Abstract
Secondary Sjogren's syndrome (sSS) is a medical condition that occurs in individuals with autoimmune diseases such as systemic lupus erythematosus (SLE) and rheumatoid arthritis. It predominantly affects females rather than males. We present a case of a 32-year-old female with a 3-year history of rheumatoid arthritis (RA) who presented to the internal medicine and rheumatology clinic with several complaints, including swelling and tenderness in her left jaw, dry mouth (xerostomia), irritated eyes (xerophthalmia), severe joint pain, and a decreased in saliva production. The blood tests demonstrate the presence of anti-SSA and anti-SSB autoantibodies and elevation of total leukocyte count (TLC), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels, indicating inflammation. A high-frequency ultrasound confirmed the diagnosis of Secondary Sjogren's syndrome grade II, specifically affecting the left parotid gland (PG).
Keywords: Sjögren's syndrome, Rheumatoid arthritis, Sonographic findings, Salivary glands
Introduction
Sjögren's Syndrome (SS) is a chronic inflammatory autoimmune disease primarily affecting exocrine glands. It is more prevalent among women in their middle age and can manifest as either primary Sjögren's Syndrome (pSS) or secondary Sjögren's Syndrome (sSS) in conjunction with other rheumatic diseases such as Rheumatoid arthritis (RA), SLE (Systemic lupus erythematosus), systemic sclerosis, polymyositis, mixed cryoglobulinemia, and polyarteritis nodosa. Among these, RA is the most common connective tissue disease associated with SS and can give rise to additional symptoms referred to as sicca symptoms, which include keratoconjunctivitis sicca (dry eyes) and xerostomia (dry mouth) [[1], [2], [3], [4]]. There is no single diagnostic test for SjD, and the clinical diagnosis is made based on compatible clinical and laboratory features, excluding other causes of dryness. Sjögren's disease (SjD) is diagnosed when there is objective evidence of ocular and oral dryness, with an underlying autoimmune basis for exocrine glandular dysfunction. The formal classification criteria set by the American College of Rheumatology/European League Against Rheumatism necessitate the presence of immunologic abnormalities, like the detection of serum anti-Sjögren's syndrome-related antigen A (SSA) antibodies or focal lymphocytic sialadenitis observed in labial salivary gland biopsies. Imaging modalities support the diagnosis of Sjögren's syndrome, in computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound (US) the manifestation of salivary glandular parenchymal abnormalities confirms the diagnosis of SjD. Salivary gland ultrasound (US) demonstrated a sensitivity of 75% and specificity of 93% for Sjögren's syndrome (SjD). However, the detection of punctate calcification in both parotid glands through CT imaging has high specificity but limited sensitivity in identifying SjD. CT imaging is not recommended as a standard diagnostic tool for SjD. Moreover, parotid scintigraphy and sialography also can identify characteristic
abnormalities in SjD, they are seldom utilized [[5], [6], [7], [8]]. Ultrasound imaging of the parotid and submandibular salivary glands might show specific features in patients with the disease and serve as a valuable tool for diagnosing or monitoring the condition over time. In this case, the sSS was diagnosed by high-frequency ultrasound which demonstrates heterogeneous left PG with multiple hypoechoic areas and hyperechoic lymphocytic infiltration, the rest of the salivary glands were normal. This case report elucidates the role of high-frequency US in the confirmation and grading of SSS in RA patients who complain of xerostomia, xerophthalmia, and increasing joint pain.
Case presentation
A 32-year-old female with a rheumatoid arthritis history presented to the internal medicine and rheumatology clinic with complaints of left tenderness, jaw swelling, dry mouth (xerostomia), irritated eyes (xerophthalmia), and increasing joint pain. She reported decreased saliva production, as evidenced by difficulties in swelling, and altered tasting ability. Anti-SSA and anti-SSB autoantibodies were positive. The patient's total leukocytosis count (TLC) erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were elevated, indicating active inflammation (Table 1).
Table 1.
