Abstract
Interleukin-8 (IL-8) is a chemotactic cytokine for neutrophils and lymphocytes. Macrophage inflammatory protein 2 (MIP-2) is a murine counterpart of IL-8. The present study was performed to determine whether MIP-2 aggravates murine myocarditis. We examined (i) the MIP-2-producing activity of encephalomyocarditis (EMC) virus-infected cultured macrophages, (ii) serial plasma MIP-2 levels in EMC virus-induced mice by enzyme-linked immunosorbent assay, and (iii) the effects of antimouse MIP-2 monoclonal antibody (MAb) in vivo upon myocarditis. The production of MIP-2 increased in an infection dose- and time-dependent manner in virus-infected RAW 264.7 macrophages. Five-week-old C3H/He mice were inoculated with EMC virus. Plasma MIP-2 levels were significantly elevated in mice on days 7 and 14 postinfection. Mice were injected subcutaneously with anti-MIP-2 MAb at 10 μg/day (group 2) or 100 μg/day (group 3) on days 0 to 5 and were observed until day 21. Uninfected control mice (group 1) were prepared. The survival rate was higher in the anti-MIP-2-treated group (group 3), but not in group 2, than in the control group. Histopathological analysis revealed that cellular infiltration and myocardial necrosis with macrophage and T-cell accumulation were less prominent in the anti-MIP-2 MAb-treated group, but not in group 2, compared to the level in the controls. MIP-2 is an important naturally occurring inflammatory cytokine in myocarditis, and anti-MIP-2 MAb treatment may prevent the inflammatory response.
A number of studies have been performed to elucidate the mechanism of myocarditis. Increasing evidence suggests that cytotoxic T cells (8, 9), neurohumoral factors (17), and free radicals (4, 5), possibly generated by infiltrating cells in the myocardium, play a significant role, separately or together, in the development of myocardial damage and dysfunction, in addition to the primary damage caused by viral infection. Inflammatory cytokines are also involved in the pathogenesis of myocardial injury in viral myocarditis (6, 11). The antiviral effects of inflammatory cytokines such as interleukin-2 (IL-2) and IL-6 have been studied (6, 11). However, those of IL-8 have not been examined.
IL-8 is a monocyte/macrophage-derived peptide that belongs to a novel cytokine family (13, 22). The predominant IL-8-producing cells are monocytes. Moreover, a variety of cells, such as endothelial cells and fibroblasts, have been shown to produce significant amounts of IL-8 on stimulation with various types of cytokines or mitogens. IL-8 has chemotactic activity for neutrophils and lymphocytes. A recent study showed that IL-8 is a potent inflammatory agent (1, 13, 19, 22). Current data suggest a possible function for IL-8 in the pathogenesis of inflammatory diseases.
Macrophage inflammatory protein 2 (MIP-2) is considered to be a murine counterpart of IL-8 (3, 14, 15, 20). Since monocyte migration is a crucial step in the development of myocarditis, we investigated the behavior of MIP-2 in encephalomyocarditis (EMC) virus infection both in vitro and in vivo and the effects of an anti-MIP-2 antibody on murine viral myocarditis (8, 10, 12).
MATERIALS AND METHODS
MIP-2 and MAb to MIP-2.
Recombinant mouse MIP-2 and anti-MIP-2 MAb were produced by recombinant DNA techniques (15). Briefly, MIP-2 cDNA was amplified by reverse transcriptase PCR in a mixture containing purified mRNA from RAW 264.7 cells (American Type Culture Collection), which were cultured in the presence of 1 μg of Escherichia coli lipopolysaccharide (LPS) per ml for 20 h at 37°C, and matching primers to amplify the whole length of MIP-2 mRNA (221 bases from alanine- to asparagine-encoding regions) (20). Murine MIP-2 was expressed as a fusion protein with staphylococcal protein A by inserting MIP-2 cDNA into HindIII and SmaI sites of the plasmid vector pRIT12. The construct was confirmed by sequencing.
The MIP-2 was injected intracutaneously into the rabbit for the preparation of hyperimmune anti-MIP-2 serum (initially injected with complete Freund's adjuvant followed by boosts with incomplete adjuvant every 2 weeks), and then anti-MIP-2 immunoglobulin G (IgG) was purified with the protein G column (Pharmacia). Purified anti-MIP-2 IgG was conjugated with CNBr-activated Sepharose 6MB (Pharmacia, Uppsala, Sweden) to examine its specificity as follows. The lyophilized conditioned medium from LPS-stimulated RAW 264.7 cells was applied to the anti-MIP-2 IgG-conjugated Sepharose column, and the binding fraction on the column was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Alternatively, Western blotting analyses of the conditioned medium were carried out.
Virus and cells.
