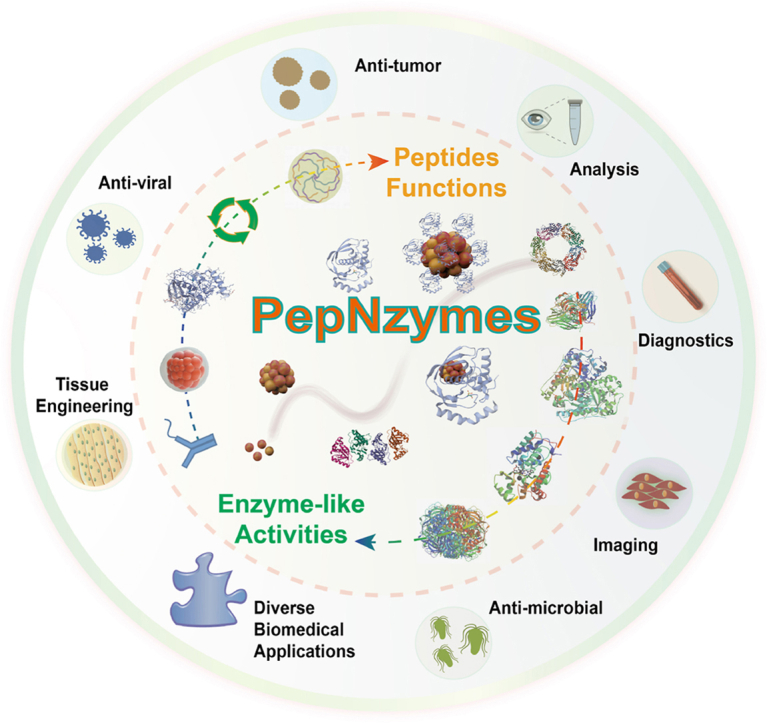
Abstract
The abundance of molecules on early Earth likely enabled a wide range of prebiotic chemistry, with peptides playing a key role in the development of early life forms and the evolution of metabolic pathways. Among peptides, those with enzyme-like activities occupy a unique position between peptides and enzymes, combining both structural flexibility and catalytic functionality. However, their full potential remains largely untapped. Further exploration of these enzyme-like peptides at the nanoscale could provide valuable insights into modern nanotechnology, biomedicine, and even the origins of life. Hence, this review introduces the groundbreaking concept of “peptide nanozymes (PepNzymes)”, which includes single peptides exhibiting enzyme-like activities, peptide-based nanostructures with enzyme-like activities, and peptide-based nanozymes, thus enabling the investigation of biological phenomena at nanoscale dimensions. Through the rational design of enzyme-like peptides or their assembly with nanostructures and nanozymes, researchers have found or created PepNzymes capable of catalyzing a wide range of reactions. By scrutinizing the interactions between the structures and enzyme-like activities of PepNzymes, we have gained valuable insights into the underlying mechanisms governing enzyme-like activities. Generally, PepNzymes play a crucial role in biological processes by facilitating small-scale enzyme-like reactions, speeding up molecular oxidation-reduction, cleavage, and synthesis reactions, leveraging the functional properties of peptides, and creating a stable microenvironment, among other functions. These discoveries make PepNzymes useful for diagnostics, cellular imaging, antimicrobial therapy, tissue engineering, anti-tumor treatments, and more while pointing out opportunities. Overall, this research provides a significant journey of PepNzymes’ potential in various biomedical applications, pushing them towards new advancements.
Keywords: Peptide, Nanozyme, Enzyme mimic, Structure-activity relationship, Biomedical applications
Graphical abstract
Highlights
-
•
The prominence of enzyme-like peptides and their nanoscale complexes is emphasized.
-
•
“Peptide nanozymes (PepNzymes)" for nanoscale biological investigation are coined.
-
•
The design and activities of PepNzymes are explored.
-
•
The biomedical applications of PepNzymes are reviewed.
-
•
The challenges and future opportunities for PepNzymes are discussed.
1. Introduction
Peptides, those concise assemblies of amino acids, boast a storied lineage stretching back to the primordial days of early Earth [1,2]. Their exploration is rooted in examining protein fragments, a pursuit notably championed by Emil Fischer during the latter part of the 19th century [3]. Yet, in the mid-20th century, peptide synthesis techniques began to advance significantly, thanks to the pioneering work of Bruce Merrifield, who developed solid-phase peptide synthesis [4]. This breakthrough allowed for the efficient production of peptides, fueling their exploration in various fields, including diagnostic tools, drug development, cancer therapy, vaccine design, agricultural biotechnology, material science, etc. [[5], [6], [7]]. Peptides could be used as therapeutics for a myriad of conditions, serving as lead compounds for novel medications, owing to their specificity, low toxicity, and high potency. Examples include insulin for diabetes and peptide hormones like oxytocin and vasopressin [[8], [9], [10]]. Beyond medicine, their influence extends into materials science, enabling versatile materials [11,12]. Amino acid side chains in peptides facilitate chemical modification, creating supramolecular nanostructures and hydrogels with properties like shear-thinning, bioactivity, self-healing, and shape memory [12].
Peptides have also been found to exhibit enzyme-like activity, revealing a profound understanding of peptides as versatile catalysts that orchestrate the intricate dance of chemical reactions necessary for life [[13], [14], [15]]. This realization has spurred scientists to push the boundaries of peptide engineering. Their goal is to replicate the catalytic abilities of enzymes by developing enzyme-like peptides. These peptides, serving as enzyme mimics, offer advantages such as enhanced stability under extreme conditions, simpler and more cost-effective synthesis, greater adjustability, lower immunogenicity, and multifunctionality compared to traditional enzymes. These attributes make them exceptionally promising for applications in biotechnology, medicine, and industry, bringing new hope for human health and sustainable development [[16], [17], [18]]. Through the intricacies of enzyme-like peptides, scientists embark on a journey of discovery that illuminates pathways to a more enlightened and prosperous future for all. Recent years have witnessed a surge in studies spotlighting ultra-small peptides' rational design and enzymatic efficacy [[19], [20], [21], [22]]. Some nanomaterials with enzyme-like catalytic activities (nanozymes) have also been reported [[23], [24], [25], [26], [27]]. When coupled with peptides, these nanozymes gain specificity, enzyme-like activity regulation, and biocompatibility, making them a novel class of multifunctional peptide-based nanozymes suitable for various biomedical applications [28,29]. Operating at the nanoscale empowers precise manipulation of molecular interactions and spatial arrangements, unlocking unprecedented prowess and selectivity, thus catalyzing the emergence of innovative solutions. Therefore, this review introduces the groundbreaking concept of “peptide nanozymes (PepNzymes)." This concept includes single peptides that exhibit enzyme-like activities, peptide-based nanostructures with enzyme-like activities, and peptide-based nanozymes. These advancements enable the investigation of biological phenomena at nanoscale dimensions.
Significantly, PepNzymes stand out as a transformative tool, with its enzyme-like activity and structure-activity relationships being of utmost importance [30,31]. Imagine a scenario where PepNzymes, engineered with precision, navigate through intricate biological pathways within the human body. With its peptide, enzyme-like activities, and nanoscale dimensions, PepNzymes seamlessly interact with cellular components, catalyzing reactions efficiently and precisely. For instance, the ability of PepNzymes to mimic enzyme-likereactions allows it to cleave specific sequences, facilitating the controlled release of therapeutic agents at the desired site [[32], [33], [34]]. Furthermore, a deep understanding of structure-activity relationships is crucial for maximizing the effectiveness of drugs, minimizing off-target effects, and improving therapeutic outcomes [[35], [36], [37]]. Moreover, by exploiting the enzyme-like activities, PepNzymes can detect subtle biomolecular changes associated with diseases [[38], [39], [40], [41]]. Through nanoscale interactions, it offers sensitive and selective detection methods, enabling early disease diagnosis and personalized treatment strategies. Therefore, PepNzymes exemplifies the integration of nanotechnology and biocatalysis, transforming biomedical applications and ushering in a new era of precision medicine.
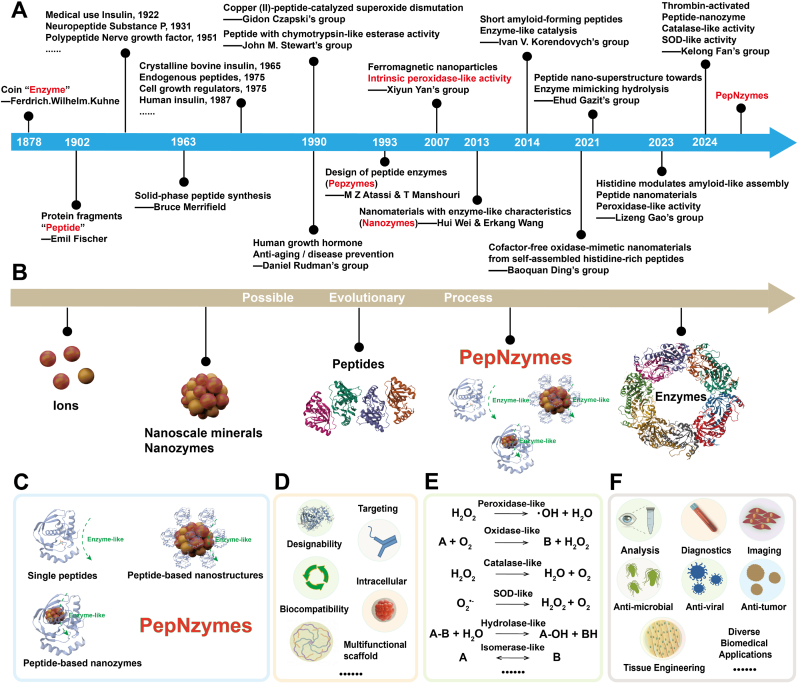
Considerable advancements have been achieved in the development of peptides, enzyme-like peptides (e.g., Pepzymes), nanozymes, etc., which have driven pivotal efforts propelling the work of PepNzymes forward and laying the groundwork for future innovations (Fig. 1A). In this context, we propose a potential evolutionary pathway from ions, nanoscale minerals/nanozymes, peptides, PepNzymes, and ultimately enzymes, inspired by the metal centers in natural enzymes (Fig. 1B). PepNzymes, in particular, may hold a unique position between peptides and enzymes, combining the structural characteristics, enzyme-like activities, and other potential features of peptides and nanomaterials, paving the way for innovative applications. Herein, PepNzymes are categorized into three distinct types: single peptides with enzyme-like activities, peptide-based nanostructures with enzyme-like activities, and peptide-based nanozymes (Fig. 1C). Single peptides, such as Pepzymes, are isolated sequences that exhibit intrinsic enzyme-like activities primarily due to their amino acid sequences and potential interactions with ions. Peptide-based nanostructures, including nanotubes, nanofibers, or nanospheres, serve mainly as carriers or scaffolds for peptides, enhancing peptide stability and catalytic efficiency by providing structural support and protection. Peptide-based nanozymes integrate peptides with nanozymes (e.g., metals, metal oxides, or carbon dots) to create composite materials, where nanozymes often serve as the primary active sites, and peptides act as scaffolds to enhance stability, facilitate functionalization, and improve overall performance. Notably, “peptides” in PepNzymes encompass both naturally occurring and artificially synthesized peptides. Together, single peptides provide the foundational enzyme-like activities, peptide-based nanostructures enhance the stability and efficacy of peptides, and peptide-based nanozymes combine peptide and enzyme-like activities with nanotechnology. These elements share fundamental characteristics of enzyme-like catalysis and nanoscale dimensions, balancing the flexibility of peptides with the rigidity of nanomaterials, not only complement each other but also advance the fields of enzymology and nanotechnology. Specifically, PepNzymes offer a customizable approach that balances enzyme-like functionality with enhanced versatility, including designability, targeting, biocompatibility, and multifunctional scaffolding, among others, distinguishing them from both traditional enzymes and conventional nanozymes (Fig. 1D). With rational design, PepNzymes can exhibit a range of enzyme-like activities, including but not limited to peroxidase, oxidase, catalase, superoxide dismutase, hydrolase, and isomerase, as well as novel activities that are either present or can be developed (Fig. 1E). These unique features allow PepNzymes to perform enzyme-like or other functions in a highly adaptable manner and be tailored for specific applications, making them promising tools across various fields (Fig. 1F). Hence, this review explores various aspects of PepNzymes, including their design, synthesis, and characterization, and delves into topics such as structure-activity relationships and enzyme-like activities. More significantly, it covers recent biomedical applications of PepNzymes, ranging from diagnostics and cellular imaging to antimicrobial therapy, tissue engineering, and anti-tumor treatments. Finally, the review provides observations on challenges and perspectives, enriching the understanding of PepNzymes.
