Abstract
Objectives
To evaluate whether embryo transfers at blastocyst stage improve the cumulative live birth rate after oocyte retrieval, including both fresh and frozen-thawed transfers, and whether the risk of obstetric and perinatal complications is increased compared with cleavage stage embryo transfers during in vitro fertilisation (IVF) treatment.
Design
Multicentre randomised controlled trial.
Setting
21 hospitals and clinics in the Netherlands, 18 August 2018 to 17 December 2021.
Participants
1202 women with at least four embryos available on day 2 after oocyte retrieval were randomly assigned to either blastocyst stage embryo transfer (n=603) or cleavage stage embryo transfer (n=599).
Interventions
In the blastocyst group and cleavage group, embryo transfers were performed on day 5 and day 3, respectively, after oocyte retrieval, followed by cryopreservation of surplus embryos. Analysis was on an intention-to-treat basis, with secondary analyses as per protocol.
Main outcome measures
The primary outcome was the cumulative live birth rate per oocyte retrieval, including results of all frozen-thawed embryo transfers within a year after randomisation. Secondary outcomes included cumulative rates of pregnancy, pregnancy loss, and live birth after fresh embryo transfer, number of embryo transfers needed, number of frozen embryos, and obstetric and perinatal outcomes.
Results
The cumulative live birth rate did not differ between the blastocyst group and cleavage group (58.9% (355 of 603) v 58.4% (350 of 599; risk ratio 1.01, 95% confidence interval (CI) 0.84 to 1.22). The blastocyst group showed a higher live birth rate after fresh embryo transfer (1.26, 1.00 to 1.58), lower cumulative pregnancy loss rate (0.68, 0.51 to 0.89), and lower mean number of embryo transfers needed to result in a live birth (1.55 v 1.82; P<0.001). The incidence of moderate preterm birth (32 to <37 weeks) in singletons was higher in the blastocyst group (1.87, 1.05 to 3.34).
Conclusion
Blastocyst stage embryo transfers resulted in a similar cumulative live birth rate to cleavage stage embryo transfers in women with at least four embryos available during IVF treatment.
Trial registration
International Clinical Trial Registry Platform NTR7034.
Introduction
In vitro fertilisation (IVF) is a successful treatment for infertility, with more than 10 million children born after assisted reproductive technology since 1978.1 Currently around one in 30 children in the US and Europe are born after assisted reproductive technology.2 3 The timing of embryo transfer is an important part of IVF treatment. Traditionally during IVF treatment or intracytoplasmic sperm injection (ICSI), embryos were transferred on day 3 after oocyte retrieval, aligning with the cleavage stage of embryo development. After improvements to in vitro culture conditions and embryo cryopreservation techniques, however, the standard practice has changed towards transferring embryos at the blastocyst stage of embryo development, usually on day 5 or day 6 after oocyte retrieval.4 5 6 7 8
The rationale for this change in practice was that as only viable embryos are thought to be able to reach the blastocyst stage in vitro, the selection of embryos for transfer would be enhanced.4 9 Additionally, this timing aligns more closely to the so called implantation window, when the endometrium is more receptive to embryos.4 5 6 7 Theoretically, therefore, a higher treatment efficacy is expected from the transfer of embryos at the blastocyst stage in terms of live birth rate for each (fresh) transfer, risk of pregnancy loss, number of embryos transfers needed, and time to conception leading to live birth. Furthermore, embryo transfers at the blastocyst stage have been proved to improve the live birth rate for each fresh transfer in women with a good prognosis.4 8 Because of those mainly theoretical advantages of the blastocyst stage, worldwide many IVF clinics rapidly changed from using cleavage stage transfer to blastocyst stage transfer without proven effectiveness and safety studies.4 5 6 7 The outcome of a live birth rate for each fresh embryo transfer does not, however, encompass the available supernumerary embryos in IVF, which are frozen for potential future transfers. Transfers and cryopreservation at the cleavage stage generally yield a higher number of embryos for future frozen-thawed embryo transfers, as some embryos will show arrested development during the cleavage to blastocyst stage in vitro. A longer period exposed to an in vitro culture condition could be detrimental compared with exposure to the uterine environment, which would favour cleavage stage embryo transfer.4 5 6 7
Nevertheless, it remains uncertain which of the two embryo transfers is superior in terms of cumulative live birth rate, encompassing live births from both fresh and frozen-thawed embryo transfers.4 5 6 7 From the patients’ perspective, the cumulative live birth rate is considered the most important outcome, as it summarises the success rate over an entire course of one IVF treatment cycle.10 11 12 13 Similarly, systematic reviews have concluded that high quality evidence on cumulative pregnancy results is currently insufficient and that well designed randomised controlled trials are needed to assess clinical value.4 5 In light of this, we conducted a multicentre, randomised, superiority controlled trial comparing cumulative live birth rates after IVF or ICSI with blastocyst or cleavage stage embryo transfers in women with a minimum of four embryos available on the day 2 after oocyte retrieval.
Methods
Study design
The Three or Five (ToF) study was designed as a multicentre randomised controlled trial at 21 Dutch hospitals and clinics, with laboratory procedures performed in 11 affiliated IVF laboratories. Trial coordination, study design, data management, and statistical analysis were conducted at Radboud University Medical Centre and Amsterdam UMC. The Netherlands Society of Obstetrics and Gynaecology Consortium provided trial support and performed independent audits and data monitoring. No interim analysis was performed. Details on rationale and design of the trial have been reported previously.14
Participants
Women aged 18-43 years, scheduled for their first, second, or third IVF or ICSI oocyte retrieval cycle were eligible for participation. Each woman could only participate in one treatment cycle. To be eligible, women needed to have four or more embryos available on the second day after oocyte retrieval. The definition of an embryo in the context of the study was all zygotes with two pronuclei, one pronuclei, or no pronuclei at day 1 (observed at 16-18 hours after insemination or injection) and at least one cell division on day 2 (embryos with ≥3 pronuclei were excluded).
Exclusion criteria included preimplantation genetic testing, the use of frozen-thawed oocytes, and the use of donor oocytes. Fertility doctors counselled the women and provided them with a patient information letter during their scheduled visits. Written informed consent was obtained from all women before oocyte retrieval.
Randomisation
Women who had provided consent were randomly assigned in a 1:1 ratio using Castor, an online computerised randomisation software, on day 2 after oocyte retrieval. Randomisation was stratified based on age (<36 years v ≥36 years). A random permuted block design with block sizes of two, four, or six was used to ensure a balanced allocation of women to both groups. Participants, doctors, embryologists, and laboratory technicians could not be masked owing to the nature of the intervention. Data analysts were masked during data cleaning and preparation of syntaxes, and they were unmasked after the inclusion and treatment phases.
