed] [Google Scholar]
- 290.Temple W.C., Nix M.A., Naik A., Izgutdina A., Huang B.J., Wicaksono G., Phojanakong P., Serrano J.A.C., Young E.P., Ramos E., et al. Framework humanization optimizes potency of anti-CD72 nanobody CAR-T cells for B-cell malignancies. J. Immunother. Cancer. 2023;11 doi: 10.1136/jitc-2023-006985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Shabani M., Asgarian-Omran H., Vossough P., Sharifian R.A., Faranoush M., Ghragozlou S., Khoshnoodi J., Roohi A., Jeddi-Tehrani M., Mellstedt H., et al. Expression profile of orphan receptor tyrosine kinase (ROR1) and Wilms' tumor gene 1 (WT1) in different subsets of B-cell acute lymphoblastic leukemia. Leuk. Lymphoma. 2008;49:1360–1367. doi: 10.1080/10428190802124000. [DOI] [PubMed] [Google Scholar]
- 292.Jiang V.C., Liu Y., Jordan A., McIntosh J., Li Y., Che Y., Jessen K.A., Lannutti B.J., Wang M. The antibody drug conjugate VLS-101 targeting ROR1 is effective in CAR T-resistant mantle cell lymphoma. J. Hematol. Oncol. 2021;14:132. doi: 10.1186/s13045-021-01143-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Balakrishnan A., Goodpaster T., Randolph-Habecker J., Hoffstrom B.G., Jalikis F.G., Koch L.K., Berger C., Kosasih P.L., Rajan A., Sommermeyer D., et al. Analysis of ROR1 Protein Expression in Human Cancer and Normal Tissues. Clin. Cancer Res. 2017;23:3061–3071. doi: 10.1158/1078-0432.CCR-16-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Labanieh L., Majzner R.G., Klysz D., Sotillo E., Fisher C.J., Vilches-Moure J.G., Pacheco K.Z.B., Malipatlolla M., Xu P., Hui J.H., et al. Enhanced safety and efficacy of protease-regulated CAR-T cell receptors. Cell. 2022;185:1745–1763.e22. doi: 10.1016/j.cell.2022.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Wang M.L., Barrientos J.C., Furman R.R., Mei M., Barr P.M., Choi M.Y., de Vos S., Kallam A., Patel K., Kipps T.J., et al. Zilovertamab Vedotin Targeting of ROR1 as Therapy for Lymphoid Cancers. NEJM Evid. 2022;1 doi: 10.1056/EVIDoa2100001. [DOI] [PubMed] [Google Scholar]
- 296.Specht J., Lee S., Turtle C., Berger C., Balakrishnan A., Srivastava S., Viollet V., Veatch J., Gooley T., Mullane E., et al. Abstract P2-09-13: A phase I study of adoptive immunotherapy for ROR1+ advanced triple negative breast cancer (TNBC) with defined subsets of autologous T cells expressing a ROR1-specific chimeric antigen receptor (ROR1-CAR) Cancer Res. 2019;79 [Google Scholar]
- 297.Srivastava S., Furlan S.N., Jaeger-Ruckstuhl C.A., Sarvothama M., Berger C., Smythe K.S., Garrison S.M., Specht J.M., Lee S.M., Amezquita R.A., et al. Immunogenic Chemotherapy Enhances Recruitment of CAR-T Cells to Lung Tumors and Improves Antitumor Efficacy when Combined with Checkpoint Blockade. Cancer Cell. 2021;39:193–208.e10. doi: 10.1016/j.ccell.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Townsend W., Leong S., Tucker D., Pottinger B., Paneesha S., El-Sharkawi D., Eyre T.A., Batten T., Shah M., Cook S., et al. First-in-Human Phase I Trial of a ROR1 Targeting Bispecific T Cell Engager (NVG-111) in Combination with Ibrutinib or As Monotherapy in Subjects with Relapsed Refractory Chronic Lymphocytic Leukaemia (CLL) and Mantle Cell Lymphoma (MCL) Blood. 2022;140:4162–4163. [Google Scholar]
- 299.Oncternal Therapeutics Oncternal Therapeutics updates the status of its phase 1/2 study of ONCT-808, a ror1-targeting autologous CAR T, in Patients with Relapsed or Refractory Aggressive B-Cell Lymphoma. 2023. https://investor.oncternal.com/news-releases/news-release-details/oncternal-therapeutics-updates-status-its-phase-12-study-onct