Fig. 1.
Overview of PepNzymes covering development, structure, functions, enzyme-like activities, and biomedical applications. (A) Timeline of critical events of peptides, enzyme-like peptides, nanozymes, PepNzymes, etc. (B) A possbile evolution from ions, nanoscale minerals/nanozymes, peptides, PepNzymes, to enzymes. (C) Types of PepNzymes. (D) Peptide functions in PepNzymes. (E) Enzyme-like activities of PepNzymes. (F) Biomedical applications.
2. Design, synthesis, and characterization of PepNzymes
PepNzymes require optimization and refinement to enhance their performance and expand their applications. Achieving this involves precise design and engineering to address their structural complexity. By fine-tuning aspects such as catalytic efficiency, stability, selectivity, and interactions with biological systems, we can significantly improve their functionality across diverse settings. Therefore, in this section, we will explore the design, synthesis, and characterization of PepNzymes to better understand how these processes contribute to their development.
2.1. Strategies of rational design
The strategies of rational design for PepNzymes are grounded in applying specific approaches that have been extensively explored in peptide chemistry. These approaches include mimicking the active sites or overall structures of natural enzymes, regulating the structural self-assembly of peptides, and incorporating ions, metals, or nanozymes to enhance functionality [[42], [43], [44], [45]]. The core strategy is to create PepNzymes with tailored activity and specificity by drawing inspiration from enzyme structures or employing theoretical models.
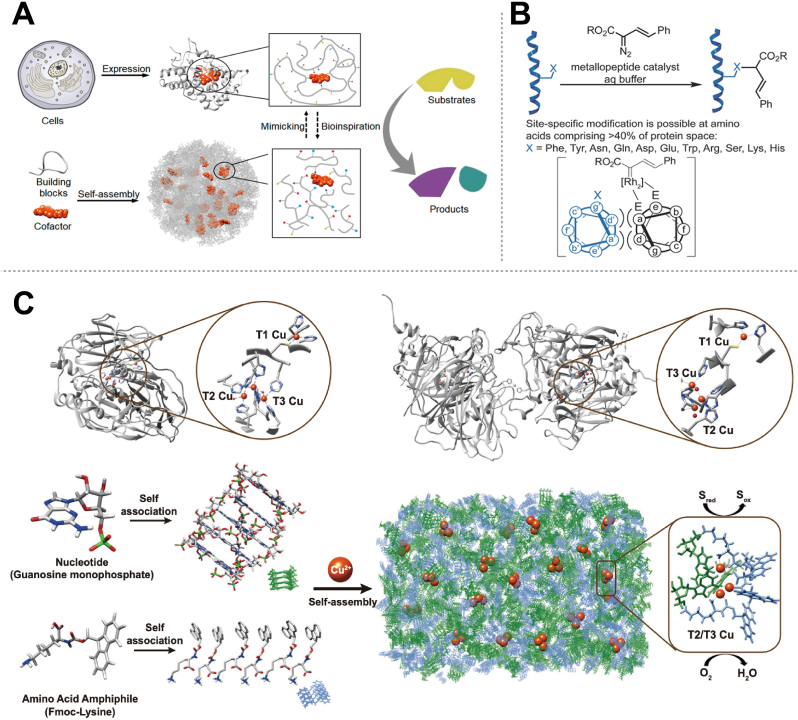
For example, mimicking enzyme structures involves designing peptide sequences that fold or assemble into active sites similar to those of natural enzymes, thereby replicating their catalytic functions. This strategy benefits from the simpler design rules of peptides compared to complex proteins, allowing for the creation of effective structures through de novo design [46]. Supramolecular catalysts, such as those formed by self-assembling designed molecular components with cofactors, illustrate this approach (Fig. 2A) [47].
Fig. 2.
Some examples of the design of PepNzymes. (A) Catalysts with enzyme-like active sites were created by self-assembly and protein folding. Reproduced with permission from Ref. [47], copyright 2020, American Chemical Society. (B) Rhodium (II) metallopeptide design. Reproduced with permission from Ref. [49], copyright 2013, American Chemical Society. (C) Copper-dependent catalysts inspired by catechol oxidases. Reproduced with permission from Ref. [50], copyright 2023, Springer Nature.
Structural self-assembly leverages the inherent properties of peptides to form customizable nanostructures with specific functions. An example is provided by Rufo et al., who designed peptides functioning as Zn2+-dependent esterases. Here, Zn2+ stabilizes the formation of prion-like fibrils, which then catalyze acyl ester hydrolysis, demonstrating a model for creating self-assembling nanostructured catalysts [48].
Insights from the design of metalloenzyme-like structures further inform the strategies for PepNzymes. For instance, Ball et al. integrated bioorganic peptide ligand design principles with nonbiological metal centers to achieve catalytic functions, addressing stability challenges with dirhodium complexes under biological conditions (Fig. 2B) [49]. Additionally, Xu et al. developed a supramolecular mimic with catalytic abilities that exceed those of reported artificial enzymes, advancing PepNzymes’ design strategies (Fig. 2C) [50].
While these strategies represent a broad spectrum of design approaches, there are numerous other methods for developing PepNzymes. Each method also requires careful integration of peptides and nanomaterials to advance biomedical applications.
2.2. Methods and strategies for synthesis
The method for synthesizing PepNzymes is crucial in defining their characteristics. Solid-phase peptide synthesis excels in adding amino acids sequentially, offering control over the peptide sequence and efficiency, which is beneficial for constructing complex peptide structures [51]. In contrast, solution-phase peptide synthesis is advantageous for simpler peptides due to its straightforward operation and milder reaction conditions [52]. Post-synthetic modifications, including chemical or bioconjugation techniques, enable the introduction of functional groups or components, broadening the scope of PepNzymes [53]. Furthermore, incorporating structural elements or templates during synthesis aids in forming PepNzymes with structures and functions [54]. Importantly, the arrangement of amino acid building blocks are critical factors that significantly influence the activity or specificity of the resulting PepNzymes [55]. Integrating peptides with nanozymes can also be achieved through various methods, each offering unique advantages. For example, surface functionalization attaches peptides to pre-synthesized nanozymes, providing control over peptide density [38,39]. Co-assembly allows the simultaneous assembly of peptides and nanozymes, resulting in well-integrated structures with synergistic properties [14]. Template-assisted synthesis uses peptides to direct nanozyme formation, enabling precise control over size and shape [32]. These methods collectively enhance the functionality, stability, and biocompatibility of peptide-integrated nanozymes.
2.3. Structural characterization techniques
Spectroscopic methods, such as nuclear magnetic resonance (NMR) spectroscopy, Fourier-transform infrared (FTIR) spectroscopy, Raman spectroscopy, and circular dichroism (CD) spectroscopy, provide information about the molecular structures of PepNzymes, including peptide sequence, secondary structure, and conformational changes [56]. For instance, NMR can reveal the arrangement of amino acids, while FTIR can detect specific bond vibrations indicative of secondary structures. Techniques like X-ray crystallography and cryo-electron microscopy (cryo-EM) offer detailed images of PepNzymes, elucidating their three-dimensional arrangements and interactions with substrates or cofactors [57]. X-ray crystallography can determine the atomic structures, whereas cryo-EM can visualize large complexes in their native states. For nanozymes, additional characterization methods such as transmission electron microscopy (TEM) and scanning electron microscopy (SEM) are crucial. TEM provides high-resolution morphology and structure images, while SEM offers detailed surface topology and composition analysis [58]. Dynamic light scattering (DLS) is used to determine the size distribution and stability of nanozyme suspensions [59]. Mass spectrometry techniques, such as matrix-assisted laser desorption/ionization and electrospray ionization, enable precise determination of the molecular weights of PepNzymes and identification of post-translational modifications, facilitating analysis [60]. In addition, computational modeling and simulation techniques also complement methods by predicting the structures and dynamic behaviors of PepNzymes, aiding rational design [61,62].
2.4. Active groups influencing activity
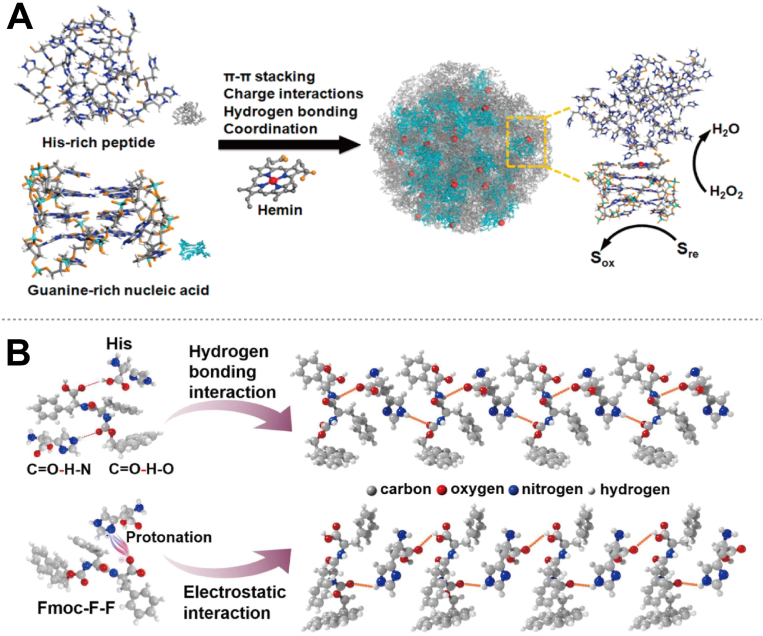
Active groups are responsible for the key interactions that govern substrate recognition, binding, and catalysis, which ultimately determine the overall activity of PepNzymes. By exploring how different functional groups influence these processes, researchers can strategically modify PepNzymes to enhance their performance. Some studies have focused on designing PepNzymes with enhanced activity by optimizing their active sites and spatial structures [63,64]. For instance, Liu et al. developed a hybrid system where a guanine-rich nucleic acid and a histidine-rich peptide self-assemble into a ligand environment tailored for hemin (Fig. 3A) [63]. In this system, the nucleic acid strand forms a stable structure by stacking its bases into quartets, while the histidine-rich peptide introduces activating groups that interact with hemin, significantly boosting its peroxidase-like activity. The presence of residues such as histidine plays a crucial role in mediating the activities of PepNzymes through mechanisms like nucleophilic attacks, proton transfers, and acid-base catalysis [28,63,65]. For example, Liu et al. reported a cofactor-free, non-conjugated supramolecular catalyst that exhibits redox activity via the cooperative action of neighboring functional groups. This catalyst is constructed by assembling oligo-histidine peptides into highly ordered nanostructures with a crystal-like lattice, demonstrating significant activity in H2O2 reduction and disproportionation reactions [28]. Remarkably, the catalytic activity can be fully restored after more than ten cycles of thermal or acid treatment, showcasing their superior robustness. Furthermore, amino acids can influence the assembly behavior of Fmoc-F-F peptides in aqueous conditions through a sonication-standing process, as demonstrated in another study (Fig. 3B) [65]. This process facilitates the formation of active sites that enable Aβ1-42 filaments to function effectively as nanozymes within physiological environments. The functional groups present within the peptide sequence play a pivotal role in substrate recognition, binding, and orientation, thereby directly affecting the substrate specificity and catalytic efficiency of PepNzymes [66,67]. Post-translational or chemical modifications introduced during PepNzymes’ synthesis, such as phosphorylation or glycosylation, can also further modulate their activity. These modifications can alter the properties of PepNzymes or their interactions with substrates, thereby fine-tuning their overall catalytic function and expanding their applicability [68].
Fig. 3.
Studies focus on designing PepNzymes with enhanced activity. (A) Nucleic acid and peptide components are designed with complementary characteristics to create peroxidase-like hierarchical sites. Reproduced with permission from Ref. [63], copyright 2017, American Chemical Society. (B) Potential interactions during the co-assembly of Fmoc-F-F and His. Reproduced with permission from Ref. [65], copyright 2023, Springer Nature.
3. Versatile enzyme-like activities and beyond
Over the past few decades, challenges related to the cost, stability, processing, storage, and recycling of enzymes have driven the search for viable alternatives. While significant progress has been made in artificial enzymes, such as nanozymes, their efficacy is often constrained by limited diversity compared to natural enzymes. The forthcoming chapter will first provide a summary of the diverse natural enzymatic activities, establishing a baseline for comparison. Following this, the focus will shift to exploring the versatile enzyme-like activities of PepNzymes and beyond. This includes their internalization mechanisms, intracellular trafficking pathways, and the complex dynamics of cellular interactions, thereby highlighting the broader potential and versatility of PepNzymes in mimicking and even surpassing natural enzyme functions.
