Abstract
For patients with hormone receptor-positive/human epidermal growth factor receptor 2 (HER2)-negative (HR+/HER2−) metastatic breast cancer (mBC) progressed on first-line endocrine therapy plus a cyclin-dependent kinase 4 and 6 inhibitor (CDK4/6i), fulvestrant, a selective estrogen receptor degrader (SERD) administered intramuscularly, represented the only monotherapy option until the approval of elacestrant. This oral SERD has been approved for patients with ESR1-mutant HR+/HER2− mBC by the European Medicines Agency, the Food and Drug Administration, and the UK Medicines and Healthcare products Regulatory Agency, according to the results of the randomized phase III EMERALD trial, which demonstrated elacestrant superiority over standard endocrine monotherapy.
Consequently, elacestrant has been incorporated in the European Society for Medical Oncology and American Society of Clinical Oncology guidelines. However, in Europe, the access to this recommended drug depends on the decision of the National Health Authorities of each state.
In this communication, we describe the main results and implications of the EMERALD trial, in the context of the biomarker-driven algorithm for patients with HR+/HER2− mBC progressed on CDK4/6i, and conclude that a subgroup of patients with ESR1-mutant tumors and specific clinical features can really derive a clinically meaningful benefit from elacestrant, sparing access to more toxic combination approaches and preserving the quality of life.
Key words: metastatic breast cancer, hormone receptor-positive/HER2-negative breast cancer, elacestrant
Highlights
-
•
HR+/HER2− mBC is the most common disease subtype.
-
•
After the progression on CDK4/6is, the choice of treatment is based on a biomarker-driven algorithm.
-
•
Elacestrant is an option for patients with ESR1-mutant disease and is well tolerated.
-
•
A subgroup of patients with ESR1-mutions and specific clinical features can derive a meaningful benefit from elacestrant.
The clinical decision context for metastatic breast cancer progressed on CDK4/6 inhibitors
In 2022, metastatic breast cancer (mBC) represented the main cause of death for solid tumors among women in Europe.1 This incurable disease requires a continuous treatment with cancer drugs, to pursue survival outcomes while preserving patients’ quality of life (QoL).2
Patients diagnosed with hormone receptor (HR)-positive/human epidermal growth factor receptor 2-negative (HR+/HER2−) mBC (70%-75% of all cases) generally receive multiple lines of therapy, with an impact on QoL depending on the frequency of clinic visits, the need for intravenous treatments, and the occurrence of treatment-related adverse events. In this setting, endocrine therapy (ET) is characterized by a favorable safety profile, the advantage of oral administration (except for fulvestrant), and the limited need for laboratory exams.2
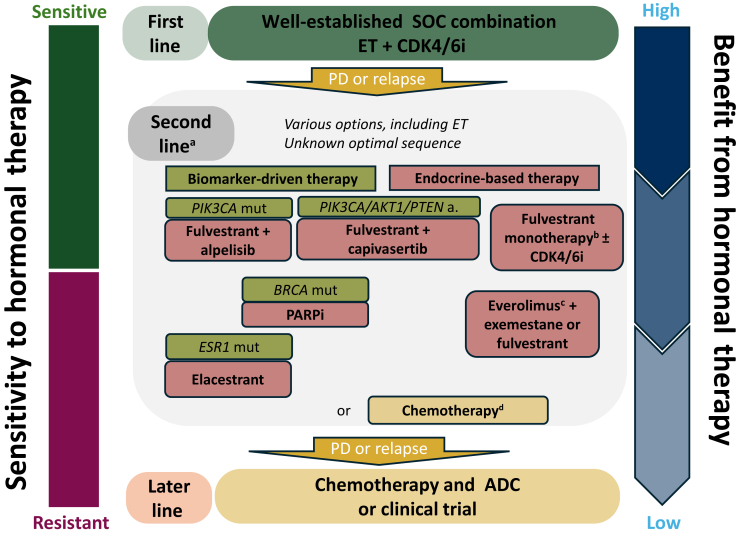
In patients who progress on first-line CDK4/6 inhibitor (CDK4/6i) plus ET, multiple ET-based and targeted treatment options have emerged in the context of a biomarker-driven algorithm (Figure 1): poly (ADP-ribose) polymerase inhibitors for patients with a germline BRCA1 or BRCA2 pathogenic variant, fulvestrant plus alpelisib in case of PIK3CA-mutant tumors, fulvestrant plus capivasertib for AKT pathway-altered (PIK3CA, AKT1, or PTEN) tumors, elacestrant for tumors with the activating gain-of-function mutations in the estrogen receptor gene ligand-binding domain (ESR1), everolimus plus exemestane, fulvestrant monotherapy, or chemotherapy (e.g. capecitabine).3, 4, 5, 6
Figure 1.