- 300.Wang M.L., Frigault M.J., Yazji S., Katz Y., Robinson J., Breitmeyer J.B., Mei M.G., Jacobson C.A. Trial-in-Progress: A Phase 1/2 Multi-Center Study of Onct-808, a ROR1-Specific CAR T, in Adult Patients with Relapsed/Refractory Aggressive B Cell Lymphoma. Blood. 2023;142:4857. [Google Scholar]
- 301.Jain N., Roberts K.G., Jabbour E., Patel K., Eterovic A.K., Chen K., Zweidler-McKay P., Lu X., Fawcett G., Wang S.A., et al. Ph-like acute lymphoblastic leukemia: a high-risk subtype in adults. Blood. 2017;129:572–581. doi: 10.1182/blood-2016-07-726588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Russell L.J., Capasso M., Vater I., Akasaka T., Bernard O.A., Calasanz M.J., Chandrasekaran T., Chapiro E., Gesk S., Griffiths M., et al. Deregulated expression of cytokine receptor gene, CRLF2, is involved in lymphoid transformation in B-cell precursor acute lymphoblastic leukemia. Blood. 2009;114:2688–2698. doi: 10.1182/blood-2009-03-208397. [DOI] [PubMed] [Google Scholar]
- 303.Vera J., Savoldo B., Vigouroux S., Biagi E., Pule M., Rossig C., Wu J., Heslop H.E., Rooney C.M., Brenner M.K., Dotti G. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006;108:3890–3897. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Xochelli A., Baliakas P., Kavakiotis I., Agathangelidis A., Sutton L.-A., Minga E., Ntoufa S., Tausch E., Yan X.-J., Shanafelt T., et al. Chronic Lymphocytic Leukemia with Mutated IGHV4-34 Receptors: Shared and Distinct Immunogenetic Features and Clinical Outcomes. Clin. Cancer Res. 2017;23:5292–5301. doi: 10.1158/1078-0432.CCR-16-3100. [DOI] [PubMed] [Google Scholar]
- 305.Pugh-Bernard A.E., Silverman G.J., Cappione A.J., Villano M.E., Ryan D.H., Insel R.A., Sanz I. Regulation of inherently autoreactive VH4-34 B cells in the maintenance of human B cell tolerance. J. Clin. Invest. 2001;108:1061–1070. doi: 10.1172/JCI12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Shaffer D.R., Savoldo B., Yi Z., Chow K.K.H., Kakarla S., Spencer D.M., Dotti G., Wu M.F., Liu H., Kenney S., Gottschalk S. T cells redirected against CD70 for the immunotherapy of CD70-positive malignancies. Blood. 2011;117:4304–4314. doi: 10.1182/blood-2010-04-278218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Deng W., Chen P., Lei W., Xu Y., Xu N., Pu J.J., Liang A., Qian W. CD70-targeting CAR-T cells have potential activity against CD19-negative B-cell Lymphoma. Cancer Commun. 2021;41:925–929. doi: 10.1002/cac2.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Abolhassani H., Edwards E.S.J., Ikinciogullari A., Jing H., Borte S., Buggert M., Du L., Matsuda-Lennikov M., Romano R., Caridha R., et al. Combined immunodeficiency and Epstein-Barr virus-induced B cell malignancy in humans with inherited CD70 deficiency. J. Exp. Med. 2017;214:91–106. doi: 10.1084/jem.20160849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Phillips T., Barr P.M., Park S.I., Kolibaba K., Caimi P.F., Chhabra S., Kingsley E.C., Boyd T., Chen R., Carret A.-S., et al. A phase 1 trial of SGN-CD70A in patients with CD70-positive diffuse large B cell lymphoma and mantle cell lymphoma. Invest. New Drugs. 2019;37:297–306. doi: 10.1007/s10637-018-0655-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Sauer T., Parikh K., Sharma S., Omer B., Sedloev D., Chen Q., Angenendt L., Schliemann C., Schmitt M., Müller-Tidow C., et al. CD70-specific CAR T cells have potent activity against acute myeloid leukemia without HSC toxicity. Blood. 2021;138:318–330. doi: 10.1182/blood.2020008221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Stein R., Mattes M.J., Cardillo T.M., Hansen H.J., Chang C.H., Burton J., Govindan S., Goldenberg D.M. CD74: a new candidate target for the immunotherapy of B-cell neoplasms. Clin. Cancer Res. 2007;13:5556s–5563s. doi: 10.1158/1078-0432.CCR-07-1167. [DOI] [PubMed] [Google Scholar]