Lab investigations.
| Parameters | Results | Reference values |
|---|---|---|
| Hemoglobin | 13.6 g/dL | 13-17 g/dL |
| Platelets | 512 | 250–400*109/L |
| WBC (White blood cell) | 11,630/µL | 4000-10,500/µL |
| Neutrophils (relative percent) | 75.2 | 40 – 70 % |
| Lymphocytes (relative percent) | 21.2 | 20 – 40 % |
| Monocytes (relative percent) | 1.4 | 2 – 8 % |
| ESR | 55 mm/hr | Male <50 years: <15 mm/hr |
| CRP | Positive |
Since there was no sialography, magnetic resonance, or salivary scintigraphy available to further evaluate the patient's symptoms with suspected (sSS). US examination of the major salivary glands and thyroid was performed using a high-frequency linear transducer (10-15 MHz) (Table 2) and (Fig. 1). US evaluation of the salivary gland based on normal salivary gland criteria developed by Jousse-Joulins [9]. When compared to nearby muscles, the normal PG has a uniform echo texture and a distinct appearance between the gland and the surrounding tissue. While the normal SMG (submandibular gland) appears uniform in echotexture (compared with adjacent muscles), with a clear demarcation between the gland and the overlying tissue, the PG's echotexture is similar to that of the normal thyroid parenchyma. The granularity of SMG echotexture is typically finer than that of the parotid gland and normal thyroid parenchyma.
Table 2.
Grades of sonographic changes in the major salivary glands in secondary Sjögren's syndrome based on (OMERACT) system [10].
| Grade 0 | Normal parenchyma. |
| Grade 1 | The gland demonstrated mild inhomogeneity with no presence of anechoic or hypoechoic areas. |
| Grade II | The gland appears moderately inhomogeneous with the presence of focal anechoic or hypoechoic areas. |
| Grade III | Severely inhomogeneity with diffuse hypoechoic or anechoic areas occupying the whole gland or fibrous gland. |
Fig. 1.
(A and B) US images show normal both thyroid lobes and right PG (R.PG) display homogenous echotexture and normal echogenicity. (C) Shows inhomogeneous left PG occupying unnamable hypoechoic areas and hyperechoic lymphocytic infiltration of sSS. Look at the heterogeneity of left PG compared to the R.PG (R.TH: right thyroid lobe, L.TH: left thyroid lobe, SHM: sternohyoid muscle, and STM: sternothyroid muscle).
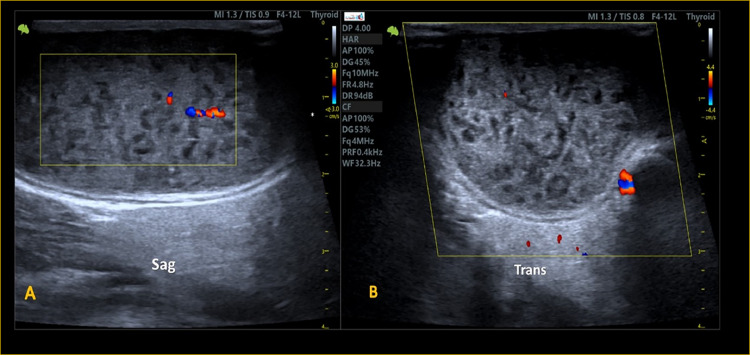
The sonographic findings revealed left PG enlargement with inhomogeneous echogenicity, predominantly hyperechoic infiltrated with innumerable hypoechoic areas (honeycomb's appearance) and decreased vascular flow within the glandular parenchyma (Fig. 1C, Figs. 2 and 3). The other salivary glands appeared relatively spared, showing normal shape and measurements with preserved echogenicity and vascularity. These sonographic findings supported the diagnosis of sSS (mostly, Grade 2) using the application of the outcome measures in rheumatology (OMERACT) scoring system [9] applying of Ultra-high frequency ultrasonography (Table 2).
Fig. 2.
