Abstract
This study aimed to investigate the interfacial behaviour of caseins in different micelle content and its effect on the stability of emulsions, including micellar casein concentrate (MCN), calcium caseinate (CaC) and sodium caseinate (NaC). Results revealed that at high protein concentrations (0.5 %–2.5 %), MCN, CaC and NaC exhibited similar interfacial behaviour as well as unfolding rate constants (k1) of 3.11–3.41 × 10−4 (s−1), 2.96–3.35 × 10−4 (s−1) and 2.75–3.27 × 10−4 (s−1), respectively. The interfacial layer formed was dominated by non-micelles, and microscopic images revealed the thickness of the interfacial layer to be 10–20 nm. By contrast, at low concentrations, the differences in the slope of E–π curves and k1 indicated that the micelle content of casein affects protein interfacial behaviour and properties and that micellar casein is involved in the formation of the interfacial layer. The formation of large numbers of droplets during emulsion preparation results in a similar low concentration environment. Cryo-TEM showed adsorption of micellar casein in all three casein-stabilised emulsions, and the amount of adsorption was proportional to the micelle content. NaC has faster adsorption and rearrangement rates due to fewer micelles and more non-micelles, so that NaC forms smaller droplets and more stable emulsions than those formed by MCN and CaC within the range of 0.5 % to 2.0 %.
Keywords: Casein, Adsorption kinetics, Micelle content, Emulsion stability
Highlights
-
•
Non-micellar casein dominates the O—W interfacial layer at high concentrations.
-
•
Diffusion limitations in emulsion production allow micellar casein to adsorb to the interface.
-
•
Casein aggregation state determines the morphology of the droplet protein layer.
-
•
Discrepancy in the percentage of micellar caseins affects the dairy cream properties greatly.
1. Introduction
The adsorption and emulsification of proteins at the oil–water interface of oil-in-water emulsion are closely related to its stabilisation properties. Previous studies have elucidated the three steps involved in protein adsorption at the oil–water interface: diffusion of proteins from the aqueous phase to the oil–water interface, unfolding and rearrangement of proteins at the interface to minimise the Gibbs free energy and formation of viscoelastic interfacial layers via intermolecular interaction among proteins (Amine et al., 2014; Day et al., 2014; Tang & Shen, 2015). The interfacial behaviour of proteins play a crucial role in determining the emulsifying properties, however, various factors such as the molecular size, structure and concentration of proteins affect their interfacial behaviour. Flexible proteins (e.g. β-casein) are more effective than rigid proteins (e.g. whey proteins) in reducing interfacial tension, indicating a better emulsifying property of the former (Dickinson, 2011; Gülseren & Corredig, 2012). Moreover, the interfacial layer formed by flexible or small proteins demonstrates a higher rate of interfacial rearrangement, and a tighter interfacial layer results in higher viscoelasticity, thereby preventing fat globule aggregation (Zhou et al., 2020).
In bovine milk, caseins primarily exist as micelles, accounting for 93 % of the four casein monomers: β-casein, αS1-casein, αS2-casein and κ-casein and 7 % of minerals, which are linked by hydrogen bonds, hydrophobic interactions and colloidal calcium phosphates (Panouille et al., 2007; Zhou et al., 2022). In general, micellar casein and non-micellar casein (casein aggregates, casein monomers, etc.) coexist in dynamic equilibrium, which is affected by pH, temperature and ionic strength of Ca2+ (Chen et al., 2016; Zhou et al., 2022). The micelles are roughly spherical in shape, with diameters ranging from 50 to 600 nm, averaging 120 nm, and masses ranging 103 from 109 kDa; the widely accepted models include the coat–core, non-micellar and Holt models. The micelle content of casein in commercially available casein powders differ owing to different preparation processes. The conventional caseinates calcium caseinate (CaC) and sodium caseinate (NaC) are isolated from whey proteins by lowering their pH and are widely used in various dairy and meat products; however, changes in pH lead to the dissolution of colloidal calcium phosphates, thereby leading to complete or partial decomposition of micellar casein (Silva et al., 2013). Micellar casein concentrate (MCN) is obtained by removing lactose, minerals and whey protein through microfiltration and ultrafiltration, and its structure is nearly identical to that of native casein (Ji et al., 2016). Our previous study reported significant differences in the properties of dairy cream prepared from different casein powders (Li et al., 2020), these differences may be related to the varying degree of dissociation of casein micelles.
Previous studies on the interfacial properties of casein mainly focused on individual caseins in model systems. For example, β-casein with a flexible conformation is always used as a model protein to examine its adsorption mechanism and interaction with small-molecule emulsifiers at the oil–water interface. At the interface, the thickness of the saturated monolayer coverage of β-casein was <2 nm in the dense inner region, with a 10-nm hydrophilic tail in the extended outer region (Dickinson, 2001). Presently, the differences in the interfacial properties of micellar/non-micellar casein in food emulsions are poorly understood and require extensive attention. Lazzaro et al. (2017) modified micellar casein using trisodium citrate to produce four casein dispersions with different aggregate states. The dispersions displayed similar interfacial properties, forming a solid-like layer around the fat droplets. In addition, Zhou et al. (2022) investigated the interfacial properties of micellar casein dispersion, pellet redispersion and supernatant adsorption at the oil–water interface and reported that the thicknesses of the interfacial layers formed by the three casein dispersions were 22.2 ± 2.3, 11.1 ± 1.5 and 27.5 ± 0.1 nm, respectively. However, the interfacial adsorption behaviour of the different micelle content of casein at different concentrations and its effect on emulsions remain unclear.
Thus, this study aimed to investigate the interfacial behaviours and emulsifying properties of different concentrations of MCN, CaC and NaC at the oil–water interface of emulsions. Determination of interfacial properties, Fourier-transform infrared spectroscopy (FTIR) and evaluation of interfacial layer micromorphology were explicitly performed. Subsequently, MCN, CaC and NaC were used to make emulsions using anhydrous milk fat (AMF). The emulsion stability was characterised in terms of the distribution of particle size and the microstructure of the fat droplets. This study provides further insight into caseins in different micelle content to better understand their applications in the dairy industry.
2. Materials and methods
2.1. Materials
AMF was purchased from Anchor (Auckland, New Zealand). The MCN powder was kindly donated by Leprino (Denver, USA). The NaC and CaC powders were purchased from Arla (Copenhagen, Denmark).
2.2. Casein dispersion properties
Before the test, casein dispersions were diluted with deionised water to 0.0025 wt% and filtered through 0.45-μm cut syringe filters. Particle sizes were determined at 25 °C using a Malvern Zetasizer Nano-ZS90 (Malvern Instruments Ltd., Worcestershire, UK). The refractive and adsorption indices of the dispersed phase were 1.460 and 0.001, respectively, whereas the refractive index of the continuous phase was 1.330. Equilibration time was 2 min.
