Abstract
Background
Idiopathic sudden sensorineural hearing loss (ISSHL) is characterised by sudden loss of hearing of cochlear or retro‐cochlear origin without an identifiable cause. Antivirals are commonly prescribed, but there is no consensus on the treatment regimen or their effectiveness.
Objectives
To determine the effectiveness and side effect profile of antivirals in the treatment of ISSHL.
Search methods
We systematically searched the Cochrane Ear, Nose and Throat Disorders Group Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 5), PubMed, EMBASE, CINAHL and other databases to 12 June 2012. We also scanned the reference lists of identified studies for further trials.
Selection criteria
Randomised controlled trials comparing different antivirals versus placebo (both with or without other treatment).
Data collection and analysis
Two authors independently extracted data, met to resolve disagreements and contacted study authors for further information. We assessed study risk of bias independently. We considered meta‐analysis inappropriate and ultimately not possible due to differing treatment protocols of varying dose and duration, together with differing inclusion criteria and outcome measures between studies. The results of each study are reported individually.
Main results
We included four randomised trials (257 participants). The overall risk of bias in the included studies was low. Two trials compared the addition of intravenous acyclovir to a steroid (prednisolone). One included 43 participants, the other 70 patients. Neither demonstrated any hearing improvement with ISSHL. Another (84 patients) did not show any statistically significant difference between groups with the addition of valacyclovir to prednisolone (compared to steroid plus placebo) with respect to change in pure‐tone audiogram. Comparing the addition of intravenous acyclovir to hydrocortisone with hydrocortisone alone, the final trial did not show any statistically significant difference between groups (60 patients). No trial documented any serious adverse effects related to the use of antiviral treatment. One study reported slight to moderate nausea equally in the acyclovir and placebo groups (one patient in each). Another reported insomnia, nervousness and weight gain with valacyclovir (number not specified). Even though no meta‐analysis was possible, evidence from the four RCTs has demonstrated no statistically significant advantage in the use of antivirals in the treatment of ISSHL.
Authors' conclusions
There is currently no evidence to support the use of antiviral drugs in the treatment of ISSHL. The four trials included in this review were, however, small and with a low risk of bias. Further randomised controlled trials with larger patient populations, using standardised inclusion criteria, antiviral regimes and outcome measures, are needed in order for adequate meta‐analysis to be performed to reach definitive conclusions. A uniform definition of ISSHL should also be established, together with what constitutes adequate recovery.
Keywords: Humans; Acyclovir; Acyclovir/analogs & derivatives; Acyclovir/therapeutic use; Antiviral Agents; Antiviral Agents/therapeutic use; Drug Therapy, Combination; Drug Therapy, Combination/methods; Glucocorticoids; Glucocorticoids/therapeutic use; Hearing Loss, Sensorineural; Hearing Loss, Sensorineural/drug therapy; Hearing Loss, Sudden; Hearing Loss, Sudden/drug therapy; Hydrocortisone; Hydrocortisone/therapeutic use; Prednisolone; Prednisolone/therapeutic use; Randomized Controlled Trials as Topic; Valacyclovir; Valine; Valine/analogs & derivatives; Valine/therapeutic use
Plain language summary
Antiviral drugs for sudden hearing loss (without known cause)
Idiopathic sudden sensorineural hearing loss (ISSHL) is sudden loss of hearing where clinical assessment has failed to reveal a cause. Patients may also suffer from additional symptoms such as tinnitus (a background ringing noise), together with dizziness and a sensation of fullness in the ear. Prompt investigation is essential to identify and treat the hearing impairment. In a large proportion of patients, however, no cause can be found.
Antiviral drugs are often used, usually in conjunction with steroids, to treat sudden hearing loss of unknown cause, based on the theory that the deafness is caused by a viral infection. We searched for randomised controlled trials (RCTs) which compared treatment of sudden hearing loss with antiviral drugs (either alone or in combination with another treatment) with placebo or no antiviral drug, in patients of any age. We found four RCTs (257 patients). The overall risk of bias in the studies was low. All four trials compared steroid treatment (either alone or plus a placebo drug) with steroid plus antiviral treatment. None of the trials found a statistically significant difference between groups. No trial documented any serious adverse effects related to using antiviral treatments. One study reported slight to moderate nausea equally in the acyclovir and placebo groups (one patient in each), both attributable to the steroid treatment. Another reported insomnia, nervousness and weight gain with valacyclovir (number not specified).
The effectiveness of antiviral drugs in the treatment of sudden hearing loss of unknown origin is questionable. Certainly, this review of the clinical trials did not identify any substantial evidence to support their use. Further research is required with larger patient numbers and standardised inclusion criteria, antiviral regimes and outcome measures.
Background
Idiopathic sudden sensorineural hearing loss (ISSHL) was first described in 1944 (DeKleyn 1944) and is characterised by sudden loss of hearing of cochlear or retro‐cochlear origin without an identifiable cause. It is considered a medical emergency (Conlin 2007b) carrying serious morbidity, affecting the patient's work and overall quality of life, with early treatment associated with a more favourable prognosis (Uri 2003).
ISSHL is usually a unilateral condition and affects approximately 5 to 20 per 100,000 individuals every year (Wu 2006). The degree of hearing loss experienced varies widely between mild, moderate and severe. A considerable proportion of cases can recover, at least partially, even without treatment. However many are left with some degree of permanent hearing loss, with or without tinnitus (a buzzing, ringing or whistling sensation of sound in one or both ears occurring without an external stimulus). The onset of ISSHL is rapid and early intervention is considered essential to prevent damage to the cochlea.
There are many theories as to the origin of this condition, including blood disorders (Gussen 1976), immune disorders (McCabe 1979), perilymphatic fistulas (Goodhill 1971) and viral infection (Wilson 1980). Each of these theories is based on certain laboratory and post‐mortem findings, but each has its own flaws. Blood disorders, such as polycythaemia, hyperviscosity and sickle cell crisis, can result in cochlear hypoxia and labyrinthine membrane necrosis by changing the viscosity and flow of the blood (Schuknecht 1986). However, experimental and clinical studies do not show enough evidence of fibrosis (the development of excess fibrous connective tissue in an organ, replacing normal tissue) or new bone formation, which would have been supportive of this theory. The eventual presence of antibodies against the inner ear suggests that sudden hearing loss pathogenesis may be autoimmune in nature, but the difficulty in establishing a correlation between the clinical aspects of the hearing loss and the antibody level also does not help this theory (Lazarini 2006).
In one study, a perilymphatic fistula was discovered in three patients with sudden hearing loss (Goodhill 1980), thus establishing round and oval window breaks as accepted aetiologies for sudden hearing loss. However, intra‐cochlear membrane breaks, with or without round or oval window involvement, have been poorly supported clinically and histopathologically as a cause for ISSHL. Another study demonstrated no active or healed membrane breaks in 12 dissected temporal bones (Schuknecht 1986).
