Abstract
Objective:
Hemodialysis patients with chronic kidney disease often exhibit inflammation characterized by elevated levels of C-reactive protein, Interleukin 6 and tumor necrosis factor-alpha, and they are shown to be associated with cardiovascular impairment and enhanced renal failure. This study aims to assess the impact of fish oil intake on inflammation indicators in adult hemodialysis patients.
Methods:
From the inception to December 2023, the datasets Cochrane Central, Google Scholar, Science Direct, Embase, and Pubmed were examined. Two authors independently searched, selected, and screened the literature. The pooled results are represented by weighted mean difference (WMD) with 95% confidence intervals. To investigate the causes of heterogeneity, subgroup analysis was done. Sensitivity analysis was then used to evaluate the validity of the combined findings.
Results:
Thirteen randomized control trials studies were included. The pooled results showed that fish oil supplementation caused a significant reduction of the C-reactive protein level (WMD, −2.92 mg/L; 95% Confidence interval, −5.23, to −0.61; p = 0.01; I2 = 99%), especially in patients with baseline C-reactive protein ⩾5 mg/L (WMD, −4.39 mg/L; 95% Confidence interval, −5.93 to 2.85; p < 0.00001; I2 = 33%). Subgroup analyses showed that C-reactive protein baseline level (C-reactive protein <5 mg/L) was the main source of heterogeneity. Fish oil intake may not reduce the level of Interleukin 6 (WMD, −2.26; 95% Confidence interval: −19.61 to 15.09; p = 0.80; I2 = 93%), nor will it reduce the level of tumor necrosis factor-alpha (random model: WMD, −2.51; 95% Confidence interval: 6.08 to 1.06; p = 0.17; I2 = 98%).
Conclusion:
Hemodialysis patients, especially those with C-reactive protein > 5 mg/L, responded to fish oil supplementation to reduce their C-reactive protein level; however, Interleukin 6 and tumor necrosis factor-alpha levels did not appear to be affected.
Keywords: Omega-3 fatty acids, inflammation, chronic kidney disease, fish oil
Introduction
With an estimated prevalence of 8–16%, chronic kidney disease (CKD) is a massive public health issue, having a surge in incidence globally. 1 Kidney disease has been ranked as the 12th most common cause of death, being responsible for 1.1 million deaths globally according to the 2015 Global Burden of Disease Study. 2 It often involves a progressive loss of kidney function necessitating renal replacement therapy (dialysis or transplantation), and the condition is called end-stage renal disease (ESRD). Patients with CKD, particularly those with ESRD and undergoing maintenance dialysis therapy have been shown to have chronic inflammation with severely elevated cytokines, proinflammatory markers, and oxidative stress mediators. Particularly those of importance are C-reactive protein (CRP), Interleukin 6 (IL-6), and tumor necrosis factor-alpha (TNF-α), as these factors are associated with cardiovascular impairment and enhanced renal failure. 1 These individuals also have a 2–3 times higher risk of death due to cardiovascular diseases (CVD) compared with the general population. 3
There is evidence that patients with high-stage CKD have lower levels of n−3 polyunsaturated fatty acids (PUFAs) in their blood than the general population, possibly due to a lower dietary intake of n−3 PUFAs, as well as changes in metabolic processes, inflammation, loss of n−3 PUFAs, and malabsorption during dialysis. 4 The omega-3 fatty acids are known to reduce the risk of developing of ESRD as they reduce platelet aggregation, restore impaired lipid metabolism, modulate blood pressure, and reduce inflammation and oxidative stress. 5 When supplemented with 1.3 gm of fish oil daily, they can improve outcomes by reducing inflammatory markers in hemodialysis patients, such as CRP, TNF-α, and IL-6. 6 Evidence shows that fish eaters who were also on maintenance hemodialysis had a reduction in cardiovascular events, which may be due to fish oil’s high content of omega-3 fatty acids. 7
We did a meta-analysis to see the effects of consuming fish oil on inflammatory markers to assess if there was mechanistic probability that its use can improve CV outcomes. Study objectives include investigating the effectiveness of fish oil on patients who are undergoing hemodialysis and plotting levels of inflammatory markers in response to fish oil supplementation.
Methods
Literature search and strategy
This systematic review and meta-analysis was performed according to the preferred reporting items for systematic review and meta-analyses (PRISMA) guidelines. 8 The literature from PubMed, EMBASE, Google Scholar, Science Direct, and Cochrane Library databases from their inception to December 2023 was systematically searched using an extensive search strategy, shown in the Supplemental material Table 1. The following MeSH terms were used: renal replacement therapy, renal dialysis, hemodiafiltration, hemofiltration, dialysis solutions, renal insufficiency, omega three fatty acids, docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), fish oil, tumor necrosis factor alpha, IL-6, and c reactive protein. In addition, we manually screened the reference list of retrieved trials, previous meta-analyses and review articles to identify any relevant studies.
Study selection
The following eligibility criteria were used to select studies: (a) published randomized controlled trials (RCT) with a follow-up of at least 2 months; (b) adult male or female patients undergoing hemodialysis; (c) fish-oil or ω-3 fatty acid being compared with placebo or plant-derived oil; and (d) change in inflammatory markers in each group before and after the intervention, including CRP, IL-6 or TNF-α. Exclusion of patients who were undergoing peritoneal dialysis or hemodialysis due to acute kidney injury was implemented.
