Abstract
Background
Malaria in pregnancy is a critical public health issue that can lead to severe adverse outcomes for both mother and fetus. This systematic review and meta-analysis evaluated the prevalence of adverse birth outcomes in malaria-infected pregnancies and examines their association with the condition.
Method
We searched databases up to January 30, 2024, for observational studies on pregnant women with malaria. Data were analyzed using a random-effects model to calculate pooled prevalence rates and risk ratios (RRs) for adverse outcomes, with statistical support from R software version 4.3.
Results
Thirty-one studies were included, showing high prevalence of low birth weight (LBW; 17.4 %), preterm birth (17.9 %), and small for gestational age (SGA; 16.1 %) in malaria-affected pregnancies. Infected mothers were significantly more likely to have LBW infants (RR = 1.755), preterm births (RR = 1.484), and SGA infants (RR = 1.554). The risk of stillbirth was not significantly increased (RR = 1.238).
Conclusion
Malaria in pregnancy significantly elevates the risk of LBW, preterm birth, and SGA, underscoring the need for effective malaria prevention and treatment strategies in endemic regions. Future research should aim to refine and implement these strategies to enhance maternal and neonatal health outcomes.
Keywords: Malaria, Plasmodium, Stillbirth, Preterm birth, Low birthweight, Meta-analysis
1. Introduction
Malaria during pregnancy is widely recognized as a significant global health challenge [1,2], presenting substantial risks to the health of both the mother and the fetus, as well as to newborns and the broader community [2,3]. The disease is attributed to infection by Plasmodium parasites, among which Plasmodium falciparum and Plasmodium vivax are the most hazardous to humans [4]. Infection is transmitted via the bite of infected female Anopheles mosquitoes, leading to a spectrum of clinical outcomes that range from mild symptoms to severe illness and even death [5,6].
Pregnant women are particularly vulnerable to malaria due to a combination of factors [[7], [8], [9]]. These include a decreased immunity to malaria, which permits higher levels of parasitemia, and the sequestration of parasites in the placenta [10]. Specific socio-economic and cultural factors may also exacerbate exposure or hinder access to preventive strategies [[11], [12], [13]]. The negative consequences of malaria in pregnancy are extensive and varied. A common pathological feature in malaria-affected pregnancies is placental malaria, which is characterized by the sequestration of Plasmodium-infected erythrocytes within the intervillous spaces of the placenta [14]. This can cause placental inflammation and impair the exchange of nutrients and gases, leading to adverse outcomes such as low birth weight (LBW), preterm birth, low birth weight, stillbirth, and neonatal death [15,16]. Malaria in pregnancy can also lead to maternal anemia, significantly elevating the risk of maternal mortality and complicating the already challenging experience of pregnancy and childbirth in areas where malaria is endemic [[17], [18], [19]].
Despite continuous efforts to control and eradicate malaria, it remains a significant burden on pregnant women and their infants, particularly in sub-Saharan Africa, the region that bears the brunt of worldwide malaria-related sickness and deaths, this condition is prevalent [20,21]. This challenge is intensified by factors like resistance to antimalarial drugs and obstacles in accessing and implementing preventive measures, such as intermittent preventive treatment in pregnancy (IPTp) and insecticide-treated nets (ITNs) [[22], [23], [24]]. The complexity of providing effective antenatal care in settings with limited resources further aggravates the situation.
Considering the profound impact of malaria on birth outcomes and the inconsistency of findings across various studies, there is a pressing need for a thorough and systematic literature review to consolidate the existing evidence on this subject. The objective of this research is to fill the existing void by undertaking a systematic review and meta-analysis that evaluates the association between malaria during pregnancy and the likelihood of negative outcomes in pregnancy. The intention is to measure the impact of malaria on various pregnancy results, such as preterm delivery, still birth, low birth weight (LBW) among other complications. The findings of this review are expected to have significant implications for public health policy and practice, especially in the development and implementation of targeted interventions to prevent and manage malaria in pregnancy.
2. Method
This study was conducted in alignment with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and outlined in Table S1 [25]. For the screening and data extraction processes, we employed the Nested Knowledge web-based software (Nested-Knowledge, MN, USA). The protocol was registered in PROSPERO: CRD42024513881.
2.1. Eligibility criteria
Original researches were eligible for the analysis if they were observational, including cohort, case-control, or cross-sectional studies that reported on pregnant women infected with malaria of any species. Eligible studies either compared outcomes between infected and non-infected pregnant women or reported the number of pregnancy-related or neonatal outcomes associated with malaria. Additionally, to be considered for inclusion, studies needed to report on at least one of the predefined adverse pregnancy outcomes. Conversely, studies were excluded from the analysis if they failed to provide specific data on pregnancy outcomes, were in the form of reviews, letters, comments, or conference abstracts that lacked comprehensive data, or were based on animal studies. The inclusion criteria are given in Table S2.
2.2. Data sources and search strategy
A search of electronic databases, including Embase, PubMed, and Web of Science, was conducted from inception to January 30, 2024. The search strategy combined terms related to "malaria," "pregnancy," and specific outcomes of interest (e.g., "preterm birth," "low birth weight"). No language restrictions were applied. After retrieving the search files, the articles were uploaded to the Nested Knowledge web application for de-duplication and screening. The complete search strategy is given in Table S3.
2.3. Screening
The screening process was conducted utilizing the Nested Knowledge web software. Two independent reviewers carried out the process. In the initial screening process, the reviewers separately examined the titles and abstracts of all retrieved articles. This preliminary assessment aimed to quickly identify studies that potentially met the eligibility criteria based on their relevance to malaria infection in pregnant women and associated adverse pregnancy outcomes. Following the title and abstract screening, studies deemed potentially relevant by either reviewer or where there was uncertainty regarding their eligibility were advanced to the second step of the screening process. During this phase, the full texts of these selected articles were reviewed. When the reviewers had differing opinions on the eligibility of a particular study, a third reviewer was consulted to mediate and provide an objective assessment.
2.4. Data extraction and quality assessment
Data were independently extracted by two reviewers using a pre-defined form, ensuring consistency in capturing key study details such as author name, publication year, country of origin, study design, and sample size. They also recorded participant demographics, specifics of malaria infection including the species and diagnostic methods used, as well as the pregnancy outcomes of interest. The Nested Knowledge web software's tagging feature aided the screening and extraction processes. Any differences between reviewers were reconciled by discussion, or if necessary, by consulting a third reviewer. Study quality was evaluated using the Newcastle-Ottawa Scale for observational studies, examining the adequacy of the population sampled, the comparability of the groups, and the assessment of outcomes.
2.5. Statistical analysis
The meta-analysis applied a random-effects model to determine the aggregated prevalence rates of adverse outcomes in pregnant women diagnosed with malaria. Events and the number of pregnant women with malaria were pooled to find the overall prevalence for each outcome [26]. Studies with a population sample size of less than one hundred (n < 100) were excluded from the meta-analysis for prevalence to minimize overestimation due to low sample size. For quantifying the association between malaria during pregnancy and each identified adverse outcome, risk ratios (RRs) accompanied by 95 % confidence intervals (CIs) were computed. The Mantel-Haenszel method estimated the pooled risk ratio by taking the number of events for each outcome and the total number of pregnant women in the malarial and non-malarial groups. Heterogeneity among studies was evaluated using the I2 statistic, categorized into levels and criteria for heterogeneity. Additionally, a 95 % prediction interval was computed. Sensitivity analyses were carried out to investigate the impact of individual studies on the overarching findings. Evaluation of publication bias was conducted through the utilization of funnel plots and Egger's test. Statistical analyses were executed using R software version 4.3 [27].
