Abstract
This study investigated the development of hybrid cheese analogues (HCA) made with fermented brewery side-stream ingredients (spent yeast and malt rootlets) and dairy milk. Different percentages of side-stream flours (3.5%, 5%, and 7.5%) were mixed with pasteurized milk, and the developed HCA were evaluated for their biochemical and textural properties. The addition of a fermentation step improved nutrient availability and led to pH (range 4.79–5.60) and moisture content (range 45.86%–61.29%) similar to traditional animal-based fresh cheeses (control). The inclusion of side-stream flours led to coagulation, even without rennet addition. The higher the concentration of the flour used, the faster the coagulation time, suggesting synergistic effect between the enzymes of the rennet and the enzymes present in the fermented side-stream flours. Nevertheless, textural properties were inferior compared to the control. Selected HCA formulations with added 3.5% flour exhibited increased counts of enterococci and enterobacteria cell densities, ranging from 7.28 ± 0.03 to 7.72 ± 0.09 log CFU/g and 4.90 ± 0.16 to 5.41 ± 0.01 log CFU/g, respectively. Compared to the control sample, HCA formulations exhibited higher concentrations of organic acids, peptides, and free amino acids (FAAs). Lactic acid reached up to 23.78 ± 0.94 g/kg of dry matter (DM), while the peptide area reached up to 22918.50 ± 2370.93 mL⋅AU. Additionally, the total concentration of individual FAAs reached up to 2809.74 ± 104.85 mg/kg of DM, contrasted with the control, which resulted in lower concentrations (847.65 ± 0.02 mg/kg of DM). The overall findings suggested that despite challenges in microbiological quality and textural properties, HCA produced with the inclusion of up to 3.5% brewery side-stream flours could be a sustainable solution to produce nutritious dairy alternatives.
Keywords: Hybrid cheese analogues, Brewery side-streams, Fermentation, Malt rootlets, Spent yeast, Plant ingredients
Graphical abstract
Highlights
-
•
Brewery side-streams effectively used to create hybrid cheese analogues.
-
•
Side-stream flours exhibited rennet-like activity.
-
•
Increased flour hastened milk coagulation but compromised texture and taste.
-
•
Microbial quality of hybrid cheese analogues was lower than a dairy-based cheese.
-
•
Hybrid cheese analogues showed high concentrations of peptides and free amino acids.
1. Introduction
Recently, a noteworthy shift in dietary patterns has emerged, mainly driven by concerns over health, environmental sustainability, and ethical considerations. Traditional dairy products, long revered for their nutritional benefits, are facing challenges as consumers seek alternatives to address issues such as milk allergies, lactose intolerance, less consumption of animal foods, and environmental impact (Short et al., 2021; Fu and Yano, 2020). In response, there is a growing trend toward the production of plant-based products. A wide range of plant-based milk alternatives has emerged, including soy, almond, coconut, oat, and rice milks, each offering unique nutritional profiles and sensory properties (Mäkinen et al., 2016). Along with milk, the dairy alternatives landscape now encompasses cheese substitutes, as well as yogurt, ice cream, butter, and cream alternatives (Lapčíková et al., 2024; Norazlina et al., 2021; Leahu et al., 2022; Kyriakopoulou et al., 2021). However, these products face different challenges. Plant-based milk often has low protein content, off-flavors, and reduced emulsifying capacity due to the presence of starch. Yogurt alternatives can exhibit undesirable off-flavors originating from the raw materials, and the flavors derived from the fermentation may differ from those of traditional dairy yogurt. Cheese alternatives, on the other hand, frequently encounter issues with melting behavior (Kyriakopoulou et al., 2021).
Within the possible dairy substitutes “precision fermentation” stands out as an innovative food technology that utilizes different microorganisms as “cell factories” to produce high-value functional food ingredients such as animal-like proteins, lipids, carbohydrates, vitamins, flavoring, etc., with high yields and purity. However, this technique raises ethical concerns due to its reliance on genetic manipulation, often for non-native products. Additionally, animal-free cheeses, produced without cows, could impact other aspects such as farmers' livelihoods, food quality (in terms of consistency and naturalness), and environmental factors (land use and climate) (Kühl et al., 2024; Chai et al., 2022). In addition to these trends, there is growing interest in developing innovative foods that integrate sustainable plant-based ingredients into traditional dairy formulations. According to Genet et al. (2023) hybrid cheese is defined as a cheese made from milk and plant-based ingredients, where both components are retained into the product matrix to various concentrations. Other used terminologies are “mixed dairy and plant-based alternatives” or “dairy supplemented with plant-based ingredients”.
The formulation of hybrid cheese analogues (HCA) that effectively replicate the sensory and nutritional attributes of traditional cheeses presents robust challenges. The complex composition and rheology of cheese, primarily governed by caseins, pose obstacles to achieving comparable properties with plant-based ingredients alone (Fox and Mulvihill, 1990). Strategies to address these challenges include optimizing processing methods, harnessing the functional properties of plant proteins, and exploring synergies between dairy and plant-based components (Hinderink et al., 2021; Grossmann and McClements, 2021).
The rise of HCA despite its challenges offers a compelling avenue to reconcile the traditional and plant-based dietary preferences (Picciotti et al., 2022). Moreover, it has the potential to reshape consumer perceptions of plant-based products, fostering a more inclusive approach to dietary choices.
Recycling side-streams from the food industry to create new products represents a potentially greener approach that remains largely unexplored. By repurposing brewing by-products like surplus yeast and malt rootlets, dairy products can be sustainably enriched, minimizing waste while enhancing their nutritional profiles. Brewer's spent yeast (BSY) offers multifunctional properties as food additive, serving as thickener, emulsifier, and water binder, with potential for fat replacement. Additionally, the nutritional value and affordability of BSY make it an ideal fermentation substrate, providing an essential nitrogen source for microbial growth (Jaeger et al., 2020). Similarly, malt rootlets, rich in protein and fiber, offer health benefits and can enhance the nutritional content of various food items, such as breads, in a cost-effective manner (Neylon et al., 2020).
Lactic acid bacteria (LAB) fermentation of side-stream products has attracted attention for its potential to enhance their properties, including nutritional aspects and techno-functional characteristics (Neylon et al., 2023a). Studies employing LAB fermentation of brewers’ spent grain have demonstrated improvements in various nutritional aspects, as well as enhanced techno-functional properties in bread and pasta, prolonged shelf life, and favorable sensory attributes (Neylon et al., 2021a, 2021b; Ktenioudaki et al., 2015). Moreover, fermentation processes involving LAB or fungi like Rhizopus oligosporus have been shown to play a crucial role in optimizing the sensory experience, enhancing flavor profiles, improving texture, and contributing to nutritional enhancements (Pua et al., 2022; Rousta et al., 2023; Olanipekun and Adelakun, 2015).
This study aimed to evaluate the feasibility of incorporating plant-based ingredients from brewery industry side-streams into dairy formulations to create HCA. By enriching milk with various percentages of plant-based flours and assessing the biochemical, textural, and sensory properties of the resulting blends, our findings provide valuable insights into the future development of sustainable and nutritious cheese analogues.
2. Materials and methods
2.1. Side-stream flours
Two different side-streams from brewery industry were selected for fungal growth: spent yeast and malt rootlets. Both were generously provided by AB InBev (Belgium). To enable fungal solid-state fermentation without compromising structural integrity upon hydration, the small-particle side-streams were utilized as co-substrates and mixed with a larger-particle substrate serving as the primary component. The selected primary substrate, soybean flour, was chosen to facilitate fungal fermentation by Rhizopus oligosporus (Putri et al., 2018). The starter was composed of powdered spores of Rhizopus oligosporus produced according to the method of Chutrtong and Bussabun (2014), with some modifications. A petri dish culture of the fungal strain in MYA medium was incubated at 30 °C for 3–5 days until clear sporulation, folowed by lyophilization using an Alpha 1–2 LDplus lyophilizer (230V, CHRIST). The resulting lyophilized cultures were then blended with sterilized rice flour (9:1 wt ratio, rice flour: lyophilized culture). Subsequently, the resulting tempeh-like starter was stored at 25 °C for a maximum duration of 60 days.
The preparation of the substrates for the solid-state fermentation involved several steps apart from the inoculum preparation. First, soybean was subjected to a thorough washing process with water and following it underwent rehydration by immersion in water, with a substrate-to-water volume ratio of 1:2, for 16 h. Once rehydrated, soybean was drained and combined with the side-stream flours, which constituted 20% (w/w DM) of the total substrate quantity. The mixture was then autoclaved at 121 °C for 15 min, ensuring optimal moisture levels for fungal growth while minimizing the presence of competing microbial organisms. After autoclaving, the substrates were cooled down to 30 °C to facilitate inoculation. The procedure for fermentation was previously described by Erkan et al. (2020), with minor adjustments. Inoculation consisted of 1.5 g of tempeh-like starter per 500 g of dry substrate, which was meticulously mixed to achieve uniform dispersion of spores. The substrate was compacted firmly onto stainless steel trays equipped with a polycarbonate lid and underwent incubation on heating mats (24 × 52 cm, 220 V, Lerway) set at 28 °C. Each heating mat was regulated by a thermostat (ITC-308, 220 V, Inkbird) with a temperature measurement probe that was inserted into the centre of the substrate bed. After 48 h of incubation, the mycelium uniformly covered the surface of the substrates, forming a dense cake that bound all the particles together. The fermented substrate was then harvested and subjected to freeze-drying.