The murine macrophage-like cell line RAW 264.7 cells and Vero African green monkey kidney cells were used. RAW 264.7 cells are murine macrophages transformed with the Abelson leukemia virus (16). The myocarditic strain of EMC virus was used. Virus stock was prepared as described previously (8, 10, 12) and stored at −80°C until it was diluted for use. Virus titers in the heart were determined by plaque formation on Vero (African green monkey kidney) cells. Cells were suspended to a concentration of 106/ml in Eagle's minimum essential medium (EMEM) with 5% fetal calf serum (FCS) in plastic plates and were allowed to grow for 2 days at 37°C in 5% CO2.
EMC virus infection in vitro.
RAW 264.7 cells were inoculated into six-well plates (3 × 105/well) and incubated at 37°C for 24 h. The cells were maintained in Dulbecco's modified minimum essential medium with 10% FCS and then infected with EMC virus at multiplicities of infection (MOI) of 0.001, 0.01, 0.1, and 1.0 PFU/cells. Twenty-four hours later, the supernatants were assayed for MIP-2 concentration with enzyme-linked immunosorbent assay (ELISA). Time-dependent production of MIP-2 at MOI of 0.1 was also studied.
Plasma MIP-2 determination.
C3H/He mice were inoculated with 102 PFU of the myocarditic strain of EMC virus on day 0. Mice were killed on days 4, 7, and 14. Blood was obtained from the retro-orbital plexus, and plasma MIP-2 levels were determined by using antibody sandwich ELISA (15). Briefly, rabbit anti-MIP-2 antibody and biotinylated anti-MIP-2 antibody were used as the capture and second-layer antibodies, respectively. Color development was continued for several minutes by addition of peroxidase-coupled streptavidin and the chromogenic substrate 3,3′-diaminobenzidine tetrahydrochloride (DAB) solution before terminating the reaction with 2 M H2SO4. The A492 was measured on a microplate reader (Bio-Rad). Three wells were used for each experimental time point to calculate the mean ± standard deviation.
Anti-MIP-2 antibody study.
Five-week-old C3H/He mice were inoculated in the same manner as for the MIP-2 assay. Mice were injected subcutaneously with anti-MIP-2 MAb diluted in 0.1 ml of saline at 10 μg/day (group 2, n = 48) and 100 μg/day (group 3, n = 43) on days 0 to 5. Controls (group 1, n = 46) were given daily subcutaneous injections of normal rabbit Ig (100 μg) on days 0 to 5. Mice were observed until day 21. Subsets of mice were killed on days 2 (n = 4 in all groups), 4 (n = 4 in all groups), 7 (n = 7 in all groups), and 14 (n = 7 in all groups); their hearts and pancreases were removed and weighed, and pathological and virological studies were performed. Thus, the survival study covered 24 mice in group 1, 26 mice in group 2, and 21 in group 3. The surviving mice were killed on day 21. Plasma MIP-2 levels were determined on day 7. Heart weight (HW) and body weight (BW) were measured, and the HW/BW ratio (HW/BW) was calculated. Pathological analysis was then performed.
All animals were cared for in accordance with the institutional policies and guidelines of Toyama Medical and Pharmaceutical University.
Pathological study.
Portions of the hearts were fixed in 10% formalin and embedded in paraffin. The sections were stained with hematoxylin and eosin and scored (0 to 4+) for myocardial necrosis and cellular infiltration by a skilled observer blind to the experimental treatments. The scores were as follows: 0 (none), no myocardial lesion; 1+, lesions involving <25% of the myocardium; 2+, lesions involving 25 to 50% of the myocardium; 3+, lesions involving 50 to 75% of the myocardium; 4+, lesions involving >75% of the myocardium. Two sections were analyzed for each sample, and each score was derived from the mean of the two sections. Some portions of the heart were snap frozen, mounted with ornithine carbamyltransferase compound, and examined immunohistologically as previously described (10, 12). Briefly, an indirect horseradish immunoperoxidase technique was used, and horseradish peroxidase activity was visualized with DAB as the chromogen. Sections 6 μm thick were cut from the frozen blocks, and endogenous peroxidase activity was blocked with cold methanol. The MAbs used were Mac-1 for macrophages (Mφ), Thy 1.2 for pan T cells, L3T4 for helper T cells (CD4; Becton Dickinson), and Lyt 2 for suppressor T cells (CD8; Becton Dickinson). In addition, MIP-2-positive cells were stained by an indirect immunoperoxidase method. Four hearts were examined in each group. We calculated the percentage of positive-stained cells, as previously described (10, 12).
Virus titers.
For infectivity assay, tissues were removed aseptically, and were weighed and homogenized in 2 ml of EMEM. After centrifugation at 1,500 × g for 15 min at 4°C, the supernatant was inoculated into Vero cells for 60 min at 37°C in 5% CO2. Cells were overlaid with 3 ml of EMEM containing 2% FCS and 1% methylcellulose. After 2 days of incubation at 37°C in a humidified atmosphere containing 5% CO2, cells were fixed with acetic acid and methanol at a ratio of 1:3 and stained with 1% crystal violet, and plaques were counted under an inverted microscope. The myocardial virus titers, which were determined from the hearts of killed mice on days 7 and 14, are expressed as PFU per milligram of tissue.
Statistical analysis.