Procedures
A gonadotrophin releasing hormone agonist or a gonadotrophin releasing hormone antagonist protocol was used to control ovarian stimulation, and the use of ICSI was at the discretion of the local investigators. The laboratories adhered to the study protocol, but otherwise applied their own laboratory procedures. Each centre always collaborated with the same laboratory.
In the blastocyst group, fresh embryos were transferred on day 5 (not day 6) after oocyte retrieval, followed by cryopreservation of surplus embryos on day 5 or day 6 using the vitrification method. If no embryo had reached the blastocyst developmental stage, an embryo could be transferred at day 5 from a delayed stage of development, such as morula or cleavage stage. In the cleavage group, fresh embryos were transferred on day 3 after oocyte retrieval, followed by cryopreservation of surplus embryos on day 3 or day 4 using a slow freezing or vitrification method depending on local protocols. In both groups, embryo selection for fresh transfer or cryopreservation was based on embryo morphology using local criteria. For ethical reasons in six centres, if women randomised to the cleavage group had embryos that did not fulfil the local freezing criteria on day 3 or day 4, those embryos were cultured up to day 6. If these remnant embryos developed into blastocysts, cryopreservation was still performed, but the results from these frozen-thawed embryo transfers were excluded from the per protocol analysis. Importantly, available cleavage stage embryos were thawed first.
In the Netherlands, single embryo transfer is commonly performed in both fresh and frozen-thawed cycles following national guidelines and local protocols.15 Double embryo transfers are, however, allowed in women aged 38 years or older as well as all women going through a third IVF cycle. For frozen-thawed embryo transfers, endometrial preparation was carried out in either a natural cycle or an artificial cycle with exogenous oestradiol and progesterone, based on characteristics of the women and their preferences.
The follow-up period included the results of the fresh embryo transfer, all frozen-thawed embryo transfers from the initial oocyte retrieval cycle, and natural conceptions within 12 months of randomisation. Owing to the covid-19 pandemic and related restrictions, some treatments were interrupted or postponed. To compensate for delays in treatment, we extended the follow-up period by five months for women with an oocyte retrieval date between 16 March 2019 and 1 September 2020.
Outcome measures
The primary outcome was the cumulative live birth rate of pregnancies arising from fresh or frozen-thawed embryo transfers from the study cycle or natural conceptions within 12 months after randomisation. Secondary outcomes included (cumulative) pregnancy rates, pregnancy loss rate, live birth rate after fresh embryo transfer, number of embryo transfers needed to achieve a live birth, cancelled transfers, number of frozen embryos, embryo utilisation rate, time to conception leading to live birth, multiple pregnancy rate, and obstetric and perinatal outcomes.
Obstetric and perinatal outcomes of gestational age at delivery, mode of delivery, sex, and birth weight were collected from records. More detailed obstetric information on hypertensive disorders of pregnancy, gestational diabetes mellitus, and abnormal placentation were included in a questionnaire administered to participants.
Serious adverse events were reported to the ethical committee and were analysed immediately.
Statistical analysis
The trial was designed as a superiority trial to prove the presence of a two sided difference. At the time of the study design and for determination of the sample size, we expected a cumulative live birth rate of 31% for each oocyte retrieval using cleavage stage embryo transfer based on cumulative results of recorded data in all patient groups in 2015 from the Dutch IVF laboratories.16 We hypothesised that the cumulative live birth rate after blastocyst stage transfer would be more than 8 percentage points higher than after cleavage stage transfer. Owing to the absence of high quality data on cumulative results for reference, our sample size was based on earlier findings of increased live birth rates after fresh transfer.4 We determined that a sample size of 1200 women would provide 80% power at an α level of 0.05, accounting for an estimated dropout rate of 2%.
We used the χ2 test to assess differences in non-continuous variables between the two study groups and the independent t test to assess differences in continuous variables, reported as means. Subsequently, we compared the cumulative and fresh embryo transfer pregnancy outcomes between the groups using log-link binomial generalised linear models, adjusted for age stratified groups. We calculated absolute differences and risk ratios along with corresponding 95% confidence intervals (CIs). A two sided P value <0.05 indicated statistical significance. Women lost to follow-up were considered not to have had a live birth. Cox proportional hazard curves adjusted for age stratified groups were constructed for both groups to summarise time to conception until live birth.17 We present hazard rates, and for each group median time to conception leading to live birth. Statistical analysis was based on the intention-to-treat principle.
Planned and prespecified subgroup analyses were performed for two age groups (<36 years v ≥36 years) to assess whether participants’ age can be used as a treatment selection marker or has prognostic value for cumulative live birth rate and cumulative pregnancy loss, including testing for interaction. We also conducted post hoc subgroup analyses by subdivision of age groups, performance by IVF centre laboratory, only single embryo transfers, fertilisation technique, stimulation protocol, cryopreservation technique, and stage of embryo development. Additionally, we conducted a per protocol analysis adjusted for age stratified groups using log-link binomial generalised linear models. The per protocol analysis excluded cycles that deviated from the protocol for any instances or cycles involving the use of frozen-thawed embryos on day 5 or day 6 in the cleavage group. Statistical analysis was performed with IBM SPSS statistics (version 28).
Patient and public involvement
Patient representatives from the Dutch patient organisation Freya (www.freya.nl) were involved in the design of this research. During the preparatory stage, priority of the research question and choice of outcome measures were chosen after consultation with a focus group from Freya. The board of the Netherlands Society of Obstetrics and Gynaecology and members of the Dutch Society of Clinical Embryologists (KLEM) also pointed out the importance of this research and high priority for clinical research in IVF.