2.2. Hybrid cheese analogues
Fermented side-stream flours, produced as described above, were provided by MOGU S.r.l within the frame of the European project Smart Protein (HORIZON, 2020, https://smartproteinproject.eu/). The first consisted of 20% spent yeast and 80% soybean flours (SYF), while the second of 20% malt rootlets and 80% soybean flours (MRF) both fermented with Rhizopus oligosporus. SYF and MRF flours had a protein content of 37.9 ± 3.8% (w/w) and 31.3 ± 3.1% (w/w), respectively, and a particle size of ≤100–200 μm. Flours were also characterized for their water absorption capacity (WAC), swelling capacity (SC), and least gelation concentration (LGC). More specifically SYF and MRF had 254 ± 4 % and 294 ± 18 % WAC, 1.86 ± 0.11 and 2.25 ± 0.12 mL/g SC and 45% and 30% LGC, respectively (Chandra et al., 2015). HCA were prepared using different percentages of SYF, both with rennet (SYR) and without rennet (SY), as well as different percentages of MRF, also with (MRR) and without rennet (MR), according to the process outlined in Fig. S1. Pasteurized and partially skimmed milk was bought from a local supermarket in Bolzano (Italy), while liquid animal rennet from Caseificio Montecristo. Briefly, the pasteurized and partially skimmed milk was heated up to 37oC, and starter cultures were inoculated to initiate the fermentation process. Different amounts (3.5%, 5%, and 7.5%) of the two distinct flours were then added, mixed at 11,000 rpm for 1.5 min using Ultra-Turrax®, and left to coagulate, with or without the addition of rennet (15 mL per 100 L). Cutting, resting (3 min), cooking (5 min), molding, pressing/turning (1 h) followed, and the HCA were let to rest until the pH reached a value close to 5.2–5.6. The whole fermentation process lasted 5.5 h, considering the time post inoculation till brining. Finally, the curds were brined (20% w/v NaCl) and the fresh HCA were placed under refrigeration (4oC). A fresh cheese produced without the addition of flour served as control.
2.3. Combination of starters based on fermentation performance
The fermentation of the HCA was conducted using the commercial starter (CS) Lyofast MOT 082 CE culture (SACCO S.r.l, Italy) in conjunction with heterofermentative strains belonging to the Culture Collection of Micro4Food (Free University of Bolzano, Italy). The CS consisted of selected strains of Lactococcus lactis subsp. lactis, Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus (fermentation at 37oC for 6 h as suggested by the manufacturer), while the heterofermentative strains included Lacticaseibacillus paracasei and Lacticaseibacillus rhamnosus, isolated from kefir and cheese respectively. Sterile falcons containing 50 mL of milk (M) were heated to 37 °C in a water bath and then mixed with 2.5 g (5%) of SYF (blend: SYF + M) or 2.5 g (5%) of MRF (blend: MRF + M) at 11,000 rpm for 1.5 min using an Ultra-Turrax®. Control contained only milk without flour (CM blend). Blends (SYF + M, MRF + M and CM) were inoculated with three combinations of starter cultures: commercial starter alone (SC1), commercial starter with Lacticaseibacillus paracasei (SC2), and commercial starter with Lacticaseibacillus rhamnosus (SC3). CS was inoculated as per producer's instructions (1 UC per 100 L), while the heterofermentative LAB were inoculated to a final cell density of 5.0 log CFU/mL. For the standardization of the two strains, LAB were firstly grown in de Man Rogosa (MRS) broth at 30oC for 16–24 h, until they reached the late exponential (LE) growth phase. After that, cells were harvested by centrifugation (10,000 rpm, 10 min at 4oC), washed twice and resuspended in sterile physiological solution (NaCl 0.9%, w/v). Cultures were brought to a final cell density of 9.0 log CFU/mL by measuring the optical density at 620 nm and then used at the selected concentration (5.0 log CFU/mL) to inoculate the blends (Di Cagno et al., 2017). Inoculated blends were fermented at 37oC for 6 h and sampled every 2 h to measure their pH and cell density. The pH was measured using a pH-meter with a food penetration probe. Enumeration of LAB on selective media MRS involved blending 1 g of the sample with 9 mL of sterile physiological solution for preparation of serial dilutions. LAB colonies were enumerated using the pour plate method, after incubation at 30 °C for 48 h. Growth kinetics were modelled using the Gompertz equation (Zwietering et al., 1990) and analyzed using Statistica 7.0 software.
2.4. Selection of flour percentages
Three different percentages of SYF and MRF flours (3.5%, 5%, and 7.5%) were assessed using a fresh dairy-based cheese as the control. The optimal percentage was selected based on the assessment of pH, moisture, coagulum strength (with and without the addition of rennet), texture (both for the coagula and the final cheese analogues), and sensory attributes. Analyses were performed for all samples the day of manufacture post-brining and seven days later. Exceptions were rheological and texture analyses of coagula.
Moisture content analysis was performed using a thermobalance (Sartorius). Initially, 4 g of fresh samples were weighed and placed in aluminum pans (80 x), then subjected to a temperature of 130 °C. Moisture content was monitored at 24-s intervals until the rate of moisture loss decreased below 2 mg within two consecutive intervals, indicating the completion of analysis.
The coagulation time and the coagulum strength were determined with a Discovery Hybrid Rheometer HR-2 from TA-Instruments Co (Milano, Italy) in an oscillatory mode using a concentric cylindrical geometry as previously described by Yu et al. (2009), with some modifications. Milk samples were heated to 37 °C in a water bath and chemically acidified by adding 10% (v/v) lactic acid. Various percentages of SYF and MRF (3.5%, 5%, and 7.5%) were then added and mixed at 11,000 rpm for 1.5 min using an Ultra-Turrax®. Twenty-five mL of the sample was transferred to the rheometer cylinder with a conical rotor (28 mm diameter) inside a temperature-controlled chamber set to 37 °C. For samples prepared with both flour and rennet, rennet was added directly to the sample inside the cylinder, followed by gentle mixing with a spatula. Sample without addition of flour served as control. Rheological properties (storage modulus (G′) and loss modulus (G”)) were measured at a frequency of 0.1 Hz and 10% strain based on previous oscillation amplitude tests. Measurements were taken at 9-s intervals for 3 h, and results were analyzed using TRIOS software. Coagulation time (CT) was defined as the time when G′ began to increase, while maximum coagulum strength was defined as the time when G′ reached its highest value (G’ max), as previously described by Frederiksen et al. (2011).
Texture analysis was performed on both the coagulum and the final fresh cheeses using two different methods. Coagula were tested with the back extrusion test, using a TA-XT PLUS Texture Analyzer equipped with a compression disk of 40 mm diameter and a 50 kg load cell. Sample preparation followed the same procedure outlined previously for rheological characterization, with the exception that after mixing the flours with the Ultra-Turrax®, 50 mL of the blends were placed in a 100 mL glass beaker and incubated at 37 °C in a water bath. The incubation time of each sample differed based on the predefined G'max value from the rheological analysis. Coagula, prepared with both flour and rennet, had the latter directly added to the sample inside the glass beaker and gently stirred with a spatula before being placed in the water bath. Sample without flour served as control. The texture of the coagula was measured directly inside the glass beakers to preserve their structure. A single compression test was conducted with the probe starting from a distance of 25 mm until it reached 15 mm at a fixed speed of 1.0 mm/s and a trigger force of 10 g. Firmness (g), consistency (g/s), cohesiveness (g), and work of cohesion (g/s) were calculated using Exponent Connect software. Samples were analyzed in triplicates.
Finally, the textural properties (gumminess (g), chewiness (g), adhesiveness (g/mm), stickiness (g) of the final fresh cheeses (HCA and control sample) were determined using a Texture Analyzer (TVT 6700 Perten Instruments) with a 35 mm diameter stainless steel cylindrical probe (probe P-672035). Texture profile analysis (TPA) was carried out by a multiple-compression cycle with parameters fixed at 30 mm distance, test speed at 1 mm/s, and compression at 3 mm. Prior to analysis, samples were cut in circles of 2 cm in diameter and 25 mm in height and analyzed in triplicates using TexCalc 5 software.