Systemictreatment options for postmenopausal women with ER-positive and HER2-negative locally advanced or metastatic breast cancer. a, altered; ADC, antibody–drug conjugate; BRCA, breast cancer gene; CDK4/6, cyclin-dependent kinase 4 and 6; ER, estrogen receptor; ESR1, estrogen receptor 1; ET, endocrine therapy; HER2, human epidermal growth factor receptor 2; m, mutation; PARP, poly (ADP-ribose) polymerase; PD, progressive disease; PFS, progression-free survival; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; SoC, standard of care.aConsider previous therapies, disease aggressiveness, extent and organ function, and toxicity profile. bFulvestrant, aromatase inhibitors, and tamoxifen are routinely and sequentially used in multiple lines. cNo controlled studies conducted in post-CDK4/6 inhibitor therapy. dChemotherapy preferred option in patients with imminent organ failure, after at least two lines of ET, and/or in patients with limited PFS after CDK4/6 inhibitor. Chemotherapy could also be an alternative to PARP inhibitor therapy. a, altered; ADC, antibody–drug conjugate; BRCA, breast cancer gene; CDK4/6, cyclin-dependent kinase 4 and 6; ER, estrogen receptor; ESR1, estrogen receptor 1; ET, endocrine therapy; HER2, human epidermal growth factor receptor 2; m, mutation; PARP, poly (ADP-ribose) polymerase; PD, progressive disease; PFS, progression-free survival; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; SoC, standard of care.
The opportunity to provide ET alone or in combination with other targeted agents is guided by cancer biology (presence of actionable molecular alterations), and patient tolerability and choices.3 Indeed, targeted agents can be associated with specific adverse effects, including diarrhea, hyperglycemia, stomatitis, asthenia and fatigue, skin rash, decreased appetite, and anemia, yielding rates of treatment discontinuation due to adverse events in clinical trials of at least ∼15%-20%.4, 5, 6
Accordingly, second-line endocrine monotherapy can be favored only in case of prior prolonged control with ET plus CDK4/6i, suggesting highly endocrine-sensitive disease. Indeed, in an unselected population previously exposed to CDK4/6i, the median progression-free survival (PFS) of fulvestrant monotherapy was 2-3 months.7
Fulvestrant, a selective estrogen receptor degrader (SERD) administered intramuscularly, represented the only monotherapy option until the approval of elacestrant for ESR1-mutant tumors. This oral SERD has been approved by the European Medicines Agency, the Food and Drug Administration, and the UK Medicines and Healthcare products Regulatory Agency, based on the clinical benefit that emerged in the randomized phase III EMERALD trial.8 Therefore, elacestrant has been incorporated in the guidelines of the European Society for Medical Oncology (ESMO) and of the American Society of Clinical Oncology as an option in patients with ESR1-mutated tumors who can still benefit from ET post-CDK4/6i.9
However, in Europe, the access to this recommended drug depends on the decision of the National Health Authorities of each state.10
This communication aims to describe the main results and implications of the EMERALD trial, providing insights for priority access in a selected population.
Emerald trial: Intended to deliver precision endocrine therapy
The EMERALD trial is an open-label, randomized phase III trial that evaluated the efficacy and safety of elacestrant in men or postmenopausal women with previously treated HR+/HER2− mBC, including patients with ESR1-mutated tumors.8
Overall, 478 patients were randomized to receive either elacestrant or endocrine monotherapy including fulvestrant, anastrozole, letrozole, or exemestane. The coprimary endpoints were PFS in all patients and in those with detectable ESR1 mutations (ESR1m) in circulating tumor DNA (ctDNA).
All patients must have received a CDK4/6i; ∼70% had visceral metastases, 43% received two prior lines of ET, and 22% one prior chemotherapy for mBC. In the control arm, 69% received fulvestrant and 31% an aromatase inhibitor (AI). Importantly, 48% of patients had detectable ESR1m.