Finally, viruses can cause sudden hearing loss either as an acute infection or as delayed onset with the latent form of the virus being possibly reactivated. Viruses implicated include herpes zoster oticus (Wilson 1980) and herpes simplex type 1 (Fukuda 1994). This theory has also been supported by the isolation of viral particles from human cochlea (Tucci 2000) and by the fact that ganciclovir (an antiviral) has a beneficial effect in preventing hearing deterioration when given to symptomatically infected infants with congenital cytomegalovirus infection (Lackner 2009). When tested on experimental herpes simplex labyrinthitis (Stokroos 1999), a combination treatment consisting of prednisolone and acyclovir resulted in earlier hearing recovery and less extensive cochlear destruction compared to prednisolone or acyclovir monotherapy.
Several treatment protocols for ISSHL exist including steroids, hyperbaric oxygen (Bennett 2007; Horn 2005), vasoactive substances (Agarwal 2009) and antiviral agents either alone or in combination (Jeyakumar 2006). Prior to treatment a thorough history is taken and a complete ear, nose and throat examination is performed. A series of investigations help to rule out any identifiable causes of hearing loss, such as a middle ear effusion or other conductive aetiology. Blood tests, including syphilis serology and even a magnetic resonance imaging (MRI) scan to rule out intracranial causes are often carried out. The pure‐tone audiogram (PTA) is the mainstay of the diagnosis and, although the hearing impairment varies in sound frequency and intensity, should show a unilateral hearing loss of 30 dB or more affecting in at least three contiguous frequencies (Wilson 1980).
Antivirals such as acyclovir and ganciclovir are commonly prescribed to treat this condition and many patients are admitted to hospital whilst receiving this treatment (Conlin 2007b). However, there is no consensus amongst otolaryngologists either on the treatment regimen or its effectiveness in clinical practice (Eisenman 2000). Antivirals are often prescribed alongside steroids such as prednisolone or hydrocortisone, whilst the patient is observed closely and hearing tests are repeated regularly. A recent Cochrane review found the value of steroids in the treatment of this condition to be unclear after identifying two randomised trials (Wei 2006).
Clinical trials (Tucci 2002; Uri 2003; Westerlaken 2003) and case series that have looked at the role of antivirals in the treatment of ISSHL are in conflict. Some studies have found encouraging results, especially when looking at steroid‐antiviral combinations and early combination therapy. However, because this is a relatively uncommon condition and the treatments vary (as well as the outcome measures used), these results do not provide strong evidence when looked at separately. A Cochrane systematic review of the evidence is therefore warranted.
Objectives
-
To assess the effectiveness of antivirals in:
improving hearing; and
reducing tinnitus in patients with sudden sensorineural hearing loss.
To determine the adverse effects of these medications.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials.
Types of participants
Patients of any age with sudden sensorineural hearing loss, meeting the following criteria:
Idiopathic sudden sensorineural hearing loss (ISSHL), defined as follows.
A history of a sudden decrease in hearing within three days.
A sensorineural hearing loss of at least 30 dB for three subsequent 1‐octave steps in frequency, unilateral or bilateral, demonstrable on a standard pure‐tone audiogram at the time of entry into the trial.
No other neurological signs except for the eighth cranial nerve defect.
Commencement of treatment within 14 days of the onset of the hearing loss.
Exclusion criteria included:
all other types of sensorineural hearing loss, or conductive forms of hearing impairment;
a history of fluctuating sensorineural hearing loss.
Types of interventions
Antivirals (oral or intravenous). Examples include acyclovir and valacyclovir, given at any dose for any duration. Comparisons were:
antiviral versus placebo;
antiviral versus no treatment;
(antiviral + other treatment) versus (placebo + same other treatment); and
(antiviral + other treatment) versus (same other treatment).
Types of outcome measures
Primary outcomes
An improvement in pure‐tone thresholds and/or speech discrimination. As the definition of improvement in hearing threshold varied between authors, we planned to dichotomise the outcome (into improvement or no improvement) according to the criteria set in each study.
Secondary outcomes
Evaluation of side effects of these treatment modalities.
Any alteration in tinnitus, disequilibrium and other symptoms was also studied.
Relevant factors to be considered in subgroup analyses were age, gender, types and route of administration of antivirals, the time interval from the initiation of therapy to audiological evaluation, degree of improvement and the time interval from the onset of symptoms to initiation of therapy.
Search methods for identification of studies
We conducted systematic searches for randomised controlled trials. There were no language, publication year or publication status restrictions. The date of the search was 12 June 2012.
Electronic searches
We searched the following databases from their inception for published, unpublished and ongoing trials: the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library 2012, Issue 5); PubMed; EMBASE; CINAHL; LILACS; KoreaMed; IndMed; PakMediNet; CAB Abstracts; Web of Science; BIOSIS Previews; ISRCTN; ClinicalTrials.gov; ICTRP; Google Scholar and Google.
We modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, we combined subject strategies with adaptations of the highly sensitive search strategy designed by the Cochrane Collaboration for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011)). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, we searched PubMed, TRIPdatabase, The Cochrane Library and Google to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. We searched for conference abstracts using the Cochrane Ear, Nose and Throat Disorders Group Trials Register.
Data collection and analysis
Selection of studies
One author scanned all articles in the search results to identify all randomised controlled trials. Two authors (ZA and CH) then separately reviewed each article to apply the inclusion criteria and assess risk of bias. The two authors then met and combined the results.
Data extraction and management
Two review authors independently extracted data using a standardised data extraction form. The authors met to resolve disagreements and contacted the authors of papers in question to obtain further information where required. The data collection sheet recorded the following:
author and year;
number of participants (total in each group, including withdrawals);
age (including range) and gender in each group;
condition(s) treated and inclusion criteria;
study design, duration and follow‐up;
intervention(s), including drug name, dose, frequency and method of administration (we also recorded the time of starting treatment after onset of symptoms together with the duration of treatment);
outcome measures (including intervals and form of assessment);
results, including statistical figures; and
withdrawals and adverse effects, including number and reason.
Assessment of risk of bias in included studies
Two authors (ZA and CH) undertook independent assessment of the risk of bias of the included trials. The following were taken into consideration, as guided by the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011):
sequence generation;
allocation concealment;
blinding;
incomplete outcome data;
selective outcome reporting; and
other sources of bias.
We used the Cochrane 'Risk of bias' tool in RevMan 5.1 (RevMan 2011), which involved describing each of these domains as reported in the trial and then assigning a judgement about the adequacy of each entry (low risk of bias, high risk of bias or unclear (or unknown) risk of bias).
Data synthesis
We extracted the data on an intention‐to‐treat basis.
Data synthesis methods for future updates
The data available in the included studies were not suitable for pooling in a meta‐analysis. If in future updates of this review suitable data are identified, we will combine data in a meta‐analysis to give a summary measure of effect. As we anticipate variation in definition, we will dichotomise the main outcome measure (recovery of hearing) into recovery or no recovery based on the study description. We will calculate statistical heterogeneity using the I2 statistic to assess the comparability of included data (I2 < 50%).
We will compare the effect of different types of antiviral agents as well as the combined effect of antiviral agents with other forms of treatment.
