Abstract
Context
Sphingolipids are linked to the pathogenesis of type 2 diabetes.
Objective
To test the hypothesis that plasma sphingolipid profiles predict incident prediabetes.
Design
A case-control study nested in the Pathobiology of Prediabetes in a Biracial Cohort study, a 5-year follow-up study.
Setting
Academic health center.
Participants
Normoglycemic adults enrolled in the Pathobiology of Prediabetes in a Biracial Cohort study. Assessments included oral glucose tolerance test, insulin sensitivity, and insulin secretion. Participants with incident prediabetes were matched in age, sex, and ethnicity with nonprogressors.
Interventions
We assayed 58 sphingolipid species (ceramides, monohexosyl ceramides, sphingomyelins, and sphingosine) using liquid chromatography/tandem mass spectrometry in baseline plasma levels from participants and determined association with prediabetes risk.
Main Outcome Measure
The primary outcome was progression from normoglycemia to prediabetes, defined as impaired fasting glucose or impaired glucose tolerance.
Results
The mean age of participants (N = 140; 50% Black, 50% female) was 48.1 ± 8.69 years, body mass index 30.1 ± 5.78 kg/m2, fasting plasma glucose 92.7 ± 5.84 mg/dL, and 2-hour plasma glucose 121 ± 23.3 mg/dL. Of the 58 sphingolipid species assayed, higher ratios of sphingomyelin C26:0/C26:1 (OR, 2.73 [95% CI, 1.172-4.408], P = .015) and ceramide C18:0/C18:1 (OR, 1.236 [95% CI, 1.042-1.466], P = .015) in baseline plasma specimens were significantly associated with progression to prediabetes during the 5-year follow-up period, after adjustments for age, race, sex, body mass index, fasting plasma glucose, 2-hour plasma glucose, insulin sensitivity, and insulin secretion.
Conclusion
We conclude that the saturated-to-monounsaturated ratios of long-chain ceramide C18:0/C18:1 and very-long-chain sphingomyelin C26:0/C26:1 are potential biomarkers of prediabetes risk among individuals with parental history of type 2 diabetes.
Keywords: sphingomyelins, biomarkers, impaired glucose tolerance, impaired fasting glucose, race/Ethnicity
Prediabetes, an intermediate dysglycemic state, can progress to type 2 diabetes (T2D) over several years and is associated with increased risks of microvascular and macrovascular complications (1–3). The risk factors for prediabetes, which overlap considerably with those of T2D, include genetic predisposition, overweight or obesity, physical inactivity, insulin resistance and β-cell dysfunction, among others (1, 4, 5). Alterations in lipid moieties precede the development of diabetes in rodents (6) and have been associated with increased risks of T2D and prediabetes in clinical studies (7–9). In addition to the classical pattern of dyslipidemia associated with T2D and prediabetes (elevated triglycerides and decreased high-density lipoprotein [HDL] cholesterol levels), alterations in sphingolipid metabolism have been reported in people with T2D (10–13).
Sphingolipids, a class of specialized lipids with an amino alcohol in their backbone, are found in all mammalian cells. Members of the sphingolipid family include ceramide, monohexosyl ceramide, sphingomyelin, and sphingosine, among others (13, 14). Besides their well-known structural function, knowledge of the biological roles of sphingolipids has expanded to include modulation of cell signaling, differentiation, growth, senescence, inflammation, and cell death (13, 14). Notably, sphingolipids have been implicated in the pathogenesis of diabetes and diabetes-related vascular complications (10–18). There is increasing evidence linking tissue accumulation of ceramide, a key sphingolipid metabolite, to insulin resistance and pancreatic β-cell apoptosis (16–18).
Most of the reports linking sphingolipids to diabetes and its complications are based on cross-sectional human data. However, the cross-sectional study design does not permit inferences as to whether alterations in sphingolipid metabolism lead to or result from diabetes. Furthermore, it is unclear whether early glucose abnormalities, such as those seen in people with prediabetes, are associated with alterations in sphingolipid metabolism.
In the Pathobiology of Prediabetes in a Biracial cohort (POP-ABC), self-identified Black or African American and White or European American offspring of parents with T2D were screened and enrolled if they had normal fasting glucose and normal glucose tolerance. The participants were followed every 3 months for 5 years for the primary outcome of incident prediabetes (19, 20). The POP-ABC study has reported numerous lifestyle, clinical, and biochemical factors associated with progression from normoglycemia to prediabetes in a diverse cohort (4, 5, 9, 21, 22).
The Ceramides and other Sphingolipids as Predictors of Incident Dysglycemia (CASPID) study investigates the role of sphingolipids in the pathogenesis of prediabetes and T2D by using existing resources from 2 established longitudinal cohorts—POP-ABC and the Diabetes Prevention Program (DPP) (23). The analysis of specimens obtained from POP-ABC participants enables an evaluation of the role of sphingolipids during transition from normoglycemia to incident prediabetes and the specimens from DPP participants provide the opportunity for assessing the role of sphingolipids during transition from prediabetes to T2D (23). In the present report, we analyzed circulating levels of 58 sphingolipid species in baseline plasma specimens obtained from normoglycemia POP-ABC study participants who were then followed up for 5 years, the primary outcome being incident prediabetes. Specifically, we tested the hypothesis that plasma sphingolipid profiles at enrollment predict the development of incident prediabetes during longitudinal follow-up.
Materials and Methods
Participants
Study subjects were participants in the POP-ABC study. Eligibility criteria included age 18 to 65 years, self-reported non-Hispanic White or non-Hispanic Black ethnicity, parental history of T2D, normal fasting plasma glucose (FPG; <100 mg/dL [5.6 mmol/L]) and normal glucose tolerance (2-hour plasma glucose [2hrPG] < 140 mg/dL [7.8 mmol/L]) during a 75-g oral glucose tolerance test (OGTT), as previously described (19, 20). Excluded from the study were individuals with a history of diabetes or prediabetes and those using medications or interventions known to alter body weight, blood glucose, lipid profile, or energy balance (19, 20).
For the present report, 140 participants who were initially normoglycemic were selected in a nested case-control design: 70 subjects who progressed to prediabetes during the 5-year follow-up were matched (age, sex, and ethnicity) with 70 nonprogressors. The sample size of 140 participants provided 90% power to detect ∼20% difference in the ratio of saturated-to-monounsaturated ceramides between progressors to prediabetes and nonprogressors (α = .05). The University of Tennessee institutional review board (IRB) approved the POP-ABC (IRB approval #8399) and the CASPID (IRB approval #21-07936-FB) studies. Written informed consent was given by all participants before initiation of the study, which was conducted at the General Clinical Research Center (GCRC) in accordance with the World Medical Association's Declaration of Helsinki. Deidentified stored plasma specimens obtained from the selected POP-ABC study participants during baseline visits were analyzed for the present report.
Assessments
Clinical Assessments
Participants arrived at the GCRC after an overnight fast for initial procedures, including a medical history, physical examination, anthropometric measurements (height, weight, waist circumference), selected biochemical measurements, and a standard 75-g OGTT. Weight was measured using a calibrated balance beam scale in duplicate and height was measured using a standard stadiometer, also in duplicate. The body mass index (BMI) was calculated as weight in kilograms divided by the height in meters squared. Study participants received written instructions to consume a normal diet and avoid strenuous exercise or alcohol consumption for 24 hours before the OGTT. After the initial (baseline) visit, POP-ABC participants made quarterly visits to the GCRC for scheduled assessments during 5 years of follow-up, as previously described (20). Insulin secretion was assessed annually, and insulin sensitivity was measured at years 1, 3, and 5.
