Abstract
The lack of adequate anti-leishmanial therapies has led to the continued suffering of millions of people from developing nations. Moreover, optimism for a therapeutic intervention by fexinidazole was dashed due to the inability to maintain cures and control unwanted side effects. To solve these shortcomings, the structural elements of fexinidazole responsible for anti-leishmanial activity and toxicities were explored. Accordingly, a systematic analog design approach was taken for the synthesis of 24 novel analogs. We established the structural features important for activity and identified modifications that improved the hERG receptor safety and liver microsomal metabolic stability. Compared to fexinidazole, the S-configured imidazolooxazole analog 51 exhibited 25-fold greater potency against miltefosine resistant L. donovani amastigotes, greater metabolic stability and little hERG receptor inhibition. Replacement of the toxicophore nitro group for a cyano group resulted in a complete loss of anti-leishmanial activity. The SAR findings should be useful in the further development of this important class of anti-leishmanial agents.
Structure-guided optimization of fexinidazole led to analog (S)-51, a promising lead compound with superior activity, improved metabolic stability, and enhanced hERG safety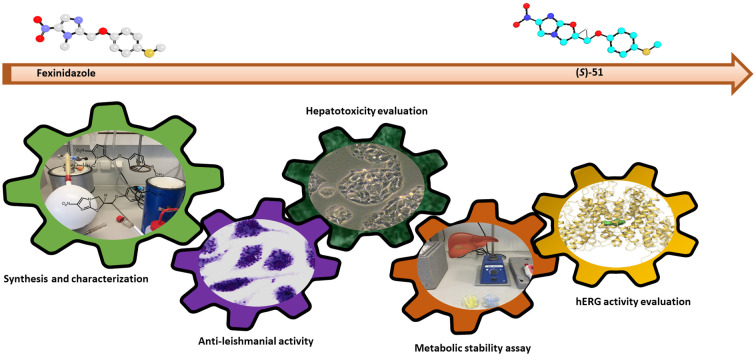
Introduction
Leishmaniasis is a protozoal disease estimated to represent the ninth most prevalent infectious disease worldwide.1 It is caused by more than 20 protozoal species from the genus Leishmania that are transmitted to humans through the bite of over 90 species of phlebotomine sand flies. Human leishmaniasis occurs in three main forms: visceral, cutaneous and mucocutaneous. Visceral leishmaniasis is the most severe form that can lead to mortality in over 95% of patients if left untreated. Conversely, cutaneous leishmaniasis is the most prevalent form with an estimated 600 000 to 1 million new infections worldwide annually.2 A specific-type of cutaneous leishmaniasis called mucocutaneous leishmaniasis results in partial or total destruction of mucous membranes and cartilages in the nose, mouth and throat. Ethiopia together with Bolivia, Brazil and Peru account for over 90% of mucocutaneous leishmaniasis.2,3
Leishmaniasis predominantly affects people in some of the most economically disadvantaged regions of the world, thus it has not received the necessary attention from the profit-driven pharmaceutical industries. However, through global warming it has been predicted that this disease will eventually spread beyond the regions of the world where it is now endemic.3 No effective vaccine has been approved for human leishmaniasis, leaving chemotherapy as the only treatment option.4 However, the available chemotherapeutic agents are inadequate and are associated with various complications. The first-line pentavalent antimonial treatments such as sodium stibogluconate (1, Fig. 1) have been used for more than eight decades, and are associated with serious adverse effects and rapid emergence of resistant strains. The alternative treatments, amphotericin B (2) or pentamidine (3), again are limited by severe toxicity and high treatment costs. The recent important advance in leishmanial therapy is miltefosine (4), the only orally active treatment, which was approved in 2014.5 Nevertheless, disadvantages such as high cost, teratogenicity, and recent reports of resistance development are impeding the widespread use of miltefosine.6–8 Unfortunately, no new drugs have been introduced to complement the currently inadequate drug therapies. Hence, the treatment gap is anticipated to widen even further.
Fig. 1. The chemical structures of anti-leishmanial compounds: sodium stibogluconate (1), amphotericin B (2), pentamidine (3), miltefosine (4) and fexinidazole (5).
In 2019, fexinidazole (5, Fig. 1) was approved by the European Medicine Agency (EMA) as a 10-day, once-a-day treatment for Human African trypanosomiasis (HAT), also known as African Sleeping Sickness.9Leishmania protozoa and Trypanosoma brucei, the causative agent of HAT, share notable similarities in their structural and biochemical features. Both are taxonomically categorized in a group named kinetoplastid.10 With this close relation in mind, the anti-trypanosomal agent fexinidazole (5) was investigated and found to have equipotent in vivo anti-leishmanial activity as 4.11 To further establish its efficacy, clinical investigations of 5 for the treatment of leishmaniasis were initiated. However, a phase II proof-of-concept trial to demonstrate the efficacy of 5 in visceral leishmaniasis was discontinued for a number of reasons.12 Despite the microscopic clearance of parasites at the end of fexinidazole treatment, relapses were noted during subsequent follow-up investigations.13 In addition, the use of 5 is contraindicated in patients with liver disease or risk of cardiac arrhythmia.14 Hence, the development of novel analogs of 5 with improved potency, broader spectrum of activity and minimal toxicities, i.e., hepato- and cardiotoxicity, was the goal of our research.
Leishmania parasites exist as motile flagellated promastigotes inside the digestive tract of transmitting sand flies. After mammalian infection, promastigotes undergo differentiation within granulocyte neutrophils and tissue macrophages into the non-motile amastigotes.15 Despite the excellent activity of 5 against axenic amastigotes, little effect on the viability of intracellular amastigotes in peritoneal mouse macrophages (PEM) was observed up to 50 mM.11 This property of 5 suggests the importance of optimizing the intracellular anti-amastigote activity of its analogs. The in vitro anti-leishmanial properties of 5 against various strains of Leishmania have been reported.11,16 The activity profile of 5 in our assay was comparable to published results.11 We have extended the activity profile of 5 and its metabolites 6–7 by including activity evaluations against L. donovani LdHU3MILR40 promastigotes and amastigotes. Developing novel drugs active against the alarming miltefosine resistant strain LdHU3MILR40 is among the main objectives of our research.
Results and discussion
Rationale behind the structural modifications and chemistry
The first set of modifications, outlined in the general scheme in Fig. 2, were aimed at establishing the importance of two structural elements in 5 to its anti-leishmania properties and toxicities. The sulfide in 5 was modified to SO, SO2, SONH and CH2 to explore the effect of different oxidation states of sulfur atom (λ2, λ4 and λ6) and the stable carbon atom on activity and toxicities. Alternately, the structural element NO2 in 5 was replaced by H or by CN to determine if the nitro moiety can be replaced without loss of activity. Subsequently, we explored analogs featuring a stable carbon bridge in lieu of the ether bridge in 5, as illustrated in type B modification. Analogs incorporating substituents adopted from other marketed or investigational anti-kinetoplastid agents, in place of the methylether linker and the 4-thiomethyl phenyl groups in 5, were synthesized as outlined in a type C modification. This modification aimed to enhance metabolic stability while maintaining or improving activity by exploring structural alternatives for the linker and phenyl moiety of 5. Analogs with a type D modification were synthesized by completely replacing the nitroimidazole group of 5 with a benzoxaborole group. This modification was a part of our pursuit to replace the nitroimidazole warhead with active and less toxic structural motif. Eventually, we incorporated the classic yet currently popular technique of bicyclic nitroimidazole to the structure of 5. This annulation with heterocycles is promoting new nitroimidazole-based drugs in the contemporary drug discovery pipeline.17 Analogues wherein the nitroimidazole group of 5 conjugated with either oxazole or pyrazinone were synthesized, represented by general structures E and F, respectively.
Fig. 2. General scheme of the types of structural modifications made to fexinidazole.
Type A
The parent compound 5 and its metabolites 6–7 were synthesized following established procedures, with minor modifications (Scheme 1). Synthesis of 5 was initiated with the hydroxylation of 1-methyl-5-nitroimidazole from paraformaldehyde.18 A rapid chlorination of the resulting hydroxyl bearing intermediate was achieved by refluxing with excess thionyl chloride.19 Williamson ether synthesis catalyzed by powdered potassium carbonate was then applied to yield 5.20 The overall yield for the synthesis of 5 was 28%. Sulfoxidation of 5 with m-chloroperoxybenzoic acid (mCPBA) yielded both metabolites 6 and 7, which were separated by flash chromatography.21 On the other hand, direct sulfoximation of the sulfide 5 catalyzed by diacetoxyl iodobenzene with ammonium carbonate as imine donor led to analog 21.22 The synthetic procedures of 5 with some changes were applied for the synthesis of analogues 17–19. The synthesis of analog 17 without an electron-withdrawing nitro group required a relatively strong reaction conditions with cesium carbonate and potassium iodide in step d of Scheme 1.23 Furthermore, an additional step for N-methylation of 4-cyanoimidazole with dimethyl sulfate was needed (step a, Scheme 1) en route to analog 18.23
Scheme 1. a) For compound 10: dimethyl sulfate, dioxane, reflux, 5 h; b) paraformaldehyde, DMSO, 110 °C, 48 h; c) SOCl2, DCM, reflux, 30 min; d) for compounds 5, 18 & 19: 4-(methylthio)phenol, DMF, K2CO3, 60 °C, 3 h; for compound 17: 4-(methylthio)phenol, Cs2CO3, KI, DMF, N2, 80 °C, overnight; for compound 20: 4-(methylthio)aniline, TEA, 2-propanol, reflux, overnight; e) for compounds 6 & 7: mCPBA, DCM, 0 °C, 30 min; for compound 21: (NH4)2CO3, PhI(OAc)2, rt, 10 min.
Type B
The acidity of the methyl moiety in the 2-methyl substituted imidazole (22 and 23) allowed a sodium methoxide catalyzed aldol addition with 4-(methylthio)benzaldehyde (Scheme 2) followed by dehydration to give 24 or 25.24,25 Selective reduction of the olefinic group in 24 or 25 was achieved with p-toluenesulfonyl hydrazide without reduction of the nitro group to give 26 or 27 with a saturated carbon linkage.24
Scheme 2. a) NaOCH3, 4-(methylthio)benzaldehyde, ethanol, 65 °C, overnight; b) p-toluenesulfonyl hydrazide, K2CO3, pyridine, 140 °C, 13 h; c) (NH4)2CO3, PhI(OAc)2, methanol, rt, 10 min.
