Abstract
Sexual differentiation of the zebra finch (Taeniopygia guttata) neural song circuit is thought to be initiated by sex differences in sex chromosome gene expression in brain cells. One theory is that Z-linked genes, present in the male's ZZ genome at double the dose of females' (ZW), are expressed at higher levels and trigger masculine patterns of development. We report here that trkB (tyrosine kinase receptor B) is Z-linked in zebra finches. trkB is the receptor for neurotrophic factors BDNF and neurotrophin 4, and mediates their influence on neuronal survival, migration, and specification. trkB mRNA is expressed at a higher level in the male telencephalon or whole brain than in corresponding regions of the female in adulthood, and at posthatch day (P) 6, when the song circuit is undergoing sexual differentiation. Moreover, this expression is higher in the song nucleus high vocal center (HVC) than in the surrounding telencephalon at P6, and in males relative to females. In addition, trkB protein is expressed more highly in male than female whole brain at P6. These results establish trkB as a candidate factor that contributes to masculine differentiation of HVC because of its Z-linkage, which leads to sex differences in expression. BDNF is known to be stimulated by estrogen and to be expressed at higher levels in males than females at later ages in HVC. Thus, the trkB-BDNF system may be a focal point for convergent masculinizing influences of Z-linked factors and hormones.
Keywords: sex determination, song, Z chromosome
The development of sex differences in the vertebrate brain is largely attributed to the differential effects of gonadal secretions. For example, in mammals, testosterone, secreted from the fetal testes, directs patterns of brain development leading to masculine behavior in adults, whereas the relative absence of testosterone leads to feminine behavior (1–3). Brain cells of males and females also contain different numbers and types of genes encoded on the sex chromosomes, which, in addition to the effects of gonadal secretions, could contribute to sex differences in neural function and behavior (4). In mammals, for example, only male cells are potentially influenced by Y genes, and the double genomic dose of X genes in females could lead to sex differences in the expressed dose of some of those genes.
Both sex hormones and sex-linked genes have been suggested as the cause of sex differences in zebra finches (Taeniopygia guttata), in which singing is a male-specific behavior mediated by a brain circuit that is much more developed in males than in females (5, 6). Because females treated with estradiol at hatching develop a more masculine song system and sing as adults (7–10), higher concentrations of estrogens in the male brain (relative to those in females) are probably required for normal masculinization of the song circuit. In males, the estrogen presumably derives from local metabolism of androgens produced by the testes or by the brain (11). In slice cultures of forebrain tissue harvested from posthatch day (P) 25 birds, estradiol is released at higher levels in tissue from males than from females (12, 13), indicating that estradiol is synthesized de novo in the telencephalon, and at a higher level in juvenile males. Nevertheless, gonadal secretions are probably not the primary determinants of brain sexual phenotype. This conclusion derives from the following findings. (i) Sex steroid hormones only partially masculinize the brain of females (14, 15). (ii) Blocking these hormones in males has not prevented the normal development of song (16, 17). (iii) Females induced to form testes during early development do not develop a masculine song system or sing, even though they are exposed to testicular hormones (18, 19). (iv) Genetically male and female brain tissue differ in their sexual phenotype even if they reside in the same bird and are exposed to the same gonadal hormonal milieu (20). Thus, in several cases the sexual character of the song system correlates with chromosomal sex of the brain, not with gonadal type. Although these studies do not eliminate gonadal hormones as agents of brain masculinization, as a group they suggest that nongonadal factors, instead, may be primary (20, 21).
We propose that genetic differences encoded on the sex chromosomes represent the brain-autonomous factor(s) that instruct cells of the zebra finch brain to develop in a sexually dimorphic manner. Because we hypothesize both a sex chromosome factor and a role for sex steroid hormones, it would be particularly interesting to find sex-linked genes that could either augment the response to steroid hormones in males relative to females or reduce the response to hormones in females. Because male birds have two Z chromosomes, whereas females have one Z and one W chromosome, sex differences in the dosage of Z and/or W genes could initiate sex-specific patterns of brain development.
Previous studies have indicated that Z-linked genes may be expressed at a higher level in male birds than in females (20, 22–25). Here, we test the hypothesis that trkB (tyrosine kinase receptor B), a Z-linked gene in chickens (26) (www.ensembl.org), is expressed at a higher level in male than female zebra finch brain. trkB is the receptor for the neurotrophins BDNF and neurotrophin 4. BDNF plays a critical role in neuronal cell survival and differentiation (27–37), and in male-specific development of song nucleus high vocal center (HVC) in songbirds (38–43). BDNF expression is regulated by steroid hormones including estradiol and testosterone (38, 40), so a sex-chromosome-linked mechanism that augments or limits BDNF's effect might provide a link between the effects of sex hormones and sex chromosomes. Sex differences in trkB expression would, therefore, be particularly well positioned to play a prominent role in song-system sexual differentiation.
Materials and Methods
Cloning of Zebra Finch trkB and zRalDH cDNAs. A 2,393-bp cDNA partially encoding zebra finch trkB (GenBank accession no. AY679520) was isolated by RT-PCR from male zebra finch brain total RNA by using the primers zfTrkB3F 5′-CAAGTGTTCCTGTGAAATCATGTGG-3′ and zfTrkB3R 5′-TTGTGRTGGGCAAACTGGAG-3′. The 2,393-bp cDNA has 91% nucleotide homology to the chicken trkB sequence (GenBank accession no. X77251) and is predicted to be missing ≈800 bp of the 5′ end based on the chicken cDNA, which includes approximately the first 100 aa of the ORF.
