Abstract
The invention in this patent application relates to 9,10,11,12-tetrahydro-8H-[1,4]diazepino[5′,6′:4,5]thieno[3,2-f]quinolin-8-one derivatives represented herein generally by Formula 1. These compounds are inhibitors of MK2 kinases and may provide useful treatments for MK2-mediated diseases or disorders such as autoimmune disorders, chronic or acute inflammatory disorders, autoinflammatory disorders, fibrotic disorders, metabolic disorders, neoplasia, or cardiovascular or cerebrovascular disorders.
Important Compound Classes
Title
MK2 Inhibitors and Uses Thereof
Patent Publication Number
WO 2024092115 A2
URL: https://patentimages.storage.googleapis.com/61/d0/13/d3ad3e173537b5/WO2024092115A2.pdf
Publication Date
May 2, 2024
Priority Application
US 63/381,195
Priority Date
October 27, 2022
Inventors
Hu, X.; Qiao, L.; Seletsky, B.; Patton, M.; Cao, M.; Van Der Mei, F.; Miao, G.; McDonald, I; Dzierba, C.
Assignee Companies
Bristol-Myers Squibb Company, Route 206 and Province Line Road, Princeton, New Jersey 08543, USA; Celgene Corporation, 86 Morris Avenue, Summit, New Jersey 07901, USA
Disease Area
MK2-mediated diseases or disorders such as autoimmune disorders, chronic or acute inflammatory disorders, autoinflammatory disorders, fibrotic disorders, metabolic disorders, neoplasia, or cardiovascular or cerebrovascular disorders
Biological Target
Mitogen-activated protein kinase-activated protein kinase 2 (MK2)
Summary
Recent advances in biological research have led to better understanding of both structures and functions of enzymes and other biomolecules associated with diseases and disorders. This understanding has greatly aided the search for and identification of new effective therapeutic agents with better pharmaceutical profiles. An important class of enzymes that has benefited from these advances is protein kinases.
Protein kinases constitute a large family of structurally related enzymes that act as master regulators responsible for the control of cellular growth and proliferation. Scientists believe that protein kinases may have evolved from a common ancestral gene based on the highly conserved structures and catalytic functions. For example, almost all protein kinases contain similar 250–300 amino acid catalytic domains. These catalytic domains share a key ability to reversibly activate and deactivate protein kinases through reversible modifications of their conformations via phosphorylation/dephosphorylation processes. Thus, a protein kinase can alternate between active phosphorylated conformation and inactive unphosphorylated conformation. While the active conformations of all kinases are remarkably structurally similar, this is not necessarily the case with the inactive conformations. In turn, activated protein kinases phosphorylate and activate particular target proteins in response to specific metabolic signals to amplify these signals into cellular growth cascades. Protein kinases may be categorized into smaller families based on the substrates they phosphorylate (e.g., protein-tyrosine kinases, protein-serine/threonine kinases, lipid kinases, etc.).
P38 mitogen-activated protein kinases (P38 MAPKs) constitute a family of mitogen-activated protein kinases (MAPKs). The members of this family respond to stress stimuli, such as cytokines, ultraviolet irradiation, heat shock, and osmotic shock, and are involved in cell differentiation, apoptosis and autophagy. Scientists have identified four members of the p38 MAPK family including p38-α (MAPK14), p38-β (MAPK11), p38-γ (MAPK12/ERK6), and p38-δ (MAPK13/SAPK4).
The p38 MAPK signaling plays a key role in the regulation and expression of the inflammatory cytokine tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), interleukin 6 (IL-6), and other inflammatory signals. For a long time, p38 MAPK signaling has been a therapeutic target for the treatment of inflammatory diseases. However, many inhibitors of p38 MAPK did not produce the promised effective treatment for diseases such as rheumatoid arthritis (RA) in clinical studies.
