Abstract
While probiotics have a wide range of beneficial properties, they can also negatively affect the taste or aroma of foods products by resulting in the phenomenon of post-acidification. Ultrasound (US) is a tool to modulate the metabolism of probiotic bacteria, counteracting post-acidification and improving the performance and functional properties of microorganisms without affecting their viability. The purpose of this paper was to evaluate the effect of 10 different combinations of power (20 and 40 %) and duration (2, 4, 6, 8 and 10 min) of US treatment on two functional strains of Lactiplantibacillus plantarum (c16 and c19) isolated from table olives, with the aim of understanding how, some of the main functional and technological traits (viability, acidification, growth profile under different conditions, antibiotic resistance, viability at pH 2.0 and 0.3 % bile salts), were affected. It was found that the effects were strain dependent, and the best results were obtained for strain c19 in the combinations at 20 % for 8 and 10 min and 40 % for 2 min, where an improvement in functional characteristics was found, with some effects on biofilm stability, inhibition of acidification, without adverse results on some technological properties.
Keywords: Ultrasound, Growth profiles, Adhesion properties, Acidification, Comprehensive overview
1. Introduction
The term probiotic is derived from the Greek word “pro bios” and means “for life”. The name has evolved in meaning over time, first used by Lilley and Stillwell [1] in 1965 to refer to cells that can promote or enhance the survival of other living microorganisms, until to the official FAO/WHO definition, which describes probiotics as live microorganisms that may contribute to human well-being, when consumed frequently [2]. Hill et al. [3] later modified this definition, although the main requisites have not been changed.
Probiotics exert beneficial effects on the gastrointestinal tract through various mechanisms, including lowering intestinal pH, reducing colonization and invasion by pathogenic organisms via competitive exclusion or the production of antimicrobial agents, and modifying the host immune response. Strains with beneficial properties, most frequently belong to the genera Bifidobacterium, Lactobacillus and the related genera introduced in the taxonomy in 2020, Propionibacterium/Acidipropionibacterium, as well as Streptococcus and yeasts [4]. Probiotic microorganisms are available in foods products or as dietary supplements; however, supplementation of probiotics in foods requires the use of specific technologies that affect their active metabolism, which could lead alterations in flavour and rheology [5]. In fact, these microorganisms can often adversely affect the taste or aroma of the product as they ferment by producing organic acids that, while creating hostile environments for the proliferation of pathogens, also act with the phenomenon of post-acidification that occurs during storage, transport, and marketing [6], [7]. Probiotication of foods therefore presents a challenge; these technologies need to address sensory acceptability while ensuring the release of live probiotics in adequate quantities at the target site, the colon [8]. Several authors [9], [10], [11] have attempted to counteract the post-acidification problem using “attenuation” through various methods i) chemical technologies: hexadecyltrimethylammonium bromide (CTAB), ethylenediaminetetraacetic acid (EDTA), isopropyl alcohol (IPA), sodium dodecyl sulfate (SDS) or n-butanol and ii) physical technologies: heat or freeze/thaw cycles, and/or mechanical treatments such as ultrasound (US), high pressure of homogenization (HPH), pulsed electric field (PEF), high intensities light pulses, microwave, radiation, and microfiltration.
Attenuation can be defined as a technological method used to increase the pool of intracellular enzymes released into the matrix, positively affecting the flavour and quality of the final product, or to produce microorganisms with a less active metabolism [11]. In this context, microorganisms can thus be attenuated to produce lysed and dormant cell populations capable of controlling the process in which they are involved [5].
This paper focuses particularly on the use of ultrasound (US), which has been widely studied for its dual effect on microorganisms: a lethal effect or growth stimulation, depending on the intensity and frequency used. The use of US in foods could delay acidification and counteract post-acidification by improving performance and some functional properties of strains, without affecting the viability [7]. US are defined as waves with frequency higher than 20 KHz. Based on frequency they are classified as i) high power US (20–100 kHz), used in the food industry to inactivate undesired and/or pathogenic microorganisms; ii) low intensity US (20 kHz–2 MHz), which alters the living state of microorganisms, inducing to accelerated cell growth and an increase in metabolic activity; indeed, an increase in membrane permeability has been reported as a result of ultrasonic pulses, thereby accelerating chemical exchange and promoting cell growth and proliferation [10], [12]; iii) diagnostic US (5–10 MHz), used for medical diagnosis. A second classification is based on intensity and frequency; in fact, a distinction can be made between destructive US (high power US, intensity 10–1000 W/cm2 and frequency 20 to 100 KHz), which produces cavitation and has an antimicrobial effect, and non-destructive US (low intensity and diagnostic US, intensity <1 W/cm2 and frequency >100 KHz), which does not produce cavitation and does not induce any changes in the materials it passes through [10]. The biological effects of ultrasound on microbial cells can thus be classified as stimulation, inactivation, or destruction. The response of microorganisms to treatment depends on the intensity of reactions generated by the cavitation phenomenon; the collapse of cavitation bubbles induces mechanical, thermal and chemical damage that is reflected on the cell wall and membrane [13]. In addition, microorganisms, exhibit varying tolerances to external pressure depending on the species. Therefore, depending on the objective, it is necessary to strategically apply the process to obtain an optimal response from different microorganisms [14]. Therefore, the purpose of this paper was to evaluate the effect of US on the attributes of two potentially probiotic strains of Lactiplantibacillus plantarum (c16 and c19) isolated from “Bella di Cerignola” table olives [15].The aim was to understand how some of the main technological and functional traits were affected by US, focusing not on a few parameters but on a comprehensive set of traits that could give a general overview.
2. Materials and methods
2.1. Microorganisms
Two strains of Lpb. plantarum, coded as c16 and c19, were used throughout this study. The microorganisms belong to the Culture Collection of the DAFNE Department, University of Foggia, were isolated from table olives and selected as potential multifunctional starter cultures [15]. The strains were stored at –20 °C in MRS broth (Oxoid, Milan, Italy) + 33 % glycerol (C. Erba, Milan, Italy), and cultured in MRS broth incubated at 30 °C for 24 h before each experiment.
2.2. Ultrasound treatments
US treatments were performed by using a VC Vibra Cell Ultrasound VC 130 (Sonics and Materials Inc., Newtown, CT, USA). Microorganisms were subjected to 10 different treatments obtained by combining power (20 and 40 % of the net power, 130 W), and duration (2, 4, 6, 8 e 10 min), while maintaining the pulse constant (2 sec), as resumed in Table 1.
Table 1.
Combination used for ultrasound treatment.
| Combination | Power | Duration | Pulse |
|---|---|---|---|
| A | 20 % | 2 min | 2 sec |
| B | 20 % | 4 min | 2 sec |
| C | 20 % | 6 min | 2 sec |
| D | 20 % | 8 min | 2 sec |
| E | 20 % | 10 min | 2 sec |
| F | 40 % | 2 min | 2 sec |
| G | 40 % | 4 min | 2 sec |
| H | 40 % | 6 min | 2 sec |
| I | 40 % | 8 min | 2 sec |
| L | 40 % | 10 min | 2 sec |
The strains (20 mL) were centrifuged at 1000g for 10 min; then, the supernatant was discarded, and the pellet was suspended in 20 mL of sterile distilled water. The treatments were performed in 50-mL plastic tube, and the US probe was placed 2 cm below the surface. Before each treatment the probe was washed with a 70 % ethanol/water solution and with sterile distilled water. Immediately after the treatment, the samples were cooled on ice.