3.1. Enzyme-like activities
Natural enzymes exhibit a vast array of catalytic activities, including those of peroxidases, oxidases, catalases, superoxide dismutases (SODs), hydrolases, isomerases, and many others. These enzymes are essential in various biological processes, facilitating specific reactions with efficiency and selectivity. To replicate these natural enzymatic functions, PepNzymes have been developed using diverse strategies aimed at mimicking the activities of these enzyme classes.
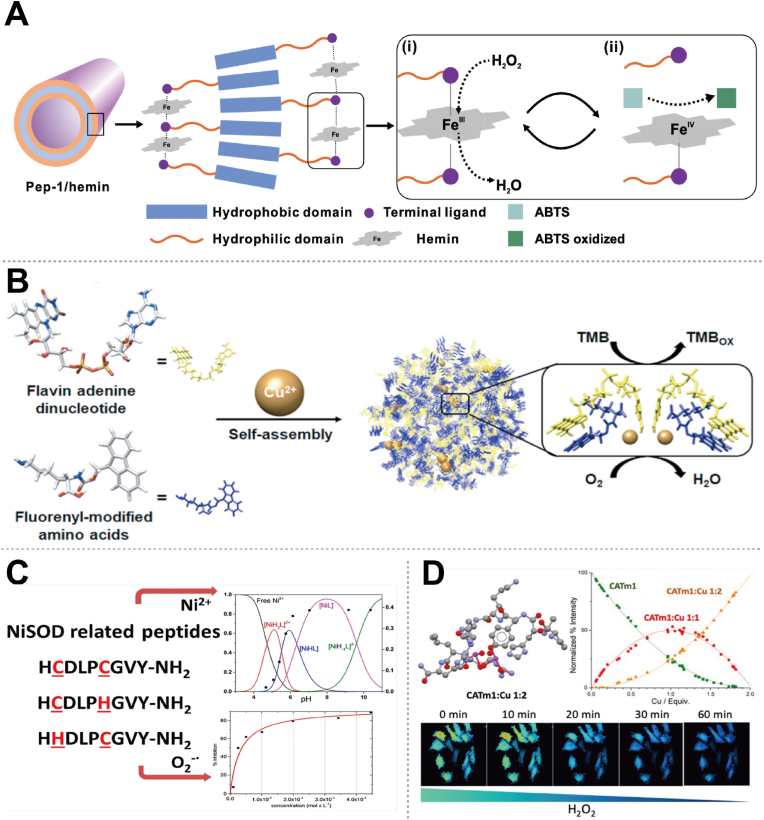
For peroxidase-like activity, as one of the most extensively studied activities among nanozymes, PepNzymes are designed to catalyze oxidation reactions using H2O2 as a co-substrate [23,63,69]. For example, Jian et al. developed peptide-based peroxidase mimics with tunable activity and high stability by constructing peptides and hemins into self-assembled crystalline nanomaterials (Fig. 4A) [69]. The peroxidase-like reaction mechanism follows a ping-pong model, where H2O2 and 2, 2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) react sequentially with the metal center. First, H2O2 oxidizes Fe3+ to Fe4+, then Fe4+ oxidizes ABTS. By systematically varying the chemical structure of peptoids, the catalytic activity of the Pep/hemin complex can be tuned. Adjusting the peptide side chain chemistry allows these peptide/hemin nanomaterials to effectively modulate their activity toward lignin model substrates, facilitating the depolymerization of biorefinery lignin. PepNzymes could also emulate oxidase-like activity, facilitating direct electron transfer from substrates to molecular oxygen and often utilizing metal cofactors [70,71]. For instance, Li et al. developed a supramolecular copper-cluster-dependent catechol oxidase by self-assembling flavin adenine dinucleotide (FAD), Fmoc-modified amino acids, and Cu2+ ions. Fmoc-amino acids form oxidase-mimetic copper clusters through fluorenyl stacking (Fig. 4B) [70]. FAD assembles with Fmoc-amino acids, creating a coordination sphere around Cu2+ and boosting the oxidase-like activity of Cu2+ by over 100-fold. There are numerous types of oxidases, each with unique structures and functions, which also suggests a multitude of possibilities for the future. Similarly, PepNzymes mimicking SOD-like activity facilitate superoxide anion (•O2-) dismutation into O2 and H2O2, typically by incorporating metal-binding sites [72,73]. Lihi et al. elucidate detailed equilibrium, spectroscopic, and SOD-like activity studies on metallopeptides (Ni2+) containing cysteine in alternative positions (Fig. 4C) [72]. Their findings underscore the importance of understanding metal-binding motifs and their impact on enzyme-like activity, which has implications for the rational design of novel catalysts and therapeutic agents targeting oxidative stress-related diseases. As another crucial antioxidant enzyme, catalase efficiently catalyzes the decomposition of H2O2, converting it into H2O and O2, thereby protecting cells from oxidative stress and damage caused by oxidation. For example, Coulibaly et al. used a combinatorial approach to discover a peptidyl di-copper (Cu) complex mimicking catalase (Fig. 4D) [74]. The complex showed catalase-like activity in both solution and on HeLa cells, demonstrating the effectiveness in finding peptidyl complexes with catalase-like activity. More significantly, PepNzymes also mimic hydrolase-like activity for substrate cleavage. Liang et al. developed oligopeptide-based nanozymes with intrinsic hydrolase-like activity through zinc (Zn)-induced self-assembly of histidine-rich heptapeptides [75]. These nanozymes effectively hydrolyzed various p-nitrophenyl esters and degraded di(2-ethylhexyl) phthalate, a common plasticizer, showing potential for environmental remediation. Extensions to other enzyme classes, such as isomerases, transferases, etc., require tailored strategies to replicate their specific catalytic mechanisms and substrate preferences. This involves a deep understanding of each enzyme's active site architecture, the dynamics of substrate binding, and the specific conditions under which they operate optimally.
Fig. 4.
Some typical enzyme-like activities of PepNzymes. (A) The assembly mechanism of Pep-1/hemin nanotubes and peroxidase-like activities displaying a ping-pong pathway for ABTS oxidation with H2O2. Reproduced with permission from Ref. [69], copyright 2022, Springer Nature. (B) Self-assembly of flavin adenine dinucleotide (FAD), Fmoc-amino acids (e.g., Fmoc-lysine), and Cu2+ to form supramolecular aggregates containing oxidase-like copper cluster active sites. Reproduced with permission from Ref. [70], copyright 2023, American Chemical Society. (C) The role of cysteine fragments in the nickel binding loop in Ni (II)-containing SOD-like activity. Reproduced with permission from Ref. [72], copyright 2020, American Chemical Society. (D) A di-copper peptidyl complex mimicking catalase activity. Reproduced with permission from Ref. [74], copyright 2021, American Chemical Society.
Currently, several methods like spectroscopy, crystallography, and computational modeling, including density functional theory (DFT), enable researchers to elucidate the structural and dynamic aspects of PepNzymes, providing valuable insights into their catalytic mechanisms [76,77]. For instance, the use of X-ray crystallography has revealed the precise atomic arrangements in PepNzymes, while DFT calculations have helped understand the electronic environments at the active sites [78]. Electron spin resonance (ESR) spectroscopy also offers a powerful tool to study the electronic properties and radical intermediates involved in PepNzyme catalysis [79]. For example, ESR has been employed to detect the formation of radical species during the catalytic cycles of PepNzymes, offering insights into reaction pathways [80]. The structural versatility of peptides allows for incorporating specific functional groups or motifs that mimic enzymatic catalysis, enhancing substrate recognition and efficiency. Techniques such as peptide synthesis and modification, coupled with DFT calculations, facilitate the design and engineering of PepNzymes with precise control over their structural and functional properties. For example, modifications of peptide side chains have been tailored to create specific microenvironments that enhance catalytic activity, as demonstrated by DFT studies showing optimal binding orientations and activation energies [81]. Moreover, by mimicking the dynamic conformational changes observed in enzymes, PepNzymes can optimize their performance in response to environmental stimuli or substrate binding. Techniques such as molecular dynamics simulations provide insights into the conformational dynamics of PepNzymes and implications for catalysis [82].
3.2. Functional diversification beyond enzyme-like activities
PepNzymes offer a range of functionalities beyond traditional enzyme-like activities due to their ability to mimic or enhance various biological processes. They exhibit biomolecular recognition capabilities similar to antibodies or aptamers, achieved through engineered peptide sequences that selectively bind to target molecules such as proteins, nucleic acids, or small molecules. This specificity is utilized in applications like biosensors, diagnostics, and targeted therapeutics [83,84]. Additionally, PepNzymes possess self-assembly properties, driven by non-covalent interactions such as hydrophobic forces, electrostatic interactions, and π-π stacking. These properties enable the formation of well-defined nanostructures with controlled architectures, which are advantageous in drug delivery systems and tissue engineering [85]. Many PepNzymes also display stimuli-responsive behavior, where they change conformation or activity in response to environmental cues like pH, temperature, or specific molecules. This adaptability is facilitated by responsive peptide sequences or functional groups incorporated into their structure [86]. Furthermore, PepNzymes are designed to be biocompatible and biodegradable, ensuring they do not provoke adverse immune responses and can break down into non-toxic byproducts after their function is complete. This makes them suitable for in vivo applications, such as supporting cell growth and tissue regeneration with biocompatible scaffolds or providing sustained drug release with biodegradable carriers [87].
3.3. Internalization mechanisms, intracellular trafficking pathways, and cellular interactions
Depending on their physicochemical properties and surface modifications, PepNzymes could be internalized via endocytic pathways, such as clathrin-mediated endocytosis, caveolae-mediated endocytosis, or macropinocytosis [88]. Understanding these endocytic mechanisms can inform strategies to enhance cellular uptake efficiency and intracellular delivery. Once inside the cell, PepNzymes may undergo trafficking through vesicular compartments like early endosomes, late endosomes, and lysosomes or be transported along microtubules and actin filaments to specific destinations [89]. Moreover, tailoring PepNzymes’ properties to exploit disease-specific internalization mechanisms, such as receptor-mediated endocytosis in cancer cells, can improve targeting specificity and therapeutic potency [90]. PepNzymes can also be engineered with stimuli-responsive functionalities for triggered release or activation within specific intracellular environments, such as acidic lysosomes or reducing cytosolic conditions [91]. Once internalized, PepNzymes may interact dynamically with various cellular organelles, cytoskeletal components, and signaling pathways, influencing their subcellular localization and functional outcomes [92]. Exploring cellular interactions and dynamics can uncover the therapeutic potential of PepNzymes in modulating cellular processes in health and disease, including applications in immunotherapy, vaccine development, and immune modulation.
4. Biomedical applications of PepNzymes
As the understanding of enzyme-like activities and their diverse functions deepens, PepNzymes emerge as a groundbreaking innovation with the potential to drive significant progress in biomedical fields. Continuous efforts are expanding their utility into emerging domains, exploring their capabilities and applications. This chapter aims to comprehensively uncover the wide-ranging capabilities of PepNzymes and their potential to revolutionize biomedical innovation, highlighting their versatility and transformative impact on diagnostics, therapeutics, and beyond.