3. Results
3.1. Search results
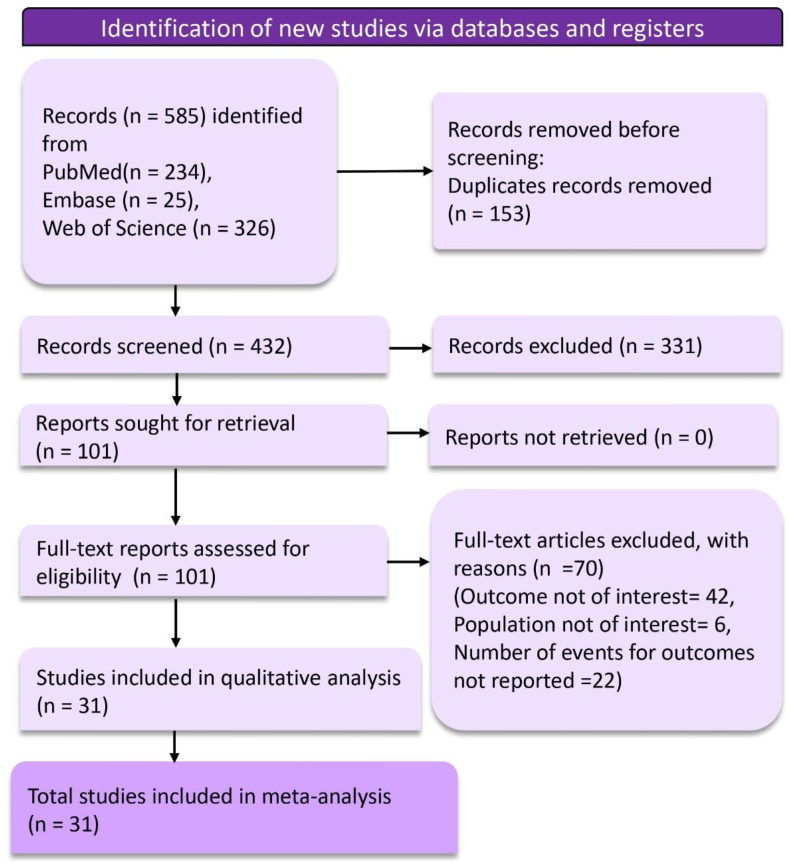
A total of 585 records were retrieved in the search from the databases. Before the screening process, duplicates were removed, resulting in the exclusion of 153 records. The screening phase then began with the evaluation of 432 records. Based on the inclusion and exclusion criteria established for this review, 331 records were excluded. Subsequently, 101 reports were identified as potentially relevant and were sought for detailed retrieval. All 101 retrieved full-text reports underwent a rigorous eligibility assessment. Seventy articles were excluded for the following reasons: the outcome was not of interest in 42 studies, the population was not of interest in 6 studies, and the number of events for the outcomes was not reported in 22 studies. A total of 31 studies were included in the final meta-analysis that informed the outcomes of this systematic review.
3.2. Characteristics of included studies
Table 1 summarizes the key characteristics of the included studies. A diverse range of study designs was represented, with observational, cross-sectional, cohort, transversal, and case-control studies. The geographical distribution of the studies spanned several continents, reflecting the global nature of malaria's impact on pregnant women. Studies were conducted in a variety of locations, including Uganda [[28], [29], [30]], Ethiopia [31,32], Brazil [[33], [34], [35]], Cameroon [19,[36], [37], [38]], Ghana [39,40], Gabon [41,42], India [[43], [44], [45], [46]], Thailand [47,48], the Democratic Republic of the Congo [49], Zimbabwe [50,51], Malawi [52], Tanzania [53], Colombia [54], Burkina Faso [55], and Nigeria [56,57]. This broad geographic coverage provides a comprehensive insight into the issue across different malaria-endemic regions. The sample sizes across the studies varied significantly, ranging from as few as 34 participants to as many as 14,487, demonstrating a broad spectrum of study scales. Such variation in sample size allows for a wide-ranging understanding of malaria's impact in different settings and populations. The type of Plasmodium species investigated also differed among the studies, with some focusing on P. falciparum, others on P. vivax, and some on both. A few studies did not specify the Plasmodium species or needed to have information available on the type of species. This distinction is crucial, as different species may affect pregnancy outcomes differently. Malaria detection methods employed in these studies were diverse. They included placenta biopsies for histological diagnosis with PCR, RT PCR, peripheral blood thin and thick blood smears, rapid diagnostic tests (RDT), and placental histopathology. Using multiple diagnostic methods enriches the robustness of the findings by incorporating a range of detection sensitivities and specificities. These outcomes included mainly low birth weight (LBW), preterm birth, stillbirth, and small for gestational age (SGA), which are critical indicators of the impact of malaria on neonatal and maternal health. The quality assessment is presented in Table S4.
Table 1.
Characteristics of included studies.
| Study | Study location | Study Design | Sample Size | Type of Plasmodium Species | Malaria detection Method |
|---|---|---|---|---|---|
| Alemayehu 2020 [31] | Ethiopia | Cross-sectional study | 897 | P. falciparum | RT-PCR |
| Aribodor 2009 [56] | Nigeria | Cross-sectional study | 500 | P. falciparum | Thick smear |
| Bassey 2015 [57] | Nigeria | Cross-sectional study | 210 | P. falciparum | Thick and thin smears |
| Bater 2020 [29] | Uganda | Prospective cohort study | 3841 | NA | RDT |
| Biteghe-Bi-Essone 2022 [41] | Gabon | Transversal study | 323 | P. falciparum | RDT, thick blood smear |
| Chawla 2007 [46] | India | Observational study | 416 | P. falciparum, P. vivax | Peripheral blood smear |
| Datta 2017 [44] | India | Prospective study | 64 | P. falciparum, P. vivax | Peripheral blood smear or ELISA test |
| Dombrowski 2018 [34] | Brazil | Observational study | 14,487 | P. falciparum, P. vivax | Thin and thick blood smear |
| Dombrowski 2019 [33] | Brazil | Cohort study | 4291 | P. falciparum, P. vivax | Peripheral blood thin and thick blood smear |
| Dombrowski 2021 [35] | Brazil | Observational cohort study | 329 | P. vivax | Thick and thin peripheral blood smears & and PCR |
| Feresu 2015 [50] | Zimbabwe | Secondary data analysis | 3110 | NA | NA |
| Gavina 2018 [54] | Colombia | Longitudinal study | 180 | P. falciparum, P. vivax | RT-qPCR, and placental histopathology |
| Gaw 2019 [49] | Democratic Republic of the Congo | Cross-sectional study | 34 | P. falciparum | Malaria symptoms and Peripheral blood smear |
| Honkpéhèdji 2021 [42] | Gabon | Cohort study | 678 | P. falciparum | Thick blood smear |
| Hussein 2022 [39] | Ghana | Prospective cohort study | 1323 | P. falciparum | RDT |
| Kamga 2024 [36] | Cameroon | Cross-sectional study | 380 | P. falciparum | PCR |
| Kumlachew 2018 [32] | Ethiopia | A cross-sectional study | 381 | NA | NA |
| Lingani 2022 [55] | Burkina Faso | Cross-sectional study | 600 | P. falciparum | Peripheral blood sample |
| Lloyd 2018 [38] | Cameroon | Observational study | 1377 | P. falciparum | Thick and thin blood smears |
| Luxemburger 2001 [48] | Thailand | Cohort study | 1495 | P. falciparum, P. vivax | Blood Smear |
| Megnekou 2023 [37] | Cameroon | Retrospective case-control study | 134 | P. falciparum | Thick and thin blood smears |
| Mikomangwa 2019 [53] | Tanzania | Cross-sectional study | 631 | NA | RDT |
| Nekaka 2020 [30] | Uganda | Cross-sectional study | 210 | P. falciparum | Thick and thin peripheral blood smears & and PCR |
| Nkwabong 2020 [19] | Cameroon | Cohort study | 3063 | NA | RDT, thick blood smear |
| Quanquin 2020 [28] | Uganda | Observational study | 40 | P. falciparum | Placenta biopsies for histological diagnosis with PCR |
| Quinn 2021 [40] | Ghana | Cohort study | 1288 | NA | Placental histopathology |
| Rijken 2014 [47] | Thailand | Population Cohort Study | 10,264 | P. falciparum, P. vivax | Giemsa stained thick and thin blood films |
| Singh 2014 [45] | India | Observational study | 203 | P. falciparum, P. vivax | Thick and thin smear |
| Singh 2020 [43] | India | Prospective cohort study | 286 | P. falciparum | Thin and thick blood smear |
| Ticconi 2003 [51] | Zimbabwe | Case-control study | 986 | NA | Blood smear |
| Weckman 2021 [52] | Malawi | Observational cohort study | 421 | P. falciparum | PCR |
Abbreviations: ELISA: Enzyme-Linked Immunosorbent Assay, NA: Not Available, PCR: Polymerase Chain Reaction, RDT: Rapid Diagnostic Test, RT PCR: Reverse Transcription Polymerase Chain Reaction, RT-qPCR: Reverse Transcription Quantitative Polymerase Chain Reaction.