- 312.Zhao S., Molina A., Yu A., Hanson J., Cheung H., Li X., Natkunam Y. High frequency of CD74 expression in lymphomas: implications for targeted therapy using a novel anti-CD74-drug conjugate. J. Pathol. Clin. Res. 2019;5:12–24. doi: 10.1002/cjp2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Chan W.K., Williams J., Sorathia K., Pray B., Abusaleh K., Bian Z., Sharma A., Hout I., Nishat S., Hanel W., et al. A novel CAR-T cell product targeting CD74 is an effective therapeutic approach in preclinical mantle cell lymphoma models. Exp. Hematol. Oncol. 2023;12:79. doi: 10.1186/s40164-023-00437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Abrahams C.L., Li X., Embry M., Yu A., Krimm S., Krueger S., Greenland N.Y., Wen K.W., Jones C., DeAlmeida V., et al. Targeting CD74 in multiple myeloma with the novel, site-specific antibody-drug conjugate STRO-001. Oncotarget. 2018;9:37700–37714. doi: 10.18632/oncotarget.26491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Kaufman J.L., Niesvizky R., Stadtmauer E.A., Chanan-Khan A., Siegel D., Horne H., Wegener W.A., Goldenberg D.M. Phase I, multicentre, dose-escalation trial of monotherapy with milatuzumab (humanized anti-CD74 monoclonal antibody) in relapsed or refractory multiple myeloma. Br. J. Haematol. 2013;163:478–486. doi: 10.1111/bjh.12565. [DOI] [PubMed] [Google Scholar]
- 316.ClinicalTrials.gov. (Identifier NCT01585688). Phase I/II Study of hLL1-DOX in Relapsed NHL and CLL. [cited 2024 March 1st]; Available from: https://clinicaltrials.gov/study/NCT01585688.
- 317.Nirav N.S., Ahmad H.M., Leslie L.P., Charalambos A., Jason M.M., Alexander I.S., Jonah S., Sudhir M., John M.B., Saurabh C., et al. Preliminary Results of an Ongoing Phase 1 Dose Escalation Study of the Novel Anti-CD74 Antibody Drug Conjugate (ADC), STRO-001, in Patients with B-Cell Non-Hodgkin Lymphoma. Blood. 2020;136:29–30. [Google Scholar]
- 318.Christopher T.R., Maria-Concetta V., Sergey G., Nadine T., Steve B., Ling H., Inzunza H.D., Hua L., Shannon T., Syd J., et al. CD32B, the human inhibitory Fc-γ receptor IIB, as a target for monoclonal antibody therapy of B-cell lymphoma. Blood. 2006;108:2384–2391. doi: 10.1182/blood-2006-05-020602. [DOI] [PubMed] [Google Scholar]
- 319.Wang G., Sun X., Zuo S., Li C., Niu Q., Xia Y., Meng Y., Liu M., Fang Z., Yang X., et al. Homogeneously high expression of CD32b makes it a potential target for CAR-T therapy for chronic lymphocytic leukemia. J. Hematol. Oncol. 2021;14:149. doi: 10.1186/s13045-021-01160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Farley C.R., Morris A.B., Tariq M., Bennion K.B., Potdar S., Kudchadkar R., Lowe M.C., Ford M.L. FcγRIIB is a T cell checkpoint in antitumor immunity. JCI Insight. 2021;6 doi: 10.1172/jci.insight.135623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Lim S.H., Vaughan A.T., Ashton-Key M., Williams E.L., Dixon S.V., Chan H.T.C., Beers S.A., French R.R., Cox K.L., Davies A.J., et al. Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood. 2011;118:2530–2540. doi: 10.1182/blood-2011-01-330357. [DOI] [PubMed] [Google Scholar]