4.1. Analysis, diagnostics, and cellular imaging
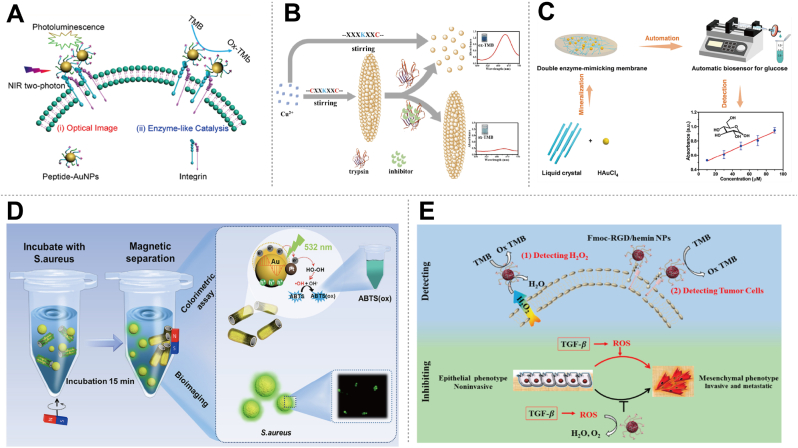
The convergence of peptides and nanozymes presents a groundbreaking approach in analysis, diagnostics, and cellular imaging [93,94]. Peptides, with their inherent specificity and ability to mimic natural biological interactions, offer precise targeting capabilities. Nanozymes, on the other hand, provide robust catalytic properties akin to natural enzymes but with enhanced stability and design flexibility. This synergistic combination leverages the strengths of both components, enabling the development of advanced tools for detecting and analyzing biological processes at a molecular level. For instance, Gao et al. demonstrated that gold nanozymes with peroxidase-like activity bioconjugated by a rationally designed peptide could selectively label and accurately quantify integrin GPIIb/IIIa on the human erythroleukemia cell line (Fig. 5A) [40]. Similarly, Cai et al. reported that the morphology of Cu nano-assemblies templated with peptides varies with the number of cysteines in the peptide chain [93]. This peptide-templated nanozyme system serves as a simple platform for rapidly monitoring trypsin activity and screening its inhibitors (Fig. 5B). For small molecules, Zhang et al. created a dual-functional enzyme-like membrane reactor by in situ mineralizing Au nanoparticles on peptide liquid crystals [94]. The resulting membrane reactor with peroxidase-like and glucose oxidase-like activities immobilized on a cellulose membrane formed a biosensor for fast, low-cost, and automatic glucose detection (Fig. 5C). Additionally, PepNzymes also demonstrate significant potential in bacterial detection and identification, serving as versatile biosensors for the rapid and specific detection of pathogenic bacteria in clinical, environmental, and food safety applications [95,96]. For instance, due to the specific recognition between S. aureus and peptides, fluorescein isothiocyanate-labeled peptide probes are captured by S. aureus and removed from Au/Pt/TiO2 magnetic nanotubes (Au/Pt/MTNTs) (Fig. 5D) [95]. This removal leads to the recovery of peroxidase-like activity and fluorescence emission of S. aureus. Benefiting from the Au-SPR effect and the magnetic properties of Au/Pt/MTNTs, the recovery of catalytic activity induces an enhanced colorimetric assay with a wider linear response for S. aureus quantification and a detection limit of four cells. Furthermore, PepNzymes hold promise in the field of cancer diagnostics. They enable targeted imaging and treatment of tumor cells through selective recognition and binding to cancer-specific biomarkers [[97], [98], [99]]. Conjugating PepNzymes with imaging probes or therapeutics enables innovative cancer diagnosis strategies. For instance, Lian et al. developed an artificial peroxidase by self-assembling hemin and fluorenylmethoxycarbonyl-arginine-glycine-aspartate (Fmoc-RGD) [97]. The resulting Fmoc-RGD/hemin nanoparticle, with RGD binding cancer cells and Fmoc enhancing catalytic activity, accurately detects cellular H2O2 and cancer cells (Fig. 5E). Moreover, it acts as a therapeutic agent by removing excess reactive oxygen species (ROS), inhibiting epithelial-mesenchymal transition induced by TGF-β. Notably, most applications of PepNzymes in analysis, diagnostics, and cellular imaging primarily leverage the recognition capabilities of peptides and the peroxidase-like activity of nanozymes. However, there is significant potential for expansion. By drawing inspiration from the diverse properties of enzymes, future research can develop new enzyme-like activities for PepNzymes. These advancements could result in more sophisticated and convenient methods for analysis and beyond.
Fig. 5.
Some typical applications of PepNzymes in analysis, diagnostics, and cellular imaging. (A) Nanozyme-linked immunosorbent assay using peptide-conjugated gold nanoprobes for integrin expression on cell membranes. Reproduced with permission from Ref. [40], copyright 2015, American Chemical Society. (B) Copper nano-assemblies regulated by peptides for trypsin assays and inhibitor screening. Reproduced with permission from Ref. [93], copyright 2022, American Chemical Society. (C) Gold nanoparticles mineralized by peptide liquid crystals with dual-functional enzyme-like activities for glucose detection. Reproduced with permission from Ref. [94], copyright 2023, American Chemical Society. (D) Plasmon-mediated peroxidase activity on asymmetric nanotubes with FITC-peptides for rapid visual bacteria detection. Reproduced with permission from Ref. [95], copyright 2022, American Chemical Society. (E) Fluorenylmethoxycarbonyl-arginine-glycine-aspartate/hemin nanoparticles for cancer cell detection and inhibition of TGF-β-induced EMT in breast cancer cells. Reproduced with permission from Ref. [97], copyright 2019, American Chemical Society.
4.2. Antibacterial or antifungal applications
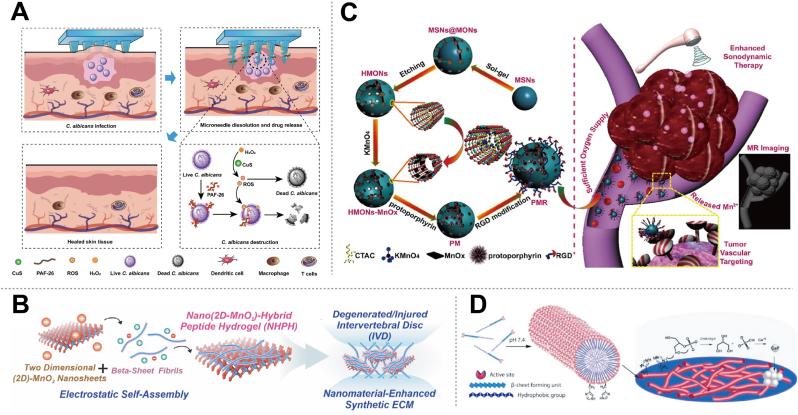
At the forefront of antibacterial innovation, PepNzymes are endowed with intrinsic antibacterial properties that enable them to combat bacterial pathogens. Remarkable PepNzymes utilize various mechanisms, including membrane disruption, cell wall degradation, and enzymatic inhibition, to neutralize bacterial threats [[100], [101], [102], [103], [104]]. With their diverse structural motifs and amino acid sequences, PepNzymes produce potent antimicrobial peptides capable of targeting a wide range of bacterial strains. Moreover, PepNzymes serve as versatile nanozymes, facilitating the catalysis of ROS or other antimicrobial agents to eradicate bacterial pathogens. For instance, Soria-Carrera et al. have revealed the peroxidase-like activity of self-assembled covalent polyoxometalates (POMlymers). These structures with peptides show increased biofilm production and ROS generation in bacteria such as Staphylococcus [101]. Similarly, Wang et al. have introduced an approach to the development of CuS/PAF-26 MN-a hyaluronic acid-based micro-needle loaded with both copper sulfide (CuS) nanoparticles and the antimicrobial peptide PAF-26 [102]. This innovative system effectively treats deep-cutaneous fungal infections by catalyzing ROS production and disrupting fungal cell envelopes against fungi (Fig. 6A). Importantly, this strategy reduces the risk of drug resistance and offers a promising therapeutic approach for combating fungal infections. Due to the emergence of antibiotic resistance and the limitations posed by ROS's short lifespan and restricted diffusion distance, conventional single-mode therapies using either antimicrobial peptides or nanozymes alone often fail to address the intricate challenges in real-world antimicrobial treatment. Enter PepNzymes-a pioneering approach that merges the antimicrobial prowess of peptides with the catalytic efficiency of nanozymes. This integration presents a novel avenue for combating microbial threats and opens up exciting prospects for advancing antimicrobial therapy to new heights.
Fig. 6.
Some typical biomedical applications of PepNzymes. (A) CuS/PAF-26 microneedles for treating deep-cutaneous fungal infections without inducing drug resistance, using hyaluronic acid (HA) and sodium carboxymethylcellulose (CMC-Na) to deliver copper sulfide (CuS) nanozyme and antimicrobial peptide (PAF-26). Reproduced with permission from Ref. [102], copyright 2023, American Chemical Society. (B) Multifunctional nanohybrid peptide hydrogel for enhanced intervertebral disc repair, created through hierarchical self-assembly of peptide amphiphile with biodegradable two-dimensional nanomaterials. Reproduced with permission from Ref. [105], copyright 2023, American Chemical Society. (C) PMR nano-sonosensitizers for MRI-guided and catalytic oxygen generation-enhanced SDT against cancer. Reproduced with permission from Ref. [108], copyright 2018, American Chemical Society. (D) Alkaline phosphatase-like peptide nanofibers for osteogenic differentiation. Reproduced with permission from Ref. [110], copyright 2015, American Chemical Society.
4.3. Tissue engineering
Peptides are also versatile in tissue engineering, enabling biomimetic scaffolds that support cell adhesion, proliferation, and differentiation [[105], [106], [107]]. Incorporating bioactive peptides mimics the native extracellular matrix (ECM) environment, promoting tissue formation and vascularization. Functionalizing peptides with ligands or growth factors enhances bioactivity and control over tissue regeneration. Therefore, researchers could create bioactive scaffolds with multifunctional properties by encapsulating PepNzymes with enzyme-like activities within biomaterials or surface-modifying scaffold materials. Combining peptide-functionalized scaffolds with nanozyme-based delivery systems creates versatile platforms for releasing bioactive molecules, growth factors, and therapeutic agents in a controlled manner, which helps improve tissue regeneration and repair. The combined effects of peptides and nanozymes can enhance the effectiveness and precision of treatments, allowing targeted delivery of therapeutic substances to specific cell populations or tissue microenvironments. For instance, Conley et al. developed a dynamic and multifunctional nanohybrid peptide hydrogel (NHPH) through the hierarchical self-assembly of peptide amphiphile modified with biodegradable two-dimensional nanomaterials exhibiting enzyme-like activities (Fig. 6B) [105]. This NHPH is injectable, biocompatible, and biodegradable while also therapeutic by catalyzing the scavenging of pro-inflammatory reactive oxygen species and promoting ECM remodeling. Their method facilitated the structural and functional recovery of the intervertebral disc (IVD) after severe injuries by delivering pro-regenerative cytokines in a sustained manner, effectively suppressing immune responses, and restoring the regenerative microenvironment of the ECM. Collectively, advanced PepNzymes-based nano-scaffold technology offers a promising alternative for tissue engineering by providing enzyme-like activity, enhanced bioactivity, precise modulation of responses, etc.
4.4. Anti-tumor strategies
PepNzymes also offer significant advantages in anti-tumor therapy [108]. Peptides inherently inhibit tumor growth and metastasis and can be modified to target tumor cells while sparing healthy tissues selectively. They enhance tumor-targeting specificity and cellular uptake, enabling precise drug delivery. As nanozymes, PepNzymes modulate the tumor microenvironment and boost anti-tumor immune responses while also exerting cytotoxic effects on tumor cells through their enzymatic activity. Encapsulation or functionalization with tumor-targeting ligands allows for direct delivery to tumor cells, inducing cytotoxic effects or activating immune cells. Thus, a synergy of peptides and nanozymes in PepNzymes combines precise targeting with potent catalytic activity, enhancing anti-tumor therapy. For example, Zhu et al. enhanced sonodynamic therapy (SDT) using catalytic PepNzymes, integrating MnOx component with hollow mesoporous organosilica nanoparticles, protoporphyrin, and cyclic RGD peptide (Fig. 6C) [108]. MnOx component acts as a nanozyme, converting H2O2 into O2, boosting tumor O2 levels and SDT-induced ROS production. This targeted approach suppressed tumor growth in U87 xenograft models, with high biocompatibility and efficient excretion, showing potential for clinical translation and providing tumor microenvironment-responsive imaging for therapeutic guidance with Mn as MRI. In another work, a tumor-targeting peptide Arg-Gly-Asp (RGD)-modified platinum nanozyme co-loaded with a glutathione (GSH)-responsive prodrug was developed [36]. This system, designed for drug release at tumor sites, enhances targeting and permeability to bladder cancer cells by peptide, alleviates tumor hypoxia, and improves photodynamic therapy. It achieved GSH-responsive drug release, enhanced drug accumulation, and increased permeability, thereby enhancing chemo-photodynamic therapy in bladder cancer models. However, despite significant advancements, cancer treatment continues to pose one of the most formidable challenges in modern medicine. In this context, PepNzymes offer promising prospects as a novel adjunctive therapy in the fight against cancer. By harnessing the combined power of peptides and nanozymes, PepNzymes hold the potential to enhance the efficacy of existing treatment modalities and overcome some of the limitations associated with traditional approaches. Nevertheless, a comprehensive understanding of the intricate mechanisms underlying tumor therapy is imperative to exploit the therapeutic potential of PepNzymes fully. Further research and exploration into the specific interactions between PepNzymes and tumor microenvironments are essential for optimizing their effectiveness and unlocking new avenues for innovative cancer treatment strategies.
