Abstract
Background
Ambient ultraviolet radiation (UVR) has been found to have a greater cardioprotective effect than previously believed. This study aimed to quantitatively measure the role of UVR in protecting against the progression of cardiovascular disease (CVD) in general on a global and regional scale.
Methods
Population‐level data on UVR, CVD incidence, aging, economic affluence, CVD genetic background (indexed with the Biological State Index, Ibs), obesity prevalence, and urbanization were collected and analysed. The correlation between UVR and CVD was examined using bivariate correlations, partial correlation, and stepwise multiple linear regression. Countries were grouped to investigate regional correlations between UVR and CVD, and Fisher's r‐to‐z transformation was used to compare correlation coefficients.
Results
UVR showed a significant inverse correlation with CVD incidence rates in bivariate correlation analyses globally (r = − 0.775 and r = − 0.760, p < 0.001), as well as within high‐income (r = −0.704, p < 0.001) and low‐ and middle‐income countries (LMIC) (r = −0.851, p < 0.001). These correlations remained significant even after controlling for confounding variables (r = −0.689 to −0.812, p < 0.001). In stepwise regression models, UVR was found to be the most significant predictor of CVD incidence. The inverse correlation between UVR and CVD was stronger in LMICs compared to high‐income countries (z = −1.96, p < 0.050).
Conclusions
Low ambient UVR may be a significant risk factor for the progression of CVD worldwide. The protective effect of UVR appears to be stronger in LMICs than in high‐income countries, suggesting a greater impact of UVR on CVD prevention in these regions. These findings emphasize the need for further research into the mechanisms underlying the cardioprotective effects of UVR and the development of public health strategies to mitigate CVD risk associated with low UVR exposure.
Keywords: ambient, cardiovascular disease (CVD), latitude, ultraviolet radiation, vitamin D
1. INTRODUCTION
Cardiovascular diseases (CVDs) are a wide range of disorders that affect the heart and blood vessels. 1 They are the leading cause of global disease burden, 1 with 55.5 million people diagnosed in 2019 alone, accounting for approximately one‐third of all deaths worldwide. 2 This prevalence highlights the significant challenge that CVDs pose to global health, affecting both developed and developing nations.
Preventative measures targeting behavioral risk factors such as unhealthy diet, obesity, and physical inactivity are crucial for reducing the incidence of CVD. 3 The risk factors for CVD are complex, involving a combination of cardiometabolic, behavioral, environmental, and social factors. 4 Understanding the interplay between these factors is essential for understanding the complex causes of CVD. 5 , 6 Cardiometabolic factors like hypertension and dyslipidaemia directly impact cardiovascular health, while behavioral factors such as diet, physical activity, and tobacco use also play important roles. 4 Environmental factors, including air quality and exposure to pollutants, further contribute to the risk, and social determinants such as socioeconomic status and healthcare access complicate the picture. 5 A comprehensive understanding of these factors is necessary to design targeted strategies for preventing and managing CVD in diverse populations.
The relationship between sunlight exposure, specifically ultraviolet radiation (UVR), and cardiovascular health has been the subject of debate in observational studies. 7 , 8 Despite the known negative effects of UVR, historical records spanning over 6,000 years suggest that sunlight has been used therapeutically for cardiocirculatory and cardiovascular disorders. 9 Natural heliotherapy is mentioned in the works of Hippocrates, ancient Roman health practices, and the practices of Islamic physicians in ancient Iran. 10 Ambient sunlight plays a vital role in the production of vitamin D, which is essential for human health. 7 When UVB light converts provitamin D3 into stable vitamin D3, it has a significant impact on our well‐being. The amount of UVR radiation we receive varies based on factors such as seasonal changes, skin pigmentation, and distance from the equator. 11 Interestingly, there is a correlation between these variations in UVR radiation and changes in blood pressure.
Studies have shown that there is an inverse relationship between exposure to UVR radiation and both blood pressure and the incidence of CVD. 7 , 12 As we move further away from the equator, the levels of ambient UVR radiation decrease, coinciding with an increase in the risk of CVD. In ecological studies, researchers have observed seasonal variations in the prevalence of conditions like CVD, hypertension, and metabolic syndrome. 13 Rates of these conditions tend to be lower in the summer but higher in the winter. 13
This intricates connection between ambient UVR exposure, vitamin D levels, and the risk of CVD highlights the importance of understanding these factors so that we can develop effective preventive strategies. 14 , 15 Previous observational studies have shown an association between UVR radiation and CVD, but it's important to note that these studies may have been influenced by other factors. We need a more comprehensive analysis to truly understand this relationship further. 8
Following previous observational studies with confounding residue, the UVR‐CVD relationship at population‐level can be depicted through the graph below 16 :
In the graph provided in Figure 1, we can see that UVR exposure statistically explains 63.11% of the variance in CVD, suggesting that it may play a main protective role against CVD‐related health issues. However, we need to approach these findings with caution, 16 as we must consider other established factors like cardiometabolic, behavioral, environmental, and social risks that also contribute significantly to CVD.
Figure 1.
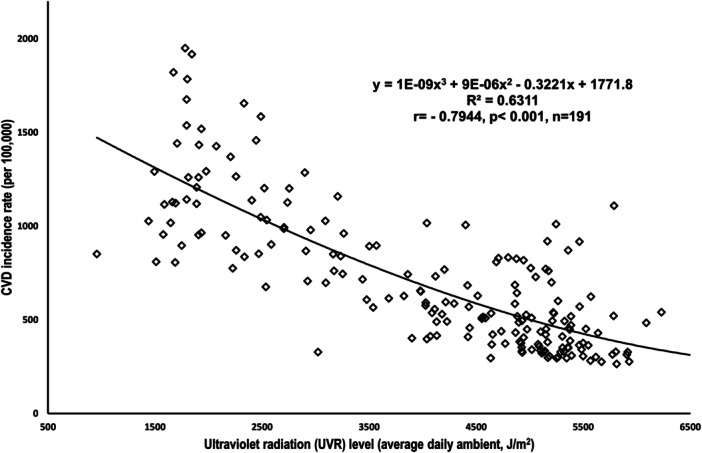
Plot to show the relationship between UV radiation and CVD incidence. Data source & definition: UV Radiation, expressed as the average daily ambient ultraviolet radiation level (in J/m2), the World Health Organization; CVD incidence, cardiovascular disease (CVD) incidence rate, the number of new cases per 100,000, the Institute for Health Metrics and Evaluation. Raw data were incorporated into the Excel for the generating the scatterplots.
Given the biases that have been observed in previous research, the aim of this study is to determine the independent influence of UVR exposure on the pathogenesis of CVD at a population level. To address potential biases in the UVR‐CVD relationship, this study first quantified the global predictive capacity of UVR exposure for CVD incidence. Then, it conducted an analysis to minimize the impact of confounding factors such as economic affluence, aging, genetic predisposition to CVD, obesity, and urbanization on the UVR‐CVD relationship. The ultimate result of this study is to reveal that UVR exposure is a significant and independent predictor for CVD incidence worldwide.
2. MATERIALS AND METHODS
2.1. Data collection and selection
This quantitative study gathered data from reputable international organizations and focused on country‐specific information. A list of WHO member countries was compiled to match the seven variables considered in the study. Throughout this article, the terms “population” and “country” are used interchangeably to refer to a geographic region or territory. 17 This approach is adoped for ease of writing and to enhance readability.
-
1.
The independent variable, the average daily ambient ultraviolet radiation (UVR) level, which encompasses solar radiation in the 280–400 nm range, was obtained as the independent variable from the WHO Global Health Observatory (GHO). 18 The UVR level is quantified in joules per square meter (J/m²). 18 In this study, population‐level exposure is represented by the annual ambient erythemal weighted UVR, which takes into account the biological effects on human skin by applying the Erythema Action Spectrum. This measure is expressed in joules effective per square meter (J/m²). 18 It is calculated either from satellite data or by using a proxy such as latitudinal position. 18
-
2.
The dependent variable, the most recent CVD incidence rate (new cases per 100,000 population), was collected from the Institute for Health Metrics and Evaluation (IHME) at the University of Washington. 2 In addition to exploring the predictive role of UVR exposure on total CVDs, this study also examined its influence on specific types of CVDs identified in previous research. The IHME provided incidence rates for all CVDs and 14 distinct types across various age groups. These types include ischemic heart disease, total stroke, ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, peripheral artery disease, atrial fibrillation and flutter, rheumatic heart disease, non‐rheumatic valvular heart disease (including non‐rheumatic degenerative mitral valve disease and non‐rheumatic calcific aortic valve disease), cardiomyopathy and myocarditis (including myocarditis), and endocarditis. All available data were integrated into the study for comprehensive analysis.