All samples were immediately plate counted on MRS agar incubated at 30 °C for 48 h under anaerobic conditions.
2.3. Acidification
The treated strains were inoculated (107 CFU/mL) in MRS broth and incubated at 15 °C for 72 h; the same conditions were used for untreated strains (control). After 24, 48 and 72 h the pH was measured twice by a pH-meter Crison (Crison Instruments, Barcellona, Spagna). The obtained data were modelled as pH decrease [11].
2.4. Growth profile
To assess the growth profile after the treatment, different modified MRS broths (supplemented with cinnamic or vanillic acids, salt, or acidified) were prepared and used as medium, as resumed in Table 2. Treated and untreated (control) samples were inoculated at 106 CFU/mL and the growth was evaluated as absorbance after 24, 48 and 72 h by a spectrophotometer UV–Vis DU 640 Beckman (Fullerton, CA, USA).
Table 2.
Parameters evaluated to assess the growth profile after treatment.
| Parameter | Value |
|---|---|
| pH | 4.5 |
| Temperature | 20 °C |
| NaCl | 0 %–1.5 %–3 %–4.5 % |
| Vanillic acid | 0.25–0.50 - 0.75 g/L |
| Cinnamic acid | 0.25–0.50- 0.75 g/L |
The results were used to calculate the Growth Index [5], [16], as follows:
where: Abss is the absorbance value of treated sample and Absc is the absorbance of the control.
GI was evaluated as follows:
GI < 25 %, complete inhibition of the strains
25 % < GI < 75 %, partial inhibition
75 % < GI < 120 %, growth similar to untreated microorganisms
GI > 120 %, growth stimulation
2.5. Antibiotic resistance
Antibiotic-resistance was evaluated using E-test (Liofilchem), with different type and different concentrations of antibiotics (Table 3). The microorganisms were spread on MRS agar and consequently an E-test strip was placed onto the middle of the plate. After 24 h incubation at 30 °C under anaerobic condition the MIC was evaluated by reading the value on the strip corresponding to the edge of the ellipse [11].
Table 3.
Antibiotics and related concentrations.
| Antibiotic | Concentration (μg/mL) |
|---|---|
| Ampicillin | 0.016–256 |
| Ciprofloxacin | 0.002–32 |
| Clarithromycin | 0.016–256 |
| Chloramfenicol | 0.016–256 |
| Erythromycin | 0.016–256 |
| Gentamicin | 0.064–1024 |
| Tetracycline | 0.016–256 |
| Trimethoprim | 0.002–32 |
| Vancomycin | 0.016–256 |
2.6. Viability at pH 2.0 and with 0.3 % bile salts
Sterile distilled water was acidified by adding HCl 1.0 N to reach pH 2.0 or adjusted by adding 0.3 % bile salts (Oxoid). Hence, the media were inoculated to 107 CFU/mL and incubated at 37 °C. The viable count was determined after 3 h [5].
2.7. Biofilm formation
Glass slides (2.5 cm × 7.6 cm) were used as adhesion support to assess the biofilm production capability of treated strains. A pretreatment was necessary to prepare the glass slides [16], summarized as follow: 1) acetone bath; 2) flush in distilled water; 3) 5 min stopover in a 3.5 % sodium hypochlorite solution (v/v) at 75° C; 4) flush in distilled water; 5) 5 min stopover in a 7.0 g/L phosphoric acid solution; 6) flush in distilled water; 7) air drying.
Coplin jars, previously filled with 40 mL of MRS, were inserted of glass slides and autoclaved (121 °C × 15 min) [17]. The microorganisms (105 CFU/mL) were inoculated in sterile Coplin jars and incubated at 30 °C. The number of attached bacteria to the glass slides were determined after 1, 3 and 7 days by plate count on MRS agar, as described by Speranza et al. [17]. Briefly, Glass slides were gently washed with sterile distilled water and placed into a 40 mL of sterile saline solution tube. The tubes were sonicated at 20 Hz × 20 W × 3 min and then plate counted.
2.8. Statistics
All the experiments were performed in duplicate over two independent batches; each batch was analysed twice. The results were preliminary analysed through one-way or multi-factorial ANOVA (Analysis of Variance), using the Tukey’s test as the post-hoc comparison test.
Additionally, data were also analysed through two-way joining using the following parameters as input variables: viability immediately after the treatment, viability at pH 2.5 after 3 h, biofilm after 7 days, acidification after 48 h, growth index with 0.75 g of vanillic or cinnamic acid or in presence of 5 % NaCl, growth index at 20 °C (all after 24 h); viability, biofilm at 7 days and acidification were preliminary standardized and reported as percentage values compared to control. Statistic was done through the software Statistica for Windows, ver. 7.0 (Statsoft, Tulsa, Okhla.).
3. Results
3.1. Viability and acidification
Table 4 shows the viable counts of Lpb. plantarum c16 and c19 (log CFU/mL) immediately after sonication. For strain c16 the combination L (40 %/10 min) caused a significant reduction in cell count (4 log CFU/mL) compared to the control (CNT, untreated strain) and other combinations. On the other hand, for the strain c19 a significant reduction in viable count (3 log CFU/mL) was found in the combinations H, I, L (40 %/6 min; 40 %/8 min; 40 %/10 min).
Table 4.
Viable count of Lpb. plantarum c16 and c19 (log CFU/mL) immediately after sonication. Mean values ± standard deviation. CNT, control. Letters indicate significant differences for each strain.