A 1,240-bp cDNA partially encoding zebra finch class I aldehyde dehydrogenase (zRalDH, nucleotides 31–1271 of Gen-Bank sequence AF16770) (44) was similarly amplified by RT-PCR (primers zRalDHF, 5′-CAACCCAAAACACAACACAGCAT-3′, and zRalDHR, 5′-CTTTCCCCCACATTCAAGTTTTG-3′).
Northern Blot Analysis. Total RNA was isolated from telencephalon of zebra finches at P2, P6, P10, and P14 (P1 is the day of hatch; n = 8 per sex per day) and separated on 1.2% agarose formaldehyde denaturing gels. Each gel and Northern blot contained 16 samples for each age analyzed. Although age and blot were therefore confounded, the blots were produced and probed at the same time, reducing interblot variability. Each lane contained 20 μg of total RNA and was probed with the 2,393-bp fragment of the trkB cDNA. Blots were exposed, and expression of the dominant 8.0-kb transcript was quantified relative to GAPDH by using a phosphorimager (Amersham Pharmacia). Group differences were analyzed by using two-way ANOVAs (main factors of sex and age), using ncss software (NCSS, Kaysville, UT). Because a main effect of sex was found, t tests were used at each age to evaluate when the sex difference occurred.
Western Blot Analysis. P6 whole brains (n = 6 per sex) were dissected out and kept in cold buffer (1% sodium deoxycholate/10 mM Tris·HCl pH 7.4/10 mM EDTA/10 mM EGTA/1 mM phenylmethylsulphonyl fluoride/1 g/ml pepstain A/1 g/ml aprotinin/1 g/ml leupeptin/1 mM NAF/1 mM navandate), homogenized with a Polytron homogenizer (Brinkmann Instruments), and centrifuged at 5,000 × g for 10 min at 4°C. Protein concentrations were quantified by Bradford assay. Twenty micrograms of protein from each brain was electrophoresed on a 4–12% Tris·HCl gel (Bio-Rad) and transferred at 4°C to a polyvinylidene difluoride membrane. The membrane was incubated with 1:5,000 rabbit anti-trkB antibody [kind gift of Louis Reichardt (University of California, San Francisco) and Frances Lefcort (University of Montana, Missoula)] at 4°C, then with 1:5,000 horseradish peroxidase-labeled goat anti-rabbit antibody at room temperature for 1 h. Immunoreactivity was detected by chemiluminescence (ECL, Amersham Pharmacia) and quantified on a phosphorimager. The antibody used here recognizes both the functional 145-kDa form of trkB and the presumably nonfunctional 95-kDa form that lacks the intracellular signal transducing domain (45). The membrane was then probed with 1:20,000 anti-tubulin antibody (Upstate Biotechnology) at 4°C, then processed and quantified as for trkB. The trkB to tubulin ratio was calculated for each animal and compared (t test).
In Situ Hybridization on Tissue Sections. Coronal sections were cut at 20 μm from frozen unfixed P6 brains, then mounted onto slides. Each slide contained sections from the same level of a male and a female brain sectioned in parallel for comparison under identical conditions of in situ hybridization and autoradiography. Sections were fixed in 4% paraformaldehyde and stored at –80°C until processing. After linearization of plasmids, sense and antisense trkB and zRalDH mRNAs were transcribed and labeled with 35S-αUTP and further purified by phenol/chloroform extraction. Hybridization was performed in 50% formamide/0.14× SSC (1× SSC = 0.15 M sodium chloride/0.015 M sodium citrate, pH 7)/25% Denhardt's solution/10% dextran sulfate at 60°C for 16 h. After a series of low- and high-stringency washes (from 4× SSC/sodium thiosulfate to 0.1× SSC/sodium thiosulfate) at 60°C, sections were exposed to Kodak BioMax film for up to 5 days. Sections were dipped in Kodak NTB2 emulsion and exposed for up to 5 weeks, then developed and fixed. Sections were stained with thionin.
Films were digitized in a flatbed scanner or with a digital camera, using a microscope. To compare the level of trkB mRNA expression in HVC (n = 5 per sex), HVC was located in tissue sections hybridized by using the zRalDH probe, a marker for HVC (44). On the immediately adjacent tissue section hybridized with the trkB antisense riboprobe, the density of trkB hybridization in HVC was measured bilaterally from digitized autoradiogram images by using scionimage software (Scion, Frederick, MD). All sections containing HVC were measured (three to six sections per bird), and the density of label was averaged across sections to yield the mean density per bird. All measurements were performed by an individual unaware of the sex of the brains.
To compare levels of trkB mRNA expression in the telencephalon based on brain sections hybridized in situ with trkB antisense riboprobes, film images of brain sections were digitized in a flatbed scanner. A person unaware of the sex of the tissue chose two or three male–female pairs of telencephalic sections for analysis from each of eight male–female pairs of brains processed in parallel. The sections were chosen to represent three rostrocaudal different levels for each brain sectioned. The mean density of pixels in the telencephalon was measured by using scionimage.
Fluorescent in Situ Hybridization (FISH). Female zebra finch metaphase chromosome sets were prepared from female embryonic fibroblast cells according to the methods of Y.I. and A.P.A. (46). The zebra finch trkB cDNA was used to probe the zebra finch bacterial artificial chromosome (BAC) library constructed by the Arizona Genome Institute (www.genome.arizona.edu) to identify three BAC clones (ZF085A14, ZF081L02, and ZF245F08) containing trkB. The identification was confirmed by probing a Southern blot of the restriction-digested BAC clones with the trkB cDNA. The BAC clones were labeled with biotin by nick translation with biotin-16-dUTP (Roche) for use as FISH probes. FISH to mitotic chromosomes was carried out as described in ref. 47. Hybridization of the probe was detected by reactions with FITC-labeled avidin (Vector Laboratories), biotinylated goat anti-avidin antibody (Vector Laboratories), and FITC-labeled avidin. Chromosomes were counterstained with 4′,6-diamidino-2-phenylindole.