Results
Participants
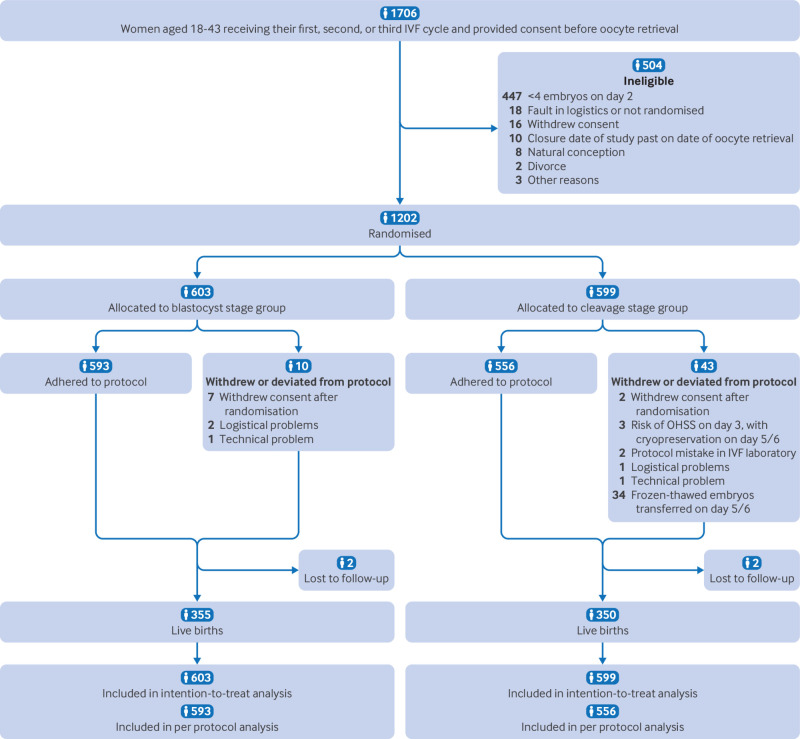
Between 18 August 2018 and 17 December 2021, a total of 1706 women provided consent and were enrolled in the trial, of whom 504 were ineligible for randomisation. Of the 1202 women who met the inclusion criteria and were eligible for randomisation, 603 (50.2%) were assigned to the blastocyst stage group and 599 (49.8%) to the cleavage stage group. Ten women (1.7%) in the blastocyst group and 43 (7.1%) in the cleavage group either withdrew from the trial or deviated from the protocol after randomisation (fig 1). Table 1 shows the baseline characteristics of the two groups. In both study arms no fresh embryo transfers were cancelled because of unsuitable embryo development.
Fig 1.
Trial profile. IVF=in vitro fertilisation; OHSS=ovarian hyperstimulation syndrome
Table 1.
Baseline characteristics of the study population, comprising women with four or more embryos available during IVF treatment. Values are number (percentage) unless stated otherwise
| Characteristics | Blastocyst stage group (n=603) | Cleavage stage group (n=599) |
|---|---|---|
| Mean (SD) age (years) | 33.9 (4.2) | 34.1 (4.2) |
| Mean (SD) BMI | 24.2 (4.4) | 24.5 (4.5) |
| Smoker | 44 (7.4) | 45 (7.6) |
| Pregnancy history: | ||
| Primary subfertility | 308 (51.2) | 333 (55.6) |
| Previous live birth | 203 (33.7) | 182 (30.4) |
| Reason for IVF or ICSI: | ||
| Male factor | 259 (43.0) | 232 (38.7) |
| Female factor | 112 (18.6) | 137 (22.9) |
| Male and female factor | 37 (6.1) | 39 (6.5) |
| Unexplained | 175 (29.1) | 155 (25.9) |
| Other | 19 (3.2) | 36 (6.0) |
| Duration of infertility (months) | 33.6 (22.3) | 33.4 (22.5) |
| Type of protocol: | ||
| GnRH antagonist | 190 (31.8) | 175 (29.3) |
| GnRH agonist | 407 (68.2) | 422 (70.7) |
| Fertilisation method: | ||
| IVF | 279 (46.3) | 271 (45.2) |
| ICSI | 316 (52.5) | 315 (52.6) |
| IVF and ICSI | 7 (1.2) | 13 (2.2) |
| No of oocyte retrievals*: | ||
| 1 | 441 (73.6) | 432 (72.4) |
| 2 | 112 (18.7) | 118 (19.8) |
| 3 | 46 (7.7) | 47 (7.9) |
| No of aspirated oocytes | 11.45 (4.9) | 11.52 (5.2) |
| Mean (SD) No of embryos on day 2 after oocyte retrieval | 7.5 (3.2) | 7.6 (3.6) |
| Type of transfer: | ||
| Fresh SET | 497 (82.4) | 494 (82.5) |
| Fresh DET | 54 (9.0) | 52 (8.8) |
| Frozen-thawed SET | 633 | 828 |
| Frozen-thawed DET | 13 | 51 |
| Fresh embryo | 52 (8.6) | 53 (8.8) |
| Risk of OHSS | 48 (8.0) | 46 (7.7) |
| Illness | 0 | 2 (0.3) |
| Technical problems | 1 (0.6) | 0 |
| Poor embryo development | 0 | 0 |
| Other reasons | 3 (0.5) | 5 (0.8) |
| Method of cryopreservation†: | ||
| Slow freeze | 2 (0.3) | 51 (8.5) |
| Vitrification | 511 (84.7) | 505 (84.3) |
BMI=body mass index; DET=double embryo transfer; GnRH=gonadotrophin releasing hormone; ICSI=intracytoplasmic sperm injection; IVF=in vitro fertilisation; OHSS=ovarian hyperstimulation syndrome; SD=standard deviation; SET=single embryo transfer.
Women could participate in their first, second, or third IVF or ICSI oocyte retrieval cycle, but they could only participate in one treatment cycle.
When possible.
Primary outcome
The intention-to-treat cumulative live birth rate did not differ between the study groups, with live births in 355 (58.9%) of 603 women in the blastocyst group and 350 (58.4%) of 599 in the cleavage group (risk ratio 1.01, 95% CI 0.84 to 1.22), corresponding to an absolute difference of 0.4 percentage points (95% CI −5.1 to−5.9) (table 2). Four women (0.7%) in the blastocyst group and 10 (1.7%) in the cleavage group had a live birth after natural conception within the follow-up period.
Table 2.