4.5. Others
PepNzymes have further extended beyond traditional applications into diverse fields such as inflammation therapy, regenerative medicine, and biotechnology [[109], [110], [111], [112], [113]]. For instance, Shi et al. developed an engineered nanosponge utilizing the stealth effect of natural erythrocytes and the blood-brain barrier crossing ability of the T7 peptide [109]. This nanosponge efficiently targeted the infarct area in ischemic stroke, exerting a therapeutic effect by regulating hypoxia and boosting O2 levels. Wang et al. introduced a novel peptide-templated manganese dioxide nanozyme designed to integrate thrombolytic activity with ROS scavenging ability [32]. These PepNzymes prolonged blood circulation, exhibited strong thrombolytic action, and reduced ischemic brain damage. Additionally, Gulseren et al. enhanced bone regeneration efficiency using peptide nanofibers carrying both catalytic and matrix-regulatory functions of alkaline phosphatase, facilitating osteogenesis for various cell lines (Fig. 6D) [110]. As research advances, it is expected that more applications leveraging the capabilities of peptides and nanozymes will emerge, leading to significant advancements in biomedical and therapeutic interventions. However, to fully explore the potential of these PepNzymes in both materials science and biomedical fields, increased interdisciplinary exchange and collaboration are imperative. This collaborative approach will facilitate the discovery and expansion of new applications, or the most suitable applications for PepNzymes, ultimately driving progress in healthcare and biotechnology.
5. Conclusion and challenge
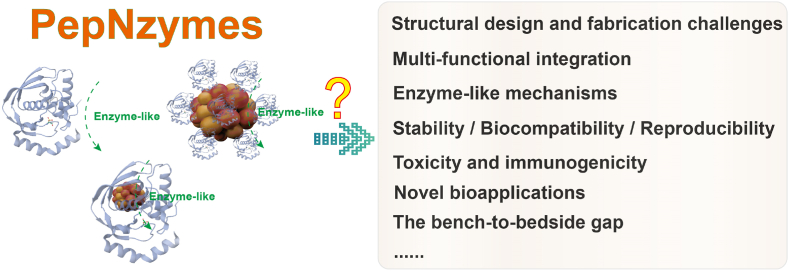
Our review highlights the crucial role of PepNzymes. By elucidating their structure-activity relationships and exploring their versatile enzyme-like architectures, we have laid the foundation for biomedical applications. These may only scratch the surface, as PepNzymes, serving as intermediaries between peptides and enzymes, likely harbor numerous novel variants yet to be discovered in nature. This realization urges us to explore and uncover new PepNzymes continuously. For instance, many naturally occurring nanozymes formed by metals ingested by organisms undergo evolution within various organisms, potentially exerting either positive or negative effects. Unveiling these potential PepNzymes hold infinite possibilities for the future. However, to fully harness the potential of PepNzymes, it is essential to overcome some existing challenges (Fig. 7). By tackling these obstacles, we can unlock fresh avenues for innovative biomedical solutions, paving the way for transformative progress.
Fig. 7.
Challenges of PepNzymes.
5.1. Addressing structural design and fabrication challenges
To tackle the challenges associated with structural design and fabrication, it is necessary to focus on nanostructure assembly details, optimize synthetic protocols, and explore innovative techniques to enhance the efficacy and reliability of PepNzymes. Researchers can optimize the self-assembly process of PepNzymes by fine-tuning various conditions during fabrication, resulting in PepNzymes with functionality, structure, and activity [114]. Methods like templated synthesis and bottom-up fabrication can be employed to control the morphology and size of PepNzymes, yielding customized structures with enhanced enzyme-like activities and reproducibility [115,116]. Furthermore, advances in synthetic biology can also facilitate the functionalization of PepNzymes, thereby enhancing their efficacy in related applications [117].
5.2. Enhancing multi-functional integration
Enhancing the multi-functional integration of PepNzymes is essential for their efficacy across various domains. Integrating multifunctional components like targeting ligands, stimuli-responsive elements, and therapeutic payloads into PepNzymes enables tailored properties to meet specific needs, facilitating precise targeting, controlled release, and synergistic therapeutic effects. For example, researchers could develop PepNzymes conjugated with new targeting ligands that recognize specific cancer cell receptors, allowing for their selective accumulation and internalization into tumor tissues while minimizing off-target effects [118]. Moreover, incorporating stimuli-responsive elements such as temperature-sensitive polymers could enable PepNzymes to undergo conformational changes in response to external cues, triggering drug release at precise locations within the body for enhanced therapeutic outcomes [119,120]. Additionally, loading PepNzymes with therapeutic payloads, like chemotherapy drugs, monoclonal drugs, or nucleic acids, could offer more functionalities, acting both as catalysts for biocatalytic reactions and carriers for therapeutic agents, thereby achieving synergistic therapeutic effects in cancer treatment or regenerative medicine [121].
5.3. Delving into enzyme-like mechanisms
A thorough investigation into the enzyme-like mechanisms governing the catalysis of PepNzymes is crucial for approaching the functionality of natural enzymes and advancing the development of complex synthetic analogs. For now, the enzyme-like mechanisms of PepNzymes may differ from each kind, and by thoroughly understanding potential processes such as substrate binding, active site chemistry, catalytic kinetics, and enzyme activity units, we can refine the design principles and engineering strategies for PepNzymes’ development [122,123].
5.4. Improving stability, biocompatibility, reproducibility, and biodegradation
Optimizing PepNzymes involves prioritizing stability and ensuring consistent enzyme-like activity in diverse environments for real-world effectiveness. Enhanced biocompatibility is crucial to prevent adverse reactions during interaction with biological systems, promoting safe use in medical settings. Moreover, reproducibility ensures consistent performance across batches, facilitating scalability for large-scale production. Designing PepNzymes with biodegradable properties minimizes long-term effects, enhancing their safety profile.
5.5. Mitigating toxicity and immunogenicity risks
To ensure the safety and regulatory compliance necessary for clinical translation, it is crucial to thoroughly address potential toxicity and immunogenicity concerns associated with PepNzymes-based therapies. This requires comprehensive research to assess their biocompatibility, pharmacokinetics, and potential immune responses. Nowadays, some peptide-based template materials still pose risks of immune reactions [124]. Therefore, rigorous preclinical evaluation and adherence to regulatory guidelines are essential to demonstrate the efficacy and safety of these PepNzymes-based therapies, paving the way for their successful translation into clinical applications [125].
5.6. Exploring novel biomedical applications and therapeutic modalities
Expanding the biomedical application scope of PepNzymes beyond traditional domains is crucial for fully harnessing their potential. By using their unique features, such as tunable enzyme-like activity, PepNzymes hold promise in addressing unmet medical needs and advancing personalized medicine. Many rare or specific diseases currently lack adequate animal models for clinical trials, presenting an opportunity for future exploration of PepNzymes’ applications. Thus, through interdisciplinary research and innovative approaches, PepNzymes-based platforms could be customized for specific medical uses, offering new avenues for diagnosis, treatment, and disease management.
5.7. Bridging the bench-to-bedside gap
Researchers can acquire pivotal data to substantiate clinical trial applications and secure regulatory approvals by establishing rigorous preclinical evaluation protocols encompassing comprehensive efficacy and toxicity assessments. Developing scalable manufacturing methods is crucial to meet the demand for large-scale production of PepNzymes while ensuring consistency and quality. Furthermore, adherence to regulatory guidelines and standards is essential for navigating the complex regulatory landscape and obtaining approval for clinical trials and commercialization. Through these concerted efforts, PepNzymes based on nanozymes is poised to effectively advance clinical translation, offering significant benefits to patients and healthcare systems worldwide.
5.8. Fostering collaboration and interdisciplinary research
Fostering collaboration among scientists, engineers, clinicians, and industry partners is essential for advancing PepNzymes and translating them into practical solutions. By pooling diverse expertise, collaborative efforts can address complex barriers and optimize PepNzymes' performance across various applications. Interdisciplinary approaches integrating insights from chemistry, biology, materials science, and medicine can drive innovation and customization of PepNzymes to meet diverse needs. Effective collaboration unlocks opportunities and drives progress in PepNzymes for societal benefit.
6. Perspectives
This work is a comprehensive review of recent advancements in PepNzyme design, enzyme-like activities, and diverse biomedical applications, revealing significant versatility and innovation. Indeed, our current surge in research focusing on PepNzymes is driven by two primary factors. Firstly, the capacity to engineer and manipulate these PepNzymes offers innovative solutions to contemporary biomedical challenges, which is evident and has been extensively discussed in this work. Secondly, we believe that PepNzymes may serve as a unique tool for investigating the fundamental principles underpinning the origin and evolution of life.
Beyond its immediate biomedical design, this convergence of peptide and nanozyme may hold far-reaching implications for the origins of life. One example involves self-assembling short peptides resembling primitive catalytic centers [2][126]. These peptide assemblies could have facilitated early biochemical reactions crucial for the emergence of life [127]. Additionally, nanoparticles, particularly metal oxide and sulfur nanoparticles, have been proposed to catalyze key prebiotic reactions involved in synthesizing essential biomolecules, such as amino acids and nucleotides [128,129]. Drawing inspiration from biomineralization processes and metal-catalyzed reactions observed in early life forms, PepNzymes integrate peptides with enzyme-like activities at the nanoscale, providing fascinating insights into the catalytic behaviors and molecular mechanisms that may have played a crucial role in the origin and evolution of life [130]. By delving deeper into the behaviors of PepNzymes, we could gain invaluable insights into the early chemical processes that may have set the stage for the emergence of life-sustaining molecules and their subsequent evolution. This is another key aspect we eagerly anticipate exploring in future research. However, further theoretical and mechanistic studies are necessary, as these aspects remain underexplored. Such investigations could potentially span interdisciplinary frontiers, encompassing paleobiology, physics, geology, and other cutting-edge research methodologies.
In essence, this review will transcend conventional boundaries in biomedical applications, offering the transformative potential of PepNzymes in addressing pressing healthcare needs while highlighting age-old questions about life's origins, thus forging a profound connection between biomedical applications and broader fields.
Ethics approval and consent to participate
Not applicable.
CRediT authorship contribution statement
Shaobin He: Writing – original draft, Validation, Resources, Conceptualization. Long Ma: Writing – original draft, Conceptualization. Qionghua Zheng: Writing – original draft, Resources. Zhuoran Wang: Validation, Conceptualization. Wei Chen: Validation, Conceptualization. Zihang Yu: Writing – review & editing. Xiyun Yan: Writing – review & editing, Conceptualization. Kelong Fan: Writing – review & editing, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was funded by the Key Project of the Joint Fund for Regional Innovation and Development of the National Natural Science Foundation of China (U23A20686), the Key Laboratory of Biomacromolecules, Chinese Academy of Sciences (ZGD-2023-03), the Joint Funds for the Innovation of Science and Technology, Fujian Province (2023Y9226), and the Introduced High-Level Talent Team Project of Quanzhou City (2023CT008).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Xiyun Yan, Email: yanxy@ibp.ac.cn.
Kelong Fan, Email: fankelong@ibp.ac.cn.