Quantitative RT-PCR. Total RNA was isolated from telencephalon (P1, P6, P10, and adult) or whole brain (P1 and adult) by using TRIzol (Invitrogen). Telencephalic group sizes were as follows: P1, 14 male, 15 female; P6, 5 each sex; P10, 6 each sex; adult, 8 each sex. Whole-brain samples were as follows: P1, 6 each sex; adult, 5 males, 8 females. After DNase treatment, 1 μg of total RNA from each sample was reverse-transcribed by SuperScript III RNase H–reverse transcriptase (Invitrogen). Real-time PCR analysis was performed on an ABI 7300 sequence detection system (Applied Biosystems) by using the SYBR Green PCR Master Mix kit with 0.3 μM each primer: trkB forward, 5′-CCATGGTATCAGCTCTCAAACAAT-3′, and trkB reverse, 5′-TCATACACTTCCTTTGGGCATGT-3′, in a total volume of 25 μl. Amplification of GAPDH was used as loading controls with 0.3 μM each primer: GAPDH forward, 5′-TGACCTGCCGTCTGGAAAA-3′, and GAPDH reverse, 5′-CCATCAGCAGCAGCCTTCA-3′. Cycling conditions were 50°C for 2 min, 95°C for 10 min, and 40 cycles at 95°C for 15 s and 60°C for 1 min. A standard curve was constructed with known concentrations of cDNA. Each sample was run in duplicate. The melting curve of PCR products was also assessed to ensure the absence of DNA contamination.
For statistical analysis, expression of trkB was divided by expression of GAPDH in each sample, and values for each age were converted to Z scores, Z = (X – m)/s, where X is the level of expression of trkB in the sample, m is the mean of all male and female samples for one assay (only samples from one age were run in each assay), and s is the standard deviation of all values in the assay. P1 telencephalic samples were run in two assays and transformed to Z scores for uniform scaling. For telencephalon or whole brain, a two-way ANOVA was calculated with factors of age and sex. Because a main effect of sex was found, t tests were used at each age to determine the ages at which the sex difference occurred.
Results
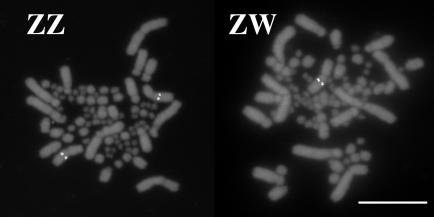
The three BAC clones encoding trkB mapped by means of FISH to the zebra finch Z chromosome (Fig. 1), which is recognized by its size and position of the centromere (46, 48). The trkB BACs hybridized to a single chromosome in females (ZW) and to two in males (ZZ). Furthermore, Southern blot hybridization with the trkB cDNA as probe showed stronger hybridization to bands in males than those in females, as expected for a Z-linked gene (data not shown). The Z-linkage of trkB in zebra finches is consistent with its Z-linkage in chickens (26) (www.ensembl.org).
Fig. 1.
Fluorescent in situ hybridization of BAC probe ZF085A14, encoding the trkB gene, to zebra finch metaphase chromosomes. The probe hybridized to the single Z chromosome of the female (Right, ZW) and to both Z chromosomes of the male (Left, ZZ). (Scale bar, 10 μm.)
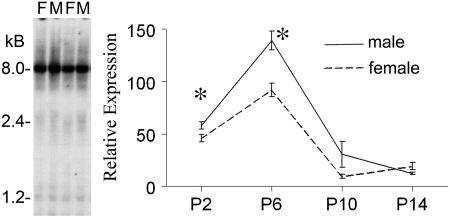
In Northern blots, the trkB cDNA hybridized to a similar pattern of transcripts in males and females. The strongest hybridization was at ≈8 kb, but smaller products at ≈2–5 kb were observed with lower expression (Fig. 2). Expression of the 8-kb transcript was quantified relative to GAPDH during early posthatch development at P2, P6, P10, and P14. In both sexes, expression was higher at the earlier two ages, increasing from P2 to P6 but then rapidly declining by P10 and P14 (Fig. 2). Analysis of the pattern of expression with a two-way ANOVA indicated a main effect of age (P < 0.000001), a main effect of sex (P < 0.0002), and an interaction of sex and age (P < 0.0008). Planned comparisons showed that the sex difference was statistically significant at P2 (P < 0.03) and P6 (P < 0.0008) but not at P10 or P14 (P > 0.05). The 8-kb mRNA product was clear at P2 and P6 but was not usually at P10 and P14, although the smaller bands increased in density at those ages. A similar two-way ANOVA of GAPDH levels alone showed a main effect of age (declining monotonically with age; P < 0.000001) and a trend (P = 0.058) toward a sex difference (M > F) that would reduce rather than account for the sex difference in the trkB/GAPDH ratio.
Fig. 2.
Northern blot analysis of trkB mRNA expression at P6. (Left) Part of Northern blot of male (M) and female (F) P6 telencephalic samples. A greater expression can be seen consistently in males at the 8-kb band and smaller bands. (Right) Graph showing quantification of expression of the 8-kb trkB band relative to GAPDH. trkB mRNA expression dropped significantly between P6 and P10. The male–female difference was statistically significant at P2 and P6 (P < 0.05, asterisks).