| Sample | c16 | c19 |
|---|---|---|
| CNT | 8.80 ± 0.15A | 9.85 ± 0.06A |
| A | 8.54 ± 0.99A | 9.55 ± 0.21A |
| B | 8.38 ± 1.11A | 9.82 ± 0.92A |
| C | 8.60 ± 0.06A | 9.93 ± 0.21A |
| D | 8.59 ± 0.00A | 9.95 ± 1.09A |
| E | 8.60 ± 0.06A | 9.83 ± 0.32A |
| F | 8.65 ± 0.02A | 9.90 ± 0.32A |
| G | 8.47 ± 0.01A | 9.58 ± 0.18A |
| H | 8.48 ± 0.00A | 6.30 ± 0,07B |
| I | 8.60 ± 0.00A | 6.20 ± 0.18B |
| L | 4.74 ± 0.00B | 6.05 ± 0.43B |
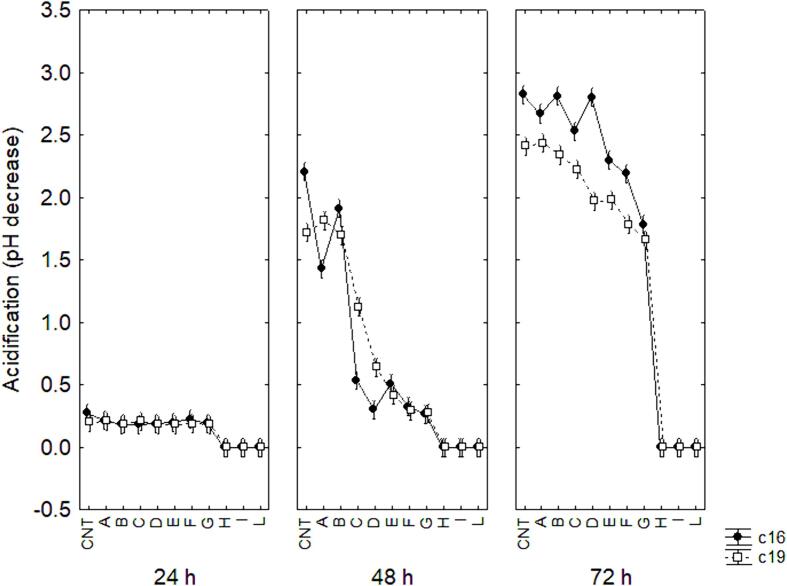
Fig. 1 illustrates the decomposition of the statistical hypothesis for the interaction “strain × time × combination” on acidification, expressed as pH decrease; after 24 h, the target strains did not show acidification in any combinations, while after 48 h in the combinations A and B the acidification was similar to that of the control sample. In sample C the extent of pH decrease relied upon the strain, as it was higher for Lpb. plantarum c19 (1.1) and lower for Lpb. plantarum c16 (0.5). In the other combinations of the design, the acidification was very low (0.3–0.5 in E, F or G) or not significant (H, I or L). After 72 h, acidification was recorded in combinations A to G, although for some, the extent of pH reduction was lower than in the control sample; no pH decrease was observed in samples H, I and L.
Fig. 1.
Decomposition of the statistical hypothesis for the effect of the interaction strain × time × sample on acidification. Vertical bars denote 95 % confidence intervals.
3.2. Growth profile
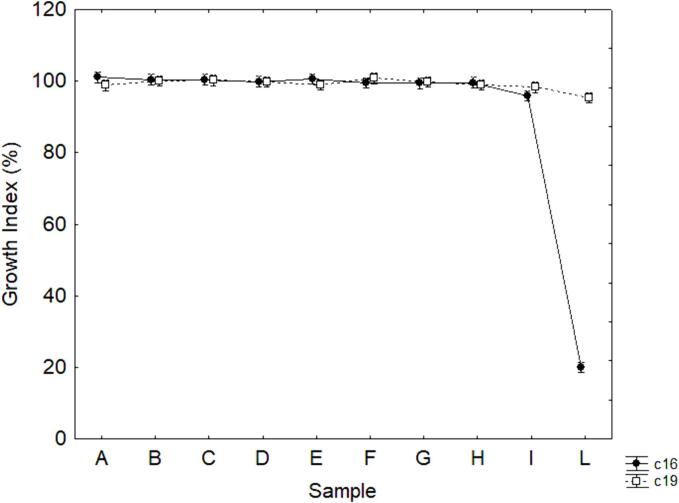
As a second step, the growth profiles were evaluated. Fig. 2 shows growth at pH 4.5/30 °C after 24 h. US-treatment did not affect the growth under sub-optimal conditions, as the GI was always at 100 %, indicating no differences between the control and the sonicated samples, except for combination L for strain c16, which exhibited a complete inhibition (GI at 19 %).
Fig. 2.
Decomposition of the statistical hypothesis for the effect of the interaction strain × sample on the Growth Index at pH 4.5 h after 24 h. Vertical bars denote 95 % confidence intervals.
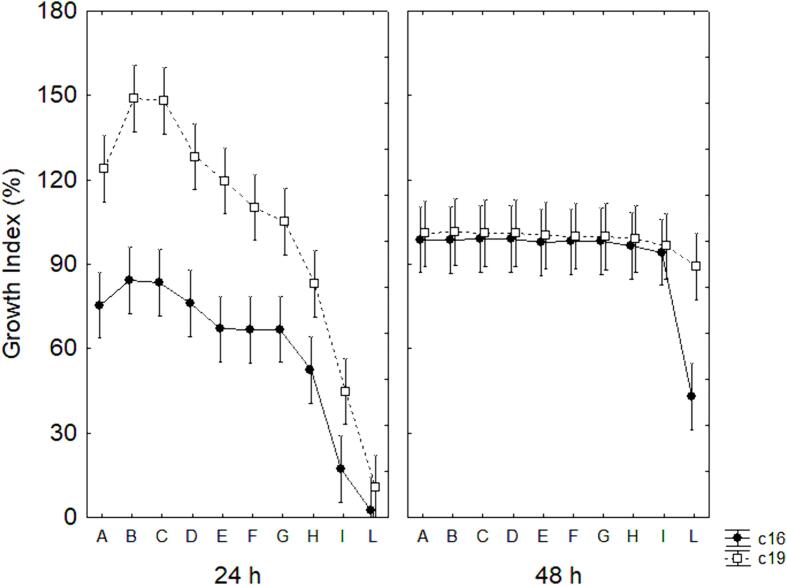
For the GI at 20 °C (Fig. 3), results indicate that for c16 strain after 24 h the combinations A, B, C, and D did not show any difference compared to untreated microorganisms, while only for I and L samples there was a complete growth inhibition (GI at 2–15 %). For the strain c19, however, combinations B, C, and D showed a stimulation of growth in the ultrasound-treated samples (GI at 150 %), while no differences were found for the other combinations, except for combinations I and H, which showed a partial inhibition (25 % < GI < 75 %) and combination L where growth was completely inhibited (GI < 25 %). After 48 h, GI was at 100 % for all combinations for both strains, except for sample L in the strain c16.
Fig. 3.
Decomposition of the statistical hypothesis for the effect of the interaction strain × time × sample on the Growth Index at 20 °C. Vertical bars denote 95 % confidence intervals.