Insulin Sensitivity
Insulin sensitivity was assessed using the hyperinsulinemic euglycemic clamp according to the method of DeFronzo et al (24). In brief, participants were fasted overnight before undergoing the clamp studies at the GCRC. After placement of IV cannulas in both forearms, a primed, continuous IV infusion of regular insulin (2 mU/kg/min; 12 pmol/kg/min) was administered for 180 minutes while maintaining blood glucose level at ∼100 mg/dL (5.6 mmol/L) with a variable-rate dextrose (20%) infusion. Arterialized blood sampling was performed every 10 minutes for glucose and insulin measurements. The total insulin-stimulated glucose disposal rate, calculated from the dextrose infusion rate during steady state (final 60 minutes of insulin infusion), was corrected for the steady-state plasma insulin levels to derive the final insulin sensitivity index (Si-clamp) as previously described (24).
Insulin Secretion
Insulin secretion was measured using the IV glucose tolerance test, as previously described (20). In brief, blood samples for the measurement of glucose and insulin were drawn 30 minutes before and at 2, 3, 4, 5, 7, and 10 minutes after an IV bolus of dextrose (25 g). The acute insulin response to glucose was calculated as the mean incremental plasma insulin concentration from 3 to 5 minutes after the dextrose bolus. The disposition index (ie, insulin secretion adjusted for ambient insulin sensitivity) was calculated as the product of Si-clamp and acute insulin response to glucose (20).
Biochemical Assays
Plasma glucose was measured at the bedside using the YSI glucose analyzer (Yellow Spring Instruments Co., Inc., Yellow Spring, OH). Plasma insulin levels were measured in our Endocrine Research Laboratory, using solid phase, 2-site sequential chemiluminescent immunometric assays on the Siemens Immulite analyzer (Siemens Healthcare Diagnostics Products, Ltd., Camberley, Surrey, UK). The within-batch variation coefficient for the insulin assay was <5%.
Lipidomic Analysis
Using established protocols, targeted lipidomic profiles of sphingolipid species in baseline fasting plasma samples were generated by liquid chromatography–tandem mass spectrometry at the Lipidomics Core at Virginia Commonwealth University, Richmond, VA (25, 26).
Materials: Internal standards were purchased from Avanti Polar Lipids (Alabaster, AL). Internal standards were added to samples in 10 µL ethanol:methanol:water (7:2:1) as a cocktail of 250 pmol each. Standards for sphingoid bases and sphingoid base 1-phosphates were 17-carbon chain length analogs: C17-sphingosine, (2S,3R,4E)-2-aminoheptadec-4-ene-1,3-diol (d17:1-So); C17-sphinganine, (2S,3R)-2-aminoheptadecane-1,3-diol (d17:0-Sa); C17-sphingosine 1-phosphate, heptadecasphing-4-enine-1-phosphate (d17:1-So1P); and C17-sphinganine 1-phosphate, heptadecasphinganine-1-phosphate (d17:0-Sa1P). Standards for N-acyl sphingolipids were C12-fatty acid analogs: C12-Cer, N-(dodecanoyl)-sphing-4-enine (d18:1/C12:0); C12-Cer 1-phosphate, N-(dodecanoyl)-sphing-4-enine-1-phosphate (d18:1/C12:0-Cer1P); C12-sphingomyelin, N-(dodecanoyl)-sphing-4-enine-1-phosphocholine (d18:1/C12:0-SM); and C12-glucosylceramide, N-(dodecanoyl)-1-β-glucosyl-sphing-4-eine.
For liquid chromatography/tandem mass spectrometry (LC-MS/MS) analyses, a Shimadzu Nexera LC-30 AD binary pump system coupled to a SIL-30AC autoinjector and DGU20A5R degasser coupled to an AB Sciex 5500 quadrupole/linear ion trap (QTrap) (SCIEX Framingham, MA) operating in a triple quadrupole mode was used. Q1 and Q3 were set to pass molecularly distinctive precursor and product ions (or a scan across multiple m/z in Q1 or Q3), using N2 to collisionally induce dissociations in Q2 (which was offset from Q1 by 30-120 eV); the ion source temperature was set to 500 °C.
Methods: Extraction of Sphingolipids. Plasma (40 µL) was transferred to 13 × 100 mm borosilicate tubes with a Teflon-lined cap (catalog #60827-453, VWR, West Chester, PA). Then 2 mL of CH3OH was added along with the internal standard cocktail (250 pmol of each species dissolved in a final total volume of 10 mL of ethanol:methanol:water 7:2:1). The contents were dispersed using an ultra sonicator at room temperature for 30 seconds. Then 1 mL of CHCl3 was added and test tubes were recapped. This single-phase mixture was incubated at 48 °C overnight. The extract was centrifuged using a table-top centrifuge, and the supernatant was removed by a Pasteur pipette and transferred to a new tube. The extract was reduced to dryness using a Speed Vac. The dried residue was reconstituted in 0.5 mL of the starting mobile phase solvent for LC-MS/MS analysis, sonicated for ∼15 seconds, then centrifuged for 5 minutes in a tabletop centrifuge before transfer of the clear supernatant to the autoinjector vial for analysis.
LC-MS/MS of Sphingoid Bases, Sphingoid Base 1-phosphates, and Complex Sphingolipids
These compounds were separated by reverse phase LC using a Supelco 2.1 (inner diameter) × 50 mm Ascentis Express C18 column (Sigma, St. Louis, MO) and a binary solvent system at a flow rate of 0.5 mL/min with a column oven set to 35 °C. Before injection of the sample, the column was equilibrated for 0.5 minutes with a solvent mixture of 95% Moble phase A1 (CH3OH/H2O/HCOOH, 58/41/1, v/v/v, with 5 mM ammonium formate) and 5% Mobile phase B1 (CH3OH/HCOOH, 99/1, v/v, with 5 mM ammonium formate). After sample injection (typically 40 mL), the A1/B1 ratio was maintained at 95/5 for 2.25 minutes, followed by a linear gradient to 100% B1 over 1.5 minutes, which was held at 100% B1 for 5.5 minutes, followed by a 0.5-minute gradient return to 95/5 A1/B1. The column was reequilibrated with 95:5 A1/B1 for 0.5 minutes before the next run.
Using an approach targeting sphingolipid species with d18:1 sphingoid base in the classes of ceramides, monohexosyl ceramides, and sphingomyelins, a total of 54 sphingolipids and 4 species of sphingoid bases were identified, based on their retention time and m/z ratio and quantified by comparing the target lipid ion of interest with the normalization of quantitated ion abundances (25, 26). Supplementary Table S1 shows the m/z ratios of complex sphingolipids (27) and Supplementary Fig. S1 shows the LC-MS/MS chromatogram of individual sphingolipid species (28).
Definition of Outcome Measures
The prespecified primary outcome was the occurrence of prediabetes, defined as impaired fasting glucose (FPG ≥ 100 mg/dL [5.6 mmol/L]) or impaired glucose tolerance (2hrPG ≥140 mg/dL [7.8 mmol/L] based on American Diabetes Association criteria (1). For each first occurrence of an endpoint, a confirmatory test using 75-g OGTT was performed within 6 weeks of the initial endpoint occurrence, as previously described (20). All prediabetes endpoints were independently adjudicated by the Institutional Data and Safety Officer (Murray Heimberg, MD, PhD). In the present report, we performed a case-control analysis of plasma levels of 58 sphingolipid species in baseline specimens obtained from POP-ABC study participants who subsequently developed prediabetes (cases) and those who maintained normoglycemia (control) during 5 years of follow-up.
Statistical Analysis
Data are reported as means ± SD (except where standard error of the mean is specified). Differences in continuous or categorical variables were analyzed using t-test, ANOVA, or chi-square test, as appropriate. Lipidomic profiles in progressors vs nonprogressors were analyzed using 2-sample t-test and analysis of covariance, adjusting for baseline differences in blood glucose, clinical, and metabolic variables. The relationship between individual ceramides and other sphingolipid species and age at enrollment, adiposity measures, insulin sensitivity, insulin secretion, and other cardiometabolic variables was analyzed using linear regression models.