Results of the cumulative live birth rates and secondary outcomes in the intention-to-treat population. Values are number (percentage) unless stated otherwise
| Blastocyst stage group* | Cleavage stage group† | Adjusted absolute difference‡ (95% CI) | Risk ratio‡ (95% CI) | |
|---|---|---|---|---|
| Cumulative rates | ||||
| Live birth§ | 355/603 (58.9) | 350/599 (58.4) | 0.4 (−5.1 to 5.9) | 1.01 (0.84 to 1.22) |
| Ongoing pregnancy¶ | 362/603 (60.0) | 357/599 (59.6) | 0.4 (−5.1 to 5.8) | 1.01 (0.84 to 1.22) |
| Clinical pregnancy** | 378/603 (62.9) | 388/599 (64.8) | −2.1 (−7.5 to 3.3) | 0.97 (0.81 to 1.16) |
| Biochemical pregnancy†† | 430/603 (71.3) | 448/599 (74.8) | −3.5 (−8.5 to 1.4) | 0.95 (0.80 to 1.14) |
| Pregnancy loss‡‡ | 98/603 (16.3) | 145/599 (24.2) | −7.9 (−12.4 to −3.4) | 0.68 (0.51 to 0.89) |
| Cumulative rates by age group | ||||
| Live birth§: | ||||
| <36.0 years | 243/388 (62.6) | 257/383 (67.1) | −4.5 (−11.2 to 2.3) | 0.93 (0.84 to 1.04) |
| ≥36.0 years | 112/215 (52.1) | 93/216 (43.1) | 9.4 (−0.4 to 18.4) | 1.21 (0.99 to 1.48) |
| Pregnancy loss‡‡: | ||||
| <36.0 years | 64/388 (16.5) | 78/383 (20.4) | −3.9 (−9.3 to 1.6) | 0.81 (0.60 to 1.09) |
| ≥36.0 years | 34/215 (15.8) | 67/216 (31.0) | −15.2 (−23.1 to −7.3) | 0.51 (0.35 to 0.74 |
| Fresh embryo transfer rates | ||||
| Live birth§ | 223/603 (37.0) | 177/599 (29.5) | 7.4 (2.1 to 12.7) | 1.26 (1.00 to 1.58) |
| Ongoing pregnancy¶ | 227/603 (37.6) | 181/599 (30.2) | 7.4 (2.1 to 12.7) | 1.26 (1.00 to 1.58) |
| Clinical pregnancy** | 243/603 (40.3) | 199/599 (33.2) | 7.0 (1.6 to 12.5) | 1.22 (0.98 to 1.52) |
| Biochemical pregnancy†† | 284/603 (47.1) | 249/599 (41.6) | 5.5 (−0.1 to 11.1) | 1.14 (0.93 to 1.40) |
| Pregnancy loss‡‡ | 61/603 (10.1) | 72/599 (12.0) | 1.9 (−5.4 to 1.6) | 0.84 (0.59 to 1.21) |
CI=confidence interval.
Definitions for cumulative pregnancy and pregnancy loss rate were the same with the addition that at least one pregnancy or pregnancy loss occurred during the follow-up period of one year. Each woman could have multiple pregnancies as a result of a one year follow-up period.
23 women had at least two pregnancies with at least one pregnancy loss and one live birth.
47 women had at least two pregnancies with at least one pregnancy loss and one live birth.
Adjusted for age group.
Delivery resulting in live birth after 24 gestational weeks.
Viable intrauterine pregnancy with fetal heartbeat 10-12 weeks after oocyte retrieval.
Presence of at least one gestational sac 5-8 weeks after oocyte retrieval.
Positive home pregnancy test result 14-17 days after oocyte retrieval.
Pregnancy loss after a positive pregnancy test result (<21 weeks).
Secondary outcomes
The cumulative pregnancy loss rate was lower in the blastocyst group compared with cleavage group (16.3% v 24.2%; risk ratio 0.68, 95% CI 0.51 to 0.89) (table 2). The live birth rate after fresh embryo transfer was higher in the blastocyst group, with live births in 223 (37.0%) of 603 women compared with 177 (29.5%) of 599 in the cleavage group (1.26, 1.00 to 1.58) (table 2).
The mean number of embryo transfers needed to result in a live birth was lower in the blastocyst group compared with cleavage group (1.55 (0.99) v 1.82 (1.24), P<0.001; table 3).
Table 3.
Results for embryo transfer procedures, number of unused embryos, number of frozen embryos, utilisation rate in the intention-to-treat population. Values are mean (standard deviation) unless stated otherwise
| Blastocyst stage group (n=603) | Cleavage stage group (n=599) | Mean difference (95% CI) | P value | |
|---|---|---|---|---|
| Embryo transfer procedures: | ||||
| Overall | 1.99 (1.38) | 2.37 (1.64) | −0.39 (−0.56 to −0.21) | <0.001 |
| Women with live birth | 1.55 (0.99) | 1.82 (1.24) | −0.27 (−0.43 to −0.10) | <0.001 |
| Women with no live birth | 2.60 (1.60) | 3.15 (1.81) | 0.54 (−0.84 to −0.24) | <0.001 |
| No of embryos transferred: | ||||
| Overall | 2.09 (1.40) | 2.54 (1.78) | −0.45 (−0.63 to −0.27) | <0.001 |
| Women with live birth | 1.62 (1.00) | 1.90 (1.30) | −0.28 (−0.45 to −0.11) | 0.002 |
| Women with no live birth | 2.76 (1.61) | 3.45 (1.96) | −0.68 (−1.00 to −0.36) | <0.001 |
| Unused embryos after 12 months: | ||||
| Overall | 2.06 (2.43) | 2.71 (3.12) | −0.65 (−0.97 to −0.33) | <0.001 |
| Women with live birth | 3.12 (2.35) | 4.10 (3.14) | −0.98 (−1.39 to −0.57) | <0.001 |
| Women with no live birth | 0.54 (1.62) | 0.76 (1.75) | −0.21 (−0.51 to 0.09) | 0.165 |
| Total No (%) of women | 354 (58.7) | 384 (64.2) | −0.05 (−0.11 to 0.001) | 0.05 |
| Women with live birth | 312 (51.7) | 325 (54.3) | −0.05 (−0.09 to −0.006) | 0.03 |
| Women with no live birth | 42 (7.0) | 59 (9.9) | −0.07 (−0.14 to 0.003) | 0.06 |
| Mean (SD) total No of frozen embryos | 3.21 (2.55) | 4.39 (3.30) | −1.18 (−1.51 to −0.85) | <0.001 |
| Embryo utilisation rate*: | ||||
| Overall | 2535/4590 (55.3) | 3226/4543 (71.0) | −0.14 (−0.17 to −0.12) | <0.001 |
| Women with live birth | 1689/2881 (58.6) | 2109/2793 (75.5) | −0.14 (−0.17 to −0.11) | <0.001 |
| Women with no live birth | 846/1709 (49.5) | 1117/1750 (63.9) | −0.14 (−0.17 to −0.12) | <0.001 |
CI=confidence interval.
Calculated by dividing number of fresh transferred embryos+number of cryopreserved embryos by number of embryos available on day 2 after oocyte retrieval.
The time to conception leading to a live birth at any given point within the 12 months’ follow-up period was comparable between the two groups (hazard ratio 1.06, 95% CI 0.92 to 1.23; P=0.35). The median time from oocyte retrieval to conception leading to a live birth was 3.1 months (95% CI 1.9 to 4.2) in the blastocyst group and 4.7 months (3.6 to 5.8) in the cleavage group (see supplementary figure 1).
The incidence of moderate preterm birth (32 to <37 weeks) in singletons was higher in the blastocyst group, with an incidence of 8.9% compared with 4.7% in the cleavage group (risk ratio 1.87, 95% CI 1.05 to 3.34). The obstetric and perinatal outcomes of birth weight, gestational age at delivery, and small or large for gestational age were similar between the two groups (table 4).
Table 4.