Study outcomes are likely to be measured in a variety of ways using continuous and categorical variables. Subgroup analysis will depend on the data extracted from the included papers and we will seek statistical advice to determine the best way of presenting and summarising the data. We may consider the following factors:
participants' age;
type of intervention, including drug, dose, treatment start and duration;
outcome measures, including level of improvement and time and method of assessment after starting treatment; and
type and severity of adverse effects.
We will identify and include future studies in the updates of this review.
Results
Description of studies
Results of the search
Of the 52 abstracts retrieved from our search, we discarded 47 as these did not focus on idiopathic sudden sensorineural hearing loss and the treatment was not targeted primarily on the use of antivirals in the treatment of idiopathic sudden sensorineural hearing loss. We excluded one of the five remaining studies as it was not a randomised controlled trial (Zadeh‐Mani 2003) (see Characteristics of excluded studies table).
The following four remaining trials: Stokroos 1998, Tucci 2002, Uri 2003 and Westerlaken 2003 satisfied the inclusion criteria.
Included studies
Full details of the included studies can be found in the Characteristics of included studies table.
Design
Stokroos 1998, Tucci 2002 and Westerlaken 2003 were prospective, multicentre, randomised, placebo‐controlled, double‐blinded trials. Uri 2003 was a prospective, randomised trial.
Sample size
Sample sizes were as follows: Stokroos 1998 43 patients (recruited 44, one excluded from final analysis); Tucci 2002 84 patients (105 recruited, 94 completed trial, 10 further then excluded from final analysis); Uri 2003 60 patients; and Westerlaken 2003 70 patients (91 recruited, 21 excluded).
Setting
Stokroos 1998 and Westerlaken 2003 took place in Griningen, The Netherlands, Tucci 2002 in the tertiary referral centre of the Duke Institute in North Carolina, USA and Uri 2003 was conducted in Haifa, Israel.
Participants
The inclusion criteria for Stokroos 1998 were (i) hearing loss of at least 30 dB for three subsequent one‐octave steps in frequency, (ii) cochlear hearing loss of unknown aetiology, (iii) hearing loss occurring within 24 hours and (iv) an unremarkable past otological history. Tucci 2002 included patients (i) with hearing loss of at least 30 dB in three contiguous frequencies in less than a three‐day period in patients with previous audiometry, (ii) seen within 10 days of onset of hearing loss and (iii) marked loss of hearing in patients with previously subjectively normal hearing and no previous audiometry record, with contralateral hearing taken as a baseline. The inclusion criteria for Uri 2003 were (i) hearing loss of at least 20 dB in at least three frequencies, (ii) patients admitted within seven days of onset of symptoms, (iii) no prior otological history and (iv) no systemic diseases with otological sequelae. Westerlaken 2003 included (i) sensorineural hearing loss of unknown aetiology, (ii) hearing loss of at least 30 dB hearing level for three subsequent one‐octave steps in frequency in the standard audiogram, (iii) hearing loss occurring within a period of 24 hours and (iv) a blank otologic history.
Interventions
Stokroos 1998 divided patients equally into two groups. Both groups were admitted for one week for intravenous administration of prednisolone at a dosage of 1 mg/kg bodyweight on day one and then reduced in equal increments over six days to 0 mg. In addition to this, one group received a dose of 10 mg/kg bodyweight of acyclovir three times daily intravenously for seven days. The second group received a placebo in similar concentration and frequency.
In Tucci 2002 patients were randomised equally two treatment arms; one received systemic steroids plus valacyclovir, the other received systemic steroid plus placebo. The steroid part of treatment consisted of a 12‐day course of prednisolone at a dose of 80 mg/day for four days and then tapered over eight days. The valacyclovir dose was 1 g administered three times daily for 10 days, replaced by placebo in the control group.
In Uri 2003 the intervention was in the form of intravenous acyclovir 15 mg/kg/day and hydrocortisone 100 mg three times a day for seven days for the intervention group consisting of 29 patients, with the control group of 31 patients receiving intravenous hydrocortisone only, at the same dose, for seven days. This was followed by a further seven days of prednisolone on a tapering regimen.
Westerlaken 2003 divided patients into two groups. Both groups were treated for one week with intravenous administration of prednisolone at a dosage of 1 mg/kg bodyweight on day one and then reduced in equal increments over the course of seven days to 0 mg. One group of 46 patients received a dose of 10 mg/kg bodyweight of acyclovir three times daily intravenously for seven days; the second group of 45 patients received a placebo in similar concentration and frequency.
Outcomes
The main outcome measures in Stokroos 1998 included (i) subjective parameters with patients being asked to judge their hearing recovery, pressure sensation and dizziness, (ii) virus serological investigations using specific IgG and IgM detection and (iii) quantitative parameters including pure‐tone audiometry, speech audiometry, brain stem‐evoked response audiometry and nystagmography. All patients were reviewed and all outcome measures were assessed at one week and then three, six and 12 months after treatment.
The primary outcome measure in Tucci 2002 was a change in pure‐tone audiogram hearing threshold, assessed at presentation, then at two and six weeks post‐treatment. Secondary outcome measures included (i) speech discrimination scores, (ii) Short Form‐12 questionnaire at presentation and week two and (iii) Hearing Screening Inventory questionnaire twice‐weekly for six weeks.
The main outcome measure in Uri 2003 was an improvement in the involved pure‐tone frequency average, or in speech reception threshold, with more than 15 dB being considered significant. Audiometry (including pure‐tone, speech reception threshold, discrimination, stapedial reflex, tympanometry and tone decay) was performed before admission and at one, three and 12 months post‐treatment.
The main outcome measures in Westerlaken 2003 included (i) patients being subjectively asked to judge their hearing recovery, pressure sensation and vertigo, (ii) quantitative parameters including pure‐tone audiometry and speech audiometry and (iii) virus serological investigations using specific IgG and IgM detection. All patients were reviewed and all outcome measures were assessed at one week and then three, six and 12 months after treatment.
Risk of bias in included studies
The overall risk of bias in the included studies was low. The details are given in the 'Risk of bias' tables (Characteristics of included studies) and in Figure 1 and Figure 2.
1.
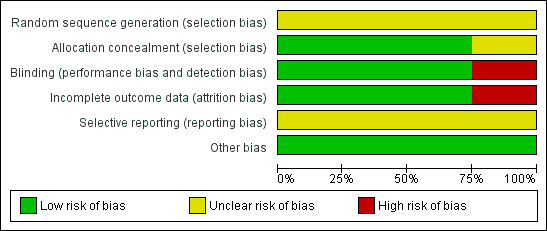
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
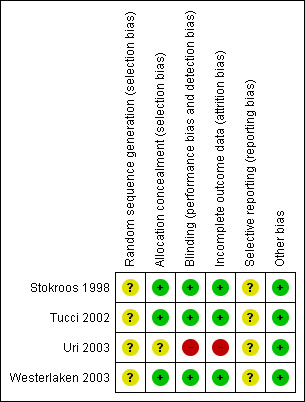
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Three out of the four included trials showed low risk of bias in allocating patients to treatment or control groups, which was performed by hospital pharmacists. In Uri 2003 the authors were not clear about allocation concealment and sequence generation.