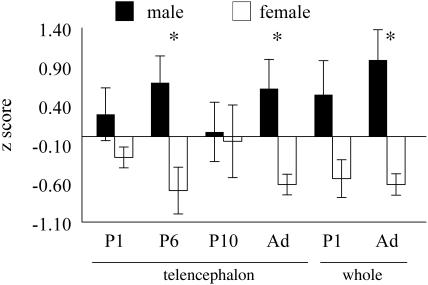
Levels of trkB mRNA were measured by using quantitative RT-PCR in telencephalon at P1, P6, P10, and adulthood (Fig. 3), and in whole brain at P1 and adulthood. trkB expression was measured relative to GAPDH and converted to Z scores for each day measured. The two-way ANOVA for telencephalic samples showed a significant main effect of sex (P < 0.002, male > female). Planned comparisons indicated that the sex difference in trkB expression was significant at P6 (P < 0.02) and in adults (P < 0.008) but not at P1 or P10 (P > 0.05). This analysis did not compare expression across ages because the comparison of samples was only valid within each age group. The two-way ANOVA for whole brain showed a significant main effect of sex (P < 0.0003, male > female), and planned comparisons found a sex difference in adults (P < 0.0008) but not at P1 (P = 0.056). None of the samples showed a sex difference in GAPDH levels (P > 0.05).
Fig. 3.
Histograms of Z scores of the level of expression of trkB mRNA, relative to GAPDH, determined by quantitative RT-PCR. Telencephalic RNA was measured at P1, P6, P10, and adulthood. Whole-brain RNA was measured at P1 and in adults. The mean level of expression was greater in males than females at all ages but was statistically significant (asterisks) in the telencephalon at P6 and in adults, and in adult whole brain.
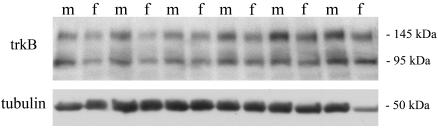
We measured trkB protein expression relative to tubulin via Western blot analysis in whole brain at P6 (n = 6 per sex) (Fig. 4). Males had ≈25% higher expression of trkB (male/female ratio range, 0.99–1.65; mean, 1.25 ± 0.118; P < 0.05, Wilcoxon signed-ranks test).
Fig. 4.
Western blot analysis of trkB expression in whole brain at P6 in six males (m) and females (f). The expression in males, relative to tubulin loading control, was on average 25% higher than in females (P < 0.05).
The sex difference in trkB expression at P6 was confirmed by in situ hybridization in side-by-side comparison of male and female brain sections (Fig. 5). trkB mRNA was expressed at consistently higher levels in the telencephalon than in most other brain regions and was higher in male telencephalon than in female telencephalon. The mean density of labeling, averaged across tissue sections, was higher in the telencephalon of all males relative to females in eight male–female brain pairs that were each processed in parallel, a result that occurs by chance with a probability of ≈0.004. Within the telencephalon, expression was widespread and in all regions, with locally heavier expression in broad areas of, for example, the nidopallium and mesopallium (for brain nomenclature, see www.avianbrain.org). Hybridization of the sense probe was much lower and showed no regional differences as with the antisense probe (data not shown).
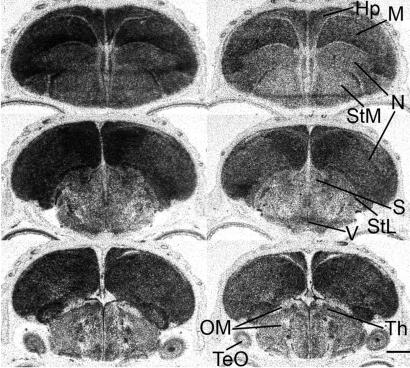
Fig. 5.
Photomicrographs of autoradiograms of representative frontal sections at three levels of the brain of a P6 male (Left) and a P6 female (Right) showing in situ hybridization of the antisense trkB probe. The middle of the sections are approximately at AP levels A2.6 (Top), A1.8, and 0.0 (Bottom) of the atlas of Stokes et al. (84). The telencephalon was labeled more than other brain regions. These sections were sectioned and processed in parallel, and hybridized on the same slide. trkB mRNA was expressed higher in the male than in the female. Hp, hippocampus; M, mesopallium; N, nidopallium; OM, occipitomesencephalic tract; S, septum; StL, lateral striatum; StM, medial striatum; TeO, optic tectum; Th, thalamus; V, third ventricle. (Scale bar, 1 mm.)
To measure expression of trkB mRNA in song nucleus HVC at P6, we located HVC by its expression of zRalDH, a marker for HVC (44), in male and female sections. trkB riboprobe hybridized clearly to HVC (Fig. 6). The labeling for trkB mRNA was higher in HVC than in the surrounding nidopallium and was consistently more prominent in males than in females. Although hybridization was variable in individual sections, in all five male–female pairs, the mean density in males was greater than that in females (male/female ratio, 1.09–2.13; mean, 1.40 ± 0.19; P < 0.05, Wilcoxon signed-ranks test). The results suggest that trkB expression was higher in male HVC than in female HVC.
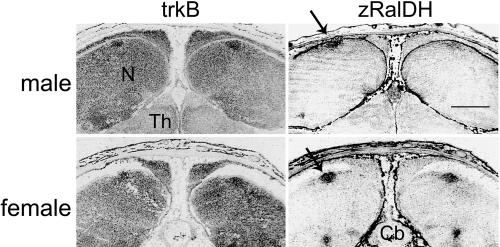
Fig. 6.
Expression of trkB mRNA in situ in HVC of a P6 male and female, based on digitized images of emulsion-dipped sections. Labeling of the trkB antisense riboprobe appears black. Adjacent sections were hybridized with antisense probes for zRalDH, a marker for HVC (arrows). The zRalDH labeling unequivocally identifies the location of HVC. Cb, cerebellum; N, nidopallium; Th, thalamus. Labeling of HVC by trkB is clearly seen. (Scale bar, 1 mm.)