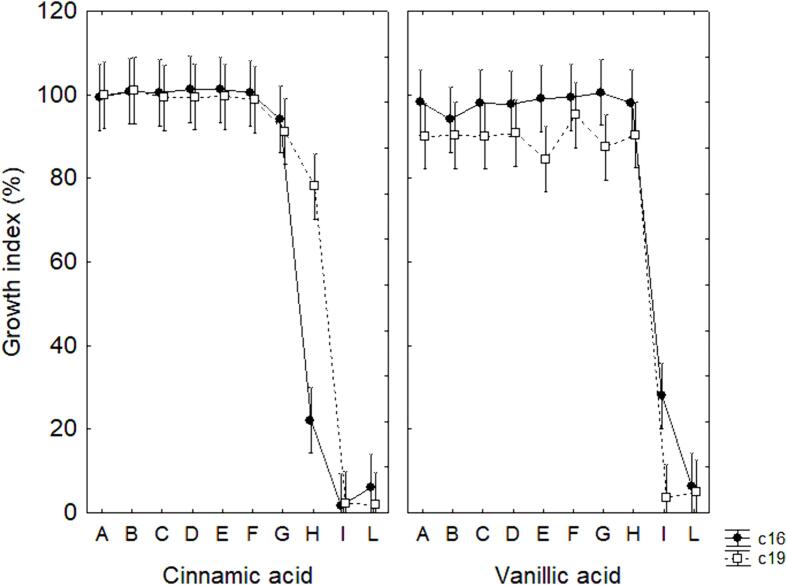
Concerning the effect of phenolic compounds (cinnamic and vanillic acids), both strains appeared inhibited in the combinations I and L; moreover, Lpb. plantarum c16 experienced a significant growth inhibition (GI at 21 %) also in the sample H in presence of cinnamic acid (Fig. 4). The growth in presence of phenolic compounds, as well as resistance to salt or growth ability at pH 4.5 or at 20 °C, are not probiotic traits, but they were chosen as a set of experiments able to define the technological robustness of the strains. Mainly, the phenolic compounds were used as representative of the classes of hydroxycinnamic and hydroxybenzoic acids (respectively, cinnamic and vanillic acids), which are the main secondary phenols of table olives, the isolation source of the test strains.
Fig. 4.
Decomposition of the statistical hypothesis for the effect of the interaction strain × phenolic compounds on the Growth Index after 24 h. Vertical bars denote 95 % confidence intervals.
3.3. Antibiotic resistance
Table 5 and Table 6 shows Minimal Inhibitory Concentrations (MIC) of several antibiotics towards c16 and c19 strains (μg/mL) after ultrasound treatments; the results show that both strains were resistant to ciprofloxacin and vancomycin. For ampicillin, clarithromycin, chloramphenicol, erythromycin, gentamicin, tetracyline and trimethoprim, US treatment determined a reduction of MICs in several combinations. For instance, in the case of gentamicin, the MIC for strain C16 was 192 µg/mL in the control and 64–32 µg/ml in the combinations H-I-L, while for the strain c19 it was 128 µg/mL in the control and 40–28 µg/mL in combinations H and I. For chloramphenicol, the MIC was 6.00 µg/mL in the control and 2.50 µg/mL in the sample L.
Table 5.
Minimal Inhibitory Concentrations of several antibiotics towards Lpb. plantarum c16 (μg/mL). Mean values ± standard deviation. Letters indicate significant differences for each antibiotic (one way ANOVA and Tukey’s test). R, resistant (no halo was found). CNT, control.
| Sample | Ampicillin | Ciprofloxacin | Clarithromycin | Chloramphenicol | Erythromycin | Gentamicin | Tetracyclines | Trimethoprim | Vancomycin |
|---|---|---|---|---|---|---|---|---|---|
| CNT | 4.50 ± 0.71B | R | 3.00 ± 0.00B | 64.0 ± 0.00A | 12.00 ± 0.00A | 192.00 ± 0.00B | 10.00 ± 2.82A | 1.25 ± 0.35A | R |
| A | 8.0 ± 0.00A | R | 2.00 ± 0.00C | 13.00 ± 1.41B | 7.00 ± 1.41B | 256.00 ± 0.00A | 12.00 ± 5.70A | 2.00 ± 0.00A | R |
| B | 0.16 ± 0.05 | R | 3.5 ± 0.71A,B | 7.00 ± 1.41C | 6.50 ± 0.71B | 128.00 ± 0.00C | 5.00 ± 1.41A,B | 2.00 ± 0.00A | R |
| C | 2,0 ± 0.00C | R | 5.0 ± 0.00A | 14.00 ± 2.83B | 5.5 ± 3.53B | 192.00 ± 0.00B | 7.00 ± 1,41A | 2.50 ± 0.71A | R |
| D | 1.0 ± 0.00D | R | 2.0 ± 0.00C | 6.00 ± 0.00C | 12.00 ± 0.00A | 192.00 ± 0.00B | 6.00 ± 0.00A,B | 1.50 ± 0.00A | R |
| E | 1.0 ± 0.00D | R | 1.5 ± 0.00C | 18.00 ± 2.48B | 3.00 ± 0.00C | 128.00 ± 0.00C | 7.00 ± 1.41A | 1.25 ± 0.35A | R |
| F | 0.5 ± 0.00D,E | R | 1.25 ± 0.35C | 8.00 ± 0.00C | 3.00 ± 0.00C | 128.00 ± 0.00C | 3.00 ± 0.00B | 0.75 ± 0.00B | R |
| G | 1.12 ± 0.53C,D | R | 0.75 ± 0.00D | 8.00 ± 0.00C | 4.00 ± 0.00C | 128.00 ± 0.00C | 3.50 ± 0.71B | 0.87 ± 0.18B | R |
| H | 0.17 ± 0.11E | R | 0.5 ± 0.00D | 4.50 ± 0.71D | 7.00 ± 1.41B | 64.00 ± 0.00D | 4.50 ± 0.71B | 1.75 ± 0.35A | R |
| I | 0.09 ± 0.04E | R | 0.25 ± 0.00E | 3.00 ± 0.00D | 1.00 ± 0.00D | 32.00 ± 0.00E | 3.00 ± 0.00B | 1.75 ± 0.35A | R |
| L | 0.09 ± 0.00E | R | 0.04 ± 0.0014F | 1.50 ± 0.00E | 1.50 ± 0.00D | 32.00 ± 0.00E | 3.00 ± 0.00B | 1.75 ± 0.35A | R |
Table 6.
Minimal Inhibitory Concentrations of several antibiotics towards Lpb. plantarum c19 (μg/mL). Mean values ± standard deviation. Letters indicate significant differences for each antibiotic (one way ANOVA and Tuke’s test). R, resistant (no halo was found). CNT, control.