Blinding
Three out of the four included trials showed a low risk of bias in specifying double‐blinding. Uri 2003 did not specify blinding.
Incomplete outcome data
Three out of the four included trials showed low risk of attrition bias by accounting for all excluded patients. Uri 2003 was not specific about excluded patients or patients lost to follow‐up.
Selective reporting
There was no clear risk of selective reporting in any of the studies as none had a pre‐published protocol.
Other potential sources of bias
No other potential sources of bias were identified apart from the failure to control for severity of initial hearing loss in Stokroos 1998.
Effects of interventions
Given differing treatment protocols of varying dose and duration, together with differing inclusion criteria and outcome measures, pooling of data and meta‐analysis was not possible. We therefore present a narrative summary of the included study results.
Improvement in pure‐tone thresholds and/or speech discrimination
Stokroos 1998 observed a hearing recovery of more than 10 dB in 34/43 patients (79%) by 12 months. This was achieved immediately after finishing treatment in 18/43 (42%) and within two weeks by 28/43 (65%). Specific patient numbers for each group were not expressed, instead being summarised as percentages. The authors concluded that the addition of acyclovir to prednisone did not impart any statistically significant difference in hearing improvement in their 43 patients with ISSHL.
In 84 patients, Tucci 2002 did not demonstrate any statistically significant difference between groups with the addition of valacyclovir to prednisone in respect to change in pure‐tone audiogram. Seventeen of 39 patients given valacyclovir and 15 of 29 given placebo recovered excellent hearing (defined as within 10 to 20 dB of the unaffected ear) ‐ about 45% of the patient total. Twenty‐one of 39 valacyclovir‐treated patients and 19 of 29 placebo‐treated patients recovered at least 50% of their hearing loss ‐ around 59% of the total study population.
Comparing the addition of acyclovir to hydrocortisone, Uri 2003 did not show any statistically significant difference between groups in 60 patients. Overall improvement, considered significant if over 15 dB in the involved frequency, was seen in 78% through both groups ‐ 78.6% of those given acyclovir and 77.4% given hydrocortisone alone.
Westerlaken 2003 was unable to show any statistically significant difference between groups when comparing the addition of acyclovir to prednisolone in their study of 70 patients. In fact, the placebo group at one year showed a greater recovery than the acyclovir group but this was not statistically significant. Specific numbers for each group were not stated.
Secondary outcome measures summary
Secondary outcome measures varied between papers and were mainly subjective, including the effect on tinnitus, vertigo, pressure sensation and hearing screening questionnaires. However, none were consistent across all papers, making collective analysis of effect of intervention impossible.
Tinnitus
The incidence of tinnitus quoted by all papers was between 73% and 87%. In Stokroos 1998 and Westerlaken 2003 the authors stated no statistical difference in tinnitus complaints between both groups (acyclovir versus placebo), with acyclovir having no influence over tinnitus prognosis, itself showing a poor prognosis being still present in 46% to 55% of patients after 12 months. Tucci 2002 observed a reduction in tinnitus in both groups irrespective of treatment given (valacyclovir or placebo). Uri 2003 did not state any direct influence of treatment on tinnitus progression but observed hearing improvement was significantly better if tinnitus was present.
Pressure sensation
Present in 35% to 49% of patients either preceding, accompanying or after the hearing loss, Stokroos 1998 and Westerlaken 2003 stated that this symptom held a favourable prognosis, reducing to around 15% of patients complaining of this symptom after 12 months. Neither acyclovir or placebo had any statistically significant influence on the sensation of pressure. This symptom was not investigated by Tucci 2002 or Uri 2003.
Dizziness/vertigo
Observed in 30% to 47%, vestibular complaints held a good prognosis and resolved quickly in the majority of patients, being present in only 10% after one year. However, none of the authors could demonstrate any difference in vertiginous symptoms between either treatment group, either at presentation or after treatment. Uri 2003 observed that hearing recovery was poorer if dizziness was present.
Side effects
In Stokroos 1998 slight to moderate nausea occurred once in the placebo group and once in the acyclovir group. In the placebo group, one patient complained of stomach pain and one patient developed a reversible high blood glucose level. These two side effects were both interpreted to be the result of prednisolone administration. No specific acyclovir side effects were observed. In Uri 2003 no side effects of acyclovir were observed. The authors of Westerlaken 2003 reported slightly raised blood glucose level, mild headache, heart palpitations and mild nausea. These side effects were interpreted to be the result of steroid therapy. No specific side effects of acyclovir were observed. Tucci 2002 reported no major side effects apart from insomnia, nervousness and weight gain, which were higher in the valacyclovir group.
No conclusions can be drawn about the effectiveness of antivirals in the treatment of idiopathic sudden sensorineural hearing loss or the effect of not using them.
Discussion
The potential success of antiviral therapy in the treatment of idiopathic sudden sensorineural hearing loss is based on the theory that viral infection is involved in the aetiology of this condition. The effectiveness of antivirals in the treatment of idiopathic sudden sensorineural hearing loss remains unproven.
We employed a comprehensive search strategy to identify as many studies of the treatment of idiopathic sudden sensorineural hearing loss as possible and to avoid the potential for selection bias. We then applied strict inclusion criteria to retain only studies that were less likely to be biased. There were only four randomised controlled trials addressing the effectiveness of antivirals in the treatment of idiopathic sudden sensorineural hearing loss. A consistent limitation of all these studies was the small number of patients included in the trials.
Summary of main results
Even though no meta‐analysis was possible, as discussed above, the evidence from all four randomised controlled trials demonstrates no statistically significant advantage in the use of antivirals in the treatment of ISSHL.
In Stokroos 1998 the authors demonstrated no statistical evidence supporting the beneficial effect of combining acyclovir with prednisolone in the treatment of idiopathic sudden sensorineural hearing loss.
Tucci 2002 was unable to demonstrate any significant difference between placebo and valacyclovir treatment groups regarding hearing or symptom recovery on the basis of the Short Form‐12 or the Hearing Screening Inventory questionnaires.
In Uri 2003 no significant difference was found between the group receiving intravenous acyclovir with hydrocortisone and the group receiving hydrocortisone alone in terms of audiometric improvement. Their data suggested that the sooner treatment is started, the better the chance of recovery, irrespective of treatment given, though this was not statistically demonstrated.
The authors of Westerlaken 2003 concluded that circumstantial evidence still points to a viral infection as an aetiological factor, however they demonstrated no statistical evidence supporting the beneficial effect of combining acyclovir with prednisolone in the treatment of idiopathic sudden sensorineural hearing loss.
With regard to secondary outcome measures, none of the authors were able to demonstrate any favourable influence of antivirals on the prognosis of tinnitus, pressure sensation or vertigo.
The available evidence does not show that antiviral agents alone are effective as first‐line treatment for ISSHL. The addition of antiviral to steroid therapy has not been demonstrated to show any obvious statistically significant improvement in outcome following ISSHL. No trial documented any serious adverse effects related to using antiviral treatments.