3.3. Qualitative summary
Many of the studies reported an association of adverse birth outcomes. Nkawabong et al. [19] in Uganda highlighted a significant correlation with a RR of 2.88 (95 % CI: 1.34–6.19) for LBW and 4.50 (95 % CI: 2.37–8.51) for preterm birth. This pattern is echoed in studies from diverse settings, showing elevated odds ratios (ORs) for LBW and preterm births; for example, Bater et al. [29] reported an OR of 2.02 (95 % CI: 1.09, 3.74) for LBW and 1.40 (95 % CI: 0.98, 2.02) for preterm births, while Husain et al. [39] from Ghana found an OR of 2.04 (95 % CI: 1.16 to 3.61) for LBW and 2.02 (95 % CI: 1.39 to 2.93) for preterm births. Biteghe-Bi-Essone et al. [57] presented notably high ORs for LBW at 9.07 (95 % CI: 2.06–45.49), indicating a severe impact of malaria on birth weight. Further contributions come from Dombrowski et al. [35] in Brazil and Kumlachew et al. [32] from Ethiopia, with findings of ORs suggesting increased risks for LBW, SGA, and prematurity. Riken et al. [47] from England provided risk differences (RDs) highlighting the risk malaria poses to neonatal health with RDs for LBW at 5.5 (95 % CI: 3.2, 7.7), preterm birth at 1.7 (95 % CI: 0.03, 3.5), and SGA at 4.8 (95 % CI: 2.7, 6.7). Additional studies from Cameroon, the Democratic Republic of the Congo, Zimbabwe, Gabon, Burkina Faso, and Thailand further reinforce the consistent trend of increased risk of LBW, SGA, and preterm births associated with malaria infection, with ORs and RRs across these studies presenting a compelling argument for the significant impact of malaria on adverse birth outcomes (see Fig. 1).
Fig. 1.
PRISMA flow diagram depicting article selection and screening process.
4. Prevalence of adverse birth outcomes with malarial infection
4.1. LBW
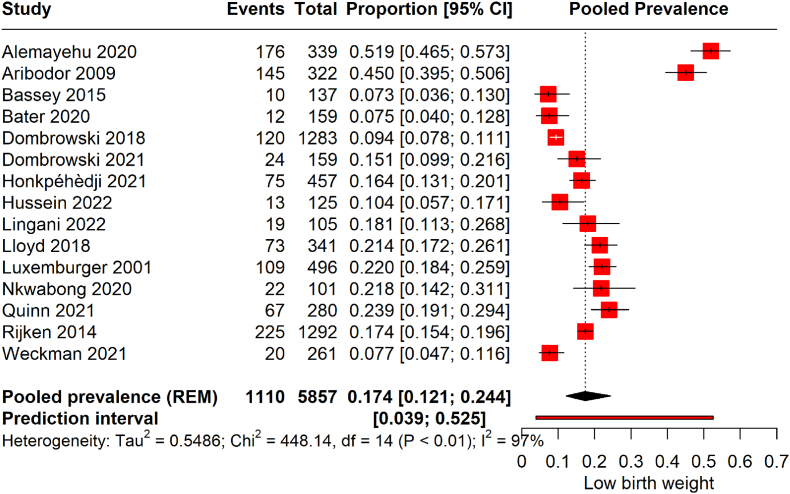
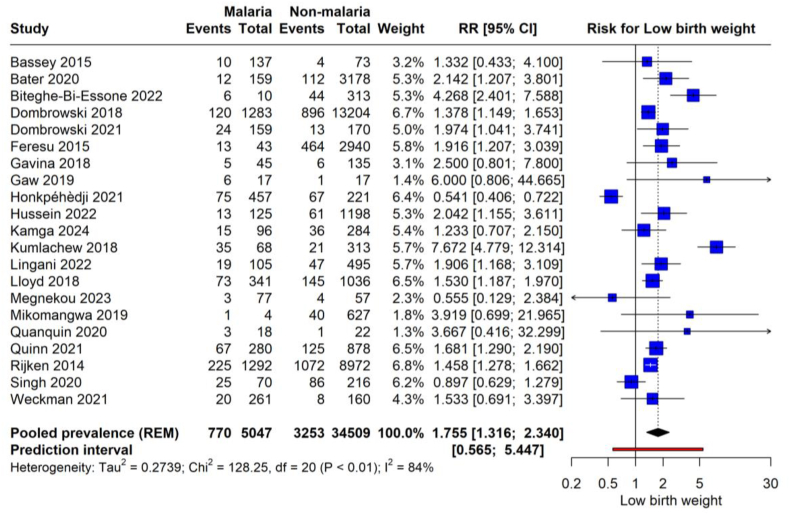
The forest plot illustrates the pooled prevalence of LBW from 15 studies, encompassing 5857 pregnancies affected by malaria. (Fig. 2). The pooled prevalence was 17.4 % (95 % CI: 12.1 to 24.4). This translates to an average of approximately 17.4 % of infants born to malaria-infected pregnant women estimated to have LBW across the included studies. The prediction interval, where the actual effect size is expected to lie in 95 % of similar future studies, spans from 0.039 to 0.525, indicating substantial heterogeneity. Sensitivity analysis was conducted to identify studies significantly affecting the overall results (Fig. S1). It was found that the studies by Alemayehu et al. [31] and Aribodor et al. [56] had a considerable impact on the overall prevalence of LBW. Thus, a re-analysis was performed excluding these studies (Fig. S2). The recalculated pooled prevalence is 14 % (95 % CI: 11 to 19). This revised prevalence suggests that around 14 % of infants born to malaria-infected mothers are at risk of LBW, which is marginally lower than the initial pooled prevalence before the influential studies were omitted. However, the heterogeneity remains high, with an I2 of 89 %. After adjusting for significant outliers, the prediction interval has also narrowed to (0.06, 0.32), reflecting a more homogeneous effect size.
