Abstract
Objective:
Development of chemotherapy-induced peripheral neuropathy (CIPN) poses significant challenges in cancer treatment, often leading to dose reductions or treatment discontinuation. Goshajinkigan (GJG), a traditional Japanese medicine, has shown promise for alleviating CIPN symptoms. This multicenter, randomized controlled trial aimed to prospectively examine the efficacy of GJG in preventing paclitaxel-induced peripheral neuropathy.
Methods:
This study enrolled 55 patients with ovarian cancer undergoing first-line chemotherapy using paclitaxel and carboplatin. The participants were randomized into Groups A (GJG initiation after onset of grade 2 neuropathy) and B (prophylactic administration of GJG from 1 week before chemotherapy). The primary endpoints were the proportion with a maximum sensory neuropathy grade and visual analog scale (VAS) scores. The secondary endpoints were the rate of chemotherapy completion and paclitaxel dose reduction due to neurotoxicity.
Results:
Prophylactic GJG administration (Group B) resulted in significant benefits. While both groups had a similar incidence of grade 2 sensory neuropathy, all patients in Group B with grade 2 neuropathy completed treatment without requiring additional analgesics. Group B exhibited lower VAS scores by the end of the study, reduced reliance on adjuvant analgesics (27.3% vs 66.7% in Group A), and significantly less frequent persistent CIPN 6 months post-chemotherapy (18.2% vs 55.6% in Group A). No differences were observed in the chemotherapy completion rates or CIPN-related changes between the groups.
Conclusion:
GJG, when administered prophylactically, showed potential for mitigating CIPN symptoms during paclitaxel chemotherapy. While promising, further research with placebo controls and objective measures is essential to comprehensively validate these findings.
Keywords: Goshajinkigan, ovarian cancer, peripheral neuropathy, taxane chemotherapy, traditional medicine, transient receptor potential vanilloid 4
Introduction
Chemotherapy is essential for treatment of malignant tumors, and recent progress in chemotherapy regimens has led to significantly improved long-term prognoses for patients with cancer. Chemotherapy-induced peripheral neuropathy (CIPN) is a challenging side effect of several chemotherapeutic agents, including platinum-based drugs, taxanes, epothilones, vinca alkaloids, and bortezomib.1 -3 CIPN develops in a dose-dependent manner and tends to worsen with continuing treatment. These events often necessitate dose reductions or drug discontinuation, undermining the efficacy of cancer treatment.3,4 CIPN is primarily a sensory neuropathy that presents in a glove-and-stocking pattern, and patients often complain of symptoms such as numbness, tingling, pain, and burning pain.5,6 Pain can also be caused by muscle spasms, or joint pain known for paclitaxel when the patients have been asked to report on those symptoms using the visual analogue scale (VAS). Additionally, the condition may be accompanied by symptoms that affect motor and autonomic nerves. These symptoms can interfere with physical function, daily activities, and work, significantly compromising the patient’s quality of life (QOL).7,8 The prevalence of CIPN has been estimated at approximately 68.1% in the first month after chemotherapy, 60.0% at 3 months, and 30.0% after 6 months. 9 Given the limited options for managing CIPN, novel therapeutic strategies to provide better care for patients should be explored.
Goshajinkigan (GJG), a traditional Japanese medicine, consists of 10 types of crude drugs: rehmannia root, dioscorea rhizome, cornus fruit, hoelen, alisma rhizome, moutan bark, cinnamon bark, aconite root, achyranthes root, and plantago seed. It has traditionally been used to manage conditions such as diabetic neuropathy, edema, lumbago, and non-specific pain, 10 but its mechanism of action is not fully understood. Several studies have suggested the effectiveness of GJG against CIPN, such as inhibition of transient receptor potential (TRP) channel function changes, involvement of descending monoaminergic systems, and suppression of TNF-α expression in the spinal cord.11 -13 Additionally, GJG is known to act on spinal kappa-opioid receptors via dynorphin release, reducing the sensation of pain.14,15 Our previous study indicated that paclitaxel (PTX)-induced hyperalgesia, which features enhanced expression of TRP vanilloid 4 (TRPV4), could be alleviated by GJG, potentially through the prevention of ganglion cell degeneration and suppression of TRPV4 expression. 16
This study prospectively examines the efficacy of GJG in preventing peripheral neuropathy induced by PTX.