Overall completeness and applicability of evidence
We identified four randomised trials out of which three were of good quality with a low risk of bias. The trials had relatively small numbers of participants and somewhat variable treatment protocols. None of the trials included showed significant benefit from adding antivirals to the treatment of ISSHL. It is doubtful that a larger trial with a similar treatment protocol will show a considerable value of using antivirals for treating ISSHL, but if such a trial is to be carried out it would be extremely important to choose a clear hearing loss inclusion threshold together with a reproducible, well‐defined outcome measure based on previous studies. It is only then, with a large number of participants, that a subtle effect maybe identified.
Quality of the evidence
Three out of the four included studies were prospective, randomised, double‐blinded, placebo‐controlled trials with a low risk of bias. However, Tucci 2002 involved only six weeks follow‐up. The authors of Stokroos 1998 and Westerlaken 2003 have confirmed that their studies were sequential and did not include any of the same patient population.
The Uri 2003 study had high risk of bias, with unclear description of allocation, blinding and patient exclusions.
Potential biases in the review process
We used an extensive search strategy for this review, which included more than international 14 databases; we also searched reference lists of retrieved articles and conducted separate searches for related systematic reviews and ongoing registered trials. Although it is possible that some studies could have been missed, this is unlikely. We were able to make contact with trial authors where information was missing or unclear.
Agreements and disagreements with other studies or reviews
We identified one systematic review and one meta‐analysis conducted by the same group which looked at treatment options for ISSHL including antiviral agents. Conlin 2007a is a systematic review and Conlin 2007b is a meta‐analyses of many treatment options for ISSHL. The authors of the non‐Cochrane review and analysis concluded that there was no difference when antivirals were added to systemic steroids in treating ISSHL. Our conclusions agree with findings of this review and since then clinical practice has started to change, with antiviral treatment no longer prescribed.
Authors' conclusions
Implications for practice.
All authors mention circumstantial evidence in the published literature pointing towards a viral cause. However, there is currently no evidence to support the use of antiviral drugs in addition to steroids in the treatment of idiopathic sudden sensorineural hearing loss (ISSHL). The four trials included in this review were, however, small and with a low risk of bias. Stokroos 1998 mentioned a previously observed synergistic effect of acyclovir and prednisolone on hearing recovery and cochlear histopathology in experimental herpes viral labyrinthitis in the guinea pig. Given that the experimental situation eliminated any treatment delay, the authors suggested that treatment of ISSHL might be divided into two phases with the possible benefit of combined therapy being helpful in the first, very early, phase. However, since patients with this condition tend to present later, these results would be extremely difficult to reproduce in clinical practice. Tucci 2002 supported this viewpoint, stating that the viral damage to the ear could be complete by time of presentation, and Uri 2003 observed better hearing recovery with earlier treatment. Tucci 2002 noted that male sex, absence of vertigo and a milder hearing loss at presentation were predictors of a better prognosis. Westerlaken 2003 suggested 'pulse therapy' with high‐dose glucocorticoid might enhance hearing recovery by suppression of the immune system but found no additive benefit of antivirals. No trial documented any serious adverse effects related to using antiviral treatments.
Implications for research.
Further randomised controlled trials with larger patient populations, using a standardised inclusion criteria, antivirals and outcome measures, are needed in order for adequate meta‐analysis to be performed to reach definitive conclusions. A uniform definition of ISSHL should also be established, together with what constitutes adequate recovery.
Acknowledgements
The authors would like to thank Ravinder S Natt for his help with data extraction.
Appendices
Appendix 1. Search Strategies
| PubMed | CENTRAL | EMBASE (Ovid) | CINAHL (EBSCO) |
| #1 "Antiviral Agents"[Mesh] #2 (antiviral* OR "anti NEXT viral" OR "anti virals") #3 (acyclovir OR acycloguanosine OR aciclobeta OR aciclovir OR acic OR aciclostad OR acifur OR "acipen solutab" OR acivir OR activir OR "acyclo V" OR avirax OR cicloferon OR clonmel OR clonorax OR cusiviral OR famciclovir OR genvir OR herpetad OR herpofug OR herpotern OR herpoviric OR isavir OR laciken OR mapox OR maynar OR milavir OR opthavir OR supraviran OR valacyclovir OR viclovir OR vipral OR virax$5 OR virherpes OR virmen OR virolex OR virupos OR virzin OR zoliparin OR zovirax OR zyclir) #4 ("BIOLF 62" OR "BW 759" OR cytovene OR ganciclovir OR gancyclovir OR "RS 21592" OR acyclovidar OR cicloviran OR cycloviran OR viropump OR viruseen OR zaclovir OR cameven OR cymevan OR cymeven OR cymevene OR cytovene OR denocin OR denosine OR vitrasert OR "bw 256u87" OR bw256u87 OR 256u87 OR vacv OR valaciclovir OR valacyclovir OR valtrex OR zelitrex) #5 #1 OR #2 OR #3 OR #4 #6 "Hearing Loss, Sudden"[Mesh] #7 "HEARING LOSS, SENSORINEURAL"[Mesh #8 "HEARING LOSS"[Mesh #9 "DEAFNESS"[Mesh #10 ((hearing AND los*) OR deaf*) #11 #7 OR #8 OR#9 OR #10 #12 (sudden* OR abrupt* OR rapid* OR acute*) #13 #11 AND #12 #14 SSHL OR SNHL OR ISHL OR ISSHL OR ISSNHL #15 "TINNITUS"[Mesh #16 tinnit #17 #6 OR #13 OR #14 OR #15 OR #16 #18 #5 AND #19 | #1 ANTIVIRAL AGENTS explode all trees (MeSH) #2 antiviral* OR anti NEXT viral* #3 acyclovir OR acycloguanosine OR aciclobeta OR aciclovir OR acic OR aciclostad OR acifur OR acipen ADJ solutab OR acivir OR activir OR acyclo NEXT V OR avirax OR cicloferon OR clonmel OR clonorax OR cusiviral OR genvir OR herpetad OR herpofug OR herpotern OR herpoviric OR isavir OR laciken OR