Data extraction and quality assessment
Using the Endnote Reference library, duplicate articles were identified and removed from the articles retrieved through the systemic search. Observational studies, review articles, conference extracts, editorials, case reports or series, and non-English studies were excluded. Two separate reviewers carried out a thorough evaluation of the remaining articles. Only those trials that fulfilled the predetermined criteria were chosen. Initially, the shortlisting of the trials was based on their title and abstract, followed by a thorough review of the full articles to ensure their relevance. A third investigator was consulted to resolve any discrepancies. From the finalized trials, the following outcomes were extracted: change in CRP levels; change in IL-6 levels; and change in TNF-α levels. The risk of bias was assessed using the Cochrane Risk of Bias tool 2.0 (Cochrane Collaboration) for assessing RCTs. 9
Statistical analysis
For the meta-analysis, the Cochrane Review Manager software RevMan 5.4.1 (Cochrane Collaboration) was used to perform the statistical calculations. The meta-analysis was performed using the Inverse–Variance random effects model. The difference between arithmetic means with a 95% confidence interval was used to measure effect size, and a p-value of <0.05 was considered significant. As the study by Ruperto et al. did not report SD of change from baseline, we first calculated the correlation coefficient (R) using another study’s which had reported its results in considerable detail and then computing them in the following formula: [(SD baseline) 2 + (SD final) 2 − (SD change) 2 ]/[2 × SD baseline × SD final]. 10 The R was calculated to be 0.5. Following that, we calculated the SD of change using the formula: [(SD baseline) 2 + (SD final) 2 − (2R × SD baseline × SD final)]½. 10 Publication bias was checked using funnel plots and asymmetry tests (Begg’s test and Egger’s test). 11 When the p-value of either Egger’s test or Begg’s test was less than 0.05, publication bias was considered significant. Heterogeneity was analyzed using the Chi-square test and the inconsistency index (I2). According to the Cochrane Collaboration tool, heterogeneity was classified as unimportant (0%–40%), moderate (30%–60%), substantial (50%–90%), and considerable (75%–100%). A p-value <0.05 was considered significant. To ensure the robustness of our findings, we conducted a leave-one-out sensitivity analysis when high heterogeneity was observed, which involved removing one study at a time.
Results
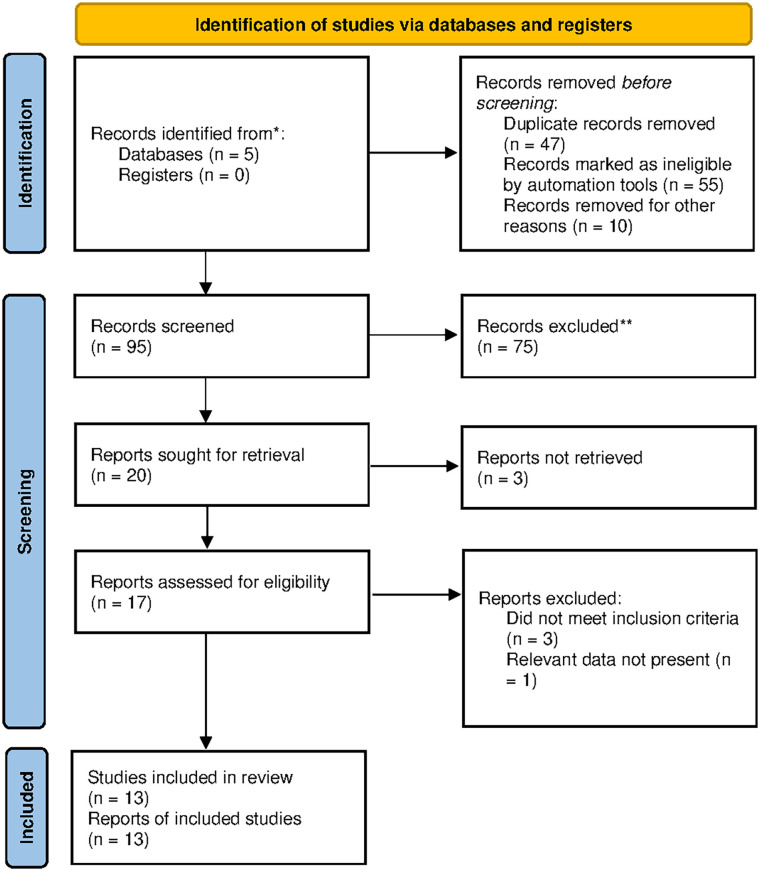
Literature was searched in Cochrane Library, PubMed, Google Scholar, Science Direct, and Embase databases. Following the removal of duplicates and the evaluation of titles and abstracts, 17 articles were screened based on eligibility criteria (Figure 1). Ultimately, a systematic review and meta-analysis were conducted on a selected pool of 13 articles with 767 participants (Table 1).12–24 The focused variables were comorbidities, CRP baseline levels, publication year, follow-up duration, age, and sex ratio. The duration period for published studies was from 2007 to 2023. The study duration of included studies was 2.5–6 months. The mean age of study participants was 52.9 (±15.9) years in the fish oil and 57.9 (±7.2) years in the placebo group. Males were more dominant than females. Mostly, subjects were found with chronic stabilized hemodialysis and diabetes mellitus complications. Some RCTs also reported CVD, severe vitamin D deficiency (<30 pg/ml), CRP ⩾5 mg/L, and albumin ⩽3.9 g/dl as other complications of studied participants. In all studies, subjects with hemodialysis were treated with EPA and DHA ranging from 1.3 to 3 g as fish oil supplementation. The control group was treated with placebo or paraffin oil, olive oil, corn oil, MCT oil, olive oil + vitamin D, and protein + olive oil. The CRP level as the main outcome was reported in all studies. Nonserious adverse events such as inability to swallow pills, fishy taste, and mild transient gastrointestinal disturbances were commonly reported.