Obstetric and perinatal outcomes in the intention-to-treat population. Values are number (percentage) unless stated otherwise
| Blastocyst stage group (n=603) | Cleavage stage group (n=599) | Absolute difference (95% CI) | Risk ratio (95% CI) | |
|---|---|---|---|---|
| Cumulative results | ||||
| Induced abortion | 2 (0.3) | 4 (0.7) | −0.3 (−1.1 to 0.5) | 0.49 (0.09 to 2.20) |
| Stillbirth or intrauterine death | 0 | 1 (0.2) | NA | NA |
| Neonatal death | 0 | 0 | NA | NA |
| Perinatal death | 0 | 0 | NA | NA |
| Live births | n=355 | n=350 | ||
| Mean (SD) gestational age at delivery (weeks)* | 38.3 (2.8) | 38.7 (2.7) | −0.4 (−15.4 to 14.6) | NA |
| Mean (SD) birth weight (g)* | 3359 (596) | 3382 (571) | −23.2 (−11.2 to 65.1) | NA |
| Small for gestational age (<10 centile)* | 28 (8.0) | 31 (9.2) | −1.2 (−5.4 to 3.1) | 0.87 (0.54 to 1.43) |
| Large for gestational age (>90 centile)* | 28 (8.0) | 30 (8.8) | −0.9 (−5.0 to 3.3) | 0.90 (0.56 to 1.48) |
| Low birth weight (<2500 g)* | 26 (7.5) | 21 (6.2) | 1.2 (−2.5 to 5.0) | 1.20 (0.69 to 2.09) |
| Very low birthweight (<1500 g)* | 3 (0.9) | 3 (0.9) | −0.0 (−1.4 to 1.4) | 0.97 (0.20 to 4.76) |
| Preterm birth (gestation)*: | ||||
| <32 weeks | 3 (0.9) | 4 (1.2) | −0.3 (−1.8 to 1.2) | 0.73 (0.16 to 3.23) |
| 32-<37 weeks | 31 (8.9) | 16 (4.7) | 4.2 (0.5 to 7.9) | 1.87 (1.05 to 3.34) |
| Girls/boys*† | 165/181 (47.7) | 170/167 (50.4) | −2.8 (−10.3 to 4.7) | 0.94 (0.81 to 1.10) |
| Caesarean delivery* | 84 (24.1) | 81 (24.0) | 0.1 (−6.3 to 6.5) | 1.00 (0.77 to 1.31) |
| No of major congenital anomalies | 4 (1.1) | 4 (1.2) | −0.0 (−1.6 to 1.5) | 0.97 (0.25 to 3.87) |
| Twin deliveries | ||||
| Overall | 7 (2.0) | 9 (2.6) | −0.6 (−2.8 to 1.6) | 0.75 (0.29 to 2.01) |
| From SET‡ | 1 (0.3) | 2 (0.6) | −0.3 (−1.3 to 0.7) | 0.49 (0.04 to 5.37) |
| From DET | 6 (1.7) | 7 (2.0) | −0.3 (−2.3 to 1.7) | 0.83 (0.28 to 2.46) |
| From natural conception | 0 | 0 | NA | NA |
CI=confidence interval; DET=double embryo transfer; NA=not applicable; SD=standard deviation; SET=single embryo transfer.
Singleton pregnancies.
Data were missing for sex in two liveborn babies in blastocyst stage group.
One intrauterine death occurred at 21 weeks.
Detailed obstetric information, particularly on hypertensive disorders of pregnancy, gestational diabetes mellitus, and abnormal placentation, was collected from administered questionnaires. Some responses were, however, incomplete. Owing to concerns about data accuracy and completeness, we did not analyse these data.
Subgroup and per protocol analyses
In a planned and prespecified subgroup analysis of pregnancy rates by age groups of the women, no interaction was found between age group and treatment group (P=0.20). In women aged 36 years and older (n=431), the cumulative live birth rate was non-significantly higher in the blastocyst group compared with cleavage group (52.1% (112 of 215) v 43.1% (93 of 216); risk ratio 1.21, 95% CI 0.99 to 1.48). In women aged <36 years (n=771), the cumulative live birth rate was lower in the blastocyst group (62.6% (243 of 388) v 67.1% (257 of 383; 0.93, 0.84 to 1.04) but did not reach a significant difference (table 2, also see supplementary figure 2). Supplementary figure 3 shows the results of a post hoc analysis for investigation of other age categories (<30 years, ≥30-35 years, 36-37 years, and ≥38 years).
The results of the per protocol analysis were generally consistent with those of the intention-to-treat analysis (supplementary table 1). In 34 women in the cleavage group, an embryo was frozen-thawed on day 5 or day 6, which led to four additional live births.
The results of the post hoc subgroup analysis on stimulation method, fertilisation technique, only single embryo transfers, embryo development stage, cryopreservation technique, and laboratory were also consistent with those of the intention-to-treat analysis (supplementary table 2). Six live births occurred in 45 women in the blastocyst group, despite the transferred embryos having not reached the blastocyst stage at day 5 of culture.
Owing to the covid-19 pandemic, an extended follow-up period was applied to 32 women (13 (2.2%) women in the blastocyst group and 19 (3.2%) in the cleavage group. During this extended follow-up period, three conceptions led to live births: one in the blastocyst group and two in the cleavage group.
Adverse events
Three adverse events were reported, none of which were deemed to be related to study procedures.
Discussion
In this randomised controlled trial involving 1202 women undergoing IVF treatment with a good prognosis for live birth, we found no difference in cumulative live birth rate with embryo transfers at the blastocyst stage or cleavage stage.
Comparison with other studies
A 2022 Cochrane review of five randomised controlled trials (632 women) reporting on cumulative live birth rates in relation to day of embryo transfer in IVF concluded that the evidence in favour of the blastocyst stage compared with cleavage stage was uncertain.4 We found no difference in outcomes in women with four or more embryos available during IVF treatment.
Our trial reported on the cumulative rate of pregnancy loss, which was lower in the blastocyst group. The results suggest that extended culture to blastocyst stage might benefit the selection of embryos with higher implantation potential and continuation of the pregnancy through the first trimester. These outcomes could possibly be linked to the chromosomal ploidy status of embryos, as the prevalence of aneuploid (abnormal number of chromosomes) embryos is higher at the cleavage stage.18 Aneuploid embryos have been regarded as the main reason for implantation failure, pregnancy loss, and recurrent miscarriages.19 The risk of pregnancy loss serves as a secondary outcome, for which our study was not specifically designed, thus requiring careful interpretation.