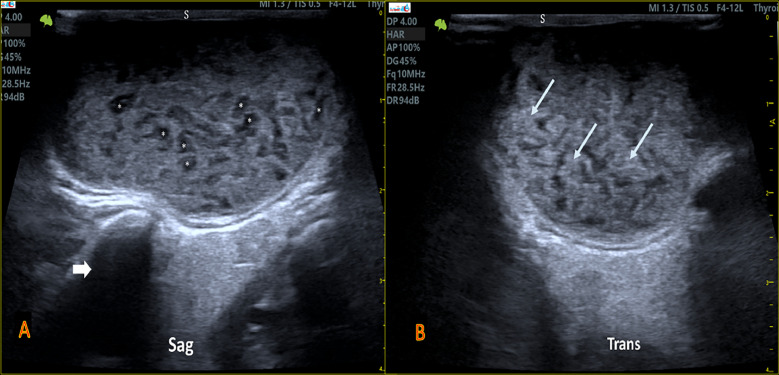
(A) US image of left SAG PG involvement of a patient with SS shows an enlarged gland that is diffusely heterogeneous in texture with a smooth outer surface and decreases in echogenicity, multiple millimetric-nodular *hypoechoic areas within for (Grade 2 of sSS). (arrow) indicted for the posterior shadowing echo from the mastoid bone. (B) Transverse image of left PG whereas the (arrows) represent the lymphocytic infiltration depositions areas within the affected parotid parenchyma. (S: skin).
Fig. 3.
US image of color Doppler shows reduced vasculature within heterogeneous parotid parenchyma of a patient with sSS.
Discussion
Sjögren's syndrome (SS) is an autoimmune condition in which the body's immune system attacks the glands responsible for producing tears and saliva. This results in reduced function of the salivary and lacrimal glands, leading to symptoms of dry mouth and dry eyes. The SS can manifest on its own as pSS, or in conjunction with other connective tissue diseases like SLE, RA, or scleroderma, in which case it is referred to as sSS. The diagnosis of SS is typically considered when individuals experience persistent daily symptoms of dry eyes and dry mouth for 3 months or longer [[11], [12], [13], [14]]. In 1993 the relationship between RA and sSS was described for the first time, the prevalence of sSS in RA is 3.8%-39.8%. [1]
An appropriate diagnosis of SS includes the following: a comprehensive physical examination, with close attention to signs of salivary gland enlargement and reduced salivary function, such as tooth decay in specific areas of the teeth, a bumpy tongue with loss of small bumps, and absence of saliva pooling under the tongue; Laboratory tests to identify any abnormalities associated with SS, including low white blood cell and platelet counts, anemia, high levels of globulins, protein or blood in the urine indicating potential kidney issues, and signs of renal tubular acidosis; and diagnostic imaging such as US or MRI to identify characteristic changes in the salivary gland tissue that support a diagnosis of SjD. Salivary gland US can reveal specific abnormalities in the gland tissue that can aid in diagnosing SjD, such as multiple areas with reduced echo, with convex borders in the affected gland [[7], [8], [9], [10], [11], [12], [13], [14], [15],16]. Hyperechoic linear bands, cysts, and calcifications may become apparent in more advanced stages of the disease. The salivary gland US results can enhance the accuracy of SS classification criteria [17]. A previous study found that salivary gland US had an 80% agreement with the 2016 ACR/EULAR classification criteria, with a sensitivity of 67 % and specificity of 94 % in diagnosis of SS [18]. Another study involving seventy-two patients with sicca syndrome showed that salivary gland US improved the sensitivity of the 2016 ACR/EULAR (American college of rheumatology/ European league against rheumatism) criteria for detecting a clinical diagnosis of SjD, with a slight decrease in specificity [19]. Salivary gland US can also replace ocular surface staining, Schirmer test, or sialometry in the ACR/EULAR (American college of rheumatology/ European league against rheumatism) classification criteria without compromising their sensitivity or specificity [20].
Abnormal salivary gland US, primarily indicated by the existence of hypoechoic foci in the glandular tissue, strongly relates to the presence of anti-Ro/SSA antibodies [19]. Nevertheless, it should not be used as a substitute for salivary gland biopsy in patients lacking these antibodies. US-guided fine-needle aspiration and core needle biopsy of the major salivary glands are important in the evaluation of solitary masses and asymmetric PG enlargement, as these findings may indicate the presence of a salivary gland lymphoma or other tumors [21,22].
MRI offers a noninvasive technique for conducting sialography on salivary glands, and it can also be beneficial in assessing patients with recurrent parotitis [[23], [24], [25]]. A CT scan of the PGs can detect variations in tissue composition, the presence of nodules, abnormal fat buildup, changes in gland size, and the existence of cysts. Diffuse punctate calcification, which is best observed through CT imaging, is a highly specific indication of SjD [26]. PG sialography involves retrograde cannulation of the major salivary gland ducts and the introduction of a nonlipid-soluble contrast medium. However, it is limited by the risk of duct rupture and is not recommended during episodes of acute parotitis [27,28]. Labial salivary gland biopsy remains a crucial diagnostic tool for suspected SS and should be obtained from an undamaged, visually normal area of the lower lip [29,30].
