Abstract
In this paper we report the regulation of Aspergillus niger growth rate during citric acid fermentation in a stirred tank bioreactor. For this, the influence of dissolved oxygen concentration in a medium on intracellular pH values and consequently on overall microbial metabolism was emphasized. Intracellular pH of mycelium grown under different concentrations of dissolved oxygen in the medium was determined. Sensitivity of proteins toward proton concentration is well recognized, therefore pH influences on the activities of key regulatory enzymes of Aspergillus niger were determined at pH values similar to those detected in the cells grown under lower dissolved oxygen concentrations. The results have shown significantly reduced specific activities of hexokinase, 6-phosphofructokinase and glucose-6-phosphate dehydrogenase in more acidic environment, while pyruvate kinase was found to be relatively insensitive towards higher proton concentration. As expected, due to the reduced specific activities of regulatory enzymes under more acidic conditions, overall metabolism should be hindered in the medium with lower dissolved oxygen concentration which was confirmed by detecting the reduced specific growth rates. From the studies, we conclude that dissolved oxygen concentration affects the intracellular pH and thus growth rate of Aspergillus niger during the fermentation process.
Keywords: Aspergillus niger, intracellular pH, dissolved oxygen concentration, citric acid fermentation, stirred tank bioreactor.
1. Introduction
An understanding of the physiology of productive microorganism is of utmost importance. With the availability of complete nucleotide sequences in increased number of microorganisms, functional genomics is becoming the prevailing method in studies of microbial physiology. However, for an understanding of the regulation of metabolic pathways and the environmental factors that affect metabolic fluxes, the information on gene expression and protein function is not sufficient. In biotechnological processes, engineers often try to achieve higher productivity by increased biomass concentration, however, under such conditions it is difficult to maintain sufficient oxygen concentration in bioreactors and therefore the corresponding specific productivity. Namely, quite often the influence of dissolved oxygen concentration in the medium on intracellular pH is ignored. Thus, the effect of dissolved oxygen concentration on changes in enzymatic activities and perturbations in overall metabolism are ignored as well. Literature has some reports on sensitivity of intracellular pH to hypoxia in filamentous fungi 1,2 that refer to reduced activity of plasma membrane H+ ATPase, which has been shown to be involved in maintenance of intracellular proton concentration by extrusion of protons from the cytoplasm at the expense of ATP 3. Reduced oxygen availability, ATP synthesis is slower and cannot cope with the requirements of proton pumps any more. One of the reasons for less interest for the effects of intracellular pH is a lack of simple and accurate method for pH detection in filamentous fungi.
Some reports have appeared in literature referring to the determination of intracellular pH values by 31P-NMR technique in perfused systems in Neurospora crassa 1 and by a airlift bioreactor in Aspergillus terreus 4 where growth conditions were difficult to maintain with respect to dissolved oxygen concentration. An advanced technique was developed using Aspergillus niger cells in a well controlled perfusion system where oxygen concentration could be monitored 5. With the results presented in this manuscript, we have tried to emphasize the often overlooked influence of dissolved oxygen concentration on intracellular pH values and consequently on overall microbial metabolism. A glass stirred tank bioreactor (7.5 L) was employed for all microbial cultivations. Samples were withdrawn every 24 h.
2. Materials and Methods
The conidia of Aspergillus niger (MNNG-115) were harvested from a 4-day old agar slants and then suspended in 25 ml of sterile 0.1 % Tween 80 solution. Conidial suspension (1×107 conidia/ml) was used to inoculate the medium containing: distilled water 1.0 L, glucose 10 g, KH2PO4 5.0 g, MgSO4.7H2O 1.0 g, NaCl 0.5 g, (NH4)2SO4 as a nitrogen source. The pH was adjusted at 5.5 with 0.1 N HCl/NaOH. For obtaining the mycelium, the fermentation was performed in a stirred glass tank bioreactor (New Brunswick, USA) of 7.5-L working volume. The temperature was kept at 30ºC and the medium was aerated with 1.0 l/l/min of air. Dissolved oxygen concentration was monitored with a sterilisable pO2 electrode. For maintaining different levels of dissolved oxygen concentrations in the fermented broth, volume of the inlet air was dosed by a mass flow controller connected with software and pO2 electrode.