We used logistic regression to analyze the association of sphingolipid levels with incident prediabetes risk, and to generate receiver operating characteristic (ROC) curves by plotting sensitivity vs 1-specificity. The space below each ROC curve was divided into rectangles and the area of each rectangle was calculated as the product of the width and height to derive the area under the curve (AUC) for each ROC plot. Significance level was set as P < .05. All analyses were carried using SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
Baseline Characteristics of Study Participants
The study subjects comprised 140 initially normoglycemic POP-ABC participants (70 Black and 70 White; 70 men, 70 women). Table 1 shows baseline characteristics of the 70 participants who progressed to prediabetes during 5 years of follow-up and 70 participants who maintained normoglycemia during the same follow-up period. Both groups were matched in age, sex, and ethnicity, but BMI (31.3 ± 6.12 vs 29.0 ± 5.22 kg/m2, P = .02) and FPG (94.0 ± 5.17 vs 91.3 ± 6.18 mg/dL, P = .006) were higher in progressors compared with nonprogressors. Both groups had similar mean values for 2hrPG, insulin sensitivity, and insulin secretion at enrollment (Table 1).
Table 1.
Baseline characteristics of study subjects
| Characteristics | All | Nonprogressors | Progressors | P value |
|---|---|---|---|---|
| Number (Black/White) |
140 (70/70) |
70 (35/35) |
70 (35/35) |
|
| Age (y) | 48.1 ± 8.69 | 47.6 ± 9.16 | 48.5 ± 8.23 | .55 |
| BMI (kg/m2) | 30.1 ± 5.78 | 29.03 ± 5.22 | 31.3 ± 6.12 | .02 |
| FPG (mg/dL) | 92.7 ± 5.84 | 91.3 ± 6.18 | 94.0 ± 5.17 | .006 |
| 2hrPG (mg/dL) | 121 ± 23.3 | 119 ± 25.02 | 123 ± 21.4 | .37 |
| Si-clamp | 0.127 ± 0.067 | 0.138 ± 0.068 | 0.118 ± 0.065 | .14 |
| AIR (µU/mL) | 84.1 ± 75.4 | 88.8 ± 89.7 | 79.6 ± 59.6 | .49 |
Abbreviations: 2hrPG, 2-hour postload plasma glucose during oral glucose tolerance test; AIR, acute insulin response to IV glucose; BMI, body mass index; FPG, fasting plasma glucose; Si-clamp, insulin sensitivity measured with hyperinsulinemic euglycemic clamp.
Demographic Distribution of Baseline Total Sphingolipid Levels
The plasma total sphingolipids levels were similar in men vs women (95.4 ± 27.5 vs 99.8 ± 28.7 nmol/mL, P = .38), as were total levels of ceramides (2.66 ± 1.24 vs 2.74 ± 1.24 nmol/mL, P = .71), monohexosyl ceramides (4.11 ± 2.41 vs 4.06 ± 2.67 nmol/mL, P = .91), sphingomyelins (88.0 ± 25.4 vs 92.4 ± 26.8 nmol/mL, P = .35), and sphingosine (92.9 ± 48.8 vs 88.3 ± 45.4 pmol/mL, P = .58) (Table 2). The mean plasma levels of total sphingolipids were similar in Black vs White participants (97.6 ± 28.3 vs 99.0 ± 28.4 nmol/mL, P = .75), as were total levels of ceramides (2.57 ± 1.10 vs 2.88 ± 1.37 nmol/mL, P = .15), monohexosyl ceramides (3.84 ± 2.66 vs 4.36 ± 2.47 nmol/mL, P = .23), sphingomyelins (90.5 ± 26.2 vs 91.3 ± 26.5 nmol/mL, P = .87), and sphingosine (95.7 ± 47.9 vs 83.2 ± 44.3 pmol/mL, P = .11) (Table 2).
Table 2.
Total levels of sphingolipids and major classes in baseline plasma specimens by sex and ethnicity
| All | Women | Men | P value | Black | White | P value | |
|---|---|---|---|---|---|---|---|
| Total sphingolipids (nmol/mL) | 98.2 ± 28.3 | 99.8 ± 28.7 | 95.4 ± 27.5 | 0.38 | 97.6 ± 28.3 | 99.0 ± 28.4 | .75 |
| Total ceramides (nmol/mL) | 2.71 ± 1.24 | 2.74 ± 1.24 | 2.66 ± 1.24 | 0.71 | 2.57 ± 1.10 | 2.88 ± 1.37 | .15 |
| Total monohexosyl ceramides (nmol/mL) | 4.08 ± 2.57 | 4.06 ± 2.67 | 4.11 ± 2.41 | 0.91 | 3.84 ± 2.66 | 4.36 ± 2.47 | .23 |
| Total sphingomyelins (nmol/mL) | 90.8 ± 26.3 | 92.4 ± 26.8 | 88.0 ± 25.4 | 0.35 | 90.5 ± 26.2 | 91.3 ± 26.5 | .87 |
| Sphingosine (pmol/mL) |
89.9 ± 46.5 | 88.3 ± 45.4 | 92.9 ± 48.8 | 0.58 | 95.7 ± 47.9 | 83.2 ± 44.3 | .11 |
Sphingolipids and Progression to Prediabetes
The mean plasma values in progressors vs nonprogressors were not significantly different for total sphingolipids (94.0 ± 30.6 vs 102 ± 25.2 nmol/mL, P = .082), total ceramides (2.91 ± 1.37 vs 2.52 ± 1.06 nmol/mL, P = .063) or total monohexosyl ceramides (4.20 ± 2.99 vs 3.96 ± 2.10 nmol/mL, P = .57) (Table 3). However, progressors to prediabetes had lower levels of total sphingomyelins (86.3 ± 28.6 vs 95.3 ± 23.0 nmol/mL, P = .043) and higher levels of sphingosine (108 ± 48.2 pmol/mL vs 72.3 ± 37.5 pmol/mL, P = .0004) compared with nonprogressors (Table 3).
Table 3.
Total levels of sphingolipids and major classes in baseline plasma specimens from participants who progressed or did not progress to prediabetes during 5 years of follow-up
| All | Progressors | Nonprogressors | P value | |
|---|---|---|---|---|
| Total sphingolipids (nmol/mL) | 98.2 ± 28.3 | 94.0 ± 30.6 | 102 ± 25.2 | .082 |
| Total ceramides (nmol/mL) | 2.71 ± 1.24 | 2.91 ± 1.37 | 2.52 ± 1.06 | .063 |
| Total monohexosyl ceramides (nmol/mL) | 4.08 ± 2.57 | 4.20 ± 2.99 | 3.96 ± 2.10 | .574 |
| Total sphingomyelins (nmol/mL) | 90.8 ± 26.3 | 86.3 ± 28.6 | 95.3 ± 23.0 | .043 |
| Sphingosine (pmol/mL) | 89.9 ± 46.5 | 108 ± 48.2 | 72.3 ± 37.5 | .0004 |
Further comparison of plasma levels of 58 sphingolipid species revealed that progressors to prediabetes and nonprogressors had similar values for most sphingolipids but significantly discordant baseline values for 3 ceramides (C18:1, C22:0, C26:0) and 5 sphingomyelins (C16:0, C18:0, C18:1, C26:0, C32:1) (Table 4 and Supplementary Table S2) (29). In addition to having higher baseline sphingosine levels (Table 3), participants who progressed from normoglycemia to prediabetes during 5 years of follow-up had a higher proportion of ceramides (3.46 ± 2.12% vs 2.51 ± 1.02%, P = .029) and a lower proportion of sphingomyelins (91.6 ± 3.15% vs 93.2 ± 1.4%, P = .003) in their sphingolipids pool (Table 4 and Supplementary Table S2) (29).
Table 4.