- 322.Mohan M., Nagavally S., Dhakal B., Radhakrishnan S.V., Chhabra S., D’Souza A., Hari P. Risk of infections with B-cell maturation antigen-directed immunotherapy in multiple myeloma. Blood Adv. 2022;6:2466–2470. doi: 10.1182/bloodadvances.2021006178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Xia J., Li H., Yan Z., Zhou D., Wang Y., Qi Y., Cao J., Li D., Cheng H., Sang W., et al. Anti-G Protein-Coupled Receptor, Class C Group 5 Member D Chimeric Antigen Receptor T Cells in Patients With Relapsed or Refractory Multiple Myeloma: A Single-Arm, Phase Ⅱ Trial. J. Clin. Oncol. 2023;41:2583–2593. doi: 10.1200/JCO.22.01824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Hsi E.D., Steinle R., Balasa B., Szmania S., Draksharapu A., Shum B.P., Huseni M., Powers D., Nanisetti A., Zhang Y., et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin. Cancer Res. 2008;14:2775–2784. doi: 10.1158/1078-0432.CCR-07-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Gogishvili T., Danhof S., Prommersberger S., Rydzek J., Schreder M., Brede C., Einsele H., Hudecek M. SLAMF7-CAR T cells eliminate myeloma and confer selective fratricide of SLAMF7+ normal lymphocytes. Blood. 2017;130:2838–2847. doi: 10.1182/blood-2017-04-778423. [DOI] [PubMed] [Google Scholar]
- 326.Tai Y.-T., Soydan E., Song W., Fulciniti M., Kim K., Hong F., Li X.-F., Burger P., Rumizen M.J., Nahar S., et al. CS1 promotes multiple myeloma cell adhesion, clonogenic growth, and tumorigenicity via c-maf–mediated interactions with bone marrow stromal cells. Blood. 2009;113:4309–4318. doi: 10.1182/blood-2008-10-183772. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 327.Kikuchi J., Hori M., Iha H., Toyama-Sorimachi N., Hagiwara S., Kuroda Y., Koyama D., Izumi T., Yasui H., Suzuki A., Furukawa Y. Soluble SLAMF7 promotes the growth of myeloma cells via homophilic interaction with surface SLAMF7. Leukemia. 2020;34:180–195. doi: 10.1038/s41375-019-0525-6. [DOI] [PubMed] [Google Scholar]
- 328.Dimopoulos M.A., Dytfeld D., Grosicki S., Moreau P., Takezako N., Hori M., Leleu X., LeBlanc R., Suzuki K., Raab M.S., et al. Elotuzumab Plus Pomalidomide and Dexamethasone for Relapsed/Refractory Multiple Myeloma: Final Overall Survival Analysis From the Randomized Phase II ELOQUENT-3 Trial. J. Clin. Oncol. 2023;41:568–578. doi: 10.1200/JCO.21.02815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Li C., Xu J., Luo W., Liao D., Xie W., Wei Q., Zhang Y., Wang X., Wu Z., Kang Y., et al. Bispecific CS1-BCMA CAR-T cells are clinically active in relapsed or refractory multiple myeloma. Leukemia. 2024;38:149–159. doi: 10.1038/s41375-023-02065-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Matsui W., Huff C.A., Wang Q., Malehorn M.T., Barber J., Tanhehco Y., Smith B.D., Civin C.I., Jones R.J. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103:2332–2336. doi: 10.1182/blood-2003-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Spencer A., Kalff A., Shortt J., Quach H., Wallington-Gates C., Reynolds J., Walker P., Harrison S.J., Dunn R., Wellard C. A sequential cohort study evaluating single-agent KappaMab and KappaMab combined with lenalidomide and low-dose dexamethasone in relapsed and/or refractory kappa light chain-restricted multiple myeloma (AMaRC 01-16) Br. J. Haematol. 2023;202:801–811. doi: 10.1111/bjh.18955. [DOI] [PubMed] [Google Scholar]
- 332.Roncador G., Puñet-Ortiz J., Maestre L., Rodríguez-Lobato L.G., Jiménez S., Reyes-García A.I., García-González Á., García J.F., Piris M., Montes-Moreno S., et al. CD229 (Ly9) a Novel Biomarker for B-Cell Malignancies and Multiple Myeloma. Cancers (Basel) 2022;14 doi: 10.3390/cancers14092154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Spanoudakis E., Hu M., Naresh K., Terpos E., Melo V., Reid A., Kotsianidis I., Abdalla S., Rahemtulla A., Karadimitris A. Regulation of multiple myeloma survival and progression by CD1d. Blood. 2009;113:2498–2507. doi: 10.1182/blood-2008-06-161281. [DOI] [PubMed] [Google Scholar]