The samples for intracellular pH determination were collected and prepared as reported previously 2. Spectroscopy was accomplished on a spectrometer (Aguarius, UK) operating at.328 MHz. Chemical shifts were related to 75 % H3PO4 contained in a capillary tube. Condensed and cooled mycelium was placed in a 10 mm tube and poured with cooled buffered methanol. During spectroscopy, the sample was kept at 42±0.2ºC by ventilating the sample with nitrogen pre-cooled on dry ice. Intracellular pH values were determined from a calibration curve by comparing the chemical shift of the ortho-phosphate peak. Dry weight of biomass was determined gravimetrically after 250 ml of fermentation broth was taken from the bioreactor, filtered over a pre-dried and weighted filter paper, rinsed extensively with three-fold volume of cold distilled water and dried in the microwave oven to the constant weight. Specific growth rates and doubling times were determined after Pirt 6.
The specific enzyme activities were determined in cell-free homogenate, which was prepared as reported previously 7. Enzyme activities were determined by following NADH-NAD+or NADP+-NADPH transformation kinetics in a double beam UV/Vis scanning spectrophotometer at 332 nm (at room temperature). They were measured in 50 mM imidazole buffer of indicated pH values in the presence of 5 mM MgCl2 and 80 mM KCl. Each specific assay mixture contained in 1.0 ml the following substrates and auxiliary enzymes (Sigma, USA): hexokinase: 10 mM glucose, 2 mM ATP, 0.4 mM NADP+and 3U glucose-6-phosphate dehydrogenase; for 6-phosphofructo-1-kinase: 10 mM fructose-6-phosphate, 1 mM ATP, 0.2 mM NADH, 5 mM DTT, 1 U aldolase, 50 U triosephosphate isomerase and 15 U glycerol-3-phosphate dehydrogenase; for piruvate kinase: 1 mM phosphoenolpiruvate, 2 mM ADP, 0.2 mM NADH, 15 U lactate dehydrogenase; for glucose-6-phosphate dehydrogenase: 0.5 NADP + and 1 mM glucose-6-phosphate. Specific enzyme activities were determined after dissolved proteins were measured in the crude enzyme preparation according to Bradford 8 with crystalline bovine serum as a standard.
3. Results and Discussion
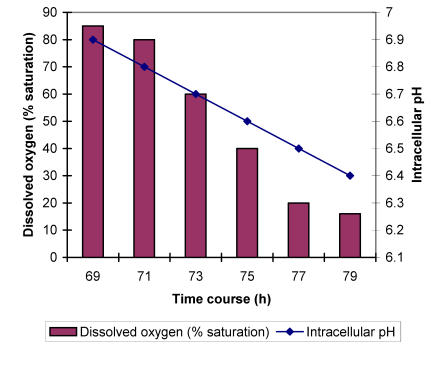
It is known that oxygen concentration in the fermentation broth influences the intracellular pH value 9,10. To check how pH values are changed during the A. niger growth under different concentrations of dissolved oxygen, the mycelium was grown in a 7.5-L bioreactor for 70 h at optimal aerating conditions to obtain sufficient biomass. The control of aeration was then switched on to maintain the level of dissolved oxygen at 75 % of saturation. Afterwards, every 2 h the dissolved oxygen concentration (DOC) was reduced for additional 20 %. Meanwhile mycelial samples were taken for pH determination. The analysis has shown that A. niger cells are relatively resistant to reductions of DOC in the range of 80 to 60 % with respect to acidification. Intracellular pH dropped for only about 0.04 units. However, by further 20 % reduction of oxygen concentration in the broth, pH dropped to the value of about 6.68 units. Even more pronounced accumulation of protons in the cells could be observed at 20 % of oxygen saturation exhibiting the reduction of intracellular pH value to 6.48 units (Fig. 1).