Mean baseline plasma levels of selected ceramides and sphingomyelins in participants who progressed or did not progress to prediabetes during 5 years of follow-up
| Sphingolipid species | Progressors (mean ± SD) | Nonprogressors (Mean ± SD) | Unadjusted P value | Adjusted P valuea |
|---|---|---|---|---|
| Ceramides | ||||
| C18:1 (pmol/mL) | 5.77 ± 3.66 | 7.03 ± 3.46 | .038 | .021 |
| C22:0 (pmol/mL) | 575 ± 323.10 | 467 ± 234.6 | .026 | .08 |
| C26:0 (pmol/mL) | 15.8 ± 9.23 | 11.4 ± 4.38 | .0005 | .007 |
| Ceramide ratios | ||||
| C18:0/C18:1 | 8.88 ± 6.74 | 5.83 ± 2.87 | .0008 | .001 |
| C26:0/C26:1 | 2.20 ± 0.99 | 1.83 ± 0.81 | .018 | .038 |
| Cer % of sphingolipids | 3.46 ± 2.12 | 2.51 ± 1.02 | .001 | .029 |
| Sphingomyelins | ||||
| C16:0 (nmol/mL) | 23.2 ± 8.85 | 26.6 ± 6.08 | .009 | .10 |
| C18:1(nmol/mL) | 5.93 ± 2.80 | 7.40 ± 2.30 | .0009 | .024 |
| C18:0 (nmol/mL) | 7.31 ± 3.07 | 8.41 ± 2.39 | .020 | .17 |
| C26:0 (pmol/mL) | 49.0 ± 20.8 | 40.5 ± 18.4 | .011 | .001 |
| Sphingomyelin ratios | ||||
| C18:0/C18:1 | 1.28 ± 0.19 | 1.16 ± 0.17 | .0002 | .002 |
| C24:0/C24:1 | 0.58 ± 0.12 | 0.52 ± 0.12 | .008 | .045 |
| C26:0/C26:1 | 0.37 ± 0.10 | 0.30 ± 0.09 | <.0001 | <.0001 |
| SM % of sphingolipids | 91.6 ± 3.15 | 93.2 ± 1.4 | .0002 | .003 |
Abbreviations: Cer, ceramides; SM, sphingomyelins.
a Adjusted for age, sex, race, body mass index, waist circumference, fasting plasma glucose, and 2-hour postload plasma glucose during oral glucose tolerance test.
Saturated and Monounsaturated Sphingolipids
The mean baseline plasma levels of sphingosine and 2 saturated sphingolipids (ceramide C26:0 and sphingomyelin C26:0) were higher in progressors to prediabetes compared with nonprogressors (Table 4). Specifically, ceramide C26:0 levels were ∼40% higher (15.8 ± 9.23 pmol/mL vs 11.4 ± 4.38 pmol/mL, P = .007) and sphingomyelin C26:0 levels were 20% higher (49.0 ± 20.8 pmol/mL vs 40.5 ± 18.4 pmol/mL, P = .011) in progressors compared with nonprogressors (Table 4). The differences in mean plasma levels of ceramide C26:0 and sphingomyelin C26:0 remained significant after adjusting for age, sex, race/ethnicity, BMI, waist circumference, FPG, and 2hrPG.
In contrast to the findings with saturated sphingolipids, we observed significantly lower mean plasma levels of monounsaturated sphingolipids in progressors to prediabetes compared with nonprogressors, after adjustments for age, sex, race/ethnicity, BMI, waist circumference, FPG, and 2hrPG (Table 4). Specifically, the mean level of monounsaturated ceramide C18:1 was 22% lower (5.77 ± 3.66 pmol/mL vs 7.03 ± 3.46 pmol/mL, P = .021) and that of monounsaturated sphingomyelin C18:1 was 25% lower (5.93 ± 2.80 nmol/mL vs 7.40 ± 2.30 nmol/mL, P = .024) in progressors compared with nonprogressors. The level of monounsaturated sphingomyelin C26:1 was not significantly different in progressors vs nonprogressors (0.13 ± 0.05 nmol/L vs 0.14 ± 0.06 nmol/L, P = .66). The differences between progressors to prediabetes and nonprogressors in the levels of other sphingolipid species (ceramide C22:0, monohexosyl ceramides C18:0 and C18:1, and sphingomyelins C16:0 and C18:0) no longer were significant after adjusting for age, sex, ethnicity, BMI, waist circumference, FPG, and 2hrPG (Table 4 and Supplementary Table S2).
Ratio of Saturated-to-monounsaturated Sphingolipids
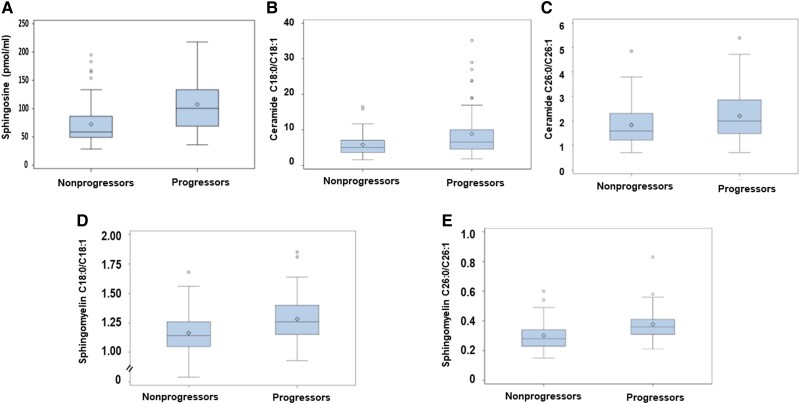
Given the discordant associations of saturated and monounsaturated sphingolipids with incident prediabetes, we examined their ratios as predictors of prediabetes risk in our prospective POP-ABC study cohort. Compared with nonprogressors, participants who progressed to prediabetes had significantly higher saturated-to-monounsaturated ratios of ceramides C18:0/C18:1 and C26:0/C26:1, and sphingomyelins C18:0/C18:1, C24:0/C24:1, and C26:0/C26:1, in baseline plasma specimens obtained 5 years before the occurrence of prediabetes (Fig. 1 and Table 4).
Figure 1.
Box-and-whisker plots of sphingosine levels (A) and the saturated-to-monounsaturated ratios of ceramides C18:0/C18:1 (B) and C26:0/C26:1(C) and sphingomyelins C18:0/C18:1 (D) and C26:0/C26:1 (E) in baseline plasma specimens obtained from participants who progressed from normoglycemia to prediabetes (progressors) and those who maintained normoglycemia (nonprogressors) during 5 years of follow-up. For each box-and-whisker plot, the diamond symbol inside the box represents the geometric mean, the horizontal line represents the median, the lower and upper ends of the box represent the 25th and 75th percentiles, respectively, and the whiskers represent the maximum or minimum values within 1.5 × the interquartile range. Progressors to prediabetes had significantly higher baseline ratios of the saturated-to-unsaturated ceramides and sphingomyelins compared with nonprogressors (P = .038-<.0001).
Logistic Regression
In both minimally adjusted and fully adjusted logistic regression models, the saturated-to-monounsaturated ratios of ceramides C18:0/C18:1 and very-long-chain sphingomyelins C26:0/C26:1 were significantly associated with the risk of incident prediabetes (Table 5). The odds ratio was 1.236 (95% CI, 1.042-1.466) for ceramides C18:0/C18:1 and 2.273 (95% CI, 1.172-4.408) for sphingomyelins C26:0/C26:1, after adjustments for age, race, sex, BMI, and other pertinent variables (Table 5). In an unadjusted logistic regression model, baseline plasma sphingosine levels were modestly associated with incident prediabetes: odds ratio, 1.021 (95% CI, 1.011-1.031), but the association was attenuated in a fully adjusted model (Table 5).
Table 5.