The casein dispersion was centrifuged at 100,000 ×g for 60 min at 25 °C using a MAX-XP ultracentrifuge (Beckman Coulter, Inc., California, USA) to determine the percentage of non-micellar casein in each dispersion (Pranata et al., 2024). The supernatant, i.e. non-micellar casein, was collected, and its nitrogen content was determined using the Kjeldahl method.
Protein surface hydrophobicity was evaluated using the fluorescence probe ANS (8-anilino-1-naphthalenesulfonic acid) via a Perkin–Elmer (LS 55) spectrometer. The casein dispersions were mixed with ANS (8 mmol/L in 0.01 mol/L phosphate buffer, pH 7.0) in a series of concentrations ranging from 0.002 % to 0.01 %. ANS solution (20 μL) was added to 4 mL of each sample, and the fluorescence intensity was measured at 25 °C. As a reference, the fluorescence intensity of the phosphate buffer with ANS was measured. The excitation and emission slits were 10 nm. The excitation and emission wavelengths were 390 and 470 nm, respectively. As an index of surface hydrophobicity, the slope of a fluorescence intensity plot as a function of protein concentration was used (Wan et al., 2018).
2.3. Interfacial properties
2.3.1. Surface pressure
A drop tensiometer was used to measure the interfacial tension (DSA100, Kruss GmbH, Hamburg, Germany). A syringe with a diameter of 1.0 mm was placed in a glass cuvette containing AMF, which was purified using a Florisil adsorbent to remove surface-active impurities as per the a previously described method (Liu et al., 2012). Next, a 15-μL casein dispersion droplet was created using the syringe, and the interfacial tension as a function of time was calculated automatically using the ADVANCE software (Kruss GmbH, Hamburg, Germany). The surface pressure (π) was determined using Eq. (1) (Zhou et al., 2020).
| (1) |
where γ0 is the interfacial tension between AMF and water after purification (30.50 ± 0.50 mN/m) and γt is the interfacial tension between AMF and casein dispersion at time t. The measurement was conducted at 42 °C as described in previous studies (Baldursdottir et al., 2010; Liu et al., 2012).
2.3.2. Interfacial rheology
DSA100 coupled with oscillating drop accessory DS4270 (Kruss GmbH, Hamburg, Germany) was used to determine interfacial dilatational rheology. During the measurement, the drop volume was subjected to eight cycles of sinusoidal compression and expansion with an amplitude of 0.5 % (dA/A) and a frequency of 0.1 Hz, which are well within the linear viscoelastic regime. The interfacial dilatational modulus (E), related to the change in interfacial tension (γ), can be described using Gibbs Eq. (2).
| (2) |
The interfacial E is described to be a complex quantity comprising real (Ed, dilatational elasticity modulus) and imaginary (Ev, dilatational viscous modulus) parts. This measurement was described in detail in a previous study (Liu et al., 2011).
2.3.3. Interfacial layer microstructure
Cryo-transmission electron microscopy (Cryo-TEM) was used to observe the micromorphology of the interfacial layers. This measurement was described in detail in a previous study (Li et al., 2020). The thickness of the interfacial layer was measured using a TEM scale tool.
2.4. Emulsion preparation
The emulsion was prepared by combining casein dispersions with AMF heated to 70 °C to completely melt the fat before mixing. Subsequently, the coarse emulsions were homogenised at 6 MPa using a two-stage homogeniser (APV-1000, Albertslund, Denmark). The dairy emulsion contained 35.5 wt% AMF and 0.5–2.5 wt% caseins.
2.5. Emulsion properties
2.5.1. Particle size distribution and microstructure of the fat droplets
The particle size distribution of fat globules in dairy emulsions was measured using an LS 230 (Beckman Coulter, Inc., California, USA). The refractive and adsorption indices of the dispersed phase were 1.460 and 0.001, respectively, and the samples were diluted 10 times with deionised water before measurement. A Leica DM IRE2 TCS SP2 (Heidelberg, Germany) inverted microscope with a 63× objective was used to acquire the images of fat droplets. The dairy emulsions were then diluted at a ratio of 1:10 (v/v) with deionised water. To label the fat, 20 μL of 0.02 % w/v Nile red (in dimethyl sulfoxide) was added. Nile red was excited using an argon laser at 488 nm and the dye was emitted at 595–648 nm.
2.5.2. Emulsion stability
2.5.2.1. Coalescence degree
The coalescence degree of the dairy emulsions was determined according to the method published by Tcholakova et al. (2002) with some modifications. Subsequently, 25 g of the dairy emulsion was centrifuged at 25 °C for 1 h at 5000 ×g, followed by 2 h at 50 °C. The percentage of oil separated from the partial coalescence droplets in the total fat was determined.
2.5.2.2. Creaming stability
The creaming stability of the emulsions was analysed using the LUMiFuge (LUM, Berlin, Germany), which monitors the transmitted intensity of near-infrared (NIR) light as a function of centrifugation time and calculates the instability index of the emulsions. As described by Li et al. (2020), 400 μL of each sample was added to a special cuvette (PC-110-131XX) and the test parameters were as follows: temperature, 25 °C; rotational speed, 4000 r/min; time interval, 10 s and number of measurements performed, 720.
2.6. Data analysis
One-way analysis of variance was performed using IBM SPSS 20 (IBM SPSS Inc., Chicago, IL, USA), and the differences were considered significant when p value was <0.05.
3. Results and discussions
3.1. Dispersion properties of the three caseins
The average protein diameters of the MCN and CaC were 162.9 ± 10.9 and 158.6 ± 3.0 nm, respectively, which were considerable close to that of native micellar casein in milk (Table S1; Chen et al., 2016). The average size of caseins in NaC was 86.7 ± 11.3 nm. Herein, we referred to the dissociated non-precipitated casein as non-micellar casein. The percentages of non-micellar casein in MCN, CaC and NaC were 16.6 % ± 1.1 %, 77.5 % ± 0.8 % and 97.1 % ± 0.4 %, respectively. The difference in non-micellar casein content between CaC and NaC dispersions is most likely due to incomplete dissociation caused by the presence of calcium ions in CaC (Ji et al., 2016). According to these results, caseins in the three dispersions were mainly present as aggregates. Table S1 also shows that the surface hydrophobicity of the three casein dispersions did not significantly differ (p > 0.05). The hydrophobic moieties in the casein aggregates may be buried within the interior, with individual hydrophobic residues randomly exposed at the protein surface, resulting in a similar affinity to ANS (Wang et al., 2014).