Ten assessors, comprising 3 men and 7 women aged between 20 and 35 years, were recruited among students and personnel in the Food Science Department of Free University of Bolzano due to their availability and willingness to participate. The assessment procedure drew inspiration from the Quantitative Descriptive Analysis (QDA®) method described by Stone et al. (2004). In an introductory session, the experimenter explained the study's objectives, the time commitment required, and the schedule of the sessions. Each session lasted approximately 45 min (total number of sessions 21). In the first two sessions, panelists evaluated a range of animal based fresh cheeses and listed words describing the sensory properties they perceived, categorizing the terms by modality: appearance, aroma, texture and taste. A qualitative screening of the terms occurred in group sessions. The terms were discussed, and their definitions were clarified, resulting in a provisional list of attributes. For quantitative screening subjects rated the intensity of attributes from 0 to 10 for 6 products. A statistical screening process produced a definitive list of attributes. The scorecard was finalized. Terms were grouped by modality, each attribute was defined, and an evaluation procedure was agreed upon. Evaluations of the entire product set were conducted in triplicate. Subjects evaluated 5 samples per session, presented sequentially and in randomized order. A 10-cm unstructured line scale, anchored with the terms “dislike extremely” and “like extremely” at each end, was used to rate the intensity of the attributes.
2.5. Characterization of the final hybrid cheese analogues
The characterization of hybrid cheese analogues was performed on a dairy-based cheese (control) and selected blends (3.5% SY, 3.5% SYR, 3.5% MR, and 3.5% MRR) one day post-production.
2.5.1. Gross chemical composition and microbiological characterization
Total nitrogen content of the samples was measured by the NDA 702 Dumas Nitrogen Analyser (VELP Scientifica Srl, Usmate (MB), Italy). Freeze-dried samples were weighed and carefully wrapped in tin foil before the analysis. Based on the nature of the sample, oxygen dosage was fixed at a rate of 400 mL/min to achieve the best combustion. Total nitrogen results were obtained using VELP DUMASoftTM 6.1.0 and converted to total protein content by multiplying with the standard conversion factor 6.38 (Rouch et al., 2008). Results were expressed as percentages of dry matter (DM). For the rest of the analyses, ash was evaluated based on AOAC Official Method 935.42 (1935), fat based on AOAC Official Method 933.05, 1933, and salt concentration based on AOAC Official Method 975.20 (1975). Results were expressed as percentages of fresh sample.
For the microbiological characterization, 10 g of sample was mixed with 90 mL of sterile physiological solution (NaCI 0.9%, w/v) and homogenized using a Stomacher 400 lab blender (Seward Medical) followed by serial dilutions. Total mesophilic bacteria were enumerated in Plate Count agar (PCA, Oxoid Ltd, Basingstoke, United Kingdom) supplemented with 1% (w/v) skimmed milk (Oxoid Ltd, Basingstoke, United Kingdom) at 30 °C for 48 h under aerobic conditions. Mesophilic and thermophilic cocci were counted in M17 agar (Oxoid Ltd, Basingstoke, United Kingdom) supplemented with 10% (w/v) lactose (Oxoid Ltd, Basingstoke, United Kingdom) at 30 °C and 42 °C for 48 h under aerobic and anaerobic conditions, respectively. Mesophilic lactobacilli were enumerated in de Man Rogosa Sharpe agar (MRS, Oxoid Ltd, Basingstoke, United Kingdom) supplemented with cycloheximide (0.1 g/L) (Sigma-Aldrich, USA) at 30 °C for 48 h under anaerobic conditions while yeasts in Sabouraud dextrose agar (SDA, Oxoid Ltd, Basingstoke, United Kingdom) supplemented with chloramphenicol (0.1 g/L) (Sigma-Aldrich, USA) at 25 °C for 48 h under aerobic conditions. Enterococci enumeration was performed in Slanetz-Bartley agar (Oxoid Ltd, Basingstoke, United Kingdom) at 37 °C for 48 h under aerobic conditions whereas enterobacteria were counted in Violet Red Bile Glucose agar (VRBGA, Oxoid Ltd, Basingstoke, United Kingdom) at 37 °C for 24 h under aerobic conditions (Fiorino et al., 2023; Galli et al., 2024; De Pasquale et al., 2014; Tornadijo et al., 2001).
Pathogen screening investigated the presence of Listeria monocytogenes, coagulase-positive Staphylococci, Escherichia coli, and Salmonella spp. in HCA samples and side-stream flours according to the respective per group international organization for standardization (ISO) protocols included in the Regulation (EC) No 2073/2005 from the European Commission (2005).
2.5.2. Biochemical characterization and phytic acid content
Organic acids, sugars, and alcohols of HCA were measured using the water-soluble extracts (WSEs). The preparation of WSEs was carried out as previously described by Tlais et al. (2022) with one modification: 1 g of lyophilized sample was suspended in 8 mL of 50 mM Tris-HCl (pH 8.8). The quantification of lactic acid, acetic acid, lactose, glucose, galactose, and ethanol concentrations was performed with a high-performance liquid chromatography (HPLC) equipped with an Aminex HPX-87H column 300 × 7.8 mm (ion exclusion, Biorad, Richmond, CA) and a UV detector operating at 210 nm. Elution was at 70 °C, with a flow rate of 0.6 mL/min, using 5 mM H2SO4 as the mobile phase.
Phytic acid concentrations (mg/g of dry matter (DM)) were also quantified using Phytic Acid (Phytate)/Total Phosphorus kit (Megazyme, Ireland) according to the manufacturer's instructions.
2.5.3. Peptides, free amino acids (FAAs) and protein digestibility
Peptide and free amino acid chromatographic profiles were evaluated using the pH 4.6-soluble nitrogen fraction. The pH 4.6-soluble nitrogen fractions of cheese analogues were extracted according to Kuchroo and Fox (1982) and lyophilized.
Reversed-phase fast-performance liquid chromatography (RP-FPLC) was used to determine the peptide profiles, which were then visualized using Unicorn software. The elution time of the peaks was used to divide the whole chromatogram area into two equal parts; the first one contained peptides that eluted between 0 and 30 min and the second one between 30 and 60 min. The overall area of each fraction was integrated to determine the total area of the peaks for each specific part of the chromatogram (Galli et al., 2024).
Free amino acids (FAAs) contained in the water-soluble extracts (pH 4.6-soluble nitrogen fractions) of the cheese analogues were analyzed with Biochrom 30 series Amino Acid Analyzer (Biochrom Ltd., Cambridge Science Park, England) equipped with a Li-cation-exchange column. Samples preparation was followed according to Rizzello et al. (2008) with the addition of L-norleucine as an internal standard.
The in vitro protein digestibility (IVPD) was evaluated using the Protein Digestibility Corrected Amino Acid Score (PDCAAS) kit (Megazyme, Ireland) according to the manufacturer's instructions. For this assay, the concentration of L-proline, L-lysine, L-histidine, and L-arginine amino acids was determined with Biochrom 30+ series Amino Acids Analyzer with a Na-cation-exchange column (20 by 0.46 cm internal diameter). Prior to the analysis, acid hydrolysis was conducted following the procedure outlined in AOAC Official Method 994.12 (1997).
2.6. Statistical analysis
Comparisons among groups was done using One-way ANOVA and subsequent post-hoc test by Tukey-Kramer test (P < 0.05). The hierarchical cluster analysis utilized the Euclidean distance and McQuitty linkage methods, visualized with a clustered heat map. All statistics were performed using R version 4.2.2 (R Development Core Team). Visualization of results was with R and Excel. All analyses were carried out considering triplicates.