Methods
Study Design and Participants
This multicenter randomized 2-group parallel controlled trial was conducted at the Tohoku Gynecologic Oncology Unit (TGCU). TGCU was established in 2003 and currently includes Iwate Medical University, Fukushima Medical University School of Medicine, Tohoku University, Akita University Graduate School of Medicine School of Medicine, Yamagata University Faculty of Medicine Graduate School of Medical Science, Tohoku Medical and Pharmaceutical University, Miyagi Cancer Center, Hokkaido University Graduate School of Medicine and Hirosaki University School of Medicine Graduate School of Medicine. The study was conducted with the approval of the central Institutional Review Board (IRB) of Hirosaki University (Approval No. 2015-241) and is registered in the University Hospital Medical Information Network (UMIN) Clinical Trials Registry in Japan (UMIN 000021361).
Participant enrollment was between February 2016 and July 2018. Patients aged 20 to 79 years, with histologically confirmed ovarian cancer (irrespective of histological subtype), an Eastern Cooperative Oncology Group performance status (ECOG PS) 0 to 2, undergoing first-line chemotherapy with 6 cycles of PTX and carboplatin, and with no impairment of hepatic, renal, or hematopoietic function were eligible for inclusion. The exclusion criteria comprised previous GJG use; experience of persistent numbness and ambulatory challenges due to peripheral neuropathy that substantially impinged on daily activities; presence of diabetic neuropathy; potentially pregnant, pregnant, or lactating women; or those deemed unsuitable for inclusion in the study by the attending physician. Patients already receiving other traditional Japanese medicines were also excluded from this study. There were no significant changes in the methods after the start of the trial. Written informed consent was obtained from all participants.
Treatment Protocol
The participants underwent central randomization in a 1:1 ratio and were allocated to 2 cohorts: Group A, commencing GJG administration after manifestation of grade 2 peripheral neuropathy, and Group B, receiving GJG from 1 week preceding chemotherapy initiation. The chemotherapeutic regimen comprised intravenous infusion of PTX at 175 mg/m² on day 1 and carboplatin at area under the curve (AUC) values of 5 to 6 on day 1, administered at 21-day intervals for a total of 6 cycles. GJG was administered at a dosage of 7.5 mg/day preceding each meal. Prevention of chemotherapy-induced nausea and vomiting (CINV) was not standardized and followed protocols established by each facility. Treatment for febrile neutropenia also followed the standards of each facility. For the patients in both groups, the choice of analgesic adjuvants when peripheral neuropathy occurred was at the discretion of each facility. The evaluation of peripheral neuropathy was conducted before each chemotherapy cycle as well as 1 and 2 weeks after chemotherapy. The evaluation was conducted by the attending physician. Prior to registration and the start of each chemotherapy course, chest radiographs and blood test results (including bone marrow function, serum aspartate transaminase, alanine transaminase, alkaline phosphatase, bilirubin, creatinine, blood urea nitrogen, sodium, chloride, and potassium) were required.
Randomization
The registration center was established at CDS JAPAN Corporation, and the patients were randomly allocated in a 1:1 ratio according to a computer-generated sequence. Assignment of patients to 1 of 2 treatment groups was implemented using dynamic allocation (minimization method). Allocation factors included institution, menopausal status, body surface area, and performance status (PS). Following the allocation notification sent from the registration center, we initiated the study by confirming whether the participants were assigned to the GJG preventive administration group (Group B) or the group to receive GJG after symptom onset (Group A).
Endpoints
The primary endpoints were the proportion of patients with maximum manifestations of sensory neuropathy per the Common Terminology Criteria for Adverse Events (CTCAE v4.0) throughout the entire treatment period and changes in the severity of peripheral neuropathy, quantified using the visual analog scale (VAS) score before the start of chemotherapy and subsequently after each cycle of chemotherapy. The secondary endpoints included the completion rate of chemotherapy and the frequency of PTX dose reduction due to neurotoxicity.
Statistical Analysis
The incidence of grade 3 or higher peripheral neuropathy due to oxaliplatin was 33% and 75% in the GJG and control groups, respectively, as reported by Nishioka et al. Based on this data, 17 we predicted the incidence of peripheral neuropathy due to PTX and carboplatin chemotherapy to be 70%, whereas it was anticipated to be 40% in the group receiving preventive GJG treatment. Based on these assumptions, an estimated 41 cases per group were required to ensure a significance level of 10% in a one-sided test and 80% power. The necessary sample size was estimated using nQuery (Statstools, Los Angeles, CA, USA). Therefore, the study planned to enroll 90 cases, with an expected dropout rate of 10%. However, due to the inability to recruit a sufficient number of cases within the trial period, we analyzed the data from patients who could be evaluated within the specified timeframe.