Discussion
The present study establishes that trkB, the membrane receptor for the neurotrophin BDNF, is expressed at a higher level in the forebrain of male zebra finches at P2–P6, ≥3 days before the earliest previously reported sex difference in the neural circuit for song (15, 49). Moreover, song nucleus HVC itself shows higher expression of trkB in males than in females. These results suggest that the sexual differentiation of HVC is already underway by P6. Moreover, the sex difference in trkB expression may contribute to subsequent sexually dimorphic development in HVC because of heightened responsiveness to BDNF in males than in females.
The Z-linkage of the trkB gene is important because current theories of sexual differentiation of the zebra finch song system attribute the origins of sexual differentiation to a sex difference in expression of sex chromosome genes within brain cells themselves (21). Thus, if the sex difference in trkB expression stems from the higher genomic dose of Z genes in males than in females, then trkB represents a strong candidate for a sex chromosome-linked factor that initiates sexual differentiation of the song nucleus HVC. Other sex-linked factors, of course, might also be required for male-specific patterns of neural development.
Among species with heteromorphic sex chromosomes, the resulting sex difference in genomic dosage of sex-linked genes is thought to represent a major problem that has led to the evolution of several distinct dosage compensation mechanisms. In mammals, flies, and worms, the dosage of X genes is adjusted via three different molecular mechanisms (50). In mammals, transcriptional silencing, or inactivation, of one of the two X chromosomes in females balances X gene dose (51). However, this mechanism is incomplete, and some genes on the inactivated X chromosome escape inactivation so that sex differences in the expression of some X genes does occur (52–54).
The mechanism for balancing Z gene dosage in birds, if any, is not understood. Some Z genes appear to be equivalently expressed in the two sexes, suggesting some compensation mechanism, but in other cases both Z alleles are expressed (23, 24, 55, 56). Three Z genes in zebra finches (trkB, CHD1Z, and PKCIZ) all show generally higher expression in male brain (20, 22), raising the possibility that Z genes are typically expressed at a higher level in males. trkB differs from CHD1Z and PKCIZ because the latter two genes have W-linked homologues. CHD1Z is highly similar to CHD1W (22), and PKCIZ is somewhat similar to the W gene ASW (57, 58). In those cases, the expression of the W homologue may offset any male-specific effect of a higher dose of the Z gene in males.
Because there are no known instances in which a genomic dose of a sex-linked gene leads to sexual differentiation of the brain of a vertebrate, it will be important to determine what causes the sex difference in trkB expression. On the one hand, Z-linkage is likely to lead to higher expression in males than in females, because several other Z-linked genes have been found to be expressed higher in males than in females just after hatching. For example, in an unbiased screen for genes that are sexually dimorphic in the zebra finch telencephalon at hatching, Wade et al. (25) found that many of the genes tested that are higher in males are Z-linked in chickens. Because of high conservation of zebra finch and chicken chromosomes (46), these genes are likely to be Z-linked also in zebra finches. This pattern of results suggests strongly that Z genes tend to be expressed higher in males than in females, a conclusion that implies that the sex difference in expression reported here may be the result of Z-linkage of the genes. On the other hand, factors other than genomic dose appear to control trkB expression as well, because we did not find a sex difference in trkB expression at all ages tested. By early posthatch ages, male and female gonads (or the brain itself) produce and secrete steroid hormones (12, 59–61), and therefore may be involved in the sex-specific expression of trkB. Indeed, estrogen has been shown to up-regulate trkB expression in cells from the hypothalamus and olfactory bulb of mice (62, 63). Thus, we cannot rule out the possibility that sex differences in trkB expression are the result of sex differences in gonadal secretions or sex steroids produced within the brain itself.
Might the sex difference in trkB expression contribute to, or even be responsible for, sex differences in the neural song circuit? Could a 2-fold sex difference in trkB expression lead to subsequent differences in HVC development? In mice, haploinsufficiency of the trkB gene has a significant impact, lowering the number and density of neurons in the hippocampal dentate gyrus (29). Like the hippocampus of mammals, the songbird HVC receives a continuous supply of new neurons in adulthood (64, 65), is important for song learning and memory, and is impacted strongly by the action of BDNF (38, 66–68). If trkB plays a similar role in these tissues, the effects of a haploinsufficiency in the HVC of zebra finches may be similar. Thus, the lower genomic dose of trkB in female zebra finches, compared with males, could limit the trophic action of BDNF and result in fewer neurons in HVC.
The present results suggest that the sex difference in trkB mRNA expression in the telencephalon is present at P2 and P6, and in adulthood, but not at P10 or P1. The expression pattern at P10 shows little bias in favor of males, but at P1 there was a consistent tendency (not statistically significant) for male samples to have a higher mean than female samples. Thus, the present results suggest that the sex difference in telencephalic trkB expression is established by P2–P6, abolished by P10, and reestablished by adulthood. Sexual differentiation of HVC is probably occurring during the first week after hatching. Sex differences in HVC can be found at P9 in expression of androgen receptor mRNA (15, 49), at P15 in number of pyknotic cells (69), and at P20–P25 in neuron number and volume (69–71). The expression of BDNF and neurotrophin 4, the ligands for trkB, has not been described for the first posthatch week, although BDNF is expressed in male HVC by P30 (38).
BDNF can increase androgen receptor expression in rat spinal motoneurons (72–75). If a similar mechanism operates in HVC, the higher expression of trkB in HVC at P6 could lead to greater BDNF signaling, which in turn increases androgen receptor expression, accounting for the higher male expression of androgen receptors in HVC found by P9 (15, 49). The greater sensitivity of HVC cells to androgen might in turn account for the importance of androgens in the growth and differentiation of HVC at later ages (76, 77). Thus, the sex difference in trkB expression at the earliest stages of formation of HVC could have a long-lasting impact on diverse cellular mechanisms that favor sexual differentiation of HVC.