In this study, we suggest that UVR generally helps protect against the development of CVDs. We have also chosen to explore the statistical predictive role of UVR because different types of CVDs have similar underlying mechanisms. While focusing on specific types of CVD could introduce bias, investigating the relationship between UVR and each individual CVD can still offer valuable evidence to support our main argument.
Previous studies have suggested that the relationship between UVR and CVD may be influenced by other factors, 16 so it is important to account for these potential confounders. This study included the following five variables that are recognized as major risk factors for CVD to isolate the independent effect of UVR exposure:
-
1.
Aging, as indexed by life expectancy at age 65 years, was obtained from the United Nations. 19 Age has been independently linked to the deterioration of cardiovascular health, significantly affecting the heart and arterial system. 20 , 21
-
2.
Gross domestic product (GDP), converted to international dollars using purchasing power parity rates (GDP PPP), was extracted from the World Bank databank. 22 Economic affluence impacts cardiovascular health through biological, behavioral, and psychosocial risk factors. 23 It is also associated with education, employment, life expectancy, and access to healthcare, which all influence the accuracy of CVD diagnosis. 24
-
3.
The level of genetic background accumulation of CVD was measured using the Biological State Index (Ibs), which ranges from 0 to 1.0. Higher Ibs values indicate reduced natural selection and have been associated with the accumulation of harmful genes/mutations [54]. 25 These genes/mutations are linked to conditions such as cancer, obesity, and type 1 diabetes. Including Ibs in the analysis helps address the impact of genetic predisposition on the UVR‐CVD correlation.
-
4.
The prevalence of obesity, defined as the percentage of the population aged 18+ with a BMI ≥ 30 kg/m², was obtained from the WHO Global Health Observatory. 26 Obesity increases the risk of CVD through factors such as atherosclerosis and comorbidities like hypertension, diabetes, and dyslipidaemia. 27 , 28 , 29 It poses a multifactorial health challenge in terms of CVD prognosis. 27
-
5.
Urbanization, measured as the percentage of the population living in urban areas, was gathered from the World Bank. 22 Urbanization has been linked to CVD morbidity and mortality, and recently, it has also been associated with vitamin D deficiency among CVD patients. 30 , 31 However, urban residents generally have better access to medical services, which aids in disease detection.
All of the above variables were extracted and matched with the WHO member list before being saved in Microsoft Excel® 2016 for further analysis. Each country was treated as an individual research subject, and the number of WHO member countries included for each variable may vary due to differences in data reporting.
2.2. Statistical analyses
An ecological study was conducted to examine the relationship between UVR exposure and the incidence of CVD at the population level. 32 , 33 , 34 , 35 , 36 , 37 To ensure accurate findings, several strategies were implemented: (1) different data analysis models were used for validation; (2) all seven variables were log‐transformed to reduce homoscedasticity; and (3) the correlation between family size and CVD incidence rate was assessed globally and regionally.
-
1.
Bivariate Correlations: Pearson's and nonparametric analyses were performed to determine the strength and direction of the associations between UVR exposure and the incidence rates of total CVDs as well as 14 specific types.
-
2.
Partial Correlation Analysis: Using the Pearson moment‐product approach, partial correlation was used to examine the independent relationships between UVR exposure and each of the 15 CVD incidence rates. This analysis considered confounding factors such as aging, GDP PPP, BMI, obesity, and urbanization to refine the examination of these relationships.
-
3.
Independent Sample t‐test: This test was conducted to compare the means of each CVD variable between countries with higher and lower UVR levels. Countries were divided into two groups based on UVR exposure, using a cut point of 4420.5 J/m², which is the world average published by IHME. The hypothesis was that significantly different UVR exposure may lead to significantly different CVD incidence rates worldwide, as well as within high‐ and upper‐middle‐income countries (HUIC) and low‐ and middle‐income countries (LMIC).
-
4.
Standard Multiple Linear Regression (stepwise): This analysis was used to identify variables that significantly predict CVD incidence rate. The independent variables included UVR exposure, aging, GDP PPP, BMI, obesity, and urbanization. A total of 15 CVD incidence variables were entered as dependent variables.
-
5.
Pearson's r Correlation: To explore regional associations between UVR and CVD incidence, countries were categorized based on different criteria:
-
–
World Bank Income Classifications: High‐income, upper‐middle‐income, low‐middle‐income, and low‐income. High‐income countries formed one grouping, while low‐ and middle‐income countries were combined into another (LMIC) as a new grouping. Pearson's r correlations were examined between UVR and CVD incidence in both groupings, and Fisher's r‐to‐z transformation was used to compare correlations.
-
–
United Nations Classifications: Developed versus developing countries. Fisher's r‐to‐z transformation was used to compare correlation coefficients between UVR and CVD incidence in developed versus developing countries.
-
–
Geographic and Socioeconomic Groupings: Seven groupings were analysed based on geographic distributions, per capita GDP levels, and cultural backgrounds: Asia Cooperation Dialog (ACD), Asia‐Pacific Economic Cooperation (APEC), the Arab World, countries with English as the official language, Latin America, Latin America and the Caribbean (LAC), and the Organization for Economic Co‐operation and Development (OECD).
SPSS v. 28 (SPSS Inc.) and Microsoft Excel 2016® were used for data analysis. The significance level was set at 0.05, with additional reporting at 0.01 and 0.001. The criteria for stepwise multiple linear regression analysis were a probability of F to enter ≤0.05 and a probability of F to remove ≥0.10.
3. RESULTS
Table 1 displays significant and consistent correlations between UVR and the incidence of CVD worldwide. These correlations are shown through both Pearson's r (r = −0.775, p < 0.001) and nonparametric analyses (r = −0.760, p < 0.001). The correlations hold true for both high‐income countries (HIC: r = −0.704, p < 0.001) and low‐ to middle‐income countries (LMIC: r = −0.851, p < 0.001). Even when controlling for five confounding variables in partial correlation analysis, the strong negative correlations persist (r = −0.689, −0.707, and −0.812, respectively, all p < 0.001), indicating that nations with higher UVR tend to have lower incidence rates of CVD, regardless of these factors.
Table 1.
Bivariate (Pearson's r, nonparametric ρ) and partial correlation analyses to examine the relationships between ultraviolet radiation exposure and cardiovascular disease incidence rates (total and 13 types).