- 334.Lameris R., Ruben J.M., Iglesias-Guimarais V., de Jong M., Veth M., van de Bovenkamp F.S., de Weerdt I., Kater A.P., Zweegman S., Horbach S., et al. A bispecific T cell engager recruits both type 1 NKT and Vγ9Vδ2-T cells for the treatment of CD1d-expressing hematological malignancies. Cell Rep. Med. 2023;4 doi: 10.1016/j.xcrm.2023.100961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Exley M., Garcia J., Wilson S.B., Spada F., Gerdes D., Tahir S.M., Patton K.T., Blumberg R.S., Porcelli S., Chott A., Balk S.P. CD1d structure and regulation on human thymocytes, peripheral blood T cells, B cells and monocytes. Immunology. 2000;100:37–47. doi: 10.1046/j.1365-2567.2000.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Canchis P.W., Bhan A.K., Landau S.B., Yang L., Balk S.P., Blumberg R.S. Tissue distribution of the non-polymorphic major histocompatibility complex class I-like molecule, CD1d, Tissue Distribution Non-polymorphic Major Histocompatibility Complex Class I-like Molecule, Cd1d. Immunology. 1993;80:561–565. [PMC free article] [PubMed] [Google Scholar]
- 337.Kotsianidis I., Silk J.D., Spanoudakis E., Patterson S., Almeida A., Schmidt R.R., Tsatalas C., Bourikas G., Cerundolo V., Roberts I.A.G., Karadimitris A. Regulation of hematopoiesis in vitro and in vivo by invariant NKT cells. Blood. 2006;107:3138–3144. doi: 10.1182/blood-2005-07-2804. [DOI] [PubMed] [Google Scholar]
- 338.Kater A.P., Van De Donk N.W., Rodríguez-Otero P., Mateos M.V., Bosch F., Tucci A., Ghia P., Adang A.E., Parren P.W.H., Tuinhof I., et al. Lava-051, a Novel Bispecific Gamma-Delta T-Cell Engager (Gammabody), in Relapsed/Refractory MM and CLL: Pharmacodynamic and Early Clinical Data. Blood. 2022;140:4608–4609. [Google Scholar]
- 339.Roy J.P., Anderson G.S., Walker I., Chapman M.A. Development of the First SEMA4A CAR-T Cell Targeting Multiple Myeloma. Blood. 2022;140:9961–9962. [Google Scholar]
- 340.John S., Chen H., Deng M., Gui X., Wu G., Chen W., Li Z., Zhang N., An Z., Zhang C.C. A Novel Anti-LILRB4 CAR-T Cell for the Treatment of Monocytic AML. Mol. Ther. 2018;26:2487–2495. doi: 10.1016/j.ymthe.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Haubner S., Perna F., Köhnke T., Schmidt C., Berman S., Augsberger C., Schnorfeil F.M., Krupka C., Lichtenegger F.S., Liu X., et al. Coexpression profile of leukemic stem cell markers for combinatorial targeted therapy in AML. Leukemia. 2019;33:64–74. doi: 10.1038/s41375-018-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Ravandi F., Walter R.B., Subklewe M., Buecklein V., Jongen-Lavrencic M., Paschka P., Ossenkoppele G.J., Kantarjian H.M., Hindoyan A., Agarwal S.K., et al. Updated results from phase I dose-escalation study of AMG 330, a bispecific T-cell engager molecule, in patients with relapsed/refractory acute myeloid leukemia (R/R AML) J. Clin. Oncol. 2020;38:7508. [Google Scholar]
- 343.Westervelt P., Cortes J.E., Altman J.K., Long M., Oehler V.G., Gojo I., Guenot J., Chun P., Roboz G.J. Phase 1 First-in-Human Trial of AMV564, a Bivalent Bispecific (2:2) CD33/CD3 T-Cell Engager, in Patients with Relapsed/Refractory Acute Myeloid Leukemia (AML) Blood. 2019;134:834. [Google Scholar]