Figure 1.
Intracellular pH dependence of A. niger on stepwise dissolved oxygen concentrations decrease during the growth in a chemically defined medium with ammonium ions as a sole source of nitrogen.
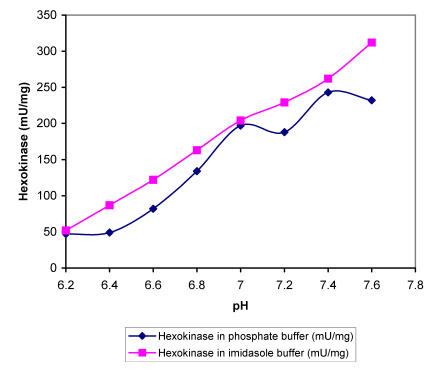
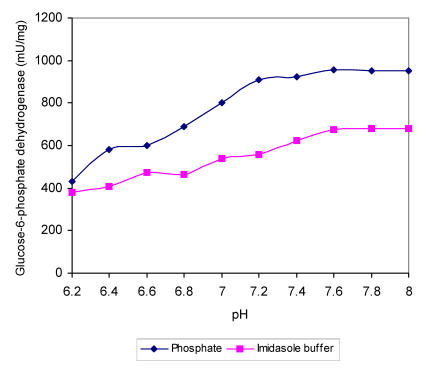
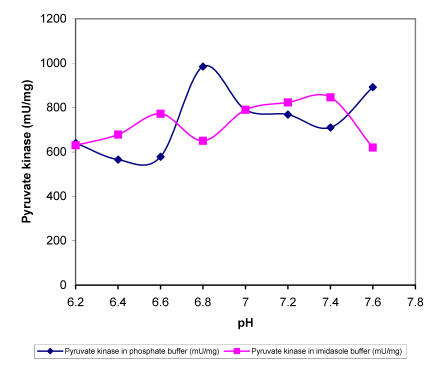
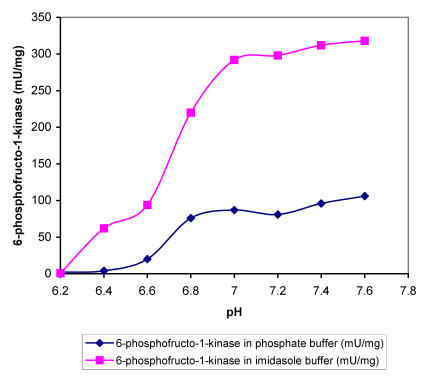
Within the range of DOC used the intracellular pH dropped for nearly 0.5 units, which must affect the activity of specific enzymes and influence the overall metabolism. In order to find out more about the sensitivity of regulatory A. niger enzymes at different proton concentrations, we determined enzyme activities in buffers with different pH values in the presence of substrates in excess. The specific activities of enzymes i.e., hexokinase, 6-phospho-fructo-1-kinase, piruvate kinase and glucose-6-phos-phate dehydrogenase were determined in two buffers (Fig. 2-5). Apart from piruvate kinase, where minor influence of different pH values on activity was recorded (Fig. 4), all other tested enzymes have shown remarkable sensitivity towards more acidic conditions. In the pH range of 6.5 to 7.0, which was determined in the cells during the incubation at DOC values between 20 and 80 %, hexokinase specific activity was reduced for about 60 % in both buffers (Fig. 2). Even more pronounced decrease in activity in the same pH range was recorded with 6-phos-phofructo-1-kinase but interestingly more expressed reduction was observed in phosphate buffer (from about 280 mU/mg to only 72 mU/mg) than in imidazole buffer, where PFK activity seemed to be relatively insensitive to changes between pH value of 7.0 and 6.8 (Fig. 3). Some influence of pH on the activity was observed also with glucose-6-phosphate dehydrogenase. However, specific activities of this enzyme were reduced for about 24.5 % in the range of physiological pH changes (Fig. 5).
Figure 2.