| All countries, n = 191 | High‐ and Upper‐Income Countries (HUIC), n = 113 | Low‐ and Middle‐Income Countries (LMIC), n = 78 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pearson's r | Nonparametric ρ | Partial correlation r | Pearson's r | Nonparametric ρ | Partial correlation r | Pearson's r | Nonparametric ρ | Partial correlation r | ||||||||||
| r | Sig. p | ρ | Sig. p | r | Sig. p | r | Sig. p | ρ | p | r | Sig. p | r | Sig. p | ρ | n | r | Sig. p | |
| Cardiovascular diseases, total | −0.775 | <0.001 | −0.760 | <0.001 | −0.689 | <0.001 | −0.704 | <0.001 | −0.700 | <0.001 | −0.707 | <0.001 | −0.851 | <0.001 | −0.679 | <0.001 | −0.812 | <0.001 |
| Atrial fibrillation and flutter | −0.764 | <0.001 | −0.754 | <0.001 | −0.648 | <0.001 | −0.755 | <0.001 | −0.738 | <0.001 | −0.729 | <0.001 | −0.707 | <0.001 | −0.631 | <0.001 | −0.505 | <0.001 |
| Cardiomyopathy and myocarditis | −0.764 | <0.001 | −0.659 | <0.001 | −0.636 | <0.001 | −0.727 | <0.001 | −0.695 | <0.001 | −0.706 | <0.001 | −0.669 | <0.001 | −0.465 | <0.001 | −0.448 | <0.001 |
| Endocarditis | −0.502 | <0.001 | −0.473 | <0.001 | −0.083 | 0.298 | −0.366 | <0.001 | −0.361 | <0.001 | −0.146 | 0.165 | −0.291 | <0.010 | −0.144 | 0.209 | 0.055 | 0.668 |
| Intracerebral hemorrhage | 0.153 | <0.050 | 0.143 | <0.050 | −0.155 | <0.050 | .206 | <0.050 | 0.231 | <0.050 | −0.278 | <0.010 | −0.269 | <0.050 | −0.243 | <0.050 | −0.191 | 0.130 |
| Ischemic heart disease | −0.647 | <0.001 | −0.657 | <0.001 | −0.517 | <0.001 | −0.507 | <0.001 | −0.469 | <0.001 | −0.479 | <0.001 | −0.785 | <0.001 | −0.670 | <0.001 | −0.769 | <0.001 |
| Ischemic stroke | −0.596 | <0.001 | −0.574 | <0.001 | −0.447 | <0.001 | −0.477 | <0.001 | −0.418 | <0.001 | −0.603 | <0.001 | −0.613 | <0.001 | −0.406 | <0.001 | −0.385 | <0.010 |
| Myocarditis | −0.764 | <0.001 | −0.659 | <0.001 | −0.636 | <0.001 | −0.727 | <0.001 | −0.695 | <0.001 | −0.706 | <0.001 | −0.669 | <0.001 | −0.465 | <0.001 | −0.448 | <0.001 |
| Nonrheumatic calcific aortic valve disease | −0.751 | <0.001 | −0.711 | <0.001 | −0.587 | <0.001 | −0.731 | <0.001 | −0.719 | <0.001 | −0.608 | <0.001 | −0.723 | <0.001 | −0.561 | <0.001 | −0.628 | <0.001 |
| Nonrheumatic degenerative mitral valve disease | −0.684 | <0.001 | −0.677 | <0.001 | −0.531 | <0.001 | −0.562 | <0.001 | −0.551 | <0.001 | −0.514 | <0.001 | −0.811 | <0.001 | −0.593 | <0.001 | −0.723 | <0.001 |
| Nonrheumatic valvular heart disease | −0.791 | <0.001 | −0.755 | <0.001 | −0.651 | <0.001 | −0.742 | <0.001 | −0.729 | <0.001 | −0.643 | <0.001 | −0.844 | <0.001 | −0.625 | <0.001 | −0.779 | <0.001 |
| Peripheral artery disease | −0.756 | <0.001 | −0.711 | <0.001 | −0.628 | <0.001 | −0.737 | <0.001 | −0.718 | <0.001 | −0.727 | <0.001 | −0.677 | <0.001 | −0.575 | <0.001 | −0.396 | <0.001 |
| Rheumatic heart disease | 0.585 | <0.001 | 0.601 | <0.001 | 0.261 | <0.001 | 0.443 | <0.001 | 0.431 | <0.001 | 0.166 | 0.115 | 0.603 | <0.001 | 0.520 | <0.001 | 0.374 | <0.010 |
| Stroke | −0.508 | <0.001 | −0.486 | <0.001 | −0.460 | <0.001 | −0.358 | <0.001 | −0.273 | <0.010 | −0.607 | <0.001 | −0.636 | <0.001 | −0.484 | <0.001 | −0.428 | <0.001 |
| Subarachnoid hemorrhage | −0.633 | <0.001 | −0.656 | <0.001 | −0.441 | <0.001 | −0.526 | <0.001 | −0.579 | <0.001 | −0.507 | <0.001 | −0.654 | <0.001 | −0.536 | <0.001 | −0.490 | <0.001 |
| Ageing e(65) | −0.560 | <0.001 | −0.550 | <0.001 | ‐ | ‐ | −0.443 | <0.001 | −0.455 | <0.001 | ‐ | ‐ | −0.375 | <0.001 | −0.315 | <0.010 | ‐ | ‐ |
| GDP PPP | −0.612 | <0.001 | −0.629 | <0.001 | ‐ | ‐ | −0.532 | <0.001 | −0.541 | <0.001 | ‐ | ‐ | −0.574 | <0.001 | −0.439 | <0.001 | ‐ | ‐ |
| Ibs | −0.467 | <0.001 | −0.630 | <0.001 | ‐ | ‐ | −0.329 | <0.001 | −0.568 | <0.001 | ‐ | ‐ | −0.489 | <0.001 | −0.497 | <0.001 | ‐ | ‐ |
| Obesity % | −0.294 | <0.001 | −0.329 | <0.001 | ‐ | ‐ | −0.008 | 0.939 | 0.029 | 0.767 | ‐ | ‐ | −0.231 | <0.050 | −0.239 | <0.050 | ‐ | ‐ |
| Urbanization | −0.439 | <0.001 | −0.466 | <0.001 | ‐ | ‐ | −0.323 | <0.001 | −0.292 | <0.010 | ‐ | ‐ | −0.253 | <0.050 | −0.163 | 0.156 | ‐ | ‐ |
Note: Significance levels: *p < 0.05, ***p < 0.01, ***p < 0.001. Control variable: Data source & definition: Ultraviolet Radiation (UVR), expressed as the average daily ambient ultraviolet radiation level (in J/m2), the World Health Organization; Cardiovascular disease (CVD) incidence rate, the number of newly diagnosed cases per 100,000 in 2019, the Institute for Health Metrics and Evaluation; Ageing indexed with life expectancy at 65 year old in 2014, United Nations; Per capita GDP PPP, measured with the per capita purchasing power parity (PPP) value of all final goods and services produced within a territory in 2019, the World Bank; Ibs indexing the detrimental CVD genetic background accumulation in the past decades, downloaded from previous publications. 33 Obesity prevalence, measured with the percentage of population aged 18+ with BMI equal to or over 30 kg/m2 in 2014, the World Health Organization. Urbanization, measured with the percentage of population living in urban area in 2019, the World Bank. All the data were log‐transformed for correlation analysis in SPSS v 28.
Regarding specific types of CVD, Table 1 demonstrates moderate to strong and statistically significant inverse relationships between UVR and each of the 13 types examined, except for intracerebral hemorrhage and rheumatic heart disease.
Overall, this bivariate correlation analysis highlights moderate to strong inverse correlations between UVR and incidence rates of CVD. These correlations remain statistically significant even after considering potential confounders such as aging, GDP per capita (PPP), obesity, smoking prevalence (Ibs), and urbanization (Table 1). This confirms the inclusion of these variables as controls when assessing the association between UVR and CVD incidence rates.
Table 2 shows that when using a cut‐off of 4420.5 J/m² (the global median for ultraviolet radiation), independent sample t‐tests revealed significant differences in mean values between country groups exposed to higher versus lower levels of ultraviolet radiation worldwide (t = 21.074, p < 0.001). This was true for both high‐ and low‐ and middle‐income ountries (HUIC: t = 15.140, p < 0.001; LMIC: t = 13.136, p < 0.001). Interestingly, countries with higher ultraviolet radiation levels above the cut‐off point had significantly lower overall incidence rates of CVD compared to those with lower ultraviolet radiation worldwide (t = −11.921, p < 0.010). This trend was observed within both HUIC (t = −7.373, p < 0.010) and LMIC (t = −7.618, p < 0.010). However, this disparity was not observed in cases of intracerebral hemorrhage and rheumatic heart disease. The findings presented in Table 2 suggest that variations in ultraviolet radiation exposure may significantly influence the incidence rates of different types of cardiovascular disease.
Table 2.