mapox OR maynar OR milavir OR opthavir OR supraviran OR valacyclovir OR viclovir OR vipral OR virax* OR virherpes OR virmen OR virolex OR virupos OR virzin OR zoliparin OR zovirax OR zyclir OR BIOLF NEXT 62 OR BW NEXT 759 OR cytovene OR ganciclovir OR gancyclovir OR RS NEXT 21592 OR acyclovidar OR cicloviran OR cycloviran OR viropump OR viruseen OR zaclovir OR cameven OR cymevan OR cymeven OR cymevene OR cytovene OR denocin OR denosine OR vitrasert OR bw NEXT 256u87 OR bw256u87 OR 256u87 OR vacv OR valaciclovir OR valacyclovir OR valtrex OR zelitrex #4 #1 OR #2 OR #3 #5 HEARING LOSS SUDDEN single term (MeSH) #6 HEARING LOSS SENSORINEURAL explode all trees (MeSH) #7 HEARING LOSS single term (MeSH) #8 DEAFNESS single term (MeSH) #9 hearing NEAR los* OR deaf* #10 #6 OR #7 OR #8 OR #9 #11 sudden* OR abrupt* OR rapid* OR acute* #12 #10 AND #11 #13 SSHL OR SNHL OR ISHL OR ISSHL OR ISSNHL #14 TINNITUS single term (MeSH) #15 tinnit* #16 #5 OR #12 OR #13 OR #14 OR #15 #17 #4 AND #16 | 1. exp ANTIVIRUS AGENT/ 2. (antiviral* or "anti viral" or "anti virals").ti,ab. 3. (acyclovir or acycloguanosine or aciclobeta or aciclovir or acic or aciclostad or acifur or "acipen solutab" or acivir or activir or "acyclo V" or avirax or cicloferon or clonmel or clonorax or cusiviral or famciclovir or genvir or herpetad or herpofug or herpotern or herpoviric or isavir or laciken or mapox or maynar or milavir or opthavir or supraviran or valacyclovir or viclovir or vipral or virax$5 or virherpes or virmen or virolex or virupos or virzin or zoliparin or zovirax or zyclir).ti,ab. 4. ("BIOLF 62" or "BW 759" or cytovene or ganciclovir or gancyclovir or "RS 21592" or acyclovidar or cicloviran or cycloviran or viropump or viruseen or zaclovir or cameven or cymevan or cymeven or cymevene or cytovene or denocin or denosine or vitrasert or "bw 256u87" or bw256u87 or 256u87 or vacv or valaciclovir or valacyclovir or valtrex or zelitrex).ti,ab. 5. 1 or 2 or 3 or 4 6. SUDDEN DEAFNESS/ 7. PERCEPTION DEAFNESS/ or HEARING LOSS/ or UNILATERAL HEARING LOSS/ or MIXED HEARING LOSS/ 8. ((hearing and los*) or deaf*).ti,ab. 9. 7 or 8 10. (sudden* or abrupt* or rapid* or acute*).ti,ab. 11. 9 and 10 12. (SSHL or SNHL or ISHL or ISSHL or ISSNHL).ti,ab. 13. TINNITUS/ 14. TINNIT*.ti,ab. 15. 6 or 11 or 12 or 13 or 14 16. 5 and 15 | S1 (MH "Tinnitus") S2 TX tinnit* S3 (MH "Hearing Loss, Sensorineural") OR (MH "Deafness") S4 TX (((hearing AND los*) OR deaf*)) S5 TX ((sudden* OR abrupt* OR rapid* OR acute*)) S6 S3 or S4 S7 S5 and S6 S8 TX (SSHL OR SNHL OR ISHL OR ISSHL OR ISSNHL) S9 S1 or S2 or S7 or S8 S10 (MH "Antiviral Agents") S11 TX ((antiviral* OR "anti viral" OR "anti virals")) S12 TX ((acyclovir OR acycloguanosine OR aciclobeta OR aciclovir OR acic OR aciclostad OR acifur OR "acipen solutab" OR acivir OR activir OR "acyclo V" OR avirax OR cicloferon OR clonmel OR clonorax OR cusiviral OR famciclovir OR genvir OR herpetad OR herpofug OR herpotern OR herpoviric OR isavir OR laciken OR mapox OR maynar OR milavir OR opthavir OR supraviran OR valacyclovir OR viclovir OR vipral OR virax$5 OR virherpes OR virmen OR virolex OR virupos OR virzin OR zoliparin OR zovirax OR zyclir)) S13 TX (("BIOLF 62" OR "BW 759" OR cytovene OR ganciclovir OR gancyclovir OR "RS 21592" OR acyclovidar OR cicloviran OR cycloviran OR viropump OR viruseen OR zaclovir OR cameven OR cymevan OR cymeven OR cymevene OR cytovene OR denocin OR denosine OR vitrasert OR "bw 256u87" OR bw256u87 OR 256u87 OR vacv OR valaciclovir OR valacyclovir OR valtrex OR zelitrex)) S14 S10 or S11 or S12 or S13 S15 S9 and S14 |
| Web of Science/BIOSIS Previews (Web of Knowledge) | Cochrane ENT Disorders Group Trials Register (ProCite database) | CAB Abstracts (Ovid) | ICTRP |
| #1 TS=((hearing AND los*) OR deaf*) #2 TS=(sudden* OR abrupt* OR rapid* OR acute*) #3 TS=(SSHL OR SNHL OR ISHL OR ISSHL OR ISSNHL) #4 #2 AND #1 #5 #4 OR #3 #6 TS=(antiviral* OR "anti viral*") #7 TS=(acyclovir OR acycloguanosine OR aciclobeta OR aciclovir OR acic OR aciclostad OR acifur OR "acipen solutab" OR acivir OR activir OR "acyclo V" OR avirax OR cicloferon OR clonmel OR clonorax OR cusiviral OR famciclovir OR genvir OR herpetad OR herpofug OR herpotern OR herpoviric OR isavir OR laciken OR mapox OR maynar OR milavir OR opthavir OR supraviran OR valacyclovir OR viclovir OR vipral OR virax* OR virherpes OR virmen OR virolex OR virupos OR virzin OR zoliparin OR zovirax OR zyclir) #8 TS=("BIOLF 62" OR "BW 759" OR cytovene OR ganciclovir OR gancyclovir OR "RS 21592" OR acyclovidar OR cicloviran OR cycloviran OR viropump OR viruseen OR zaclovir OR cameven OR cymevan OR cymeven OR cymevene OR cytovene OR denocin OR denosine OR vitrasert OR "bw 256u87" OR bw256u87 OR 256u87 OR vacv OR valaciclovir OR valacyclovir OR valtrex OR zelitrex) #9 #8 OR #7 OR #6 #10 #9 AND #5 | ((hear* OR deaf) AND (sudden* OR abrupt* OR rapid* OR acute*)) OR SSHL OR SNHL OR ISHL OR ISSHL OR ISSNHL OR tinnit* | 1. (antiviral* or "anti viral" or "anti virals").ti,ab. 2. (acyclovir or acycloguanosine or aciclobeta or aciclovir or acic or aciclostad or acifur or "acipen solutab" or acivir or activir or "acyclo V" or avirax or cicloferon or clonmel or clonorax or cusiviral or famciclovir or genvir or herpetad or herpofug or herpotern or herpoviric or isavir or laciken or mapox or maynar or milavir or opthavir or supraviran or valacyclovir or viclovir or vipral or virax$5 or virherpes or virmen or virolex or virupos or virzin or zoliparin or zovirax or zyclir).ti,ab. 3. ("BIOLF 62" or "BW 759" or cytovene or ganciclovir or gancyclovir or "RS 21592" or acyclovidar or cicloviran or cycloviran or viropump or viruseen or zaclovir or cameven or cymevan or cymeven or cymevene or cytovene or denocin or denosine or vitrasert or "bw 256u87" or bw256u87 or 256u87 or vacv or valaciclovir or valacyclovir or valtrex or zelitrex).ti,ab. 4. 1 or 2 or 3 5. ((hearing and los*) or deaf*).ti,ab. 6. (sudden* or abrupt* or rapid* or acute*).ti,ab. 7. 5 AND 6 8. (SSHL or SNHL or ISHL or ISSHL or ISSNHL).ti,ab. 9. TINNITUS/ 10. TINNIT*.ti,ab. 11. 7 OR 8 OR 9 OR 10 12. 4 and 11 | sudden AND deaf* OR sudden AND hear* OR SSHL OR SNHL OR ISHL OR ISSHL OR ISSNHL OR tinnit* |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Stokroos 1998.