Figure 1.
Flow diagram of literature search and study selection.
Table 1.
Basic characteristics of the included studies.
| Study | Study design | Country | No. of patients | Gender M/F | Mean age | Intervention | Control | Outcome | Duration (months) |
|---|---|---|---|---|---|---|---|---|---|
| Ruperto et al. 12 | RCT | Spain | 42 | A:16/5 B:13/8 |
A:65.7±16.7 B:67.7±14.6 |
645 mg DHA 3/week | Placebo | CRP | 2 |
| Valle Flores et al. 13 | RCT | Venezuela | 93 | A:30/16 B:32/15 |
A:52.1±8.2 B:49.6±8.3 |
2.4 g ω-3 (360 mg EPA + 240 mg DHA) | Paraffin oil | CRP, IL-6, TNF- α | 3 |
| Zakaria et al. 14 | RCT | United States | 40 | A:12/8 B:11/9 |
A:50.25±14.44 B:46.40±15.62 |
3 soft gel capsules (each capsule 203 mg EPA and 148 mg DHA) + 100 mg wheat germ oil (0.255 mg vitamin E) |
Placebo | CRP | 4 |
| Asemi et al. 15 | RCT | Iran | 120 | A:20/10 B:20/10 |
A:55.2±17.0 B:59.9±15.7 |
1.25 g ω-3 (600 mg EPA, 300 mg DHA and 350 mg of other w-3 fatty acids) |
Placebo | CRP | 3 |
| Harving et al. 16 | RCT | Denmark | 162 | A:55/28 B:51/28 |
A:65.5±11 B:68±11 |
1.7 g ω-3 (45% EPA and 37.5% DHA) | Olive oil | CRP | 3 |
| Lee et al. 17 | RCT | Korea | 15 | A:2/6 B:3/4 |
A:60.0±7.3 B:64.4±8.5 |
2.4 g ω-3 + Vit D | Olive oil—Vit D | CRP | 3 |
| Gharekhani et al. 18 | RCT | Iran | 45 | A:13/12 B:12/8 |
A:56.8±13.09 B:57.2±15.19 |
1.8 g ω-3 (0.36 g EPA and 0.24 g DHA) | Paraffin oil | CRP, IL-6, TNF- α | 4 |
| Hung et al. 19 | RCT | United States | 38 | A:14/3 B:13/4 |
A:50 (38,58) B:53 (45,65) |
2.9 g ω-3 (EPA+DHA) | Placebo | CRP, IL-6, TNF-α | 3 |
| Daud et al. 20 | RCT | United States | 63 | A:20/11 B:12/20 |
A:59±13 B:58±13 |
30 ml liquid protein + 2.4 g ω-3 3/week (1.8 g EPA + 0.6 g DHA) | Protein – olive oil | CRP | 6 |
| Kooshki et al. 21 | RCT | Iran | 34 | A:10/7 B:11/6 |
A:50±18 B:50±17 |
2.08 g ω-3 (4 capsules, each containing 310 mg EPA and 210 mg DHA) | MCT oil | CRP, IL-6, TNF- α | 2.5 |
| Bowden et al. 22 | RCT | United States | 33 | A:11/7 B:8/7 |
A:57.2±12.8 B:64.3±14.2 |
3 g fish oil supplement (0.96 g EPA + 0.6 g DHA) | Corn oil | CRP | 6 |
| Saifullah et al. 23 | RCT | United States | 23 | A:11/4 B:7/1 |
A:58±12 B:57±14 |
1.3 g (EPA + DHA) | Soybean/corn oil | CRP | 3 |
| de Lima et al. 24 | RCT | Brazil | 59 | 36/23 | 54±11 | 2 g ω-3 (0.36 g EPA + 0.24 g DHA) | Placebo | CRP | 2 |
A: Fish oil; B: Placebo; CRP; c-reactive protein; IL-6; interleukin-6; RCT: randomized controlled trial; TNF-α: tumor necrosis factor alpha; w-3: omega-three fatty acids.
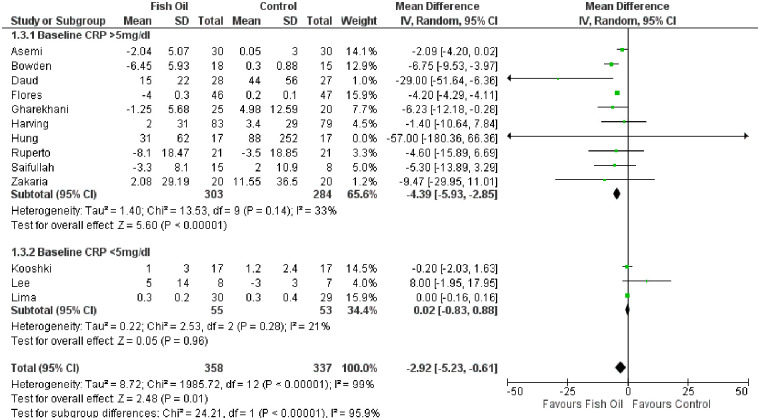
By comparing different studies, this meta shows that CRP level decreases significantly by using fish oil supplementation (WMD, −2.92 mg/L; 95% CI: −5.23 to −0.61; p = 0.01; I2 = 99%) (Figure 2). Heterogeneity sources and fish oil effects on specific subpopulation was determined by further analyzing the subgroups. Subgroup analysis revelaed that CRP levels significantly decrease in patients with baseline CRP ⩾5 mg/L (WMD, −4.39 mg/L; 95% CI: −5.93 to 2.85; p < 0.00001; I2 = 33%), as compared to those with a lower CRP baseline level (Figure 2). The main source of heterogeneity in studies with CRP baseline level <5 mg/L was observed in the studies by Kooshki et al. 21 and de Lima et al. 24 (Supplemental material file). While comorbidity (albumin ⩽3.9 g/dl and/or chronic inflammation) and duration to follow-up (<3 months) were the potential heterogeneity sources (Figure 2). Publication bias was statistically insignificant (p-value for Egger’s regression test: 0.727).