Elacestrant improved PFS in both the overall study population [hazard ratio: 0.70; 95% confidence interval (CI) 0.55-0.88; P = 0.002] and patients with ESR1m in ctDNA (n = 228; median PFS: 3.8 versus 1.9 months; hazard ratio: 0.55; 95% CI 0.39-0.77; P = 0.0005). In cases with ESR1m, 6-month PFS rates were 41% for elacestrant versus 19% in the control arm, as per landmark analysis. Among patients without detectable ESR1m, there was no significant improvement in PFS (hazard ratio: 0.86; 95% CI 0.63-1.19; P = 0.308), clearly showing in the metastatic setting that the therapeutic effect of elacestrant monotherapy is biomarker driven.
Compared with the control arm, elacestrant therapy was associated with more grade 1-2 toxicity, including nausea (35% versus 19%) and vomiting (19% versus 8%); however, treatment-related adverse events which led to treatment discontinuation were limited (3% versus 1%).
Elacestrant in clinical practice: Who can derive a real clinical benefit?
On the basis of these data, a debate on elacestrant efficacy has arisen in Europe, with potential implications for the priority access.10 The main concerns were the limited absolute gain in median PFS (inferior to 2 months), and that the control arm was substandard and did not include other regimens (e.g. everolimus + exemestane, everolimus + fulvestrant, or even mono-chemotherapy). Furthermore, no overall survival (OS) improvement was demonstrated in the final analyses.
The real question here to debate may not be whether the overall results of EMERALD should yield a priority access to a new treatment in a setting of clinical need, but whether special programs of access can be tailored to specific subgroups who can derive large benefits.
The sense of priority access programs is to ensure that therapeutic advancements reach patients in need as soon, while the formal pathway of approval is undertaken. As such, high-level evidence is usually utilized based on rigorous methodology and from primary outcome analyses. Still, post hoc evaluations may sometimes refine the comprehension of key mechanisms, to generate hypothesis in specific subgroups of patients.
Key points of discussion should be addressed, to give a context:
-
1.
Although the absolute median PFS benefit in the ESR1m subgroup was 2 months, the PFS curves were characterized by early drops, indicating other mechanisms of endocrine resistance beyond ESR1m (observed in 40% of patients), followed by a subsequent separation, driven by the most sensitive population. Accordingly, the landmark analysis showed 6- and 12-month PFS rates were doubled in the elacestrant arm (6 months: 41% versus 19%; 12 months: 27% versus 8%). In this context of an endocrine-resistant and endocrine-sensitive mixed population, hazard ratio as a continuous variable is more appropriate to assess the primary endpoint than the difference in median PFS, which is limited to a single time point;
-
2.
Data on QoL have been presented at the ESMO Breast Meeting 2023 and demonstrated that QoL was maintained between treatment groups (measured by the EORTC-QLQ-C30, PRO-CTCAE and EQ-5D-5L questionnaires), highlighting the clinically meaningful benefit derived from elacestrant.11 In fact, some patients may favor oral administration as opposed to repeated injections, where oral SERD would fit as an option12;
-
3.
the presence of a substandard control (without everolimus or alpelisib) should be contextualized in a setting where combination therapies are associated with higher toxicities and discontinuation rates, without any evidence of OS benefit. Indeed, elacestrant has a space in the context of endocrine monotherapy. Of note, exemestane alone in ESR1-mutated cancers appears essentially ineffective, and there is no reason to believe that it will synergize with everolimus now that the role of ESR1 in determining the resistance to AIs has been well characterized. Furthermore, in the post-CDK4/6i setting, the PFS of everolimus + exemestane is 3-5 months in retrospective studies, lower than the PFS of 8 months that emerged in the BOLERO-2 trial5,13;
-
4.
EMERALD results represented the first attempt to enrich clinical criteria of endocrine resistance with molecular alterations, paving the way to precision medicine also in the context of endocrine resistance. In clinical practice, the identification of ESR1m provides the direction to select the appropriate ET: that is the real legacy from EMERALD. Indeed, SERDs had been developed to overcome resistance to first-line monotherapy with AI, which is characterized in 20%-40% of patients by the acquired mutations of ESR1. These alterations result in the estrogen-independent activation of the estrogen receptor (ER) and, consequently, drive the resistance to AIs but not ER inhibitors (e.g. fulvestrant, elacestrant, tamoxifen).14 However, fulvestrant monotherapy in unrestricted and in ESR1m population has not shown to be very promising.7,15
Despite the selection per ESR1m, 40% of patients from the elacestrant arm experienced a progression within 2 months. Therefore, the most important question is how to upfront select potential responders beyond ESR1m.