| Methods | Double‐blinded, randomised controlled trial | |
| Participants | 44 patients recruited; 1 excluded from final analysis Inclusion criteria: (i) Hearing loss of at least 30 dB for 3 subsequent one‐octave steps in frequency (ii) Cochlear hearing loss of unknown aetiology (iii) Hearing loss occurring within 24 hours and an unremarkable past otological history |
|
| Interventions | Patients were divided equally into 2 groups. Both groups were admitted for 1 week for intravenous administration of prednisone at a dosage of 1 mg/kg body weight on day 1 and then reduced in equal increments over a 6 days to 0 mg. One group received a dose of 10 mg/kg body weight of acyclovir 3 times daily intravenously for 7 days. The second group received a placebo in similar concentration and frequency. | |
| Outcomes | The main outcome measures included:
(i) Patients were asked to judge their hearing recovery, pressure sensation and dizziness subjectively
(ii) Virus serological investigations using specific IgG and IgM detection
(iii) Quantitative parameters including pure‐tone audiometry, speech audiometry, brain stem‐evoked response audiometry and nystagmography All patients were reviewed and all outcome measures were assessed at 1 week and then 3, 6 and 12 months after treatment |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Low risk | Principal author stated that the "randomisation performed by the hospital pharmacist", but specifics regarding the allocation were not discussed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons given for exclusion of one patient |
| Selective reporting (reporting bias) | Unclear risk | The study did not have a pre‐published protocol for comparison |
| Other bias | Low risk | Authors admitted "failure to control for severity of initial hearing loss" |
Tucci 2002.
| Methods | Double‐blinded, randomised controlled trial | |
| Participants | 105 patients from 32 study centres were recruited but only 94 completed the trial; 10 were then deleted from the final analysis resulting in 84 patients being evaluated Inclusion criteria: (i) Loss of at least 30 dB in 3 contiguous frequencies over a period of < 3 days in patients who have been monitored previously for hearing loss (ii) Subjective marked loss of hearing in patients with subjectively normal baseline hearing and no previous record of audiometry (in these patients hearing in the contralateral ear was taken as “baseline”) (iii) Patients seen within 10 days of onset of hearing loss (iv) No underlying disease that could be associated with sudden sensorineural hearing loss as an aetiologic factor |
|
| Interventions | 50 patients received prednisolone (80 mg/day for 4 days then tapered over 8 days) with placebo and 44 patients received prednisone with valacyclovir (1 g, 3 times a day for 10 days) | |
| Outcomes | (i) Audiometric assessment at presentation, week 2 and week 6 (ii) Hearing Screening Inventory questionnaire twice weekly for 6 weeks (iii) Acute Short Form‐12 questionnaire at presentation and week 2 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Low risk | Principal author stated that allocation was concealed by the pharmacist at their clinical research institute |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Excluded patients accounted for |
| Selective reporting (reporting bias) | Unclear risk | The study did not have a pre‐published protocol for comparison |
| Other bias | Low risk | No other sources of bias identified |
Uri 2003.
| Methods | Randomised controlled trial | |
| Participants | 60 patients Inclusion criteria: (i) Sensory hearing impairment of at least 20 dB in at least 3 frequencies |
|
| Interventions | Patients were randomly assigned into 2 groups. Group A, the study group, included 29 patients put on bed rest and treated with intravenous acyclovir 15 mg/kg/day and hydrocortisone 100 mg 3 times a day for 7 days. Group H, the control group, consisted of 31 patients put on bed rest and treated with intravenous hydrocortisone 100 mg 3 times a day. After intravenous treatment, the patients were put on a taper regimen of prednisolone for 7 days. | |
| Outcomes | The main outcome measure included: (i) Speech reception threshold (ii) Mean hearing level at each frequency (iii) Speech reception threshold improvement (iv) Tinnitus (v) Balance complaints | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Principal author has stated that the trial was randomised but has neither stated whether this was double‐blinded nor whether the allocation was concealed |
| Blinding (performance bias and detection bias) All outcomes | High risk | The study was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The authors were not specific about excluded patients or patients lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | The study did not have a pre‐published protocol for comparison |
| Other bias | Low risk | No other sources of bias identified |
Westerlaken 2003.
| Methods | Double‐blinded, randomised controlled trial | |
| Participants | 91 patients recruited; 21 excluded from final analysis resulting in 70 being analysed Inclusion criteria: (i) Sensorineural hearing loss of unknown aetiology (ii) Hearing loss of at least 30 dB hearing level for 3 subsequent one‐octave steps in frequency in the standard audiogram (iii) Hearing loss occurring within a period of 24 hours (iv) A blank otologic history |
|
| Interventions | Patients were divided into 2 groups. Both groups were treated for 1 week with intravenous administration of prednisolone at a dosage of 1 mg/kg body weight on day 1 and then reduced in equal increments over the course of 7 days to 0 mg. One group received a dose of 10 mg/kg body weight of acyclovir 3 times daily intravenously for 7 days and consisted of 46 patients. The second group of 45 patients received a placebo in similar concentration and frequency. | |
| Outcomes | The main outcome measures included:
(i) Patients were asked to judge their hearing recovery, pressure sensation and vertigo subjectively
(ii) Quantitative parameters including pure‐tone audiometry and speech audiometry
(iii) Virus serological investigations using specific IgG and IgM detection All patients were reviewed and all outcome measures were assessed at 1 week and then 3, 6 and 12 months after treatment |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Low risk | Principal author stated that randomisation was performed by the hospital pharmacist, but specifics regarding the allocation were not discussed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Principal author stated that the trial was "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Summary of reasons for 21 excluded patients was given |
| Selective reporting (reporting bias) | Unclear risk | The study did not have a pre‐published protocol for comparison |
| Other bias | Low risk | No other sources of bias identified |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Zadeh‐Mani 2003 | ALLOCATION: Non‐randomised study |
Differences between protocol and review
We added the following to our secondary outcome measures: "Any alteration in tinnitus, disequilibrium and other symptoms was also studied". We have adopted the Cochrane 'Risk of bias' tool (Handbook 2011).
Contributions of authors
Zaid Awad:
main author of protocol and review;
team leader;
all communications within the team and with the Cochrane Ear, Nose and Throat Disorders Group;
review of study search;
identification and assessment of studies;
extraction of data according to the protocol.
Charlie Huins:
second author;
administration;
extraction of data according to the protocol;
identification and assessment of the selected studies independently of the first author.
David D Pothier:
strategy advice;
background research;
analysis and statistics;
protocol and review writing;
review of study search.