| Sample | Ampicillin | Ciprofloxacin | Clarithromycin | Chloramphenicol | Erythromycin | Gentamicin | Tetracyclines | Trimethoprim | Vancomycin |
|---|---|---|---|---|---|---|---|---|---|
| CNT | 0.25 ± 0.00A | R | 1.50 ± 0.00A | 6.00 ± 0.00A | 3.00 ± 0.00A | 128.00 ± 0.00A | 48.00 ± 0.00A | 4.00 ± 0.00A | R |
| A | 0.25 ± 0.00A | R | 1.50 ± 0.00A | 6.00 ± 0.00A | 2.00 ± 0.00A | 128.00 ± 0.00A | 48.00 ± 0.00A | 4.00 ± 0.00A | R |
| B | 0.125 ± 0.00B | R | 2.00 ± 0.00A | 4.00 ± 0.00B | 2.00 ± 0.00A | 96.00 ± 0.00B | 48.00 ± 0.00A | 4.00 ± 0.00A | R |
| C | 0.19 ± 0.00A | R | 1.50 ± 0.00A | 4.00 ± 0.00B | 2.00 ± 0.00A | 96.00 ± 0.00B | 32.00 ± 0.00B | 4.00 ± 0.00A | R |
| D | 0.19 ± 0.00A | R | 2.00 ± 0.00A | 4.00 ± 0.00B | 1.50 ± 0.00A | 96.00 ± 0.00B | 48.00 ± 0.00A | 4.00 ± 0.00A | R |
| E | 0.50 ± 0.00A | R | 2.00 ± 0.00A | 4.00 ± 0.00B | 0.75 ± 0.00B | 128.00 ± 0.00A | 48.00 ± 0.00A | 1.50 ± 0.00B | R |
| F | 0.37 ± 0.18A | R | 1.25 ± 0.35A | 4.00 ± 0.00B | 2.50 ± 0.71A | 128.00 ± 0.00A | 32.00 ± 0.00B | 1.50 ± 0.00B | R |
| G | 0.25 ± 0.00A | R | 1.00 ± 0.00A | 4.00 ± 0.00B | 1.75 ± 0.35A | 112.00 ± 22.63A,B | 48.00 ± 0.00A | 1.50 ± 0.00B | R |
| H | 0.08 ± 0.02B | R | 0.22 ± 0.04B | 3.00 ± 0.00C | 0.75 ± 0.00B | 40.00 ± 11.31C,D | 32.00 ± 0.00B | 1.50 ± 0.00B | R |
| I | 0.09 ± 0.00B | R | 0.16 ± 0.05B | 2.50 ± 0.71C | 0.37 ± 0.18C | 28.00 ± 5.66D | 2.00 ± 0.00C | 1.50 ± 0.00B | R |
| L | 0.09 ± 0.00B | R | 0.16 ± 0.05B | 2.50 ± 0.71C | 1.00 ± 0.18A,B | 48.00 ± 0.00C | 1.25 ± 0.35C | 1.50 ± 0.00B | R |
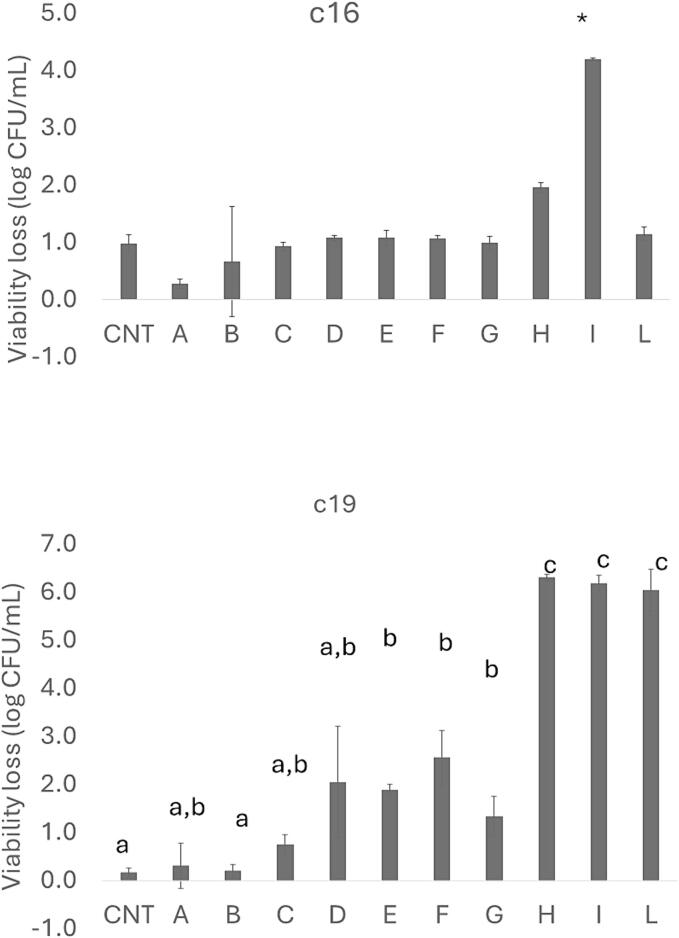
3.4. Viability loss at pH 2.5 and in presence of bile salts
Fig. 5 shows the viability loss at pH 2.5; for the strain c16, the combinations A to G showed a similar trend to control with a reduction in viable count of approximately 1 log CFU/mL, while a higher viability loss was observed in sample I where the target strain experienced a 4 log-reduction. For the strain c19, in the combinations E-G and H-L the viability loss was 1 and 6 log CFU/mL, respectively, vs 0.2 log CFU/mL in the control.
Fig. 5.
Viability loss (log CFU/mL) at pH 2.5 after 3 h. Mean values ± standard deviation. Letters or the symbol “*” indicate significant differences (one way ANOVA and Tukey’s test).
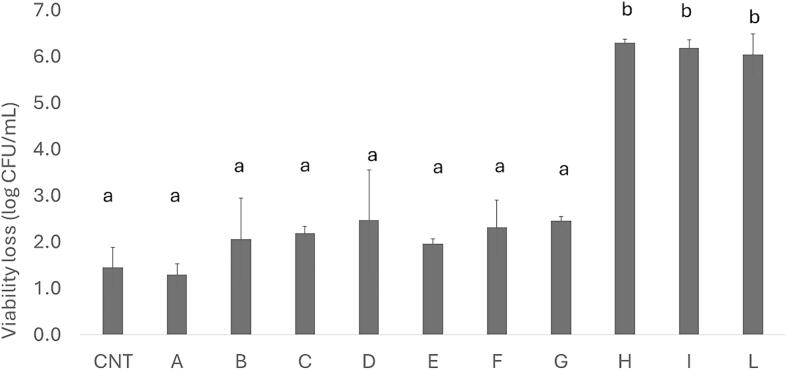
Concerning the trend in presence of bile salts, the strains showed a similar response; thus, Fig. 6 shows the results for Lpb. plantarum c19. The viability loss was 1.7–2.2 log CFU/mL in the control and samples A-G, whereas a stronger reduction was found in the combinations H, I, and L with a viability loss of 6 log CFU/mL.
Fig. 6.
Viability loss of Lpb. plantarum c19 (log CFU/mL) with 0.3 % bile salts after 3 h. Mean values ± standard deviation. Letters “*” indicate significant differences (one way ANOVA and Tukey’s test).