3. Results
3.1. Combination of starters based on fermentation performance
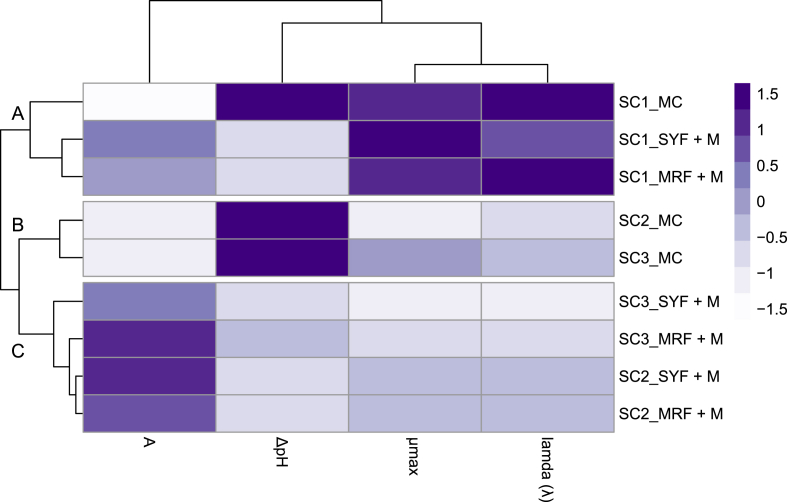
Acidification and growth kinetics of three different combinations of starter cultures (SC1, SC2, and SC3) were assessed on blends (SYF + M, MRF + M, and CM) using both side-stream flours (SYF and MRF). CM and SYF + M samples showed no significant differences in ΔpH values among the different combinations of starters. In contrast, MRF + M blend demonstrated significant differences (P < 0.05) among the starters, with the combination SC3 resulting in a final ΔpH value higher (1.48 ± 0.00) than that of SC1 and SC2 (1.41 ± 0.01 and 1.42 ± 0.01, respectively). Growth data were modelled using the Gompertz equation, and subsequently, the parameters cell density (A), maximum growth rate (μmax), and lamda (λ) were extrapolated and compared for each condition. The maximum cell density ranged from 0.97 to 1.89 units (corresponding to approximately 8 Log CFU/g) with SC3 presenting higher values in MRF + M matrix while SC2 in SYF + M. The μmax was achieved with SC1, in all three different blends. Lower lamda values were achieved with SC3 combination in SYF + M and MRF + M blends. Based on the collective findings detailed above, a heatmap analysis (Fig. 1) was performed, leading to the categorization of samples into three distinct clusters (A-C). Cluster A was characterized only by the SC1 combination across all blends, but was deemed unsuitable for our objectives as it consisted of only the commercial starter. Cluster B comprised CM blends fermented with SC2 and SC3 combinations, while cluster C encompassed HCA samples fermented with the same combinations. Consequently, our attention shifted towards cluster C, which included only HCA samples. Following an assessment based on lambda values, SC3 (lower lambda) was selected as the final combination to ferment the cheese analogues.
Fig. 1.
Clustered heatmap displaying the results of acidification capacity (ΔpH) and growth kinetics (A, λ, and μmax) during fermentation of substrates using three different combinations of starter cultures. The starters used were commercial starter Lyofast MOT 082 CE (SC1), commercial starter together with Lacticaseibacillus paracasei (SC2), and commercial starter together with Lacticaseibacillus rhamnosus (SC3). All combinations were tested in pasteurized partially skimmed milk (MC), pasteurized partially skimmed milk with added 5% spent yeast flour (SYF + M), and pasteurized partially skimmed milk with 5% malt rootlets flour (MRF + M) during 6 h of fermentation at 37oC. Growth kinetics were determined and modelled according to the Gompertz equation using the Statistica 7.0 software. The data are the means of three independent analyses ± standard deviations. Rows were clustered using Euclidean distance and McQuitty linkage, revealing distinct groups (A–C). The color scale shows the differences between the standardized data, with darker colors indicating higher values and lighter colors indicating lower values. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2. Selection of flour percentages
The pH and moisture content values, of the control and the HCA samples made with different percentages of flour (3.5%, 5%, and 7.5%), are presented in Table S1. Results were retained for the first and the seventh day post-production. The pH values ranged between 4.95 ± 0.12 and 5.51 ± 0.09 after one day, while they slightly decreased after seven days, ranging from 4.88 ± 0.09 to 5.31 ± 0.01. Similarly, moisture content was within the range of 52.08 ± 1.13–61.29 ± 0.71% the first day, while it decreased after seven days with values ranging from 40.21 ± 1.55–56.29 ± 0.89%.
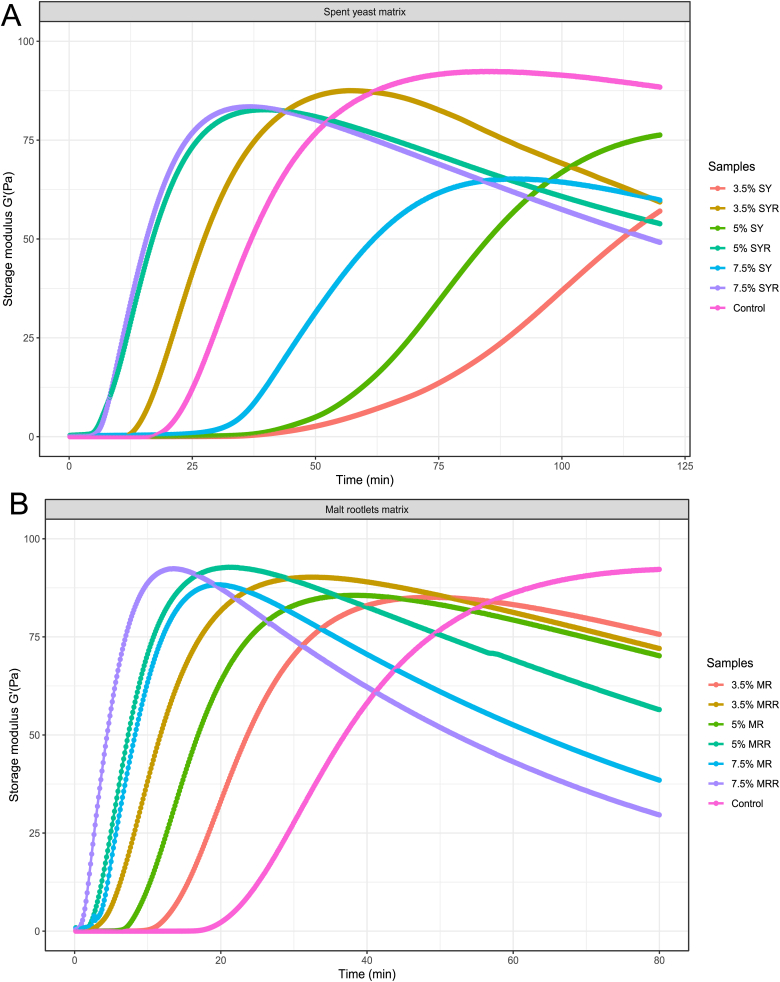
The G'max (maximum coagulum strength) of the different samples are given in Fig. 2. Concerning the blends with spent yeast substrate, the CT values were 70, 40 and 30 min for 3.5%, 5% and 7.5%, respectively, and 12, 9 and 7 min for 3.5%, 5% and 7.5% SYR, respectively. Different results were observed concerning the blends with malt rootlets substrate in which CT values were 10, 7 and 3 min for 3.5%, 5% and 7.5% MR, respectively and 2, 2, and 1 min for 3.5%, 5% and 7.5% MRR, respectively. Control sample began to coagulate after 20 min. In general, although the G'max values did not differ between the blends and the control, variations were observed in the time required to achieve it. The time needed, for blends with SY substrate, to reach G'max values was 170, 130 and 85 min for 3.5%, 5% and 7.5% SY, respectively, while values were decreased to 60, 40 and 45 min for 3.5%, 5% and 7.5% SYR, respectively. Concerning blends with MR substrate, the time needed to reach G'max values once again decreased after the use of rennet from 50, 40 and 20 min for 3.5%, 5% and 7.5% MR, respectively, to 35, 20 and 15 min for 3.5%, 5% and 7.5% MRR. Control sample needed 80 min to reach the G'max value.
Fig. 2.
Rheological properties of cheese blends over 3 h at 37oC. Cheese blends included dairy-based fresh cheese (control) and hybrid cheese analogues made with added 3.5%, 5% and 7.5% SYF flour (20% spent yeast and 80% soybean flours), with rennet (SYR) and without (SY), and 3.5%, 5% and 7.5% MRF flour (20% malt rootlets and 80% soybean flours), with rennet (MRR) and without (MR). Storage modulus G′ of spent yeast (A) and malt rootlets (B) blends was acquired using TRIOS software at 0.1 Hz frequency and 10% strain using a Discovery Hybrid Rheometer HR-2. Data are means of each blend, analyzed in triplicate.
Texture analysis on coagula, in the presence or absence of rennet, was evaluated for firmness (g), consistency (g/s), cohesiveness (g), and work of cohesion (g/s) (Fig. S2). Concerning blends with spent yeast substrate and addition of rennet, 3.5% SYR, 5% SYR and control samples showed the highest values of firmness (172.55 ± 3.65 g, 151.32 ± 1.30 g and 140.04 ± 3.06 g, respectively), consistency (1949.90 ± 54.96 g/s, 1632.80 ± 8.20 g/s, 1757.37 ± 62.60 g/s, respectively), and work of cohesion (−33.35 ± 1.16 g/s, −30.63 ± 1.94 g/s, −31.35 ± 1.35 g/s, respectively). However, 7.5% SYR sample showed higher value in terms of cohesiveness (−36.68 ± 0.55 g) when compared to the control sample (−33.10 ± 2.60 g) while the highest value was obtained by 3.5% SYR (−40.52 ± 1.87 g). Control sample presented the highest firmness and consistency with values up to 133.16 ± 7.56 g and 1757.37 ± 62.60 g/s, respectively when compared to the blends with malt rootlets substrate (MR and MRR). Samples of 5% MR and MRR showed also high values concerning firmness (128.54 ± 7.08 g and 122.78 ± 12.66 g, respectively) while 3.5% MRR and 7.5% MRR concerning consistency (1542.83 ± 58.34 g/s and 1524.71 ± 33.70 g/s, respectively). Both 7.5% MR and MRR samples had the highest values concerning cohesiveness (−47.09 ± 0.51 and −54.40 ± 0.95 g, respectively) and work of cohesion (−47.61 ± 4.00 and −46.84 ± 5.89 g/s, respectively).