Fig. 2.
Prevalence of LBW among malarial-infected pregnancies.
4.2. Preterm birth
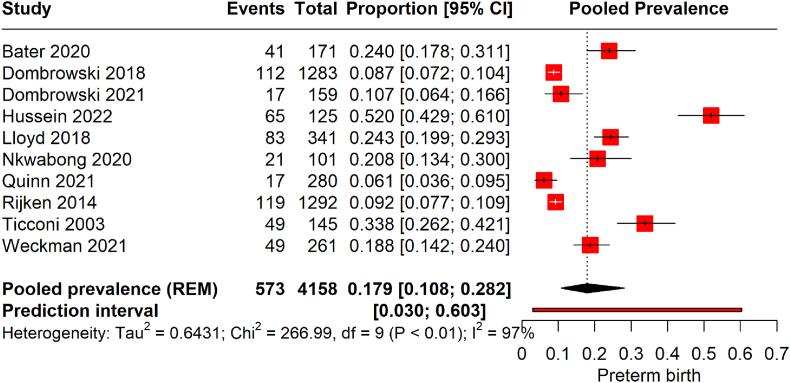
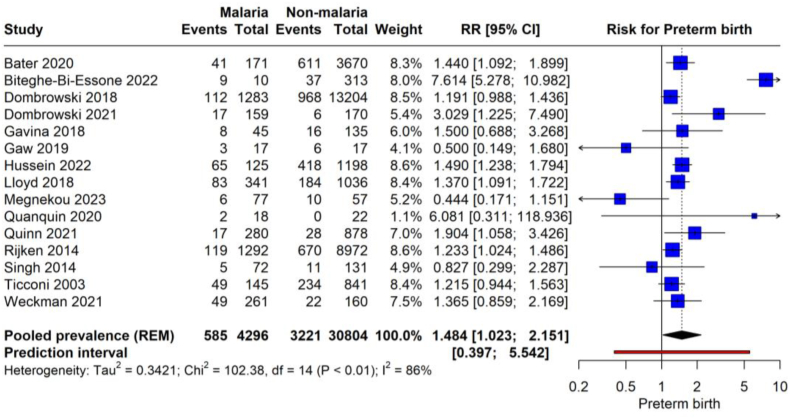
The forest plot illustrates the pooled prevalence of preterm birth from 10 studies encompassing 4158 pregnant women infected with malaria (Fig. 3). The meta-analysis yielded a pooled prevalence 17.9 (95 % CI of 10.8–28.2) with significant heterogeneity (97 %). This indicates that the estimated average prevalence of preterm births among the malaria-infected pregnant population across these studies is approximately 17.9 %. The studies exhibit considerable variability, reflected by the wide prediction interval of 0.030–0.603.
Fig. 3.
Prevalence of preterm birth among malarial infected pregnancies.
4.3. SGA births
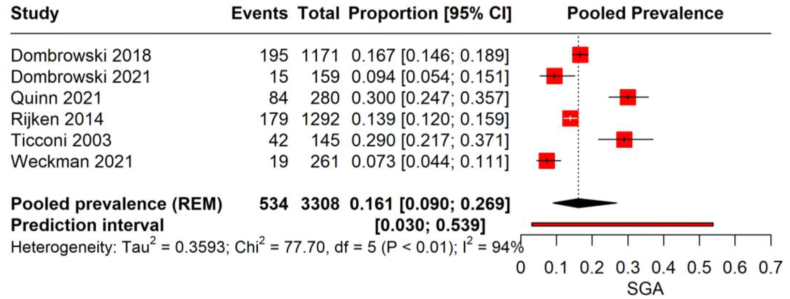
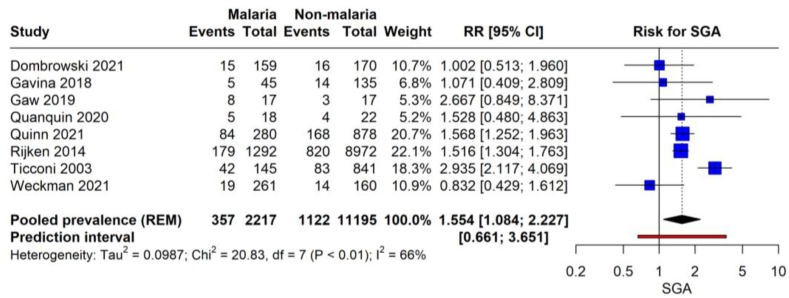
The meta-analysis on the prevalence of SGA births from 6 studies with 3380 malaria-infected pregnant women revealed a pooled prevalence rate of 16.1 %, with a 95 % CI (9.0–26.9) with high heterogeneity (I2 = 94 %). This suggests that in the studied populations, approximately 1 in 6 infants born to women who had malaria during pregnancy were SGA. The individual study proportions range significantly, from as low as 7.3 % to as high as 30.0 %, and the prediction interval extends from 3.0 % to 53.9 %, indicating a wide range of outcomes across different study settings (Fig. 4).
Fig. 4.
Prevalence of SGA birth among malarial infected pregnancies.
5. Risk of adverse birth outcomes with malaria
5.1. LBW
The meta-analysis assesses the risk of LBW associated with malaria infection during pregnancy (Fig. 5) from 21 studies, encompassing a cohort of 50,447 pregnant women, of whom 7770 were infected with malaria and 42,677 were not. The results demonstrate a significant increase in the risk of LBW when the mother is infected with malaria, with a pooled RR of 1.755 (95 % CI: 1.316 to 2.340, p = 0006. This increased risk is substantiated by a high degree of heterogeneity (I2 = 84 %), indicating that women with malaria have an estimated 75.5 % higher likelihood of delivering LBW infants compared to uninfected women. The prediction interval ranges from 0.565 to 5.447, reflecting the potential variation in the effect size in similar future studies and confirming the substantial heterogeneity observed in the current analysis.
Fig. 5.
Risk of LBW with malarial infection.
5.2. Preterm birth
The meta-analysis aggregates data from 15 studies, comprising 8296 malaria events in pregnant women and 32,204 events in non-malaria-affected pregnancies (Fig. 6). The pooled RR for preterm birth in women with malaria is 1.484 (95 % CI: 1.023 to 2.151, p = 0.039), with high heterogeneity (86 %). This indicates that malaria infection during pregnancy is associated with a 48.4 % increased risk of preterm birth compared to non-infected pregnancies. The total sample size of these studies amounts to 40,500 pregnancies, providing a substantial basis for the estimated association. The prediction interval of 0.397–5.542 further acknowledges this variability and implies that the actual effect size could vary significantly in similar studies conducted in the future.
Fig. 6.
Risk of preterm birth with malarial infection.
5.3. SGA births
The forest plot presents the risk of SGA births associated with malaria infection during pregnancy (Fig. 7). The meta-analysis synthesizes data from eight studies, totaling 11,338 pregnant women, 3527 with malaria and 7811 without. The pooled RR for SGA in women with malaria is 1.554 (95 % CI: 1.084 to 2.227, p = 0.021). This suggests that pregnant women with malaria are 55.4 % more likely to have SGA infants than those without malaria. The studies included in the analysis reflect moderate heterogeneity (I2 = 66 %), indicating some variability in the association between malaria and SGA across the different populations studied. The prediction interval ranges from 0.661 to 3.651, which is pretty broad, reflecting the uncertainty in the effect size that could be expected in similar future studies.