References
- 1.Muchowska K.B., Moran J. Peptide synthesis at the origin of life. Science. 2020;370:767–768. doi: 10.1126/science.abf1698. [DOI] [PubMed] [Google Scholar]
- 2.Frenkel-Pinter M., Samanta M., Ashkenasy G., Leman L.J. Prebiotic peptides: molecular hubs in the origin of life. Chem. Rev. 2020;120:4707–4765. doi: 10.1021/acs.chemrev.9b00664. [DOI] [PubMed] [Google Scholar]
- 3.Laps S., Metanis N. Organic solvent enhances oxidative folding of disulfide-rich proteins. Nat. Chem. 2024:1–2. doi: 10.1038/s41557-024-01518-9. [DOI] [PubMed] [Google Scholar]
- 4.Yan H., Chen F.-E. Recent progress in solid-phase total synthesis of naturally occurring small peptides. Adv. Synth. Catal. 2022;364:1934–1961. doi: 10.1002/adsc.202200079. [DOI] [Google Scholar]
- 5.Boyle A.L., Woolfson D.N. De novo designed peptides for biological applications. Chem. Soc. Rev. 2011;40:4295–4306. doi: 10.1039/C0CS00152J. [DOI] [PubMed] [Google Scholar]
- 6.Li Q., Wang Y., Zhang G., Su R., Qi W. Biomimetic mineralization based on self-assembling peptides. Chem. Soc. Rev. 2023;52:1549–1590. doi: 10.1039/D2CS00725H. [DOI] [PubMed] [Google Scholar]
- 7.Hamley I.W. Small bioactive peptides for biomaterials design and therapeutics. Chem. Rev. 2017;117:14015–14041. doi: 10.1021/acs.chemrev.7b00522. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez A.M., Torres-Alemán I. The many faces of insulin-like peptide signalling in the brain. Nat. Rev. Neurosci. 2012;13:225–239. doi: 10.1038/nrn3209. [DOI] [PubMed] [Google Scholar]
- 9.Meyer-Lindenberg A., Domes G., Kirsch P., Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat. Rev. Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 10.Muttenthaler M., King G.F., Adams D.J., Alewood P.F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 2021;20:309–325. doi: 10.1038/s41573-020-00135-8. [DOI] [PubMed] [Google Scholar]
- 11.Huo Y., Hu J., Yin Y., Liu P., Cai K., Ji W. Self-assembling peptide-based functional biomaterials. Chembiochem. 2023;24 doi: 10.1002/cbic.202200582. [DOI] [PubMed] [Google Scholar]
- 12.Chen J., Zou X. Self-assemble peptide biomaterials and their biomedical applications. Bioact. Mater. 2019;4:120–131. doi: 10.1016/j.bioactmat.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lou Y., Zhang B., Ye X., Wang Z.-G. Self-assembly of the de novo designed peptides to produce supramolecular catalysts with built-in enzyme-like active sites: a review of structure-activity relationship. Mater. Today Nano. 2023;21 doi: 10.1016/j.mtnano.2023.100302. [DOI] [Google Scholar]
- 14.Han J., Gong H., Ren X., Yan X. Supramolecular nanozymes based on peptide self-assembly for biomimetic catalysis. Nano Today. 2021;41 doi: 10.1016/j.nantod.2021.101295. [DOI] [Google Scholar]
- 15.Chatterjee A., Reja A., Pal S., Das D. Systems chemistry of peptide-assemblies for biochemical transformations. Chem. Soc. Rev. 2022;51:3047–3070. doi: 10.1039/D1CS01178B. [DOI] [PubMed] [Google Scholar]
- 16.Sharma K., Sharma K.K., Sharma A., Jain R. Peptide-based drug discovery: current status and recent advances. Drug Discov. Today. 2023;28 doi: 10.1016/j.drudis.2022.103464. [DOI] [PubMed] [Google Scholar]
- 17.Karimzadeh A., Hasanzadeh M., Shadjou N., de la Guardia M. Peptide based biosensors. TrAC, Trends Anal. Chem. 2018;107:1–20. doi: 10.1016/j.trac.2018.07.018. [DOI] [Google Scholar]
- 18.Sathya R., MubarakAli D., MohamedSaalis J., Kim J.-W. A systemic review on microalgal peptides: bioprocess and sustainable applications. Sustainability. 2021;13:3262. doi: 10.3390/su13063262. [DOI] [Google Scholar]
- 19.Omosun T.O., Hsieh M.-C., Childers W.S., Das D., Mehta A.K., Anthony N.R., Pan T., Grover M.A., Berland K.M., Lynn D.G. Catalytic diversity in self-propagating peptide assemblies. Nat. Chem. 2017;9:805–809. doi: 10.1038/nchem.2738. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y., Yang Y., Orr A.A., Makam P., Redko B., Haimov E., Wang Y., Shimon L.J.W., Rencus-Lazar S., Ju M., Tamamis P., Dong H., Gazit E. Self-assembled peptide nano-superstructure towards enzyme mimicking hydrolysis. Angew. Chem. Int. Ed. 2021;133:17301–17307. doi: 10.1002/ange.202105830. [DOI] [PubMed] [Google Scholar]
- 21.Liu Q., Kuzuya A., Wang Z.-G. Supramolecular enzyme-mimicking catalysts self-assembled from peptides. iScience. 2023;26 doi: 10.1016/j.isci.2022.105831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rink W.M., Thomas F. De Novo Designed α-Helical Coiled-coil peptides as scaffolds for chemical reactions. Chem. Eur J. 2019;25:1665–1677. doi: 10.1002/chem.201802849. [DOI] [PubMed] [Google Scholar]
- 23.Zandieh M., Liu J. Nanozymes: definition, activity, and mechanisms. Adv. Mater. 2024;36 doi: 10.1002/adma.202211041. [DOI] [PubMed] [Google Scholar]
- 24.Fan H., Zhang R., Fan K., Gao L., Yan X. Exploring the specificity of nanozymes. ACS Nano. 2024;18:2533–2540. doi: 10.1021/acsnano.3c07680. [DOI] [PubMed] [Google Scholar]
- 25.Meng X., Zare I., Yan X., Fan K. Protein-protected metal nanoclusters: an emerging ultra-small nanozyme. WIREs Nanomed. Nanobi. 2020;12 doi: 10.1002/wnan.1602. [DOI] [PubMed] [Google Scholar]
- 26.Zuo L., King H., Hossain M.A., Farhana F., Kist M.M., Stratton R.L., Chen J., Shen H. Single-molecule spectroscopy reveals the plasmon-assisted nanozyme catalysis on AuNR@TiO2. Chem. Biomed. Imaging. 2023;1:760–766. doi: 10.1021/cbmi.3c00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang S., Cheng M., Wang S., Jiang W., Yang F., Shen X., Zhang L., Yan X., Jiang B., Fan K. A self-catalytic NO/O₂ gas-releasing nanozyme for radiotherapy sensitization through vascular normalization and hypoxia relief. Adv. Mater. 2024 doi: 10.1002/adma.202403921. Early View. [DOI] [PubMed] [Google Scholar]
- 28.Liu Q., Wan K., Shang Y., Wang Z.-G., Zhang Y., Dai L., Wang C., Wang H., Shi X., Liu D., Ding B. Cofactor-free oxidase-mimetic nanomaterials from self-assembled histidine-rich peptides. Nat. Mater. 2021;20:395–402. doi: 10.1038/s41563-020-00856-6. [DOI] [PubMed] [Google Scholar]
- 29.Yi J., Deng Q., Liu Z., Wang H., Liu X., Ren J., Qu X. Nanozyme-based supramolecular self-assembly as an artificial host defense system for treatment of bacterial infections. Small. 2023;19 doi: 10.1002/smll.202301096. [DOI] [PubMed] [Google Scholar]
- 30.Chen J., Shi K., Chen R., Zhai Z., Song P., Chow L.W., Chandrawati R., Pashuck E.T., Jiao F., Lin Y. Supramolecular hydrolase mimics in equilibrium and kinetically trapped states. Angew. Chem. Int. Ed. 2024;63 doi: 10.1002/anie.202317887. [DOI] [PubMed] [Google Scholar]
- 31.Li Z., Joshi S.Y., Wang Y., Deshmukh S.A., Matson J.B. Supramolecular peptide nanostructures regulate catalytic efficiency and selectivity. Angew. Chem. Int. Ed. 2023;62 doi: 10.1002/anie.202303755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z., Zhao Y., Hou Y., Tang G., Zhang R., Yang Y., Yan X., Fan K. A thrombin-activated peptide-templated nanozyme for remedying ischemic stroke via thrombolytic and neuroprotective actions. Adv. Mater. 2024;36 doi: 10.1002/adma.202210144. [DOI] [PubMed] [Google Scholar]
- 33.Perdomo Y., Slocik J.M., Phillips D.M., Knecht M.R. Peptide/nanoparticle biointerfaces for multistep tandem catalysis. J. Am. Chem. Soc. 2023;145:16650–16657. doi: 10.1021/jacs.3c04097. [DOI] [PubMed] [Google Scholar]
- 34.Yang X., Wu B., Zhou J., Lu H., Zhang H., Huang F., Wang H. Controlling intracellular enzymatic self-assembly of peptide by host-guest complexation for programming cancer cell death. Nano Lett. 2022;22:7588–7596. doi: 10.1021/acs.nanolett.2c02612. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y., Zhan J., Huang J., Wang X., Chen Z., Yang Z., Li J. Dynamic responsiveness of self-assembling peptide-based nano-drug systems, Interdiscip. Méd. 2023;1 doi: 10.1002/INMD.20220005. [DOI] [Google Scholar]
- 36.Hao Y., Chen Y., He X., Han R., Yang C., Liu T., Yang Y., Liu Q., Qian Z. RGD peptide modified platinum nanozyme Co-loaded glutathione-responsive prodrug nanoparticles for enhanced chemo-photodynamic bladder cancer therapy. Biomaterials. 2023;293 doi: 10.1016/j.biomaterials.2022.121975. [DOI] [PubMed] [Google Scholar]
- 37.Zhu X., Sun Q., Chen J., Liang C., Chen L., Qi Y., Luo H., Chen L., Chen J. Nanozyme-based guanidinium peptides mediate surface reactive oxygen species for multidrug resistant bacterial infection management. J. Mater. Chem. B. 2023;11:6393–6403. doi: 10.1039/D3TB01104F. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X., Liu Y., Guo Q., Yang S., Lan F., Du J., Qiao D., Zheng P., Xu S., Pan Q., Zhu W. Light-responsive Au@Zn-TCPP nanozyme functionalized with cell-penetrating peptide and antisense oligonucleotide for sensing living bacteria and synergistic therapy of diabetic wounds. Chem. Eng. J. 2024;488 doi: 10.1016/j.cej.2024.150945. [DOI] [Google Scholar]
- 39.Mansur A.A.P., Carvalho S.M., Oliveira L.C.A., Souza-Fagundes E.M., Lobato Z.I.P., Leite M.F., Mansur H.S. Bioengineered carboxymethylcellulose-peptide hybrid nanozyme cascade for targeted intracellular biocatalytic-magnetothermal therapy of brain cancer cells. Pharmaceutics. 2022;14:2223. doi: 10.3390/pharmaceutics14102223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao L., Liu M., Ma G., Wang Y., Zhao L., Yuan Q., Gao F., Liu R., Zhai J., Chai Z., Zhao Y., Gao X. Peptide-conjugated gold nanoprobe: intrinsic nanozyme-linked immunsorbant assay of integrin expression level on cell membrane. ACS Nano. 2015;9:10979–10990. doi: 10.1021/acsnano.5b04261. [DOI] [PubMed] [Google Scholar]
- 41.Wei G., Liu S., Peng Y.-K., Wei H. On the specificity of nanozymes: a perspective. Chin. J. Chem. 2024;42:1515–1522. doi: 10.1002/cjoc.202300755. [DOI] [Google Scholar]
- 42.Sinha N.J., Langenstein M.G., Pochan D.J., Kloxin C.J., Saven J.G. Peptide design and self-assembly into targeted nanostructure and functional materials. Chem. Rev. 2021;121:13915–13935. doi: 10.1021/acs.chemrev.1c00712. [DOI] [PubMed] [Google Scholar]
- 43.Sheehan F., Sementa D., Jain A., Kumar M., Tayarani-Najjaran M., Kroiss D., Ulijn R.V. Peptide-based supramolecular systems chemistry. Chem. Rev. 2021;121:13869–13914. doi: 10.1021/acs.chemrev.1c00089. [DOI] [PubMed] [Google Scholar]
- 44.Lampel A., Ulijn R.V., Tuttle T. Guiding principles for peptide nanotechnology through directed discovery. Chem. Soc. Rev. 2018;47:3737–3758. doi: 10.1039/C8CS00177D. [DOI] [PubMed] [Google Scholar]
- 45.He J., Hou Y., Zhang Z., Zhang J., Yan X., Fan K., Liang M. Carbon-based nanozymes: how structure affects performance. Nano Biomed. Eng. 2024;16:28–47. doi: 10.26599/NBE.2024.9290053. [DOI] [Google Scholar]
- 46.Hamley I.W. Biocatalysts based on peptide and peptide conjugate nanostructures. Biomacromolecules. 2021;22:1835–1855. doi: 10.1021/acs.biomac.1c00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu S., Du P., Sun H., Yu H.-Y., Wang Z.-G. Bioinspired supramolecular catalysts from designed self-assembly of DNA or peptides. ACS Catal. 2020;10:14937–14958. doi: 10.1021/acscatal.0c03753. [DOI] [Google Scholar]