The sex difference in trkB expression occurs during a sensitive period (first week posthatch) when estrogen has its greatest influence on masculinizing the song system in females (78). Although estrogen could potentially regulate trkB expression, it is better known for its ability to regulate BDNF expression in mammalian systems and, importantly, in the HVC of zebra finches (38, 40, 79–83). For example, BDNF expression is higher in HVC of males between 30 and 35 days of age and can be induced in females if treated with estrogen at P5–P10 (38). In addition, estrogen can be used to increase BDNF expression prematurely in the HVC of P15–P25 males, which normally express little BDNF in HVC (38). Thus, estrogen's effect on BDNF expression during early development may be its more important role in masculinizing female HVC. Because females express less trkB receptor mRNA in HVC, sensitivity to BDNF signaling may be reduced relative to males and result in a sex difference in HVC neuron number. Treatment of females with estradiol, which masculinizes female HVC, could increase BDNF signaling and partially overcome the deficit in trkB receptors in females. This explanation is attractive because it would help resolve the question of how sex chromosome-linked factors interact with estradiol, which has an established role in partially masculinizing the song system. If Z-linkage causes sexually dimorphic expression of the trkB receptor, and if estradiol, synthesized at a higher level in male forebrain (12), increases BDNF in HVC, then the trkB/BDNF system provides a focal point for the convergence of sex chromosome and sex steroid influences on the brain. The factors leading to higher synthesis of estradiol in the brain, of course, are not yet known.
How might the action of Z-linked factors such as trkB cause sex differences in a discrete brain region like HVC but not in other areas of the telencephalon, where trkB is also expressed? Why might some species respond to a Z-linked factor(s) with sexually dimorphic development, whereas other species that have the same Z-linkage show less dimorphic development? To answer each of these questions, one must postulate other factors that modulate the effects of the dimorphic Z-linked signal. In this respect, the Z-linked hypothesis discussed here is similar to the idea that all sex differences in Drosophila development are initiated by a measurement of the X to autosome ratio in each cell, even though only some cells respond to that signal in a sexually dimorphic manner (50). Future studies are needed to resolve these interesting questions.
Acknowledgments
We thank Jeff Thompson, Yong-Hwan Kim, Esther Melamed, and Kathy Kampf for valuable assistance. This work was supported by National Institutes of Health Grant DC 000217.
Author contributions: X.C., R.J.A., Y.I., and A.P.A. designed research; X.C., R.J.A., and Y.I. performed research; X.C., R.J.A., Y.I., and A.P.A. analyzed data; and X.C., R.J.A., Y.I., and A.P.A. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: HVC, high vocal center; Pn, posthatch day n; trkB, tyrosine kinase receptor B.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AY679520).
References
- 1.Arnold, A. (2002) in Hormones, Brain, and Behavior, eds. Pfaff, D. W., Arnold, A. P., Etgen, A., Fahrbach, S. & Rubin, R. (Academic, San Diego), Vol. 4, pp. 105–135. [Google Scholar]
- 2.Arnold, A. P. & Gorski, R. A. (1984) Annu. Rev. Neurosci. 7, 413–442. [DOI] [PubMed] [Google Scholar]
- 3.Maclusky, N. J. & Naftolin, F. (1981) Science 211, 1294–1303. [DOI] [PubMed] [Google Scholar]
- 4.Arnold, A. P., Xu, J., Grisham, W., Chen, X. Q., Kim, Y. H. & Itoh, Y. (2004) Endocrinology 145, 1057–1062. [DOI] [PubMed] [Google Scholar]
- 5.Nottebohm, F. & Arnold, A. P. (1976) Science 194, 211–213. [DOI] [PubMed] [Google Scholar]
- 6.Bottjer, S. W., Miesner, E. A. & Arnold, A. P. (1986) Neurosci. Lett. 67, 263–268. [DOI] [PubMed] [Google Scholar]
- 7.Gurney, M. E. & Konishi, M. (1980) Science 208, 1380–1383. [DOI] [PubMed] [Google Scholar]
- 8.Simpson, H. B. & Vicario, D. S. (1991) J. Neurobiol. 22, 777–793. [DOI] [PubMed] [Google Scholar]
- 9.Grisham, W. & Arnold, A. P. (1995) J. Neurobiol. 26, 163–170. [DOI] [PubMed] [Google Scholar]
- 10.Arnold, A. P. (1996) Horm. Behav. 30, 495–505. [DOI] [PubMed] [Google Scholar]
- 11.London, S. E., Boulter, J. & Schlinger, B. A. (2003) J. Comp. Neurol. 467, 496–508. [DOI] [PubMed] [Google Scholar]
- 12.Holloway, C. C. & Clayton, D. E. (2001) Nat. Neurosci. 4, 170–175. [DOI] [PubMed] [Google Scholar]