Survival of mice were analyzed by the Kaplan-Meier method (7). Statistical comparisons of plasma MIP-2 levels, BW, HW, the HW/BW ratio, and histopathological data were performed by one-way analysis of variance. Differences were considered statistically significant at P < 0.05. Results are expressed as mean ± standard deviation.
RESULTS
Western blotting analysis of purified MIP-2.
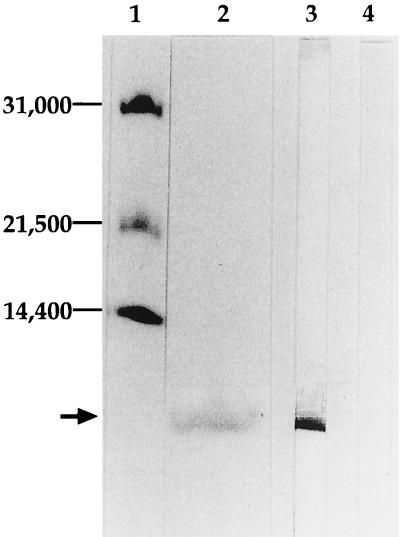
Anti-MIP-2 IgG reacted with single molecule with molecular weight of 6,000, which is identical to that of murine MIP-2 in the conditioned medium from LPS-stimulated cells (Fig. 1, lane 3), but did not react with the conditioned medium from unstimulated cells (lane 4), confirming the specificity of anti-MIP-2 IgG. Lane 2 of Fig. 1 showed the binding fraction of the conditioned medium from LPS-stimulated RAW 254.7 cells to the anti-MIP-2 IgG. An electrophoresis calibration kit was used for molecular weight standards (31,000, 21,500, and 14,400) (lane 1).
FIG. 1.
Western blotting analysis of purified MIP-2. Purified anti-MIP-2 IgG was conjugated with CNBr-activated Sepharose 6MB (Pharmacia, Uppsala, Sweden) to examine its specificity as follows. The lyophilized conditioned medium from LPS-stimulated RAW 264.7 cells was applied to the anti-MIP-2 IgG-conjugated Sepharose column, and the binding fraction on the column was analyzed by SDS-PAGE followed by visualization of protein with dye (lane 2). Alternatively, Western blotting analyses of the conditioned medium were carried out. As shown in the figure, anti-MIP-2 IgG reacted with single molecule with molecular weight of 6,000, which is identical to that of murine MIP-2 in the conditioned medium from LPS-stimulated cells (lane 3), but did not react with the conditioned medium from unstimulated cells (lane 4), confirming the specificity of anti-MIP-2 IgG. An electrophoresis calibration kit was used for molecular weight standards (31,000, 21,500, and 14,400) (lane 1).
MIP-2 production in RAW 264.7 cells in response to EMC virus infection.
As shown in Table 1, a significant infection dose-dependent increase in MIP-2 level was detected in the supernatants of EMC virus-infected RAW 264.7 cells. The time-dependent production of MIP-2 at an MOI of 10−1 PFU is also shown. The MIP-2 level increased with time from 6 h until 72 h after virus infection.
TABLE 1.
Changes in MIP-2 production in EMC virus-infected macrophagesa
| MOI | Change in MIP-2 production (ng/ml) atb:
|
|||||
|---|---|---|---|---|---|---|
| 0 h | 6 h | 12 h | 24 h | 48 h | 72 h | |
| 1 | 77.8 ± 11.2 | 93.0 ± 17.9 | 90.2 ± 17.3 | 93.2 ± 17.7 | ||
| 10−1 | 101.0 ± 12.3* | 126.6 ± 9.2** | 154.2 ± 31.1** | 202.4 ± 15.2** | 217.6 ± 12.5** | |
| 10−2 | 133.0 ± 21.5** | |||||
| 10−3 | 110.0 ± 30.9 | |||||
The RAW 264.7 cells were infected with EMC virus at the indicated MOI and then cultured. Culture supernatants were collected at the indicated time points after infection to determine MIP-2 levels by ELISA. Four to five wells were used for each experimental time point to calculate the mean ± standard deviation. The MIP-2 level increased in an MOI-dependent manner and with time from 6 until 72 h after virus infection. Time 0 values represent the noninfected control.
∗ and ∗∗, P < 0.05 and P < 0.01, respectively, versus control.
Plasma MIP-2 levels in EMC viral myocarditis.