Mitogen-activated protein kinase-activated protein kinase 2 (MK2) is a serine-threonine kinase encoded in humans by the MAPKAPK2 gene. MK2 is the key downstream substrate of p38 MAPK that mediates several of the p38 MAPK-dependent cellular responses. It is also an essential intracellular regulator of the production of the cytokines, TNF-α, IL-6 and interferon gamma (IFNγ), which are involved in many acute and chronic inflammatory diseases such as RA and inflammatory bowel disease. MK2 resides in the nucleus of nonstimulated cells. Upon stimulation, it moves to the cytoplasm then phosphorylates and activates tuberin and heat shock protein 27 (HSP27). MK2 has been implicated in heart failure, brain ischemic injury, the regulation of stress resistance and the production of TNF-α.
The inhibition of MK2 may lead to a significant reduction in the level of several proinflammatory cytokine production. The inhibitors of MK2 may also provide an attractive alternative therapeutic approach to blocking the p38 MAPK signaling pathway with similar efficacy to the p38 inhibitors while producing fewer toxicity and side effects.
The compounds of formula 1 in this patent application are inhibitors of MK2. They can potentially be useful as treatment for a variety of MK2-mediated diseases or disorders including autoimmune disorders, chronic inflammatory disorders, acute inflammatory disorders, autoinflammatory disorders, fibrotic disorders, metabolic disorders, neoplasias, or cardiovascular or cerebrovascular disorders. The inventors have included 69 embodiments describing structural details of compounds as well as long lists of potential examples of diseases and disorders representing each of the aforementioned therapeutic classes.
Key Structures
The inventors reported the synthesis
procedures and structures of 274 examples of formula 1, including the following representative examples: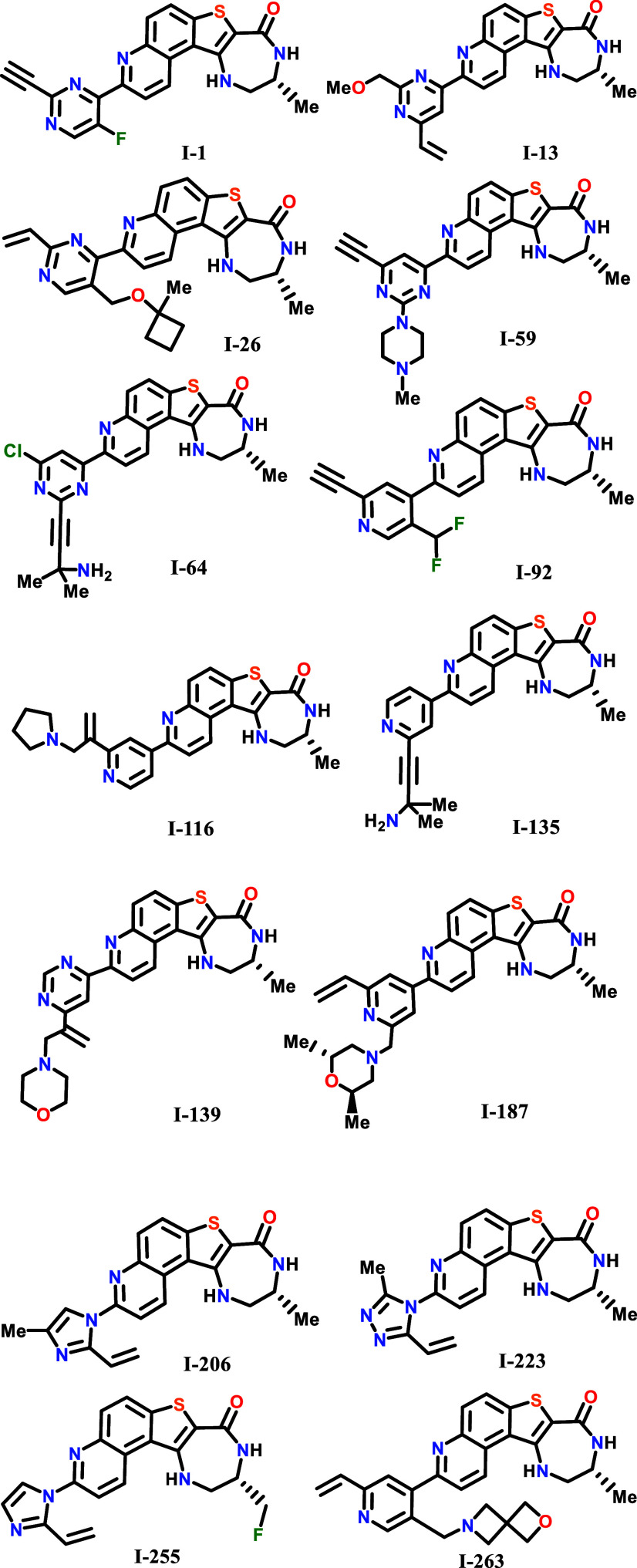
Biological Assay
The in vitro Mitogen-Activated Protein Kinase-Activated Protein Kinase 2 Omnia Assay for Compound Potency Assessment was used to measure the biological activity of the compounds of formula 1 as selective inhibitors of MK2.