- 13.Schlinger, B. A., Soma, K. K. & London, S. E. (2001) Trends Neurosci. 24, 429–431. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs, E. C., Grisham, W. & Arnold, A. P. (1995) J. Neurobiol. 27, 513–519. [DOI] [PubMed] [Google Scholar]
- 15.Gahr, M. & Metzdorf, R. (1999) J. Neurosci. 19, 2628–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathews, G. A. & Arnold, A. P. (1990) Gen. Comp. Endocrinol. 80, 48–58. [DOI] [PubMed] [Google Scholar]
- 17.Wade, J. & Arnold, A. P. (1994) Brain Res. 639, 347–350. [DOI] [PubMed] [Google Scholar]
- 18.Wade, J. & Arnold, A. P. (1996) Proc. Natl. Acad. Sci. USA 93, 5264–5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wade, J. (2001) Microsc. Res. Tech. 54, 354–363. [DOI] [PubMed] [Google Scholar]
- 20.Agate, R. J., Grisham, W., Wade, J., Mann, S., Wingfield, J., Schanen, C., Palotie, A. & Arnold, A. P. (2003) Proc. Natl. Acad. Sci. USA 100, 4873–4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wade, J. & Arnold, A. P. (2004) Ann. N.Y. Acad. Sci. 1016, 540–559. [DOI] [PubMed] [Google Scholar]
- 22.Agate, R. J., Choe, M. & Arnold, A. P. (2004) Mol. Biol. Evol. 21, 384–386. [DOI] [PubMed] [Google Scholar]
- 23.McQueen, H. A., McBride, D., Miele, G., Bird, A. P. & Clinton, M. (2001) Curr. Biol. 11, 253–257. [DOI] [PubMed] [Google Scholar]
- 24.Kuroda, Y., Arai, N., Arita, M., Teranishi, M., Hori, T., Harata, M. & Mizuno, S. (2001) Chromosome Res. 9, 457–468. [DOI] [PubMed] [Google Scholar]
- 25.Wade, J., Tang, Y. P., Peabody, C. &Tempelman, R. J. (2005) J. Neurobiol., in press. [DOI] [PubMed]
- 26.Schmid, M., Nanda, I., Guttenbach, M., Steinlein, C., Hoehn, M., Schartl, M., Haaf, T., Weigend, S., Fries, R., Buerstedde, J. M., et al. (2000) Cytogenet. Cell Genet. 90, 169–218. [DOI] [PubMed] [Google Scholar]
- 27.Oppenheim, R. W., Yin, Q. W., Prevette, D. & Yan, Q. (1992) Nature 360, 755–759. [DOI] [PubMed] [Google Scholar]
- 28.Luikart, B. W., Nef, S., Shipman, T. & Parada, L. F. (2003) Neuroscience 117, 847–858. [DOI] [PubMed] [Google Scholar]
- 29.von Bohlen und Halbach, O., Minichiello, L. & Unsicker, K. (2003) Eur. J. Neurosci. 18, 2319–2325. [DOI] [PubMed] [Google Scholar]
- 30.Hyman, C., Hofer, M., Barde, Y. A., Juhasz, M., Yancopoulos, G. D., Squinto, S. P. & Lindsay, R. M. (1991) Nature 350, 230–232. [DOI] [PubMed] [Google Scholar]
- 31.Lindsay, R. M. (1996) Philos. Trans. R. Soc. London B 351, 365–373. [DOI] [PubMed] [Google Scholar]
- 32.Frade, J. M., Bovolenta, P., MartinezMorales, J. R., Arribas, A. & RodriguezTebar, A. (1997) Development (Cambridge, U.K.) 124, 3313–3320. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz, P. M., Borghesani, P. R., Levy, R. L., Pomeroy, S. L. & Segal, R. A. (1997) Neuron 19, 269–281. [DOI] [PubMed] [Google Scholar]
- 34.Kirschenbaum, B. & Goldman, S. A. (1995) Proc. Natl. Acad. Sci. USA 92, 210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu, Z. Z., Zhu, L. Q. & Eide, F. F. (1997) J. Neurosci. 17, 8749–8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alderson, R. F., Alterman, A. L., Barde, Y. A. & Lindsay, R. M. (1990) Neuron 5, 297–306. [DOI] [PubMed] [Google Scholar]
- 37.Mizuno, K., Carnahan, J. & Nawa, H. (1994) Dev. Biol. 165, 243–256. [DOI] [PubMed] [Google Scholar]
- 38.Dittrich, F., Feng, Y., Metzdorf, R. & Gahr, M. (1999) Proc. Natl. Acad. Sci. USA 96, 8241–8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nottebohm, F. (2004) Ann. N.Y. Acad. Sci. 1016, 628–658. [DOI] [PubMed] [Google Scholar]
- 40.Rasika, S., Alvarez-Buylla, A. & Nottebohm, F. (1999) Neuron 22, 53–62. [DOI] [PubMed] [Google Scholar]
- 41.Alvarez-Borda, B., Haripal, B. & Nottebohm, F. (2004) Proc. Natl. Acad. Sci. USA 101, 3957–3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson, F., Hohmann, S. E., DiStefano, P. S. & Bottjer, S. W. (1997) J. Neurosci. 17, 2101–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li, X. C., Jarvis, E. D., Alvarez-Borda, B., Lim, D. A. & Nottebohm, F. (2000) Proc. Natl. Acad. Sci. USA 97, 8584–8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denisenko-Nehrbass, N. I., Jarvis, E., Scharff, C., Nottebohm, F. & Mello, C. V. (2000) Neuron 27, 359–370. [DOI] [PubMed] [Google Scholar]
- 45.Nakagawara, A. (2001) Cancer Lett. 169, 107–114. [DOI] [PubMed] [Google Scholar]
- 46.Itoh, Y. & Arnold, A. P. (2005) Chromosome Res. 13, 1–10. [DOI] [PubMed] [Google Scholar]
- 47.Saitoh, Y. & Mizuno, S. (1992) Chromosoma 101, 474–477. [DOI] [PubMed] [Google Scholar]