In our study, we observed a higher rate of moderate preterm birth (32 to <37 weeks) in singleton pregnancies in the blastocyst group, whereas studies have not reported an increased risk of preterm birth.20 21 However, our findings align with two recent cohort studies and two systematic reviews that reported an increased risk of preterm birth with extended culture to the blastocyst stage.22 23 24 25 This could be explained by the effect of suboptimal conditions of extended in vitro culture compared with in vivo culture for implantation and placentation.22 The outcome of preterm birth (<37 weeks’ gestation) was divided into two categories: very preterm birth (<32 weeks) and moderate preterm birth (32 to <37 weeks), because of the significantly higher risks of mortality and morbidity associated with very preterm birth.26 We found no significant differences in the risk of very preterm birth. Other suggested differences in obstetric and perinatal outcomes reported in the literature, such as a higher male to female ratio,20 21 large for gestational age,24 25 27 and monozygotic twins after single embryo transfers,22 28 did not differ between the groups in our study. However, the number of live births with complications in our study was small compared with the numbers in cohort studies and reviews. Additionally, these obstetric and perinatal results were secondary outcomes for which our study was not explicitly designed, necessitating prudent interpretation. As a policy for embryo transfer at blastocyst stage is standard in many IVF centres, future research should focus on differences in obstetric and perinatal outcomes between blastocyst and cleavage stages.
A previous smaller randomised controlled trial suggested that maternal age influences the cumulative ongoing pregnancy rate with embryo transfer during the blastocyst stage versus cleavage stage.29 Our findings suggest a similar, although non-significant, effect of age favouring embryo transfer during the cleavage stage in younger women and during the blastocyst stage in older women. A possible explanation might be that among woman of advanced maternal age with four or more embryos, a blastocyst stage policy offers a clearer advantage from selection of viable embryos by extended in vitro culture. Maternal ageing is known to result in reduced oocyte quality and embryo competence, as shown by increased oxidative damage, mitochondrial dysfunction, and number of age related chromosomal aneuploidies.30 31 Careful interpretation is necessary, however, as these results are subgroup findings and require validation in future trials.
Strengths and limitations of this study
Our trial has some limitations. We only included women with a minimum of four embryos available on day 2 after oocyte retrieval, limiting generalisability to women with fewer than four embryos. When designing the study, we were concerned that women and centres might hesitate to participate in the trial as the likelihood that none of the embryos would develop to blastocyst stage increases when fewer embryos are present. However, it may be justified to invite women to participate in such a trial now.32 33
During the design of the study, we expected a cumulative live birth rate of 31% in the cleavage group but found a rate of 58% in both groups. This discrepancy can be explained by the group selection (good prognosis with at least four embryos) and improvement of cryopreservation methods. European data from 1997 to 2019 suggested a higher live birth rate up to 35% after frozen-thawed embryo transfer.34 The higher-than-expected cumulative live birth rates slightly decrease the power of the results—consequently uncertainties remain around the risk estimates. With an absolute difference of 0.4 percentage points (95% CI −5.1 to 5.9) in our study, clinically relevant differences are unlikely but small differences cannot be ruled out.
To ensure the generalisability of our findings, we applied broad inclusion criteria and enrolled women from multiple centres. This study encompassed various IVF protocols on ovarian stimulation, embryo culture, endometrial preparation, and embryo cryopreservation methods, reflecting local protocols of participating centres. The results of post hoc analyses were consistent with those of the intention-to-treat analysis, suggesting findings are broadly generalisable. Noticeably, the study was not powered for these specific subgroup analyses, therefore a larger sample size would be needed to prove our findings in specific subgroups. Despite this, the variation in protocols should be considered a strength of our study as it represents the variations in daily clinical IVF practice. Furthermore, the difference in outcome between the 11 IVF laboratories showed no interaction between laboratories and cumulative live birth rate, indicating similar performance of the interventions under different laboratory circumstances.
Our study was designed as a superiority trial. As a protocol for embryo transfer at blastocyst stage is often regarded as a selection-deselection tool, it has been argued that the cumulative live birth rate could theoretically never be higher than that from using a cleavage stage transfer protocol, suggesting that a non-inferiority design should have been considered. However, the timing of a blastocyst stage transfer aligns more closely with the so called implantation window, which suggests the cumulative live birth rate could potentially be higher.4 5 6 7 A superiority trial allows for a more straightforward interpretation of whether the intervention under evaluation offers a meaningful improvement in clinical practice.
Policy implications
In assisted reproduction, many innovations and techniques are introduced into routine clinical practice with little or no evidence of safety and efficacy.35 Worldwide, IVF laboratories anticipated improving the efficacy of treatments by prolonging embryo culture to the blastocyst stage. Our results suggest that women and healthcare professionals should consider various other factors alongside live birth rates. The chance of a live birth should be individually balanced against outcomes such as pregnancy loss; risk of preterm birth; emotional and psychological burden, including the burden of time to pregnancy and implantation failure; and financial implications. A cost effectiveness analysis is currently being performed on our data.
Conclusions
This study found that an IVF protocol for transfer of embryos at the blastocyst stage in women with four or more embryos results in cumulative live birth rates comparable to that of embryo transfer at the cleavage stage.
Blastocyst stage embryo transfer was also associated with higher efficiency for secondary outcomes: a lower risk of pregnancy loss, higher live birth rate per fresh transfer, and reduced number of embryo transfers until live birth. A blastocyst stage transfer, however, raises concerns about the safety of preterm birth. Careful interpretation of the secondary outcomes is necessary, since these results are subgroup findings for which the study was not powered. They require validation in future trials.
What is already known on this topic
In vitro fertilisation (IVF) centres expected better treatment efficacy by extending embryo culture to the blastocyst stage, assuming only viable embryos reach this stage
Focus has been on higher live birth rates after the first embryo transfer, with blastocyst transfers showing better outcomes than cleavage stage transfers
The cumulative live birth rate, including both fresh and frozen-thawed embryo transfers, is considered most relevant but is missing in studies
What this study adds
This study found no difference in cumulative live birth rate between blastocyst and cleavage stage transfers in women with at least four embryos
Blastocyst transfers may reduce pregnancy loss and require fewer embryo transfers, but they might increase moderate preterm births (32 to <37 weeks)
Acknowledgments
We thank the following people who participated in project management, data collection, and site monitoring: S Gommans, M Peters-Knuiman, J Terken, A Schulten, and G Zijderveld (Department of Obstetrics and Gynaecology, Radboud university medical centre, Nijmegen); M de Reus (Department of Obstetrics and Gynaecology, University Medical Centre Utrecht, Utrecht); E van der Vaart and Nij Barrahûs (Centre for Fertility, Wolvega); E de Vaan and H Grens (Jeroen Bosch Ziekenhuis, 's-Hertogenbosch); H Hulsebos (Isala Fertility Centre, Zwolle); J Gerards (Diakonessenhuis, Utrecht); R Hiltermann-Tilanus (University Medical Centre Groningen, Groningen); B Kruitbosch-Groen (NoordWest Ziekenhuisgroep, Alkmaar); I van Hooff (Maxima Medical Centre, Veldhoven/Eindhoven); A Ritman (Gelre Ziekenhuizen, Apeldoorn); I Martens (Amphia Ziekenhuis, Breda); A P van Haaps and F Pronk (Amsterdam UMC location Vrije Universiteit Amsterdam, IVF Centre, Amsterdam); N van Dekken (OLVG Oost); S Van Gelderen-Boele and N F Klijn (Leiden University Medical Centre, Leiden); S Saaed (Elisabeth Twee Steden Ziekenhuis, IVF Centre, Tilburg); M Karskens (Haaglanden Medical Centre, The Hague); and T de Vries (Amsterdam UMC location, University of Amsterdam, Centre for Reproductive Medicine, Amsterdam). We thank the staff members of the Dutch Consortium for Healthcare Evaluation and Research in Obstetrics and Gynaecology-Trialbureau Zorgevaluatie Nederland. We also thank the patient representatives from the Dutch national patient organisation Freya for their active input and feedback on the study protocol. Furthermore, we are grateful to all participants willing to participate in this study.