For comparisons between patient groups, we used the Wilcoxon rank-sum test to analyze continuous data and the chi-square test or Fisher’s exact test for categorical data. If the chi-square test revealed a significant difference, we performed the adjusted residuals to determine which cell caused the significant difference. Assessment of the incidence of peripheral neuropathy of grade 2 or higher was based on the Kaplan–Meier method, and the 2 groups were compared using the log-rank test. A P value < .05 or less was considered to indicate significance. All statistical analyses were performed using SPSS version 27 (IBM SPSS Inc, Chicago, IL).
Results
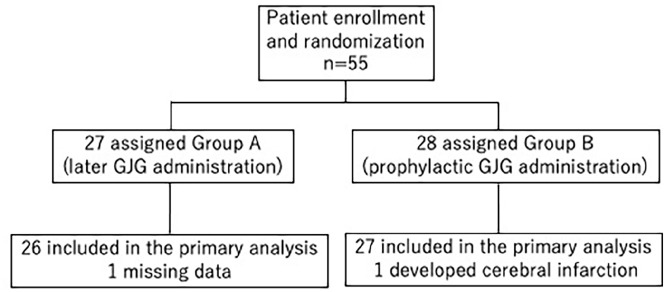
Between February 2016 and July 2018, we enrolled 55 patients across TGCU and allocated them randomly into Groups A (n = 27) and B (n = 28). One patient from each group was excluded: a patient in group A developed cerebral infarction immediately after chemotherapy, and another in group B had missing data and could not be evaluated (Figure 1).
Figure 1.
CONSORT diagram: study flow for the two intervention groups.
No significant differences were found in the patient background factors (Table 1). Notably, in Group B, 8 patients (30.8%) did not experience peripheral neuropathy and did not require GJG. During chemotherapy, no significant differences were found between the groups regarding the maximum CTCAE grade for sensory neuropathy or VAS score (Tables 2 and 3).
Table 1.
Patient Characteristics.
| Group A(later GJG administration) | Group B (prophylactic GJG administration) | P value | |
|---|---|---|---|
| Variables | n = 26 | n = 27 | |
| Age (years, mean ± SD) | 58.7 ± 11.4 | 60.4 ± 8.9 | .545 |
| BMI (kg/m2, mean ± SD) | 22.6 ± 0.6 | 23.4 ± 0.3 | .731 |
| BSA (m 2 , mean ± SD) | 1.53 ± 0.13 | 1.54 ± 0.13 | .754 |
| Menopausal status | |||
| Premenopausal | 7 (26.9%) | 6 (21.4%) | .757 |
| Menopausal | 19 (73.1%) | 21 (78.6%) | |
| Performance status | |||
| 0 | 26 (100%) | 27 (100%) | - |
| Chemotherapy cycle | |||
| 1 | 1 (3.8%) | 1 (3.7%) | .268 |
| 2 | 2 (7.7%) | 1 (3.7%) | |
| 3 | 0 | 2 (7.4%) | |
| 4 | 0 | 1 (3.7%) | |
| 5 | 3 (11.5%) | 0 | |
| 6 | 20 (76.9%) | 22 (81.5%) | |
| Time to start GJG | |||
| Not administered | 8 (30.8%) | ||
| 1 cycle | 7 (26.9%) | ||
| 2 cycles | 6 (23.1%) | ||
| 3 cycles | 3 (11.5%) | ||
| 4 cycles | 0 | ||
| 5 cycles | 1 (3.8%) | ||
| 6 cycles | 1 (3.8%) | ||
| Total dose of paclitaxel (mg, mean ± SD) | 1390.7 ± 397.8 | 1391.3 ± 388.8 | .995 |
| Total dose of carboplatin (mg, mean ± SD) | 3010.8 ± 1062.1 | 3011.1 ± 860.6 | .999 |
Abbreviations: BMI, body mass index; BSA, body surface area; GJG, Goshajinkigan; SD, standard deviation.
Table 2.