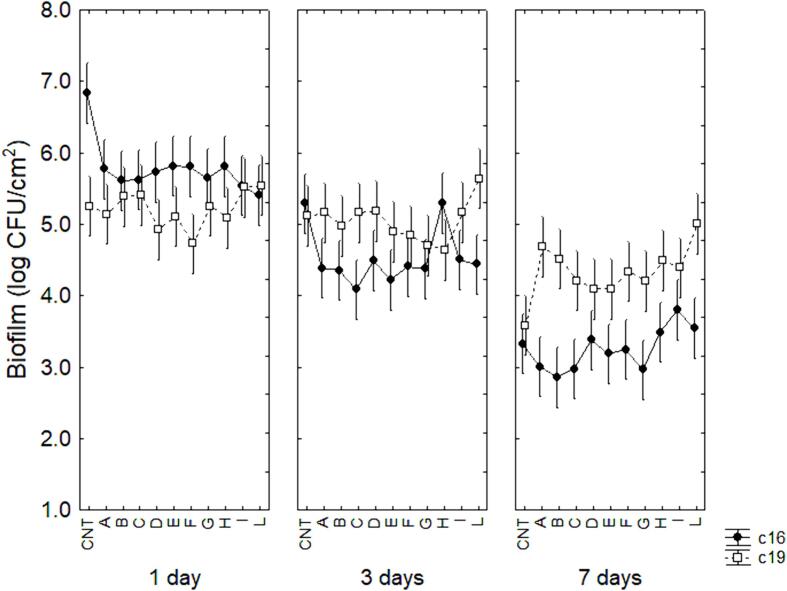
3.5. Biofilm formation
The results for biofilm formation are presented in Fig. 7. The decomposition of the statistical hypothesis highlighted the significance of the interaction “time × treatment × strain”. After 7 days, the most notable results were found for the strain c19, as in the control sample biofilm was at 3.6 log CFU/cm2, while US-treatment improved biofilm stability with a higher concentration of sessile cells in the combinations A to L (4.49–5.01 log CFU/cm2) (P < 0.05).
Fig. 7.
Decomposition of the statistical hypothesis for the interaction strain × samples × time on biofilm formation. Vertical bars denote 95 % confidence intervals.
3.6. Two way joining
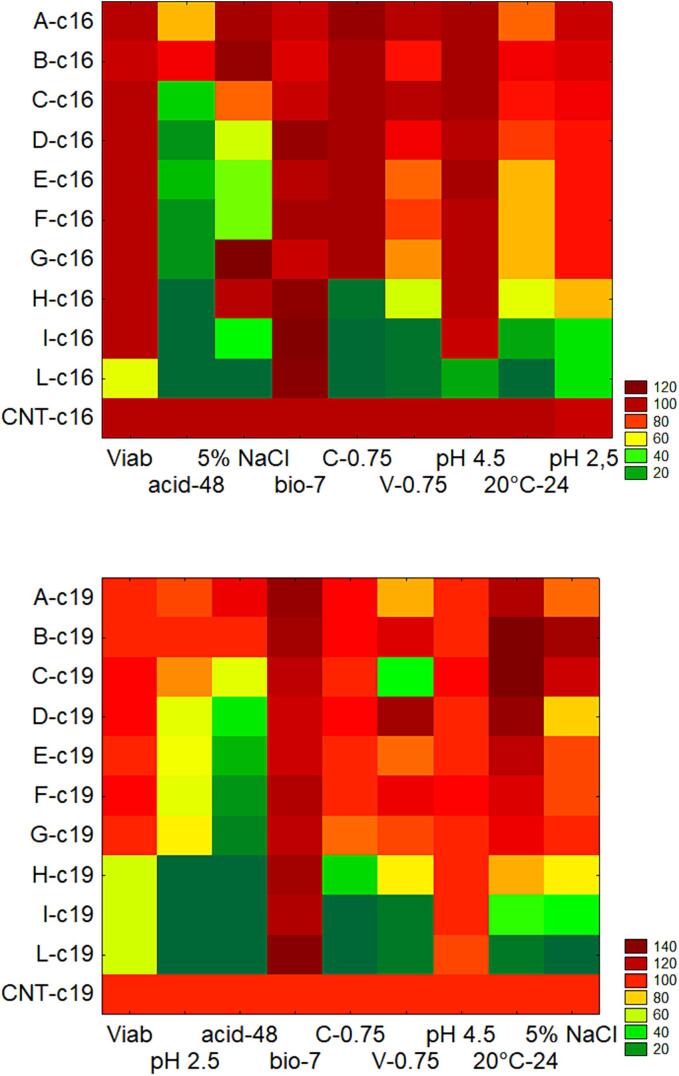
As a final step, a two-way joining (or heat-map) analysis was conducted to gain a comprehensive overview of the attenuation effect on the target strains (Fig. 8); all the parameters used as input value are percentages compared to the control sample (untreated microorganism).
Fig. 8.
Two way joining for the combinations of the design (from A to L, US-treatments; CNT, control). Viab, viability compared to control after the treatment (%); acid-48, acidification at 15 °C after 48 h compared to control (%); pH 2.5, viability at pH 2.5 after 3 h compared to control (%); bio-7, biofilm formation after 48 h compared to control (%); C-0.75, Growth Index after 24 h with 0.75 g of cinnamic acid; V-0.75, Growth Index after 24 h with 0.75 g of vanillic acid; pH 4.5, Growth Index after 24 h at pH 4.5; 20 °C-24, Growth Index at 20 °C after 24 h; 5 % NaCl, Growth Index after 24 h with 5 % NaCl.
This approach is useful to point out the optimal combinations for the attenuation of the microorganisms; however, a preliminary step is to set the criteria.
Considering the main aims of attenuation, the prerequisite are the viability (no viability loss compared to untreated microorganisms) and the reduction of acidification ability; after that, some secondary criteria could be pointed out on some possible beneficial effects (improvement of biofilm formation, viability at pH 2.5, growth profiles).
In the case of the strain c19 a good compromise could be the combinations from D to F, for which the viability after the treatment was similar to the control sample (95 %), the acidification appeared reduced (15–37 % of the control), the viability at pH 2.5 was good although lower than untreated microorganism (ca. 70 % of the control), biofilm stability after 7 days appeared improved (the concentration of sessile cells was 115–120 % of the control), and the growth index with salt, phenolic compounds, at pH 4.5 or at 20 °C was >75 %.
In the case of the strain c16, the good compromise could be the combination C, although the Growth Index in presence of 5 % NaCl was more affected; as a secondary choice, combination D could be proposed, but in this case the Growth Index with 5 % of salt was 48 % which means a retarded growth compared to the control.
4. Discussion
Probiotics play an important role in human health, and the interest in these bacteria is growing year by year, leading to an increasing number of studies on the mechanisms that enhance properties, like growth and resistance to gastrointestinal conditions, as well as those which regulate negative characteristics like acidification [7], [13], [18].
US treatments directly affect cell viability enhancing or reducing the growth of the treated bacteria and it mainly depends on the type of injury occurred to the cell wall or membrane, which can be lethal or sublethal. When the treatment is prolonged or too powerful (with high energy loaded into the matrix), the injuries become irreparable, resulting in cell death. In contrast, sub-lethal injuries do not cause death but delay certain metabolitic processes, including lactic fermentation.
In addition, the metabolic pathway can be affected by the cavitation process, as reported by Gholamhosseinpour et al. [19], both positively (stimulation) and negatively (attenuation). Focusing on stimulation, membrane pores can release in the medium a higher number of enzymes that are responsible for transformation of complex sugars in simple sugars. Consequently the microorganisms can accellerate their metabolic activity, leading to a decrease in pH. This study, otherwise, aligns more closely with the findings of Giordano et al. [20] on the metabolism’s attenuation of Limosilactobacillus reuterii DSM 17938 in tomato juice. It is likely that damage to the external surface forces the bacteria to focus energy on repair mechanisms rather than other metabolic pathways, and this idea could explain the effect on acidification and slowing of metabolism, the main aim of this technology.