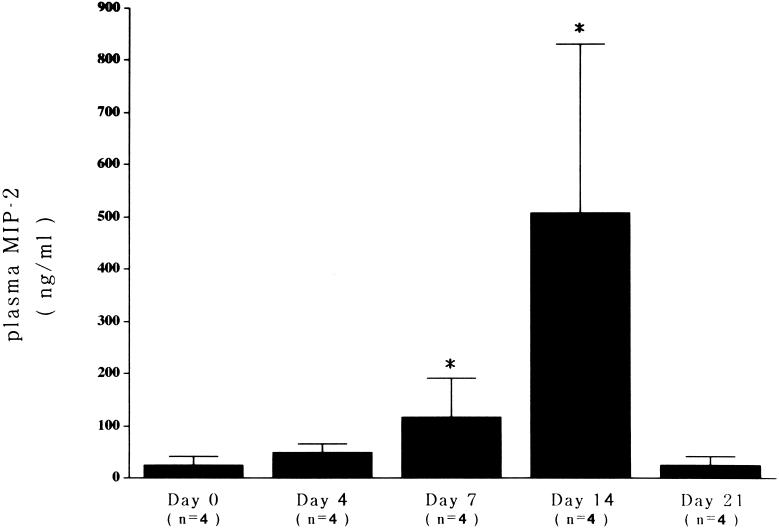
As shown in Fig. 2, plasma MIP-2 levels were significantly elevated in the blood of infected mice on days 7 and 14, but not on day 4 or 21, in comparison with uninfected mice. There was a rough correlation between plasma MIP-2 levels and the severity of the pathological grade of mice.
FIG. 2.
MIP-2 levels in mice with myocarditis. Plasma MIP-2 levels were elevated significantly on days 7 and 14 (each P < 0.05) in comparison with that of the control (day 0).
Effects of anti-MIP-2 MAb administration on myocarditis.
As shown in Fig. 3, 4, and 5 and Tables 2 and 3, in another set of experiments, we found that administration of the preparations after absorption of anti-MIP-2 activity did not influence the disease course, plasma MIP-2 levels, nor myocarditis score when compared with the results from normal rabbit Ig-treated or nontreated mice (Table 2).
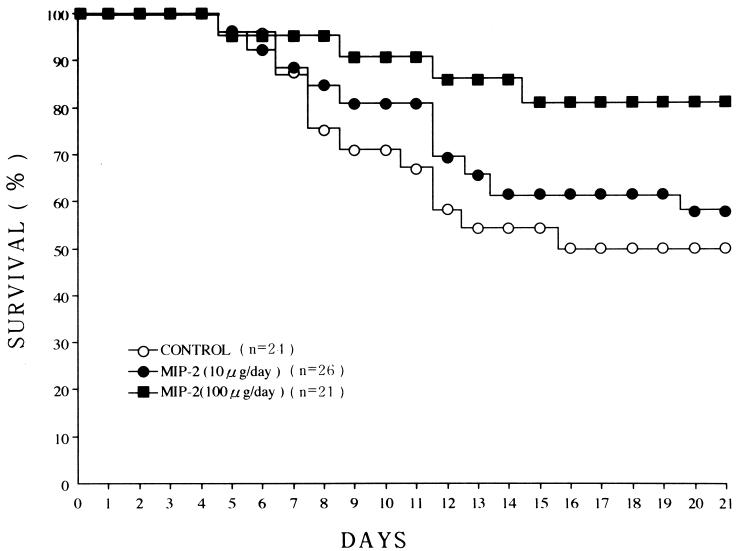
FIG. 3.
Plots of effects of anti-MIP-2 MAb treatment on survival in mice with EMC viral myocarditis. C3H/He mice were infected subcutaneously with anti-MIP-2 MAb on days 0 to 5 and were observed until day 21. Significant increases (P < 0.05) in survival were found in the anti-MIP-2 MAb group (group 3; ■) in comparison with the controls (group 1; ○). ○, group 1, control; ●, group 2, anti-MIP-2 at 10 μg/day; ■, group 3, anti-MIP-2 at 100 μg/day.
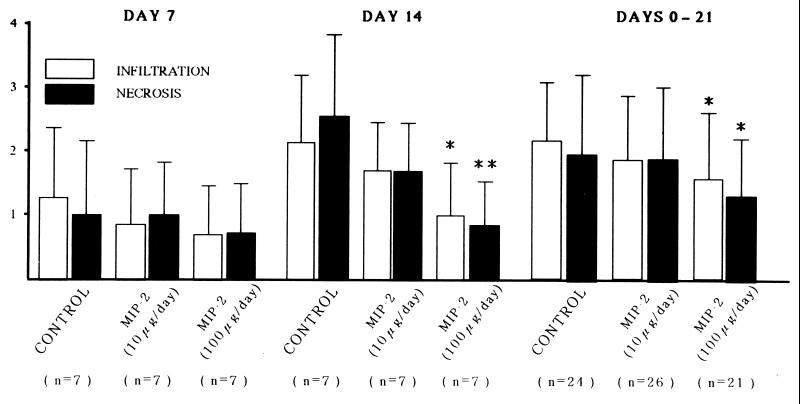
FIG. 4.