- 48.Rufo C.M., Moroz Y.S., Moroz O.V., Stöhr J., Smith T.A., Hu X., DeGrado W.F., Korendovych I.V. Short peptides self-assemble to produce catalytic amyloids. Nat. Chem. 2014;6:303–309. doi: 10.1038/nchem.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ball Z.T. Designing enzyme-like catalysts: a rhodium(II) metallopeptide case study. Acc. Chem. Res. 2013;46:560–570. doi: 10.1021/ar300261h. [DOI] [PubMed] [Google Scholar]
- 50.Xu S., Wu H., Liu S., Du P., Wang H., Yang H., Xu W., Chen S., Song L., Li J., Shi X., Wang Z.-G. A supramolecular metalloenzyme possessing robust oxidase-mimetic catalytic function. Nat. Commun. 2023;14:4040. doi: 10.1038/s41467-023-39779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laps S., Satish G., Brik A. Harnessing the power of transition metals in solid-phase peptide synthesis and key steps in the (semi) synthesis of proteins. Chem. Soc. Rev. 2021;50:2367–2387. doi: 10.1039/D0CS01156H. [DOI] [PubMed] [Google Scholar]
- 52.Sharma A., Kumar A., de la Torre B.G., Albericio F. Liquid-phase peptide synthesis (LPPS): a third wave for the preparation of peptides. Chem. Rev. 2022;122:13516–13546. doi: 10.1021/acs.chemrev.2c00132. [DOI] [PubMed] [Google Scholar]
- 53.Lim D., Lee W., Hong J., Gong J., Choi J., Kim J., Lim S., Yoo S.H., Lee Y., Lee H.-S. Versatile post-synthetic modifications of helical β-peptide foldamers derived from a thioether-containing cyclic β-amino acid. Angew. Chem. Int. Ed. 2023;135 doi: 10.1002/ange.202305196. [DOI] [PubMed] [Google Scholar]
- 54.Callmann C.E., Thompson M.P., Gianneschi N.C. Poly (peptide): synthesis, structure, and function of peptide-polymer amphiphiles and protein-like polymers. Acc. Chem. Res. 2020;53:400–413. doi: 10.1021/acs.accounts.9b00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tao K., Levin A., Adler-Abramovich L., Gazit E. Fmoc-modified amino acids and short peptides: simple bio-inspired building blocks for the fabrication of functional materials. Chem. Soc. Rev. 2016;45:3935–3953. doi: 10.1039/C5CS00889A. [DOI] [PubMed] [Google Scholar]
- 56.Becker E.D., Farrar T.C. Fourier transform spectroscopy. Science. 1972;178:361–368. doi: 10.1126/science.178.4059.361. [DOI] [PubMed] [Google Scholar]
- 57.Wang H.-W., Wang J.-W. How cryo-electron microscopy and X-ray crystallography complement each other. Protein Sci. 2017;26:32–39. doi: 10.1002/pro.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng S., Erich M., Matthias M., Dagmar G. On the progress of scanning transmission electron microscopy (STEM) imaging in a scanning electron microscope. Microsc. Microanal. 2018;24:99–106. doi: 10.1017/S1431927618000181. [DOI] [PubMed] [Google Scholar]
- 59.Hu S., Yang C., Li Y., Luo Q., Luo H. Nanozyme sensor array based on manganese dioxide for the distinction between multiple amyloid β peptides and their dynamic aggregation process. Biosens. Bioelectron. 2022;199 doi: 10.1016/j.bios.2021.113881. [DOI] [PubMed] [Google Scholar]
- 60.Chaurand P., Luetzenkirchen F., Spengler B. Peptide and protein identification by matrix-assisted laser desorption ionization (MALDI) and MALDI-post-source decay time-of-flight mass spectrometry. J. Am. Soc. Mass Spectrom. 1999;10:91–103. doi: 10.1016/S1044-0305(98)00145-7. [DOI] [PubMed] [Google Scholar]
- 61.Jiang Y., Chen Z., Sui N., Zhu Z. Data-driven evolutionary design of multienzyme-like nanozymes. J. Am. Chem. Soc. 2024;146:7565–7574. doi: 10.1021/jacs.3c13588. [DOI] [PubMed] [Google Scholar]
- 62.Zhuang J., Midgley A.C., Wei Y., Liu Q., Kong D., Huang X. Machine-learning-assisted nanozyme design: lessons from materials and engineered enzymes. Adv. Mater. 2024;36 doi: 10.1002/adma.202210848. [DOI] [PubMed] [Google Scholar]
- 63.Liu Q., Wang H., Shi X., Wang Z.-G., Ding B. Self-assembled DNA/peptide-based nanoparticle exhibiting synergistic enzymatic activity. ACS Nano. 2017;11:7251–7258. doi: 10.1021/acsnano.7b03195. [DOI] [PubMed] [Google Scholar]
- 64.Liu Y., Li N., Su K., Du J., Guo R. Arginine-rich peptide–rhodium nanocluster@reduced graphene oxide composite as a highly selective and active uricase-like nanozyme for the degradation of uric acid and inhibition of urate crystal. Inorg. Chem. 2024;63:13602–13612. doi: 10.1021/acs.inorgchem.4c01801. [DOI] [PubMed] [Google Scholar]
- 65.Yuan Y., Chen L., Kong L., Qiu L., Fu Z., Sun M., Liu Y., Cheng M., Ma S., Wang X., Zhao C., Jiang J., Zhang X., Wang L., Gao L. Histidine modulates amyloid-like assembly of peptide nanomaterials and confers enzyme-like activity. Nat. Commun. 2023;14:5808. doi: 10.1038/s41467-023-41591-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Y., Qin Y., Zhang Q., Zou W., Jin L., Guo R. Arginine-rich peptide/platinum hybrid colloid nanoparticle cluster: a single nanozyme mimicking multi-enzymatic cascade systems in peroxisome. J. Colloid Interf. Sci. 2021;600:37–48. doi: 10.1016/j.jcis.2021.05.025. [DOI] [PubMed] [Google Scholar]
- 67.Fan K., Wang H., Xi J., Liu Q., Meng X., Duan D., Gao L., Yan X. Optimization of Fe3O4 nanozyme activity via single amino acid modification mimicking an enzyme active site. Chem. Commun. 2016;53:424–427. doi: 10.1039/C6CC08542C. [DOI] [PubMed] [Google Scholar]
- 68.Madsen T.D., Hansen L.H., Hintze J., Ye Z., Jebari S., Andersen D.B., Joshi H.J., Ju T., Goetze J.P., Martin C., Rosenkilde M.M., Holst J.J., Kuhre R.E., Goth C.K., Vakhrushev S.Y., Schjoldager K.T. An atlas of O-linked glycosylation on peptide hormones reveals diverse biological roles. Nat. Commun. 2020;11:4033. doi: 10.1038/s41467-020-17473-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jian T., Zhou Y., Wang P., Yang W., Mu P., Zhang X., Zhang X., Chen C.-L. Highly stable and tunable peptoid/hemin enzymatic mimetics with natural peroxidase-like activities. Nat. Commun. 2022;13:3025. doi: 10.1038/s41467-022-30285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li S., Wu H., Liu Y., Zhang B., Xu S., Wang Z.-G. Oxidase-mimetic nanocatalyst based on geometry-dependent biomolecular self-assembly. Chem. Mater. 2023;35:10515–10523. doi: 10.1021/acs.chemmater.3c02028. [DOI] [Google Scholar]
- 71.Xia N., Liu G., Zhang S., Shang Z., Yang Y., Li Y., Liu L. Oxidase-mimicking peptide-copper complexes and their applications in sandwich affinity biosensors. Anal. Chim. Acta. 2022;1214 doi: 10.1016/j.aca.2022.339965. [DOI] [PubMed] [Google Scholar]
- 72.Lihi N., Kelemen D., May N.V., Fábián I. The role of the cysteine fragments of the nickel binding loop in the activity of the Ni(II)-containing SOD enzyme. Inorg. Chem. 2020;59:4772–4780. doi: 10.1021/acs.inorgchem.0c00057. [DOI] [PubMed] [Google Scholar]
- 73.Székely E., Molnár M., Lihi N., Várnagy K. Characterization of copper(II) and zinc(II) complexes of peptides mimicking the CuZnSOD enzyme. Molecules. 2024;29:795. doi: 10.3390/molecules29040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coulibaly K., Thauvin M., Melenbacher A., Testard C., Trigoni E., Vincent A., Stillman M.J., Vriz S., Policar C., Delsuc N. A di-copper peptidyl complex mimics the activity of catalase, a key antioxidant metalloenzyme. Inorg. Chem. 2021;60:9309–9319. doi: 10.1021/acs.inorgchem.0c03718. [DOI] [PubMed] [Google Scholar]
- 75.Liang S., Wu X.-L., Zong M.-H., Lou W.-Y. Construction of Zn-heptapeptide bionanozymes with intrinsic hydrolase-like activity for degradation of di (2-ethylhexyl) phthalate. J. Colloid Interf. Sci. 2022;622:860–870. doi: 10.1016/j.jcis.2022.04.122. [DOI] [PubMed] [Google Scholar]
- 76.Shen X., Wang Z., Gao X.J., Gao X. Reaction mechanisms and kinetics of nanozymes: insights from theory and computation. Adv. Mater. 2024;36 doi: 10.1002/adma.202211151. [DOI] [PubMed] [Google Scholar]
- 77.Kløve M., Sommer S., Iversen B.B., Hammer B., Dononelli W. A machine-learning-based approach for solving atomic structures of nanomaterials combining pair distribution functions with density functional theory. Adv. Mater. 2023;35 doi: 10.1002/adma.202208220. [DOI] [PubMed] [Google Scholar]
- 78.Wang G., Feng N., Zhao S., Song L., Zhang Y., Tong J., Liu Y., Kang X., Hu T., Ahmad Khan I., Lu K., Wu H., Xie J. Synthesis and DFT calculation of microbe-supported Pd nanocomposites with oxidase-like activity for sensitive detection of nitrite. Food Chem. 2024;434 doi: 10.1016/j.foodchem.2023.137422. [DOI] [PubMed] [Google Scholar]
- 79.Roessler M.M., Salvadori E. Principles and applications of EPR spectroscopy in the chemical sciences. Chem. Soc. Rev. 2018;47:2534–2553. doi: 10.1039/C6CS00565A. [DOI] [PubMed] [Google Scholar]
- 80.Li F., Xu B., Lu Z., Chen J., Fu Y., Huang J., Wang Y., Li X. Hollow CoFe nanozymes integrated with oncolytic peptides designed via machine-learning for tumor therapy. Small. 2024 doi: 10.1002/smll.202311101. [DOI] [PubMed] [Google Scholar]
- 81.Hesamzadeh P., Seif A., Mahmoudzadeh K., Ganjali Koli M., Mostafazadeh A., Nayeri K., Mirjafary Z., Saeidian H. De novo antioxidant peptide design via machine learning and DFT studies. Sci. Rep. 2024;14:6473. doi: 10.1038/s41598-024-57247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dutta S., Corni S., Brancolini G. Molecular dynamics simulations of a catalytic multivalent peptide-nanoparticle complex. Int. J. Mol. Sci. 2021;22:3624. doi: 10.3390/ijms22073624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jiang B., Yan L., Zhang J., Zhou M., Shi G., Tian X., Fan K., Hao C., Yan X. Biomineralization synthesis of the cobalt nanozyme in SP94-ferritin nanocages for prognostic diagnosis of hepatocellular carcinoma. ACS Appl. Mater. Interfaces. 2019;11:9747–9755. doi: 10.1021/acsami.8b20942. [DOI] [PubMed] [Google Scholar]
- 84.Chen Q., Duan X., Yu Y., Ni R., Song G., Yang X., Zhu L., Zhong Y., Zhang K., Qu K., Qin X., Wu W. Target functionalized carbon dot nanozymes with dual-model photoacoustic and fluorescence imaging for visual therapy in atherosclerosis. Adv. Sci. 2024;11 doi: 10.1002/advs.202307441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li H., Qian X., Mohanram H., Han X., Qi H., Zou G., Yuan F., Miserez A., Liu T., Yang Q., Gao H., Yu J. Self-assembly of peptide nanocapsules by a solvent concentration gradient. Nat. Nanotechnol. 2024:1–9. doi: 10.1038/s41565-024-01654-w. [DOI] [PubMed] [Google Scholar]
- 86.Mu R., Zhu D., Abdulmalik S., Wijekoon S., Wei G., Kumbar S.G. Stimuli-responsive peptide assemblies: design, self-assembly, modulation, and biomedical applications. Bioact. Mater. 2024;35:181–207. doi: 10.1016/j.bioactmat.2024.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang F., Xia W., Zhang M., Wu R., Song X., Hao Y., Feng Y., Zhang L., Li D., Kang W., Liu C., Liu L. Engineering of antimicrobial peptide fibrils with feedback degradation of bacterial-secreted enzymes. Chem. Sci. 2023;14:10914–10924. doi: 10.1039/D3SC01089A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.He W., Xing X., Wang X., Wu D., Wu W., Guo J., Mitragotri S. Nanocarrier-mediated cytosolic delivery of biopharmaceuticals. Adv. Funct. Mater. 2020;30 doi: 10.1002/adfm.201910566. [DOI] [Google Scholar]