Maximum CTCAE Grade of Sensory Neuropathy During Chemotherapy.
| Group A (later GJG administration) | Group B (prophylactic GJG administration) | P value | |
|---|---|---|---|
| CTCAE Grades | n = 26 | n = 27 | |
| Maximum grade | .263 | ||
| 0 | 6 (23.1%) | 3 (11.1%) | |
| 1 | 5 (19.2%) | 8 (29.6%) | |
| 2 | 13 (50.0%) | 16 (59.3%) | |
| 3 | 2 (7.7%) | 0 |
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; GJG, Goshajinkigan.
Table 3.
Maximum VAS Scores of Sensory Neuropathy During Chemotherapy.
| Group A (later GJG administration) | Group B (prophylactic GJG administration) | P value | |
|---|---|---|---|
| VAS Scores | n = 26 | n = 27 | |
| Maximum score | .333 | ||
| 0 | 6 (23.1%) | 3 (11.1%) | |
| 1-2 | 5 (19.2%) | 11 (40.7%) | |
| 3-4 | 7 (26.9%) | 3 (11.1%) | |
| ≥5 | 8 (31.0%) | 10 (37.0%) |
Abbreviations: GJG, Goshajinkigan; VAS, visual analog scale.
The incidence rate of grade 2 sensory neuropathy was similar in both groups (Figure 2, P = .445, log-rank test). However, a remarkable observation in the GJG group (Group B) was that all patients with grade 2 sensory neuropathy after the first chemotherapy cycle completed the treatment without requiring additional analgesics.
Figure 2.
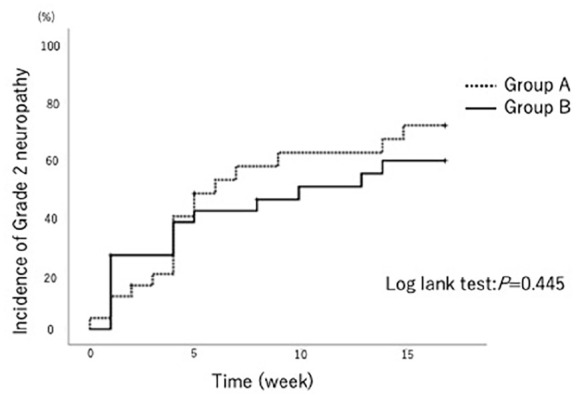
Incidence rate of grade 2 sensory neuropathy.
By the end of the 6 chemotherapy cycles, the patients in Group B exhibited significantly lower VAS scores than those in Group A. Adjusted residuals showed that Group B had significantly more frequent VAS scale scores in the range of 1 to 2 (Table 4). Comparison of adjuvant analgesic usage for CIPN revealed that 6 patients (27.3%) in Group B versus 12 patients (66.7%) in Group A required analgesics (Table 5; data from cases with a CTCAE grade 0 for the entire period have been excluded). This observation highlights the reduced reliance on adjuvant analgesics after the preventive GJG intervention (Group B).
Table 4.
VAS Scores at the End of Treatment.
| Group A (later GJG administration) | Group B (prophylactic GJG administration) | P value | |
|---|---|---|---|
| VAS Scores | n = 26 | n = 27 | |
| Scores at the end of treatment | .033 | ||
| 0 | 11 (44.0%) | 5 (19.2%) | <.10 |
| 1-2 | 5 (20.0%) | 14 (53.8%) | <.05 |
| 3-4 | 7 (28.0%) | 3 (11.5%) | n.s. |
| ≥5 | 2 (8.0%) | 4 (15.4%) | n.s. |
Abbreviations: GJG, Goshajinkigan; VAS, Visual Analog Scale; n.s.; not significant.
Table 5.
Use of Adjuvant Analgesics and Persistence of CIPN.
| Group A (later GJG administration) | Group B (prophylactic GJG administration) | P value | |
|---|---|---|---|
| n = 18 | n = 22 | ||
| Adjuvant analgesics | 12 (66.7%) | 6 (27.3%) | .024 |
| NSAIDs | 9 | 1 | |
| Pregabalin | 1 | 2 | |
| Mirogabalin | 1 | 1 | |
| NSAIDs+VitB12 | 0 | 1 | |
| NSAIDs + Pregabalin | 1 | 0 | |
| NSAIDs + Mirogabalin | 0 | 1 | |
| Persistence of CIPN | 10 (55.6%) | 4 (18.2%) | .021 |
Abbreviations: CIPN, chemotherapy-induced peripheral neuropathy; GJG, Goshajinkigan; NSAIDs, non-steroidal anti-inflammatory drugs.