Fig. 7.
Risk of SGA birth with malarial infection.
5.4. Stillbirth
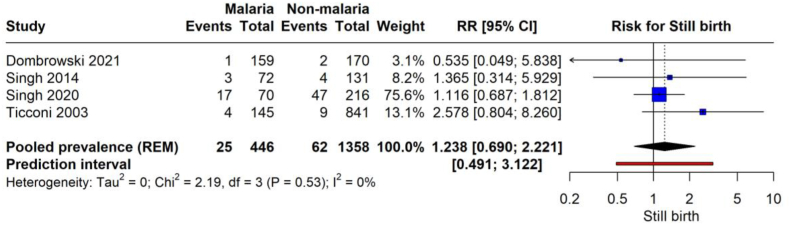
The forest plot represents the risk of stillbirth in the context of malaria infection during pregnancy (Fig. 8). The meta-analysis combines data from four studies involving 2004 pregnancies, 446 with malaria and 1558 without malaria. The analysis yields a pooled RR of 1.238 (95 % CI: 0.690 to 2.221, p = 0.329) for stillbirth when the mother is infected with malaria. This shows no significant association. The studies demonstrate low heterogeneity (I2 = 0 %). The prediction interval ranges from 0.491 to 3.122, accommodating potential variability in the effect size in future studies with similar settings.
Fig. 8.
Risk of stillbirth with malarial infection.
6. Publication bias
The evaluation of publication bias within this analysis was carried out through the use of funnel plots and Egger's test, as depicted in Figs. S3–S7. The presence of publication bias was not suspected in the analysis across all outcomes (Egger, p > 0.1).
7. Discussion
Our systematic review and meta-analysis have synthesized data from multiple studies to elucidate the relationship between malarial infection in pregnancy and the risk of adverse pregnancy outcomes. Malaria in pregnancy emerged as an apparent risk factor for negative consequences, including LBW, preterm birth, and SGA infants. The pooled prevalence of LBW and preterm births among women with malaria underscores the critical need for targeted malaria prevention strategies during pregnancy.
Incorporating data from 31 studies with a broad geographical distribution, our analysis highlights the substantial burden of malaria during pregnancy. This reflects the diversity of malaria's impact across different regions and its consistent association with adverse outcomes. The analysis reveals notably high pooled prevalence rates of LBW, preterm birth, and SGA among malaria-infected pregnant women. Specifically, approximately 17.4 % of infants born to mothers infected with malaria are at risk of LBW, with further analysis suggesting a slightly lower, yet significant, prevalence of 14 %. Alarmingly high estimated prevalences of preterm birth and SGA births, at approximately 17.9 % and 16.1 %, respectively, were also observed. These outcomes are particularly concerning due to their significant implications for neonatal survival and long-term health. LBW and preterm birth are leading risk factors for neonatal mortality, and they can have lasting effects on health and development. SGA infants face increased risks of immediate health issues and long-term developmental challenges. The analysis revealed a significant association between malaria infection during pregnancy and an increased risk of LBW, preterm birth, and SGA births was also observed. Pregnant women infected with malaria are estimated to have a 75.5 % higher likelihood of delivering LBW infants, a 48.4 % increased risk of preterm birth, and a 55.4 % higher likelihood of having SGA infants compared to uninfected women. These findings highlight the urgent need for effective malaria prevention and treatment strategies during pregnancy to mitigate these risks. Our analysis did not find a significant relationship between malaria infection and stillbirth. This may be due to limitations in the available data or true variability in malaria's impact on stillbirth across different settings. Further research is necessary to clarify this relationship and identify factors that may influence the risk of stillbirth in malaria-infected pregnancies.
A previous systematic review showed similar findings [58]. Their meta-analysis revealed that women with Plasmodium-associated malaria had a 63 % higher risk of LBW and a 23 % higher risk of preterm birth compared to women without malaria. However, they included a smaller number of studies [58]. Our study strengthens these findings with a more significant number of studies. Another systematic review also showed similar results [59]. But they indicated significant association for stillbirths as well. Additionally, we calculated the prevalence rate as well. Another systematic review analyzing 35 studies estimated the prevalence of asymptomatic Plasmodium infection to be 26.1 % [60]. When breaking down the prevalence by specific Plasmodium species, Plasmodium falciparum was identified as the most prevalent at 22.1 %, followed by Plasmodium vivax, Plasmodium malaria, and Plasmodium ovale, with respective prevalences of 3 %, 0.8 %, and 0.2 %. It was found that pregnant women with asymptomatic malaria infections were 2.28 times more likely to be anemic compared to those without infections. Furthermore, the risk of asymptomatic malaria infection was 1.54 times higher in women experiencing their first pregnancy than in those with subsequent pregnancies. These findings, along with our own, highlight the significant consequences of malaria infection during pregnancy.
While our analysis shows the need for enhanced strategies in this area, it is essential to recognize that this is an extension of ongoing efforts documented in seminal studies [61,62]. These prior studies have laid the foundation for the progress observed in recent decades through targeted anti-gestational malaria programs [61,62]. Insufficient management of malaria in endemic areas can impact the incidence of imported malaria, necessitating robust measures to prevent re-establishment of transmission in areas that are malaria-free. Improved surveillance and examination of genetic variations in Plasmodium species will aid in the effective diagnosis and treatment of malaria going forward [63]. Additionally, innovative approaches to integrate a One Health strategy for malaria control should be reinforced [63]. Economically benefiting strategies should be implemented in endemic regions [63]. Research should focus more on the identifying and mitigating barriers of malaria elimination [64]. Efforts to eliminate malaria face significant challenges, including the growing resistance of insects to insecticides, the prevalence of malaria infections that are too low to be detected by routine tests, and the difficulty of reaching and treating communities living in remote regions [64].
Clinically, there is an imperative need for healthcare systems to adopt enhanced screening and monitoring protocols for malaria in pregnant women, particularly in areas with high prevalence. Early identification and management of malaria can markedly diminish the risk of unfavorable pregnancy outcomes. This necessitates the availability of safe and effective antimalarial treatments for pregnant women, requiring clinicians to be well-informed about the safety profiles of antimalarial medications during pregnancy to safeguard maternal and fetal well-being. Moreover, integrating malaria prevention and treatment strategies into standard prenatal care practices, such as offering insecticide-treated nets (ITNs), providing intermittent preventive treatment in pregnancy (IPTp), and educating about malaria prevention, is crucial. From a public health perspective, the evidence calls for the creation and execution of malaria prevention programs explicitly targeting pregnant women. These initiatives should minimize malaria exposure through comprehensive community efforts, including vector control measures and the widespread distribution of ITNs. Furthermore, policy decisions and the allocation of resources need to prioritize malaria prevention and treatment among pregnant women as an essential aspect of maternal and child health initiatives. This includes investing in developing new, more effective prevention and treatment modalities and enhancing educational and awareness campaigns about the dangers of malaria during pregnancy. Such campaigns should inform communities, especially in endemic regions, about preventive measures and the importance of early testing and treatment. Ongoing research and surveillance are vital to assess the effectiveness of existing malaria prevention and treatment interventions for pregnant women and to explore new antimalarial drugs that are safe during pregnancy. Surveillance activities should complement this effort to continually monitor malaria prevalence and its impact on pregnancy outcomes. Addressing malaria in pregnancy effectively requires a collaborative approach beyond national borders, necessitating global and regional cooperation. Sharing knowledge, strategies, and resources through international partnerships is essential for efficiently combating this public health challenge, ensuring the well-being of mothers and their infants worldwide.