Different outcomes were observed in TPA in the final fresh cheeses (HCA and control) when compared after one- and seven-days post-production (Table S2). After one day, control sample showed the highest value in gumminess and chewiness (873.0 ± 169.3 g and 872.3 ± 170.3 g, respectively) while the lowest in adhesiveness (−60.0 ± 28.1 g/mm). Samples of 3.5% SYR, MR and MRR resulted as well in high values of gumminess (137.6 ± 12.6 g, 177.6 ± 18.5 g and 191.6 ± 44.5 g, respectively) and chewiness (137.3 ± 13.1 g, 177.6 ± 18.5 g and 191.6 ± 44.6 g), while 5% SY sample, showed the highest values for both the above-mentioned characteristics (224.3 ± 10.2 g for both gumminess and chewiness).
After seven days of production, the adhesiveness and stickiness of the 5% SY sample exhibited negligible variations (from −169.3 ± 21.2 to −159.3 ± 64.8 g/mm and from −77.3 ± 11.5 to −85.6 ± 7.2 g, respectively), whereas those of 5% MR sample decreased from −408.6 ± 106.4 to −71.3 ± 17.1 g/mm and from −137.6 ± 23.1 to −87.6 ± 6.4 g, respectively. Conversely, the remaining samples displayed an overall increase in all examined textural parameters after seven days of storage.
The sensory attributes including Appearance/Color, Aroma/Odour, Texture/Mouthfeel, Taste/Flavor and Global impression were assessed for all the different blends (3.5%, 5% and 7.5% added side-stream flour) of HCA. Compared to the control, all HCA showed inferior sensory attributes. HCA with and without rennet did not show any differences for the sensory indicators analyzed. Samples of 3.5% and 5% SY garnered higher preference scores across all evaluated parameters, followed by 3.5% and 5% MR samples. Specifically, 3.5% SY sample exhibited scores, for all the above-mentioned sensory attributes, ranging from 4.79 ± 2.28–5.72 ± 2.34. Similarly, the 5% SY sample yielded scores within a comparable range (3.88 ± 3.00 to 5.81 ± 1.64). In both cases, the highest score was given to the Appearance/Color while the lowest to the Taste/Flavor attributes. Conversely, 3.5% and 5% of MR samples demonstrated lower preference, scoring between 2.78 ± 1.37 to 4.54 ± 1.65 and 2.68 ± 1.99 to 4.07 ± 1.94, respectively. Aroma/Odour received the highest score within the MR samples, while Taste/Flavor attributes the lowest. HCA produced with 7.5% added side-stream flours were characterized by a granny mouthfeel with a bitter aftertaste and were rejected by the panelists.
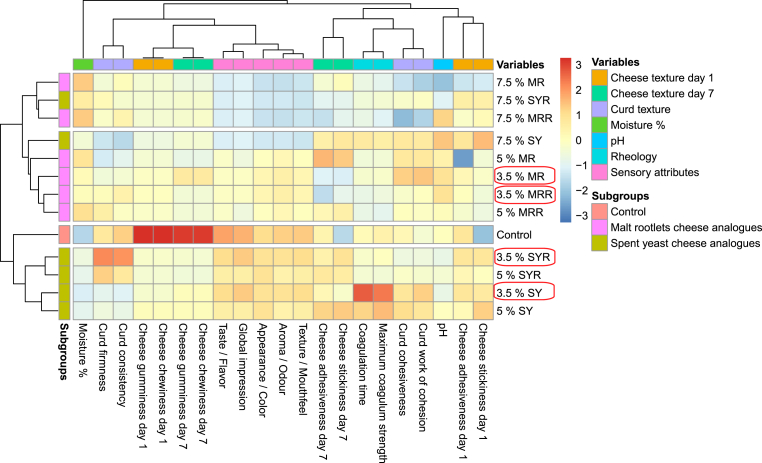
Cumulative evaluation of all above-mentioned results was presented in a heatmap including cluster analysis (Fig. 3). Cluster analysis grouped samples in two main clusters, denoted as A and B. Notably, 3.5% and 5% of SY and SYR samples were grouped in the same cluster with control sample (cluster B) while HCA made with malt rootlets flour (both MR and MRR) and 7.5% SY and SYR samples were grouped separately (cluster A). Control sample, 3.5% SY and SYR samples from cluster B, and 3.5% MR and MRR samples from cluster A, were selected for final characterization. The final evaluation was performed on the cheese curds one day after their production.
Fig. 3.
Clustered heatmap of dairy-based fresh cheese (control), and hybrid cheese analogues made with added 3.5%, 5% and 7.5% SYF flour (20% spent yeast and 80% soybean flours), with rennet (SYR) and without (SY), and 3.5%, 5% and 7.5% MRF flour (20% malt rootlets and 80% soybean flours), with rennet (MRR) and without (MR). The data are the means of three independent analyses ± standard deviations. Rows were clustered using Euclidean distance and McQuitty linkage, revealing distinct groups (A and B). The color scale shows the differences between the standardized data, with warmer colors indicating higher values and cooler colors indicating lower values. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. Characterization of the final hybrid cheese analogues
3.3.1. Gross chemical composition and microbiological characterization
Gross chemical composition and microbial counts of control and HCA samples are presented in Table 1. HCA had no significant differences in the total protein content when compared to the control sample (48.48 ± 0.57% w/w of DM). Exception was 3.5% MR sample which demonstrated a significantly (P < 0.05) lower value (43.61 ± 0.52% w/w of DM).
Table 1.
Gross chemical composition (moisture, fat, protein, NaCl, ash and pH) and cell density (log CFU/g) of total bacterial count, mesophilic cocci, thermophilic cocci, mesophilic lactobacilli, yeasts, enterococci, and enterobacteria determined by a culture-dependent method.
| Samplesa | Gross chemical composition |
Microbial groups log CFU/g |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture %g | Fat %g | Protein %h | NaCl %g | Ash %g | pH | Total bacterial count | Mesophilic cocci | Thermophilic cocci | Mesophilic lactobacilli | Yeasts | Enterococci | Enterobacteria | |
| Controlb | 61.87 ± 0.17bc | 9.92 ± 0.56a | 48.48 ± 0.57a | 1.23 ± 0.03b | 3.18 ± 0.02ab | 5.52 ± 0.01a | 8.46 ± 0.02a | 8.62 ± 0.02a | 8.72 ± 0.02a | 7.10 ± 0.12d | 2.25 ± 0.05c | 3.50 ± 0.06c | 1.54 ± 0.09c |
| SYc | 61.67 ± 0.05c | 9.75 ± 1.03a | 46.63 ± 0.59ab | 1.40 ± 0.01b | 3.12 ± 0.02bc | 5.17 ± 0.01b | 7.74 ± 0.18b | 7.33 ± 0.01c | 7.35 ± 0.18c | 7.45 ± 0.03c | 3.62 ± 0.22a | 7.28 ± 0.03b | 5.41 ± 0.01a |
| SYRd | 62.27 ± 0.20b | 9.03 ± 0.29a | 47.15 ± 0.26a | 1.73 ± 0.02a | 3.28 ± 0.02a | 5.03 ± 0.01c | 7.59 ± 0.03b | 7.36 ± 0.00c | 7.50 ± 0.01c | 7.43 ± 0.26bc | 3.92 ± 0.07a | 7.29 ± 0.02b | 5.37 ± 0.09a |
| MRe | 60.58 ± 0.08d | 10.04 ± 0.23a | 43.61 ± 0.52b | 1.39 ± 0.01b | 2.98 ± 0.01c | 5.11 ± 0.03bc | 7.67 ± 0.09b | 7.81 ± 0.16b | 7.83 ± 0.11b | 7.86 ± 0.15ab | 3.09 ± 0.09b | 7.70 ± 0.01a | 5.02 ± 0.03b |
| MRRf | 63.15 ± 0.07a | 9.00 ± 1.00a | 45.96 ± 1.48ab | 1.73 ± 0.14a | 3.12 ± 0.06bc | 5.05 ± 0.04bc | 7.81 ± 0.26b | 7.89 ± 0.18b | 7.83 ± 0.16b | 8.02 ± 0.21a | 2.81 ± 0.03b | 7.72 ± 0.09a | 4.90 ± 0.16b |
Statistical analysis was performed with One-way ANOVA and the pairwise comparison by Tukey's post-hoc analysis using R version 4.2.2. Data in the same column with different letters are significantly different (P < 0.05). All the control and hybrid cheese analogues (HCA) samples were fermented with a combination (SC3) of a commercial starter Lyofast MOT 082 CE (1 UC/100 L) and Lacticaseibacillus rhamnosus (105 CFU/mL).