Giordano et al. [20], [21] analysed the transcriptomic profile of US-attenuated Lacticaseibacillus casei and found impacts on several biological functions, includinghigher ATP accumulation anddecreased biosynthetic capacity. They suggested that the effect on acidification was not the mere action on the enzymes involved in lactic fermentation but the result of a general perturbation.
Otherwise, structural alterations of the membrane and increased permeability [11], [13], [22] could partially account for the lowered Minimal Inhibitory Concentrations for several antibiotics (erythromycin, tetracyclines, gentamycin, trimethoprim, clarithormycin), which act on targets inside the cells. Therefore, increased permeability leads to increased diffusion of these compounds into the cells. On the other hand, increased sensitivity to ampicillin, which has the cell wall as its primary target, is probably due to morphological changes to occur in the outer layers as a result of cavitation and acoustic streaming [23].
Concerning the effect on the growth profile, GI values similar to the control in some combinations highlight the ability of the attenuated cells to repair potential injuries and to obtain an active metabolism; however, this ability is probably related to the energy loaded into the system, which is a function of power, pulse, and duration [24]. At high energy levels, such as in combination L, the extent of injury probably does not make possible a repair and the microorganisms could not growth under stress conditions (e.g., as in presence of phenolic compounds).
Biofilm formation is a fundamental feature for potential probiotic strain because it enhances adhesion to mucosa and the persistence in the human gut, maximizing their beneficial effects [25]. US treatment can be used to either improve the adhesion of biofilm or to detach biofilm from abiotic surfaces [26], [27]. The enhancement activity, as showed by Bevilacqua et al. [11], could be due to a higher availability of nutrients in the deeper layers of the biofilm which led to a stronger and more stable structure or to an increased hydrophobicity of cell surfaces, as also suggested by the same authors for propionibacteria and by other authors for Lcb. casei, Lim. reuteri [5], [13] and the same strain of Lpb. plantarum at 50 % [28]. It is plausible that the increased nutrient diffusion across the different layers of biofilm could delay or counteract the senescence, thereby positively influencing biofilm stability [26], [28].
Another plausible reason beyond this increased biofilm stability, is a potential shift of Lpb. plantarum to aerobic metabolism [29] due to the increased oxygen diffusion rate in the deeper layers of biofilm [26]. This shift is generally linked to stress resistance [30].
5. Conclusion
The attenuation of the metabolism of probiotic/functional microorganisms is a complex process, that depends on several variables. This paper provides, for the first time, a comprehensive overview not only on acidification and probiotic properties (such as biofilm formation, viability at pH 2.5 and with bile salts, and antibiotic resistance), as already explored by authors or other researchers in the past, but also on some technological/functional profiles (like the growth in presence of phenols, sub-lethal pH or sub-optimal temperatures).The data obtained offer some key-points.
First, the energy loaded into the system, and thus power, duration and pulse of the treatment, is a primary factor to control, as if too high it could determine an irreversible injury and some functional traits, like growth in presence of stressful elements, could be strongly affected. Another point is the strong strain-dependence of the treatment, apart the species-effect, as the two strains of the species Lpb. plantarum exhibited different behaviors for certain properties, like biofilm stability.
Attenuation could be used to improve some surface properties, and for the strain c19 it determined an amelioration of biofilm stability. Finally, selecting the appropriate combination for attenuation involves a kind of risk/benefit analysis, as evidenced by the heat-map. It is up to the end-user to decide which parameter is the most important for an efficient and useful treatment, as different parameters could be affected in a different way, some of them with positive effects and other with negative outcomes.
Further investigations are required to focus on the transcriptomic profiles of the two strains of Lpb. plantarum, and their behaviour in a complex food matrix after attenuation.
CRediT authorship contribution statement
Antonio Bevilacqua: Writing – review & editing, Writing – original draft, Supervision, Investigation, Formal analysis, Conceptualization. Barbara Speranza: Writing – review & editing, Investigation. Daniela Campaniello: Writing – review & editing, Investigation. Angela Racioppo: Writing – review & editing, Investigation. Alessandra Accettulli: Writing – review & editing, Writing – original draft, Investigation. Alessandro De Santis: Writing – review & editing, Writing – original draft, Investigation. Milena Sinigaglia: Writing – review & editing, Funding acquisition, Formal analysis, Conceptualization. Maria Rosaria Corbo: Writing – review & editing, Funding acquisition, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Lilly D.M., Stillwell R.H. Probiotics: growth-promoting factors produced by microorganisms. Science. 1965;147(3659):747–748. doi: 10.1126/science.147.3659.747. [DOI] [PubMed] [Google Scholar]
- 2.Food and Agriculture Organization of the United Nations, World Health Organization, eds., Probiotics in food: health and nutritional properties and guidelines for evaluation, Food and Agriculture Organization of the United Nations: World Health Organization, Rome, 2006.