Logistic regression of plasma sphingosine and saturated/monounsaturated ceramide and sphingomyelin ratios as predictors of incident prediabetes
| Sphingolipids | Odds ratio | 95% CI | P value | |
|---|---|---|---|---|
| Model 1a | ||||
| Sphingosine | 1.021 | 1.011-1.031 | <.0001 | |
| C18:0/C18:1 ceramides | 1.177 | 1.061-1.304 | .0020 | |
| C26:0/C26:1 sphingomyelins | 2.579 | 1.608-4.134 | <.0001 | |
| Model 2b | ||||
| Sphingosine | 1.020 | 1.010-1.031 | .0001 | |
| C18:0/C18:1 ceramides | 1.176 | 1.061-1.303 | .0020 | |
| C26:0/C26:1 sphingomyelins | 2.716 | 1.632-4.521 | .0001 | |
| Model 3c | ||||
| Sphingosine | 1.016 | 1.000-1.032 | .0512 | |
| C18:0/C18:1 Cer | 1.236 | 1.042-1.466 | .0152 | |
| C26:0/C26:1 SM | 2.273 | 1.172-4.408 | .0151 |
Abbreviations: 2hrPG, 2-hour postload plasma glucose during oral glucose tolerance test; BMI, body mass index; Cer, ceramides; FPG, fasting plasma glucose; SM, sphingomyelins.
a Unadjusted.
b Adjusted for age, race, sex, BMI.
c Adjusted for age, race, sex, BMI, FPG, 2hrPG, insulin sensitivity, insulin secretion.
Potential Mechanisms
To explore potential mechanisms linking sphingolipids to prediabetes risk, we examined the association of sphingosine levels and sphingolipid ratios with several cardiometabolic variables. Plasma sphingosine levels and the saturated-to-monounsaturated ratios of ceramides C18:0/C18:1 and C26:0/C26:1 and sphingomyelins C18:0/C18:1 and C26:0/C26:1 exhibited variable correlations with measures of adiposity (BMI, fat mass, waist circumference), lipid profile, glycemia, and glucoregulation (insulin sensitivity and insulin secretion) (Fig. 2 and Table 6). Plasma sphingosine levels and the saturated-to-monounsaturated sphingomyelin C26:0/C26:1 ratio showed the strongest associations with adiposity measures; the sphingomyelin C26:0/C26:1 ratio also showed the strongest associations with lean mass and HDL cholesterol levels (Fig. 2 and Table 6). We also observed significant correlations between plasma levels of the sphingosine metabolite, sphingosine-1-phosphate, and weight (r = 0.19, P = .02), BMI (r = 0.20, P = .02), and waist circumference (r = 0.18, P = .04). However, baseline plasma sphingosine-1-phosphate levels were not significantly different in progressors to prediabetes vs nonprogressors (402 ± 186 pmol/mL vs 398 ± 157 pmol/mL, P = .88) (Supplementary Table S2) (29).
Figure 2.
Bubble correlogram of plasma sphingosine levels and the saturated-to-monounsaturated ratios of ceramides (Cer) and sphingomyelins (SM) with age and various cardiometabolic variables. 2hrPG, two-hour post-load plasma glucose; AIR, acute insulin response to intravenous glucose; FPG, fasting plasma glucose; Si-clamp, insulin sensitivity measured with hyperinsulinemic euglycemic clamp; Bubble size represents the relative correlation coefficients; larger circle indicates higher correlation. Color intensity represents the magnitude of positive (red) or inverse (blue) correlation between individual sphingolipid markers and the various cardiometabolic measures (see additional data in Table 6). *P < .05; **P = .01; ***P < .001.
Table 6.
Association of age and metabolic variables with plasma levels of sphingosine and the saturated-to-monounsaturated ratios of ceramides and sphingomyelins
| Sphingosine (pmol/mL) | Ceramides C18:0/C18:1 | Ceramides C26:0/C26:1 | Sphingomyelins C18:0/C18:1 | Sphingomyelins C26:0/C26:1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | r | P | r | P | r | P | r | P | r | P |
| Age at enrollment | 0.04 | .68 | −0.07 | .43 | −0.003 | .97 | 0.16 | .05 | −0.02 | .79 |
| BMI (kg/m2) | 0.22 | .01 | −0.005 | .96 | 0.08 | .35 | −0.07 | .45 | 0.07 | .39 |
| Waist circumference | 0.34 | <.0001 | 0.03 | .74 | 0.14 | .11 | −0.02 | .80 | 0.11 | .18 |
| Total fat mass (kg) | 0.20 | .02 | −0.06 | .50 | 0.07 | .45 | −0.14 | .12 | 0.12 | .17 |
| Lean mass (kg) | 0.12 | .18 | −0.006 | .99 | 0.05 | .53 | 0.17 | .05 | 0.31 | .0003 |
| FPG (mg/dL) | 0.07 | .40 | −0.01 | .95 | 0.08 | .33 | 0.20 | .02 | 0.02 | .86 |
| 2hrPG (mg/dL) | 0.19 | .03 | 0.10 | .26 | 0.09 | .32 | 0.10 | .26 | 0.10 | .22 |
| Total cholesterol (mg/dL) | 0.05 | .58 | 0.11 | .21 | −0.066 | .44 | −0.064 | .46 | −0.09 | .31 |
| HDL cholesterol (mg/dL) | −0.15 | .07 | −0.02 | .80 | −0.091 | .29 | −0.18 | .036 | −0.36 | <.0001 |
| Triglycerides (mg/dL) | 0.13 | .14 | 0.02 | .82 | 0.012 | .89 | 0.11 | .21 | 0.11 | .19 |
| Si-clamp | −0.09 | .36 | 0.04 | .71 | −0.08 | .44 | 0.13 | .19 | −0.15 | .13 |
| AIR (µU/mL) | 0.10 | .26 | −0.02 | .81 | −0.008 | .93 | −0.12 | .17 | 0.13 | .13 |
| Disposition index | −0.07 | .47 | 0.04 | .72 | 0.15 | .13 | −0.02 | .86 | 0.17 | .10 |
Abbreviations: 2hrPG, 2-hour postload plasma glucose during oral glucose tolerance test; AIR, acute insulin response to IV glucose; BMI, body mass index; Cer, ceramides; FFM, fat-free mass; FPG, fasting plasma glucose; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Si-clamp (µmol/kgFFM/min−1/pM), insulin sensitivity measured with hyperinsulinemic euglycemic clamp; SM, sphingomyelins.
Receiver Operating Characteristic Curve Analysis
We undertook ROC curve analysis to assess the performance of baseline plasma sphingosine levels and the ratios of ceramide C18:0/C18:1 and sphingomyelin C26:0/C26:1 as predictors of incident prediabetes (Fig. 3). The AUC of plasma sphingosine level was 0.7394 compared with 0.6338 for ceramide C18:0/C18:1 ratio and 0.7314 for sphingomyelin C26:0/C26:1 ratio. The combined ratios of ceramide C18:0/C18:1 and sphingomyelin C26:0/C26:1 generated a greater AUC (0.7736) compared with the AUC of individual ratios; the inclusion of sphingosine further increased the AUC to 0.8173 (Fig. 3). Thus, baseline plasma levels of sphingosine, the ratio of ceramides C18:0/C18:1, or the ratio of sphingomyelin C26:0/C26:1 each performed better than would be expected by chance in predicting progression to prediabetes, but their combination was a better predictor than individual tests. The addition of clinical variables (age, BMI, and glucose) to the regression model further increased the AUC to 0.8387 (Fig. 3).
Figure 3.
Receiver operating characteristics (ROC) curves for classifying participants as progressors or nonprogressors to prediabetes using baseline plasma levels of sphingosine (A), saturated-to-monounsaturated ceramide and sphingomyelin ratios (B, C, D), and the latter in combination with sphingosine (E) along with clinical variables (F) in the Pathobiology of Prediabetes in a Biracial Cohort study.
We compared the c-statistic between model 3 (Table 5) and a model comprising clinical predictors (age, race, sex, BMI, FPG, 2hrPG, insulin sensitivity, insulin secretion) only, excluding the 3 sphingolipid markers (sphingosine, ceramides C18:0/C18:1, and sphingomyelin C26:0/C26:1), using ROC analysis. The result showed that the addition of the 3 sphingolipid markers significantly increased the prediction of incident prediabetes compared with clinical parameters alone (P = .0068).