Figure 2.
Forest plot for CRP.
CI: Confidence interval; CRP: C-reactive protein; IV: inverse variance.
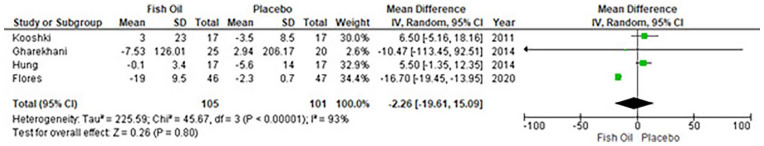
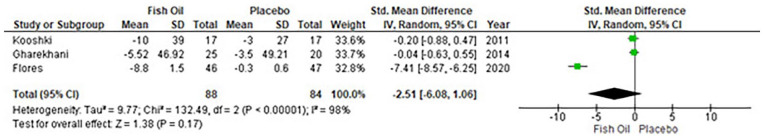
Overall, four studies reported the IL-6 levels with no effect of fish oil supplementation [WMD: −2.26 ng/L (95% CI: −19.61 to 15.09); p = 0.80; I2 = 93%] (Figure 3). Publication bias was statistically insignificant (p-value for Egger’s regression test: 0.367). Only three studies reported the TNF-α levels and suggested that TNF-α levels do not reduce with fish oil supplementation [WMD: −2.51 ng/L (95% CI: 6.08–1.06); p = 0.17; I2 = 98%] (Figure 4). Publication bias was statistically insignificant (p-value for Egger’s regression test: 0.12). Sensitivity analyses were removed in each study and showed that there is no change in pooled results (Supplemental material file).
Figure 3.
Forest plot for IL-6.
CI: Confidence interval; IL-6; Interleukin 6; IV: inverse variance.
Figure 4.
Forest plot for TNF-α.
CI: Confidence interval; TNF-α; tumor necrosis factor alpha; IV: inverse variance.
Quality assessment
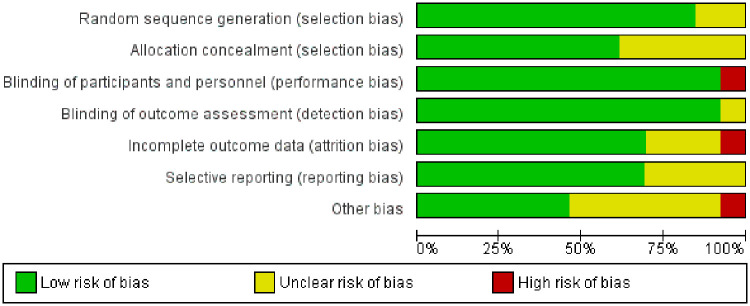
For the quality assessment using the Cochrane tool for assessing the risk of bias in RCTs, 9 out of 11 studies were at low risk of bias, 3 had a moderate risk, and one was of high risk. Other than that, 11 studies are at low risk of random sequence generation. Eight are at low risk for allocation concealment. Eleven are at a low risk of performance bias and detection bias. Nine are at low risk of attrition bias (Figure 5 and Supplemental material Figure 1).
Figure 5.
Risk of bias for included randomized controlled trials in the study.
Discussion
This pooled analysis of 13 RCTs gathered data from 767 participants treated with omega-3 fatty acids supplements or fish oils. We found that supplementation with either fish oil or omega-3 fatty acids supplements in patients with CKD, undergoing hemodialysis, is associated with reduced levels of CRP, whereas this association was not found with IL-6 and TNF-α. The results were consistent with a previous meta-analysis carried out by Zhou et al. 25 These findings support a promising role for increased supplementation of omega-3 or fish oil for decreasing inflammatory markers like CRP in patients with CKD.