Type C
Oxidation of the 2-methylhydroxyl moiety of intermediate 12 with potassium permanganate initiated the synthesis of analogues 33–35 that bear an amide linker (Scheme 3a). Different types of amidation reactions were necessary for each analog due to differences in the nucleophilicity of the amine reagents. HATU amide coupling was effective for the synthesis of analogs 32 and 34.21 Triethylamine catalyzed amidation required a prior reaction of 29 with oxalyl chloride to the corresponding acid chloride to give analog 33.26 With the less nucleophilic 4-fluoro-2-(trifluoromethyl)aniline, activation of 29 with methanesulfonyl chloride was required for the synthesis of analog 35.27
Scheme 3. Synthesis of chemotherapeutic hybrids: a) KMnO4, acetone, ice-cold water bath to rt, 5 h; b) for compound 32: 4-(methylthio)aniline, HATU, and TEA, MeCN, 40 °C, overnight; for compound 33: oxalyl chloride, anhydrous DCM, N2, ice-cold water bath, DMF (2 drops), 2 h, then 4-(methylthio)aniline, TEA, MeCN, 40 °C, overnight; for compound 34: thiomorpholine 1,1-dioxide, HATU, and DIPEA, DCM, rt, 2 h; for compound 35: 1-methylimidazole, methanesulfonyl chloride, DMF, 0 °C, 20 min, then 2-trifluromethyl-4-fluoroaniline, rt, 5 h; c) conc. H2SO4, HNO3, 80 °C, 6 h; d) TEA, MeCN, HATU, rt, 15 min, then 4-(methylthio)aniline, 40 °C, overnight. b: a) Conc. H2SO4, −20 °C, then HNO3, −15 °C, 10 min; b) Pd/C (10%), ethyl acetate, H2, overnight; c) 4-(methylthio)benzoic acid for analog 38 or intermediate 39 for analog 40, DIPEA, HATU, DCM, rt, overnight.
Type D
Prior to amidation in the synthesis of benzoxaborole analogues 38 and 40 (Scheme 3b), nitration of the aromatic ring of benzoxaborole by using the conventional method involving HNO3 and H2SO4 was performed (intermediate 36), which was followed by reduction of the resulting nitro group to an amine (intermediate 37) by Pd/C catalyzed hydrogenation.28 Boronic acid is susceptible to oxidative degradation by peroxides, thus the oxidative stability was improved by caging boronic acid into a borolactone.29 However, to avoid any oxidative hydrolysis of the borolactone 38 from mCPBA, the sulfoxide intermediate 4-(methylsulfinyl)benzoic acid 39 was synthesized by oxidizing 4-(methylthio)benzoic acid separately, followed by amidation to yield 40.
Type E
The synthesis of imidazolooxazole rac-49 was commenced from epibromohydrine (Scheme 4). In contrast, oxiran-2-ylmethyl 3-nitrobenezenesulfonate containing the corresponding configuration was used as a starting material in the synthesis of (R)-50 and (S)-51 enantiomers.8 The enantiomeric purity of (R)-50 and (S)-51 was confirmed by using a chiral HPLC method (see ESI†). Oxidation of rac-49 with mCPBA did not proceed smoothly as 5 and most of the starting material was left unoxidized. Hence, additional equivalent of the oxidizing agent and longer reaction times were needed in order to achieve the sulfone derivative 53. As a result, a separate synthesis of the sulfoxide analogue 52 was initiated by sulfoxidation of 42 to 44.
Scheme 4. a) For compound 41: 4-(methylthio)phenol, epibromohydrine, K2CO3, butanone, 80 °C, 36 h; for compound 42 and 43: 4-(methylthio)phenol, CsF, anhydrous DMF, N2, rt, 1 h, then (R)-oxiran-2-ylmethyl 3-nitrobenzenesulfonate for 42/(S)-oxiran-2-ylmethyl 3-nitrobenzenesulfonate for 43, N2, rt, 36 h; for compound 44: intermediate 42, mCPBA, DCM, 0 °C, 1.5 h; b) 2-bromo-4-nitroimidazole, DIPEA, 107 °C, 16 h; c) dry NaH, anhydrous DMF, N2, 0 °C, 70 min; d) compound 49, mCPBA, DCM, rt, overnight.
Type F
The route followed for the synthesis of nitroimidazopyrazinone 58–59 is given in Scheme 5. After nitration of the imidazole ring in 30, the 2-carboxylic group in 31 was chlorinated and amidated to yield intermediates 54–55. Finally, alkylation of 54–55 with bromoacetaldehyde diethyl acetal followed by acidic conditions with heating to 100 °C led to a cyclization between the free amide nitrogen and the diethylacetal substituent attached to the N1 of imidazole, resulting in either 58 or 59 in overall yields of 29% and 27%, respectively.30
Scheme 5. a) Conc. H2SO4, HNO3, 80 °C, 6 h; b) oxalyl chloride, DMF, DCM, overnight, rt, then 4-methoxybenzylamine for 54 or 4-(methylthio)benzylamine for 55, TEA, DCM, 0 °C, 15 min; c) bromoacetaldehyde diethyl acetal, K2CO3, DMF, MW 180 °C, 15 min, 2 times; d) H2O, HCl, 100 °C, 4.5 h.
Anti-promastigote profiles and structure–activity relationships
Replacing putative toxicophores with less reactive structural features is a commonly employed technique to identify lead structural moieties responsible for activity, toxicity and to elucidate the mechanism of toxicity. The nitroaromatic substructure was hypothesized as the cause of the hepatotoxicity associated with 5. To this end, structural modification was commenced by synthesizing compound 17 (Scheme 1), an analog of 5, that does not bear a nitro group. However, activity was lost against all tested Leishmania strains (Table 1). The loss of activity might be due to such incomparable stereo-electronic properties. Therefore, a switch from the nitro moiety in 5 to the bioisosteric cyano moiety was applied in analog 18. This type of switch retained the stereo-electronic properties while improving the hepatotoxicity property in flutamide derivatives.31 However, similar to 17, the anti-leishmanial efficacy was lost in 18. Unfortunately, the nitro group appears to be essential for anti-leishmanial activity. The lack of activity of 17 and 18 is supporting evidence to the postulated mechanism of action of 5; i.e., the activation of the nitro group in 5 by specific enzymes within the parasites generates reactive species that are toxic to and capable of inhibiting the DNA synthesis of the parasites.9
Summary of the types of modifications, structure, activity, toxicity and metabolic stability of 1, its metabolites 2–3, and synthesized analogs.

| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Substituents | Anti-promastigote IC50 (μM) | Cytotoxicity IC50 (μM) | hERG IC25 (μM) | Metabolic stability | |||||||||
| R1 | X | R2 | LV78 | UA847 | FV1 | HU3 | LdHU3MILR40 | L929 | RAW 264.7 | Micro-somesa (%) | S9b (%) | |||
| Fexinidazolec | A | NO2 | S | — | 7.0 | 3.9 | 1.9 | 3.3 | 5.5 | >100 | >100 | 8.6 | 0 | 73.4 |
| 6 | A | NO2 | SO | — | 7.8 | 4.3 | 1.4 | 3.4 | 4.3 | >100 | >100 | >100 | 100 | 100 |
| 7 | A | NO2 | SO2 | — | 9.4 | 5.9 | 1.7 | 3.8 | 5.6 | >100 | >100 | 42.2 | 100 | 100 |
| 17 | A | H | S | — | >20 | >20 | >20 | >20 | >20 | >100 | >91.1 | 34.5 | 100 | 100 |
| 18 | A | CN | S | — | >20 | >20 | >20 | >20 | >20 | >100 | >99.8 | 27.1 | 8.2 | 82.6 |
| 19 | A | NO2 | C | — | 4.5 | 3.9 | 1.4 | 5.0 | 5.3 | 71.8 | >68.5 | 14.9 | 0 | 96.4 |
| 20d | A | NO2 | S | — | >20 | >20 | 9.5 | >20 | 11.9 | >100 | >100 | 3.1 | 0 | 73.4 |
| 21 | A | NO2 | SONH | — | 14.6 | 9.6 | 2.7 | 12.2 | 12.3 | >100 | >100 | >100 | 100 | 100 |
| 26 | B | CH3 | S | — | 27.2 | 17.5 | 9.1 | 12.5 | 7.1 | >100 | >91.7 | 9.8 | 0 | 47.5 |
| 27 | B | (CH2)2OH | S | — | >20 | >20 | >20 | >20 | >20 | >100 | >100 | 6.0 | 20.5 | 76.8 |
| 28 | B | CH3 | SONH | — | >20 | >20 | 11.8 | >20 | >20 | >100 | >100 | 49.4 | 100 | 100 |
| 32 | C | H | — | NH(C6H4)SCH3 | >20 | >20 | >20 | >20 | >13.9 | 84.7 | >68.7 | >12.5 | 18.4 | 68.2 |
| 33 | C | CH3 | — | NH(C6H4)SCH3 | >20 | >20 | 6.5 | >20 | >20 | >100 | >100 | >3.1 | 0 | 6.9 |
| 34 | C | CH3 | — | (NC4H8S)O2 | 13.3 | 12.2 | 3.4 | 7.5 | 13.7 | >100 | >100 | >6.2 | 100 | 100 |
| 35 | C | CH3 | — | NH(C6H3)CF3F | 4.5 | 2.3 | 1.2 | 8.1 | 12.2 | >100 | >100 | >6.2 | 100 | 100 |
| 38 | D | — | S | — | 3.6 | 4.4 | 3.9 | 11.9 | 15.4 | >100 | 73.6 | >6.2 | 8.0 | 61.3 |
| 40 | D | — | SO | — | >20 | >20 | >20 | >16.8 | >20 | >100 | >100 | 25.7 | 95.2 | 100 |
| 49 | E | — | S | — | 1.8 | 1.5 | 3.4 | 1.0 | 0.4 | >100 | >100 | 2.9 | 55.8 | 90.4 |
| 50 | E | — | S | — | 1.9 | 1.4 | 5.7 | 1.1 | 0.4 | >100 | >95.3 | 3.1 | 64.7 | 92.1 |
| 51 | E | — | S | — | 1.2 | 1.4 | 3.1 | 1.1 | 0.5 | >92.2 | >68.5 | >6.2 | 67.5 | 97.0 |
| 52 | E | — | SO | — | >20 | >20 | >20 | 10.2 | 7.1 | >100 | >100 | 30.1 | 100 | 100 |
| 53 | E | — | SO2 | — | >20 | >20 | >20 | >20 | 4.5 | >100 | >100 | 15.2 | 100 | 100 |
| 58 | F | — | O | — | >20 | >20 | 6.9 | >12.4 | >18.5 | >100 | >100 | >2 | 100 | 100 |
| 59 | F | — | S | — | >20 | >20 | 8.9 | >16.6 | >20 | >57.5 | 48.1 | >2 | 93.1 | 100 |
| Miltefosinee | — | — | — | — | 22.04 | 17.8 | 7.0 | 6.9 | >40 | 81.5 | 9.9 | — | — | — |
Percentage left after 32 min of incubations in rat microsomes.
Percentage of test compounds left after 32 min of incubation in rat S9 cells in the presence of GSH.
The parent compound 5 was used as a control for metabolic incubation assays.
Analog 20 has an NH in place of O moiety.