- 344.Tettamanti S., Marin V., Pizzitola I., Magnani C.F., Giordano Attianese G.M.P., Cribioli E., Maltese F., Galimberti S., Lopez A.F., Biondi A., et al. Targeting of acute myeloid leukaemia by cytokine-induced killer cells redirected with a novel CD123-specific chimeric antigen receptor. Br. J. Haematol. 2013;161:389–401. doi: 10.1111/bjh.12282. [DOI] [PubMed] [Google Scholar]
- 345.Ngai L.L., Ma C.Y., Maguire O., Do A.D., Robert A., Logan A.C., Griffiths E.A., Nemeth M.J., Green C., Pourmohamad T., et al. Bimodal expression of potential drug target CLL-1 (CLEC12A) on CD34+ blasts of AML patients. Eur. J. Haematol. 2021;107:343–353. doi: 10.1111/ejh.13672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Kikushige Y., Yoshimoto G., Miyamoto T., Iino T., Mori Y., Iwasaki H., Niiro H., Takenaka K., Nagafuji K., Harada M., et al. Human Flt3 Is Expressed at the Hematopoietic Stem Cell and the Granulocyte/Macrophage Progenitor Stages to Maintain Cell Survival1. J. Immunol. 2008;180:7358–7367. doi: 10.4049/jimmunol.180.11.7358. [DOI] [PubMed] [Google Scholar]
- 347.Cancer Genome Atlas Research Network. Ley T.J., Miller C., Ding L., Raphael B.J., Mungall A.J., Robertson A.G., Hoadley K., Triche T.J., Jr., Laird P.W., et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Masao M., Regina F., Hartmut H., Itaru M., Rainer S., Carsten M., Wolfram G., Karsten K.-A., Susanne S., Claudia S., et al. Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood. 2000;96:3907–3914. [PubMed] [Google Scholar]
- 349.Warda W., Larosa F., Neto Da Rocha M., Trad R., Deconinck E., Fajloun Z., Faure C., Caillot D., Moldovan M., Valmary-Degano S., et al. CML Hematopoietic Stem Cells Expressing IL1RAP Can Be Targeted by Chimeric Antigen Receptor–Engineered T Cells. Cancer Res. 2019;79:663–675. doi: 10.1158/0008-5472.CAN-18-1078. [DOI] [PubMed] [Google Scholar]
- 350.Herrmann H., Sadovnik I., Eisenwort G., Rülicke T., Blatt K., Herndlhofer S., Willmann M., Stefanzl G., Baumgartner S., Greiner G., et al. Delineation of target expression profiles in CD34+/CD38- and CD34+/CD38+ stem and progenitor cells in AML and CML. Blood Adv. 2020;4:5118–5132. doi: 10.1182/bloodadvances.2020001742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Mitchell K., Barreyro L., Todorova T.I., Taylor S.J., Antony-Debré I., Narayanagari S.R., Carvajal L.A., Leite J., Piperdi Z., Pendurti G., et al. IL1RAP potentiates multiple oncogenic signaling pathways in AML. J. Exp. Med. 2018;215:1709–1727. doi: 10.1084/jem.20180147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Maria A., Helena Å., Nils H., Sandra G., Alexandros A., Marianne R., Gunnar J., Johan R., Marcus J., Thoas F. Selective killing of candidate AML stem cells by antibody targeting of IL1RAP. Blood. 2013;121:3709–3713. doi: 10.1182/blood-2012-09-458935. [DOI] [PubMed] [Google Scholar]
- 353.Clémentine N., Mathieu N., Walid W., Xavier R., Rafik H., Evan S., Rim T., Lucie B., Mathieu G., Léa B., et al. CAR-T cells targeting IL-1RAP produced in a closed semiautomatic system are ready for the first phase I clinical investigation in humans. Curr. Res. Translational Med. 2023;71 doi: 10.1016/j.retram.2023.103385. [DOI] [PubMed] [Google Scholar]
- 354.Riether C., Schürch C.M., Bührer E.D., Hinterbrandner M., Huguenin A.L., Hoepner S., Zlobec I., Pabst T., Radpour R., Ochsenbein A.F. CD70/CD27 signaling promotes blast stemness and is a viable therapeutic target in acute myeloid leukemia. J. Exp. Med. 2017;214:359–380. doi: 10.1084/jem.20152008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Riether C., Pabst T., Höpner S., Bacher U., Hinterbrandner M., Banz Y., Müller R., Manz M.G., Gharib W.H., Francisco D., et al. Targeting CD70 with cusatuzumab eliminates acute myeloid leukemia stem cells in patients treated with hypomethylating agents. Nat. Med. 2020;26:1459–1467. doi: 10.1038/s41591-020-0910-8. [DOI] [PubMed] [Google Scholar]