Many clinical features can be considered to enrich the patient selection: previous treatments, limiting the rechallenge with drugs of the same class; sites of disease, prioritizing for endocrine monotherapy in patients without visceral involvement: in this regard, only patients without visceral disease derived a higher benefit in the FALCON trial, which compared fulvestrant to anastrozole in the first-line setting16; and PFS on CDK4/6i ≥12 months. Indeed, an ad hoc subgroup analysis carried out on patients from the EMERALD trial with ESR1m and a PFS on CDK4/6i ≥12 months showed a median PFS delta of 7 months (mPFS: 8.6 versus 9.1 months; hazard ratio: 0.41; 95% CI 0.26-0.63) and an increase in the 12-month PFS rate from 8.4% to 35.8% with elacestrant.17 Interestingly, in this subgroup the early drop of curves decreased from 40% to 30%. This analysis represents an excellent example of the combination of clinical and molecular data to predict the activity of targeted therapies, and the PFS on CDK4/6i ≥12 months should be considered when prescribing elacestrant.
Future efforts are required to further improve the selection of patients potentially candidate to endocrine monotherapy after CDK4/6i, including the detection of mechanisms of resistance other than ESR1m, such as acquired RB1 mutations or HR expression loss (observed in ∼10% of cases), which could be a priori re-assessed by novel nuclear imaging tracers.18,19
In conclusion, a subgroup of patients with HR+/HER2− ESR1m mBC, enriched per clinical features, can really derive a clinically meaningful benefit from elacestrant, sparing access to more toxic combination approaches and preserving the QoL.
Acknowledgments
Funding
None declared.
Disclosure
GC reports financial interests with AstraZeneca, Celcuity, Daiichi Sankyo, Exact Sciences, Lilly, Merck, Novartis, Pfizer, Roche, Veracyte, Ellipsis, Astellas, Blueprint Medicine, BMS, Kymab, Merck, Novartis, Philogen, Relay Therapeutics, Sanofi; and non-financial interests with the Italian National Health Council as Advisor for Ministry of Health ESMO, ESMO as Clinical Practice Guidelines Chair, Europa Donna as Member of the Scientific Council, EUSOMA as member of the Advisory Council, Fondazione Beretta, Lega Italiana Lotta ai Tumori as member of Board of Directors. All the competing interests were outside the submitted work. FCB reports conflicts of interest with Roche (advisory board), Lilly (advisory board), AstraZeneca (advisory board, travel support, research grant), Pfizer (advisory board, travel support, research grant), Menarini/stemline (advisory board). FPL reports personnal honoraria: AbbVie, Amgen, Astellas, AstraZeneca, Bayer, BMS, Clovis, Daiichy Sankyo, EISAI, Exact Science, GSK, Illumina, Invitae, Lilly, Menarini-Stemline, MSD, Myriad, Novartis, Pfizer, Pierre-Fabre, Roche, Sanofi, Veracyte; funding to institution for translational research: AbbVie, Astellas, AstraZeneca, Bayer, BMS, Genomic Health, Illumina, Lilly, MSD, Myriad, Nanostring, Novartis, Pfizer, Pierre-Fabre, Roche; travel grants: AstraZeneca, Bayer, BMS, Gilead, MSD, Novartis, Pfizer, Roche. All other authors have declared no conflicts of interest.
References
- 1.Eurostat Cancer statistics—specific cancers. 2024. https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Cancer_statistics_-_specific_cancers#Breast_cancer Available at.