Additionally, persistent CIPN 6 months post-chemotherapy was significantly less frequent in Group B, with 4 patients (18.2%) showing symptoms compared to 10 patients (55.6%) in Group A (Table 5). There were no differences in the groups regarding the completion rate of chemotherapy or changes due to CIPN (Table 6). Three cases in Group A and 5 in Group B discontinued the study for reasons other than peripheral neuropathy. In Group A, 1 patient had anaphylaxis due to PTX, and 2 had disease progression. In Group B, 2 patients had anaphylaxis due to PTX, 2 had disease progression, and 1 discontinued treatment due to cellulitis.
Table 6.
Completion Rate of Chemotherapy and Dose Reduction or Discontinuation Due to CIPN.
| Group A (later GJG administration) | Group B (prophylactic GJG administration) | P value | |
|---|---|---|---|
| n = 26 | n = 27 | ||
| Completion rate of chemotherapy | 21 (80.8%) | 22 (81.5%) | 1.0 |
| Dose reduction | 6 (23.1%) | 4 (14.8%) | .773 |
| Neurotoxicity | 4 (15.4%) | 2 (7.4%) | |
| Other than neurotoxicity | 2 (7.7%) | 2 (7.4%) | |
| Discontinuation | 5 (19.2%) | 5 (18.5%) | .472 |
| Neurotoxicity | 2 (7.7%) | 0 | |
| Other than neurotoxicity | 3 (11.5%) | 5 (18.5%) |
Abbreviations: CIPN, chemotherapy-induced peripheral neuropathy; GJG, Goshajinkigan.
No deaths were recorded during the study period. There were no differences in the incidence of hematological or non-hematological adverse events between the 2 groups, other than peripheral neuropathy (Table 7). The side effects of GJG include liver dysfunction, jaundice, interstitial pneumonia, and gastrointestinal symptoms, but they did not occur in this study. There was no difference in the occurrence of liver dysfunction between the 2 groups. Al l patients who took GJG followed the prescribed dosage and administration method.
Table 7.
Hematologic and Nonhematologic Adverse Events.
| All grades (%) | Grade 3/4 (%) | |||||
|---|---|---|---|---|---|---|
| Adverse Events | Group A | Group B | P value | Group A | Group B | P value |
| Leukopenia | 18 (69.2) | 20 (74.1) | .766 | 7 (26.9) | 6 (22.2) | .757 |
| Neutropenia | 18 (69.2) | 25 (92.6) | .039 | 16 (61.5) | 19 (70.4) | .569 |
| Anemia | 13 (50.0) | 15 (55.6) | .786 | 0 (0.0) | 2 (7.4) | .491 |
| Thrombocytopenia | 3 (11.5) | 2 (7.4) | .669 | 0 (0.0) | 1 (3.7) | 1.000 |
| Anorexia | 17 (65.4) | 14 (51.9) | .406 | 1 (3.8) | 2 (7.4) | 1.000 |
| Fatigue | 14 (53.8) | 10 (37.0) | .275 | 0 (0.0) | 0 (0.0) | N.E. |
| Nausea | 12 (46.2) | 17 (63.0) | .275 | 2 (7.7) | 1 (3.7) | .61 |
| Diarrhea | 3 (11.5) | 9 (33.3) | .099 | 0 (0.0) | 0 (0.0) | N.E. |
| Allergic reaction | 5 (19.2) | 4 (14.8) | .728 | 0 (0.0) | 0 (0.0) | N.E. |
| AST, ALT levels | 7 (26.9) | 11 (40.7) | .387 | 1 | 0 (0.0) | .491 |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; N.E., not evaluated.
Discussion
This multicenter, prospective randomized controlled trial provides new insights into the management of CIPN using GJG. Notably, prophylactic administration of GJG demonstrated significant benefits in terms of reduced analgesic adjuvant use and reduced persistence of CIPN symptoms 6 months post-treatment. Although the primary and secondary outcome data did not show a reduced incidence of grade 2 neuropathy or differences in the VAS scores during treatment, the VAS scores decreased significantly by the end of chemotherapy in the group that received prophylactic GJG.