In ESRD patients, there is evidence that proinflammatory cytokines are involved in malnutrition and CVD pathogenesis.26,27 In terms of cardiovascular events, these cytokines are more prognostic than traditional risk factors. 28 Chronic hemodialysis patients experience inflammation for a wide variety of reasons, some of which include infections of grafts or fistulas, bioincompatible dialysis membranes, dialysate exposure, endotoxins, back filtration, chronic infections, and malnutrition.29,30
The level of CRP increases during the inflammatory process, with the plasma concentration fluctuating by at least 25%.31,32 The level of CRP in the blood, which is provoked by IL-6, is a predictor of cardiovascular morbidity and death in hemodialysis patients.33–36 CRP has been shown to bind to injured cell membranes, such as infarcted heart tissue, and participate in local complement activation, causing further damage, for example, to heart tissue. 37 TNF-α and IL-6 are key cytokines that are implicated in both acute and chronic inflammation and lead to cardiovascular morbidity and mortality in predialysis and dialysis patients.38,39 Furthermore, plasma levels of IL-6 are a better predictor of death in dialysis patients with CKD than other inflammatory markers.27,40
Several mechanisms have been proposed to explain how EPA and DHA found in fish oil can reduce inflammation in the body. Early studies have shown that EPA and DHA can inhibit the production of IL-6 and IL-8 by human endothelial cells when stimulated by endotoxins. 41 Additionally, EPA or fish oil can inhibit endotoxin-induced TNF production by cultured monocytes. 42 Fish oil has been shown to reduce the production of inflammatory cytokines and down-regulate the expression of genes associated with inflammation (via NFκB, PPAR-γ, GPR120 etc.). 43 Moreover, enzymatic oxidation of EPA and DHA can produce resolvins and protectins, which help with inflammation resolution; however, studies have shown that to achieve this mechanism, omega-3 fatty acid concentrations as high as 3 g may be needed. 44
A prior meta-analysis found that fish oil supplementation significantly lowered CRP levels in trials with baseline CRP levels of more than 5 mg/L (WMD −4.43 mg/L; 95% CI: −6.10 to −2.76. p = .00001: p = 41%), which is similar with our pooled findings. 25 This meta-analysis supports the conclusion that supplementing fish oil to reduce CRP levels is an effective strategy when CRP is greater than 5 mg/dl at baseline. This meta-analysis also demonstrates consistent findings for TNF-α and IL-6 levels after fish oil consumption. Nevertheless, we included the results by Ruperto et al. 12 to explore the effects of fish oil on CRP (13 RCTs), IL-6 (4 RCTs), and TNF-α (3 RCTs). Subsequently, to explore the sources of heterogeneity, subgroup, and sensitivity analysis was conducted.
Our meta-analysis is in agreement with a recent meta-analysis done by Liu et al, in that it showed decreased levels of CRP by omega-3 supplementation, but it failed to show a significant decrease in TNF-α levels as demonstrated by de Abreu et al. 45 Another study which used vegetable and other dietary sources with high levels of alpha-linolenic acid (ALA) in CKD patients going through dialysis also showed that CRP levels were significantly reduced after the intervention. 46 There are several possible reasons that could have influenced the outcome, including the number of patients who were studied, the duration of the treatment, the dose of omega-3 fatty acids that were used (including the ratio of EPA/DHA), and the baseline level of inflammation markers. It is also possible that the patient’s current medical condition or other treatments they were receiving could have affected the outcome.
In terms of inflammatory biomarkers, this meta-analysis found conflicting results from numerous RCTs, showing that fish oil was not significantly different from the control. A nonsignificant increase in CRP levels was reported by Zakaria et al. 14 in the fish oil and control groups, the control group being higher. According to Asemi et al. 15 and de Lima et al., 24 omega-3 fatty acid supplements did reduce CRP levels, but the drop was not significant compared to the placebo and alpha-tocopherol groups. In a study by Harving et al., 16 omega-3 fatty acid supplementation did not significantly affect the level of CRP after 3 months. It is possible that, since Ruperto et al.’s 12 study followed the two groups for only 8 weeks, they failed to detect any significant difference in s-CRP levels between the treatment and control groups despite substantial declines in the treatment group. Lee et al. 17 observed that 3 months of n−3 PUFA supplementation increased mean CRP in the omega-3 fatty acid group while decreasing mean CRP in the placebo group. Hung et al.’s 19 study found that the average CRP levels in the placebo group were higher than those in the treatment group; however, the CRP level difference between the two groups was not statistically significant. Additionally, there was no noteworthy shift in IL-6 levels from baseline levels. During the 6-month study conducted by Daud et al., 20 the omega-3 group had lower mean CRP levels compared to the placebo group, but when the median CRP values were taken into account, the differences between the two groups were not statistically significant. Kooshki et al. 21 found that there were no significant variations in the serum concentrations of CRP, TNF-α, and IL-6 in either group over time.
According to a quasi-experimental study by Moreira et al., 47 low-dose omega-3 didn’t affect CRP in the treatment group, but when patients were stratified according to CRP tertiles, CRP levels fell in those with higher levels, similar to what we observed in our results. Furthermore, in mice with adenine-induced CKD, fish oil supplementation significantly decreased IL-6, and other pro-inflammatory markers; however, renal histo-morphological changes such as tubular dilatation and interstitial infiltration persisted after treatment, indicating it had no significant influence on renal function. 48
Marine omega-3 fatty acids, which include EPA, DHA, and docosapentaenoic acid, among others, is an important element found in significant amounts in fish and different types of seafood. In a typical fish oil supplement, EPA and DHA collectively contribute to roughly 30% of the total fatty acid content. Therefore, a standard 1 g fish oil capsule typically delivers around 0.3 g of EPA + DHA. 43 Studies on fish oil often fail to take into account the effects of other types of fatty acids and do not measure plasma levels. This makes it difficult to determine the correct dosage due to the varying concentrations and ratios of supplements. It is important to conduct more research, with a focus on isolating EPA, DHA, and other omega fatty acids, in order to gain a better understanding of their specific impacts on inflammation.