In our case the patient complained of left tender, jaw swelling, dry mouth (xerostomia), irritated eyes (xerophthalmia), and increasing joint pain, Anti-SSA and anti-SSB autoantibodies were positive, in Andonopoulos et al, no patient complained of xerophthalmia or xerostomia and Anti-Ro (SSA) antibodies, detected in 23.5% of the RA patients with sSS, correlated well with positive labial salivary gland biopsy [31]. Hui Zhang et al. [32] reported sSS in a 70‐year-old female with long-term rheumatoid arthritis led to heart failure, Santosh Jadhav et al. [33] described another case in a 55-year female who suffered from an inability to eat completely due to loss of teeth, along with dryness of mouth, and dryness of eyes for several years, on clinical examination both PGs enlarged. The Schirmer and Rose Bengal dye tests were positive. Serum immunoglobulin - SS-A RO is positive for Sjögren's syndrome. The RA factor tested positive for rheumatoid arthritis. US reveals bilateral submandibular and PG enlargement with several hypoechoic lesions containing very high vascularity, indicating Sjögren's syndrome. Sialography reveals scattered foci of sialectasis. In our case only 1 PG is involved despite the positive serum immunoglobulin-SS-A RO, the other salivary gland was normal.
The management of SS varies depending on the severity of symptoms and is best addressed through a team-based approach. Treatment typically involves addressing symptoms with artificial tears and salivary substitutes to alleviate discomfort and prevent complications such as conjunctivitis, and dental, and periodontal disease. Corticosteroid therapy is only recommended for cases with organ damage, severe symptoms, or significant leukopenia [33].
Conclusion
sSS occurs as a complication of several conditions such as rheumatoid arthritis, in addition to clinical and laboratory findings, high-frequency US is important to confirm the diagnosis, in the US the affected gland appears inhomogeneous in echotexture, with multiple discrete hypoechoic areas and hyperechoic lymphocytic infiltration.
Patient consent
The patient provided written informed consent for the publication of this case report and the accompanying image.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments: The authors would like to thank the patient for agreeing to publish this case report. We also express our gratitude to the medical and nursing staff involved in the patient's care. This work did not receive any particular funding from public, commercial, or not-for-profit organizations.
References
- 1.Hajiabbasi A, Shenavar Masooleh I, Alizadeh Y, Banikarimi AS, Ghavidel Parsa P. Secondary Sjogren's syndrome in 83 patients with rheumatoid arthritis. Acta Med Iran. 2016;54(7):448–453. [PubMed] [Google Scholar]
- 2.Koopman WJ, Moreland LW. Arthritis and allied conditions: a textbook of rheumatology. 15 ed. Lippincott William and Wilkins; Philadelphia: 2005. Arthritis and allied conditions: a textbook of rheumatology. [Google Scholar]
- 3.Gilboe I, Kvien T, Uhlig T, Husby G. Sicca symptoms and secondary Sjögren's syndrome in systemic lupus erythematosus: comparison with rheumatoid arthritis and correlation with disease variables. Ann Rheumat Dis. 2001;60(12):1103. doi: 10.1136/ard.60.12.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah A. In: Harrison's Principles of Internal Medicine. 18th ed. Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, editors. McGraw-Hill Professional; New York, NY: 2012. EWSC. Rheumatoid arthritis; pp. 2738–2770. [Google Scholar]
- 5.Sun Z, Zhang Z, Fu K, Zhao Y, Liu D, Ma X. Diagnostic accuracy of parotid CT for identifying Sjögren's syndrome. Eur J Radiol. 2012;81(10):2702–2709. doi: 10.1016/j.ejrad.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 6.Zhou M, Song S, Wu S, Duan T, Chen L, Ye J, et al. Diagnostic accuracy of salivary gland ultrasonography with different scoring systems in Sjögren's syndrome: a systematic review and meta-analysis. Scientific Rep. 2018;8(1):17128. doi: 10.1038/s41598-018-35288-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.AN Baer. Diagnosis and classification of Sjögren's disease. https://www.uptodate.com/contents/diagnosis-and-classification-of-sjogrens-disease. Accessed December 28, 2024.