- 48.Pigozzi, M. I. & Solari, A. J. (1998) Chromosome Res. 6, 105–113. [DOI] [PubMed] [Google Scholar]
- 49.Kim, Y. H., Perlman, W. R. & Arnold, A. P. (2004) J. Comp. Neurol. 469, 535–547. [DOI] [PubMed] [Google Scholar]
- 50.Cline, T. W. & Meyer, B. J. (1996) Annu. Rev. Genet. 30, 637–702. [DOI] [PubMed] [Google Scholar]
- 51.Lyon, M. F. (1999) Curr. Biol. 9, R235–R237. [DOI] [PubMed] [Google Scholar]
- 52.Brown, C. J. & Greally, J. M. (2003) Trends Genet. 19, 432–438. [DOI] [PubMed] [Google Scholar]
- 53.Carrel, L., Cottle, A. A., Goglin, K. C. & Willard, H. F. (1999) Proc. Natl. Acad. Sci. USA 96, 14440–14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu, J., Burgoyne, P. S. & Arnold, A. P. (2002) Hum. Mol. Genet. 11, 1409–1419. [DOI] [PubMed] [Google Scholar]
- 55.Ellegren, H. (2002) Trends Genet. 18, 25–28. [DOI] [PubMed] [Google Scholar]
- 56.Teranishi, M., Shimada, Y., Hori, T., Nakabayashi, O., Kikuchi, T., Macleod, T., Pym, R., Sheldon, B., Solovei, I., Macgregor, H. & Mizuno, S. (2001) Chromosome Res. 9, 147–165. [DOI] [PubMed] [Google Scholar]
- 57.O'Neill, M., Binder, M., Smith, C., Andrews, J., Reed, K., Smith, M., Millar, C., Lambert, D. & Sinclair, A. (2000) Dev. Genes Evol. 210, 243–249. [DOI] [PubMed] [Google Scholar]
- 58.Hori, T., Asakawa, S., Itoh, Y., Shimizu, N. & Mizuno, S. (2000) Mol. Biol. Cell 11, 3645–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schlinger, B. A. & Arnold, A. P. (1992) Endocrinology 130, 289–299. [DOI] [PubMed] [Google Scholar]
- 60.Hutchison, J. B., Wingfield, J. C. & Hutchison, R. E. (1984) J. Endocrinol. 103, 363–369. [DOI] [PubMed] [Google Scholar]
- 61.Adkins-Regan, E., Abdelnabi, M., Mobarak, M. & Ottinger, M. A. (1990) Gen. Comp. Endocrinol. 78, 93–109. [DOI] [PubMed] [Google Scholar]
- 62.Carrer, H. F., Cambiasso, M. J., Brito, V. & Gorosito, S. (2003) Steroids Nerv. Syst. 1007, 306–316. [DOI] [PubMed] [Google Scholar]
- 63.Jezierski, M. K. & Sohrabji, F. (2001) Neurobiol. Aging 22, 309–319. [DOI] [PubMed] [Google Scholar]
- 64.Goldman, S. A. & Nottebohm, F. (1983) Proc. Natl. Acad. Sci. USA 80, 2390–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paton, J. A. & Nottebohm, F. N. (1984) Science 225, 1046–1048. [DOI] [PubMed] [Google Scholar]
- 66.Nottebohm, F., Kasparian, S. & Pandazis, C. (1981) Brain Res. 213, 99–109. [DOI] [PubMed] [Google Scholar]
- 67.Ward, B. C., Nordeen, E. J. & Nordeen, K. W. (1998) Proc. Natl. Acad. Sci. USA 95, 1277–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Airey, D. C., Kroodsma, D. E. & DeVoogd, T. J. (2000) Neurobiol. Learn. Mem. 73, 274–281. [DOI] [PubMed] [Google Scholar]
- 69.Kirn, J. R. & DeVoogd, T. J. (1989) J. Neurosci. 9, 3176–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bottjer, S. W., Glaessner, S. L. & Arnold, A. P. (1985) J. Neurosci. 5, 1556–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nordeen, E. J. & Nordeen, K. W. (1988) J. Neurosci. 8, 2869–2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Al-Shamma, H. A. & Arnold, A. P. (1997) Proc. Natl. Acad. Sci. USA 94, 1521–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang, L. Y. & Arnold, A. P. (2000) Brain Res. 852, 127–139. [DOI] [PubMed] [Google Scholar]
- 74.Yang, L. Y. & Arnold, A. P. (2000) J. Neurobiol. 44, 308–319. [DOI] [PubMed] [Google Scholar]
- 75.Yang, L. Y., Verhovshek, T. & Sengelaub, D. R. (2004) Endocrinology 145, 161–168. [DOI] [PubMed] [Google Scholar]
- 76.Grisham, W., Lee, J., McCormick, M. E., Yang-Stayner, K. & Arnold, A. P. (2002) J. Neurobiol. 51, 1–8. [DOI] [PubMed] [Google Scholar]
- 77.Bottjer, S. W. & Hewer, S. J. (1992) J Neurobiol. 23, 337–353. [DOI] [PubMed] [Google Scholar]
- 78.Adkins-Regan, E., Mansukhani, V., Seiwert, C. & Thompson, R. (1994) J. Neurobiol. 25, 865–877. [DOI] [PubMed] [Google Scholar]
- 79.Fusani, L., Metzdorf, R., Hutchison, J. B. & Gahr, M. (2003) J. Neurobiol. 54, 370–379. [DOI] [PubMed] [Google Scholar]
- 80.Solum, D. T. & Handa, R. J. (2002) J. Neurosci. 22, 2650–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ivanova, T., Kuppers, E., Engele, J. & Beyer, C. (2001) J. Neurosci. Res. 66, 221–230. [DOI] [PubMed] [Google Scholar]
- 82.Berchtold, N. C., Kesslak, J. P., Pike, C. J., Adlard, P. A. & Cotman, C. W. (2001) Eur. J. Neurosci. 14, 1992–2002. [DOI] [PubMed] [Google Scholar]
- 83.Gibbs, R. B. (1999) Brain Res. 844, 20–27. [DOI] [PubMed] [Google Scholar]
- 84.Stokes, T. M., Leonard, C. M. & Nottebohm, F. (1974) J. Comp Neurol. 156, 337–374. [DOI] [PubMed] [Google Scholar]