Independent samples t‐test to compare the differences between the means in two country groups with the cut point of 4420.5 J/m2(median of ultraviolet radiations).
|
All countries, n = 191, cut point 4420.5 |
High‐ and upper‐income Countries (HUIC), n = 113, cut point 4420.5 |
Low‐ and middle‐income Countries (LMIC), n = 68, cut point 4420.5 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UVR | UVR | UVR | ||||||||||
| Mean >4420.5 | Mean <4420.5 | Mean difference, t | Sig. p | Mean > 4420.5 | Mean <4420.5 | Mean difference t | Sig. p | Mean >4420.5 | Mean (<4420.5) | Mean difference t | Sig. p | |
| UVR | 5220.52 | 2791.07 | 21.074 | <0.01 | 5185.66 | 2609.77 | 15.140 | <0.01 | 5247.44 | 3386.76 | 13.136 | <0.010 |
| CVD incidence rate | CVD incidence rate | CVD incidence rate | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean > 4420.5, | Mean <4420.5 | Mean difference t | Sig. p | Mean > 4420.5 | Mean <4420.5 | Mean difference t | Sig. p | Mean >4420.5 | Mean (<4420.5) | Mean difference t | Sig. p | |
| Cardiovascular diseases, total | 479.51 | 973.68 | −11.921 | <0.001 | 603.72 | 1031.81 | −7.373 | <0.001 | 383.63 | 782.68 | −7.618 | <0.001 |
| Atrial fibrillation and flutter | 26.82 | 82.02 | −11.483 | <0.001 | 37.38 | 91.85 | −7.973 | <0.001 | 18.66 | 49.73 | −5.984 | <0.001 |
| Cardiomyopathy and myocarditis | 12.42 | 18.60 | −10.006 | <0.001 | 14.04 | 20.19 | −7.329 | <0.001 | 11.17 | 13.38 | −3.866 | <0.001 |
| Endocarditis | 12.28 | 19.22 | −5.448 | <0.001 | 17.47 | 22.03 | −2.572 | <0.050 | 8.28 | 10.00 | −2.082 | <0.050 |
| Intracerebral hemorrhage | 43.23 | 38.08 | 1.497 | 0.136 | 44.04 | 34.09 | 2.274 | <0.050 | 42.60 | 51.17 | −1.385 | 0.170 |
| Ischemic heart disease | 172.70 | 424.43 | −9.736 | <0.001 | 249.20 | 433.21 | −4.894 | <0.001 | 113.64 | 395.56 | −8.389 | <0.001 |
| Ischemic stroke | 62.89 | 113.28 | −7.365 | <0.001 | 78.88 | 120.07 | −3.985 | <0.001 | 50.55 | 90.97 | −5.257 | <0.001 |
| Myocarditis | 12.42 | 18.60 | −10.006 | <0.001 | 14.04 | 20.19 | −7.329 | <0.001 | 11.17 | 13.38 | −3.866 | <0.001 |
| Nonrheumatic calcific aortic valve disease | 1.63 | 20.42 | −7.923 | <0.001 | 3.21 | 24.35 | −6.111 | <0.001 | 0.40 | 7.50 | −2.406 | <0.050 |
| Nonrheumatic degenerative mitral valve disease | 1.88 | 19.88 | −6.417 | <0.001 | 3.03 | 22.82 | −4.274 | <0.001 | 0.99 | 10.21 | −4.895 | <0.001 |
| Nonrheumatic valvular heart disease | 3.51 | 40.29 | −8.174 | <0.001 | 6.24 | 47.17 | −5.923 | <0.001 | 1.39 | 17.70 | −3.506 | <0.001 |
| Peripheral artery disease | 78.44 | 198.14 | −10.738 | <0.001 | 106.78 | 226.76 | −7.903 | <0.001 | 56.57 | 104.10 | −2.105 | <0.050 |
| Rheumatic heart disease | 57.03 | 19.28 | 9.946 | <0.001 | 35.77 | 14.17 | 5.834 | <0.001 | 73.43 | 36.05 | −4.582 | <0.001 |
| Stroke | 116.32 | 171.70 | −5.982 | <0.001 | 136.83 | 176.42 | −2.875 | <0.010 | 100.48 | 156.17 | 5.968 | <0.001 |
| Subarachnoid hemorrhage | 10.20 | 20.34 | −6.831 | <0.001 | 13.92 | 22.26 | −3.639 | <0.001 | 7.33 | 14.03 | −4.667 | <0.001 |
Note: Significance levels: *p < 0.05, ***p < 0.01, ***p < 0.001. Data source and definition: Ultraviolet radiation (UVR), expressed as the average daily ambient ultraviolet radiation level (in J/m2), the World Health Organization; Cardiovascular disease (CVD) incidence rate, the number of newly diagnosed cases per 100,000 in 2019, the Institute for Health Metrics and Evaluation. All the data were not log‐transformed for mean compassion in SPSS v 28.
Table 3 presents the results of the stepwise linear regression model, which highlights UVR as the most significant predictor of total CVD incidence globally. This finding holds true for both high‐income and low‐ and middle‐income countries, with adjusted R2 values of 0.629, 0.519, and 0.748 respectively. The analysis shows a strong inverse relationship between UVR and CVD incidence rates worldwide, as well as in both high‐income and low‐ and middle‐income countries. The beta coefficients for these relationships are −0.607, −0.716, and −0.709 respectively, with p‐values less than 0.010.
Table 3.
Stepwise multiple linear regression to identify the significant predictors of cardiovascular disease incidence rates (cardiovascular disease incidence rates [total and 14 types]).
| All Countries, n = 191 | High‐ and Upper‐middle Income Countries (HUIC), n = 113 | Low‐ and Low‐middle Income Countries (LMIC), n = 68 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Independent predictor | Adjusted R2 | Beta | Sig. p | Independent predictor | Adjusted R2 | Beta | Sig. p | Independent predictor | Adjusted R2 | Beta | Sig. p | |
| Cardiovascular diseases, total | UV radiation | 0.629 | −0.607 | <0.001 | UV radiation | 0.519 | −0.716 | <0.001 | UV radiation | 0.748 | −0.709 | <0.001 |
| Ibs | 0.742 | 0.387 | <0.001 | Urbanization | 0.561 | −0.233 | <0.001 | Ibs | 0.829 | 0.327 | <0.001 | |
| Ageing e(65) | Insignificant | Ibs | 0.605 | 0.231 | <0.001 | Ageing e(65) | Insignificant | |||||
| GDP PPP | Insignificant | Ageing e(65) | Insignificant | GDP PPP | Insignificant | |||||||
| Obesity % | Insignificant | GDP PPP | Insignificant | Obesity % | Insignificant | |||||||
| Urbanization | Insignificant | Obesity % | Insignificant | Urbanization | Insignificant | |||||||
| Atrial fibrillation and flutter | UV radiation | 0.597 | −0.559 | <0.001 | UV radiation | 0.577 | −0.734 | <0.001 | GDP PPP | 0.485 | 0.243 | <0.050 |
| Ibs | 0.747 | 0.443 | <0.001 | Ibs | 0.628 | 0.255 | <0.001 | UV radiation | 0.605 | −0.384 | <0.001 | |
| Ageing e(65) | Insignificant | Urbanization | 0.658 | −0.193 | <0.010 | Ibs | 0.664 | 0.347 | <0.001 | |||
| GDP PPP | Insignificant | Ageing e(65) | Insignificant | Ageing e(65) | Insignificant | |||||||
| Obesity % | Insignificant | GDP PPP | Insignificant | Obesity % | Insignificant | |||||||
| Urbanization | Insignificant | Obesity % | Insignificant | Urbanization | Insignificant | |||||||
| Cardiomyopathy and myocarditis | UV radiation | 0.589 | −0.505 | <0.001 | UV radiation | 0.523 | −0.617 | <0.001 | GDP PPP | 0.442 | 0.293 | <0.050 |
| Ageing e(65) | 0.759 | 0.444 | <0.001 | Ageing e(65) | 0.658 | 0.401 | <0.001 | UV radiation | 0.535 | −0.369 | <0.001 | |
| Obesity % | 0.766 | −0.138 | <0.010 | Obesity % | 0.693 | −0.177 | <0.010 | Ibs | 0.573 | 0.335 | <0.010 | |
| Ibs | 0.772 | 0.138 | <0.050 | Urbanization | 0.706 | −0.136 | <0.050 | Obesity % | 0.607 | −0.210 | <0.050 | |
| GDP PPP | Insignificant | GDP PPP | Insignificant | Ageing e(65) | Insignificant | |||||||
| Urbanization | Insignificant | Ibs | Insignificant | Urbanization | Insignificant | |||||||
| Endocarditis | Ageing e(65) | 0.616 | 0.697 | <0.001 | Ageing e(65) | 0.440 | 5.325 | <0.001 | GDP PPP | 0.216 | 0.374 | <0.010 |
| Obesity % | 0.657 | 0.224 | <0.001 | Ibs | 0.485 | 3.054 | 0.003 | Urbanization | 0.253 | 0.241 | <0.050 | |
| UV radiation | Insignificant | UV radiation | Insignificant | UV radiation | Insignificant | |||||||
| GDP PPP | Insignificant | GDP PPP | Insignificant | Ageing e(65) | Insignificant | |||||||
| Ibs | Insignificant | Obesity % | Insignificant | Ibs | Insignificant | |||||||
| Urbanization | Insignificant | Urbanization | Insignificant | Obesity % | Insignificant | |||||||