Maximal specific activities of A. niger hexokinase measured at different pH values in phosphate and imidasole buffer
Figure 5.
Maximal specific activities of A. niger glucose-6-phosphate dehydrogenase measured at different pH values in phosphate and imidasole buffer
Figure 4.
Maximal specific activities of A. niger pyruvate kinase measured at different pH values in phosphate and imidasole buffer
Figure 3.
Maximal specific activities of A. niger 6-phosphofructokinase measured at different pH values in phosphate and imidasole buffer
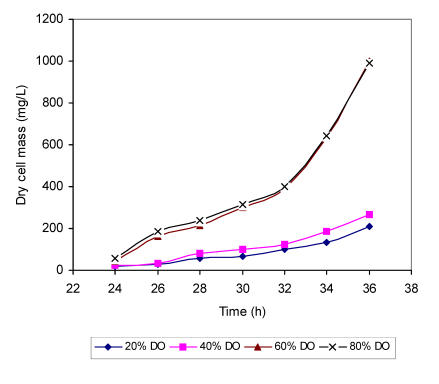
The data obtained by measuring specific activities of regulatory enzymes in different pH environment shows significant reduction in metabolic fluxes under lower dissolved oxygen concentrations, which must affect the specific growth rate of the fungus in the same medium. The increase of dry weight of biomass was monitored from 24-36 h in batch fermentation runs under defined dissolved oxygen concentrations (Fig. 6). In glucose medium with ammonium ions as a sole nitrogen source, a transition from lag phase to exponential phase took place before 24 h of fermentation regardless of the level of dissolved oxygen. However, distinct correlation between oxygen availability and specific growth rate could be observed later. In the medium with 80 % of oxygen saturation the cells grew most rapidly with value of 0.202 h having a doubling time of 3.26 h. Negligible difference in specific growth rate (m=0.211 h ) and doubling time (t/d =3.28 h) was recorded in the same medium with 60 % of oxygen saturation. In the medium with further reduction of dissolved oxygen (40 % of saturation), dry weight doubled in 3.5 h (0.198 h). Even slower growth was recorded with 20 % of dissolved oxygen giving the doubling time of 3.91 h and specific growth rate of 0.177 h, respectively.
Figure 6.
Growth curves of A. niger mycelium grown in a chemically defined substrate with ammonium ions as a sole nitrogen source at different concentrations of dissolved oxygen (DO).
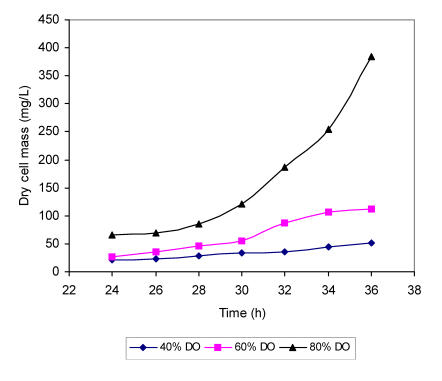
A. niger cells showed more sensitivity towards the lower dissolved oxygen concentrations in the medium where ammonium ions were replaced by glutamic acid (Fig. 7). Again the most rapid growth rate was recorded at higher dissolved oxygen level although the specific growth rate values were lower and m value of only 0.19 h was detected in the medium with 80 % of oxygen saturation. The doubling time was accordingly extended to 3.64 h. As expected, more reduced specific growth rate was determined in the medium with dissolved oxygen concentration of 60 % with doubling time of 3.74 h and m value of 0.185 h . Markedly slower growth with specific rate of 0.139 h and doubling time of 4.9 h were observed in the substrate with 40 % of oxygen saturation. In the medium with only 20 % of dissolved oxygen, the increase of biomass could not be followed adequately.
Figure 7.
Growth curves of A. niger mycelium grown in a chemically defined substrate with glutamic acid as a sole nitrogen source at different concentrations of dissolved oxygen (DO).