Control is referred to a dairy-based fresh cheese.
SY is referred to a hybrid cheese analogue made with added 3.5% SYF flour (20% spent yeast and 80% soybean flours), without rennet.
SYR is referred to a hybrid cheese analogue made with added 3.5% SYF flour (20% spent yeast and 80% soybean flours), with rennet.
MR is referred to a hybrid cheese analogue made with added 3.5% MRF flour (20% malt rootlets and 80% soybean flours), without rennet.
MRR is referred to a hybrid cheese analogue made with added 3.5% MRF flour (20% malt rootlets and 80% soybean flours), with rennet.
Results are expressed in g per 100g of fresh sample.
Results are expressed in g per 100g of dry matter (DM).
Moreover, no significant differences were observed for the gross chemical parameters between samples made with rennet (SYR and MYR) and without (SY and MY). Fat content was between 9.00 ± 1.00% w/w and 10.04 ± 0.23% w/w of fresh sample, without significant differences among the samples, while NaCl content between 1.23 ± 0.03% w/w and 1.73 ± 0.14% w/w of fresh sample, with the highest salt concentration found in the samples contained rennet (3.5% SYR and 3.5% MRR). Finally, ash and pH ranged between 2.98 ± 0.01–3.28 ± 0.02% w/w of fresh sample, and 5.03 ± 0.01–5.52 ± 0.01, respectively. Total bacterial count and mesophilic and thermophilic cocci showed significantly (P < 0.05) higher values in control sample (8.46 ± 0.02, 8.62 ± 0.02 and 8.72 ± 0.02 log CFU/g, respectively) when compared to the HCA samples. Conversely, mesophilic lactobacilli were significantly (P < 0.05) higher in HCA when compared to the control sample; up to 7.45 ± 0.03 and 7.43 ± 0.26 log CFU/g for 3.5% SY and 3.5% SYR, respectively, and 7.86 ± 0.15 and 8.02 ± 0.21 log CFU/g for 3.5% MR and 3.5% MRR. No significant differences were observed in yeast cell density between the 3.5% SY and 3.5% SYR samples (3.62 ± 0.22 and 3.92 ± 0.07 log CFU/g, respectively), and between 3.5% MR and 3.5% MRR samples (3.09 ± 0.09 and 2.81 ± 0.03 log CFU/g, respectively), while control sample had significantly lower value (P < 0.05) (2.25 ± 0.05 log CFU/g). Enterococci and enterobacteria cell densities were significantly higher (P < 0.05) in HCA samples with values ranging from 7.28 ± 0.03–7.72 ± 0.09 log CFU/g and 4.90 ± 0.16–5.41 ± 0.01 log CFU/g, respectively, when compared to the control (3.50 ± 0.06 log CFU/g and 1.54 ± 0.09 log CFU/g, respectively). The pathogen screening of SYF, MRF, and all HCA samples resulted in absence of Salmonella, L. monocytogenes, Staphylococci and E.coli.
3.3.2. Biochemical characterization and phytic acid content
Results of sugars and organic acids are presented in Table S3. Glucose exhibited the highest concentration in both the control (10.51 ± 1.66 g/kg of dry matter [DM]) and 3.5% SY samples (11.23 ± 0.27 g/kg of DM). Lactose, maltose, cellobiose and trehalose could not be distinguished with the method used since they were eluted at the same retention time. The same applied to galactose and fructose. Therefore, these compounds were considered tentative.
Notably, the peak associated with lactose, maltose, cellobiose, and trehalose was significantly elevated (P < 0.05) in the 3.5% SY sample (37.43 ± 1.64 g/kg of DM) compared to the control sample (30.98 ± 6.91 g/kg of DM), while the peak representing galactose and fructose demonstrated similar values across all samples, ranging from 14.20 ± 0.50 to 17.63 ± 0.53 g/kg of DM. Lactic acid levels varied between 15.79 ± 3.11 and 23.78 ± 0.94 g/kg of DM, with the control sample demonstrating the lowest concentration and the 3.5% SY sample showing the highest. Regarding acetic acid concentration, the 3.5% SY sample (2.56 ± 0.31 g/kg of DM) exhibited a significantly higher level (P < 0.05) compared to the rest of the samples.
Phytic acid concentration showed significant differences (P < 0.05) among the different blends, but no effect was observed by the addition of rennet. Control sample had the lowest value (0.15 ± 0.12 mg/g of DM), followed by 3.5% SY and SYR samples (2.90 ± 0.61 and 3.63 ± 0.37 mg/g of DM, respectively) and 3.5% MR and MRR samples (8.09 ± 1.45 and 8.54 ± 2.18 mg/g of DM, respectively).
3.3.3. Peptides, free amino acids (FAAs) and protein digestibility
RP-FPLC chromatographic analysis (214 nm) highlighted significant differences in the peptide fraction among all five different samples (Fig. S3). The total area of peptides significantly increased when rennet was used from 11653.00 ± 2.83 to 14230.50 ± 116.67 mL⋅AU for 3.5% SY and 3.5% SYR, respectively, and from 20020.00 ± 1494.82 to 22918.50 ± 2370.93 mL⋅AU for 3.5% MR and 3.5% MRR, respectively, while control sample had the lowest total area (11154.75 ± 58.75 mL⋅AU). Fractions were classified as hydrophilic peptides with early elution time (HPEE: fractions 0–30 min) and hydrophobic peptides with late elution time (HPLE: fractions 30–60 min), and the results were expressed as the sum of the areas of the detected peaks for each fraction. The results of hydrophilic peptides didn't show significant differences when rennet was added with values up to 2653.50 ± 65.76 and 3139.50 ± 68.59 mL⋅AU, for 3.5% SY and SYR samples, respectively. The same was for 3.5% MR and MRR samples with values up to 8166.00 ± 690.14 and 8603.00 ± 694.38 mL⋅AU, respectively. The value of the control sample (910.25 ± 94.40 mL⋅AU) was significantly lower (P < 0.05) from those reported for the HCA. Conversely, the results of hydrophobic peptides showed significant differences (P < 0.05) within the blends prepared with the same substrate; increased from 8999.50 ± 62.93 to 11141.00 ± 22.63 mL⋅AU for 3.5% SY and SYR samples, respectively, and from 11854.00 ± 804.69 to 14315.50 ± 1676.55 mL⋅AU for 3.5% MR and MRR samples, respectively. Control sample (10244.50 ± 177.48 mL⋅AU) didn't show significant differences when compared to the spent yeast matrices (SY and SYR). However, it exhibited significantly lower values (P < 0.05) compared to malt rootlets (MR and MRR).