Contributors: SC was research coordinator. KF, LR, and SM were principal investigators of the study and responsible for the study design, logistics, discussion, and drafting of the manuscript. LW, JPB, CV, CK, JD, JV, JEA, ES, BA, MZ, AD, JBV, MH, MC, HV, MTH, YW, PM, and QP were local primary investigators. SC was responsible for seeking ethical approval, overall logistics, data analysis, and writing of the manuscript. MvW was the study methodologist and responsible for study design, discussion, statistical power calculations, and data analysis. SC and MvW did the data analysis, and data were interpreted by all authors. SC, KF, DB, MvW, LR, and SM had access to and verified the underlying study data. The executive writing committee (SC, KF, DB, MvW, LR, and SM) was responsible for the decision to submit the manuscript. All authors contributed to the execution of the study, review of the text, and final approval of the manuscript. The last authors, LR and SM, contributed equally. The authors are fully responsible for the content of this manuscript, and the views and opinions described in the publication reflect solely those of the authors. SC, LR, and SM are the guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Web extra.
Extra material supplied by authors
Supplementary information: Additional figures 1-3 and tables 1-3
Funding: The trial received a grant from the Leading the Change programme from Stichting Zorgevaluatie Nederland (project No 80-87600-98-19501), a Dutch foundation that consists of the Dutch Medical Specialists Federation, Dutch Health Insurers, and Dutch Patient Federation. Before receiving this grant, the Netherlands Organisation for Health Research and Development (ZonMw) externally peer reviewed the study protocol. The funder of the study had no role in study design; data collection, analysis, or interpretation; or writing of the report.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from Stichting Zorgevaluatie Nederland; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Transparency: SC, KF, DB, MvW, LR, and SM affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned have been explained.
Dissemination to participants and related patient and public communities: A plain language summary of the results of the study will be made available to research participants on the trial website. This summary will be assessed by the Dutch patient organisation FREYA and also distributed among its members and through its website. The results will be internally disseminated through the media departments and websites of the authors’ institutes. We plan on reaching a wider audience through presentations at international conferences, press releases, and social media.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
The trial protocol was approved by the Central Committee on Research involving Human Subjects (The Hague, Netherlands) on 16 June 2018 (CCMO NL 64060.000.18), and by the board of directors of each participating clinic before recruitment. The authors assure accuracy and completeness of the data and fidelity of the trial to the protocol.
Data availability statement
Restricted access to the study data can be arranged on request to the corresponding author. Written proposals will be assessed by the ToF study group. A data sharing agreement including terms and conditions for authorship and publication will need to be signed before data are available.
References
- 1.European Society of Human Reproduction and Embryology (ESHRE). ART Fact Sheet; 2022. www.eshre.eu/Press-Room/Resources/Fact-sheets. [DOI] [PMC free article] [PubMed]
- 2. Wyns C, De Geyter C, Calhaz-Jorge C, et al. European IVF Monitoring Consortium (EIM), for the European Society of Human Reproduction and Embryology (ESHRE) . ART in Europe, 2018: results generated from European registries by ESHRE. Hum Reprod Open 2022;2022:hoac022. 10.1093/hropen/hoac022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2019 assisted reproductive technology fertility clinic and national summary report. 2019. https://www.cdc.gov/art/reports/2019/fertility-clinic.html (accessed Sept 30, 2023).
- 4. Glujovsky D, Quinteiro Retamar AM, Alvarez Sedo CR, Ciapponi A, Cornelisse S, Blake D. Cleavage-stage versus blastocyst-stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev 2022;5:CD002118. 10.1002/14651858.CD002118.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martins WP, Nastri CO, Rienzi L, van der Poel SZ, Gracia C, Racowsky C. Blastocyst vs cleavage-stage embryo transfer: systematic review and meta-analysis of reproductive outcomes. Ultrasound Obstet Gynecol 2017;49:583-91. 10.1002/uog.17327. [DOI] [PubMed] [Google Scholar]
- 6. Practice Committee of the American Society for Reproductive Medicine. Practice Committee of the Society for Assisted Reproductive Technology. Electronic address: asrm@asrm.org . Blastocyst culture and transfer in clinically assisted reproduction: a committee opinion. Fertil Steril 2018;110:1246-52. 10.1016/j.fertnstert.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 7. Glujovsky D, Farquhar C. Cleavage-stage or blastocyst transfer: what are the benefits and harms? Fertil Steril 2016;106:244-50. 10.1016/j.fertnstert.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 8. Papanikolaou EG, Camus M, Kolibianakis EM, Van Landuyt L, Van Steirteghem A, Devroey P. In vitro fertilization with single blastocyst-stage versus single cleavage-stage embryos. N Engl J Med 2006;354:1139-46. 10.1056/NEJMoa053524. [DOI] [PubMed] [Google Scholar]
- 9. Gardner DK, Sakkas D. Assessment of embryo viability: the ability to select a single embryo for transfer--a review. Placenta 2003;24(Suppl B):S5-12. [DOI] [PubMed] [Google Scholar]
- 10. Wong KM, Mastenbroek S, Repping S. Cryopreservation of human embryos and its contribution to in vitro fertilization success rates. Fertil Steril 2014;102:19-26. 10.1016/j.fertnstert.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 11. Cornelisse S, Vos MS, Groenewoud H, et al. Women’s preferences concerning IVF treatment: a discrete choice experiment with particular focus on embryo transfer policy. Hum Reprod Open 2022;2022:hoac030. 10.1093/hropen/hoac030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duffy JMN, Bhattacharya S, Bhattacharya S, et al. Core Outcome Measure for Infertility Trials (COMMIT) initiative . Standardizing definitions and reporting guidelines for the infertility core outcome set: an international consensus development study. Fertil Steril 2021;115:201-12. 10.1016/j.fertnstert.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 13. Maheshwari A, McLernon D, Bhattacharya S. Cumulative live birth rate: time for a consensus? Hum Reprod 2015;30:2703-7. 10.1093/humrep/dev263. [DOI] [PubMed] [Google Scholar]