3.2. Interfacial properties
3.2.1. Surface pressure
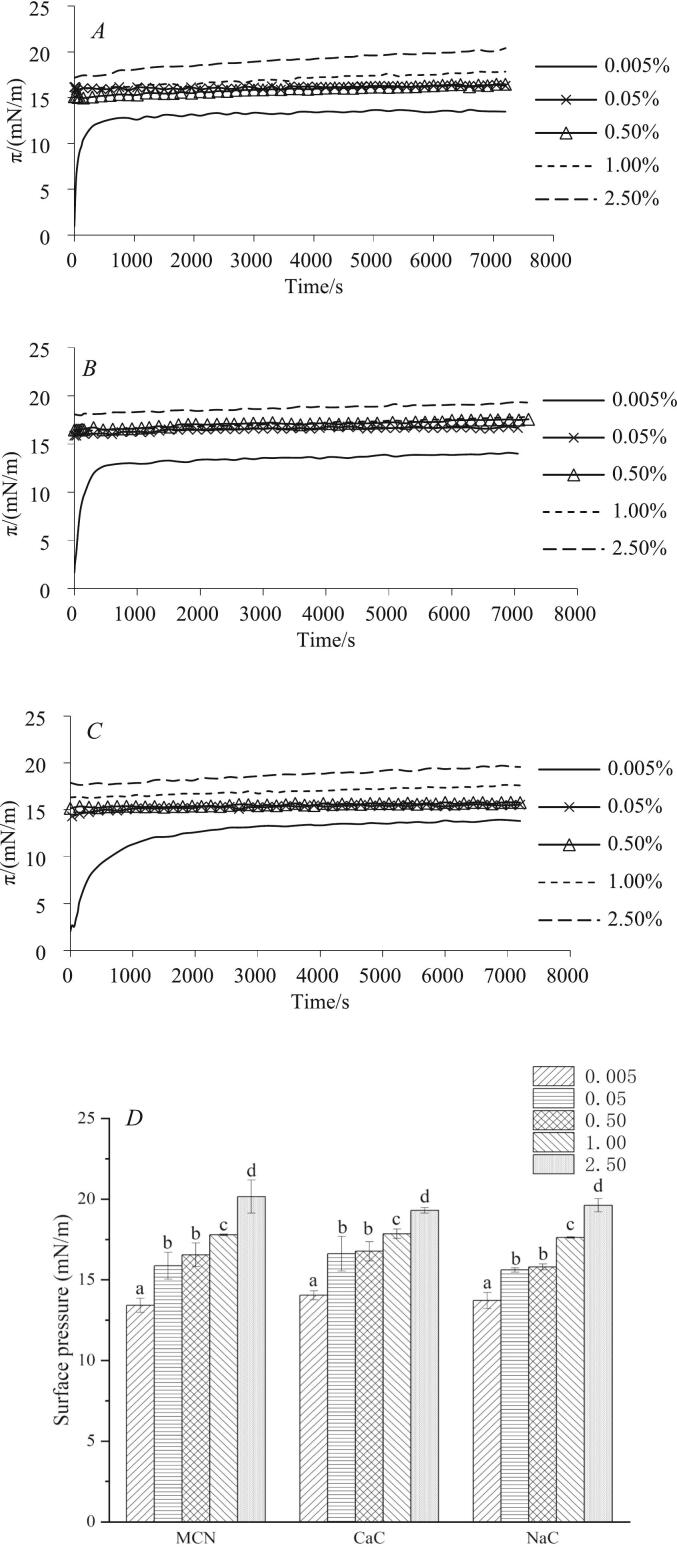
Herein, the surface pressure (π) was measured as a function of time until it reached a pseudo-equilibrium value, which took ∼7200 s. Fig. 1 A–C show that the π increased sharply during the first 150 s at 0.005 % protein concentration, followed by a slight increase. At higher concentrations, π increased immediately to >15 mN/m (<0.5 s), increasing gradually thereafter. When considering the adsorption process, the initial increase in surface pressure was directly related to the protein diffusion process (Liu et al., 2014). Thus, at 0.005 % concentration, the adsorption of scarce casein in the continuous phase at the oil–water interface was limited by diffusion kinetics, thereby elongating the time required for π to increase. By contrast, at higher concentrations (>0.005 %), the instantaneous surface pressure at the onset of adsorption was >15 mN/m (<0.5 s), which may be due to the fact that there is sufficient protein near the interface and protein adsorption is not limited by the rate of diffusion, and therefore is completed instantaneously and cannot be monitored (Zhou et al., 2021).
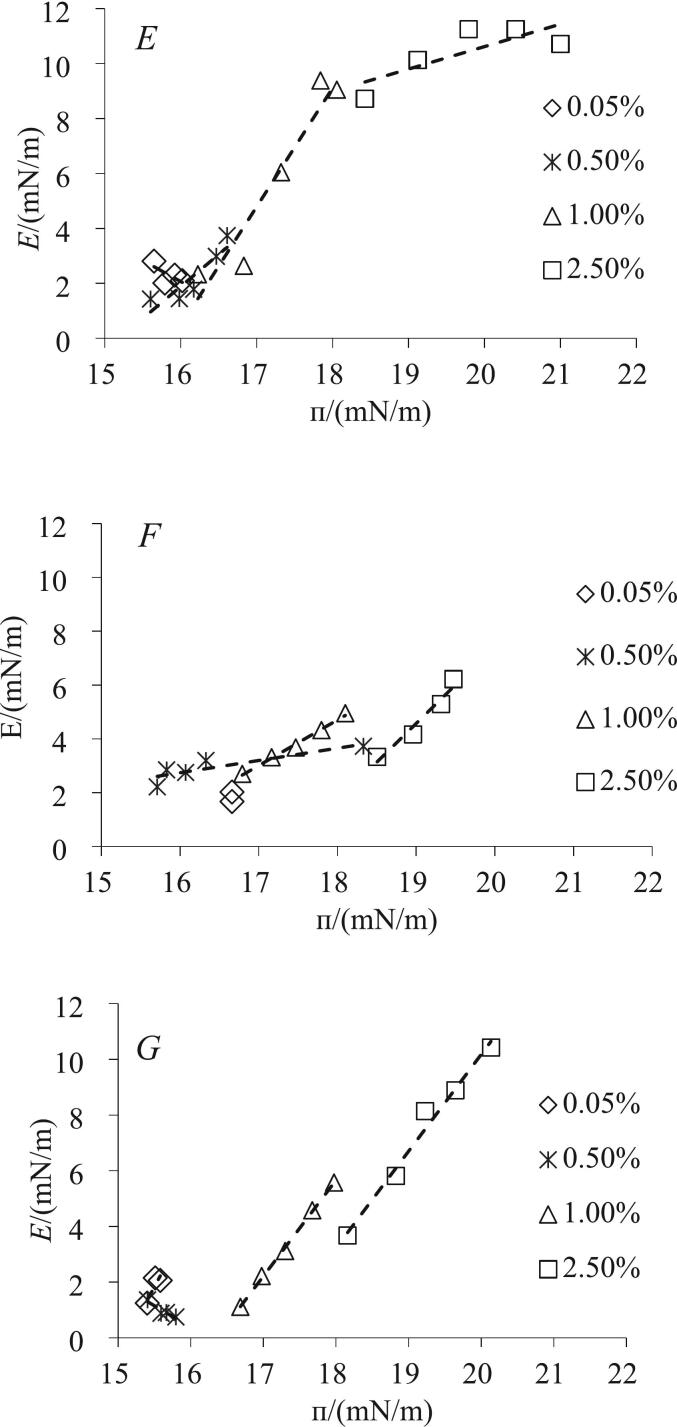
Fig. 1.