Our study has several strengths. Combining the results of 13 RCTs gave us a large sample size that reduced potential effect modifiers that may be present in individual RCTs. Our findings are generalizable because we conducted analyses across RCTs that included diverse demographic backgrounds and had different incident CKD rates. The outcomes of the studies were measured in a laboratory setting, which reduces the likelihood of bias. To detect any publication bias, funnel plots were utilized, and no bias was observed as the plot was almost symmetrical.
Our study also has some limitations. There were marked differences among baseline levels in individual RCTs and measurement of variables occurred once only at baseline and then once at follow-up times which could affect the results. Follow-up times of the studies were very different as well so it cannot be determined whether there is a given time duration at which significant or nonsignificant results are obtained. Fish oil intake may have different effects on pro-inflammatory markers based on gender. A preliminary study found men maintained or lowered CRP and IL-6 levels, while women increased inflammatory markers; without exceeding the reference level. 49 In the present meta-analysis, no adverse events serious enough to warrant attention were identified. Adverse reactions were typically mild, consisting of brief episodes of gastrointestinal discomfort, fishy odors, and difficulty swallowing pills. Finally, there is minimal information available about the impact of omega-3 fatty acids on IL-6 and TNF-α levels; IL-6 has only been studied in four studies, and TNF-α in three. In light of this, the findings should be regarded with caution. Moreover, this study wasn’t registered in PROSPERO.
Conclusion
In our pooled analysis of 13 RCTs with 767 participants, higher supplementation of omega-3 fatty acid and fish oil was associated with lowering CRP levels resulting in the reduced inflammatory effect of CRP in patients with CKD on hemodialysis. Two other inflammatory markers, TNF-α and IL-6 failed to show significant reductions. Additionally, further RCTs and cohort studies are required to prove the potential benefits of fish oil supplementation to include this in the treatment regimen of CKD patients.
Supplemental Material
Supplemental material, sj-doc-2-smo-10.1177_20503121241275467 for Effectiveness of fish oil in controlling inflammation in adult patients undergoing hemodialysis: A systematic review and meta-analysis by Kaneez Fatima, Aysal Mahmood, Faiza Zafar Sayeed, Maryam Raza, Rahima Azam, Nazish Waris, Muttia Abdul Sattar, Teesha Rani, Zainab Wahaj, Danisha Kumar and Simra Nadeem Siddiqui in SAGE Open Medicine
Supplemental material, sj-docx-1-smo-10.1177_20503121241275467 for Effectiveness of fish oil in controlling inflammation in adult patients undergoing hemodialysis: A systematic review and meta-analysis by Kaneez Fatima, Aysal Mahmood, Faiza Zafar Sayeed, Maryam Raza, Rahima Azam, Nazish Waris, Muttia Abdul Sattar, Teesha Rani, Zainab Wahaj, Danisha Kumar and Simra Nadeem Siddiqui in SAGE Open Medicine
Acknowledgments
None.
Footnotes
Author contributions: Conceptualization, KF, AM, and FZS; Data curation, FZS, MR, RA, and NW; Formal analysis, AM, RA, DK, and SNS; Methodology, AM, MAS, ZW, TR, and DK; Writing—original draft, FZS, MAS, RA, TR, and ZW; Writing review and editing, SNS, AM, KF, and DK. All authors have read and agreed to the submitted version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Not applicable as it is a systematic review and meta-analysis.
Informed consent: Not applicable as it is a systematic review and meta-analysis.
Grant number: None.
ORCID iD: Aysal Mahmood  https://orcid.org/0000-0001-9924-8038
https://orcid.org/0000-0001-9924-8038
Supplemental material: Supplemental material for this article is available online.
References
- 1. Rapa SF, Di Iorio BR, Campiglia P, et al. Inflammation and oxidative stress in chronic kidney disease-potential therapeutic role of minerals, vitamins and plant-derived metabolites. Int J Mol Sci 2019; 21(1): 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang H, Naghavi M, Allen C, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388(10053): 1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roberts MA, Polkinghorne KR, McDonald SP, et al. Secular trends in cardiovascular mortality rates of patients receiving dialysis compared with the general population. Am J Kidney Dis 2011; 58(1): 64–72. [DOI] [PubMed] [Google Scholar]
- 4. Saglimbene VM, Wong G, Ruospo M, et al. Dietary n-3 polyunsaturated fatty acid intake and all-cause and cardiovascular mortality in adults on hemodialysis: the DIET-HD multinational cohort study. Clin Nutr 2019; 38(1): 429–437. [DOI] [PubMed] [Google Scholar]
- 5. Zhang C, Ge C, Wang J, et al. Effects of fish oil during hemodialysis on nutritional status and quality of life: a randomized double-blinded trial. Food Nutr Res 2020; 64: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tayyebi-Khosroshahi H, Houshyar J, Dehgan-Hesari R, et al. Effect of treatment with omega-3 fatty acids on C-reactive protein and tumor necrosis factor-alfa in hemodialysis patients. Saudi J Kidney Dis Transpl 2012; 23(3): 500–506. [PubMed] [Google Scholar]
- 7. He L, Li MS, Lin M, et al. Effect of fish oil supplement in maintenance hemodialysis patients: a systematic review and meta-analysis of published randomized controlled trials. Eur J Clin Pharmacol 2016; 72(2): 129–139. [DOI] [PubMed] [Google Scholar]
- 8. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015; 162(11): 777–784. [DOI] [PubMed] [Google Scholar]
- 9. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: 4898. [DOI] [PubMed] [Google Scholar]
- 10. Chapter 6: choosing effect measures and computing estimates of effect | Cochrane Training, https://training.cochrane.org/handbook/current/chapter-06#section-6-5-2-8 (accessed 21 May 2024).