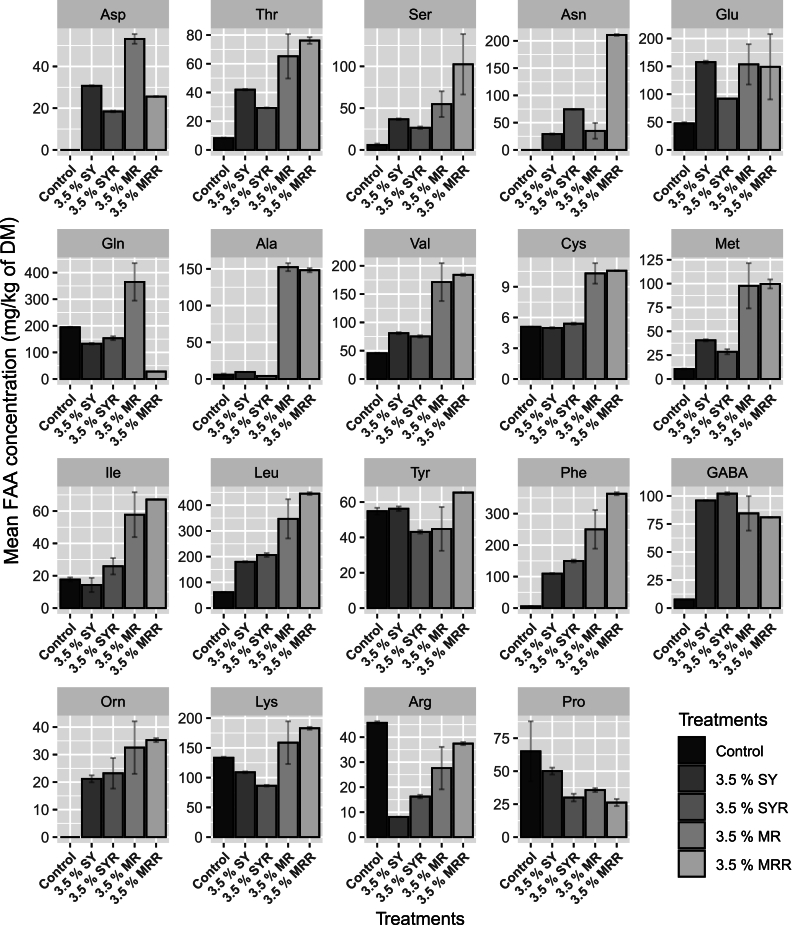
The sum of the individual FAAs (TFAA) was significantly higher (P < 0.05) in blends made with MR substrate, followed by blends made with SY substrate and control. However, no significant differences were observed, between blends made with the same substrate, with or without the presence of rennet. In more detail, TFAA concentrations were 2614.03 ± 463.40 and 2809.74 ± 104.85 mg/kg of DM for 3.5% MR and MRR, respectively, 1470.29 ± 13.77 and 1437.78 ± 33.79 mg/kg of DM for 3.5% SY and SYR respectively, and 847.65 ± 0.02 mg/kg of DM for control sample. The concentration of the individual FAAs for all samples is presented in Fig. 4. Asp, Thr, Ala, Val, Cys, Met, Ile, Leu, and Phe were significantly higher in malt rootlets (in both MR and MRR) when compared to spent yeast and control samples. Ser, Asn, Leu and Phe were significantly higher (P < 0.05) in 3.5% MRR sample (102.49 ± 36.17, 210.83 ± 1.61, 445.02 ± 5.38 and 363.27 ± 4.77 mg/kg of DM, respectively) than 3.5% MR sample (54.78 ± 15.42, 34.97 ± 14.41, 346.97 ± 76.18 and 250.15 ± 61.43 mg/kg of DM, respectively). Conversely, Asp and Gln values were significantly higher (P < 0.05) in 3.5% MR sample (53.18 ± 2.32 and 365.11 ± 70.51 mg/kg of DM, respectively) when compared to 3.5% MRR (25.56 ± 0.16 and 28.02 ± 0.22 mg/kg of DM, respectively). Asp and Asn amino acids showed significant differences (P < 0.05) also in spent yeast samples, when compared with and without the presence of rennet. In more detail, Asp was higher in 3.5% SY sample (30.72 ± 0.24 mg/kg of DM) than 3.5% SYR sample (18.42 ± 0.39 mg/kg of DM), while Asn was higher in 3.5% SYR sample (74.42 ± 0.32 mg/kg of DM) than 3.5% SY sample (29.08 ± 1.12 mg/kg of DM). Other FAAs like Glu, Tyr and GABA were present in high values in all HCA. Control sample displayed the lowest concentrations in most of FAAs except Arg and Pro in which it presented the highest values (45.75 ± 0.59 and 65.03 ± 22.79 mg/kg of DM, respectively) while Asp, Asn and Orn were totally absent.
Fig. 4.
Free amino acids concentration (mg/kg of DM) of dairy-based fresh cheese (control), and hybrid cheese analogues made with added 3.5% SYF flour (20% spent yeast and 80% soybean flours), with rennet (SYR) and without (SY), and 3.5% MRF flour (20% malt rootlets and 80% soybean flours), with rennet (MRR) and without (MR). All the control and hybrid cheese analogues (HCA) samples were fermented with a combination (SC3) of a commercial starter Lyofast MOT 082 CE (1 UC/100 L) and Lacticaseibacillus rhamnosus (105 CFU/mL). Statistical analysis was performed with One-way ANOVA and the pairwise comparison by Tukey's post-hoc analysis using R version 4.2.2. Lowercase letters indicate the significant differences (P < 0.05) among different samples for each amino acid.
The IVPD values showed no significant differences among the samples with values ranged from 82.73 ± 0.63% to 83.92 ± 0.08%.
4. Discussion
The utilization of industry by-products aims to reduce waste and add value to underutilized resources. Brewing and malting side-streams (spent yeast and malt rootlets), have found diverse applications in the food industry, including baked goods, meat products, and dairy formulations, as sources of bioactive compounds and functional ingredients (Neylon et al., 2020, 2023b; Mejri et al., 2014). Studies have reported that incorporating these side-streams resulted in higher levels of antifungal compounds, fibers, and proteins. However, in bread formulations, these components can weaken the gluten network due to the variety of charged amino acids and fibers, which impact the essential bonds needed for gluten structure and affect the final product's technological characteristics (Neylon et al., 2023b). In sausage formulations, the addition of malt rootlets decreased cooking losses, thereby reducing production costs (Neylon et al., 2020). While beta-glucans isolated from brewer's spent yeast and used as a fat replacer in non-fat yogurt formulations resulted in physicochemical and rheological properties similar to those of regular yogurt (Mejri et al., 2014). Hybrid cheese represents the amalgamation of milk and plant-based ingredients in the final product, at varying concentrations (Genet et al., 2023). As industry stakeholders pursue sustainability goals, the incorporation of food side-streams in hybrid systems, like hybrid cheeses seems a promising avenue. This study aims to produce HCA by utilizing brewery side-streams, specifically spent yeast and malt rootlets, combined with dairy milk. Through the fermentation of these raw materials and their designed analogues, we seek to expand the application of such substrates in food production. The primary goal is to demonstrate the feasibility of incorporating these substrates at various ratios to develop hybrid cheese prototypes.
Side-stream flours were prepared by combining soybeans (80%) with either spent yeast or malt rootlets (20%) as co-substrates, followed by fermentation using a tempeh-like starter culture involving Rhizopus oligosporus. The decision to mix these two substrates was motivated by the concern that the small particle size of the side-streams could pose challenges for solid-state fermentation processes. Small particle size generally provides a larger surface area for microbial activity. However, excessively smaller particles can lead to substrate agglomeration, potentially impeding microbial respiration and aeration (Krishna, 2005). Hence, by incorporating co-substrates such as spent yeast or malt rootlets with substrates such as soybean flour, can mitigate potential issues related to particle size and ensure optimal conditions for microbial growth and activity during fermentation. Evidently the selection of these substrates was based on sustainability but also for their functional properties since they could absorb twice their weight in water and showed positive gelling properties. Despite their good functional properties when compared to other plant-based flours (Chandra et al., 2015), their integration presents distinct challenges, particularly concerning the maintenance of food prototype quality, especially at higher inclusion levels (Badia-Olmos et al., 2023; Neylon et al., 2023a).
To enhance nutrient availability and ensure quality, the hybrid cheeses underwent fermentation with LAB. Typically, cheese-making starters consist of starter LAB, tasked with rapidly acidifying the substrate, and non-starter LAB, which contributes to the proteolytic process, enriching the substrate with peptides and amino acids (Galli et al., 2023; Gobbetti et al., 2015). In line, a commercial cheese starter (SACCO, Lyofast MOT 082 CE) was tested for performance on each blend (MC, SY + M, and MR + M) as a main starter, coupled with a heterofermentative LAB (Lacticaseibacillus paracasei and Lacticaseibacillus rhamnosus), previously isolated from dairy products. Fermentation protocol (37 °C for 6 h) referred to the standard conditions for testing the suitability of a strain for cheese-making process (Law and Tamime, 2011). The short lag phase and the high acidification capacity of SC3 (commercial starter Lyofast MOT 082 CE (1 UC/100 L) and Lacticaseibacillus rhamnosus (105 CFU/mL)) indulged its selection for expediting the cheese production process (Vinicius De Melo Pereira et al., 2020).
To determine the amount of flour that could be incorporated in the blend without compromising key biochemical and textural properties of HCA, cheeses were assessed on the first- and seventh-day post-production. The pH and moisture content resembled those of traditional fresh semi-hard cheeses (Nájera et al., 2021), with minimal deviations observed between control cheese and HCA, consistent with previous research that used diverse food industry side-streams to produce fresh cheeses (Costa et al., 2018). Over time, both pH and moisture content decreased. Notably, moisture content increased with higher flour addition to the blends, yet after seven days, the control sample exhibited a more significant decrease compared to HCA. This variation may stem from factors such as the water-holding capacity of the flour (Shams El Din et al., 2022), potential structural changes due to a forming barrier impeding moisture loss from the cheese matrix (Ali et al., 2022), and chemical interactions affecting emulsion stability (Bobade et al., 2021). Additionally, particle size of the added material can influence moisture content, with smaller particles exhibiting higher absorption potential. During HCA production, a high-shear homogenizer (Ultra Turrax®) was utilized, potentially breaking down larger particles into smaller ones, contributing to a product with higher moisture content (Bobade et al., 2021).