Biological Data
The data obtained from testing the
representative examples using the above assay are listed in the following
table: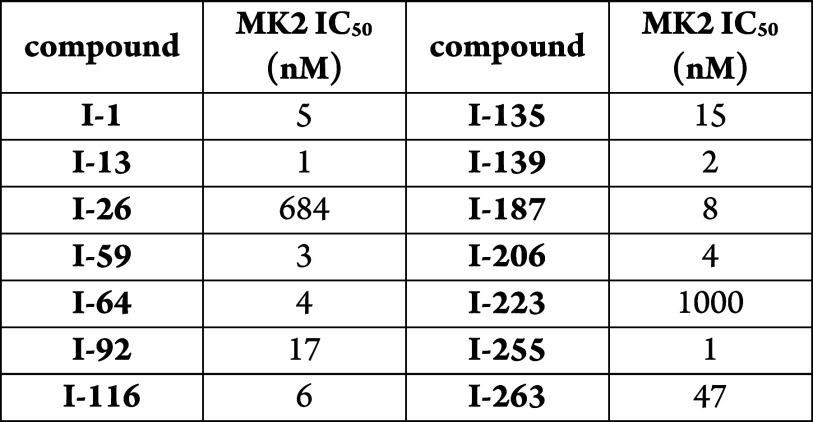
Recent Review Articles
The author declares no competing financial interest.
References
- Alimbetov D.; Umbayev B.; Tsoy A.; Begimbetova D.; Davis T.; Kipling D.; Askarova Sh. Small molecule targeting of the p38/Mk2 stress signaling pathways to improve cancer treatment. BMC Cancer 2023, 23 (1), 895. 10.1186/s12885-023-11319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D.; Kivitz A.; Singhal A.; Burt D.; Bangs M. C.; Huff E. E.; Hope H. R.; Monahan J. B. Selective Inhibition of the MK2 Pathway: Data from a Phase IIa Randomized Clinical Trial in Rheumatoid Arthritis. ACR Open Rheumatology 2023, 5 (2), 63–70. 10.1002/acr2.11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canovas B.; Nebreda A. R. Diversity and versatility of p38 kinase signalling in health and disease. Nat. Rev. Mol. Cell. Biol. 2021, 22, 346–366. 10.1038/s41580-020-00322-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni S.; Anand P.; Padwad Y. S. MAPKAPK2: the master regulator of RNA-binding proteins modulates transcript stability and tumor progression. J. Exp. Clin. Cancer Res. 2019, 38 (2019), 121. 10.1186/s13046-019-1115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. K.; Najmi A. Novel Therapeutic Potential of Mitogen-Activated Protein Kinase Activated Protein Kinase 2 (MK2) in Chronic Airway Inflammatory Disorders. Curr. Drug Targets 2019, 20 (4), 367–379. 10.2174/1389450119666180816121323. [DOI] [PubMed] [Google Scholar]