A-C: Changes in the surface pressure (π) between AMF and casein dispersions as a function of time. A: MCN; B: CaC; C: NaC. D: The Pseudo-equilibrium values of surface pressure of the three casein dispersions as a function of concentrations. E-G: Interfacial modulus (E) as a function of surface pressure (п) of the interfacial layers formed by caseins in different aggregate states. E: MCN; F: CaC; G: NaC; dashed line, trendline.
Fig. 1D showed the pseudo-equilibrium values of π as a function of concentrations. In each system, the surface pressure tended to increase with increasing casein concentration. For example, at a concentration of 0.005 %, the surface pressure between MCN and AMF was ∼13.42 ± 0.44 mN/m, whereas at a concentration of 2.5 %, it increased to 20.16 ± 1.02 mN/m (p < 0.05). This is due to the fact that the content of non-micellar casein in MCN is elevated with increasing casein concentration, and non-micellar casein is considered to be more effective in reducing interfacial tension than micelles (Zhou et al., 2022). Furthermore, no substantial difference was observed between the pseudo-equilibrium surface pressures of the three proteins at a special concentration (p > 0.05). It may indicate that non-micellar casein preferentially adsorbed and dominated the interfacial layer at sufficient content, in agreement with the report of Zhou et al. (2022).
3.2.2. Dynamic adsorption properties
The adsorption of proteins at the oil–water interface involves the diffusion, structural unfolding and rearrangement of protein molecules, which consequently aggregate at the oil–water interface to form an interfacial layer (Day et al., 2014; Patra & Somasundaran, 2014; Tang & Shen, 2015). Casein migrated to the oil–water interface within 0.5 s (Fig. 1), indicating that the diffusion phase was completed instantaneously. The penetration and rearrangement processes can be monitored using a first-order phenomenological equation, i.e. , where π7200, πt and π0 are the surface pressures at the final adsorption time (7200 s) of each step at time t and t0, respectively. A typical plot of ln [(π7200-πt)/(π7200-π0)] against time for interfacial layers exhibits two linear regions; the first and second slopes are considered the first-order rate constant of unfolding (k1) and rearrangement (k2) for the adsorbed caseins (Liu et al., 2012; Tang & Shen, 2015; Wang et al., 2020). Fig. S1 shows the plot of ln [(π7200–πt)/(π7200–π0)] against time for the interfacial layers formed by MCN, CaC and NaC, all of which exhibited a short second linear region.
The k1 value of MCN, CaC and NaC at ranges of 3.11–4.86 × 10−4 (s−1), 2.79–4.10 × 10−4 (s−1) and 2.75–5.21 × 10−4 (s−1), respectively, decreased considerably and then remained almost constant with increasing casein concentration (See Table 1). These results indicate that the unfolding of adsorbed caseins at the interface became restricted at higher concentrations (>0.05 %). In this case, more caseins migrated to the interface at the beginning, resulting in a spatial site barrier or multilayer adsorption that prevents other caseins from unfolding (Zhou et al., 2021). Therefore, the area per adsorbed protein molecule was deemed smaller, resulting in a larger amount of casein adsorption to completely cover the interface (Rouimi et al., 2005). When compared with MCN and CaC, NaC displayed a higher k1 at concentrations of 0.005 % and 0.05 %, indicating that that NaC was more unfolded at the interface, which is due to its more flexible conformation as it contains more non-micellar casein and fewer α-helices (Table S2). However, with increasing casein concentration, no substantial difference was observed in k1 (p > 0.05); moreover, at higher concentrations (>0.5 %), the three caseins exhibited similar structural changes at the interface.
Table 1.
The unfolding parameters (k1 × 104 s−1) of caseins at the oil-water interface.
| Concentration (%) | MCN | CaC | NaC |
|---|---|---|---|
| 0.005 | 4.86 ± 0.61b | 4.10 ± 0.10b | 5.21 ± 0.30b |
| 0.05 | 3.59 ± 0.22ab | 2.79 ± 0.34a | 4.82 ± 0.48b |
| 0.5 | 3.41 ± 0.11ab | 3.35 ± 0.22ab | 2.80 ± 0.49a |
| 1.0 | 3.11 ± 0.74a | 3.02 ± 0.20a | 2.75 ± 0.20a |
| 2.5 | 3.13 ± 0.10a | 2.96 ± 0.14a | 3.27 ± 0.32a |
Values not sharing the same superscript letters in one column differ significantly (p < 0.05).
3.2.3. Interfacial adsorbed caseins
The evolution of E with π in the interfacial layer is dependent on the concentration and interaction of the adsorbed proteins (Liu et al., 2011; Tang & Shen, 2015). Because of the insufficient coverage of the interface at a concentration of 0.005 %, the E of the MCN, CaC and NaC layers was undetectable (data not shown). At higher concentrations, the curves of E–π are shown in Fig. 1 E–G. In a previous study, it was reported that when the slope is ∼1, the amount of adsorbed molecules dominates E, whereas when the slope is >1, the interaction between the adsorbed molecules dominates E (Wan et al., 2014). However, at concentrations of >0.5 %, k1 no longer showed significant change with increasing concentration, thus implying similar unfolding of the interfacial proteins. Therefore, we hypothesised that E might be explained by changes in the amount of adsorbed casein over time.