- 356.Sempowski G.D., Lee D.M., Kaufman R.E., Haynes B.F. Structure and function of the CD7 molecule. Crit. Rev. Immunol. 1999;19:331–348. [PubMed] [Google Scholar]
- 357.Pan J., Tan Y., Wang G., Deng B., Ling Z., Song W., Seery S., Zhang Y., Peng S., Xu J., et al. Donor-Derived CD7 Chimeric Antigen Receptor T Cells for T-Cell Acute Lymphoblastic Leukemia: First-in-Human, Phase I Trial. J. Clin. Oncol. 2021;39:3340–3351. doi: 10.1200/JCO.21.00389. [DOI] [PubMed] [Google Scholar]
- 358.Cao X., Dai H., Cui Q., Li Z., Shen W., Pan J., Shen H., Ma Q., Li M., Chen S., et al. CD7-directed CAR T-cell therapy: a potential immunotherapy strategy for relapsed/refractory acute myeloid leukemia. Exp. Hematol. Oncol. 2022;11:67. doi: 10.1186/s40164-022-00318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Dai Z., Mu W., Zhao Y., Jia X., Liu J., Wei Q., Tan T., Zhou J. The rational development of CD5-targeting biepitopic CARs with fully human heavy-chain-only antigen recognition domains. Mol. Ther. 2021;29:2707–2722. doi: 10.1016/j.ymthe.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Hill L.C., Rouce R.H., Wu M., Wang T., Ma R., Zhang H., Mehta B., Lapteva N., Mei Z., Smith T.S., et al. Anti-tumor Efficacy and Safety of Unedited Autologous CD5.CAR T cells in Relapsed/Refractory Mature T-cell Lymphomas. Blood. 2023;143:1231–1241. doi: 10.1182/blood.2023022204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Peter J.M., Stuart J.K., editors. Kidney Transplantation–Principles and Practice. Seventh Edition. W.B. Saunders; 2014. Chapter 20 - Antilymphocyte Globulin, Monoclonal Antibodies, and Fusion Proteins; pp. 287–313. [Google Scholar]
- 362.Melenhorst J.J., Chen G.M., Wang M., Porter D.L., Chen C., Collins M.A., Gao P., Bandyopadhyay S., Sun H., Zhao Z., et al. Decade-long leukaemia remissions with persistence of CD4(+) CAR T cells. Nature. 2022;602:503–509. doi: 10.1038/s41586-021-04390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Fang K.K.-L., Lee J., Khatri I., Na Y., Zhang L. Targeting T-cell malignancies using allogeneic double-negative CD4-CAR-T cells. J. Immunother. Cancer. 2023;11 doi: 10.1136/jitc-2023-007277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Savoldo B., Rooney C.M., Di Stasi A., Abken H., Hombach A., Foster A.E., Zhang L., Heslop H.E., Brenner M.K., Dotti G. Epstein Barr virus specific cytotoxic T lymphocytes expressing the anti-CD30zeta artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease. Blood. 2007;110:2620–2630. doi: 10.1182/blood-2006-11-059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Sotomayor E.M., Young K.H., Younes A. Clinical roundtable monograph: CD30 in lymphoma: its role in biology, diagnostic testing, and targeted therapy. Clin. Adv. Hematol. Oncol. 2014;12:1–22. [PubMed] [Google Scholar]
- 366.Zheng W., Medeiros L.J., Young K.H., Goswami M., Powers L., Kantarjian H.H., Thomas D.A., Cortes J.E., Wang S.A. CD30 expression in acute lymphoblastic leukemia as assessed by flow cytometry analysis. Leuk. Lymphoma. 2014;55:624–627. doi: 10.3109/10428194.2013.820293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Hombach A.A., Görgens A., Chmielewski M., Murke F., Kimpel J., Giebel B., Abken H. Superior Therapeutic Index in Lymphoma Therapy: CD30(+) CD34(+) Hematopoietic Stem Cells Resist a Chimeric Antigen Receptor T-cell Attack. Mol. Ther. 2016;24:1423–1434. doi: 10.1038/mt.2016.