Miltefosine (4) was used as a positive control in the evaluation of anti-promastigote activity. The structure of 4 is given in Fig. 1. Synthesized compounds were tested against the cutaneous strains Leishmania amazonensis (LV78), Leishmania braziliensis (UA847), and Leishmania major (FV1), as well as the visceral strains Leishmania donovani (HU3) and miltefosine (MIL)-resistant Leishmania donovani s (HU3MILR40).
The metabolism of 5 is characterized by the rapid oxidation of the sulfide moiety, as elaborated later. Enhanced exposure of parasites to 5 could be attained if this metabolic pathway were to be blocked. Accordingly, a stable methylene analog 19 was synthesized. Replacing the sulfide for methylene preserved the in vitro anti-leishmanial profile across all tested Leishmania strains, with the exception of L. donovani HU3 (Table 1).
Taking the near equipotent activity of metabolites 6 and 7 formed by oxidation of sulfide group into consideration (Table 1), analogs bearing other oxidized forms of sulphur were designed. Sulfoximines are mono-aza analogs of sulfones and are currently attracting much interest due to favourable properties such as better water solubility, hydrogen bond donor or acceptor abilities and improved chemical and metabolic stability compared to sulfones.32 However, the potency of the sulfoximine analog 21, apart from L. major FV1 strain, was consistently lower in tested promastigotes (Table 1).
Multiple attempts to chemically reduce the nitro moiety in 5 with Pd/C or RANEY®-nickel catalysts led to the isolation of 4-(methylthio)phenol, revealing the cleavage of the ether bridge. If a similar disjunction occurs during in vivo metabolic processes, the generated phenol could be oxidized to quinone, which are well known to induces cellular damages including hepatotoxicity. Paracetamol -induced hepatotoxicity is the well-known example, involving depletion of intracellular glutathione (GSH) following detoxification of the notorious N-acetyl-p-benzoquinone imine (NAPQI) metabolite. NAPQI is generated from oxidation of the phenol moiety in paracetamol.33 Accordingly, stable bridging alternatives could overcome this safety concern. Analog 20 was designed with an amine in lieu of the ether group. The antileishmanial property of 20 was moderate compared to 5 and only limited to FV1 and HU3MILR40.
The more stable carbon bridge was designed in analog 26 (Scheme 2). The antileishmanial activity of 26 against HU3MILR40 (IC50 = 7.1 μM) was comparable to that of 5, but substantially diminished against the remaining tested Leishmania strains. Analog 26 was further derivatized by sulfoximation to compensate for the reduced water solubility when switching from the ether to the carbon linker. However, the resulting analog 28, was not active except against FV1 with reduced IC50 of 11.8 μM (Table 1).
Combination therapy is an integral part of the treatment of leishmaniasis, including the first-line combination of 1 and 3.34,35 A hybrid strategy to produce a synthetic construct by linking the pharmacophores of individual drugs together through a stable bond is attractive since it functions as a single molecule pertaining to pharmacokinetic parameters.36 Analog 27 may be considered as a hybrid of metronidazole (23) and 5 through a stable carbon bridge. Metronidazole shared the 5-nitroimidazole motif with 5 and multiple clinical evidences37,38 indicating resolution of cutaneous lesions following treatment with 23. Analog 27 was, paradoxically, inactive against all tested strains.
Additional hybrids between 5 and the anti-trypanosomal agents nifurtimox (60, Fig. 3) and acoziborole (61) were designed because of the anti-leishmanial property of 60 (ref. 39) and the potent anti-leishmania properties of analogues of 61,40 as well as their similar structural architecture to 5, a bridge about similar length to the methyl ether bridge in 5 connecting two aromatic moieties. Initially, the suitability of an amide bridge that provides more stability than the ether was tested in analogs 32 and 33. Unfortunately, the anti-leishmanial properties were not preserved in these analogs. An additional amide bridge containing hybrid in analog 34 was designed by joining the N1-methyl-5-nitrimidazole in 5 to a thiomorpholino1,1-dioxide in 60 (Scheme 3a). Nifurtimox–eflornithine combination therapy (NECT) is recommended in the WHO list of essential medicines as the first-line treatment for the meningo-encephalitic stage of HAT.41 Analog 34 showed good activity against FV1 and HU3 strains with IC50 values of 3.4 and 7.5 μM, respectively.
Fig. 3. The chemical structures of nifurtimox (60), acoziborole (61), delamanid (62) and pretomanid (63).
Acoziborole is a benzoxaborole with a single oral dose of 960 mg showing comparable efficacy to NECT in patients with the meningo-encephalitic stage of gHAT.42 The 4-fluoro-2-trifluoromethylphenyl moiety of 61 was hybridized with the N1-methyl-5-nitroimidazole of 5via an amide bridge. The resulting hybrid 35 showed better activity than 5 against LV78, UA847 and FV1 strains (Table 1). The activity of 35 against the resistant LdHU3MILR40, however, was reduced to IC50 value of 12.2 μM. Acoziborole is also adaptable to our pursuit to eliminate the dubious nitroaromatic moiety and accordingly, hybrid 38 was designed in which the benzoxaborole group in 61 supersedes the N1-methyl-5-nitroimidazole moiety in 5. This germinal trait was appealing as analog 38 showed moderate to good activity against all tested Leishmania strains (Table 1). Considering the slightly higher activity and better safety profile observed with the sulfoxide metabolite 6 compared to the parent compound 5, we pursued the sulfoxidation of analog 38. However, this strategy was in vain as the biological activity of the sulfoxide derivative 40 was completely lost against all tested Leishmania strains.
Bicyclic nitroimidazoles, in which a nitroimidazole is fused to another heterocycle, are promising as new nitroaromatic-based drugs in the modern drug pipeline; delamanid (62) and pretomanid (63) are recently approved anti-tubercular drugs containing 4-nitroimidazo-oxazole and 4-nitroimidazo-oxazine scaffolds, respectively.43 Derivatization of 62 and 63 has inspired the development of anti-leishmanial agents such as DNDi-0690 and DNDi-VL-2098.44 We synthesized novel bicyclic nitroimidazoles by fusing oxazole (Scheme 4) or piperazin-2-one into the structure of 5 (Scheme 5). The sulfide moiety was targeted to improve the solubility of these analogs by sulfoxidation.
Exceptional anti-leishmanial activities were achieved with analog rac-49. The IC50 of rac-49 against LV78 and LdHU3 strains were 1.8 and 1.0 μM, respectively. Furthermore, a 14-fold decrease in IC50 value of rac-49 was noted against the resistant LdHU3MILR40 strain as compared to 5. To understand the importance of chirality for the activity profile, the (R)-50 and (S)-51 enantiomers of rac-49 were synthesized from chiral starting materials. The anti-leishmanial activities against both wild-type and resistant leishmania strains were similar for each enantiomer as well as the racemate, indicating that chirality in the imidazolooxazole conjugates is not a decisive structural feature for anti-leishmanial properties. However, it may be critical for the hERG receptor activity, as discussed later. The solubility of the imidazolooxazole conjugates was improved by oxidizing the sulfide moiety. Unfortunately, the excellent anti-leishmanial properties were not retained in the oxidized counterparts 52 and 53, but relatively good activity was noted against the resistant strain LdHU3MILR40. The sulfoxide derivative 52 displayed activity only against the LdHU3MILR40 strain with IC50 of 7.1 μM. The sulfone analog 53, akin to 52, only showed activity against the LdHU3MILR40 strain with an IC50 of 4.5 μM.
Nitroimidazopyrazinones are relatively new bicyclic imidazoles with promising anti-trypanosomal and anti-tubercular activities.45 The anti-leishmanial activities of the fexinidazole analogs with a nitroimidazopyrazinone core were investigated with analogs 58–59 (Scheme 5). Unfortunately, the modest anti-leishmanial profile of these analogs precluded further studies; activity only against strain FV1 was realized with analogs 58 and 59 with respective IC50 of 6.9 and 8.9 μM.
Anti-amastigote and cytotoxicity profiles
The in vitro anti-amastigote assay was conducted in mouse PEM infected with either LV78, UA847, HU3, or HU3MILR40 (Table 2). The selected panel of analogs are compounds that displayed notable anti-promastigote activity. The anti-amastigote activity of analogs was evaluated in comparison to both fexinidazole (5) and the positive control, miltefosine (4). In agreement with the aforementioned report mentioned earlier,11 the IC50 of 5 against cellular amastigotes was greater than 10 μM. Likewise, the analogs 19, 35 and the borolactone 38 were inactive.
Anti-amastigote activity of fexinidazole analogues in peritoneal mouse macrophages.
| Anti-amastigote activity IC50 (μM) | Cytotoxicity IC50 (μM) | Tox./acta | ||||
|---|---|---|---|---|---|---|
| LV78 | UA847 | HU3 | HU3MILR40 | PEM | HU3MILR40 | |
| Fexinidazole | >10 | >10 | >10 | >10 | >82 | — |
| 19 | >10 | >10 | >10 | >10 | >100 | — |
| 35 | >10 | >10 | >10 | >10 | >100 | — |
| 38 | >10 | >10 | >10 | >10 | >100 | — |
| Rac-49 | 0.7 | 0.7 | 0.3 | 0.4 | >100 | >250 |
| (R)-50 | 0.7 | 1.0 | 0.4 | 0.6 | >86 | >143 |
| (S)-51 | 0.6 | 0.5 | 0.4 | 0.4 | >88 | >220 |
| Miltefosineb | 9.6 | 3.2 | 2.7 | >20 | >40 | — |
Determined by dividing the IC50 of cytotoxicity in PEM to the IC50 against HU3MILR40.
Miltefosine (4) was used as a positive control in the evaluation of anti-amastigote properties of analogs.
The imidazolooxazoles rac-49, (R)-50, and (S)-51 not only display the most potent activity against multiple promastigotes but also demonstrate remarkable efficacy against intracellular amastigotes with high safety indices (Table 2). The activity of these analog surpasses the agreed key criterion for hits for VL, an IC50 value less than 10 μM against intracellular L. donovani.46 The anti-amastigote profile of the racemate and enantiomers were similar and was at least 14-fold more active compared to miltefosine against LV78 strain. Across all strains tested, these analogs were several folds more potent than 4 and equipotent activities (0.3–0.6 μM) were noted against wild-type HU3 and resistant HU3MILR40. Thousand-fold magnified microscopic images of HU3MILR40 amastigotes in DMSO control, and HU3MILR40 amastigotes under treatment of rac-49, (S)-51 and 4 are shown in Fig. 4. The high number of the amastigotes was nearly eradicated at 1.5 μM of rac-49 and (S)-51. HU3MILR40 amastigotes were nearly unaffected by a high 20 μM concentration of 4. In addition, the number of LV78 and HU3MILR40 amastigotes per 100 macrophages left after treatment is provided in Fig. 5.
Fig. 4. Microscopic images of HU3MILR40 amastigotes, stained with Giemsa and magnified 1000×. A) DMSO control of HU3MILR40 amastigote and PEM macrophages; B) HU3MILR40 amastigote under rac-49 treatment (1.5 μM); C) HU3MILR40 amastigote under (S)-51 treatment (1.5 μM); D) HU3MILR40 amastigote under treatment with miltefosine (20 μM).