| Intracerebral hemorrhage | Ageing e(65) | 0.203 | −0.818 | <0.001 | Ageing e(65) | 0.307 | −0.630 | <0.001 | UV radiation | Insignificant | ||
| Ibs | 0.312 | 0.601 | <0.001 | Urbanization | 0.384 | −0.205 | 0.019 | Ageing e(65) | Insignificant | |||
| Obesity % | 0.338 | −0.203 | <0.010 | Ibs | 0.416 | 0.305 | 0.002 | GDP PPP | Insignificant | |||
| UV radiation | Insignificant | Obesity % | 0.459 | −0.262 | 0.001 | Ibs | Insignificant | |||||
| GDP PPP | Insignificant | UV radiation | 0.478 | −0.238 | 0.007 | Obesity % | Insignificant | |||||
| Urbanization | Insignificant | GDP PPP | 0.500 | −0.263 | <0.050 | Urbanization | Insignificant | |||||
| Ischemic heart disease | UV radiation | 0.460 | −0.472 | <0.001 | UV radiation | 0.280 | −0.501 | <0.001 | UV radiation | 0.714 | −0.724 | <0.001 |
| Ibs | 0.581 | 0.327 | <0.001 | Ibs | 0.322 | 0.227 | <0.010 | Ibs | 0.761 | 0.255 | <0.001 | |
| Obesity % | 0.595 | 0.151 | <0.050 | Obesity % | 0.349 | 0.202 | <0.050 | Ageing e(65) | Insignificant | |||
| Ageing e(65) | Insignificant | Urbanization | 0.374 | −0.185 | <0.050 | GDP PPP | Insignificant | |||||
| GDP PPP | Insignificant | Ageing e(65) | Insignificant | Obesity % | Insignificant | |||||||
| Urbanization | Insignificant | GDP PPP | Insignificant | Urbanization | Insignificant | |||||||
| Ischemic stroke | UV radiation | 0.367 | −0.483 | <0.001 | UV radiation | 0.235 | −0.604 | <0.001 | UV radiation | 0.354 | −0.438 | <0.001 |
| Ibs | 0.468 | 0.495 | <0.001 | Ageing e(65) | 0.302 | −0.575 | <0.001 | Ibs | 0.437 | 0.342 | <0.010 | |
| Ageing e(65) | 0.483 | −0.207 | <0.050 | Ibs | 0.367 | 0.402 | <0.001 | Ageing e(65) | Insignificant | |||
| GDP PPP | Insignificant | Obesity % | 0.421 | −0.251 | 0.003 | GDP PPP | Insignificant | |||||
| Obesity % | Insignificant | GDP PPP | Insignificant | Obesity % | Insignificant | |||||||
| Urbanization | Insignificant | Urbanization | Insignificant | Urbanization | Insignificant | |||||||
| Myocarditis | UV radiation | 0.589 | −0.505 | <0.001 | UV radiation | 0.523 | −0.617 | <0.001 | GDP PPP | 0.442 | 0.293 | <0.050 |
| Ageing e(65) | 0.759 | 0.444 | <0.001 | Ageing e(65) | 0.658 | 0.401 | <0.001 | UV radiation | 0.535 | −0.369 | <0.001 | |
| Obesity % | 0.766 | −0.138 | <0.010 | Obesity % | 0.693 | −0.177 | <0.010 | Ibs | 0.573 | 0.335 | <0.010 | |
| Ibs | 0.772 | 0.138 | <0.050 | Urbanization | 0.706 | −0.136 | <0.050 | Obesity % | 0.607 | −0.210 | <0.050 | |
| GDP PPP | Insignificant | GDP PPP | Insignificant | Ageing e(65) | Insignificant | |||||||
| Urbanization | Insignificant | Ibs | Insignificant | Urbanization | Insignificant | |||||||
| Nonrheumatic calcific aortic valve disease | GDP PPP | 0.676 | 0.343 | <0.001 | UV radiation | 0.526 | −8.573 | <0.001 | UV radiation | 0.574 | −0.572 | <0.001 |
| UV radiation | 0.783 | −0.405 | <0.001 | Ageing e(65) | 0.608 | 4.548 | <0.001 | GDP PPP | 0.626 | 0.251 | <0.010 | |
| Ageing e(65) | 0.793 | 0.209 | <0.010 | GDP PPP | Insignificant | Obesity % | 0.647 | 0.170 | <0.050 | |||
| Obesity % | 0.800 | 0.106 | <0.050 | Ibs | Insignificant | Ageing e(65) | Insignificant | |||||
| Ibs | Insignificant | Obesity % | Insignificant | Ibs | Insignificant | |||||||
| Urbanization | Insignificant | Urbanization | Insignificant | Urbanization | Insignificant | |||||||
| Nonrheumatic degenerative mitral valve disease | UV radiation | 0.473 | −0.528 | <0.001 | UV radiation | 0.307 | −0.575 | <0.001 | UV radiation | 0.670 | −0.686 | <0.001 |
| Ibs | 0.557 | 0.336 | <0.001 | Obesity % | 0.336 | −0.263 | <0.010 | Ibs | 0.726 | 0.280 | <0.001 | |
| Ageing e(65) | Insignificant | Ibs | 0.366 | 0.369 | <0.001 | Ageing e(65) | Insignificant | |||||
| GDP PPP | Insignificant | Ageing e(65) | 0.412 | −0.305 | <0.010 | GDP PPP | Insignificant | |||||
| Obesity % | Insignificant | GDP PPP | Obesity % | Insignificant | ||||||||
| Urbanization | Insignificant | Urbanization | Urbanization | Insignificant | ||||||||
| Nonrheumatic valvular heart disease | UV radiation | 0.626 | −0.502 | <0.001 | UV radiation | 0.528 | −0.652 | <0.001 | UV radiation | 0.729 | −0.717 | <0.001 |
| GDP PPP | 0.762 | 0.386 | <0.001 | Ibs | 0.578 | 0.258 | <0.001 | Ibs | 0.790 | 0.287 | <0.001 | |
| Ibs | 0.778 | 0.201 | <0.001 | Obesity % | 0.598 | −0.155 | <0.050 | Ageing e(65) | Insignificant | |||
| Urbanization | 0.782 | −0.102 | <0.050 | Ageing e(65) | GDP PPP | Insignificant | ||||||
| Ageing e(65) | Insignificant | GDP PPP | Obesity % | Insignificant | ||||||||
| Obesity % | Insignificant | Urbanization | Urbanization | Insignificant | ||||||||
| Peripheral artery disease | UV radiation | 0.584 | −0.463 | <0.001 | UV radiation | 0.555 | −0.661 | <0.001 | Ibs | 0.546 | 0.422 | <0.001 |
| Ageing e(65) | 0.755 | 0.304 | <0.001 | Ageing e(65) | 0.638 | 0.364 | <0.001 | UV radiation | 0.645 | −0.280 | <0.010 | |
| Ibs | 0.789 | 0.282 | <0.001 | Urbanization | 0.669 | −0.198 | <0.010 | GDP PPP | 0.675 | 0.272 | <0.010 | |
| GDP PPP | Insignificant | GDP PPP | Insignificant | Ageing e(65) | Insignificant | |||||||
| Obesity % | Insignificant | Ibs | Insignificant | Obesity % | Insignificant | |||||||
| Urbanization | Insignificant | Obesity % | Insignificant | Urbanization | Insignificant | |||||||
| Rheumatic heart disease | GDP PPP | 0.576 | −0.466 | <0.001 | GDP PPP | 0.363 | −0.325 | <0.001 | GDP PPP | 0.443 | −0.280 | <0.050 |
| UV radiation | 0.603 | 0.209 | <0.001 | Ibs | 0.410 | −0.299 | <0.001 | Ageing e(65) | 0.501 | −0.331 | <0.010 | |
| Ageing e(65) | 0.612 | −0.196 | <0.050 | Urbanization | 0.467 | −0.286 | <0.001 | UV radiation | 0.560 | 0.310 | <0.010 | |
| Ibs | Insignificant | UV radiation | Insignificant | Ibs | Insignificant | |||||||
| Obesity % | Insignificant | Ageing e(65) | Insignificant | Obesity % | Insignificant | |||||||
| Urbanization | Insignificant | Obesity % | Insignificant | Urbanization | Insignificant | |||||||
| Stroke | UV radiation | 0.276 | −6.860 | <0.001 | UV radiation | 0.153 | −0.628 | <0.001 | UV radiation | 0.361 | −0.419 | <0.001 |
| Ibs | 0.359 | 7.092 | <0.001 | Ageing e(65) | 0.268 | −0.562 | <0.001 | Ibs | 0.472 | 0.392 | <0.001 | |
| Ageing e(65) | 0.442 | −5.021 | <0.001 | Ibs | 0.353 | 0.477 | <0.001 | Ageing e(65) | Insignificant | |||
| GDP PPP | Obesity % | 0.451 | −0.340 | <0.001 | GDP PPP | Insignificant | ||||||
| Obesity % | GDP PPP | 0.476 | −0.264 | <0.050 | Obesity % | Insignificant | ||||||
| Urbanization | Urbanization | Urbanization | Insignificant | |||||||||
| Subarachnoid hemorrhage | Ibs | 0.624 | 0.626 | <0.001 | Ibs | 0.363 | 0.502 | <0.001 | Ibs | 0.615 | 0.613 | <0.001 |
| UV radiation | 0.713 | −0.342 | <0.001 | UV radiation | 0.479 | −0.380 | <0.001 | UV radiation | 0.711 | −0.360 | <0.001 | |
| Ageing e(65) | Insignificant | Obesity % | 0.558 | −0.288 | <0.001 | Ageing e(65) | Insignificant | |||||
| GDP PPP | Insignificant | Ageing e(65) | Insignificant | GDP PPP | Insignificant | |||||||
| Obesity % | Insignificant | GDP PPP | Insignificant | Obesity % | Insignificant | |||||||
| Urbanization | Insignificant | Urbanization | Insignificant | Urbanization | Insignificant | |||||||
Note: Significance levels: * p < 0.05, *** p < 0.01, *** p < 0.001.