Pathological scores. The pathological scores of cellular infiltration and necrosis on day 14 and on days from 0 to 21 were less in the anti-MIP-2 group (100 μg/day treated) compared with those of the control. n indicates the number of mice sacrificed on days 7 and 14 in each group, and the 0- to 21-day group represents those animals used in Fig. 2. The pathological scores of cellular infiltration and necrosis on day 21 were also significantly less in group 3 (infiltration = 1.71 ± 0.92; P < 0.05; necrosis = 1.41 ± 0.80; P < 0.01; n = 17), but not in group 2 (infiltration = 2.27 ± 0.80; necrosis = 2.20 ± 1.08; n = 15), as compared with group 1 (infiltration = 2.50 ± 0.80; necrosis = 2.67 ± 0.98; n = 12). ∗ and ∗∗, P < 0.05 and P < 0.01, respectively, versus the control.
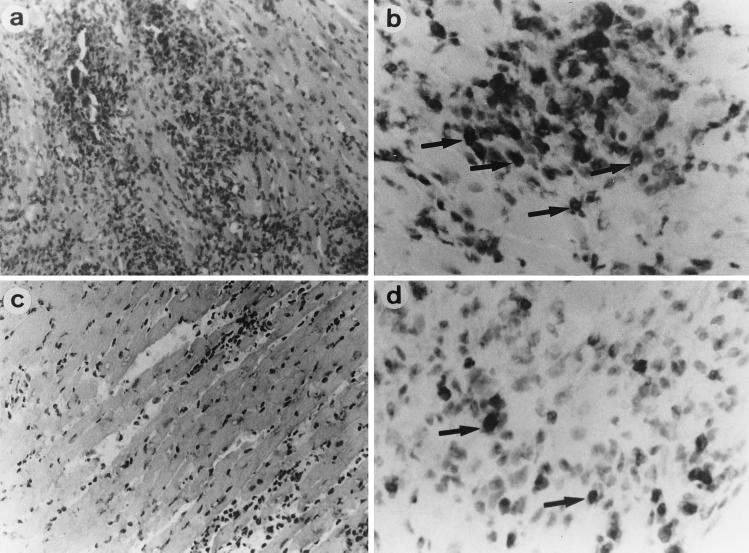
FIG. 5.
Cardiac histopathology. Tissues were sampled on day 14. In untreated control mice (left), severe myocardial necrosis (a) with MIP-2-positive cells (b) was observed. However, in anti-MIP-2 MAb (100 μg/day)-treated mice (right), myocardial necrosis (c), and MIP-2-positive cell infiltrations (d) were less severe. Arrows and arrowheads indicate cells positively stained for MIP-2. (a and c) hematoxylin and eosin staining; magnification, ×80. (b and d) MIP-2 staining; magnification, ×150.
TABLE 2.
Results of absorption studya
| Treatment (n) | No. of survivors | Pathological scoreb
|
Plasma MIP-2 levels (μg/ml) (n = 5) | |
|---|---|---|---|---|
| Infiltration (n) | Necrosis (n) | |||
| Controls (16) | 9 | 2.2 ± 1.1 (9) | 2.3 ± 1.2 (9) | 323.2 ± 138.9 |
| Normal rabbit Ig (100 μg/day) (12) | 7 | 2.3 ± 0.8 (7) | 2.0 ± 1.0 (7) | 299.4 ± 161.9 |
| Absorbed anti-MIP-2 MAb (100 μg/day)c (10) | 7 | 2.3 ± 1.0† (7) | 2.0 ± 1.2† (7) | 333.2 ± 204.1 |
Mice were injected subcutaneously with saline (controls), normal rabbit Ig, and absorbed anti-MIP MAb on days 0 to 5. Mice were observed until day 14. The surviving mice were killed, and the pathological study was performed. Also, plasma MIP-2 levels were determined on day 14. There were no statistical differences in survival rates, pathological scores, or plasma MIP-2 levels among the three groups. Values are means ± standard deviations.
†, P < 0.05 versus group 3 (anti-MIP-2 MAb treatment at 100 μg/day).
The data of this group on day 14 were compared with those of anti-MIP-2 MAb-treated groups (groups 2 and 3) on day 14. As a result, the survival rate on day 14 was not statistically (by χ2 test) different. The pathological scores of cellular infiltration and necrosis on day 14 were significantly less in group 3 than those of the absorbed anti-MIP-2 MAb-treated group.
TABLE 3.