Fig. 5. The number of LV78 and HU3MILR40 amastigotes per 100 macrophages in DMSO solvent, or under treatment of rac-49, (R)-50 or (S)-51. The data was represented as mean ± standard deviation of three independent experiments. Student's t-test was performed between the treatments and the control miltefosine (4). *** p < 0.001.
The cytotoxic properties of all reported analogs were evaluated with murine fibroblast L929 and murine macrophage RAW 264.7 cell lines. The incubation period lasted for 72 hours. In general, the reported compounds did not exhibit cytotoxic properties (Table 1). For almost all analogs, IC50 values could not be determined and exceeded 100 μM. In the L929 cytotoxicity assay, IC50 of 71.8 and 84.7 μM were determined for analogs 19 and 32, respectively. Moreover, IC50 values against RAW 264.7 cells were determined for the borolactone 38 and the nitroimidazopyrazinone 59, with respective concentrations of 73.6 and 48.1 μM. On the other hand, 4, that was originally developed as anti-neoplastic agent,4 displayed strong cytotoxicity against RAW 264.7 mouse cells with an IC50 of 9.9 μM.
In vitro hepatotoxicity evaluations
The use of 5 is contraindicated in patients with symptoms of liver disease.16,47,48 The mechanism(s) of liver toxicity of 5 is (are) not yet known but appear to be class-specific. Cases of drug-induced hepatotoxicity have been reported following the use of drugs of the nitroimidazoles class, including metronidazole,49,50 albendazole51,52 and ornidazole.53 In addition, the formation of reactive oxygen species, reactive nitrogen species or electrophilic intermediates following bioreduction of aromatic nitro groups is well-documented and accounts for the infrequent use of nitroaromatic moieties in modern drug discovery and development paradigm.54
Evaluation of the hepatotoxic properties of the synthesized analogs of 5 is necessary to identify structural manipulations that are germane to safe hepatotoxicity profile. Accordingly, the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide) cell viability assay was evaluated with the mouse TAMH and human HEP-G2 hepatic cell lines at incubation times of 24 and 48 h. The ability to maintain a stable phenotype, and express drug-metabolizing enzymes such as cytochromes make the TAMH cell line an attractive model for the assessment of drug-induced livery injury (DILI).33 The hepatotoxicity profiles of drugs such as paracetamol, flutamide and flupirtine have been studied in varying depth with the TAMH cell line.21,33 The HEP-G2 cell line is another popular tool to study DILI due to its interesting hepatocyte features such as albumin secretion, insulin-stimulated glycogen synthesis and glutathione-based detoxification.55
The effect of 5, metabolites 6–7, and the synthesized analogs on the viability of the two liver cell lines was evaluated at the highest soluble concentrations (see ESI,† S9). At 62.5 μM of 5, the viability of TAMH and HEP-G2 cell lines compared to the negative control, was at least 90% and 95% respectively; these values indicate no direct hepatotoxic effect of 5 on these cell lines after 48 h of incubation. Similarly, the metabolites 6–7 were almost harmless to both cell lines at concentration of 125 μM. Torrico et al.48 agreed with experts consulted on the hepatotoxicity profile of 5 during the phase II clinical trial. Given the delayed onset of liver damage, a direct toxic effect of 5 was considered unlikely. Rather, an adaptive immune response to the hepatocyte was suspected. Accordingly, this in vitro hepatocellular assay with a maximum of 48 h of incubations could be experimental evidence that acute liver damage is not associated with 5 and its metabolites 6–7.
The synthesized analogs have generally shown safe hepatotoxicity profiles (see ESI†). In particular, the viability of TAMH cells was not reduced below 50% for any of compounds tested. The lowest TAMH viability of 56–59% was observed with the incubations of compounds 32 and 52. However, in HEP-G2 cell lines, viabilities below 50% compared to the negative control were observed for few analogs.
Viability was consistently abated in both the TAMH and HEP-G2 cell lines incubated with analog 52; at 125 μM the viability of TAMH cells after 24 h incubation was the second lowest at 58%. After 24 h of incubation of HEP-G2 cells with 52, the viability was the lowest detected at 40.8%. Although the viability of TAMH cells incubated with analog 40 was high, the viability of HEP-G2 cells incubated with 40 was reduced to 54.2%. It is essential to note that the reduced viability observed following incubation with 40 and 52 occurred at the highest concentrations evaluated, 125 μM. Interestingly, reduced viability of 54% was noted in HEP-G2 cells incubated with the other borolactone derivative 38. This implies the need to closely monitor the hepatotoxicity profile of borolactone-based anti-leishmanial agents.
Reduced viability of HEP-G2 cells were also observed with incubations of analogs 27, 28 and 58. The N1-methyl of 5-nitroimidazole appears optimal for the hepatotoxicity profile of 5 since the cell viability was steadily abated in 32, an analog without the N1-methyl moiety. Moreover, reduced viability of TAMH and HEP-G2 cell lines was observed in analog 27, the only other 5-nitroimidazole analog with an exclusive N1-ethylhydroxyl substituent.
Microsomes and S9 incubation assays
The bioavailability of small molecules is affected by their metabolic stability, and if less stable, therapeutic effectiveness in patients could be compromised. On the other hand, toxicity can be increased if metabolism generates toxic metabolites. The rapid oxidation of the sulfide moiety in 5 to generate metabolites 6 and 7 as the main metabolic route has been reported.56,57 An N1-desmethyl metabolite was also assigned depending on mass spectra.57 Thus, it was of interest to evaluate the metabolic and toxic potentials of 6 and 7 in in vitro assays. A similar pattern was also expected for analogs of 5.
In our case with 5, the main metabolite in rat liver microsomes was the sulfoxide 6 while no evidence for the formation of the sulfone 7 from either 5 or 6 was observed by reversed-phase HPLC analysis. In fact, only the corresponding sulfoxide metabolites of fexinidazole analogs with sulfide groups were observed. The reasons for this claim include: (1) the only metabolites generated from incubations of 5, 38 and 49 were analogs 6, 40 and 52, which are their corresponding sulfoxide derivatives, respectively; (2) only a single metabolite was observed in the HPLC following the incubation of all sulfide analogs. Conventional sulfides are known to undergo rapid oxidative metabolism to yield the more water soluble sulfoxide derivatives;58 (3) the incubation of analogs where the sulfide moiety was already oxidized to a sulfoxide, sulfone or sulfoximine moiety were found to be stable to further oxidation; (4) the reduced metabolism in S9 compared to microsomes incubation implies only the oxidative system is responsible for metabolism of fexinidazole analogs, leaving out the occurrence of phase II metabolism.
As expected, compound 5 as well as all analogs bearing a sulfide moiety were found labile to enzymes in rat liver microsomes (Fig. 6A). Short-lived compounds include 5, 19, 26 and 33, whose starting amounts were completely consumed in the first 32 min of the incubation. It is interesting to note that the 4-ethyl substituent in 19 adopted in lieu of the liable 4-methylthio moiety was just as labile to metabolism as the sulfide; the metabolite generated from incubation of 19 could be the result of oxidation of the terminal ethyl moiety, which appears as an accessible and activated substrate for oxidative pathway of cytochrome P450 monooxygenase (CYP). Conversely, analogs 49–51 and 59 containing an imidazooxazole nucleus and nitroimidazopyrazinone nucleus, respectively, showed relatively good metabolic stability. This improved stability is a decided advantage to the potent activities of analogs 49–51.
Fig. 6. Results of metabolic stability studies with rat liver microsomes and S9 liver fractions (n = 2). A) the relative percentage of fexinidazole (5) and oxidizable analogs from rat liver microsomes (2 mg mL−1) incubation assay: the percentage of all compounds at 0 min of incubation is taken to be 100%. The graph shows the relative percentage decline of each compound at 16 min and 32 min of incubation at 37 °C; B) the percentage of each oxidizable analog left after 16 min and 32 min of S9 (2 mg mL−1) incubation in the presence of GSH (5.5 μM) at 37 °C: the addition of GSH to S9 incubations made little to none difference on the metabolism of tested analogs. The S9 incubation without GSH is given in the ESI.† One-way ANOVA analysis revealed no significance differences among the analogues. However, one-way ANOVA showed statistical significance (P < 0.05) for incubation times of microsomes. The post hoc analysis (adjusted P < 0.0167) identified significance differences between 0 min and 16 min, as well as between 0 min and 32 min of incubation.
Phase II enzymes including glutathione S-transferase (GST) are more abundant in the S9 fraction than are in microsomes.59 Thus, the metabolism of synthesized analogs by the cytosolic phase II enzymes was explored through incubation with rat liver S9 fractions in the presence of GSH (Fig. 6B). GSH was included in the S9 incubation in order to evaluate the involvement of GST in the hepatotoxicity of 5. However, no new metabolites other than those identified in the microsomes incubations were detected, regardless of the presence or absence of GSH.
For each analog found susceptible to metabolism, the metabolized portion from the liver S9 incubation was far slower than with the liver microsomes (Fig. 6B). This implies the major role of microsomes in the hepatic metabolism of 5 and its analogs because S9 fraction is a mixture of microsomes and cytosol and the CYP activity in S9 fraction is only 20–25% of those in the microsomes.60
Methods that selectively inhibit the enzyme flavin monooxygenase (FMO) activity can be used to discriminate the contributions of CYP to FMO enzymes in microsomes. Consequently, we attempted to selectively abate the FMO activity by using methimazole as an inhibitor.61 The presence of methimazole led to a major reduction in the metabolism of the tested analogs 5, 19 and 49 (Fig. 7A). However, findings62–64 indicated that methimazole is not highly selective to FMO and is capable of inhibiting various CYP isoenzymes at certain concentrations. Consequently, the observed major reduction in metabolism could be due to the inhibition of both CYP and FMO activities. We then employed the heat inactivation method to preferentially degrade the temperature sensitive FMO enzymes over CYPs (Fig. 7B). Accordingly, a brief heating treatment of microsomes for 90 s at 50 °C in the absence of NADPH regenerating system was applied to eliminate the activity of FMO.45,61 The degree of metabolism of tested analogs was only marginally reduced compared to the incubation without heating treatment. For instance, only 11% of 5 after 32 min and 16% of 19 after 16 min were detected under heat treatment, whereby the same compounds were metabolized 100% in incubations without heating. Furthermore, only a minor difference of approximately 5% was observed in the metabolism of 49 between incubations with heat and without heat treatment. It thus appears that CYP are likely the major enzymes involved in the metabolism of fexinidazole and its analogs.
Fig. 7. Inhibition studies of rat liver microsomal metabolism of select compounds. The percentage of all compounds at 0 min of incubation is taken to be 100%. The graph shows the relative percentage decline of each compound at 16 min and 32 min of incubation at 37 °C. A) Microsomes incubation of 5, 19 and 49 in the presence of methimazole (n = 2). Analog 49 was not metabolized; B) microsomes incubation of 5, 19 and 49 at 50 °C heat treatment (n = 2); C) incubation of 5, 38 and 49 in human microsomes (n = 3). One-way ANOVA followed by post hoc analysis showed statistically significant differences between 0 min and 16 min of incubation under heat treatment, as well as between 0 min and 16 min and between 0 min and 32 min of incubation in human microsomes.