Data source and definition: Ultraviolet radiation (UVR), expressed as the average daily ambient ultraviolet radiation level (in J/m2), the World Health Organization; Cardiovascular disease (CVD) incidence rate, the number of newly diagnosed cases per 100,000 in 2019, the Institute for Health Metrics and Evaluation; Ageing indexed with life expectancy at 65 year old in 2014, United Nations; Per capita GDP PPP, measured with the per capita purchasing power parity (PPP) value of all final goods and services produced within a territory in 2019, the World Bank; Ibs indexing the detrimental CVD genetic background accumulation in the past decades, downloaded from previous publications 34 ; Obesity prevalence, measured with the percentage of population aged 18+ with BMI equal to or over 30 kg/m2 in 2014, the World Health Organization. Urbanization, measured with the percentage of population living in urban area in 2019, the World Bank.
All the data were log‐transformed for correlation analysis in SPSS v 28.
With the exception of endocarditis and intracerebral hemorrhage, UVR is found to be a significant predictor for all 12 types of CVD analysed. However, it is not always the most influential predictor. Additionally, there is a significant correlation between UVR and each of these 12 types of CVD.
Table 4 presents the correlation between UVR and CVD incidence across different country clusters, using Pearson's r. Generally, there was a consistent negative correlation between UVR and CVD incidence within each country grouping. However, the strength and significance of these correlations may vary depending on sample size and the predictive role of UVR in CVD incidence.
Table 4.
Ultraviolet radiation (UVR) level determining total cardiovascular disease incidence rate in different country groupings.
| Country groupings | Pearson r | p |
|---|---|---|
| World Bank income classifications | ||
| Low Income, n = 30 | −0.684** | <0.001 |
| Low Middle Income, n = 49 | −0.846** | <0.001 |
| Upper Middle Income, n = 51 | −0.740** | <0.001 |
| Low‐ and middle‐income countries (LMIC), n = 130 | −0.785** | <0.001 |
| High Income, n = 60 | −0.633** | <0.001 |
| Fisher r‐to‐z transformation | LMIC versus High: z = − 1.96, p < 0.050 | |
| United Nations common practice | ||
| Developed, n = 44 | 0.025 | 0.872 |
| Developing, n = 146 | −0.582** | <0.001 |
| Fisher r‐to‐z transformation | LMIC versus High: z = − 3.90, p < 0.001 | |
| Countries grouped with various factors | ||
| Asia Cooperation Dialog, n = 33 | −0.776** | <0.001 |
| Asia‐Pacific Economic Cooperation, n = 19 | −0.900** | <0.001 |
| Arab World, n = 21 | −0.872** | <0.001 |
| English as Official Language, n = 54 | −0.441** | <0.001 |
| Latin America, n = 20 | −0.632** | <0.010 |
| Latin America and Caribbean, n = 33 | −0.124 | 0.494 |
| Organization for Economic Co‐operation and Development, n = 38 | −0.598** | <0.001 |
Note: Pearson r and nonparametric correlations within country groupings were reported.
Data source & definition: Ultraviolet Radiation (UVR), expressed as the average daily ambient ultraviolet radiation level (in J/m2), the World Health Organization; Cardiovascular disease (CVD) incidence rate, the number of new cases per 100,000, the Institute for Health Metrics and Evaluation.
All the data were log‐transformed for correlation analysis.
By applying Fisher's r‐to‐z transformation, it was found that the correlation between UVR and CVD incidence was significantly stronger in low‐ and middle‐income countries (LMIC) compared to high‐income countries (z = −1.96, p < 0.050). Additionally, the correlation in developing countries was significantly stronger than in developed countries (z = −3.90, p < 0.001). These results suggest that UVR may have a more pronounced impact on CVD incidence in LMIC than in high‐income countries (Table 4).
4. DISCUSSION
CVDs are a significant public health challenge influenced by various factors, including exposure to UVR. This study examines the relationship between UVR exposure and the incidence of 14 specific types of CVD [53], highlighting UVR as a notable risk factor in their development. 38
-
1.
UVR exposure is significantly correlated with a reduction in the overall incidence of CVD. When accounting for confounding factors such as aging, economic status, obesity, and urbanization, UVR explains 63.11% of the variance. This statistical association underscores the protective role of UVR across different types of CVD.
-
2.
However, after adjusting for established confounders like aging and economic status, the impact of UVR on the incidence of total CVD slightly diminishes. Stepwise linear regression reveals that UVR explains 36.84% of the variance, while partial correlation analysis attributes 47.47% to UVR, indicating a persistent albeit reduced association.
-
3.
Interestingly, the protective effect of UVR appears to be more pronounced in developing countries compared to developed ones, suggesting varying impacts across different geographic and economic contexts.
Throughout human evolution, there has been significant exposure to sunlight, approximately half of each day. 39 While research has highlighted the adverse effects of UVR, such as skin cancer and photo‐aging, recent insights challenge conventional wisdom. 40 Contrary to prevailing beliefs, reduced UVR exposure, rather than increased exposure, may contribute to the development of skin cancer, prompting a re‐evaluation of the complex relationship between UVR and human health. 36 , 41
Ultraviolet radiation from sunlight is believed to provide cardiovascular benefits and has been used for over 6,000 years in the treatment of cardiocirculatory disorders. 42 , 43 However, there is no scientific consensus on the consistent cardioprotective effects of UVR.
One established mechanism links UVR exposure to the synthesis of vitamin D in the skin, 44 which helps protect against CVD and high blood pressure. Another suggests that UVR stimulates the conversion of skin nitrogen oxides to nitric oxide (NO), promoting coronary vasodilation and exhibiting cardioprotective and antihypertensive properties. 8 Although UV‐A radiation does not promote vitamin D synthesis, 45 it has been associated with lower blood pressure. 40 Moreover, while greater UVR exposure may degrade bioactive folate metabolites, 46 including 5‐methyltetrahydrofolate, known for its cardioprotective properties, this study focuses on the role of natural ambient UVR in maintaining and improving cardiovascular health, without delving into the specifics of the UVR spectrum and its controversial impact on vitamin D and folate synthesis.
Scragg conducted an ecological study that found a possible link between seasonal variations in UVR and the observed patterns of CVD. 47 In contrast to this descriptive observational approach, this study took a quantitative approach using population‐level data. As the lead author of a recent systematic review, Scragg et al. raised concerns about previous studies that linked UVR to CVD, suggesting that other influencing risk factors may have confounded the results. 47 This study addresses this concern by using partial correlation and stepwise linear regression to independently investigate the role of UVR in predicting CVD incidence globally.