- 8.Sjogren Disease. American Dental Association. https://www.ada.org/resources/ada-library/oral-health-topics/sjogren-disease.
- 9.Jousse-Joulin S, D’agostino MA, Nicolas C, Naredo E, Ohrndorf S, Backhaus M, et al. Video clip assessment of a salivary gland ultrasound scoring system in Sjögren’s syndrome using consensual definitions: an OMERACT ultrasound working group reliability exercise. Ann Rheum Dis. 2019;78(7):967–973. doi: 10.1136/annrheumdis-2019-215024. [DOI] [PubMed] [Google Scholar]
- 10.Finzel S, Jousse-Joulin S, Costantino F, Hánová P, Hocevar A, Iagnocco A, et al. Patient-based reliability of the Outcome Measures in Rheumatology (OMERACT) ultrasound scoring system for salivary gland assessment in patients with Sjögren's syndrome. Rheumatology (Oxford, England), 2021;60(5):2169–2176. doi: 10.1093/rheumatology/keaa471. [DOI] [PubMed] [Google Scholar]
- 11.VanSchaik JT, Jain P, Rajapuri A, Cheriyan B, Thyvalikakath TP, Chakraborty S. Using transfer learning-based causality extraction to mine latent factors for Sjögren's syndrome from biomedical literature. Heliyon. 2023;9:e19265. doi: 10.1016/j.heliyon.2023.e19265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishna S, Dhir V, Singh S, Sood A, Gupta A, Sharma A, Sharma S. Prevalence of secondary Sj€ogren’s syndrome in Indian patients with rheumatoid arthritis: a single-center study. Int J Rheum Dis. 2017;20(7):870–874. doi: 10.1111/1756-185X.13017. [DOI] [PubMed] [Google Scholar]
- 13.Vitali C, Moutsopoulos HM, Bombardieri S. The European Community Study Group on diagnostic criteria for Sjögren's syndrome. Sensitivity and specificity of tests for ocular and oral involvement in Sjögren's syndrome. Ann Rheum Dis. 1994;53:637. doi: 10.1136/ard.53.10.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox PC, Busch KA, Baum BJ. Subjective reports of xerostomia and objective measures of salivary gland performance. J Am Dent Assoc. 1987;115:581. doi: 10.1016/s0002-8177(87)54012-0. [DOI] [PubMed] [Google Scholar]
- 15.https://fgdpscotland.org.uk/wp-content/uploads/2018/09/Challacombe-Scale-oral-dryness-ENG.pdf (Accessed on May 08, 2022).
- 16.Jousse-Joulin S, Nowak E, Cornec D, Brown J, Carr A, Carotti M, et al. Salivary gland ultrasound abnormalities in primary Sjögren’s syndrome: consensual US-SG core items definition and reliability. RMD Open. 2017;3;000364 doi: 10.1136/rmdopen-2016-000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornec D, Jousse-Joulin S, Pers JO, Marhadour T, Cochener B, Boisramé-Gastrin S, et al. Contribution of salivary gland ultrasonography to the diagnosis of Sjögren’s syndrome: toward new diagnostic criteria? Arthritis Rheum. 2013;65:216. doi: 10.1002/art.37698. [DOI] [PubMed] [Google Scholar]
- 18.Mossel E, Delli K, van Nimwegen JF, Stel AJ, Kroese FGM, Spijkervet FKL, et al. Ultrasonography of major salivary glands compared with parotid and labial gland biopsy and classification criteria in patients with clinically suspected primary Sjögren’s syndrome. Ann Rheum Dis. 2017;76:1883. doi: 10.1136/annrheumdis-2017-211250. [DOI] [PubMed] [Google Scholar]
- 19.Robin F, Albert JD, Lescoat A, Martel A, Perdriger A, DeBandt M, et al. Diagnostic performances of ultrasound evaluation of major salivary glands according to the 2019 outcome measures in rheumatology ultrasound scoring system. Arthritis Care Res (Hoboken) 2022;74:1924. doi: 10.1002/acr.24631. [DOI] [PubMed] [Google Scholar]