Our study has several strengths, including a multidisciplinary expert team of reviewers, the large sample size, the comprehensive nature of the search strategy, and the systematic approach to data synthesis. However, there are limitations to consider. The inherent heterogeneity among included studies in terms of study design, geographical locations, and methodologies for diagnosing malaria and assessing pregnancy outcomes may influence the pooled estimates and their interpretation. The potential for publication bias cannot be entirely ruled out despite no significant evidence of such bias in our analysis. Although our analysis did not reveal significant evidence of publication bias, the nature of published research, favoring significant or positive results, can skew the understanding of the true effects of malaria on pregnancy outcomes. This study synthesizes data that reflect specific times, geographical settings, and healthcare contexts, each of which can vary widely in terms of malaria prevalence and the medical care available to pregnant women. Therefore, caution should be exercised when generalizing these results to regions or populations that differ from those included in our review. The variability in malaria transmission intensity and healthcare infrastructure across the study locations might result in different health outcomes that are not fully captured in this meta-analysis. This underlines the need for localized studies and suggests that our findings should be viewed as indicative rather than definitive across different global contexts. The variability in the definitions and measurement of outcomes across studies, such as the criteria for LBW, preterm birth, and SGA, might contribute to the observed heterogeneity and affect the comparability of results. These limitations underscore the need for further research with standardized methodologies to better understand malaria's impact on pregnancy outcomes.
8. Conclusion
Our study revealed a considerable prevalence and risk of adverse birth outcomes associated with malaria infection in pregnancy. This underscores the need for continued efforts to prevent and treat malaria in pregnant women. Addressing this challenge is crucial for improving maternal and neonatal health and achieving broader public health goals in malaria-endemic regions. Further researches are required to assess the effectiveness and implementation of strategies to prevent malaria infection in this population.
Funding
This study received no funding.
Ethical approval
Not required.
Data availability
The data is with the authors and available on request.
Funding declaration
None.
Ethic approval
Ethics approval was not required for the study as it is a secondary analysis of existing data that previously published.
Consent for publication
All authors gave consent for publication.
Availability of data and materials
All data are presented within the manuscript and are available by contacting the corresponding author.
CRediT authorship contribution statement
Prakasini Satapathy: Data curation, Conceptualization. Mahalaqua Nazli Khatib: Resources, Project administration. Shilpa Gaidhane: Validation, Supervision. Quazi Syed Zahiruddin: Writing – review & editing. Rakesh Kumar Sharma: Writing – original draft, Visualization, Validation. Sarvesh Rustagi: Resources, Methodology. Jumana M. Al-Jishi: Visualization, Validation. Hawra Albayat: Writing – review & editing. Mona A. Al Fares: Project administration, Methodology. Mohammed Garout: Validation, Supervision. Hayam A. Alrasheed: Software, Resources, Project administration. Maha F. Al-Subaie: Supervision, Resources. Ali A. Rabaan: Writing – review & editing, Conceptualization. Ranjit Sah: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors acknowledge the Nested-Knowledge, MN, USA for providing the access to the software.
Handling Editor: Patricia Schlagenhauf
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nmni.2024.101474.
Contributor Information
Prakasini Satapathy, Email: prakasini.satapathy@gmail.com.
Mahalaqua Nazli Khatib, Email: nazli.786@rediffmail.com.
Shilpa Gaidhane, Email: drshilpagaidhane@gmail.com.
Quazi Syed Zahiruddin, Email: zahirquazi@gmail.com.
Rakesh Kumar Sharma, Email: prochancellor@geu.ac.in.
Sarvesh Rustagi, Email: sarveshrustagi@uumail.in.
Jumana M. Al-Jishi, Email: dr.jmsj@gmail.com.
Hawra Albayat, Email: H.albayat@ksmc.med.sa.
Mona A. Al Fares, Email: maalfares@kau.edu.sa.
Mohammed Garout, Email: Magarout@uqu.edu.sa.
Hayam A. Alrasheed, Email: Haalrasheed@pnu.edu.sa.
Maha F. Al-Subaie, Email: maha7id@gmail.com.
Ali A. Rabaan, Email: arabaan@gmail.com.
Ranjit Sah, Email: ranjitsah57@gmail.com, ranjitsah@iom.edu.np.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Whittaker C., Slater H., Nash R., Bousema T., Drakeley C., Ghani A.C., et al. Global patterns of submicroscopic Plasmodium falciparum malaria infection: insights from a systematic review and meta-analysis of population surveys. The Lancet Microbe. 2021;2(8):e366–e374. doi: 10.1016/S2666-5247(21)00055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reddy V., Weiss D.J., Rozier J., Ter Kuile F.O., Dellicour S. Global estimates of the number of pregnancies at risk of malaria from 2007 to 2020: a demographic study. Lancet Global Health. 2023;11(1):e40–e47. doi: 10.1016/S2214-109X(22)00431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakken L., Iversen P.O. The impact of malaria during pregnancy on low birth weight in East-Africa: a topical review. Malar J. 2021;20(1):1–9. doi: 10.1186/s12936-021-03883-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sato S. Plasmodium—a brief introduction to the parasites causing human malaria and their basic biology. J Physiol Anthropol. 2021;40(1):1–13. doi: 10.1186/s40101-020-00251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Awadhi M., Ahmad S., Iqbal J. Current status and the epidemiology of malaria in the Middle East Region and beyond. Microorganisms. 2021;9(2):338. doi: 10.3390/microorganisms9020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White N.J. Severe malaria. Malar J. 2022;21(1):284. doi: 10.1186/s12936-022-04301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gontie G.B., Wolde H.F., Baraki A.G. Prevalence and associated factors of malaria among pregnant women in Sherkole district, Benishangul Gumuz regional state, West Ethiopia. BMC Infect Dis. 2020;20:1–8. doi: 10.1186/s12879-020-05289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahadzie-Soglie A., Addai-Mensah O., Abaka-Yawson A., Setroame A.M., Kwadzokpui P.K. Prevalence and risk factors of malaria and anaemia and the impact of preventive methods among pregnant women: a case study at the Akatsi South District in Ghana. PLoS One. 2022;17(7) doi: 10.1371/journal.pone.0271211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almaw A., Yimer M., Alemu M., Tegegne B. Prevalence of malaria and associated factors among symptomatic pregnant women attending antenatal care at three health centers in north-west Ethiopia. PLoS One. 2022;17(4) doi: 10.1371/journal.pone.0266477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chua C.L.L., Khoo S.K.M., Ong J.L.E., Ramireddi G.K., Yeo T.W., Teo A. Malaria in pregnancy: from placental infection to its abnormal development and damage. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.777343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naserrudin N.A., Lin P.Y.P., Monroe A., Culleton R., Baumann S.E., Sato S., et al. Exploring barriers to and facilitators of malaria prevention practices: a photovoice study with rural communities at risk to Plasmodium knowlesi malaria in Sabah, Malaysia. BMC Publ Health. 2023;23(1):1316. doi: 10.1186/s12889-023-16173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Towoju F.V. Manchester Metropolitan University; 2023. Exploring the role of socio-economic and cultural factors influencing the occurrence of VVF in Northern Nigeria. [Google Scholar]