Several analogs were also incubated with human liver microsomes to compared the pattern of metabolism with rat liver microsomes (Fig. 7C). The sulfoxide (6) and sulfone (7) derivatives of 5 were stable as was observed in incubation in rat liver microsomes and are not shown in the figure. On the other hand, compared to rat liver microsomes, relatively increased stability for 5 and 38, and decreased stability for 49 were observed with human microsomes.
hERG channel activity profile
The use of 5 is contraindicated in patients at risk of cardiac arrhythmias.14 There are many examples where the blockage of the potassium channel in cardiac tissue, Kv11.1 (hERG) by a drug can result in prolonged QT intervals, leading to cardiac arrhythmias and can result in death of the patient.65 Previous in vitro studies with HEK-293 human kidney cells stably expressing the hERG channels indicated 5 and metabolite 6 do not interfere with the hERG ion-channel activity at concentrations up to 30 μM. The sulfone metabolite 7, however, reduced 32.6% of hERG activity at 30 μM.57,66 A clinical finding of significant QTc prolonging effect of 5 was associated with the plasma concentration of metabolite 7. Accordingly, intrinsic and extrinsic factors such as co-administration of CYP inducers that can increase the concentration of this metabolites may lead to problems with cardiac function.66,67
The hERG channel opening activities were determined with HEK-293 cells stably overexpressing the hERG channel. This evaluation utilized a thallium uptake FLIPR® fluorescence microtiter-based assay. Fig. 8A–C shows the relative opening of the hERG channel at 100, 25 and 6 μM of test compounds. Our results appear to be in conflict with some of the above-mentioned findings;66i.e., 5 is indeed capable of reducing hERG activity with an approximate IC50 value of 10.7 μM (Fig. 9A). The sulfone metabolite 7 also demonstrated the ability to reduce hERG activity, but with a higher IC50 value of ca. 100 μM (Fig. 9C) in contrast to the aforementioned reports. Our data suggests that 5 and metabolite 7 may be responsible in the prolongation of QT interval. On the other hand, the sulfoxide metabolite 6 displayed no tendency to hERG channel inhibition even at 100 μM (Fig. 9B). In fact, this metabolite was the safest of all analogs reported here with regards to hERG activity inhibition profile. More evidence that sulfoxidation provides a safe route to avoid hERG inhibition were the analogs 40 and 52. Compared to hERG activity of 66% and 81% of their parent compounds 38 and 49, respectively (Fig. 8C), the hERG activity was also unaffected by analogs 40 and 52.
Fig. 8. The hERG activity for 3 concentrations of the test compounds depending on their solubility. A) Activities of highly soluble analogs at 100 μM; B) fexinidazole (5) and analog 27 showed the lowest hERG channel opening at 25 μM. On the other hand, metabolite 6 showed 115% hERG channel opening at 25 μM; C) activity of nearly all synthesized analogs at 6 μM. The hERG activity is expressed relative to the solvent control, which was set at 100%. The maximum inhibition was indicated by a 1 μM astemizole control. All data are given as mean of at least three independent determinations with standard deviation.
Fig. 9. Abilities of compounds 5–7 to block the opening of the hERG channel expressed in HEK-293 cells. A) the IC50 and IC25 values of 5; B) the sulfoxide metabolite 6 shows no tendency to reduce the hERG current; C) the hERG activity of the sulfone metabolite 7. Data are given as mean of at least three independent determinations with standard deviation.
The aromatic nitro moiety could be a factor contributing to the blockage of the hERG channel. A comparison at 25 μM indicates analog 17 (83%) has a relatively improved hERG activity than 5 (34%, Fig. 8B). The isosteric cyano moiety in analog 18 showed a reduced tendency to block hERG opening (Fig. 8C). Similar to sulfoxidation, sulfoximation of analogue 21 resulted in very little blockage of hERG activity. The strong contrast in hERG inhibition between analogs 26 and 28 likewise supports this argument (Fig. 8B). On the other hand, the switch from sulfide in 5 to methylene analog 19 had no noticeable effect on hERG activity.
Replacing the ether bridge with a carbon or amino bridge had minimal adverse effect on the hERG activity. The 78% hERG opening activity at 6 μM of 5 was slightly reduced to 64% and 48% for analogs 26 and 20, respectively. The other bridge variation, the amide functionality in lieu of the ether in analogs 32 exhibited no tendency to reduce the hERG opening at 6 μM (Fig. 8C).
The hybrid 27 that joined structural elements of 5 with the versatile chemotherapeutic agent metronidazole resulted in the most potent hERG channel inhibitor, with complete hERG inhibition at 100 μM (Fig. 7A). Analog 27 at 100 μM reduced the hERG activity more strongly than the positive control astemizole at 1 μM; this effect could be due to the N1-ethylhydroxyl substituent, which was used only in 27. The mean IC50 value of astemizole in our assay was determined to be 23.44 nM.
To gain a better understanding of how analog 27 interacts with the hERG channel, we performed an induced-fit molecular docking of the compound into the Cryo-EM structure of hERG channel (7CN1) (Fig. 10).68 A large hydrophobic pocket in the lower end of the pore-forming domain formed by F557, Y652, and F656 aligned with the 4-thiomethylphenyl group of 5. On the top of this hydrophobic interactions, the hydroxyethyl group in compound 27 forms hydrogen bond interactions with the potassium binding residue S624 in at least one subunit (Fig. 10C).
Fig. 10. Predicted binding modes of selected compounds by induced-fit docking to the cryo-EM structure of the human hERG potassium channel (PDB: 7CN1). (A) Top (left) and side view (right) of the homotetrameric hERG channel with bound ligand in surface representation (colored green); (B) the 4-thiomethylphenyl group of 5 binds to a large hydrophobic pocket formed by F557, Y652, and F656; (C) the hydroxyethyl group of 27 forms hydrogen bond interactions with the potassium binding residue S624; (D) R-50 allows binding with the hydrophobic pocket, S624′ and Y656′.
Another interesting observation was the difference in hERG blockage between R- and S-enantiomers 50 and 51. Although chirality in the imidazolooxazole-based analogs was found to be trivial for anti-leishmanial activities, the hERG activity was sensitive to their stereochemistry, with the R-enantiomer 50 being a stronger inhibitor (46% residual activity) than the S-enantiomer 51 (87%) at 6 μM (Fig. 8C). The rather planar configuration in 50 allows for binding within the hydrophobic pocket along with S624′ interaction and a favorable π–π interaction with Y656′ (Fig. 10D). A similar binding pose was not possible for 51 as this configuration results in a steric clash with S624 and S649′. This could be a reason for the observed disparity in hERG inhibition. As expected, the racemic mixture 49 has a hERG current activity of 66%, midway between 50 and 51.
Conclusions
Important structure–activity-relationships for the anti-leishmanial activities of fexinidazole analogues were revealed in this project (Fig. 11). For example, replacement of the 5-nitro toxicophore group in fexinidazole for the isosteric nitrile group is detrimental to the anti-leishmanial activities. Additionally, the N1-methyl groups and the ethyl bridge proved to be optimal for anti-leishmanial properties since multiple corresponding structural modifications led to reduction or loss of activity. Thus, the imidazole end of the molecule would appear to contain the pharmacophore structure. The 4-(methylthio)phenol sub-structure of fexinidazole, on the other hand, offers various possibilities to improve the hepatic metabolic stability, lower hERG inhibition and increase solubility of analogs without compromising the anti-leishmanial properties. Moreover, the oxazole ring is a highly suitable heterocycle to be incorporated into the structure of fexinidazole since it increased substantially the anti-leishmanial activity of fexinidazole while improving the hERG safety and metabolic stability.
Fig. 11. Summary of the structure–activity relationships of fexinidazole.
Experimental methods
All chemical reagents and solvents were used without prior purifications. The progress of chemical reactions was evaluated via TLC on silica gel 60 F254 aluminum plates from Merck using UV-light (λ = 254 nm and 366 nm) for visualization. Compounds were purified using either recrystallization or flash chromatography. Following purifications, structural confirmation of synthesized compounds was done by 1D- and 2D-NMR, which were measured in DMSO-d6 solution with tetramethylsilane as an internal standard. 1H-NMR and 13C-NMR spectra were recorded with a Bruker Avance III at 400 MHz and 101 MHz respectively. Melting point ranges were measured automatically using a Melting Point M-565 instrument (Büchi). The IR spectra of solid compounds were recorded on a Bruker optics Alpha FT-IR spectrometer equipped with a diamond single ATR probehead. HR-MS spectra of analogues were determined by electrospray ionization technique of LCMS-IT-TOF compact (Bruker). Compound purity was determined by a RP-HPLC with DAD detector fixed at wavelength of 254 and 280 nm, ProntoSIL 120-5 C18H column at temperature of 35° and gradient elution of phosphate buffer (50 mM, pH 7) and acetonitrile at a flow rate of 1 ml min−1. All compounds are >95% pure by HPLC analysis. Moreover, the enantiomeric purity of (R)-50 and (S)-51 was determined by using chiral HPLC (see ESI†).
General synthetic procedures
General procedure A: hydroxymethylation
In a pressurized reactor, a mixture of 1-methylimidazole (100 mmol, 8 mL), paraformaldehyde (170 mmol, 5.11 g), and DMSO (25 mL) were heated at 110 °C for 48 h. DMSO was then removed at reduced pressure.
General procedure B: chlorination
A solution of compound 11 (10 mmol, 1.12 g) in DCM (20 mL) was added dropwise to a solution of thionyl chloride (2.65 mL) in DCM (10 mL). After complete addition, the mixture was heated at 40 °C for 30 min. The reaction mixture was evaporated to dryness. Ethanol (10 mL) was then added, and the reaction mixture was stirred at 40 °C for 10 min and evaporated to dryness to remove the excess thionyl chloride and to give the intermediate 14.
General procedure C: etherification
To a solution of 4-(methylthio)phenol (11 mmol, 1.55 g) in DMF (15 mL), K2CO3 (11 mmol, 1.52 g) was added and the mixture was stirred for 10 min. A solution of intermediate 14 (11 mmol, 1.94 g) in DMF (10 mL) was added slowly while stirring at room temperature. The reaction was stirred at 60 °C for 3 h. The reaction mixture was then cooled and added to an ice-cold water (100 mL). The product was collected by filtration.
General procedure D: sulfoxidation
Compound 5 (3 mmol, 840 mg) was dissolved in DCM (10 mL). The solution was cooled in ice-cold water bath. A solution of 3-chloroperoxybenzoic acid (3.3 mmol, 1.04 g) in DCM (20 mL) was added over a period of 1 h. After complete addition, the mixture was stirred for an additional 30 min in an ice-cold water bath. The reaction mixture was then washed with NaHCO3 (10%, 20 mL). The products 6 and 7 were separated using flash chromatography.