- 20.van Nimwegen JF, Mossel E, Delli K, van Ginkel MS, Stel AJ, Kroese FGM, et al. Incorporation of salivary gland ultrasonography into the American College of Rheumatology/European League Against Rheumatism criteria for Primary Sjögren’s Syndrome. Arthritis Care Res (Hoboken) 2020;72:583. doi: 10.1002/acr.24017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baer AN, Grader-Beck T, Antiochos B, Birnbaum J, Fradin JM. Ultrasound-guided biopsy of suspected salivary gland lymphoma in Sjögren’s syndrome. Arthritis Care Res (Hoboken) 2021;73:849. doi: 10.1002/acr.24203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zabotti A, Zandonella Callegher S, Lorenzon M, Pegolo E, Scott CA, Tel A, et al. Ultrasound-guided core needle biopsy compared with open biopsy: a new diagnostic approach to salivary gland enlargement in Sjögren’s syndrome? Rheumatology (Oxford) 2021;60:1282. doi: 10.1093/rheumatology/keaa441. [DOI] [PubMed] [Google Scholar]
- 23.Jungehülsing M, Fischbach R, Schröder U, Kugel H, Damm M, Eckel HE. Magnetic resonance sialography. Otolaryngol Head Neck Surg. 1999;121:488. doi: 10.1016/S0194-5998(99)70243-3. [DOI] [PubMed] [Google Scholar]
- 24.Tonami H, Ogawa Y, Matoba M, Kuginuki Y, Yokota H, Higashi K, et al. MR sialography in patients with Sjögren syndrome. AJNR Am J Neuroradiol. 1998;19:1199. [PMC free article] [PubMed] [Google Scholar]
- 25.André R, Becker M, Lombardi T, Buchholzer S, Marchal F, Seebach JD. Comparison of clinical characteristics and magnetic resonance imaging of salivary glands with magnetic resonance sialography in Sjögren’s syndrome. Laryngoscope. 2021;131:E83. doi: 10.1002/lary.28742. [DOI] [PubMed] [Google Scholar]
- 26.Sun Z, Zhang Z, Fu K, Zhao Y, Liu D, Ma X. Diagnostic accuracy of parotid CT for identifying Sjögren’s syndrome. Eur J Radiol. 2012;81:2702. doi: 10.1016/j.ejrad.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 27.Kalk WW, Vissink A, Spijkervet FK, Möller JM, Roodenburg JL. Morbidity from parotid sialography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:572. doi: 10.1067/moe.2001.117300. [DOI] [PubMed] [Google Scholar]
- 28.Daniels TE, Benn DK. Is sialography effective in diagnosing the salivary component of Sjögren's syndrome? Adv Dent Res. 1996;10:25. doi: 10.1177/08959374960100010301. [DOI] [PubMed] [Google Scholar]
- 29.Lee M, Rutka JA, Slomovic AR, McComb J, Bailey DJ, Bookman AA. Establishing guidelines for the role of minor salivary gland biopsy in clinical practice for Sjögren’s syndrome. J Rheumatol. 1998;25:247. [PubMed] [Google Scholar]
- 30.Varela Centelles P, Sánchez-Sánchez M, Costa-Bouzas J, et al. Neurological adverse events related to lip biopsy in patients suspicious for Sjögren's syndrome: a systematic review and prevalence meta-analysis. Rheumatology (Oxford) 2014;53:1208. doi: 10.1093/rheumatology/ket485. [DOI] [PubMed] [Google Scholar]
- 31.Andonopoulos AP, Drosos AA, Skopouli FN, Acritidis NC, Moutsopoulos HM. Secondary Sjögren's syndrome in rheumatoid arthritis. J Rheumatol. 1987;14(6):1098–1103. [PubMed] [Google Scholar]
- 32.Zhang H, Kong F, Yu F, Hao S. First report of rheumatoid arthritis and secondary Sjögren's syndrome complicated with heart failure. Clin Case Rep. 2021;9:e04581. doi: 10.1002/ccr3.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jadhav S, Jadhav A, Thopte S, Marathe S, Vhathakar P, Chivte P, et al. Sjögren's syndrome: a case study. J Int Oral Health. 2015;7(3):72–74. [PMC free article] [PubMed] [Google Scholar]