- 2.Loibl S., Poortmans P., Morrow M., Denkert C., Curigliano G. Breast cancer. Lancet. 2021;397(10286):1750–1769. doi: 10.1016/S0140-6736(20)32381-3. [DOI] [PubMed] [Google Scholar]
- 3.Gennari A., André F., Barrios C.H., et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32(12):1475–1495. doi: 10.1016/j.annonc.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Rugo H.S., Lerebours F., Ciruelos E., et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021;22(4):489–498. doi: 10.1016/S1470-2045(21)00034-6. [DOI] [PubMed] [Google Scholar]
- 5.Baselga J., Campone M., Piccart M., et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med. 2012;366(6):520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner N.C., Oliveira M., Howell S.J., et al. Capivasertib in hormone receptor–positive advanced breast cancer. N Engl J Med. 2023;388(22):2058–2070. doi: 10.1056/NEJMoa2214131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindeman G.J., Fernando T.M., Bowen R., et al. VERONICA: randomized phase II study of fulvestrant and venetoclax in ER-positive metastatic breast cancer post-CDK4/6 inhibitors–efficacy, safety, and biomarker results. Clin Cancer Res. 2022;28(15):3256–3267. doi: 10.1158/1078-0432.CCR-21-3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bidard F.-C., Kaklamani V.G., Neven P., et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: results from the randomized phase III EMERALD trial. J Clin Oncol. 2022;40(28):3246–3256. doi: 10.1200/JCO.22.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burstein H.J., DeMichele A., Somerfield M.R., Henry N.L. Testing for ESR1 mutations to guide therapy for hormone receptor–positive, human epidermal growth factor receptor 2–negative metastatic breast cancer: ASCO guideline rapid recommendation update. J Clin Oncol. 2023;41(18):3423–3425. doi: 10.1200/JCO.23.00638. [DOI] [PubMed] [Google Scholar]
- 10.Haute Autorité de Santé. Decision n°2024.0031/DC/SEM of February 8, 2024 of the college of the High Authority of Health refusing the request for early access authorization for the specialty ORSERDU. 2024. https://www.has-sante.fr/jcms/p_3495000/fr/decision-n2024-0031/dc/sem-du-8-fevrier-2024-du-college-de-la-haute-autorite-de-sante-portant-refus-de-la-demande-d-autorisation-d-acces-precoce-de-la-specialite-orserdu Available at.
- 11.Cortés J., Bidard F.C., Bardia A., et al. 188O EMERALD trial analysis of patient-reported outcomes (PROs) in patients with ER+/HER2− advanced or metastatic breast cancer (mBC) comparing oral elacestrant vs standard of care (SoC) endocrine therapy. ESMO Open. 2023;8(1) [Google Scholar]
- 12.Fallowfield L., Atkins L., Catt S., et al. Patients’ preference for administration of endocrine treatments by injection or tablets: results from a study of women with breast cancer. Ann Oncol. 2006;17(2):205–210. doi: 10.1093/annonc/mdj044. [DOI] [PubMed] [Google Scholar]
- 13.Cook M.M., Al Rabadi L., Kaempf A.J., Saraceni M.M., Savin M.A., Mitri Z.I. Everolimus plus exemestane treatment in patients with metastatic hormone receptor-positive breast cancer previously treated with CDK4/6 inhibitor therapy. Oncologist. 2021;26(2):101–106. doi: 10.1002/onco.13609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brett J.O., Spring L.M., Bardia A., Wander S.A. ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res. 2021;23(1):85. doi: 10.1186/s13058-021-01462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner N.C., Kingston B., Kilburn L.S., et al. Circulating tumour DNA analysis to direct therapy in advanced breast cancer (plasmaMATCH): a multicentre, multicohort, phase 2a, platform trial. Lancet Oncol. 2020;21(10):1296–1308. doi: 10.1016/S1470-2045(20)30444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robertson J.F.R., Bondarenko I.M., Trishkina E., et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet. 2016;388(10063):2997–3005. doi: 10.1016/S0140-6736(16)32389-3. [DOI] [PubMed] [Google Scholar]
- 17.Bardia A., Cortes J., Bidard F.-C., et al. Elacestrant in ER+, HER2− MBC with ESR1-mutated tumors: subgroup analyses from the phase III EMERALD trial by prior duration of endocrine therapy plus CDK4/6 inhibitor and in clinical subgroups. Clin Cancer Res. 2024 doi: 10.1158/1078-0432.CCR-24-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Leary B., Hrebien S., Morden J.P., et al. Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nat Commun. 2018;9(1):896. doi: 10.1038/s41467-018-03215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Geel J.J.L., Boers J., Elias S.G., et al. Clinical validity of 16α-[18F]Fluoro-17β-estradiol positron emission tomography/computed tomography to assess estrogen receptor status in newly diagnosed metastatic breast cancer. J Clin Oncol. 2022;40(31):3642–3652. doi: 10.1200/JCO.22.00400. [DOI] [PubMed] [Google Scholar]