Results of anti-MIP-2 MAB studya
| Anti-MIP-2 MAB treatment | Day | % of mononuclear cell subset (n = 4)
|
Virus titer (PFU/mg of tissue) (n) | BW (g) (n = 7) | HW/BW ratio (103) (n = 7) | |||
|---|---|---|---|---|---|---|---|---|
| Mφ | Pan T | CD4 | CD8 | |||||
| Group 1 (none) | 2 | (4.6 ± 2.5) × 105 (4) | ||||||
| 4 | (4.6 ± 1.8) × 106 (4) | |||||||
| 7 | 6.5 ± 2.4 | 16.5 ± 3.1 | 9.5 ± 2.1 | 7.3 ± 1.0 | (3.3 ± 2.7) × 103 (7) | 20.8 ± 1.7 | 7.2 ± 0.6 | |
| 14 | 14.0 ± 3.9 | 24.3 ± 4.4 | 17.8 ± 2.5 | 13.0 ± 2.5 | NDb (3) | 25.5 ± 1.5 | 7.7 ± 0.5 | |
| Group 2 (10 μg/day) | 2 | (3.6 ± 2.9) × 105 (4) | ||||||
| 4 | (3.8 ± 2.9) × 106 (4) | |||||||
| 7 | 4.0 ± 1.4 | 15.0 ± 2.2 | 9.5 ± 1.9 | 6.3 ± 1.7 | (4.5 ± 3.2) × 103 (7) | 20.8 ± 1.1 | 7.2 ± 0.7 | |
| 14 | 8.8 ± 1.5 | 21.0 ± 3.2 | 16.3 ± 1.9 | 12.8 ± 5.6 | ND (3) | 24.5 ± 1.1 | 7.5 ± 0.6 | |
| Group 3 (100 μg/day) | 2 | (4.7 ± 1.6) × 105 (4) | ||||||
| 4 | (3.9 ± 1.5) × 106 (4) | |||||||
| 7 | 3.0 ± 0.8* | 9.0 ± 1.8** | 5.3 ± 1.3* | 3.0 ± 0.8** | (2.9 ± 2.6) × 103 (7) | 20.8 ± 1.9 | 6.4 ± 0.7 | |
| 14 | 6.8 ± 2.1* | 14.8 ± 3.2* | 9.8 ± 1.3** | 7.0 ± 2.5* | ND (3) | 25.3 ± 1.0 | 6.7 ± 0.6** | |
Mice were injected subcutaneously with anti-MIP-2 MAb at 10 μg/day (group 2) and at 100 μg/day (group 3) on days 0 to 5. Subsets of mice were killed on days 7 and 14. The BW and HW/BW ratio were calculated. The percentages of positively stained mononuclear cells were calculated as previously described (16, 17). Myocardial virus titers were determined by a plaque assay. Values are means ± standard deviations. ∗ and ∗∗, P < 0.05 and P < 0.01, respectively, versus control.
ND, not detected.
The survival rates on day 21 were 50.0% (12 of 24) in group 1 (the control group), 57.7% (15 of 26) in group 2 (10 μg/day treated), and 81.0% (17 of 21) in group 3 (100 μg/day treated), respectively. A significant increase in survival (P < 0.05) was observed in group 3, but not in group 2, in comparison with that of group 1. There was a significant decrease in the HW/BW ratio in group 3 on day 14 in comparison with that of group 1. The pathological scores of cellular infiltration and necrosis on days 14 and 21 and on from day 0 to day 21 were significantly less in group 3, but not in group 2, compared with group 1. The distribution of MIP-2-positive cells in the myocardium on days 7 (6.8% ± 2.2%; n = 4) and 14 (15.0% ± 3.6%; n = 4) in the control group (group 1) was very similar to that of Mac-1-positive cells (day 7, 6.5% ± 2.4%; day 14, 14.0% ± 3.9%). Fibroblast and vascular endothelial cells were negative for MIP-2 staining. Thus, the in vivo cellular source of MIP-2 seemed to be macrophages in this model. The percentages of macrophages and T-lymphocyte subsets in the anti-MIP-2 MAb group (group 3, but not group 2) were lower than in the control group (group 1). The myocardial virus titers did not differ significantly among the three groups. Also, there were no significant differences in the pancreatic virus titers among the three groups on days 2, 4, and 7 (data not shown). The serum MIP-2 levels on day 7 in the anti-MIP-2 groups (group 2, 61.2 ± 33.5 ng/ml, n = 5; group 3, 53.0 ± 27.4 ng/ml, n = 5) were significantly lower (both P < 0.01) than in the control (group 1, 142.2 ± 40.0 ng/ml, n = 5).
DISCUSSION
In the present study, we demonstrated that the production of MIP-2 increased significantly in virus-infected RAW 264.7 macrophages in an infection dose- and time-dependent manner, that plasma MIP-2 levels were elevated in the blood of the virus-infected mice, and that the anti-MIP-2 MAb prevented myocardial inflammatory and necrotic responses resulting in reduced macrophage and T-cell accumulation in the myocardium compared with controls. Thus, MIP-2 is an important naturally occurring inflammatory cytokine in murine myocarditis, and anti-MIP-2 antibody treatment may prevent the myocardial damage in this condition.
EMC, an enterovirus of the family Picornaviridae, can infect many mammals (8). We reported previously that EMC infection in the mouse may be followed by dilated cardiomyopathy and congestive heart failure, with pathological changes similar to those seen in humans (8, 12). These severe myocardial lesions were not seen in athymic mice. Thus, the severity and the development of myocarditis are dependent upon T cells in this model (8, 12).
IL-8 is a monocyte/macrophage-derived peptide that belongs to a novel cytokine family (1, 13, 18, 22) and MIP-2 is considered to be a murine counterpart of IL-8 (3, 14, 15, 20). This cytokine has chemotactic activity for neutrophils and lymphocytes. A recent study showed that IL-8 and MIP-2 are potent inflammatory agents. Indeed, T-lymphocyte recruitment by IL-8 administration was reported in severe combined immunodeficiency (SCID) mice (18). Accordingly, it may be of value to investigate the changes of MIP-2 in murine myocarditis and the effects of anti-MIP-2 MAb upon this condition by analysis of myocardial infiltrating macrophages and T lymphocytes.