The measurements of intracellular pH in A. niger cells grown at different oxygen concentrations revealed relative insensitivity of pH changes towards decreased oxygen concentration to about 60 %, while further decrease of oxygen concentration caused more rapid accumulation of protons in the cells. The data is in accordance with the theory of H+-ATPases 3 that plasma membrane located enzymes play a crucial role in cellular pH regulation, al-though their activity depends on ATP availability and is diminished by reduced ATP synthesis under oxygen limitation. The recorded values of intracellular pH of the strain in a well aerated system presented in the present paper are about 0.62 units lower than those detected in Ng13 strain of the same species 5. MNNG-115 strain is known to be a good citric acid producer able to convert up to 75 % of sucrose into citric acid within 6 days under optimal conditions 11, while strain Ng13 is a less efficient producer 12. Its intracellular citric acid concentration measured at 24 h was 2.5 mM 13, which is about 4-fold less than that detected in MNNG-115 strain 14. At neutral pH values citric acid is present in ionic form releasing one more proton, therefore higher acid concentrations in the cells most probably contribute to more acidic conditions.
No data have been presented for A. niger enzymes so far, in spite of the fact that this is one of the important commercial microorganisms 15. Interestingly, pH optima of all enzymes tested were in slightly alkaline region but declined rapidly under the pH value of 6.2. The activity of pyruvate kinase alone was relatively insensitive towards more acidic environment. These data are in accordance with the measurements of specific growth rates and intracellular pH values under different dissolved oxygen concentration, where slower growth rate was recorded at values below 60 % of saturation. The specific growth rate that reflects the efficiency of overall metabolism was less affected by dissolved oxygen concentrations above 60 % and again it fits the data about the changes of intracellular pH values that were less affected by decreased oxygen concentration. The enzyme 6-phosphofructo-1-kinase showed significantly higher specific activities in phosphate buffer while with other enzymes the values measured in buffer were comparable. It is known that PFK of Bf12 strain is activated by phosphorylation and it seems that phosphate ions present in buffer prevent dephosphorylation and deactivation of the activated form 16.
It is important to realize that enzymatic activities were measured with substrate concentrations in excess, which are comparable to those previously detected in A. niger. Substrate levels are, however, much lower and adequately decreased levels of specific enzymatic activities that would cope with calculations of metabolic fluxes 17. The activation of plasma H+-ATPases by the addition of ammonium ions to the medium is described in A. niger 18. It was found that H+-ATPase is activated by phosphorylation, which is triggered through the phosphatidyl-inositol signalling pathway. Since plasma membrane proton pumps play a crucial role in the regulation of intracellular pH, it was expected that under the conditions of inactivated mechanism for pH control the specific growth rate would be more sensitive towards the decreased dissolved oxygen concentration. The measurements of specific growth rate in less aerated media with glutamic acid as a sole nitrogen source confirmed our results.
4. Conclusion
Considerable changes in intracellular pH values were recorded in Aspergillus niger cells during the growth in a medium with decreased oxygen concentration. More acidic conditions affect the activity of key regulatory enzymes resulting in changes of overall metabolisms that are reflected as altered specific growth rate.
Biographies
Ikram-ul Haq is a professor in Department of Botany, G.C. University Lahore, Pakistan. He was the Vice President of Pakistan Botanical Society and member of Editorial Board of Biologia, Pakistan Journal of Botany, and Pakistan Journal of Biotechnology. He was awarded a productive scientist of Pakistan by PCS&T for Year 2002. Current research projects include process development for the production of cellulases for industrial , process development for the production of enzyme invertase by Saccharomyces cerevisiae, and the development of mutant strain of Aspergillus niger for citric acid fermentation.
Sikander Ali is a lecturer in Department of Botany, G.C. University Lahore. Between 2002 and 2003, he was the Fulbright Scholar under the supervision of Prof. David A. Lightfoot, participating in the research projects in Plant Molecular Biology and Genomic Core Facility at PSGA, SIUC, IL, USA.