Many studies have examined the statistical relationship between UVR and blood pressure. 12 However, it is important to note that while high blood pressure is a significant risk factor for CVD, 48 , 49 it is not a disease in itself. Blood pressure readings can be influenced by various factors, such as medical interventions, emotional states, physical activities, and environmental conditions. Previous research has mainly focused on high blood pressure as a risk factor and has often overlooked the fact that low blood pressure can also pose risks for CVD. 48 , 49 Therefore, using blood pressure as a dependent variable to explore the role of UVR exposure in predicting CVD may not provide accurate insights.
The presence of biases in previous research may have led to null or positive correlations between UVR and CVD. For example, in a study involving 17,773 American stroke patients over the age of 45, 50 Kent et al. did not observe a correlation between daily ambient solar radiation and brachial blood pressure. 50 Similarly, Scragg et al. conducted a clinical trial with 119 individuals in New Zealand who had low vitamin D levels and found that ultraviolet exposure did not lead to a reduction in blood pressure. 51 These studies may have been affected by biases such as limited latitude range exposure and biased blood pressure readings from subjects who were already unhealthy. 51 Similar biases were also observed in a study involving male cyclists and triathletes in the United Kingdom. 52
A quartile correlation analysis showed a weak positive correlation between UVR and CVD mortality in a cohort study of individuals aged 51‐70 in the United States. 53 However, this method only measured relationships between quartiles and not individual data points, which limits its accuracy. Additionally, using CVD mortality as a measure of UVR exposure's role as a risk factor may be flawed, as CVD is preventable and involves distinct phases before and after diagnosis. 54 This improper measure may bias the findings suggesting a detrimental role of UVR in the development of CVD. The inverse correlation between UVR and CVD incidence has been observed in various countries, which has led to an examination of UVR's effects on CVD in LMICs and high‐income countries (1). This study shows that the correlations between UVR and CVD incidence are much stronger in LMICs compared to high‐income countries. This difference may be influenced by economic factors and disparities in healthcare education between these regions.
4.1. Strength and limitation of this study
Population‐level data, including second‐hand data, are utilized in this study despite potential random errors during collection and aggregation. This enhances the repeatability of our analysis by minimizing subjective biases often found in individual‐based quantitative studies. However, several limitations should be acknowledged:
First, this study is quantitative and focuses on correlating variables, which does not imply causation even if associations are observed concurrently.
Second, as a population‐level study, each population serves as the research unit, potentially leading to the intrinsic ecological fallacy in data analysis. Therefore, correlations identified at the country level may not necessarily be applicable at the individual level.
Third, the incidence of CVD is influenced by the availability of formal medical diagnoses, closely tied to economic prosperity and urbanization levels. The country‐specific CVD incidence rates from the University of Washington data set may be incomplete, particularly in LMICs. Economic affluence (indexed by GDP PPP) and urban advantages (measured by urbanization rates) have been integrated into our analyses to mitigate potential confounding effects on CVD incidence accuracy, yet residual confounders may persist.
Fourth, the total population‐level exposure to UVR is included as an independent variable to assess its statistical role in determining CVD incidence rates. However, real‐life UVR exposure is influenced by various behavioral factors, such as sunglasses usage, sunblock application, outdoor recreational activities, clothing choices, and duration of outdoor exposure. These factors significantly modify UVR exposure levels, but their exact impact has not been quantified, posing a challenge in incorporating these potential confounders into the statistical models in this study. To address this limitation, future studies could consider innovative methods, such as using Satellite Earth Observation for near real‐time monitoring of UV‐A solar radiation, 55 which offers more precise measurements. 55 , 56 These advancements could support further research into the relationship between personal solar exposure and its implications for cardiovascular health.
5. CONCLUSION
Ambient UVR may play a significant and independent role in protecting against CVD progression worldwide. This protective effect appears to be more prominent in LMIC. However, since there is a lack of evidence to support the cardioprotective role of vitamin D supplementation, it is worth exploring whether ambient UVR can also protect against CVD independently of vitamin D supplementation.
AUTHOR CONTRIBUTIONS
Wenpeng You: Conceptualization; Investigation; Funding acquisition; Writing—original draft; Methodology; Validation; Visualization; Writing—review and editing; Software; Formal analysis; Project administration; Data curation; Resources.
CONFLICT OF INTEREST STATEMENT
The sole author declares that there is no conflict of interest regarding the publication of this article. The author has read and approved the final version of the manuscript (corresponding author: WY) had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
ETHICS STATEMENT
All variables used in this study were collected from reputable sources, such as international organizations like United Nations agencies and the Institute for Health Metrics and Evaluation (IHME). The data set comprises solely of non‐identifiable, pre‐existing data about human subjects. It is not possible to trace individual persons or their communities using this data. Therefore, the study poses minimal ethical risk and does not necessitate ethical approval or written informed consent from participants.
TRANSPARENCY STATEMENT
The lead author Wenpeng You affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
ACKNOWLEDGMENTS
The author thanks Mr Hao You from Glenunga International High School, South Australia for his editing assistance. This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors. Open access publishing facilitated by The University of Adelaide, as part of the Wiley ‐ The University of Adelaide agreement via the Council of Australian University Librarians.
You W. Ambient ultraviolet radiation as a cardioprotective factor: a global and regional analysis. Health Sci Rep. 2024;7:e70065. 10.1002/hsr2.70065
DATA AVAILABILITY STATEMENT
The data used for this study were obtained from publicly available repositories hosted by United Nations (UN) Agencies and the Institute for Health Metrics and Evaluation at the University of Washington. Our use of this data complies with the terms and conditions set by the respective UN agencies, eliminating the need for formal permission to access and analyse the datasets. More information about the data sources can be found in the “Materials and Methods” section.
REFERENCES
- 1. WHO (2021). “Cardiovascular diseases (CVDs).” Retrieved December 21 2022, from https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- 2. IHME . 2020. Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Results. http://ghdx.healthdata.org/gbd-results-tool
- 3. Elagizi A, Kachur S, Carbone S, Lavie CJ, Blair SN. A review of obesity, physical activity, and cardiovascular disease. Curr Obes Rep. 2020;9:571‐581. [DOI] [PubMed] [Google Scholar]
- 4. Yusuf S, Hawken S, Ôunpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case‐control study. Lancet. 2004;364(9438):937‐952. [DOI] [PubMed] [Google Scholar]
- 5. Brook RD, Rajagopalan S, Pope CA, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331‐2378. [DOI] [PubMed] [Google Scholar]
- 6. Stringhini S. Association of socioeconomic position with health behaviors and mortality. JAMA. 2010;303(12):1159‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Juzeniene A, Moan J. Beneficial effects of UV radiation other than via vitamin D production. Dermat Endocrinol. 2012;4(2):109‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weller RB. Sunlight has cardiovascular benefits independently of vitamin D. Blood Purif. 2016;41(1‐3):130‐134. [DOI] [PubMed] [Google Scholar]
- 9. Roelandts R. The history of phototherapy: something new under the sun? J Am Acad Dermatol. 2002;46(6):926‐930. [DOI] [PubMed] [Google Scholar]
- 10. Al‐Ghazal SK. The valuable contributions of al‐Rāzī (Rhazes) in the history of pharmacy. Foundation for Science Technology and Civilization; 2007. [Google Scholar]
- 11. Brennan P, Greenberg G, Miall W, et al. Seasonal variation in arterial blood pressure. British Med J Clin Res. 1982;285(6346):919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rostand SG. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension. 1997;30(2):150‐156. [DOI] [PubMed] [Google Scholar]
- 13. Marti‐Soler H, Gubelmann C, Aeschbacher S, et al. Seasonality of cardiovascular risk factors: an analysis including over 230 000 participants in 15 countries. Heart. 2014;100(19):1517‐1523. [DOI] [PubMed] [Google Scholar]
- 14. Judd SE, Tangpricha V. Vitamin D deficiency and risk for cardiovascular disease. Am J Med Sci. 2009;338(1):40‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wimalawansa SJ. Vitamin D deficiency: effects on oxidative stress, epigenetics, gene regulation, and aging. Biology. 2019;8(2):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scragg R, Rahman J, Thornley S. Association of sun and UV exposure with blood pressure and cardiovascular disease: a systematic review. J Steroid Biochem Mol Biol. 2019;187:68‐75. [DOI] [PubMed] [Google Scholar]
- 17. The World Bank (2017). “World Bank Country and Lending Groups.” Retrieved 10 May 2017, from https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
- 18. WHO (2015). “Global Health Observatory, the data repository.” WHO. Retrieved 11.26.2015, from https://www.who.int/data/gho/data/indicators/indicator-details/GHO/uv-radiation
- 19. United Nations‐Department of Economic and Social Affairs‐Population Division (2021). File MORT/05‐1: Life expectancy at exact age, e(x), for both sexes combined, by region, subregion and country, annually for 1950‐2100 (Estimates, 1950 ‐ 2021). Online edition The United Nations.