In the present study, the production of MIP-2 increased in the supernatants of EMC virus-infected murine macrophages in vitro in a infection dose-dependent manner. The time-dependent production of MIP-2 suggested that MIP-2 may operate early in the course of infection. In addition, increases in MIP-2 production were detected in the plasma of the mice. The MIP-2 concentration decreased late in the course of infection, possibly because the peak of inflammatory changes occurred around 10 to 14 days after viral infection in this model, and the change in MIP-2 levels may reflect the course of the disease. These observations suggested that EMC virus infection has the potential to accelerate the production of MIP-2 in vitro and in vivo. Furthermore, the therapeutic efficacy of various doses of anti-MIP-2 MAb was confirmed in vivo; macrophage and T-lymphocyte infiltrations and associated myocardial cell necrosis were reduced in antibody-treated mice in a dose-dependent manner. While anti-MIP-2 treatment reduced the severity of myocarditis on day 14, it had no effects on day 7, even though plasma MIP-2 levels were increased at this time. This indicates that the primary effect of MIP-2 might be at the late stage of the disease, which is probably due to the time lag between the activities of inflammatory cytokines and disease development. Thus, MIP-2 is a major neutrophil and lymphocyte chemoattractant contributing to myocardial influx or infiltration in viral myocarditis.
Recently, Cook et al. demonstrated in vivo the involvement of MIP-1 α, a member of the MIP-2 family, in the inflammatory response in murine coxsackievirus B3 myocarditis using knockout mice (2). That is, homozygous MIP-1 α mutant (−/−) mice were resistant to coxsackievirus B3-induced myocarditis compared with wild-type (+/+) controls. These results demonstrated that MIP-2 and MIP-1 α are important mediators of virus-induced myocarditis.
Study limitations.
In the anti-MIP-2 MAb study, the treatment began simultaneously with viral infection to obtain effects, and the lower dose of anti-MIP-2 MAb (group 2, 10 μg/day treated) was ineffective. Hence, the results of the present study cannot necessarily be extrapolated to other experimental designs.
At the initiation of an inflammatory response, a number of cytokines are released. Chemoattractants released from infiltrating cells in the diseased myocardium specifically attract T lymphocytes (19). While there are a number of chemotactic proteins produced at the sites of inflammation, infiltrating cell-mediated macrophage and T-cell migratory events indeed occur early in the initiation of viral myocarditis. That is, MIP-2 is a major macrophage and lymphocyte chemoattractant contributing to myocardial influx or infiltration in viral myocarditis. In conclusion, MIP-2, a murine counterpart of IL-8, is an important naturally occurring inflammatory cytokine in murine myocarditis, and anti-MIP-2 MAb treatment may prevent the inflammatory response in this condition.
ACKNOWLEDGMENTS
This work was supported in part by research grants from the Conference on Coronary Artery Disease, Japanese Education of Science and Welfare (no. 08877110 and 09470164), Kanae Shinyaku Foundation, and Japan Cardiovascular Research Foundation.
REFERENCES
- 1.Brennan F M, Zachariae C O C, Chantry D, Larsen C G, Turner M, Maini R N, Matsushima K, Feldmann M. Detection of IL-8 biological activity in synovial fluids from patients with rheumatoid arthritis. Eur J Immunol. 1990;20:2141–2144. doi: 10.1002/eji.1830200938. [DOI] [PubMed] [Google Scholar]
- 2.Cook D N, Beck M A, Coffman T M, Kirby S L, Sherida J F, Pragnell I B, Smithies O. Requirement of MIP-1 α for an inflammatory response to viral infection. Science. 1995;269:1583–1585. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 3.Feng L, Xia Y, Yoshimura T, Wilson C B. Modulation of neutrophil influx in glomerulonephritis in the rat with anti-macrophage inflammatory protein-2 (MIP-2) antibody. J Clin Investig. 1995;95:1009–1017. doi: 10.1172/JCI117745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiraoka Y, Kishimoto C, Kurokawa M, Ochiai H, Sasayama S. Effects of polyethylene glycol conjugated superoxide dismutase on coxsackievirus B3 myocarditis in mice. Cardiovasc Res. 1992;26:956–961. doi: 10.1093/cvr/26.10.956. [DOI] [PubMed] [Google Scholar]
- 5.Hiraoka Y, Kishimoto C, Takada H, Kurokawa M, Ochiai H, Shiraki K. Role of oxygen derived free radicals in the pathogenesis of coxsackievirus B3 myocarditis in mice. Cardiovasc Res. 1993;27:957–961. doi: 10.1093/cvr/27.6.957. [DOI] [PubMed] [Google Scholar]