At a concentration of 0.05 %, E of the three caseins was considerably low (<5 mN/m) and the slope of E–π in the three interfacial layers was <1, suggesting that the formed interfacial layers were weak and incapable of resisting dilatational deformation (Fig. 1 E–G). With further increase in the MCN concentration to 0.5 % and 1.0 %, the slope of E–π increased to 2.33 and 4.32, respectively. However, the slope decreased to 0.82 at 2.5 % MCN despite exhibiting the highest E. Similar results were also observed for bovine serum albumin at the interface (Tang & Shen, 2015). This suggests that the amount of adsorbed casein first increases and then decreases with increasing concentration, with the amount of non-micellar casein being low at low concentrations and the adsorption of micellar casein increasing the amount of interfacial proteins. Moreover, as the concentration increases, the non-micelles break through the limitations of the diffusion kinetics and dominate the interfacial layer. The non-micellar contents of CaC and NaC were higher than MCN; therefore, the slope of E–π in the interfacial layer increased with casein concentration of >1 for concentrations up to 1.0 % and 2.5 %. At 1.0 % protein concentration, the slope of the MCN interfacial layer was the largest (4.32), followed by the slope of the NaC interfacial layer (3.45), at which time the amount of possible interfacially adsorbed casein was in the order of MCN > NaC > CaC.
3.2.4. FTIR
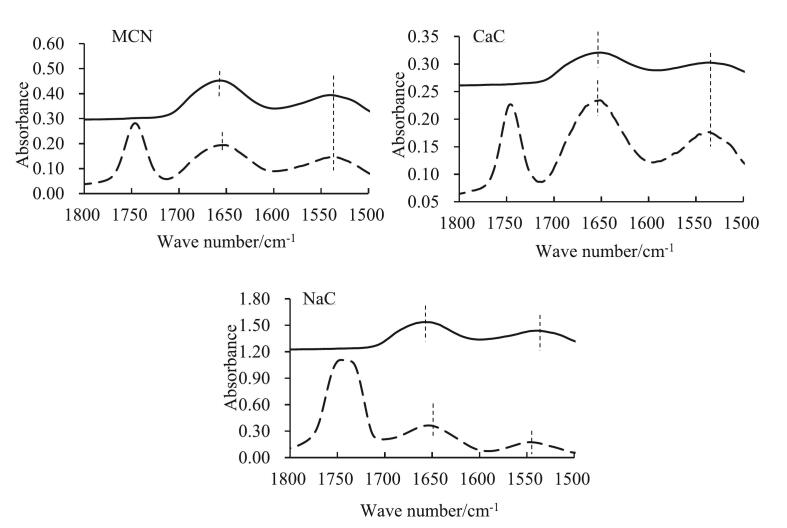
FTIR was used to compare the structure of caseins at the interface and in the dispersion. The infrared spectrum of proteins primarily comprises two amide bands, namely, amide I bands (80 % C O stretching vibration, 1700–1600 cm−1) and amide II band (60 % N—H bending vibration and 40 % C—N stretching vibration, 1450–1550 cm−1) (Carbonaro & Nucara, 2010). When caseins adsorbed at the oil–water interface, the wave number of amide I band in MCN and NaC decreased from 1657 ± 1 cm−1 and 1658 ± 0 cm−1 to 1652 ± 1 cm−1 and 1655 ± 1 cm−1, respectively (Fig. 2). By contrast, the wave number of amide bands of caseins in CaC was ∼1655 cm−1 and exhibited no discernible shift after adsorption. The wave number of the amide II band of caseins in MCN and CaC was ∼1540 cm−1, which remained constant after adsorption. However, it exhibited a substantial shift from 1541 ± 1 cm−1 in the dispersion to 1547 ± 0 cm−1 at the oil–water interface in NaC, indicating that the N—H and C—N groups in NaC also participated in interfacial adsorption (Zhang et al., 2024). When proteins migrated to the interface, their hydrophobic chains favoured at least a portion of the oil phase and interacted with the hydrophobic chains of the AMF (Herrero et al., 2011; Tian et al., 2020). Therefore, the results suggest that NaC is more efficient in reducing interfacial tension and is more conducive to the formation and dispersion of fat globules, most likely by preventing the exchange between adsorbed protein molecules at the interface and in the water phase, compared with CaC and MCN.
Fig. 2.
Amide I band and amide II band of the caseins in the dispersions and at interfacial layers. Solid line, caseins in the dispersion; dashed line, caseins at the interface.
3.2.5. Micromorphology of the interfacial layers
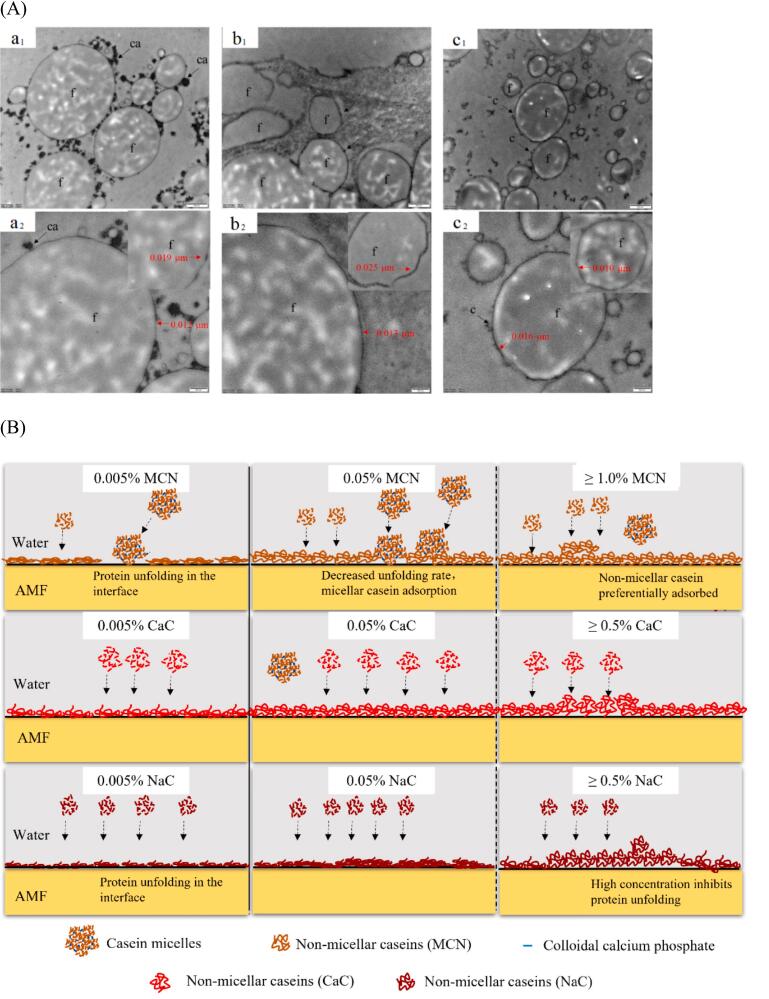
Cryo-TEM was used to observe the micromorphology of the interfacial layers. The morphology of the interfacial layers formed by 1.5 % MCN, 1.5 % CaC and 1.5 % NaC differed considerably (Fig. 3A). In MCN, the interfacial layer was intact and uniform, with a thickness ranging from 10 to 20 nm; micellar casein was preferentially distributed in the serum phase or attached loosely and non-uniformly to the interfacial layers. In CaC and NaC, interfacial layers exhibited intact and uniform boundaries and their thickness varied in different parts in the range of 10–30 nm, resembling that of MCN. These regions may be interfacial layers composed of non-micellar casein (Zhou et al., 2021). In addition, micelle casein was also adsorbed on the surface of different microdroplets, consistent with the hypothesis that micelle casein is involved in the composition of the protein layer at low protein concentrations. This may be due to the relative lack of non-micellar casein due to the large number of droplets formed instantaneously during homogenisation and the limitation of the diffusion rate, which involves micelle casein in the formation of the interfacial layer. The higher amount of micelle casein on the surface of MCN emulsion microdroplets indicates a positive correlation between interfacial composition and micellar casein content, consistent with the findings of our previous study, which reported that MCN-prepared emulsions exhibit high interfacial protein loadings (Li et al., 2020).