- 13.Soni M., Shilpa D.M., Mirza Adil B. Exploring secure pathways: finding the most reliable malaria prophylaxis strategies for HIV-positive pregnant women. The Evidence. 2024;2(1) [Google Scholar]
- 14.Zakama A.K., Ozarslan N., Gaw S.L. Placental malaria. Current Tropical Medicine Reports. 2020;7:162–171. doi: 10.1007/s40475-020-00213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein J.A., Gallagher K., Beck C., Kumar R., Gernand A.D. Maternal-fetal inflammation in the placenta and the developmental origins of health and disease. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.531543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chua C.L., Hasang W., Rogerson S.J., Teo A. Poor birth outcomes in malaria in pregnancy: recent insights into mechanisms and prevention approaches. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.621382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fondjo L.A., Addai-Mensah O., Annani-Akollor M.E., Quarshie J.T., Boateng A.A., Assafuah S.E., et al. A multicenter study of the prevalence and risk factors of malaria and anemia among pregnant women at first antenatal care visit in Ghana. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0238077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ssentongo P., Ba D.M., Ssentongo A.E., Ericson J.E., Wang M., Liao D., et al. Associations of malaria, HIV, and coinfection, with anemia in pregnancy in sub-Saharan Africa: a population-based cross-sectional study. BMC Pregnancy Childbirth. 2020;20:1–11. doi: 10.1186/s12884-020-03064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nkwabong E., Mayane D.N., Meka E., Essiben F. Malaria in the third trimester and maternal‐perinatal outcome. Int J Gynecol Obstet. 2020;151(1):103–108. doi: 10.1002/ijgo.13261. [DOI] [PubMed] [Google Scholar]
- 20.Aguzie I. Pregnancy-associated malaria, challenges and prospects in sub-Saharan Africa. Clin Mother Child Health. 2018;15(1):1–8. [Google Scholar]
- 21.van Eijk A.M., Hill J., Noor A.M., Snow R.W., ter Kuile F.O. Prevalence of malaria infection in pregnant women compared with children for tracking malaria transmission in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Global Health. 2015;3(10):e617–e628. doi: 10.1016/S2214-109X(15)00049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisenberg S.-L., Krieger A.E. A comprehensive approach to optimizing malaria prevention in pregnant women: evaluating the efficacy, cost-effectiveness, and resistance of IPTp-SP and IPTp-DP. Glob Health Action. 2023;16(1) doi: 10.1080/16549716.2023.2231257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ippolito M.M., Moser K.A., Kabuya J.-B.B., Cunningham C., Juliano J.J. Antimalarial drug resistance and implications for the WHO global technical strategy. Current Epidemiology Reports. 2021;8:46–62. doi: 10.1007/s40471-021-00266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sudathip P., Saejeng A., Khantikul N., Thongrad T., Kitchakarn S., Sugaram R., et al. Progress and challenges of integrated drug efficacy surveillance for uncomplicated malaria in Thailand. Malar J. 2021;20(1):1–16. doi: 10.1186/s12936-021-03791-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page M.J., Moher D., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. bmj. 2021;372 doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shabil M., Yadav A., Shamim M.A., Ahmed M., Satapathy P., Zaidan A.A., et al. Prevalence of hepatitis B and C infections among HIV‐positive men who have sex with men: a systematic review and meta‐analysis. Health Science Reports. 2024;7(6) doi: 10.1002/hsr2.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shamim M.A., Gandhi A.P., Dwivedi P., Padhi B.K. How to perform meta-analysis in R: a simple yet comprehensive guide. The Evidence. 2023;1(1):93–113. [Google Scholar]
- 28.Quanquin N.M., Barres L.G., Aliyari S.R., Day N.T., Gerami H., Fisher S.J., et al. Gravidity-dependent associations between interferon response and birth weight in placental malaria. Malar J. 2020;19:1–8. doi: 10.1186/s12936-020-03351-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bater J., Lauer J.M., Ghosh S., Webb P., Agaba E., Bashaasha B., et al. Predictors of low birth weight and preterm birth in rural Uganda: findings from a birth cohort study. PLoS One. 2020;15(7) doi: 10.1371/journal.pone.0235626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nekaka R., Nteziyaremye J., Oboth P., Iramiot J.S., Wandabwa J. Malaria preventive practices and delivery outcomes: a cross-sectional study of parturient women in a tertiary hospital in Eastern Uganda. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0237407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alemayehu G.M., Chernet A.G., Dumga K.T. Determinants of child size at birth and associated maternal factor in gurage zone. J Reproduction Infertil. 2020;21(2):138. [PMC free article] [PubMed] [Google Scholar]
- 32.Kumlachew W., Tezera N., Endalamaw A. Below normal birth weight in the Northwest part of Ethiopia. BMC Res Notes. 2018;11(1):1–7. doi: 10.1186/s13104-018-3723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dombrowski J.G., de Souza R.M., Lima F.A., Bandeira C.L., Murillo O., de Sousa Costa D., et al. Association of malaria infection during pregnancy with head circumference of newborns in the Brazilian Amazon. JAMA Netw Open. 2019;2(5) doi: 10.1001/jamanetworkopen.2019.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dombrowski J.G., Souza RMd, Silva N.R.M., Barateiro A., Epiphanio S., Gonçalves L.A., et al. Malaria during pregnancy and newborn outcome in an unstable transmission area in Brazil: a population-based record linkage study. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0199415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dombrowski J.G., Barateiro A., Peixoto E.P.M., Barros ABCdS., Souza RMd, Clark T.G., et al. Adverse pregnancy outcomes are associated with Plasmodium vivax malaria in a prospective cohort of women from the Brazilian Amazon. PLoS Neglected Trop Dis. 2021;15(4) doi: 10.1371/journal.pntd.0009390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamga S.L.S., Ali I.M., Ngangnang G.R., Ulucesme M.C., Keptcheu L.T., Keming E.M., et al. Uptake of intermittent preventive treatment of malaria in pregnancy and risk factors for maternal anaemia and low birthweight among HIV-negative mothers in Dschang, West region of Cameroon: a cross sectional study. Malar J. 2024;23(1):6. doi: 10.1186/s12936-023-04816-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Megnekou R., Nana C.M.M., Djontu J.C., Bitye B.M.Z., Nana B.C., Zangue B.K.T., et al. Chemokine modulation in microscopic and submicroscopic Plasmodium falciparum malaria infection in women at delivery in Yaoundé, Cameroon. PLoS One. 2023;18(1) doi: 10.1371/journal.pone.0280615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lloyd Y.M., Fang R., Bobbili N., Vanda K., Ngati E., Sanchez-Quintero M.J., et al. Association of antibodies to VAR2CSA and merozoite antigens with pregnancy outcomes in women living in Yaoundé, Cameroon. Infect Immun. 