- 20. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107(1):139‐146. [DOI] [PubMed] [Google Scholar]
- 21. Rodgers JL, Jones J, Bolleddu SI, et al. Cardiovascular risks associated with gender and aging. J Cardiovasc Develop Disease. 2019;6(2):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. The World Bank (2018). “Indicators|Data.” from https://data.worldbank.org/indicator
- 23. Schultz WM, Kelli HM, Lisko JC, et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137(20):2166‐2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gaziano TA, Bitton A, Anand S, Abrahams‐Gessel S, Murphy A. Growing epidemic of coronary heart disease in low‐and middle‐income countries. Curr Probl Cardiol. 2010;35(2):72‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. You W, Henneberg M. Modern medical services, a double‐edged sword manages symptoms, but accumulates genetic background of cardiovascular diseases: A cross populational analysis of 217 countries. Health Sci Rep. 2024. Jan 22;7(1):e1828. 10.1002/hsr2.1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. WHO Global Health Observatory (2022). “The data repository.” WHO. Retrieved November 11 2022, from http://www.who.int/gho/database/en/
- 27. Powell‐Wiley TM, Poirier P, Burke LE, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143(21):e984‐e1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schwartz MW, Seeley RJ, Zeltser LM, et al. Obesity pathogenesis: an endocrine society scientific statement. Endocr Rev. 2017;38(4):267‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Unamuno X, Gómez‐Ambrosi J, Rodríguez A, Becerril S, Frühbeck G, Catalán V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Invest. 2018;48(9):e12997. [DOI] [PubMed] [Google Scholar]
- 30. Yusuf S, Reddy S, Ôunpuu S, Anand S. Global burden of cardiovascular diseases: part II: variations in cardiovascular disease by specific ethnic groups and geographic regions and prevention strategies. Circulation. 2001;104(23):2855‐2864. [DOI] [PubMed] [Google Scholar]
- 31. Zittermann A, Schleithoff SS, Koerfer R. Putting cardiovascular disease and vitamin D insufficiency into perspective. Br J Nutr. 2005;94(4):483‐492. [DOI] [PubMed] [Google Scholar]
- 32. Mahajan H, Mallinson PAC, Lieber J, et al. The Association of Total Meat Intake with Cardio‐Metabolic Disease Risk Factors and Measures of Sub‐Clinical Atherosclerosis in an Urbanising Community of Southern India: A Cross-Sectional Analysis for the APCAPS Cohort. Nutrients. 2024. Mar 5;16(5):746. 10.3390/nu16050746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. You W, Henneberg M. Cereal crops are not created equal: wheat consumption associated with obesity prevalence globally and regionally. AIMS Pub Health. 2016;3(2):313‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. You W, Henneberg M. Prostate cancer incidence is correlated to total meat intake–a cross‐national ecologic analysis of 172 countries. Asian Pacific J Cancer Preve. 2018;19(8):2229‐2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. You W, Henneberg R, Henneberg M. Advanced healthcare services leading to relax natural selection may have been contributing to global and regional increase of dementia incidence. Res Square. 2021:1091190. [Google Scholar]
- 36. You W, Henneberg R, Henneberg M. Healthcare services relaxing natural selection may contribute to increase of dementia incidence. Sci Rep. 2022;12(1):8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. You W, Henneberg R, Saniotis A, Ge Y, Henneberg M. Total meat intake is associated with life expectancy: a cross‐sectional data analysis of 175 contemporary populations. Int J Gen Med. 2022;15:1833‐1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. IHME . Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Results. 2020. http://ghdx.healthdata.org/gbd-results-tool
- 39. Feelisch M, Kolb‐Bachofen V, Liu D, et al. Is sunlight good for our heart? Eur Heart J. 2010;31(9):1041‐1045. [DOI] [PubMed] [Google Scholar]
- 40. Weller RB. The health benefits of UV radiation exposure through vitamin D production or non‐vitamin D pathways. Blood pressure and cardiovascular disease. Photochem Photobiol Sci. 2017;16:374‐380. [DOI] [PubMed] [Google Scholar]
- 41. Krause R, Dobberke J, Buehring M, et al. Biologic Effects of Light. The role of ultraviolet radiation on cardiocirculatory regulation and on cardiovascular risk. Springer; 2002:219‐229. [Google Scholar]
- 42. Zittermann A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol. 2006;92(1):39‐48. [DOI] [PubMed] [Google Scholar]
- 43. You W, Henneberg R, Coventry BJ, Henneberg M. Cutaneous malignant melanoma incidence is strongly associated with European depigmented skin type regardless of ambient ultraviolet radiation levels: evidence from Worldwide population‐based data. AIMS Public Health. 2022;9(2):378‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. de la Guía‐Galipienso F, Martínez‐Ferran M, Vallecillo N, Lavie CJ, Sanchis‐Gomar F, Pareja‐Galeano H. Vitamin D and cardiovascular health. Clin Nutr. 2021;40(5):2946‐2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6):1678S‐1688S. [DOI] [PubMed] [Google Scholar]
- 46. Wolf ST, Kenney WL. Skin pigmentation and vitamin D–folate interactions in vascular function: an update. Curr Opin Clin Nutr Metab Care. 2021;24(6):528‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Scragg R. Seasonality of cardiovascular disease mortality and the possible protective effect of ultra‐violet radiation. Int J Epidemiol. 1981;10(4):337‐341. [DOI] [PubMed] [Google Scholar]
- 48. Gu D, Kelly TN, Wu X, et al. Blood pressure and risk of cardiovascular disease in Chinese men and women. Am J Hypertens. 2008;21(3):265‐272. [DOI] [PubMed] [Google Scholar]
- 49. Stokes J, Kannel WB, Wolf PA, et al. Blood pressure as a risk factor for cardiovascular disease. Hypertension. 1989;13(suppl 1):113‐118. [DOI] [PubMed] [Google Scholar]
- 50. Kent ST, Cushman M, Howard G, et al. Sunlight exposure and cardiovascular risk factors in the REGARDS study: a cross‐sectional split‐sample analysis. BMC Neurol. 2014;14:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Scragg R, Wishart J, Stewart A, et al. No effect of ultraviolet radiation on blood pressure and other cardiovascular risk factors. J Hypertens. 2011;29(9):1749‐1756. [DOI] [PubMed] [Google Scholar]
- 52. Muggeridge DJ, Sculthorpe N, Grace FM, et al. Acute whole body UVA irradiation combined with nitrate ingestion enhances time trial performance in trained cyclists. Nitric oxide. 2015;48:3‐9. [DOI] [PubMed] [Google Scholar]
- 53. Lin S‐W, Wheeler DC, Park Y, et al. Prospective study of ultraviolet radiation exposure and mortality risk in the United States. Am J Epidemiol. 2013;178(4):521‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shojania KG, Forster AJ. Hospital mortality: when failure is not a good measure of success. Can Med Assoc J. 2008;179(2):153‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Morelli M, Michelozzi B, Simeone E, Khazova M. Validation of a satellite‐based solar UV‐A radiation dosimeter for mobile healthcare applications. J Atmos Solar‐Terrestrial Physics. 2021;215:105529. [Google Scholar]
- 56. Parisi A, Igoe D, Downs N, Turner J, Amar A, A Jebar M. Satellite monitoring of environmental solar ultraviolet A (UVA) exposure and irradiance: a review of OMI and GOME‐2. Remote Sens. 2021;13(4):752. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used for this study were obtained from publicly available repositories hosted by United Nations (UN) Agencies and the Institute for Health Metrics and Evaluation at the University of Washington. Our use of this data complies with the terms and conditions set by the respective UN agencies, eliminating the need for formal permission to access and analyse the datasets. More information about the data sources can be found in the “Materials and Methods” section.