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Ramos C.A., Grover N.S., Beaven A.W., Lulla P.D., Wu M.F., Ivanova A., Wang T., Shea T.C., Rooney C.M., Dittus C., et al. Anti-CD30 CAR-T Cell Therapy in Relapsed and Refractory Hodgkin Lymphoma. J. Clin. Oncol. 2020;38:3794–3804. doi: 10.1200/JCO.20.01342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Ramos C.A., Quach D.H., Lulla P.D., Rouce R.H., Sharma S., Ganesh H.R., Nouraee N., Briones Y.D., Becerra-Dominguez L., Thakkar S.G., et al. OFF-THE-SHELF CD30.CAR-MODIFIED EPSTEIN-BARR VIRUS-SPECIFIC T CELLS (CD30.CAR EBVSTS) PROVIDE A SAFE AND EFFECTIVE THERAPY FOR PATIENTS WITH HODGKIN LYMPHOMA (HL) Hematological Oncol. 2023;41:83–85. [Google Scholar]
- 370.Hombach A.A., Rappl G., Abken H. Blocking CD30 on T Cells by a Dual Specific CAR for CD30 and Colon Cancer Antigens Improves the CAR T Cell Response against CD30(-) Tumors. Mol. Ther. 2019;27:1825–1835. doi: 10.1016/j.ymthe.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Wang C.-M., Wu Z.-Q., Wang Y., Guo Y.-L., Dai H.-R., Wang X.-H., Li X., Zhang Y.-J., Zhang W.-Y., Chen M.-X., et al. Autologous T Cells Expressing CD30 Chimeric Antigen Receptors for Relapsed or Refractory Hodgkin Lymphoma: An Open-Label Phase I Trial. Clin. Cancer Res. 2017;23:1156–1166. doi: 10.1158/1078-0432.CCR-16-1365. [DOI] [PubMed] [Google Scholar]
- 372.Castellino S.M., Pei Q., Parsons S.K., Hodgson D., McCarten K., Horton T., Cho S., Wu Y., Punnett A., Dave H., et al. Brentuximab Vedotin with Chemotherapy in Pediatric High-Risk Hodgkin's Lymphoma. N. Engl. J. Med. 2022;387:1649–1660. doi: 10.1056/NEJMoa2206660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Wang D., Zeng C., Xu B., Xu J.H., Wang J., Jiang L.J., Wang Q.X., Li C.R., Wang N., Huang L., et al. Anti-CD30 chimeric antigen receptor T cell therapy for relapsed/refractory CD30+ lymphoma patients. Blood Cancer J. 2020;10:8. doi: 10.1038/s41408-020-0274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Natalie S.G., Anastasia I., Dominic T.M., Catherine Joyce Arago C., Caroline B., John W., Tammy C., Morrison J.K., Faith Brianne B., Edith B., et al. CD30-Directed CAR-T Cells Co-Expressing CCR4 in Relapsed/Refractory Hodgkin Lymphoma and CD30+ Cutaneous T Cell Lymphoma. Blood. 2021;138:742. [Google Scholar]
- 375.Paul S., Pearlman A.H., Douglass J., Mog B.J., Hsiue E.H.C., Hwang M.S., DiNapoli S.R., Konig M.F., Brown P.A., Wright K.M., et al. TCR β chain-directed bispecific antibodies for the treatment of T cell cancers. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abd3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Zhang C., Palashati H., Rong Z., Lin N., Shen L., Liu Y., Li S., Yu B., Yang W., Lu Z. Pre-depletion of TRBC1+ T cells promotes the therapeutic efficacy of anti-TRBC1 CAR-T for T-cell malignancies. Mol. Cancer. 2020;19:162. doi: 10.1186/s12943-020-01282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Nichakawade T.D., Ge J., Mog B.J., Lee B.S., Pearlman A.H., Hwang M.S., DiNapoli S.R., Wyhs N., Marcou N., Glavaris S., et al. TRBC1-targeting antibody–drug conjugates for the treatment of T cell cancers. Nature. 2024;628:416–423. doi: 10.1038/s41586-024-07233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Shaw L.C., Poussin M., Rodriguez-Garcia A., Eggold J., Minutolo N.G., Wang J., Rook A.H., Schuster S.J., Powell D.J., Jr. TCRvβ-CART therapy mediates high-precision targeting of malignant T-cell clones. Blood Adv. 2023;7:1885–1898. doi: 10.1182/bloodadvances.2022008798. [DOI] [PMC free article] [PubMed] [Google Scholar]