- 6.Kanda T, McManus J E W, Nagai R, Imai R, Suzuki T, Yang D, McManus B M, Kobayashi I. Modification of viral myocarditis in mice by interleukin-6. Circ Res. 1996;78:848–856. doi: 10.1161/01.res.78.5.848. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan E L, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–462. [Google Scholar]
- 8.Kishimoto C, Kuribayashi K, Masuda T, Tomioka N, Kawai C. Immunologic behavior of lymphocytes in experimental viral myocarditis: significance of T lymphocytes in the severity of myocarditis and silent myocarditis in BALB/c-nu/nu mice. Circulation. 1985;71:1247–1254. doi: 10.1161/01.cir.71.6.1247. [DOI] [PubMed] [Google Scholar]
- 9.Kishimoto C, Misaki T, Crumpacker C S, Abelmann W H. Serial immunologic identification of lymphocyte subsets in murine coxsackievirus B3 myocarditis: different kinetics and significance of lymphocyte subsets in the heart and in peripheral blood. Circulation. 1988;77:645–653. doi: 10.1161/01.cir.77.3.645. [DOI] [PubMed] [Google Scholar]
- 10.Kishimoto C, Abelmann W H. In vivo significance of T cells in the development of coxsackievirus B3 myocarditis in mice: immature but antigen-specific T cells aggravate cardiac injury. Circ Res. 1990;67:589–598. doi: 10.1161/01.res.67.3.589. [DOI] [PubMed] [Google Scholar]
- 11.Kishimoto C, Kuroki Y, Hiraoka Y, Ochiai H, Kurokawa M, Sasayama S. Cytokine and murine coxsackievirus B3 myocarditis: interleukin-2 suppressed myocarditis in the acute stage but enhanced the condition in the subsequent stage. Circulation. 1994;89:2836–2842. doi: 10.1161/01.cir.89.6.2836. [DOI] [PubMed] [Google Scholar]
- 12.Kishimoto C, Kuribayasi K, Fukuma K, Masuda T, Tomioka N, Abelmann W H, Kawai C. Immunologic identification of lymphocyte subsets in experimental murine myocarditis with encephalomyocarditis virus: different kinetics of lymphocyte subsets between the heart and the peripheral blood, and significance of Thy 1.2+ (pan T) and Lyt 1+, 23+ (immature T) subsets in the development of myocarditis. Circ Res. 1997;61:715–725. doi: 10.1161/01.res.61.5.715. [DOI] [PubMed] [Google Scholar]
- 13.Koch A E, Polverini P J, Kunkel S L, Harlow L A, Dipietro L A, Elner V M, Elner S G, Strieter R M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 14.Ochiai H, Ikesue A, Kurokawa M, Nakajima K, Nakagawa H. Enhanced production of rat interleukin-8 by in vitro and in vivo infections with influenza A NWS virus. J Virol. 1993;67:6811–6814. doi: 10.1128/jvi.67.11.6811-6814.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ochiai H, Sakai S, Kogure T, Hirabayashi T, Nakajima K, Terasawa K. Development and some applications of enzyme linked immunosorbent assay system for murine macrophage inflammatory protein-2 (MIP-2) Mediat Inflamm. 1996;5:206–209. doi: 10.1155/S0962935196000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raschke W C, Baird S, Ralph P, Nakoinz I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 1978;15:261–267. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]
- 17.Rezkalla S, Khatib G, Khatib R. Coxsackievirus B3 murine myocarditis: deleterious effects of nonsteroidal antiinflammatory agents. J Lab Clin Med. 1986;107:93–95. [PubMed] [Google Scholar]
- 18.Sakai S, Kawamata H, Mantani N, Kogure T, Shimada Y, Terasawa K, Sakai T, Imanishi N, Ochiai H. Therapeutic effect of anti-macrophage inflammatory protein 2 antibody on influenza virus-induced pneumonia in mice. J Virol. 2000;74:2472–2476. doi: 10.1128/jvi.74.5.2472-2476.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taub D D, Anver M, Oppenheim J J, Longo D L, Murphy W J. T lymphocyte recruitment by interleukin-8 (IL-8). IL-8-induced degranulation of neutrophils releases potent chemoattractants for human T lymphocytes. J Clin Investig. 1996;97:1931–1941. doi: 10.1172/JCI118625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tekamp-Olson P, Gallegos C, Bauer D, McClain J, Sherry B, Fabre M, van Deventer S, Cerami A. Cloning and characterization of cDNAs for murine macrophage inflammatory protein 2 and its human homologues. J Exp Med. 1990;172:911–919. doi: 10.1084/jem.172.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolpe S D, Sherry B, Jueres D, Davatelis G, Yurt R W, Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci USA. 1989;86:612–616. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yue T L, Wang X, Sung C P, Olson B, McKenna P J, Gu J L, Feuerstein G Z. Interleukin-8: a mitogen and chemoattractant for vascular smooth muscle cells. Circ Res. 1994;76:1–7. doi: 10.1161/01.res.75.1.1. [DOI] [PubMed] [Google Scholar]