Discussion
Among initially normoglycemic participants in our prospective POP-ABC study, we found that baseline plasma levels of certain sphingolipids were significantly different in participants who developed prediabetes during 5 years of follow-up compared with those who maintained normoglycemia during the same follow-up period. Specifically, we observed that progressors to prediabetes had higher baseline levels of saturated long chain ceramide (C18:0) and saturated very-long-chain sphingomyelin C26:0 and lower levels of unsaturated long-chain ceramide C18:1 and unsaturated very-long-chain sphingomyelin C26:1 compared with nonprogressors. The progressors also had ∼50% higher mean baseline plasma sphingosine level than nonprogressors.
Our analysis of baseline plasma specimens further showed a lower abundance of sphingomyelin as a percentage of total sphingolipids in individuals who developed prediabetes vs those who maintained normoglycemia during follow-up. Lower sphingomyelin levels have been associated with higher risk of T2D among obese individuals, and weight loss has been reported to increase circulating sphingomyelin levels (30). Carlsson et al (30) measured serum sphingomyelin levels before Roux-en-Y gastric bypass surgery and followed postoperative levels for approximately 2 years in 220 patients with obesity. The study participants were divided into those with or without diabetes. Lower preoperative serum sphingomyelin levels were reported in participants with diabetes compared with those without diabetes. Standardized for body weight, Roux-en-Y gastric bypass appeared to induce a relative increase in postoperative serum sphingomyelin levels (30).
In a prospective analysis of the association between serum metabolites and type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition-Potsdam study, Floegel et al (31) identified baseline levels of sphingomyelin 16:1 as protective of incident diabetes, with a relative risk (per SD) of 0.80 (95% CI, 0.72-0.88), after adjusting for BMI, waist circumference, and several demographic and behavioral variables. Thus, the previous reports, in accord with our present findings, suggest a possible link between alterations in sphingomyelin metabolism and the pathogenesis of diabetes and prediabetes.
Although we observed that lower plasma sphingomyelin levels were associated with a higher risk of incident prediabetes, the exact origin(s) of circulating sphingomyelin are unclear. Ingested sphingolipids and ceramides are poorly absorbed, and dietary sphingomyelin is digested by intestinal sphingomyelinase and neutral ceramidase to the more absorbable sphingosine (32, 33). Sphingomyelin in cell membranes can be degraded to ceramide by acidic or neutral sphingomyelinases; the increased ceramide accumulation is associated with insulin resistance and apoptosis of pancreatic β cells (34, 35). The lower plasma sphingomyelin levels observed in people at risk for prediabetes in the present study, and similar findings by others in relation to diabetes risk (31), can be explained by either decreased synthesis or increased catabolism. Increased hydrolysis of sphingomyelin would lower sphingomyelin abundance while increasing the abundance of ceramides (34, 35). Ceramide synthesis occurs in the endoplasmic reticulum, and nascent molecules are transported from the endoplasmic reticulum to the Golgi apparatus by the ceramide transporter CERT and subsequently converted to other sphingolipid species. There is evidence of decreased CERT protein expression in experimental models of insulin resistance (36). Furthermore, overexpression of CERT reduces ceramide accumulation in muscle leading to improved insulin signaling, whereas CERT inhibition accentuates lipotoxicity (36). These reports suggest that impaired intracellular trafficking of ceramides generated from sphingomyelin could lead to ceramide accumulation and muscle insulin resistance.
Our finding of higher plasma sphingosine levels in progressors to prediabetes vs nonprogressors is consistent with the physiology of ceramide production. In addition to de novo biosynthesis and hydrolysis of complex sphingolipids, ceramides can be produced via salvage of sphingosine generated in the lysosome (35). Alternatively, sphingosine can be derived from ceramides through the action of ceramidases. Sphingosine can be phosphorylated to sphingosine-1-phosphate, a bioactive lipid that has been linked to obesity and T2D (37). Congruently, we found a significant correlation between plasma levels of sphingosine and its metabolite, sphingosine-1-phosphate, and measures of adiposity among our POP-ABC study participants.
Previous studies had reported associations between sphingolipids and diabetes risk (11–18). Our present study extends such associations to prediabetes, an earlier stage in T2D pathogenesis. Unlike cross-sectional studies, the data from our prospective POP-ABC study participants showed that alterations in sphingolipid metabolism preceded dysregulation of glucose metabolism, consistent with a possible pathophysiological role. Remarkably, baseline plasma sphingosine levels and the saturated-to-monounsaturated ratios of long-chain fatty acid ceramide C18:0/C18:1 and very-long-chain fatty acid sphingomyelin C26:0/C26:1 emerged as strong predictors of incident prediabetes risk after adjustments for clinical variables. The specificity of those findings (of 58 sphingolipid species assayed in the present study) is noteworthy.
Our findings are in general accord with previous reports linking certain sphingolipid species to diabetes incidence (38–42). In the study by Wigger et al, elevated plasma levels of stearic acid ceramide and other long chain dihydroceramides preceded the onset of T2D by 3 to 9 years in Europeans (41). Similarly, the study by Hilvo et al found that stearic acid ceramide [Cer(d18:1/18:0)] and the stearic acid to palmitic acid ceramide ratio [Cer(d18:1/18:0)/Cer(d18:1/16:0)] each significantly predicted incident diabetes during 13 years of follow up of participants in the FINRISK 2002 study after adjusting for pertinent variables including BMI and glycemia (40). The FINRISK 2002 population had 445 people with prevalent diabetes and 593 individuals who developed incident diabetes during follow-up. Interestingly, the elevated stearic ceramide levels were observed in participants with prevalent diabetes and those with incident diabetes compared with participants without diabetes during follow-up (40). Importantly, those findings were validated in an independent cohort of 3344 initially diabetes-free individuals who were assessed for incident diabetes during a median follow-up duration of 7.6 years (40). Furthermore, Hilvo et al reported that weight loss of 5% or greater decreased the elevated stearic to palmitic acid ceramide ratio and diabetes risk scores during 2 years of lifestyle intervention in a group of 371 adults (40). The latter finding is consistent with a previous report showing significantly decreased plasma C14:0, C16:0, C18:1, and C24:0 ceramide levels following exercise intervention that also induced weight loss and improved insulin sensitivity in individuals with obesity and normal glucose tolerance (43).
In the present study, we show that higher baseline ratios of stearic acid to oleic acid ceramide (C18:0/C18:1) and sphingomyelin C26:0/C26:1 preceded the development of prediabetes by 5 years, among initially normoglycemia African Americans and European Americans. These findings suggest that elevated saturated-to-monounsaturated ratios of ceramide C18:0/18:1 and sphingomyelin C26:0/C26:1 could serve as predictive biomarkers for prediabetes and early glucose dysregulation. Furthermore, taken together with previous reports linking elevated stearic acid ceramide to incident diabetes risk (40, 41), our findings suggest that elevated stearic acid ceramide [Cer(d18:1/18:0)] might be a biomarker of longitudinal risk across the spectrum from prediabetes to T2D. Accumulation of saturated stearic acid ceramide is associated with skeletal muscle and whole-body insulin resistance and pancreatic β-cell dysfunction (10, 17, 40–45). Our observation that higher baseline levels of saturated sphingolipids and lower levels of monounsaturated sphingolipids were associated with incident prediabetes risk supports the merits of current recommendations regarding dietary fat intake. Those recommendations generally promote reduction in saturated fat intake and substitution with mono- and polyunsaturates for diabetes prevention and cardiovascular health (46, 47). In exploring possible mechanisms linking circulating sphingolipids to glucose dysregulation, we found variable, but generally congruent, associations between sphingosine and certain sphingolipid ratios and measures of adiposity, lipidemia, insulin sensitivity, and insulin secretion. Plasma levels of sphingosine (higher in progressors to prediabetes vs nonprogressors) correlated positively with BMI, waist circumference, fat mass, and glucose, and inversely with HDL cholesterol levels. The saturated-to-monounsaturated ratio of sphingomyelin C26:0/C26:1 (also higher in progressors to prediabetes vs nonprogressors) correlated positively with lean and fat mass and inversely with insulin sensitivity and HDL cholesterol levels. These findings indicate that the sphingolipid species and ratios linked to incident prediabetes in the present study exhibited discernible associations with anthropometric and biochemical markers of insulin resistance.