- 11. Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315(7109): 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruperto M, Rodríguez-Mendiola N, Díaz-Domínguez M, et al. Effect of oral administration of docohexanoic acid on anemia and inflammation in hemodialysis patients: a randomized controlled clinical trial. Clin Nutr ESPEN 2021; 41: 129–135. [DOI] [PubMed] [Google Scholar]
- 13. Valle Flores JA, Fariño Cortéz JE, Mayner Tresol GA, et al. Oral supplementation with omega-3 fatty acids and inflammation markers in patients with chronic kidney disease in hemodialysis. Appl Physiol Nutr Metab 2020; 45(8): 805–811. [DOI] [PubMed] [Google Scholar]
- 14. Zakaria H, Mostafa TM, El-Azab GA, et al. The impact of fish oil and wheat germ oil combination on mineral-bone and inflammatory markers in maintenance hemodialysis patients: a randomized, double-blind, placebo-controlled clinical trial. Int Urol Nephrol 2017; 49(10): 1851–1858. [DOI] [PubMed] [Google Scholar]
- 15. Asemi Z, Soleimani A, Shakeri H, et al. Effects of omega-3 fatty acid plus alpha-tocopherol supplementation on malnutrition-inflammation score, biomarkers of inflammation and oxidative stress in chronic hemodialysis patients. Int Urol Nephrol 2016; 48(11): 1887–1895. [DOI] [PubMed] [Google Scholar]
- 16. Harving F, Svensson M, Flyvbjerg A, et al. N-3 polyunsaturated fatty acids and adiponectin in patients with end-stage renal disease. Clin Nephrol 2015; 83(5): 279–285. [DOI] [PubMed] [Google Scholar]
- 17. Lee SM, Son YK, Kim SE, et al. The effects of omega-3 fatty acid on vitamin D activation in hemodialysis patients: a pilot study. Mar Drugs 2015; 13(2): 741–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gharekhani A, Khatami MR, Dashti-Khavidaki S, et al. Effects of oral supplementation with omega-3 fatty acids on nutritional state and inflammatory markers in maintenance hemodialysis patients. J Ren Nutr 2014; 24(3): 177–185. [DOI] [PubMed] [Google Scholar]
- 19. Hung AM, Booker C, Ellis CD, et al. Omega-3 fatty acids inhibit the up-regulation of endothelial chemokines in maintenance hemodialysis patients. Nephrol Dial Transplant 2015; 30(2): 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Daud ZAM, Tubie B, Adams J, et al. Effects of protein and omega-3 supplementation, provided during regular dialysis sessions, on nutritional and inflammatory indices in hemodialysis patients. Vasc Health Risk Manag 2012; 8: 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kooshki A, Taleban FA, Tabibi H, et al. Effects of marine omega-3 fatty acids on serum systemic and vascular inflammation markers and oxidative stress in hemodialysis patients. Ann Nutr Metab 2011; 58(3): 197–202. [DOI] [PubMed] [Google Scholar]
- 22. Bowden RG, Wilson RL, Deike E, et al. Fish oil supplementation lowers C-reactive protein levels independent of triglyceride reduction in patients with end-stage renal disease. Nutr Clin Pract 2009; 24(4): 508–512. [DOI] [PubMed] [Google Scholar]
- 23. Saifullah A, Watkins BA, Saha C, et al. Oral fish oil supplementation raises blood omega-3 levels and lowers C-reactive protein in haemodialysis patients—A pilot study. Nephrol Dial Transplant 2007; 22(12): 3561–3567. [DOI] [PubMed] [Google Scholar]
- 24. de Lima K, Mazur CE, Vicente Cavagnari MA, et al. Omega-3 supplementation effects on cardiovascular risk and inflammatory profile in chronic kidney disease patients in hemodialysis treatment: an intervention study. Clin Nutr ESPEN 2023; 58: 144–151. [DOI] [PubMed] [Google Scholar]
- 25. Zhou J, Tang G, Tang S, et al. The effect of fish oil on inflammation markers in adult patients undergoing hemodialysis: a meta-analysis. Semin Dial 2022; 35(1): 6–14. [DOI] [PubMed] [Google Scholar]
- 26. Yao Q, Pecoits-Filho R, Lindholm B, et al. Traditional and non-traditional risk factors as contributors to atherosclerotic cardiovascular disease in end-stage renal disease. Scand J Urol Nephrol 2004: 405–416. [DOI] [PubMed] [Google Scholar]
- 27. Honda H, Qureshi AR, Heimbürger O, et al. Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis 2006; 47(1): 139–148. [DOI] [PubMed] [Google Scholar]
- 28. Libby P, Ridker PM. Inflammation and atherothrombosis: from population biology and bench research to clinical practice. J Am Coll Cardiol 2006; 48: A33–A46. [Google Scholar]
- 29. Noack B, Genco RJ, Trevisan M, et al. Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol 2001; 72(9): 1221–1227. [DOI] [PubMed] [Google Scholar]
- 30. Pejcic A, Kesic LJ, Milasin J. C-reactive protein as a systemic marker of inflammation in periodontitis. Eur J Clin Microbiol Infect Dis 2011; 30(3): 407–414. [DOI] [PubMed] [Google Scholar]