Fig. 3.
A: Microcosmic images of the interface of fat droplets in emulsions formed with caseins in different casein aggregate states. a:1.5 % MCN; b: 1.5 % CaC; c: 1.5 %NaC; a1-c1: scale bar = 500 nm, a2-c2: scale bar = 200 nm; f: fat droplets; ca: micelle caseins; c: smaller casein aggregates. B: Schematic diagram of the interfacial layer between AMF and water formed by the three casein dispersions (Dashed line: unadsorbed casein; solid line: adsorbed casein).
To better understand how the aggregate states of caseins affect the interfacial layers, as shown in Fig. 3B. In the MCN system, at a concentration of 0.005 %, non-micellar casein migrated to the interface, exhibited a greater unfolding degree and then interacted to form an incomplete interfacial layer. With increasing casein concentration, the unfolding rate decreased, consequently increasing the adsorption of casein at the interface in the form of non-micelles and micelles, resulting in a complete but weak interface. Further increase in MCN concentration did not alter the extent of casein unfolding but may lead to preferential adsorption of non-micelles. The interfacial behaviour of CaC and NaC was concentration-dependent, similar to that of MCN. However, at low concentrations (0.005 % and 0.05 %), NaC exhibited a higher unfolding coefficient, whereas the opposite was true at high concentrations, probably because excessive non-micelles dominated the interfacial layer and inhibited protein unfolding.
3.3. Emulsion properties
3.3.1. Particle size distribution of fat droplets
Fig. 4 demonstrates the particle size distribution of fat droplets in emulsion created using the three casein dispersions. The 0.5 % and 1.0 % MCN-stabilised emulsions immediately destabilised after production and were not analysed in the following study. Particle size distributions were bimodal between the concentrations of 1.5 % and 2.0 %, with the main peak between 1.6 and 19.0 μm and the smaller peak between 0.4 and 1.5 μm. The range of particle size distribution remained constant at 2.5 % concentration, but the main peak shifted to the left, indicating smaller fat droplets in the 2.5 % MCN emulsion. However, large aggregated fat droplets were present in all the MCN emulsions (Fig. 4A). Our previous study reported that increase in MCN concentration increased the emulsion viscosity, which may be related to the aggregation of fat globules (Li et al., 2020). Fat globule distribution in 0.5 % CaC emulsion was bimodal, and fat droplet aggregates were also observed (Fig. 4B). With increasing concentration, the fat droplets became well-dispersed, ranging in size from 0.4 to 13.0 μm without aggregation (Fig. 4B). The particle size distribution in the NaC emulsions was unimodal between 0.4 and 10.0 μm, and fat droplet aggregates were not observed at any concentration levels (Fig. 4B). It was evident with increasing concentration of caseins in each system, the particle size distribution became more uniform, and higher surface pressure produced smaller fat droplets. Previous studies have elucidated the underlying mechanism in detail (Dickinson, 2010; Zhou et al., 2020).
Fig. 4.
Particle size distribution and microscopic images of fat droplets in emulsions formed with caseins in different aggregate states. A1-A3: 1.5 % MCN, 2.0 % MCN, and 2.5 % MCN, respectively; B1-B5: 0.5 % CaC, 1.0 % CaC, 1.5 % CaC, 2.0 % CaC, and 2.5 % CaC, respectively; C1-C5: 0.5 % NaC, 1.0 % NaC, 1.5 % NaC, 2.0 % NaC, and 2.5 % NaC; scale bar = 50 μm.
However, the surface pressure measured using DSA100 was similar for the NaC, MCN and CaC emulsions, but the fat droplets in the NaC emulsions were smaller and more uniformly distributed at a specific concentration compared with those in the MCN and CaC emulsions (Fig. 1). The oil–water interface area in the interfacial test was ∼20 cm2/g, and non-micellar casein at concentrations of >0.5 % in the three casein dispersions were adequate for covering the interface instantly. However, the specific surface area of fat globules (Table S1) formed the basis for the newly formed oil–water interface during homogenisation, which is almost three orders of magnitude higher than the specific interface area in the pendant drop analysis (Schestkowa et al., 2020). As a key component of the interfacial layer, non-micellar casein was more likely to be deficient during emulsion preparation, thus enabling micelles to simultaneously adsorb.
3.3.2. Emulsion stability
3.3.2.1. Coalescence degree
The collision of fat droplets during storage may result in the rupture of the oil–water interface, releasing the oil phase, followed by phase separation. Tcholakova et al. (2002) reported that centrifugation could be used to evaluate the long-term stability of emulsions. The oil separation content decreased considerably with increasing casein concentration in each system (Fig. 5A). In particular, in the NaC emulsions, no oil was separated at a concentration of 2.0 % and 2.5 %. These results indicate a close correlation between coalescence stability and interfacial properties when emulsions were formed from a single type of casein; higher casein concentration promoted the adsorption of casein at the interface, thus preventing damage to the interfacial layer.
Fig. 5.
Degree of partial coalescence (A) and instability index (B) of emulsions formed from casein in different aggregation states; different letters represent a significant difference in instability index of emulsions formed with a specific protein at different casein concentration levels (p < 0.05).