Our study has several strengths, including the enrollment of high-risk individuals with parental diabetes, equal representation of male and female as well as Black and White participants, and rigorous ascertainment of prediabetes endpoints. Other strengths include the longitudinal design of the POP-ABC study, the extensive follow-up period, and the employment of robust methodology for measuring insulin sensitivity and insulin secretion. Importantly, the prospective design of our study enables insight into the direction of the association between alterations in sphingolipid levels and glucose dysregulation. Furthermore, we were able to explore potential mechanistic associations between candidate sphingolipid predictors of prediabetes and several cardiometabolic variables. Notably, the sphingolipid levels observed in the present study, including percentage composition of sphingomyelin, ceramides, monohexosylceramides, and sphingoid bases, were in line with values reported in pooled human plasma from healthy men and women with an ethnic distribution that is representative of the US population (48).
One limitation of our study is the restriction of participation in the POP-ABC study, by design, to Black and White adults with parental history of diabetes. Thus, our findings may not be generalizable to the wider population. Further, by limiting our targeted analysis to sphingolipids containing d18:1 sphingoid base, we cannot exclude possible contributions of unmeasured sphingolipids (such as dihydro species of ceramide, monohexosyl ceramide, and sphingomyelins, and other sphingoid base-containing sphingolipids) to our overall findings. Moreover, we assessed sphingolipids only at baseline and cannot exclude the possible contribution of temporal changes in sphingolipid levels to our findings. Another weakness is the lack of data on dihydroceramides in our study cohort. It has been suggested that dihydroceramide desaturase 1-catalyzed conversion of dihydroceramides to ceramides might play a role in the pathogenesis of insulin resistance and hepatic steatosis (49). Thus, inclusion of data on dihydroceramides could have provided additional mechanistic insights on the relationship between sphingolipids and prediabetes risk.
Finally, although our results are in general accord with previous reports linking saturated sphingolipids to increased risk of T2D (37–39), our present findings relative to incident prediabetes risk would need to be validated in an independent cohort.
In conclusion, of 58 sphingolipid species examined in the present study, the saturated-to-monounsaturated ratio of 2 species (C18:0/C18:1 ceramide and C26:0/C26:1 sphingomyelin) emerged as significant predictors of progression to prediabetes among initially normoglycemic Black and White offspring of parents with T2D. Thus, C18:0/C18:1 ceramide and C26:0/C26:1 sphingomyelin ratios may be specific biomarkers of prediabetes and early glucose dysregulation.
Acknowledgments
The authors thank the research volunteers who participated in the POP-ABC study and the University of Tennessee Clinical Research Center staff for their assistance.
Abbreviations
- 2hrPG
2-hour postload plasma glucose during oral glucose tolerance test
- AUC
area under the curve
- BMI
body mass index
- FPG
fasting plasma glucose
- GCRC
General Clinical Research Center
- HDL
high-density lipoprotein
- IRB
institutional review board
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- OGTT
oral glucose tolerance test
- ROC
receiver operating characteristic
- T2D
type 2 diabetes
Contributor Information
Samuel Dagogo-Jack, Department of Medicine, Division of Endocrinology, Diabetes and Metabolism, University of Tennessee Health Science Center, Memphis, TN 38163, USA; General Clinical Research Center, University of Tennessee Health Science Center, Memphis, TN 38163, USA.
Peace Asuzu, Department of Medicine, Division of Endocrinology, Diabetes and Metabolism, University of Tennessee Health Science Center, Memphis, TN 38163, USA.
Jim Wan, Department of Preventive Medicine, University of Tennessee Health Science Center, Memphis, TN 38163, USA.
Richard Grambergs, Department of Medicine, College of Medicine, University of Tennessee Health Science Center, Memphis, TN 38163, USA.
Frankie Stentz, Department of Medicine, Division of Endocrinology, Diabetes and Metabolism, University of Tennessee Health Science Center, Memphis, TN 38163, USA.
Nawajes Mandal, Departments of Ophthalmology, Anatomy and Neurobiology, University of Tennessee Health Science Center, Memphis, TN 38163, USA; Memphis VA Medical Center, Memphis, TN 38104, USA.
Funding
Supported by grants from the National Institute of Diabetes Digestive and Kidney Diseases (R01 R01DK067269, R01DK128129). The CASPID study is supported by a grant from the National Institute of Diabetes Digestive and Kidney Diseases (R01DK128129). The POP-ABC study was supported by a grant from the National Institute of Diabetes Digestive and Kidney Diseases (R01 DK067269). The funding source had no role in the execution of the study or the decision to submit the findings for publication. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Author Contributions
S.D.-J. and N.M. were involved in the conceptualization and design of the study and the analysis and interpretation of the results. F.S. and P.A. were involved in the conduct of the study, R.G. was involved in analysis and interpretation of the results, and J.W. performed statistical analysis. S.D.-J. wrote the first draft of the manuscript and all authors edited, reviewed, and approved the final version of the manuscript.
Disclosures
The authors report no conflicts of interest related to the content of the present manuscript.
Data Availability
The source data analyzed in the current study are provided with this paper. Any other data that support the findings of this study can be obtained from the corresponding author on request.
Declaration of Generative AI and AI-assisted Technologies in the Writing Process
The authors did not use any AI tool or service during the preparation of this work.