- 14. Cornelisse S, Ramos L, Arends B, et al. Comparing the cumulative live birth rate of cleavage-stage versus blastocyst-stage embryo transfers between IVF cycles: a study protocol for a multicentre randomised controlled superiority trial (the ToF trial). BMJ Open 2021;11:e042395. 10.1136/bmjopen-2020-042395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Achten NMJ, Mol BW, Dondorp WJ. Plaatsing van een enkel embryo per ivf: antwoord op elke hulpvraag? [Single embryo implantation per IVF cycle: the answer to every request for assisted reproduction?]. Ned Tijdschr Geneeskd 2017;161:D1887. [PubMed] [Google Scholar]
- 16.Stichting Landelijke Infertiliteit Registratie, Landelijk IVF cijfers 2015, IVFlandelijk2015.pdf (degynaecoloog.nl)
- 17. Aalberts J, van Wely M. How to deal with time-to-pregnancy data? Fertil Steril 2023;119:907-9. 10.1016/j.fertnstert.2023.03.017. [DOI] [PubMed] [Google Scholar]
- 18. Staessen C, Platteau P, Van Assche E, et al. Comparison of blastocyst transfer with or without preimplantation genetic diagnosis for aneuploidy screening in couples with advanced maternal age: a prospective randomized controlled trial. Hum Reprod 2004;19:2849-58. 10.1093/humrep/deh536. [DOI] [PubMed] [Google Scholar]
- 19. Cornelisse S, Zagers M, Kostova E, Fleischer K, van Wely M, Mastenbroek S. Preimplantation genetic testing for aneuploidies (abnormal number of chromosomes) in in vitro fertilisation. Cochrane Database Syst Rev 2020;9:CD005291. 10.1002/14651858.CD005291.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Siristatidis C, Papapanou M, Karageorgiou V, et al. Congenital anomaly and perinatal outcome following blastocyst- vs cleavage-stage embryo transfer: systematic review and network meta-analysis. Ultrasound Obstet Gynecol 2023;61:12-25. 10.1002/uog.26019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marconi N, Raja EA, Bhattacharya S, Maheshwari A. Perinatal outcomes in singleton live births after fresh blastocyst-stage embryo transfer: a retrospective analysis of 67 147 IVF/ICSI cycles. Hum Reprod 2019;34:1716-25. 10.1093/humrep/dez133. [DOI] [PubMed] [Google Scholar]
- 22. Spangmose AL, Ginström Ernstad E, Malchau S, et al. Obstetric and perinatal risks in 4601 singletons and 884 twins conceived after fresh blastocyst transfers: a Nordic study from the CoNARTaS group. Hum Reprod 2020;35:805-15. 10.1093/humrep/deaa032. [DOI] [PubMed] [Google Scholar]
- 23. Ginström Ernstad E, Spangmose AL, Opdahl S, et al. Perinatal and maternal outcome after vitrification of blastocysts: a Nordic study in singletons from the CoNARTaS group. Hum Reprod 2019;34:2282-9. 10.1093/humrep/dez212. [DOI] [PubMed] [Google Scholar]
- 24. Alviggi C, Conforti A, Carbone IF, Borrelli R, de Placido G, Guerriero S. Influence of cryopreservation on perinatal outcome after blastocyst- vs cleavage-stage embryo transfer: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2018;51:54-63. 10.1002/uog.18942. [DOI] [PubMed] [Google Scholar]
- 25. Marconi N, Allen CP, Bhattacharya S, Maheshwari A. Obstetric and perinatal outcomes of singleton pregnancies after blastocyst-stage embryo transfer compared with those after cleavage-stage embryo transfer: a systematic review and cumulative meta-analysis. Hum Reprod Update 2022;28:255-81. 10.1093/humupd/dmab042 [DOI] [PubMed] [Google Scholar]
- 26. Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 2019;7:e37-46. 10.1016/S2214-109X(18)30451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. De Vos A, Dos Santos-Ribeiro S, Tournaye H, Verheyen G. Birthweight of singletons born after blastocyst-stage or cleavage-stage transfer: analysis of a data set from three randomized controlled trials. J Assist Reprod Genet 2020;37:127-32. 10.1007/s10815-019-01641-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ikemoto Y, Kuroda K, Ochiai A, et al. Prevalence and risk factors of zygotic splitting after 937 848 single embryo transfer cycles. Hum Reprod 2018;33:1984-91. 10.1093/humrep/dey294. [DOI] [PubMed] [Google Scholar]
- 29. Fernández-Shaw S, Cercas R, Braña C, Villas C, Pons I. Ongoing and cumulative pregnancy rate after cleavage-stage versus blastocyst-stage embryo transfer using vitrification for cryopreservation: impact of age on the results. J Assist Reprod Genet 2015;32:177-84. 10.1007/s10815-014-0387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet 2001;2:280-91. 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- 31. Smits MAJ, Schomakers BV, van Weeghel M, et al. Human ovarian aging is characterized by oxidative damage and mitochondrial dysfunction. Hum Reprod 2023;38:2208-20. 10.1093/humrep/dead177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. De Croo I, Colman R, De Sutter P, Stoop D, Tilleman K. No difference in cumulative live birth rates between cleavage versus blastocyst transfer in patients with four or fewer zygotes: results from a retrospective study. Hum Reprod Open 2022;2022:hoac031. 10.1093/hropen/hoac031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Braakhekke M, Mol F, Mastenbroek S, Mol BW, van der Veen F. Equipoise and the RCT. Hum Reprod 2017;32:257-60. 10.1093/humrep/dew286. [DOI] [PubMed] [Google Scholar]
- 34. Smeenk J, Wyns C, De Geyter C, et al. European IVF Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE) . ART in Europe, 2019: results generated from European registries by ESHRE. Hum Reprod 2023;38:2321-38. 10.1093/humrep/dead197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mastenbroek S, de Wert G, Adashi EY. The Imperative of Responsible Innovation in Reproductive Medicine. N Engl J Med 2021;385:2096-100. 10.1056/NEJMsb2101718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: Additional figures 1-3 and tables 1-3
Data Availability Statement
Restricted access to the study data can be arranged on request to the corresponding author. Written proposals will be assessed by the ToF study group. A data sharing agreement including terms and conditions for authorship and publication will need to be signed before data are available.