However, on comparing the emulsions stabilised by different caseins, no correlation was observed between the interfacial properties and coalescence stability. For example, the aggregation stability of NaC was considerably higher than that of MCN at a concentration of 2.5 %, although surface pressure measurements indicate that both have similar interfacial properties. This may be due to the fact that during homogenisation, NaC adsorbs more quickly due to its smaller size, forming smaller droplets. Moreover, the MCN and CaC emulsions exhibited similar amounts of oil separation, probably because of micellar casein adsorption in the MCN emulsions, which decelerates the movement of fat globules and acts as a spatial barrier. This can further affect the physical quality of the emulsion according to the findings of our previous study (Li et al., 2020).
3.3.2.2. Creaming stability
The creaming stability (in terms of instability index) of emulsions was evaluated using LUMiFuge, wherein a lower instability index suggests a slower creaming rate of fat droplets. The instability index of the MCN and CaC emulsions decreased slightly but gradually with increasing casein concentration, consistent with their appearance after 30 days of storage at 4 °C (Figs. 5B and S2). Further increase in casein concentrations resulted in more non-micellar casein forming interfacial layers and smaller fat droplet particle sizes, thereby enhancing creaming stability. Moreover, a different trend was observed in the NaC emulsions. At 0.5 %–2.0 % NaC concentration, the instability index decreased with increasing concentration, with 2.0 % NaC exhibiting better creaming stability with an instability index of 0.511 ± 0.005. However, when the NaC concentration increased to 2.5 %, the instability index increased to 0.545 ± 0.002, most likely because of the depletion flocculation that occurs when large amounts of non-micellar casein are present, as reported previously (Radford & Dickinson, 2004).
In addition, compared with the MCN or CaC emulsions, the NaC emulsions at concentrations between 0.5 % and 2.0 % exhibited a lower instability index, suggesting improved creaming stability. The results were attributed to the highest percentage of non-micellar casein, which formed more interfacial layers, leading to the smallest particle size and an increase in emulsion stability. However, at a concentration of 2.5 %, the instability index of the MCN emulsion was slightly lower than those of the CaC and NaC emulsions, despite the presence of a certain amount of fat droplet aggregates. Fat droplets in the MCN emulsion aggregated to form a strong gel-like network structure, inhibiting the movement of fat globules (Dickinson, 2019) despite the instability of the emulsions. Furthermore, the micellar casein-formed network considerably reduced serum loss in whipped cream (Li et al., 2020).
4. Conclusions
Herein, our results indicate that the micelle content of casein influences the structure of the interfacial layer, but it is regulated by concentration. At higher concentrations (>0.5 %), the three caseins MCN, CaC and NaC exhibit similar interfacial behaviour during adsorption at the oil–water interface, forming an interfacial layer dominated by non-micelles. As the interfacial behaviour of the proteins during emulsion formation appears to be closer to that at low concentrations, micellar casein may be involved in the formation of the interfacial layer. This may be because a large number of interfaces are created at the instant of microdroplet formation and the diffusion rate is limited, which consequently leads to a relative lack of non-micellar casein. The adsorption of micellar casein on the droplet surface was also observed via cryo-TEM of the emulsion, and the amount of adsorption was positively correlated with the micelle content of the casein, suggesting that the composition of the interfacial layer of the fat globule is influenced by the micelle content of the casein, which could potentially further affect the stability of the emulsions. In the concentration range of 0.5 %–2.5 %, the emulsion fat droplets formed by NaC were uniformly distributed with minimum size and better emulsion stability, whereas the droplets stabilised by MCN and CaC tended to agglomerate. This may be because of the micellar casein at the fat globule surface affecting the stability of the droplets. Herein, we closely investigated the interfacial properties of casein in different micelle content and combined them with the state of the emulsion to obtain a more fundamental understanding of the role of micellar casein in multiphase systems.
CRediT authorship contribution statement
Guosen Yan: Writing – review & editing, Writing – original draft, Visualization. Shiran Wang: Formal analysis, Conceptualization. Yan Li: Resources, Project administration, Funding acquisition, Data curation. Liebing Zhang: Resources, Funding acquisition. Jianguo Yan: Resources. Yanfang Sun: Resources.
Declaration of competing interest
The authors declare that neither of them has a conflict of interest or personal relationships that could have appear to influence the work reported in this paper. The work described is original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part.
Acknowledgments
This work was supported by the Agriculture Research System of China of MOF and MARA (grant no. CARS-36), the Key Research and Development Program of Ningxia (NO. 2021BEF02031) and the National Natural Science Foundation of China (NO. 32302137).
Data availability
Data will be made available on request.
References
- Amine C., Dreher J., Helgason T., Tadros T. Investigation of emulsifying properties and emulsion stability of plant and milk proteins using interfacial tension and interfacial elasticity. Food Hydrocolloids. 2014;39:180–186. [Google Scholar]
- Baldursdottir S.G., Fullerton M.S., Nielsen S.H., Jorgensen L. Adsorption of proteins at the oil/water interface--observation of protein adsorption by interfacial shear stress measurements. Colloid Surface B. 2010;79(1):41–46. doi: 10.1016/j.colsurfb.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Carbonaro M., Nucara A. Secondary structure of food proteins by Fourier transform spectroscopy in the mid-infrared region. Amino Acids. 2010;38(3):679–690. doi: 10.1007/s00726-009-0274-3. [DOI] [PubMed] [Google Scholar]
- Chen M., Bleeker R., Sala G., Meinders M.B.J., Van Valenberg H.J.F., Van Hooijdonk A.C.M., Van Der L.E. Particle size determines foam stability of casein micelle dispersions. International Dairy Journal. 2016;56:151–158. [Google Scholar]