2018;86(9) doi: 10.1128/iai.00166-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hussein H., Shamsipour M., Yunesian M., Hassanvand M.S., Agordoh P.D., Seidu M.A., et al. Prenatal malaria exposure and risk of adverse birth outcomes: a prospective cohort study of pregnant women in the Northern Region of Ghana. BMJ Open. 2022;12(8) doi: 10.1136/bmjopen-2021-058343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quinn A.K., Adjei I.A., Ayuurebobi K., Agyei O., Boamah-Kaali E.A., Burkart K., et al. Prenatal household air pollutant exposure is associated with reduced size and gestational age at birth among a cohort of Ghanaian infants. Environ Int. 2021;155 doi: 10.1016/j.envint.2021.106659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biteghe-Bi-Essone J.C., Imboumy-Limoukou R.K., Ekogha-Ovono J.J., Maghendji-Nzondo S., Sir-Ondo-Enguier P.N., Oyegue L.S., et al. Intermittent preventive treatment and malaria amongst pregnant women who give birth at the Centre Hospitalier Regional Paul Moukambi de Koula-Moutou in southeastern Gabon. Malar J. 2022;21(1):315. doi: 10.1186/s12936-022-04305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Honkpehedji Y.J., Adegbite B.R., Zinsou J.F., Dejon‐Agobé J.C., Edoa J.R., Zoleko Manego R., et al. Association of low birth weight and polyparasitic infection during pregnancy in Lambaréné, Gabon. Trop Med Int Health. 2021;26(8):973–981. doi: 10.1111/tmi.13591. [DOI] [PubMed] [Google Scholar]
- 43.Singh P.P., Bhandari S., Sharma R.K., Singh N., Bharti P.K. Association of angiopoietin dysregulation in placental malaria with adverse birth outcomes. Dis Markers. 2020;2020 doi: 10.1155/2020/6163487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Datta M., BiSwaS J., DaSGupta S., BanerJee K., Choudhury S., Sengupta S.K., et al. Comparative study on antenatal and perinatal outcome of vivax and falciparum malaria in a tertiary care hospital of Kolkata, India. J Clin Diagn Res: J Clin Diagn Res. 2017;11(1):QC01. doi: 10.7860/JCDR/2017/23051.9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh J., Soni D., Mishra D., Singh H., Bijesh S. Placental and neonatal outcome in maternal malaria. Indian Pediatr. 2014;51:285–288. doi: 10.1007/s13312-014-0402-3. [DOI] [PubMed] [Google Scholar]
- 46.Chawla S., Manu V. Malaria in pregnancy. Med J Armed Forces India. 2007;63(2):147–148. doi: 10.1016/S0377-1237(07)80060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rijken M.J., De Livera A.M., Lee S.J., Boel M.E., Rungwilailaekhiri S., Wiladphaingern J., et al. Quantifying low birth weight, preterm birth and small-for-gestational-age effects of malaria in pregnancy: a population cohort study. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0100247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luxemburger C., McGready R., Kham A., Morison L., Cho T., Chongsuphajaisiddhi T., et al. Effects of malaria during pregnancy on infant mortality in an area of low malaria transmission. Am J Epidemiol. 2001;154(5):459–465. doi: 10.1093/aje/154.5.459. [DOI] [PubMed] [Google Scholar]
- 49.Gaw S.L., Hromatka B.S., Ngeleza S., Buarpung S., Ozarslan N., Tshefu A., et al. Differential activation of fetal hofbauer cells in primigravidas is associated with decreased birth weight in symptomatic placental malaria. Malaria research and treatment. 2019;2019 doi: 10.1155/2019/1378174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feresu S.A., Harlow S.D., Woelk G.B. Risk factors for low birthweight in Zimbabwean women: a secondary data analysis. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0129705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ticconi C., Mapfumo M., Dorrucci M., Naha N., Tarira E., Pietropolli A., et al. Effect of maternal HIV and malaria infection on pregnancy and perinatal outcome in Zimbabwe. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2003;34(3):289–294. doi: 10.1097/00126334-200311010-00005. [DOI] [PubMed] [Google Scholar]
- 52.Weckman A.M., Conroy A.L., Madanitsa M., Gnaneswaran B., McDonald C.R., Kalilani-Phiri L., et al. Neurocognitive outcomes in Malawian children exposed to malaria during pregnancy: an observational birth cohort study. PLoS Med. 2021;18(9) doi: 10.1371/journal.pmed.1003701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mikomangwa W.P., Oms M., Aklillu E., Kamuhabwa A.A. Adverse birth outcomes among mothers who received intermittent preventive treatment with Sulphadoxine-Pyrimethamine in the low malaria transmission region. BMC Pregnancy Childbirth. 2019;19(1):1–11. doi: 10.1186/s12884-019-2397-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gavina K., Gnidehou S., Arango E., Hamel-Martineau C., Mitran C., Agudelo O., et al. Clinical outcomes of submicroscopic infections and correlates of protection of VAR2CSA antibodies in a longitudinal study of pregnant women in Colombia. Infect Immun. 2018;86(4) doi: 10.1128/iai.00797-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lingani M., Zango S.H., Valéa I., Somé G., Sanou M., Samadoulougou S.O., et al. Low birth weight and its associated risk factors in a rural health district of Burkina Faso: a cross sectional study. BMC Pregnancy Childbirth. 2022;22(1):1–8. doi: 10.1186/s12884-022-04554-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aribodor D.N., Nwaorgu O.C., Eneanya C.I., Okoli I., Pukkila-Worley R., Etaga H.O. Association of low birth weight and placental malarial infection in Nigeria. The Journal of Infection in Developing Countries. 2009;3(8):620–623. doi: 10.3855/jidc.554. [DOI] [PubMed] [Google Scholar]
- 57.Bassey G., Nyengidiki T., John C. Prevalence of placenta Plasmodium parasitemia and pregnancy outcome in asymptomatic patients at delivery in a University Teaching Hospital in Nigeria. Niger J Clin Pract. 2015;18(1):27–32. doi: 10.4103/1119-3077.146975. [DOI] [PubMed] [Google Scholar]
- 58.Thompson J.M., Eick S.M., Dailey C., Dale A.P., Mehta M., Nair A., et al. Relationship between pregnancy-associated malaria and adverse pregnancy outcomes: a systematic review and meta-analysis. J Trop Pediatr. 2020;66(3):327–338. doi: 10.1093/tropej/fmz068. [DOI] [PubMed] [Google Scholar]
- 59.Das J.K., Lakhani S., Rahman A.R., Siddiqui F., Padhani Z.A., Rashid Z., et al. Malaria in pregnancy: meta-analyses of prevalence and associated complications. Epidemiol Infect. 2024;152:e39. doi: 10.1017/S0950268824000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Danwang C., Bigna J.J., Nzalie R.N.T., Robert A. Epidemiology of clinical congenital and neonatal malaria in endemic settings: a systematic review and meta-analysis. Malar J. 2020;19(1):312. doi: 10.1186/s12936-020-03373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brabin B.J., Romagosa C., Abdelgalil S., Menéndez C., Verhoeff F.H., McGready R., et al. The sick placenta-the role of malaria. Placenta. 2004;25(5):359–378. doi: 10.1016/j.placenta.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 62.Desai M., Ter Kuile F.O., Nosten F., McGready R., Asamoa K., Brabin B., et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7(2):93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 63.González-Sanz M., Berzosa P., Norman F.F. Updates on malaria epidemiology and prevention strategies. Curr Infect Dis Rep. 2023;25(7):131–139. doi: 10.1007/s11908-023-00805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ranjha R., Sharma A. Forest malaria: the prevailing obstacle for malaria control and elimination in India. BMJ Glob Health. 2021;6(5) doi: 10.1136/bmjgh-2021-005391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data is with the authors and available on request.
All data are presented within the manuscript and are available by contacting the corresponding author.