Sources of support
Internal sources
None, Not specified.
External sources
None, Not specified.
Declarations of interest
None known.
New
References
References to studies included in this review
Stokroos 1998 {published data only}
- Stokroos RJ, Albers FW, Tenvergert EM. Antiviral treatment of idiopathic sudden sensorineural hearing loss: a prospective, randomized, double‐blind CT. Acta Oto‐laryngologica 1998;118(4):488‐95. [DOI] [PubMed] [Google Scholar]
Tucci 2002 {published data only}
- Tucci DL, Farmer JC Jr, Kitch RD, Witsell DL. Treatment of sudden sensorineural hearing loss with systemic steroids and valacyclovir. Otology & Neurotology 2002;23(3):301‐8. [DOI] [PubMed] [Google Scholar]
Uri 2003 {published data only}
- Uri N, Doweck I, Cohen‐Kerem R, Greenberg E. Acyclovir in the treatment of idiopathic sudden sensorineural hearing loss. Otolaryngology ‐ Head and Neck Surgery 2003;128(4):544‐9. [DOI] [PubMed] [Google Scholar]
Westerlaken 2003 {published data only}
- Westerlaken BO, Stokroos RJ, Dhooge IJM, Wit HP, Albers FWJ. Treatment of idiopathic sudden sensorineural hearing loss with antiviral therapy: a prospective, randomized, double‐blind clinical trial. Annals of Otology, Rhinology and Laryngology 2003;112(11):993‐1000. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Zadeh‐Mani 2003 {published data only}
- Zadeh MH, Storper IS, Spitzer JB. Diagnosis and treatment of sudden‐onset sensorineural hearing loss: a study of 51 patients. Otolaryngology ‐ Head and Neck Surgery 2003;128(1):92‐8. [DOI] [PubMed] [Google Scholar]
Additional references
Agarwal 2009
- Agarwal L, Pothier DD. Vasodilators and vasoactive substances for idiopathic sudden sensorineural hearing loss. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD003422.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bennett 2007
- Bennett M, Kertesz T, Yeung P. Hyperbaric oxygen for idiopathic sudden sensorineural hearing loss and tinnitus. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD004739.pub3] [DOI] [PubMed] [Google Scholar]
Conlin 2007a
- Conlin AE, Parnes LS. Treatment of sudden sensorineural hearing loss: I. A systematic review. Archives of Otolaryngology ‐ Head and Neck Surgery 2007;133(6):573‐81. [DOI] [PubMed] [Google Scholar]
Conlin 2007b
- Conlin AE, Parnes LS. Treatment of sudden sensorineural hearing loss: II. A meta‐analysis. Archives of Otolaryngology ‐ Head and Neck Surgery 2007;133(6):582‐6. [DOI] [PubMed] [Google Scholar]
DeKleyn 1944
- DeKleyn A. Sudden complete or partial loss of function of the octavus‐system in apparently normal person. Acta Oto‐laryngologica 1944;32:407‐29. [Google Scholar]
Eisenman 2000
- Eisenman DJ, Arts HA. Effectiveness of treatment for sudden sensorineural hearing loss. Archives of Otolaryngology ‐ Head and Neck Surgery 2000;126:1161‐4. [DOI] [PubMed] [Google Scholar]
Fukuda 1994
- Fukuda S, Furuta Y, Takasu T, Suzuki S, Inuyama Y, Nagashima K. The significance of herpes viral latency in the spiral ganglia. Acta Oto‐Laryngologica. Supplement 1994;514:108‐10. [DOI] [PubMed] [Google Scholar]
Goodhill 1971
- Goodhill V. Sudden deafness and round window rupture. Laryngoscope 1971;81(9):1462‐74. [DOI] [PubMed] [Google Scholar]
Goodhill 1980
- Goodhill W. The "idiopathic group" and the "labyrinthine window rupture group" approaches to sudden sensorineural hearing loss. In: Snow JB Jr editor(s). Controversy in Otolaryngology. Philadelphia: Saunders, 1980:12‐20. [Google Scholar]
Gussen 1976
- Gussen R. Sudden deafness of vascular origin: a human temporal bone study. Annals of Otology, Rhinology and Laryngology 1976;85(1 Pt 1):94‐100. [DOI] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Horn 2005
- Horn CE, Himel HN, Selesnick SH. Hyperbaric oxygen therapy for sudden sensorineural hearing loss: a prospective trial of patients failing steroid and antiviral treatment. Otology & Neurotology 2005;26(5):882‐9. [DOI] [PubMed] [Google Scholar]
Jeyakumar 2006
- Jeyakumar A, Francis D, Doerr T. Treatment of idiopathic sudden sensorineural hearing loss. Acta Oto‐laryngologica 2006;126(7):708‐13. [DOI] [PubMed] [Google Scholar]
Lackner 2009
- Lackner A, Acham A, Alborno T, Moser M, Engele H, Raggam RB, et al. Effect on hearing of ganciclovir therapy for asymptomatic congenital cytomegalovirus infection: four to 10 year follow up. Journal of Laryngology and Otology 2009;123(4):391‐6. [DOI] [PubMed] [Google Scholar]
Lazarini 2006
- Lazarini PR, Camargo AC. Idiopathic sudden sensorineural hearing loss: etiopathogenic aspects. Brazilian Journal of Otorhinolaryngology 2006;72(4):554‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCabe 1979
- McCabe BF. Autoimmune sensorineural hearing loss. Annals of Otology, Rhinology and Laryngology 1979;88(5 Pt 1):585‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Schuknecht 1986
- Schuknecht HF, Donovan ED. The pathology of idiopathic sudden sensorineural hearing loss. Archives of Oto‐rhino‐laryngology 1986;243(1):1‐15. [DOI] [PubMed] [Google Scholar]
Stokroos 1999
- Stokroos RJ, Albers FW, Schirm J. Therapy of idiopathic sudden sensorineural hearing loss: antiviral treatment of experimental herpes simplex virus infection of the inner ear. Annals of Otology, Rhinology and Laryngology 1999;108(5):423‐8. [DOI] [PubMed] [Google Scholar]
Tucci 2000
- Tucci DL. Sudden sensorineural hearing loss: a viral etiology?. Archives of Otolaryngology ‐ Head and Neck Surgery 2000;126(9):1164‐5. [DOI] [PubMed] [Google Scholar]
Wei 2006
- Wei BPC, Mubiru S, O'Leary S. Steroids for idiopathic sudden sensorineural hearing loss. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD003998.pub2] [DOI] [PubMed] [Google Scholar]
Wilson 1980
- Wilson WR, Byl FM, Laird N. The efficacy of steroids in the treatment of idiopathic sudden hearing loss. A double‐blind clinical study. Archives of Otolaryngology 1980;106(12):772‐6. [DOI] [PubMed] [Google Scholar]
Wu 2006
- Wu CS, Lin HC, Chao PZ. Sudden sensorineural hearing loss: evidence from Taiwan. Audiology and Neuro‐otology 2006;11(3):151‐6. [DOI] [PubMed] [Google Scholar]