In our previous evaluation of hyperalgesia in rats, we compared a group that received GJG for 1 week before PTX administration (PTX + GJG group) to a group that received PTX alone (PTX group). In the PTX + GJG group, a sustained decrease in the pain threshold was recorded initially, in the first week; the pain later increased and eventually reached the level of the control group. We recorded increased gene and protein expression of TRPV4 in the dorsal root ganglion (DRG) cells in the PTX group compared to those in the control group. Conversely, significant suppression of the TRPV4 gene and protein expression was demonstrated in the PTX + GJG group relative to the PTX group. 16
Kuriyama and Endo conducted a systematic review and meta-analysis on the preventive effects of GJG on CIPN, collecting data up to 2013. Their analysis included 5 clinical trials with 397 patients. 18 Three trials involved FOLFOX chemotherapy,17,19,20 one used weekly PTX therapy, 21 and the other used docetaxel-based therapy. 22 Four trials evaluated CIPN severity using the National Cancer Institute (NCI)-CTCAE, and 3 used the Neurotoxicity Criteria of Debiopharm (DEB-NTC). The results from the trials that followed the NCI-CTCAE grading showed that GJG was not effective in lowering the incidence of CIPN of any grade compared to the disease status in controls. However, in the trials that used DEB-NTC, treatment with GJG led to a significant decrease in the occurrence of grade 1 or higher CIPN (relative risk [RR] 0.43; 95% confidence interval [CI], 0.27-0.66) and grade 3 CIPN (RR 0.42; 95% CI, 0.25-0.71), but not of grade 2 or higher CIPN (RR 0.78; 95% CI, 0.36-1.72).
The distinction between this and prior studies lies in the administration method of GJG, which, based on our animal experiments, 16 involves commencing treatment 1 week before PTX administration. One salient advantage of prophylactic GJG administration was that the patients completed chemotherapy with minimal use of additional analgesics and showed significant recovery from neuropathy symptoms at treatment conclusion. This suggests that GJG may have prevented irreversible changes in the peripheral nerves or that the neuropathy experienced was mild despite reaching grade 2. There is a need to develop objective evaluation methods. Additionally, approximately 30% of patients in the prophylactic GJG group experienced grade 2 numbness from the first week of the TC therapy course. This result is similar to the results of animal experiments. However, the underlying reasons and mechanisms need to be explored in future studies. Further challenges remain, such as understanding why approximately 30% of patients did not develop PTX-induced neuropathy from the first course. This could be attributed to patient background, metabolic pathways, genetic factors, or gut microbiota, all of which warrant further investigation.
Additionally, the mechanisms of CIPN differs depending on the type of drug. 5 Taxane-based drugs primarily cause axonal damage, while platinum-based drugs cause neuronal damage. GJG has been reported to be effective in peripheral neuropathy induced by taxane-based drugs, whereas another Kampo medicine Ninjin’yoeito was effective in peripheral neuropathy induced by platinum-based drugs. 23 Due to the different causes of peripheral neuropathy depending on the type of chemotherapy, it is believed that the appropriate traditional herbal medicine should be selected to alleviate symptoms based on the anticancer drugs used.
It is important to acknowledge that while subjective scales such as the VAS are commonly used in clinical research to measure symptoms like pain and discomfort, they may carry inherent biases due to their subjective nature. However, these measures are validated tools that provide essential insights into a patient’s experience and symptom severity, which are otherwise difficult to quantify objectively. In this study, despite relying on subjective scales, the methodology was robust, including subjective assessments and objective endpoints such as the rate of chemotherapy completion and dose adjustments due to neurotoxicity. This combination helps balance the subjective nature of some data with more objective, measurable outcomes.
Furthermore, to minimize bias, the study design included randomization and a control group, which are standard methods for reducing the impact of confounding variables and bias in clinical trials. Nonetheless, including additional objective measures, such as nerve conduction studies or biomarkers, in future studies would provide more quantitative data on the neuroprotective effects of GJG.
This study has some limitations. The subjective nature of the assessments—relying on patient and physician evaluations—points to the need for more objective measures. This study did not use a placebo, and information bias might be present. Future trials should compare GJG with a placebo in a double-blind setting to validate our findings. The grouping method in this study is reasonable, and the findings suggest that prophylactic intervention could be effective. However, further research is necessary to obtain more objective data. Additionally, while some predictability of the results exists, determining whether this leads to a bias in the study requires additional detailed analysis. Furthermore, understanding the mechanism underlying the early onset of grade 2 neuropathy in the prophylactic group is a crucial area of research. Another limitation of the study is the sample size. This can increase the risk of type II errors. Therefore, there is a need for further research with larger sample sizes to confirm the findings of this study. This could include larger, possibly multicentric studies to replicate and extend the findings to enhance their reliability and applicability to a broader population. Overall, although GJG shows promise, a comprehensive and objective evaluation of its benefits requires further exploration.