- 3.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Berni Canani R., Flint H.J., Salminen S., Calder P.C., Sanders M.E. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 4.Soccol C.R., Vandenberghe L.D.S., Spier M.R., Medeiros A.B.P., Yamaguishi C.T., Lindner J.D.D., Pandey A., Thomaz-Soccol V. The potential of probiotics: a review. Food Technol. Biotechnol. 2010;48:413–434. [Google Scholar]
- 5.Racioppo A., Corbo M.R., Piccoli C., Sinigaglia M., Speranza B., Bevilacqua A. Ultrasound attenuation of lactobacilli and bifidobacteria: Effect on some technological and probiotic properties. Int. J. Food Microbiol. 2017;243:78–83. doi: 10.1016/j.ijfoodmicro.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Deshwal G.K., Tiwari S., Kumar A., Raman R.K., Kadyan S. Review on factors affecting and control of post-acidification in yoghurt and related products. Trends Food Sci. Tech. 2021;109:499–512. doi: 10.1016/j.tifs.2021.01.057. [DOI] [Google Scholar]
- 7.Campaniello D., Corbo M.R., Speranza B., Sinigaglia M., Bevilacqua A. Ultrasound-Attenuated microorganisms inoculated in vegetable beverages: effect of strains, temperature, ultrasound and storage conditions on the performances of the treatment. Microorganisms. 2020;8:1219. doi: 10.3390/microorganisms8081219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giordano I., Maresca D., Mauriello G. Microencapsulation and sonication: A multiple physical approach to attenuate the probiotic Lacticaseibacillus casei ATCC 393. Heliyon. 2023;9:12. doi: 10.3390/microorganisms8081219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yarlagadda A.B., Wilkinson M.G., O'sullivan M.G., Kilcawley K.N. Utilisation of microfluidisation to enhance enzymatic and metabolic potential of lactococcal strains as adjuncts in Gouda type cheese. Int. Dairy J. 2014;38:124–132. doi: 10.1016/j.idairyj.2014.01.007. [DOI] [Google Scholar]
- 10.Bevilacqua A., Speranza B., Iorio M.C., Loi M., Sinigaglia M., Corbo M.R. US-inactivation of foodborne bacteria: Screening in distilled water and combination with citrus extract in skim milk. LWT. 2016;70:135–141. doi: 10.1016/j.lwt.2016.02.042. [DOI] [Google Scholar]
- 11.A. Bevilacqua, A. Racioppo, M. Sinigaglia, B. Speranza, D. Campaniello, M.R. Corbo, A low-power ultrasound attenuation improves the stability of biofilm and hydrophobicity of Propionibacterium freudenreichii subsp. freudenreichii DSM 20271 and Acidipropionibacterium jensenii DSM 20535, Food Microbiol. 78 (2019) 104-109, https://doi.org/10.1016/j.fm.2018.10.010. [DOI] [PubMed]
- 12.Pagnossa J.P., Rocchetti G., Ribeiro A.C., Piccoli R.H., Lucini L. Ultrasound: Beneficial biotechnological aspects on microorganisms-mediated processes. Curr. Opin. Food Sci. 2020;31:24–30. doi: 10.1016/j.cofs.2019.10.006. [DOI] [Google Scholar]
- 13.Giordano I., Mauriello G. Ultrasound attenuation improves some surface properties of the probiotic strain Lacticaseibacillus casei ATCC 393. Microorganisms. 2023;11:142. doi: 10.3390/microorganisms11010142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W.S., Yang C.Y., Fang T.J. Strategic ultrasound-induced stress response of lactic acid bacteria on enhancement of β-glucosidase activity for bioconversion of isoflavones in soymilk. J. Microbiol. Methods. 2018;148:145–150. doi: 10.1016/j.mimet.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Bevilacqua A., Altieri C., Corbo M.R., Sinigaglia M., Ouoba L.I.I. Characterization of lactic acid bacteria isolated from Italian Bella di Cerignola table olives: selection of potential multifunctional starter cultures. J. Food Sci. 2010;75:536–544. doi: 10.1111/j.1750-3841.2010.01793.x. [DOI] [PubMed] [Google Scholar]
- 16.Bevilacqua A., Perricone M., Cannarsi M., Corbo M.R., Sinigaglia M. Technological and spoiling characteristics of the yeast microflora isolated from Bella di Cerignola table olives. Int. J. Food Sci. Tech. 2009;44:2198–2207. doi: 10.1111/j.1365-2621.2009.02060.x. [DOI] [Google Scholar]
- 17.Speranza B., Corbo M.R., Sinigaglia M. Effects of nutritional and environmental conditions on Salmonella sp. biofilm formation. J. Food Sci. 2011;76:12–16. doi: 10.1111/j.1750-3841.2010.01936.x. [DOI] [PubMed] [Google Scholar]
- 18.Shokri S., Terefe N.S., Shekarforoush S.S., Hosseinzadeh S. Ultrasound-assisted fermentation for enhancing metabolic and probiotic activities of Lactobacillus brevis. Chem. Eng. Process. 2021;166 doi: 10.1016/j.cep.2021.108470. [DOI] [Google Scholar]
- 19.A. Gholamhosseinpour, S.M.B. Hashemi, F. Safari, K. Kerboua, Impact of ultrasonicated Lactobacillus delbrueckii subsp. bulgaricus, Streptococcus thermophilus and Lactiplantibacillus plantarum AF1 on the safety and bioactive properties of stirred yoghurt during storage, Ultrason. Sonochem. 102 (2024) 106726, https://doi.org/10.1016/j.ultsonch.2023.106726. [DOI] [PMC free article] [PubMed]
- 20.Giordano I., Abuqwider J., Altamimi M., Di Monaco R., Puleo S., Mauriello G. Application of ultrasound and microencapsulation on Limosilactobacillus reuteri DSM 17938 as a metabolic attenuation strategy for tomato juice probiotication. Heliyon. 2022;8:10. doi: 10.1016/j.heliyon.2022.e10969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giordano I., Pasolli E., Mauriello G. Transcriptomic analysis reveals differential gene expression patterns of Lacticaseibacillus casei ATCC 393 in response to ultrasound stress. Ultrason. Sonochem. 2024;107:106939. doi: 10.1016/j.ultsonch.2024.106939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai C., Xiong F., He R., Zhang W., Ma H. Effects of low-intensity ultrasound on the growth, cell membrane permeability and ethanol tolerance of Saccharomyces cerevisiae. Ultrason. Sonochem. 2017;36:191–197. doi: 10.1016/j.ultsonch.2016.11.035. [DOI] [PubMed] [Google Scholar]
- 23.Ojha K.S., Mason T.J., O’Donnell C.P., Kerry J.P., Tiwari B.K. Ultrasound technology for food fermentation applications. Ultrason. Sonochem. 2017;34:410–417. doi: 10.1016/j.ultsonch.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Bevilacqua A., Sinigaglia M., Corbo M.R. Ultrasound and antimicrobial compounds: a suitable way to control Fusarium oxysporum in juices. Food Bioprocess Tech. 2013;6:1153–1163. doi: 10.1007/s11947-012-0782-0. [DOI] [Google Scholar]
- 25.Plaza-Diaz J., Ruiz-Ojeda F.J., Gil-Campos M., Gil A. Mechanisms of action of probiotics. Adv. Nutr. 2019;10:49–66. doi: 10.1093/advances/nmy063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erriu M., Blus C., Szmukler-Moncler S., Buogo S., Levi R., Barbato G., Madonnaripa D., Denotti G., Piras V., Orrù G. Microbial biofilm modulation by ultrasound: current concepts and controversies. Ultrason. Sonochem. 2014;21:15–22. doi: 10.1016/j.ultsonch.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 27.B. Speranza, A. Liso, V. Russo, M.R. Corbo, Evaluation of the potential of biofilm formation of Bifidobacterium longum subsp. infantis and Lactobacillus reuteri as competitive biocontrol agents against pathogenic and food spoilage bacteria, Microorganisms 8 (2020) 177, https://doi.org/10.3390/microorganisms8020177. [DOI] [PMC free article] [PubMed]
- 28.Racioppo A., Speranza B., Altieri C., Sinigaglia M., Corbo M.R., Bevilacqua A. Ultrasound can increase biofilm formation by Lactiplantibacillus plantarum and Bifidobacterium spp. Front. Microbiol. 2023;14:1094671. doi: 10.3389/fmicb.2023.1094671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zotta T., Ricciardi A., Guidone A., Sacco M., Muscariello L., Mazzeo M.F., Cacace G., Parente E. Inactivation of ccpA and aeration affect growth, metabolite production and stress tolerance in Lactobacillus plantarum WCFS1. Int. J. Food Microbiol. 2012;155:51–59. doi: 10.1016/j.ijfoodmicro.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 30.Guidone A., Ianniello R.G., Ricciardi A., Zotta T., Parente E. Aerobic metabolism and oxidative stress tolerance in the Lactobacillus plantarum group. World J. Microb. Biot. 2013;29:1713–1722. doi: 10.1007/s11274-013-1334-0. [DOI] [PubMed] [Google Scholar]