- 89.Beenken A. Endocytosis begins inside the cell. J. Am. Soc. Nephrol. 2022;33:661. doi: 10.1681/ASN.2022020155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jafari B., Pourseif M.M., Barar J., Rafi M.A., Omidi Y. Peptide-mediated drug delivery across the blood-brain barrier for targeting brain tumors, Expert Opin. Drug Deliv. 2019;16:583–605. doi: 10.1080/17425247.2019.1614911. [DOI] [PubMed] [Google Scholar]
- 91.Zhao Y., Jiang H., Yu J., Wang L., Du J. Engineered histidine-rich peptides enhance endosomal escape for antibody-targeted intracellular delivery of functional proteins. Angew. Chem. Int. Ed. 2023;62 doi: 10.1002/anie.202310355. [DOI] [PubMed] [Google Scholar]
- 92.Xie W., Gan Y., Wang L., Si Y., Li Q., Song T., Wei P., Wu Z., Zhang G. Tumor microenvironment-activated nanostructure to enhance MRI capability and nanozyme activity for highly tumor-specific multimodal theranostics. Small. 2024;20 doi: 10.1002/smll.202306446. [DOI] [PubMed] [Google Scholar]
- 93.Cai Y., Dong T., Zhang X., Liu A. Morphology and enzyme-mimicking activity of copper nanoassemblies regulated by peptide: mechanism, ultrasensitive assaying of trypsin, and screening of trypsin inhibitors. Anal. Chem. 2022;94:18099–18106. doi: 10.1021/acs.analchem.2c04767. [DOI] [PubMed] [Google Scholar]
- 94.Zhang G., Li Q., Li J., Pan M., Wang Y., Su R., Qi W., Zhang W. Gold nanoparticles mineralized by peptide liquid crystals with dual-functional enzyme-like activities: an automatic membrane reactor for glucose detection. Langmuir. 2023;39:8779–8786. doi: 10.1021/acs.langmuir.3c00792. [DOI] [PubMed] [Google Scholar]
- 95.Zhao C., Jian X., Gao Z., Song Y.-Y. Plasmon-mediated peroxidase-like activity on an asymmetric nanotube architecture for rapid visual detection of bacteria. Anal. Chem. 2022;94:14038–14046. doi: 10.1021/acs.analchem.2c03471. [DOI] [PubMed] [Google Scholar]
- 96.Wang X.-Y., Ji R.-Y., Lang W.-W., Qin K.-X., Bai F.-Y., Xi H.-Y., Zheng Y., Xia B.-X., Dong L.-Y., Wang X.-H. Integrating PEGylated peptide-oriented bacteria-imprinted matrix and PdPt bimetallic-doped imidazolium zeolite framework-8 for sensitive detection of Escherichia coli with smartphone readout system. Sensor Actuat. B-Chem. 2024;411 doi: 10.1016/j.snb.2024.135749. [DOI] [Google Scholar]
- 97.Lian M., Zhang S., Chen J., Liu X., Chen X., Yang W. Self-assembling peptide artificial enzyme as an efficient detection prober and inhibitor for cancer cells. ACS Appl. Bio Mater. 2019;2:2185–2191. doi: 10.1021/acsabm.9b00160. [DOI] [PubMed] [Google Scholar]
- 98.Zhu X., Wang X., Liu Z., Jiang B., He Z., Liu S., Wu Y., Wu Z., Zhang T., Liu M., Li K., Niu X., Gao Y. Peroxidase-like nanozyme activates the cGAS-STING pathway via ROS-induced mtDNA release for cancer immunotherapy. Adv. Funct. Mater. 2024 doi: 10.1002/adfm.202401576. [DOI] [Google Scholar]
- 99.Lee J., Liao H., Wang Q., Han J., Han J.-H., Shin H.E., Ge M., Park W., Li F. Exploration of nanozymes in viral diagnosis and therapy. Exploration. 2022;2 doi: 10.1002/EXP.20210086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhou C., Wang Q., Jiang J., Gao L. Nanozybiotics: nanozyme-based antibacterials against bacterial resistance. Antibiotics. 2022;11:390. doi: 10.3390/antibiotics11030390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Soria-Carrera H., Atrián-Blasco E., de la Fuente J.M., Mitchell S.G., Martín-Rapún R. Polyoxometalate-polypeptide nanoassemblies as peroxidase surrogates with antibiofilm properties. Nanoscale. 2022;14:5999–6006. doi: 10.1039/D1NR08223J. [DOI] [PubMed] [Google Scholar]
- 102.Wang B., Zhang W., Pan Q., Tao J., Li S., Jiang T., Zhao X. Hyaluronic acid-based CuS nanoenzyme biodegradable microneedles for treating deep cutaneous fungal infection without drug resistance. Nano Lett. 2023;23:1327–1336. doi: 10.1021/acs.nanolett.2c04539. [DOI] [PubMed] [Google Scholar]
- 103.Geng Z., Cao Z., Liu J. Recent advances in targeted antibacterial therapy basing on nanomaterials. Exploration. 2023;3 doi: 10.1002/EXP.20210117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yuan Y., Chen L., Song K., Cheng M., Fang L., Kong L., Yu L., Wang R., Fu Z., Sun M., Wang Q., Cui C., Wang H., He J., Wang X., Liu Y., Jiang B., Jiang J., Wang C., Yan X., Zhang X., Gao L. Stable peptide-assembled nanozyme mimicking dual antifungal actions. Nat. Commun. 2024;15:5636. doi: 10.1038/s41467-024-50094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Conley B.M., Yang L., Bhujel B., Luo J., Han I., Lee K.-B. Development of a nanohybrid peptide hydrogel for enhanced intervertebral disc repair and regeneration. ACS Nano. 2023;17:3750–3764. doi: 10.1021/acsnano.2c11441. [DOI] [PubMed] [Google Scholar]
- 106.Chen Z., Zhang Q., Li H., Wei Q., Zhao X., Chen F. Elastin-like polypeptide modified silk fibroin porous scaffold promotes osteochondral repair. Bioact. Mater. 2021;6:589–601. doi: 10.1016/j.bioactmat.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao C., Li X., Bian S., Zeng W., Ronca A., D'Amora U., Raucci M.G., Liang J., Sun Y., Jiang Q., Fan Y., Ambrosio L., Zhang X. Nanofibrous polypeptide hydrogels with collagen-like structure as biomimetic extracellular matrix. Collagen Leather. 2023;5:1–13. doi: 10.1186/s42825-022-00110-6. [DOI] [Google Scholar]
- 108.Zhu P., Chen Y., Shi J. Nanoenzyme-augmented cancer sonodynamic therapy by catalytic tumor oxygenation. ACS Nano. 2018;12:3780–3795. doi: 10.1021/acsnano.8b00999. [DOI] [PubMed] [Google Scholar]
- 109.Shi J., Yu W., Xu L., Yin N., Liu W., Zhang K., Liu J., Zhang Z. Bioinspired nanosponge for salvaging ischemic stroke via free radical scavenging and self-adapted oxygen regulating. Nano Lett. 2020;20:780–789. doi: 10.1021/acs.nanolett.9b04974. [DOI] [PubMed] [Google Scholar]
- 110.Gulseren G., Yasa I.C., Ustahuseyin O., Tekin E.D., Tekinay A.B., Guler M.O. Alkaline phosphatase-mimicking peptide nanofibers for osteogenic differentiation. Biomacromolecules. 2015;16:2198–2208. doi: 10.1021/acs.biomac.5b00593. [DOI] [PubMed] [Google Scholar]
- 111.Lu Y., Li Z., Li L., Chen J., Xu X., Lin Z., Zhang T., Zhu Y., Ding C., Mao C. Highly effective rheumatoid arthritis therapy by peptide-promoted nanomodification of mesenchymal stem cells. Biomaterials. 2022;283 doi: 10.1016/j.biomaterials.2022.121474. [DOI] [PubMed] [Google Scholar]
- 112.Chawla V., Sharma S., Singh Y. Yttrium oxide nanoparticle-loaded, self-assembled peptide gel with antibacterial, anti-inflammatory, and proangiogenic properties for wound healing. ACS Biomater. Sci. Eng. 2023;9:2647–2662. doi: 10.1021/acsbiomaterials.3c00134. [DOI] [PubMed] [Google Scholar]
- 113.Bai Z., Ge K., Fu J., Yu D., Hua Z., Xue S., Li Z., Sheng W., Wu X., Gao F., Geng D., Gao F. Engineered urinary-derived extracellular vesicles loaded nanoenzymes as Trojan horses to regulate the inflammatory microenvironment for treatment of Alzheimer's disease. Chem. Eng. J. 2023;465 doi: 10.1016/j.cej.2023.142955. [DOI] [Google Scholar]
- 114.Chen H., Zhang T., Tian Y., You L., Huang Y., Wang S. Novel self-assembling peptide hydrogel with pH-tunable assembly microstructure, gel mechanics and the entrapment of curcumin. Food Hydrocolloid. 2022;124 doi: 10.1016/j.foodhyd.2021.107338. [DOI] [Google Scholar]
- 115.Wang C., Fei J., Wang K., Li J. A dipeptide-based hierarchical nanoarchitecture with enhanced catalytic activity. Angew. Chem., Int. Ed. 2020;59:18960–18963. doi: 10.1002/anie.202006994. [DOI] [PubMed] [Google Scholar]
- 116.Chen K., Hu Y., Dong X., Sun Y. Molecular insights into the enhanced performance of EKylated PETase toward PET degradation. ACS Catal. 2021;11:7358–7370. doi: 10.1021/acscatal.1c01062. [DOI] [Google Scholar]
- 117.Liu Q., Tian J., Liu J., Zhu M., Gao Z., Hu X., Midgley A.C., Wu J., Wang X., Kong D., Zhuang J., Liu J., Yan X., Huang X. Modular assembly of tumor-penetrating and oligomeric nanozyme based on intrinsically self-assembling protein nanocages. Adv. Mater. 2021;33 doi: 10.1002/adma.202103128. [DOI] [PubMed] [Google Scholar]
- 118.Kong F., Bai H., Ma M., Wang C., Xu H., Gu N., Zhang Y. Fe3O4@Pt nanozymes combining with CXCR4 antagonists to synergistically treat acute myeloid leukemia. Nano Today. 2021;37 doi: 10.1016/j.nantod.2021.101106. [DOI] [Google Scholar]
- 119.Amin M., Lammers T., ten Hagen T.L.M. Temperature-sensitive polymers to promote heat-triggered drug release from liposomes: towards bypassing EPR. Adv. Drug Deliv. Rev. 2022;189 doi: 10.1016/j.addr.2022.114503. [DOI] [PubMed] [Google Scholar]
- 120.Liu J., Zhou Y., Lyu Q., Yao X., Wang W. Targeted protein delivery based on stimuli-triggered nanomedicine. Exploration. 2023 doi: 10.1002/EXP.20230025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shao N., Yuan L., Liu L., Cong Z., Wang J., Wu Y., Liu R. Reversing anticancer drug resistance by synergistic combination of chemotherapeutics and membranolytic antitumor β-peptide polymer. J. Am. Chem. Soc. 2024;146:11254–11265. doi: 10.1021/jacs.4c00434. [DOI] [PubMed] [Google Scholar]
- 122.Kozuch S., Martin J.M.L. “Turning over” definitions in catalytic cycles. ACS Catal. 2012;2:2787–2794. doi: 10.1021/cs3005264. [DOI] [Google Scholar]
- 123.Zandieh M., Liu J. Surface science of nanozymes and defining a nanozyme unit. Langmuir. 2022;38:3617–3622. doi: 10.1021/acs.langmuir.2c00070. [DOI] [PubMed] [Google Scholar]
- 124.Wei L., Ye X., Sakurai T., Mu Z., Wei L. ToxIBTL: prediction of peptide toxicity based on information bottleneck and transfer learning. Bioinformatics. 2022;38:1514–1524. doi: 10.1093/bioinformatics/btac006. [DOI] [PubMed] [Google Scholar]
- 125.Dai H., Fan Q., Wang C. Recent applications of immunomodulatory biomaterials for disease immunotherapy. Exploration. 2022;2 doi: 10.1002/EXP.20210157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Carny O., Gazit E. A model for the role of short self-assembled peptides in the very early stages of the origin of life. FASEB J. 2005;19:1051–1055. doi: 10.1096/fj.04-3256hyp. [DOI] [PubMed] [Google Scholar]
- 127.Kuila S., Nanda J. Cysteine-based dynamic self-assembly and their importance in the origins of life. Syst. Chem. 2024;6 doi: 10.1002/syst.202400022. [DOI] [Google Scholar]
- 128.Huang X.-L., Harmer J.R., Schenk G., Southam G. Inorganic Fe-O and Fe-S oxidoreductases: paradigms for prebiotic chemistry and the evolution of enzymatic activity in biology. Front. Chem. 2024;12 doi: 10.3389/fchem.2024.1349020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang X.-L. Unveiling the role of inorganic nanoparticles in Earth's biochemical evolution through electron transfer dynamics. iScience. 2024;27 doi: 10.1016/j.isci.2024.109555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hlouchová K. Peptides en route from prebiotic to biotic catalysis. Acc. Chem. Res. 2024;57:2027–2037. doi: 10.1021/acs.accounts.4c00137. [DOI] [PMC free article] [PubMed] [Google Scholar]