General procedure E: sulfoximation
Compound 5 (0.65 mmol, 180 mg) was suspended in methanol (5 mL). Ammonium carbonate (0.95 mmol, 90 mg) and diacetoxyl iodobenzene (1.5 mmol, 445 mg) were added respectively. The mixture was stirred for 10 min at room temperature. The product was isolated using flash chromatography.
General procedure F: base-catalyzed aldol condensation
To a solution of 1,2-dimethyl-5-nitroimidazole (4 mmol, 565 mg) in ethanol (22 mL), sodium ethoxide (10 mmol, 785 μL) was added. The mixture was stirred vigorously for 10 min. 4-(thiomethyl)benzaldehyde (5.2 mmol, 690 μL) was then added dropwise. The reaction mixture was stirred at 65 °C overnight.
General procedure G: reduction of alkenes
A mixture of compound 24 (0.5 mmol, 140 mg), p-toluenesulfonyl hydrazide (2.5 mmol, 470 mg), K2CO3 (2.5 mmol, 350 mg) in pyridine (10 mL) was refluxed for 13 h. The reaction mixture was cooled and filtered. Ethyl acetate (30 mL) was added to the filtrate, which was then washed with aqueous CuSO4 solution (5%, 3 × 15 mL). The product 26 was isolated using flash chromatography.
General procedure H: basic epoxide ring-opening
A sealed tube was charged with intermediate 41 (8 mmol, 644 mg), 2-bromo-4-nitro-1H-imidazole (8 mmol, 1.54 g) and N,N-diisopropylethylamine (40 mmol, 7 mL). The sealed tube was heated at 107 °C for 16 h. The tube was cooled and the residue was dissolved in DCM (50 mL), and the resulting solution was washed with brine. The product 45 was separated using flash chromatography.
General procedure I: intramolecular Williamson ether synthesis
A solution of compound 45 (0.44 mmol, 172 mg) in anhydrous DMF (5 mL) was put in ice-cold water bath. After adding dry sodium hydride 90% (0.7 mmol, 17 mg), the reaction suspension was set under N2 atmosphere and stirred for 70 min. The reaction was quenched by the addition of ice-cold conc. NaHCO3 aqueous solution (15 mL). Brine was added to the reaction mixture and the product 49 was extracted using DCM (3 × 20 mL).
General procedure J: stereochemistry-controlled etherification
Cesium fluoride (15.5 mmol, 2.36 g) was added to a round-bottom flask charged with anhydrous dimethylformamide (8 mL). 4-(Methylthio)phenol (5 mmol, 700 mg) was added to the reaction mixture, which was then set under N2 atmosphere. After 1 h of stirring, (R)-oxiran-2-ylmethyl 3-nitrobenzenesulfonate (purity 98%+, 5 mmol, 1.3 g) was added. The reaction mixture was resealed under nitrogen atmosphere and stirred at room temperature for 36 h. The mixture was added to water and the product was extracted using DCM (3 × 20 mL). The combined organic extract was washed with brine. The compound 42 was isolated using flash chromatography.
General procedure K: HATU-mediated amide coupling
To a suspension of compound 29 (3 mmol, 633 mg) and thiomorpholine 1,1-dioxide (3.3 mmol, 446 mg) in anhydrous DCM (15 mL), N,N-diisopropylethylamine (11.5 mmol, 2 mL) was added. HATU (3.6 mmol, 1.4 g) was added to the reaction solution which was then stirred at room temperature overnight. The reaction solution was washed with HCl (1 N, 10 mL), conc. NaHCO3 (10 mL) and brine (10 mL).
The chemical structures, the labeling of atoms, the whole synthetic and purification procedures, and characterization of each compound are provided in the ESI.†
Evaluation of anti-leishmanial properties
Cell lines and cell culture
Anti-leishmanial activities were evaluated according to protocols published previously.69–71 Four wild-type strains (L. amazonensis LV78, L. brazilensis UA847, L. major FV1 and L. donovani HU3) and a miltefosine resistant leishmanial strain (L. donovani LdHU3MILR40) were included for comprehensive evaluations of the anti-leishmanial profile of synthesized analogs. Leishmania promastigotes were cultured in Schneider's Drosophila medium (Invitrogen) supplemented with 4 mM glutamine (Sigma), 25 μg mL−1 gentamicin solution (Invitrogen) and 10% (v/v) heat-inactivated FBS (Hyclone). The medium was maintained at pH 6.9 and 27 °C.
In vitro anti-promastigote activity
Cell viability was determined using Cell Titer 96® Aqueous Assay (Promega) that employed MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, Sigma) and PMS (phenazine methosulfate, Sigma) as a coupling agent as previously described.69–71 Briefly, 1.5 × 105 promastigote parasites per well were seeded into 96-well plate in a final volume of 100 μL supplemented Schneider medium and incubated with different concentrations of fexinidazole derivatives or miltefosine at 27 °C for 72 h. 10 μL of MTS/PMS mixture (MTS : PMS = 20 : 1) was added to each well and incubated for 45 min. Absorbance at 490 nm was measured using automatic microtiter plate reader (Bio-Rad) and the IC50 was determined using the PRISM software. The IC50 values are given as means ± SD of a minimum of three independent experiments.
In vitro anti-amastigote activity
Each well in a 24-well plate were planted with a round coverslip of 12 mm in diameter. Mouse PEM were collected as described previously.72 Mouse PEMs were resuspended in the DMEM media supplemented with 10% (v/v) heat-inactivated FBS, 100 U mL−1 penicillin and 100 μg mL−1 streptomycin. 1 × 105 PEM cells were then seeded per well and were left overnight to adhere to the coverslip at 37 °C and 5% CO2. Non-adherent cells were washed away gently with unsupplemented DMEM media.
Adherent macrophages were infected overnight with late-log promastigotes at a parasite-to-macrophage ratio of 20 : 1 for LV78 and UA847, and 40 : 1 for HU3 and HU3MILR40 as reported previously.69–71 Infected macrophages were further treated with test or reference compounds in 500 μL of supplemented DMEM media at 37 °C for 72 h. After incubation, coverslips were stained with Giemsa and the number of amastigotes per 100 macrophages was enumerated to determine the IC50.73,74 The IC50 values presented are means ± SD of 3–4 independent experiments.
In vitro cytotoxicity of macrophages
Mouse fibroblasts L929 cells and mouse macrophage RAW264.7 cells were cultured in DMEM medium supplemented with 10% (v/v) heat-inactivated FBS and 100 U mL−1 penicillin and 100 μg mL−1 streptomycin at 37 °C in a humidified atmosphere with 5% CO2. The L929 and RAW264.7 cells were seeded into a 96 well plate at a cell density of 1 × 104 cells per well and incubated with different concentrations of fexinidazole analogs in a final volume of 100 μL at 37 °C for 72 h. Cell viability was determined by MTS proliferation assay as described above.
MTT cell viability assay
Cell culturing
MTT cell viability assay with TAMH and HEP-G2 cells was evaluated according to previously published protocols.21 TAMH mouse liver cells, generously provided by Dr. Sidney Nelson, Institute of Pharmacy, University of Washington, USA, were cultured in a T75 flasks (Sarstedt) with FCS-free DMEM/F12 (1 : 1) mix (PAN Biotech), which was supplemented with 5% PANEXIN NTA (PAN Biotech), 10 mM nicotinamide (Sigma Aldrich) and 10 μg mL−1 gentamicin sulfate (PAN Biotech). In 200 μL media per well, 20 000 cells were seeded into 96-well microtiter plates (Sarstedt). HEP-G2 liver cancer cells, obtained from the DSMZ, Braunschweig, Germany, were cultured in a T75 flask (Sarstedt) with RPMI 1640 (PAN Biotech), which was supplemented with 10% heat-inactivated FBS (Sigma Aldrich) and 1% penicillin/streptomycin (PAN Biotech). In 200 μL media per well, 15 000 cells were seeded into 96-well microtiter plates (Sarstedt). The cells were allowed to attach for 24 h at 37 °C in a humidified 5% CO2 atmosphere.
Compound testing
Desired concentrations of test compounds were prepared by serial dilutions in DMSO. After 24 or 48 h exposure to the test compounds, 25 μL of 2.5 mg mL−1 of MTT (Alfa Aesar) aqueous solution was added. The plates were incubated for an additional 4 h in a humidified incubator at 37 °C. The supernatant was removed and the formed formazan crystals were dissolved in DMSO (50 μL) by a brief shaking with a plate shaker. Absorbance was measured at λ = 570 nm with a SpectraMax 190 microplate reader (Molecular Devices, San Jose, USA).
Data analysis
Blank controls of wells lacking cells but with DMSO were used to determine the background signal. On the other hand, wells not treated with test compounds but containing cells and DMSO in the respective media were used as controls. This controls represent the maximum viability of the TAMH or HEP-G2 cells. Each absorbance values were adjusted by reducing the mean of background signal from each plate. The mean of the adjusted absorbance values of each treatment was then calculated. The corrected cell viability (T/Ccorr) was calculated by dividing the mean of the adjusted absorbance values to the mean of the control from each plate, and then multiplying by 100%. The determined values are the mean of at least three independent experiments ± standard deviation (SD).
Microsomes and S9 incubation assays
Incubation suspension
An incubation suspension was prepared by rehomogenizing the rat or human liver microsomes (0.5 mL) or S9 fractions (1 mL), both purchased from Thermo Fischer Scientific (Darmstadt, Germany) with a preincubation solution (4.5 mL) containing the below listed reagents. The final concentration of each reagent in the incubation suspension was as follows: rat or human microsomes (2 mg mL−1) or S9 fraction (4 mg mL−1), NADP+ (0.3 mM), glucose-6-phosphate (5.3 mM), glucose-6-phosphate dehydrogenase solution (12.5 IU), MgCl2·6H2O (10 mM), EDTA disodium dihydrate (5 mM) and KH2PO4 phosphate buffer (50 mM pH = 7.4).
The suspension was divided into four aliquots (1.1 mL) and then incubated at 37 °C for 10 min. A test compound (final concentration 20 μM) was added to each aliquot, which was then vortexed and returned to the water bath. Fexinidazole was used as a control. For incubations in the presence of GSH, addition of test compounds was followed by the addition of GSH (5.5 μM). At 0, 16 min and 32 min, incubation suspension (350 μL) was removed immediately after vortexing the test compound and added to a vial of ice-cold acetonitrile (350 μL). The vials were kept on ice for 30–50 min to allow precipitation of proteins. Then, the vials were centrifuge at 14 000g at 0 °C for 10 min and store in a freezer. Before HPLC analysis, the vials were centrifuged again and the clear supernatant was carefully transferred to HPLC vials, which were kept at 4 °C in the autosampler until analysis.