References
- 1.Pilatus U, Techel D. Production of citric acid. Biochim. Biophys. Acta. 1991;1091:349–355. doi: 10.1016/0167-4889(91)90199-8. [DOI] [PubMed] [Google Scholar]
- 2.Legisa M, Grdadolnik G. Molecular biology and biotechnological applications for large-scale synthesis of recombinant proteins. Acta Chim. Slov. 2000;47:389–400. [Google Scholar]
- 3.Serrano R. New developments in oxidative fermentation. Biochim. Biophys. Acta. 1988;947:1–28. doi: 10.1016/0304-4157(88)90017-2. [DOI] [PubMed] [Google Scholar]
- 4.Lyngstad M, Grasdalen H. The use of image analysis for morphological measurements on filamentous microorganisms. J. Biochem. Biophys. Methods. 1993;27:105–116. doi: 10.1016/0165-022x(93)90054-r. [DOI] [PubMed] [Google Scholar]
- 5.Hesse SJ. et al. Attempts at improving citric acid fermentation by Aspergillus niger in beet molasses medium. J. Biotechnol. 2000;77:5–15. doi: 10.1016/s0960-8524(02)00007-x. [DOI] [PubMed] [Google Scholar]
- 6.Pirt SJ. Principles of Microbe and Cell Cultivation. London: Blackwell Scientific Publications; 1975. [Google Scholar]
- 7.Legisa M. Effect of some metabolic inhibitors on citric acid production by Aspergillus niger. FEMS Microbiol. Lett. 1994;118:327–334. doi: 10.1016/0378-1097(94)90524-x. [DOI] [PubMed] [Google Scholar]
- 8.Bradford MM. Effect of alcohol on the production of citric acid by Aspergillus niger KG-20. Anal. Biochem. 1976;72:248–254. [Google Scholar]
- 9.Chance B. et al. Mitochondrial activity during citric acid production by Aspergillus niger. Proc. Natl. Acad. Sci. USA. 1978;75:4925–4929. [Google Scholar]
- 10.Ikai I. et al. Submerged fermentation of citric acid: Counter-act effect of Cu2+ on the deleterious effect of Fe2+ in black strap molasses. Biochim. Biophys. Acta. 1991;1074:289–293. [Google Scholar]
- 11.Legisa M, Gradisnik-Grapulin M. Enhancement in citrate production by alcoholic limitation. Appl. Environ. Microbiol. 1995;61:2732–2737. doi: 10.1128/aem.61.7.2732-2737.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruijter GJ. et al. Hydrodynamics and mass transfer in Aspergillus niger fermentation in bubble column and loop bioreactors. Food Technol. Biotechnol. 1998;36:185–188. doi: 10.1002/bit.260340602. [DOI] [PubMed] [Google Scholar]
- 13.Ruijter GJ. et al. Measurement of rheological properties of filamentous fermentation broths. FEMS Microbiol. Lett. 2000;184:35–40. doi: 10.1111/j.1574-6968.2000.tb08986.x. [DOI] [PubMed] [Google Scholar]
- 14.Legisa M, Kidri J. Metabolism of citric acid production by Aspergillus niger model definition, steady state analysis and constrained optimisation of the citric acid production rate. Appl. Microbiol. Biotechnol. 1989;31:453–457. [Google Scholar]
- 15.Kurkdjian A, Guern J. Production and purification of citric acid by Aspergillus niger. Ann. Rev. Plant Physiol. 1989;40:271–303. [Google Scholar]
- 16.Ruijter GJ. et al. Estimating cell and product yields. Biochim. Biophys. Acta. 1997;1334:317–326. doi: 10.1016/s0304-4165(96)00110-9. [DOI] [PubMed] [Google Scholar]
- 17.Steinboeck F. Food additives solvent extraction process for citric acid. Biochem. Biophys. Acta. 1994;1200:215–223. doi: 10.1016/0304-4165(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 18.Legisa M, Jernejc K. Selectivity in citric acid production by Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2001;57:368–373. doi: 10.1007/s002530100697. [DOI] [PubMed] [Google Scholar]