References
- 1. ElSayed NA, Aleppo G, Aroda VR, et al. American Diabetes Association. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S19‐S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brannick B, Dagogo-Jack S. Prediabetes and cardiovascular disease: pathophysiology and interventions for prevention and risk reduction. Endocrinol Metab Clin North Am. 2018;47(1):33‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. White NH, Pan Q, Knowler WC, et al. Risk factors for the development of retinopathy in prediabetes and type 2 diabetes: the Diabetes Prevention Program experience. Diabetes Care. 2022;45(11):2653‐2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boucher AB, Adesanya EAO, Owei I, et al. Dietary habits and leisure-time physical activity in relation to adiposity, dyslipidemia, and incident dysglycemia in the pathobiology of prediabetes in a biracial cohort study. Metabolism. 2015;64(9):1060‐1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Owei I, Umekwe N, Provo C, Wan J, Dagogo-Jack S. Insulin-sensitive and insulin-resistant obese and non-obese phenotypes: role in prediction of incident pre-diabetes in a longitudinal biracial cohort. BMJ Open Diabetes Res Care. 2017;5(1):e000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51(1):7‐18. [DOI] [PubMed] [Google Scholar]
- 7. Schmidt MI, Duncan BB, Bang H, et al. Atherosclerosis risk in communities investigators. Identifying individuals at high risk for diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care. 2005;28(8):2013‐2018. [DOI] [PubMed] [Google Scholar]
- 8. Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, D’Agostino RB. Prediction of incident diabetes mellitus in middle-aged adults: the Framingham offspring study. Arch Intern Med. 2007;167(10):1068‐1074. [DOI] [PubMed] [Google Scholar]
- 9. Owei I, Umekwe N, Wan J, Dagogo-Jack S. Plasma lipid levels predict dysglycemia in a biracial cohort of nondiabetic subjects: potential mechanisms. Exp Biol Med (Maywood). 2016;241(17):1961‐1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mandal N, Grambergs R, Mondal K, Basu SK, Tahia F, Dagogo-Jack S. Role of ceramides in the pathogenesis of diabetes mellitus and its complications. J Diabetes Complications. 2021;35(2):107734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meikle PJ, Summers SA. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat Rev Endocrinol. 2017;13(2):79‐91. [DOI] [PubMed] [Google Scholar]
- 12. Neeland IJ, Singh S, McGuire DK, et al. Relation of plasma ceramides to visceral adiposity, insulin resistance and the development of type 2 diabetes mellitus: the Dallas heart study. Diabetologia. 2018;61(12):2570‐2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J Lipid Res. 2009;50(Suppl):S91‐S96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hannun YA, Obeid LM. Sphingolipids and their metabolism in physiology and disease. Nat Rev Mol Cell Biol. 2018;19(3):175‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roszczyc-Owsiejczuk K, Zabielski P. Sphingolipids as a culprit of mitochondrial dysfunction insulin resistance and type 2 diabetes. Front Endocrinol (Lausanne). 2021;12:635175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sokolowska E, Blachnio-Zabielska A. The role of ceramides in insulin resistance. Front Endocrinol (Lausanne). 2019;10:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yun H, Sun L, Wu Q, et al. Associations among circulating sphingolipids, β-cell function, and risk of developing type 2 diabetes: a population-based cohort study in China. PLoS Med. 2020;17(12):e1003451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Turpin-Nolan SM, Brüning JC. The role of ceramides in metabolic disorders: when size and localization matters. Nat Rev Endocrinol. 2020;16(4):224‐233. [DOI] [PubMed] [Google Scholar]
- 19. Ebenibo S, Edeoga C, Ammons A, Egbuonu N, Dagogo-Jack S. Pathobiology of prediabetes in a biracial cohort (POP-ABC) research group. Recruitment strategies and yields for the pathobiology of prediabetes in a biracial cohort: a prospective natural history study of incident dysglycemia. BMC Med Res Methodol. 2013;13(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dagogo-Jack S, Edeoga C, Ebenibo S, Nyenwe E, Wan J. Lack of racial disparity in incident prediabetes and glycemic progression among black and white offspring of parents with type 2 diabetes: the pathobiology of prediabetes in a biracial cohort (POP-ABC) study. J Clin Endocrinol Metab. 2014;99(6):E1078‐E1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jiang Y, Owei I, Wan J, Ebenibo S, Dagogo-Jack S. Adiponectin levels predict prediabetes risk: the pathobiology of prediabetes in A biracial cohort (POP-ABC) study. BMJ Open Diabetes Res Care. 2016;4(1):e000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Owei I, Umekwe N, Stentz F, Wan J, Dagogo-Jack S. Association of plasma acylcarnitines with insulin sensitivity, insulin secretion, and prediabetes in a biracial cohort. Exp Biol Med (Maywood). 2021;246(15):1698‐1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mandal N, Asuzu P, Stentz F, Wan J, Dagogo-Jack S. Ceramides and other sphingolipids as predictors of incident dysglycemia (CASPID): design, methods, and baseline characteristics. Exp Biol Med (Maywood). 2023:248(16):1393‐1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214‐E223. [DOI] [PubMed] [Google Scholar]
- 25. Wilmott LA, Grambergs RC, Allegood JC, Lyons TJ, Mandal N. Analysis of sphingolipid composition in human vitreous from control and diabetic individuals. J Diabetes Complications. 2019;33(3):195‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shaner RL, Allegood JC, Park H, et al. Quantitative analysis of sphingolipids for lipidomics using triple quadrupole and quadrupole linear ion trap mass spectrometers. J Lipid Res. 2009;50(8):1692‐1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Supplemental Figure S1. Chromatogram illustrating the separation of different sphingolipid species by the retention time and m/z ratios. lcms-figure-s1.pdf (uthsc.edu).
- 28. Supplemental Table S1. Comparison of plasma sphingolipids levels in baseline plasma specimens from participants who progressed or did not progress to prediabetes during 5 years of follow-up. sphingolipids-table-s1.pdf (uthsc.edu).
- 29.Supplemental Table S2. AB Sciex 5500 QTrap Mass Spectrometer Settings for Complex Sphingolipids. sphingolipids-table-s2.pdf (uthsc.edu).
- 30. Carlsson ER, Grundtvig JLG, Madsbad S, Fenger M. Changes in serum sphingomyelin after Roux-en-Y gastric bypass surgery are related to diabetes status. Front Endocrinol (Lausanne). 2018;9:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Floegel A, Stefan N, Yu Z, et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes. 2013;62(2):639‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ohlsson L, Hertervig E, Jönsson BAG, et al. Sphingolipids in human ileostomy content after meals containing milk sphingomyelin. Am J Clin Nutr. 2010;91(3):672‐678. [DOI] [PubMed] [Google Scholar]
- 33. Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv Exp Med Biol. 2010;688:1‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wigger D, Gulbins E, Kleuser B, Schumacher F. Monitoring the sphingolipid de novo synthesis by stable-isotope labeling and liquid chromatography-mass spectrometry. Front Cell Dev Biol. 2019;7:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Galadari S, Rahman A, Pallichankandy S, Galadari A, Thayyullathil F. Role of ceramide in diabetes mellitus: evidence and mechanisms. Lipids Health Dis. 2013;12(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bandet CL, Mahfouz R, Véret J, et al. Ceramide transporter CERT is involved in muscle insulin signaling defects under lipotoxic conditions. Diabetes. 2018;67(7):1258‐1271. [DOI] [PubMed] [Google Scholar]
- 37. Kowalski GM, Carey AL, Selathurai A, Kingwell BA, Bruce CR. Plasma sphingosine-1-phosphate is elevated in obesity. PLoS One. 2013;8(9):e72449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wigger D, Schumacher F, Schneider-Schaulies S, Kleuser B. Sphingosine 1-phosphate metabolism and insulin signaling. Cell Signal. 2021;82:109959. [DOI] [PubMed] [Google Scholar]
- 39. Chen GC, Chai JC, Yu B, et al. Serum sphingolipids and incident diabetes in a US population with high diabetes burden: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Am J Clin Nutr. 2020;112(1):57‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hilvo M, Salonurmi T, Havulinna AS, et al. Ceramide stearic to palmitic acid ratio predicts incident diabetes. Diabetologia. 2018;61(6):1424‐1434. [DOI] [PubMed] [Google Scholar]
- 41. Wigger L, Cruciani-Guglielmacci C, Nicolas A, et al. Plasma dihydroceramides are diabetes susceptibility biomarker candidates in mice and humans. Cell Rep. 2017;18(9):2269‐2279. [DOI] [PubMed] [Google Scholar]
- 42. Haus JM, Kashyap SR, Kasumov T, et al. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58(2):337‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kasumov T, Solomon TP, Hwang C, et al. Improved insulin sensitivity after exercise training is linked to reduced plasma C14:0 ceramide in obesity and type 2 diabetes. Obesity (Silver Spring). 2015;23(7):1414‐1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tonks KT, Coster AC, Christopher MJ, et al. Skeletal muscle and plasma lipidomic signatures of insulin resistance and overweight/obesity in humans. Obesity (Silver Spring). 2016;24(4):908‐916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab. 2012;15(5):585‐594. [DOI] [PubMed] [Google Scholar]
- 46. Jaacks LM, Ma Y, Davis N, et al. Long-term changes in dietary and food intake behavior in the Diabetes Prevention Program outcomes study. Diabet Med. 2014;31(12):1631‐1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hooper L, Martin N, Jimoh OF, Kirk C, Foster E, Abdelhamid AS. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev. 2020;19(5):CD011737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Quehenberger O, Armando AM, Brown AH, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. 2010;51(11):3299‐3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chaurasia B, Tippetts TS, Mayoral Monibas R, et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science. 2019;365(6451):386‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The source data analyzed in the current study are provided with this paper. Any other data that support the findings of this study can be obtained from the corresponding author on request.