FMO versus CYP contribution to metabolism
Contribution of CYP and FMO enzymes to the metabolism of a test compound can be determined by selectively inhibiting either FMO or CYP activity of the microsomes. In this study, FMO activity of microsomes was selectively inhibited using chemical and heat method. Chemical inhibition was evaluated by adding methimazole (6.6 μL) to the incubation suspension. Heat inactivation of FMO was achieved by heating of the microsomes at 50 °C for 90 s before adding NADP+, G-6-P and G-6-P-DH.
HPLC analysis
Analysis was done by using a Merck-Hitachi D-7000 HPLC system equipped with HPLC pump (L-7100), autosampler (L-7200), DAD detector (L-7450) and column oven (L-7350). A ProntoSIL 120-5 C18H 125 × 4.0 mm (Bischoff Chromatography, Germany) column proceeded by a guard column of same material was employed for all analysis and held at 35 °C. Chromatograms were collected and analyzed with the D-7000 HPLC system manager (D-7000 HSM). The method involved a gradient elution (see ESI†) of phosphate buffer (20 mM, pH = 3) and acetonitrile with a flow rate of 1 ml mL−1 and detections at wavelengths λ = 254 and 280 nm. The analysis focused on quantifying the relative decline in the concentration of each analog from 0 min to 32 min. Accordingly, the peak area of each analog at 0 min was taken to be 100% and the relative peak area of analogs left at 16 min and 32 min, respectively, were plotted in bar graphs. Control incubations without microsomes showed no losses of substances.
In vitro hERG assay
Procedure
The FLIPR® Potassium Assay Kit from Molecular Devices (San Jose, USA) was used to measure hERG channel activity in a HEK-293 cell line; this cell line overexpressing the hERG (KV11.1) channel from BPS Bioscience (San Diego, USA) with accession number NM_000238, was obtained from tebu-bio GmbH (Offenbach, Germany). The cells were grown in MEM medium with non-essential amino acids (Thermo Fisher Scientific), supplemented with 10% heat-inactivated FBS, sodium pyruvate (1 mM), 1% penicillin/streptomycin and G418 sulfate (0.4 μg mL−1). In 100 μL media per well, 60 000 cells were seeded into a sterile black 96-well (Vision Plate™) with tissue-culture treatment and optical bottom (order no. 4ti-0221, 4titude). After incubating the plate for 24 h (37 °C, 5% CO2), the cells were further treated according to the assay instructions provided by the manufacturer. Serial dilutions of test compounds, dissolved in DMSO, were added to wells according to the plate loading schedule in the ESI,† resulting in a final DMSO concentration of 0.5%. The plate was then incubated in the dark for 30 min and subsequently placed in an Infinite® F200 Pro plate reader (Tecan). The baseline fluorescence was measured for 20 s, followed by the addition of a stimulus buffer containing Tl+ (3 mM Tl+ per well). Fluorescence intensity was then recorded every 2.5–3 s for 2.5 min at excitation and emission wavelengths of 485 and 535 nm, respectively.
Data analysis
Each compound set contained one well with only 0.5% DMSO as a negative control, indicating the maximum activity of the hERG channel, and one with astemizole (1 μM) as a positive control, to indicate the maximum inhibition. The mean background fluorescence was calculated for each well, then the relative fluorescence (F/F0) was calculated by dividing the fluorescence intensity measured after adding the stimulus buffer by the mean of the background fluorescence. The correction for basal flux value (corr. F/F0) was calculated by subtracting the mean F/F0 value of the positive control from each F/F0 value at 120 s. The corr. F/F0 value of the negative control at 120 s was defined as 100% activity, all other corr. F/F0 values at 120 s were related to this value.
The relative activities were plotted versus log(concentration) of a compound and interpolated to the 50% and 75% activity to obtain the absolute IC50 and IC25 values. In cases when 75 or 50% inhibition could not be reached due to poor compound solubility, the highest tested concentrations are reported. The data is the mean of at least three independent experiments ± standard deviation. IC50 and IC25 values are given as geometric mean with CI 95%.
Induced fit molecular docking
All calculations were performed with the Schrödinger small molecule drug discovery suite version 2023.3 (New York, USA). The cryo-EM structure of the human hERG channel (PDB: 7CN1)68 was initially prepared by using the Protein Preparation Wizard75 in Maestro without any bound ligand, as astemizole is not available in the structure. The preparation was performed in three steps by first adding hydrogen atoms, assigning bond orders, and predicting missing loops with Prime,76 followed by an optimization of the hydrogen bond assignment and finally, a restrained minimization after applying OPLS4 force field parameters.77 To obtain a reference ligand structure, the 3D structure of astemizole was first generated from SMILES, prepared by LigPrep, and then docked into the lower pore domain by Glide78 with XP scoring.79 The final binding pose was selected by best fit with the cryo-EM map data. Subsequently, all putative hERG inhibitors presented in this study were also prepared via LigPrep and docked using induced fit docking (SP scoring).80 The binding pocket was assigned based on the bound astemizole conformation. All docking results were first processed by cluster analysis and then visually analyzed.
Data availability
The data that supports the findings of this study are available in the ESI† of this manuscript.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
A. S. S. thanks the Alexander von Humboldt Foundation for the generous postdoctoral fellowship (ETH 1218330 GF-P) to perform this project at the University of Greifswald. The activity and toxicity evaluation were performed in collaboration with the Polytechnic University of Hong Kong. We wish to acknowledge the excellent technical assistance of Dr. Anja Botdke, Maria Hühr and Tobias Oergel for performing the NMR and HR-MS.
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4md00426d
References
- Ngouateu O. B. Dondji B. Curr. Res. Parasitol. Vector-Borne Dis. 2022;2:100077. doi: 10.1016/j.crpvbd.2022.100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, Leishmaniasis, https://who.int/news-room/fact-sheets/detail/leishmaniasis, (accessed 7 October 2023)
- Jones C. M. Welburn S. C. Front. Vet. Sci. 2021;8:618766. doi: 10.3389/fvets.2021.618766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvolti S. Malhame M. Mantel C. F. Le Rutte E. A. Kaye P. M. PLoS Neglected Trop. Dis. 2021;15:e0009742. doi: 10.1371/journal.pntd.0009742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surur A. S. Fekadu A. Makonnen E. Hailu A. ACS Med. Chem. Lett. 2020;11:2058–2062. doi: 10.1021/acsmedchemlett.0c00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnielli J. B. T. Monti-Rocha R. Costa D. L. Molina Sesana A. Pansini L. N. N. Segatto M. Mottram J. C. Costa C. H. N. Carvalho S. F. G. Dietze R. Am. J. Trop. Med. Hyg. 2019;101:789–794. doi: 10.4269/ajtmh.18-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanra S. Sarraf N. R. Das A. K. Roy S. Manna M. Sci. Rep. 2017;7:10330. doi: 10.1038/s41598-017-09720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A. M. O'Connor P. D. Blaser A. Yardley V. Maes L. Gupta S. J. Med. Chem. 2016;59:2530–2550. doi: 10.1021/acs.jmedchem.5b01699. [DOI] [PubMed] [Google Scholar]
- Deeks E. D. Drugs. 2019;79:215–220. doi: 10.1007/s40265-019-1051-6. [DOI] [PubMed] [Google Scholar]
- Barrett M. P. Croft S. L. Br. Med. Bull. 2012;104:175–196. doi: 10.1093/bmb/lds031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie S. Patterson S. Stojanovski L. Simeons F. R. Norval S. Kime R. Read K. D. Fairlamb A. H. Sci. Transl. Med. 2012;4:119re1. doi: 10.1126/scitranslmed.3003326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA, Trial to determine efficacy of fexinidazole in visceral leishmaniasis patients in Sudan, https://clinicaltrials.gov/study/NCT01980199?tab=table, (accessed 10 October 2023)
- DNDi, R&D status November 2015: DNDi leishmaniasis programme, https://dndi.org/news/2015/leish-rndstatus-19112015/, (accessed 12 October 2023)
- EMA, Fexinidazole Winthorp, https://www.ema.europa.eu/en/opinion-medicine-use-outside-EU/human/fexinidazolewinthrop#:~:text=Fexinidazole%20Winthrop%20is%20indicated%20for,and%20weighing%20%E2%89%A5%2020%20kg, (accessed 15 October 2023)
- Clos J. Grünebast J. Holm M. Pathogens. 2022;11:1052. doi: 10.3390/pathogens11091052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Morais-Teixeira E. Rabello A. Aguiar M. M. G. J. Antimicrob. Chemother. 2019;74:2318–2325. doi: 10.1093/jac/dkz172. [DOI] [PubMed] [Google Scholar]
- Ang C. W. Jarrad A. M. Cooper M. A. Blaskovich M. A. T. J. Med. Chem. 2017;60:7636–7657. doi: 10.1021/acs.jmedchem.7b00143. [DOI] [PubMed] [Google Scholar]
- Vanelle P. Maldonado J. Crozet M. Savornin B. Delmas F. Gasquet M. Timon-David P. Eur. J. Med. Chem. 1992;27:551–553. doi: 10.1016/0223-5234(92)90190-C. [DOI] [Google Scholar]
- Hoff D. R. and Henry D. W., US3299090, 1967
- Winkelmann E. and Raether W., US4042705A, 1976
- Surur A. S. Bock C. Beirow K. Wurm K. Schulig L. Kindermann M. K. Siegmund W. Bednarski P. J. Link A. Org. Biomol. Chem. 2019;17:4512–4522. doi: 10.1039/C9OB00511K. [DOI] [PubMed] [Google Scholar]
- Lohier J.-F. Glachet T. Marzag H. Gaumont A.-C. Reboul V. Chem. Commun. 2017;53:2064–2067. doi: 10.1039/C6CC09940H. [DOI] [PubMed] [Google Scholar]
- Samant B. S. Sukhthankar M. G. Bioorg. Med. Chem. Lett. 2011;21:1015–1018. doi: 10.1016/j.bmcl.2010.12.040. [DOI] [PubMed] [Google Scholar]