Conclusion
In this multicenter trial, we assessed the effects of GJG on CIPN in patients undergoing PTX chemotherapy. While GJG did not prevent neuropathy onset, its administration was associated with fewer incidents of long-term CIPN and reduced analgesic use, suggesting a potential protective effect. Notably, subjective measures such as VAS may introduce bias, indicating a need for objective assessments. Future studies should consider double-blind designs with placebo controls to further validate these findings.
Acknowledgments
We thank all patients who participated in this study. This clinical trial was performed as the Tohoku Gynecologic Cancer Unit (TGCU) study, and we thank all TGCU investigators. In addition, the authors would like to thank Editage (www.honyakucenter.jp) for English language review.
Footnotes
Data Availability Statement: The datasets used and analyzed during the current study are available from the corresponding author upon request.
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a Grant-in-Aid for Cancer Research from the Ministry of Education, Culture, Sports, Science and Technology (Tokyo, Japan) (No. 20K09589 to Dr. Y. Yokoyama).
Trial Registration: UMIN Clinical Trials Registry (UMIN-CTR) registration No UMIN 000021361
ORCID iD: Yukiko Matsumura  https://orcid.org/0009-0008-0560-3923
https://orcid.org/0009-0008-0560-3923
References
- 1. Gutiérrez-Gutiérrez G, Sereno M, Miralles A, Casado-Sáenz E, Gutiérrez-Rivas E. Chemotherapy-induced peripheral neuropathy: clinical features, diagnosis, prevention and treatment strategies. Clin Transl Oncol. 2010;12:81-91. doi: 10.1007/S12094-010-0474-z [DOI] [PubMed] [Google Scholar]
- 2. Yu A, Street D, Viney R, et al. Clinical assessment of chemotherapy-induced peripheral neuropathy: a discrete choice experiment of patient preferences. Support Care Cancer. 2021;29:6379-6387. doi: 10.1007/s00520-021-06196-8 [DOI] [PubMed] [Google Scholar]
- 3. Hu L-Y, Mi W-L, Wu G-C, Wang Y-Q, Mao-Ying Q-L. Prevention and treatment for chemotherapy-induced peripheral neuropathy: therapies based on CIPN mechanisms. Curr Neuropharmacol. 2019;17:184-196. doi: 10.2174/1570159X15666170915143217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boyette-Davis JA, Hou S, Abdi S, Dougherty PM. An updated understanding of the mechanisms involved in chemotherapy-induced neuropathy. Pain Manag. 2018;8:363-375. doi: 10.2217/pmt-2018-0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ibrahim EY, Ehrlich BE. Prevention of chemotherapy-induced peripheral neuropathy: a review of recent findings. Crit Rev Oncol Hematol. 2020;145:102831. doi: 10.1016/j.critrevonc.2019.102831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jaggi AS, Singh N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology. 2012;291:1-9. doi: 10.1016/j.tox.2011.10.019 [DOI] [PubMed] [Google Scholar]
- 7. Mols F, Beijers T, Vreugdenhil G, van de Poll-Franse L. Chemotherapy-induced peripheral neuropathy and its association with quality of life: a systematic review. Support Care Cancer. 2014;22:2261-2269. doi: 10.1007/s00520-014-2255-7 [DOI] [PubMed] [Google Scholar]
- 8. Wang C, Chen S, Jiang W. Treatment for chemotherapy-induced peripheral neuropathy: a systematic review of randomized control trials. Front Pharmacol. 2022;13:1080888. doi: 10.3389/fphar.2022.1080888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155:2461-2470. doi: 10.1016/j.pain.2014.09.020 [DOI] [PubMed] [Google Scholar]