- 31. Du Clos TW, Mold C. C-reactive protein: an activator of innate immunity and a modulator of adaptive immunity. Immunol Res 2004; 30(3): 261–277. [DOI] [PubMed] [Google Scholar]
- 32. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999; 340(6): 448–454. [DOI] [PubMed] [Google Scholar]
- 33. Van Der Sande FM, Kooman JP, Leunissen KML. The predictive value of C-reactive protein in end-stage renal disease: is it clinically significant? Blood Purif 2006; 24(4): 335–341. [DOI] [PubMed] [Google Scholar]
- 34. Bazeley J, Bieber B, Li Y, et al. C-reactive protein and prediction of 1-year mortality in prevalent hemodialysis patients. Clin J Am Soc Nephrol 2011; 6(10): 2452–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arici M, Walls J. End-stage renal disease, atherosclerosis, and cardiovascular mortality: is C-reactive protein the missing link? Kidney Int 2001; 59(2): 407–414. [DOI] [PubMed] [Google Scholar]
- 36. Bermudez EA, Rifai N, Buring J, et al. Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. Arterioscler Thromb Vasc Biol 2002; 22(10): 1668–1673. [DOI] [PubMed] [Google Scholar]
- 37. Lagrand WK, Niessen HWM, Wolbink GJ, et al. C-reactive protein colocalizes with complement in human hearts during acute myocardial infarction. Circulation 1997; 95(1): 97–103. [DOI] [PubMed] [Google Scholar]
- 38. Barreto DV, Barreto FC, Liabeuf S, et al. Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int 2010; 77(6): 550–556. [DOI] [PubMed] [Google Scholar]
- 39. Pecoits-Filho R, Bárány P, Lindholm B, et al. Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transplant 2002; 17(9): 1684–1688. [DOI] [PubMed] [Google Scholar]
- 40. Tripepi G, Mallamaci F, Zoccali C. Inflammation markers, adhesion molecules, and all-cause and cardiovascular mortality in patients with ESRD: searching for the best risk marker by multivariate modeling. J Am Soc Nephrol 2005; 16(Suppl 1): S83–S88. [DOI] [PubMed] [Google Scholar]
- 41. Khalfoun B, Thibault G, Bardos P, et al. Docosahexaenoic and eicosapentaenoic acids inhibit in vitro human lymphocyte-endothelial cell adhesion. Transplantation 1996; 62(11): 1649–1657. [DOI] [PubMed] [Google Scholar]
- 42. Zhao Y, Joshi-Barve S, Barve S, et al. Eicosapentaenoic acid prevents LPS-induced TNF-alpha expression by preventing NF-kappaB activation. J Am Coll Nutr 2004; 23(1): 71–78. [DOI] [PubMed] [Google Scholar]
- 43. Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta 2015; 1851(4): 469–484. [DOI] [PubMed] [Google Scholar]
- 44. Ibrahim ES. Enteral nutrition with omega-3 fatty acids in critically ill septic patients: a randomized double-blinded study. Saudi J Anaesth 2018; 12(4): 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. de Abreu AM, Copetti CLK, Hauschild DB, et al. Effects of supplementation with vegetable sources of alpha-linolenic acid (ALA) on inflammatory markers and lipid profile in individuals with chronic kidney disease: a systematic review and meta-analysis. Clin Nutr 2022; 41(6): 1434–1444. [DOI] [PubMed] [Google Scholar]
- 46. Liu R, Jiang J, Fu Z, et al. Effects of omega-3 fatty acid intake in patients undergoing dialysis: a systematic review and meta-analysis of randomized controlled trials. J Am Nutr Assoc 2022; 41(7): 697–712. [DOI] [PubMed] [Google Scholar]
- 47. Moreira AC, Gaspar A, Serra MA, et al. Effect of a sardine supplement on C-reactive protein in patients receiving hemodialysis. J Ren Nutr 2007; 17(3): 205–213. [DOI] [PubMed] [Google Scholar]
- 48. Henao Agudelo JS, Baia LC, Ormanji MS, et al. Fish oil supplementation reduces inflammation but does not restore renal function and klotho expression in an adenine-induced CKD model. Nutrients 2018; 10(9): 1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zabel R, Ash S, King N, et al. Gender differences in the effect of fish oil on appetite, inflammation and nutritional status in haemodialysis patients. J Hum Nutr Diet 2010; 23(4): 416–425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-2-smo-10.1177_20503121241275467 for Effectiveness of fish oil in controlling inflammation in adult patients undergoing hemodialysis: A systematic review and meta-analysis by Kaneez Fatima, Aysal Mahmood, Faiza Zafar Sayeed, Maryam Raza, Rahima Azam, Nazish Waris, Muttia Abdul Sattar, Teesha Rani, Zainab Wahaj, Danisha Kumar and Simra Nadeem Siddiqui in SAGE Open Medicine
Supplemental material, sj-docx-1-smo-10.1177_20503121241275467 for Effectiveness of fish oil in controlling inflammation in adult patients undergoing hemodialysis: A systematic review and meta-analysis by Kaneez Fatima, Aysal Mahmood, Faiza Zafar Sayeed, Maryam Raza, Rahima Azam, Nazish Waris, Muttia Abdul Sattar, Teesha Rani, Zainab Wahaj, Danisha Kumar and Simra Nadeem Siddiqui in SAGE Open Medicine