- Valdez C. A. Tripp J. C. Miyamoto Y. Kalisiak J. Hruz P. Andersen Y. S. Brown S. E. Kangas K. Arzu L. V. Davids B. J. Gillin F. D. Upcroft J. A. Upcroft P. Fokin V. V. Smith D. K. Sharpless K. B. Eckmann L. J. Med. Chem. 2009;52:4038–4053. doi: 10.1021/jm900356n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyachi H. Nagatsu Y. Chem. Pharm. Bull. 2002;50:1137–1140. doi: 10.1248/cpb.50.1137. [DOI] [PubMed] [Google Scholar]
- Jarrad A. M. Debnath A. Miyamoto Y. Hansford K. A. Pelingon R. Butler M. S. Eur. J. Med. Chem. 2016;120:353–362. doi: 10.1016/j.ejmech.2016.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangarapu N. R. Reddy E. K. Sajith A. M. Yellappa S. Chandrasekhar K. B. ChemistrySelect. 2017;2:7706–7710. doi: 10.1002/slct.201700801. [DOI] [Google Scholar]
- Liu C., Zhang Y.-K., Liu C. Y. and Zhou Y., WO2020/051575A1, 2020
- Graham B. J. Windsor I. W. Gold B. Raines R. T. Proc. Natl. Acad. Sci. U. S. A. 2021;118:e2013691118. doi: 10.1073/pnas.2013691118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrad A. M. Ang C. W. Debnath A. Hahn H. J. Woods K. Tan L. Sykes M. L. Jones A. J. Pelingon R. Butler M. S. Avery V. M. West N. P. Karoli T. Blaskovich M. A. T. Cooper M. A. J. Med. Chem. 2018;61:11349–11371. doi: 10.1021/acs.jmedchem.8b01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe K. J. Jia Y. Ho H. K. Rademacher P. Bammler T. K. Beyer R. P. Farin F. M. Woodke L. Plymate S. R. Fausto N. Nelson S. D. Chem. Res. Toxicol. 2007;20:1277–1290. doi: 10.1021/tx7001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresini M. Tota A. Degennaro L. Bull J. A. Luisi R. Chemistry. 2021;27:17293–17321. doi: 10.1002/chem.202102619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Stamper B. D. Biomed. Res. Int. 2016;2016:4780872. doi: 10.1155/2016/4780872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uliana S. R. B. Trinconi C. T. Coelho A. C. Parasitology. 2018;145:464–480. doi: 10.1017/S0031182016002523. [DOI] [PubMed] [Google Scholar]
- Musa A. M. Mbui J. Mohammed R. Olobo J. Ritmeijer K. Alcoba G. Clin. Infect. Dis. 2023;76:e1177–e1185. doi: 10.1093/cid/ciac643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surur A. S. Sun D. Front. Chem. 2021;9:659845. doi: 10.3389/fchem.2021.659845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen J. K. Sawicki S. Arch. Dermatol. 1975;111:1343–1344. doi: 10.1001/archderm.1975.01630220107012. [DOI] [PubMed] [Google Scholar]
- Long P. I. JAMA, J. Am. Med. Assoc. 1973;223:1378–1379. doi: 10.1001/jama.1973.03220120044011. [DOI] [PubMed] [Google Scholar]
- Marsden P. D. Cuba C. C. Barreto A. C. Sampaio R. N. Rocha R. A. A. Trans. R. Soc. Trop. Med. Hyg. 1979;73:391–394. doi: 10.1016/0035-9203(79)90161-5. [DOI] [PubMed] [Google Scholar]
- Mowbray C. E. Braillard S. Glossop P. A. Whitlock G. A. Jacobs R. T. Speake J. J. Med. Chem. 2021;64:16159–16176. doi: 10.1021/acs.jmedchem.1c01437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, https://list.essentialmeds.org/recommendations/823, (accessed 10 September 2023)
- Betu Kumeso V. K. Kalonji W. M. Rembry S. Valverde Mordt O. Ngolo Tete D. Pretre A. Lancet Infect. Dis. 2023;23:463–470. doi: 10.1016/S1473-3099(22)00660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudde S. E. Upton A. M. Lenaerts A. Bax H. I. De Steenwinkel J. E. M. J. Antimicrob. Chemother. 2022;77:880–902. doi: 10.1093/jac/dkab505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A. M. O'Connor P. D. Marshall A. J. Blaser A. Yardley V. Maes L. J. Med. Chem. 2018;61:2329–2352. doi: 10.1021/acs.jmedchem.7b01581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. C. Srivastava A. Colclough N. Wilson J. Reddy V. P. Amberntsson S. Li D. Drug Metab. Dispos. 2017;45:1060–1067. doi: 10.1124/dmd.117.077396. [DOI] [PubMed] [Google Scholar]
- Katsuno K. Burrows J. N. Duncan K. van Huijsduijnen R. H. Kaneko T. Kita K. Mowbray C. E. Schmatz D. Warner P. Slingsby B. T. Nat. Rev. Drug Discovery. 2015;14:751–758. doi: 10.1038/nrd4683. [DOI] [PubMed] [Google Scholar]
- Watson J. A. Strub-Wourgraft N. Tarral A. Ribeiro I. Tarning J. White N. J. Antimicrob. Agents Chemother. 2019;63:e02515–e02518. doi: 10.1128/AAC.02515-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrico F. Gascon J. Ortiz L. Pinto J. Rojas G. Palacios A. Clin. Infect. Dis. 2023;76:e1186–e1194. doi: 10.1093/cid/ciac579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kancherla D. Gajendran M. Vallabhaneni P. Vipperla K. Case Rep. Gastrointest. Med. 2013;2013:568193. doi: 10.1155/2013/568193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunaut T. Boulagnon-Rombi C. Thorn H. Doco-Fenzy M. Thiefin G. Eur. J. Med. Genet. 2022;65:104388. doi: 10.1016/j.ejmg.2021.104388. [DOI] [PubMed] [Google Scholar]
- Dragutinovic N. Barac A. Stevanovic G. Dordic I. Paglietti B. Micic J. Aleksic E. Stojnic J. Nestorov J. M. J. Infect. Dev. Ctries. 2022;16:1660–1663. doi: 10.3855/jidc.16594. [DOI] [PubMed] [Google Scholar]
- LiverTox, https://www.ncbi.nlm.nih.gov/books/NBK547852/, (accessed 23 October 2023)
- Ersoz G. Vardar R. Akarca U. S. Tekin F. Yilmaz F. Gunsar F. Karasu Z. Turk. J. Gastroenterol. 2011;22:494–499. doi: 10.4318/tjg.2011.0245. [DOI] [PubMed] [Google Scholar]
- Boelsterli U. A. Ho H. K. Zhou S. Leow K. Y. Curr. Drug Metab. 2006;7:715–727. doi: 10.2174/138920006778520606. [DOI] [PubMed] [Google Scholar]
- Moeller T. A. Shukla S. J. Xia M. Assay Drug Dev. Technol. 2012;10:78–87. doi: 10.1089/adt.2010.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahia M. T. Nascimento A. F. Mazzeti A. L. Marques L. F. Goncalves K. R. Mota L. W. Antimicrob. Agents Chemother. 2014;58:4362–4370. doi: 10.1128/AAC.02754-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torreele E. Bourdin Trunz B. Tweats D. Kaiser M. Brun R. Mazue G. Bray M. A. Pecoul B. PLoS Neglected Trop. Dis. 2010;4:e923. doi: 10.1371/journal.pntd.0000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surur A. S. Schulig L. Link A. Arch. Pharm. 2019;352:e1800248. doi: 10.1002/ardp.201800248. [DOI] [PubMed] [Google Scholar]
- Wang X. He B. Shi J. Li Q. Zhu H. J. Drug Metab. Dispos. 2020;48:31–40. doi: 10.1124/dmd.119.089235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L. Liu X. Curr. Drug Metab. 2007;8:822–889. doi: 10.2174/138920007782798207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R. L. Curr. Protoc. Toxicol. 2002;4:4.9.1–4.9.11. doi: 10.1002/0471140856.tx0409s13. [DOI] [PubMed] [Google Scholar]
- Guo Z. Raeissi S. White R. B. Stevens J. C. Drug Metab. Dispos. 1997;25:390–393. [PubMed] [Google Scholar]
- Li J. Hussain Z. Zhu J. Lei S. Lu J. Ma X. Chem. Res. Toxicol. 2021;34:2534–2539. doi: 10.1021/acs.chemrestox.1c00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagmann L. Meyer M. R. Maurer H. H. Toxicol. Lett. 2016;258:55–70. doi: 10.1016/j.toxlet.2016.06.013. [DOI] [PubMed] [Google Scholar]
- Yap Y. G. Cam A. G. Heart. 2003;89:1363. doi: 10.1136/heart.89.11.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMA, https://www.ema.europa.eu/en/documents/outside-eu-assessment-variation/fexinidazole-winthrop-h-w-2320-ii-0016-assessment-report-variation_en.pdf, (accessed 03 September 2023)
- FDA, https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/214429Orig1s000OtherActionLtrs.pdf, (accessed 06 September 2023)
- Asai T. Adachi N. Moriya T. Oki H. Maru T. Kawasaki M. Structure. 2021;29:203–212.e4. doi: 10.1016/j.str.2020.12.007. [DOI] [PubMed] [Google Scholar]
- Wong I. L. K. Chan K.-F. Chan T. H. Chow L. M. C. J. Med. Chem. 2012;55:8891–8902. doi: 10.1021/jm301172v. [DOI] [PubMed] [Google Scholar]
- Wong I. L. K. Chan K.-F. Chen Y.-F. Lun Z.-R. Chan T. H. Chow L. M. C. Antimicrob. Agents Chemother. 2014;58:3379–3388. doi: 10.1128/AAC.02425-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C.-F. Liu Z. Wong I. L. K. Zhao X. Yang Z. Zheng J. Lee M. M. Chan M. K. Chan T. H. Chow L. M. C. Antimicrob. Agents Chemother. 2021;65:e02165-20. doi: 10.1128/AAC.02165-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. Goncalves R. Mosser D. M. Curr. Protoc. Immunol. 2008;14:14.1.1–14.1.14. doi: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal R. A. Croft S. L. J. Antimicrob. Chemother. 1984;14:463–475. doi: 10.1093/jac/14.5.463. [DOI] [PubMed] [Google Scholar]
- Inocêncio Da Luz R. Vermeersch M. Dujardin J.-C. Cos P. Maes L. Antimicrob. Agents Chemother. 2009;53:5197–5203. doi: 10.1128/AAC.00866-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavi Sastry G. Adzhigirey M. Day T. Annabhimoju R. Sherman W. J. Comput.-Aided Mol. Des. 2013;27:221–234. doi: 10.1007/s10822-013-9644-8. [DOI] [PubMed] [Google Scholar]
- Jacobson M. P. Pincus D. L. Rapp C. S. Day T. J. F. Honig B. Shaw D. E. Friesner R. A. Proteins. 2004;55:351–367. doi: 10.1002/prot.10613. [DOI] [PubMed] [Google Scholar]
- Lu C. Wu C. Ghoreishi D. Chen W. Wang L. Damm W. J. Chem. Theory Comput. 2021;17:4291–4300. doi: 10.1021/acs.jctc.1c00302. [DOI] [PubMed] [Google Scholar]
- Halgren T. A. Murphy R. B. Friesner R. A. Beard H. S. Frye L. L. Pollard W. T. Banks J. L. J. Med. Chem. 2004;47:1750–1759. doi: 10.1021/jm030644s. [DOI] [PubMed] [Google Scholar]
- Friesner R. A. Murphy R. B. Repasky M. P. Frye L. L. Greenwood J. R. Halgren T. A. Sanschagrin P. C. Mainz D. T. J. Med. Chem. 2006;49:6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- Sherman W. Day T. Jacobson M. P. Friesner R. A. Farid R. J. Med. Chem. 2006;49:534–553. doi: 10.1021/jm050540c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that supports the findings of this study are available in the ESI† of this manuscript.


