- 10. Watanabe K, Shimada A, Miyaki K, et al. Long-term effects of goshajinkigan in prevention of diabetic complications: a randomized Open-Labeled Clinical Trial. Evid Based Complement Alternat Med. 2014;2014:8. doi: 10.1155/2014/128726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mizuno K, Kono T, Suzuki Y, et al. Goshajinkigan, a traditional Japanese medicine, prevents oxaliplatin-induced acute peripheral neuropathy by suppressing functional alteration of TRP channels in rat. J Pharmacol Sci. 2014;125:91-98. doi: 10.1254/jphs.13244fp [DOI] [PubMed] [Google Scholar]
- 12. Kitamura R, Andoh T, Fushimi H, et al. Involvement of descending monoaminergic systems in antiallodynic effect of goshajinkigan in oxaliplatin-treated mice. J Tradit Med. 2013;30:183-189. doi: 10.11339/jtm.30.183 [DOI] [Google Scholar]
- 13. Nakanishi M, Nakae A, Kishida Y, et al. Go-sha-jinki-Gan (GJG) ameliorates allodynia in chronic constriction injury-model mice via suppression of TNF-α expression in the spinal cord. Mol Pain. 2016;12:174480691665638. doi: 10.1177/1744806916656382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suzuki Y, Goto K, Ishige A, Komatsu Y, Kamei J. Antinociceptive effect of gosha-jinki-gan, a Kampo medicine, in streptozotocin-induced diabetic mice. Jpn J Pharmacol. 1999;79:169-175. doi: 10.1254/jjp.79.169 [DOI] [PubMed] [Google Scholar]
- 15. Higuchi H, Yamamoto S, Ushio S, Kawashiri T, Egashira N. Goshajinkigan reduces bortezomib-induced mechanical allodynia in rats: Possible involvement of kappa opioid receptor. J Pharmacol Sci. 2015;129:196-199. doi: 10.1016/j.jphs.2015.09.004 [DOI] [PubMed] [Google Scholar]
- 16. Matsumura Y, Yokoyama Y, Hirakawa H, et al. The prophylactic effects of a traditional Japanese medicine, Goshajinkigan, on paclitaxel-induced peripheral neuropathy and its mechanism of action. Mol Pain. 2014;10:61. doi: 10.1186/1744-8069-10-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nishioka M, Shimada M, Kurita N, et al. The Kampo medicine, goshajinkigan, prevents neuropathy in patients treated by FOLFOX regimen. Int J Clin Oncol. 2011;16:322-327. doi: 10.1007/s10147-010-0183-1 [DOI] [PubMed] [Google Scholar]
- 18. Kuriyama A, Endo K. Goshajinkigan for prevention of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Support Care Cancer. 2018;26:1051-1059. doi: 10.1007/s00520-017-4028-6 [DOI] [PubMed] [Google Scholar]
- 19. Kono T, Hata T, Morita S, et al. Goshajinkigan oxaliplatin neurotoxicity evaluation (GONE): a phase 2, multicenter, randomized, double-blind, placebo-controlled trial of goshajinkigan to prevent oxaliplatin-induced neuropathy. Cancer Chemother Pharmacol. 2013;72:1283-1290. doi: 10.1007/s00280-013-2306-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oki E, Emi Y, Kojima H, et al. Preventive effect of Goshajinkigan on peripheral neurotoxicity of FOLFOX therapy (GENIUS trial): a placebo-controlled, double-blind, randomized phase III study. Int J Clin Oncol. 2015;20:767-775. doi: 10.1007/s10147-015-0784-9 [DOI] [PubMed] [Google Scholar]
- 21. Kawabata K, Kawajiri H, Takashima T, et al. Reduction of paclitaxel-related peripheral sensory neuropathy by Gosha-Jinki-Gan or carbon dioxide feet and hand bathing. Ann Oncol. 2013;24:ix80. doi: 10.1093/annonc/mdt460.68 [DOI] [Google Scholar]
- 22. Abe H, Kawai Y, Mori T, et al. The Kampo medicine Goshajinkigan prevents neuropathy in breast cancer patients treated with docetaxel. Asian Pac J Cancer Prev. 2013;14:6351-6356. doi: 10.7314/apjcp.2013.14.11.6351 [DOI] [PubMed] [Google Scholar]
- 23. Motoo Y, Tomita Y, Fujita H. Prophylactic efficacy of ninjin'yoeito for oxaliplatin-induced cumulative peripheral neuropathy in patients with colorectal cancer receiving postoperative adjuvant chemotherapy: a randomized, open-label, phase 2 trial (HOPE-2). Int J Clin Oncol. 2020;25:1123-1129. doi: 10.1007/s10147-020-01648-3 [DOI] [PubMed] [Google Scholar]