- Day L., Zhai J., Xu M., Jones N.C., Hoffmann S.V., Wooster T.J. Conformational changes of globular proteins adsorbed at oil-in-water emulsion interfaces examined by synchrotron radiation circular dichroism. Food Hydrocolloids. 2014;34:78–87. [Google Scholar]
- Dickinson E. Milk protein adsorbed layers and the relationship to emulsion stability and rheology. Studies in Surface Science and Catalysis. 2001;132:973–978. [Google Scholar]
- Dickinson E. Food emulsions and foams: Stabilization by particles. Curr. Opin. Colloid In. 2010;15(1–2):40–49. [Google Scholar]
- Dickinson E. Mixed biopolymers at interfaces: Competitive adsorption and multilayer structures. Food Hydrocolloids. 2011;25(8):1966–1983. [Google Scholar]
- Dickinson E. Strategies to control and inhibit the flocculation of protein-stabilized oil-in-water emulsions. Food Hydrocolloids. 2019;96:209–223. [Google Scholar]
- Gülseren İ., Corredig M. Interactions at the interface between hydrophobic and hydrophilic emulsifiers: Polyglycerol polyricinoleate (PGPR) and milk proteins, studied by drop shape tensiometry. Food Hydrocolloids. 2012;29(1):193–198. [Google Scholar]
- Herrero A.M., Carmona P., Pintado T., Jiménez-Colmenero F., Ruíz-Capillas C. Olive oil-in-water emulsions stabilized with caseinate: Elucidation of protein–lipid interactions by infrared spectroscopy. Food Hydrocolloids. 2011;25(1):12–18. [Google Scholar]
- Ji J., Fitzpatrick J., Cronin K., Maguire P., Zhang H., Miao S. Rehydration behaviours of high protein dairy powders: The influence of agglomeration on wettability, dispersibility and solubility. Food Hydrocolloids. 2016;58:194–203. [Google Scholar]
- Lazzaro F., Saint-Jalmes A., Violleau F., Lopez C., Gaucher-Delmas M., Madec E., Frédéric B., Gaucheron F. Gradual disaggregation of the casein micelle improves its emulsifying capacity and decreases the stability of dairy emulsions. Food Hydrocolloids. 2017;63:189–200. [Google Scholar]
- Li Y., Li Y., Yuan D., Wang Y., Li M., Zhang L. The effect of caseins on the stability and whipping properties of recombined dairy creams. International Dairy Journal. 2020;105 [Google Scholar]
- Liu H., Wang B., Barrow C.J., Adhikari B. Relating the variation of secondary structure of gelatin at fish oil-water interface to adsorption kinetics, dynamic interfacial tension and emulsion stability. Food Chemistry. 2014;143:484–491. doi: 10.1016/j.foodchem.2013.07.130. [DOI] [PubMed] [Google Scholar]
- Liu L., Zhao Q., Liu T., Long Z., Kong J., Zhao M. Sodium caseinate/xanthan gum interactions in aqueous solution: Effect on protein adsorption at the oil-water interface. Food Hydrocolloids. 2012;27(2):339–346. [Google Scholar]
- Liu L., Zhao Q., Liu T., Zhao M. Dynamic surface pressure and dilatational viscoelasticity of sodium caseinate/xanthan gum mixtures at the oil–water interface. Food Hydrocolloids. 2011;25(5):921–927. [Google Scholar]
- Panouille M., Nicolai T., Benyahia L., Durand D. Royal Society of Chemistry press; Microstructure and Processing: 2007. Food colloids: Interactions. [Google Scholar]
- Patra P., Somasundaran P. Evidence of conformational changes in oil molecules with protein aggregation and conformational changes at oil-'protein solution' interface. Colloid Surface B. 2014;120:132–141. doi: 10.1016/j.colsurfb.2014.03.045. [DOI] [PubMed] [Google Scholar]
- Pranata J., Hoyt H., Drake M., Barbano D.M. Effect of dipotassium phosphate addition and heat on proteins and minerals in milk protein beverages. Journal of Dairy Science. 2024;107(2):695–710. doi: 10.3168/jds.2023-23768. [DOI] [PubMed] [Google Scholar]
- Radford S.J., Dickinson E. Depletion flocculation of caseinate-stabilised emulsions: What is the optimum size of the non-adsorbed protein nano-particles? Colloid Surface A. 2004;238(1–3):71–81. [Google Scholar]
- Rouimi S., Schorsch C., Valentini C., Vaslin S. Foam stability and interfacial properties of milk protein–surfactant systems. Food Hydrocolloids. 2005;19(3):467–478. [Google Scholar]
- Schestkowa H., Drusch S., Wagemans A.M. FTIR analysis of β-lactoglobulin at the oil/water-interface. Food Chemistry. 2020;302 doi: 10.1016/j.foodchem.2019.125349. [DOI] [PubMed] [Google Scholar]
- Silva N.N., Piot M., de Carvalho A.F., Violleau F., Fameau A., Gaucheron F. pH-induced demineralization of casein micelles modifies their physico-chemical and foaming properties. Food Hydrocolloids. 2013;32(2):322–330. [Google Scholar]
- Tang C., Shen L. Dynamic adsorption and dilatational properties of BSA at oil/water interface: Role of conformational flexibility. Food Hydrocolloids. 2015;43:388–399. [Google Scholar]
- Tcholakova S., Denkov N., Campbell B. Coalescence in β-Lactoglobulin-stabilized emulsions: Effects of protein adsorption and drop size. Langmuir. 2002;18(23):8960–8971. [Google Scholar]
- Tian Y., Zhang Z., Zhang P., Taha A., Pan S. The role of conformational state of pH-shifted β-conglycinin on the oil/water interfacial properties and emulsifying capacities. Food Hydrocolloids. 2020;108 [Google Scholar]
- Wan Y., Liu J., Guo S. Effects of succinylation on the structure and thermal aggregation of soy protein isolate. Food Chemistry. 2018;245:542–550. doi: 10.1016/j.foodchem.2017.10.137. [DOI] [PubMed] [Google Scholar]
- Wan Z.L., Wang L.Y., Wang J.M., Zhou Q., Yuan Y., Yang X.Q. Synergistic interfacial properties of soy protein–stevioside mixtures: Relationship to emulsion stability. Food Hydrocolloids. 2014;39:127–135. [Google Scholar]
- Wang P., Tao H., Wu F., Yang N., Chen F., Jin Z., Xu X. Effect of frozen storage on the foaming properties of wheat gliadin. Food Chemistry. 2014;164:44–49. doi: 10.1016/j.foodchem.2014.05.010. [DOI] [PubMed] [Google Scholar]
- Wang S., Yang J., Shao G., Qu D., Zhao H., Yang L., Zhu L., He Y., Zhu D. Soy protein isolated-soy hull polysaccharides stabilized O/W emulsion: Effect of polysaccharides concentration on the storage stability and interfacial rheological properties. Food Hydrocolloids. 2020;101(5) [Google Scholar]
- Zhang X., Wang Y., Li Z., Li Y., Qi B. Effects of polysaccharide type on the structure, interface behavior, and foam properties of soybean protein isolate hydrolysate-polysaccharide Maillard conjugates. Food Hydrocolloids. 2024;151 [Google Scholar]
- Zhou B., Tobin J.T., Drusch S., Hogan S.A. Interfacial properties of milk proteins: A review. Advances in Colloid and Interface Science. 2021;295 doi: 10.1016/j.cis.2020.102347. [DOI] [PubMed] [Google Scholar]
- Zhou X., Sala G., Sagis L.M.C. Bulk and interfacial properties of milk fat emulsions stabilized by whey protein isolate and whey protein aggregates. Food Hydrocolloids. 2020;109 [Google Scholar]
- Zhou X., Yang J., Sala G., Sagis L.M.C. Are micelles actually at the interface in micellar casein stabilized foam and emulsions? Food Hydrocolloids. 2022;129 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.