The rheological properties of the samples were evaluated with and without the addition of rennet on the diverse blends. The coagulation time (CT) values of the control sample remained consistent at 20 min at 37 °C, aligned with the findings reported by Cipolat-Gotet et al. (2012). Notably, with the addition of higher percentages of flour in blends, there was a corresponding decrease in CT, which was more pronounced when also rennet was added, suggesting a synergistic effect. The side-stream flours were previously fermented with Rhizopus oligosporus, a fungus known for its ability to produce enzymes that exhibit rennet-like activity (Thakur et al., 1990; Sternberg, 1976). In fact, fungal fermentation of brewing industry side-streams has previously shown not only to increase the nutrient availability, but also to release multiple enzymes, such as xylanases, β-glucanases and proteases (Marcus and Fox, 2021; Shahryari and Niknezhad, 2022; Banerjee et al., 2024). Previously, Chen et al. (2010) identified optimal conditions (40 °C and pH 6) for milk-clotting time, around 7.6 ± 1.1 min, using the enzyme from soybean produced by Rhizopus oligosporus, which align closely with the conditions and results of coagulating time of the HCA analogues. Quicker CT was observed in blends utilizing co-substrate malt rootlets compared to spent yeast. Malt rootlets flour is anticipated to possess a higher enzyme content compared to spent yeast flour due to the inherent physiological processes of germination in barley grains. During germination, the barley grain activates various enzymes, including amylases and proteases, to facilitate the degradation of starches and proteins into simpler forms. Conversely, spent yeast flour primarily consists of residual yeast cells resulting from fermentation processes. While yeast cells do contain enzymes involved in fermentation, their abundance and diversity may be comparatively lower than those found in malt rootlets (Marson et al., 2020; Olivares-Galván et al., 2022).
The maximum coagulum strength (G'max value) was similar for the control and the HCA samples, a positive indicator of the rigidity of the coagulum and thus the quality of the product (Landfeld et al., 2002). The time when the G'max values were obtained was used for the textural evaluation of the coagulum. Coagulum analysis revealed that the addition of rennet improved the textural properties of the samples when compared to those without rennet. In a previous study López et al. (2012) compared the coagulant properties in fresh cheeses made of calf, microbial, and vegetable rennet. The study showed no significant differences (P < 0.05) in terms of hardness, gumminess, chewiness, cohesiveness, and springiness when samples were treated with animal or microbial rennet. However, in our case, samples treated with rennet included indigenous enzymes derived from the plant-based flours, which resulted most probably in a synergetic effect and thus, in a better textural profile. Exception was when 7.5% of flour was added to the blend, which demonstrates once more the difficulty of including high amounts of such ingredients in hybrid systems (Badia-Olmos et al., 2023; Neylon et al., 2023a). Nevertheless, TPA analysis on the final HCA showed inferior properties compared to the control cheese, similar to other studies attempting to reinforce the addition of plant matrices into dairy matrices. (Fu and Yano, 2020). After the coagulum is formed, the gel is naturally prone to spontaneous syneresis (whey expulsion). Therefore, cutting and stirring are employed that assist forming a curd-whey mixture that after pressing results to a final curd with specific firmness (Walstra et al., 2005). Despite the positive textural properties found in the coagulum of HCA, the textural properties of final cheeses could be inferior due to weak interactions of animal and plant protein during the whey expulsion step. According to Chavan and Jana (2007), in a mixed system of both dairy and plant proteins, when more than 20% (w/w) of the total protein consists of vegetable protein, the resulting cheese analogue is often worse than a dairy cheese. Considering that milk consisted of 3.6% (w/w) protein and spent yeast and malt rootlets flours consisted of 37.9 ± 3.8% (w/w) and 31.3 ± 3.1% (w/w), respectively, cheese analogues made with 5% added flour or more tend to have inferior properties compared to fresh cheese. A similar trend was observed also during sensory evaluation in which HCA up to 5% were partially acceptable by the panelist while the 7.5% HCA were rejected due to the grainy texture and bitter taste (Kerby and Vriesekoop, 2017; Waters et al., 2013).
Evaluation of the results in a cluster heatmap led to the selection of the final percentage of flour (3.5%) for the analogues. The final HCA cheeses, made with up to 3.5% of side-stream flours, were tested one post-production for microbiological, biochemical, and antinutritional properties, with and without the addition of rennet. Presumptive mesophilic and thermophilic cocci as well mesophilic lactobacilli were the most dominant populations like in other fresh cheeses (Busetta et al., 2022). Enterobacteria and enterococci were found in higher numbers in HCA compared to the control. Enterococci in cheese curd may range from 104 to 106 CFU/g CFU/g, and their positive influence on cheese appears to be attributed to specific biochemical traits such as lipolytic activity, citrate utilization, and the production of aromatic volatile compounds (Giraffa, 2003). According to the criteria in EC Regulation 2005/2073/EC (European Commission, 2005) previously reported by Giammanco et al. (2011), the hygienic quality of cheese is considered good when the number of Enterobacteriaceae is ≤ 105 CFU/g and poor when the number is between 105 ≤ x 107 CFU/g. Our HCA fall in the second category. This can be attributed to post–thermal treatment contaminations, originally derived from manufacturing environments (Giammanco et al., 2011).
Possibly, a ripening process could influence and deplete the presence of this group since the use of LAB has been proven to be a possible solution to this problem, not only in milk (Gaya et al., 1983) but also in both brewery side-streams (Jaeger et al., 2024). Further, the European Regulation (EC) No. 2073/2005 establishes a tolerance of a maximum of 100 CFU/g of L. monocytogenes in ready to eat foods, including cheese, absence of Salmonella spp. in 25 g of product, <1000 CFU/g for E. coli and coagulase-positive staphylococci <100 CFU/g. In our flours and final HCA cheeses these pathogens were not detected. Fresh cheese analogues showed noticeable differences in the concentrations of sugars and organic acids, particularly when compared to the control. However, our analytical method presented certain limitations. For instance, it was not possible to differentiate between lactose, maltose, cellobiose, and trehalose as they eluted at the same retention time, nor between galactose and fructose for the same reason. While lactose is the predominant sugar in milk (Bezerra et al., 2017), the higher cumulative area corresponding to that peak in the HCA analogues suggests the presence of additional sugars. Malt rootlets and spent yeast are potential sources of maltose (Langenaeken et al., 2020) and in case of spent yeast trehalose (Rachwał et al., 2020), while soybean contains various sugars such as sucrose, fructose, and glucose (Eldridge et al., 1979; Bainy et al., 2008). Moreover, fermentation of the substrates after blending and recovering the flour leads to the release of different enzymes that could increase the availability of sugars (Tsakona et al., 2014). Therefore, the higher area of that peak in HCA compared to the control could be attributed to the additional sugars derived from the side-stream flour sources. Additionally, the lower concentration of glucose and higher concentration of lactic acid in HCA suggest better utilization of the carbon sources by the LAB compared to the control. The presence of heterofermentative strains from the starter also resulted in the production of acetic acid, albeit in low concentrations. Typically, homofermentative lactic acid bacteria metabolize lactose into glucose and galactose, ultimately yielding lactic acid. However, Lacticaseibacillus rhamnosus, can perform both hexose and pentose fermentations using the homo-lactic and hetero-lactic pathways (Bintsis, 2018).
Despite the antioxidant properties of phytic acid in humans, is also known that it has antinutritional effects with respect to mineral bioavailability (Neylon et al., 2020). The side-stream co-substrates (spent yeast and malt rootlets) used to prepare the HCA may be a source of phytic acid, with exception the main substrate (soybeans). Soybeans contain a relevant amount that is affected by processing (Rasha Mohamed et al., 2011). In our work, side-stream flours were first fermented and then used to prepare HCA. Fermentation process can reduce phytic acid since some LAB and yeast possess phytase activity (Lynch et al., 2016; Feizollahi et al., 2021). In fact, the phytic acid content in all HCA was low and comparable to other studies (Rasha Mohamed et al., 2011) in which bacterial fermentation reduced its content significantly.
Soybeans and beer industry side-streams are rich sources of nutrients, protein, bioactive peptides, amino acids and are excellent substrates for fermentation (Ogrodowczyk and Drabińska, 2021; Rachwał et al., 2020; Kim et al., 2021). In fact, the HCA had higher total area of peptides when compared to the dairy-based cheese (control), with a significant increase in the hydrophobic fraction. The prior fungal fermentation of the side-streams flours by Rhizopus oligosporus may have enhanced the presence of enzymes such as proteases (Awasthi et al., 2022), which in turn affected positively the process of proteolysis increasing the peptide and free amino acid concentrations (Rousta et al., 2023; Verni et al., 2019). However, Sousa et al. (2001) reported that hydrophobic peptides can be responsible for the bitter taste in cheeses, reenforcing the results of the sensory analysis. The primary source of bitter peptides in cheese is the action of coagulant and starter proteinases and thus, excessive concentrations of these peptides can arise from either overproduction or insufficient breakdown by microbial enzymes (Sousa et al., 2001).
Limited studies have been conducted on the FAAs profiles of fresh cheeses. However, similar FAAs profiles like ours was previously observed (Peralta et al., 2016), but with different proportions for some free amino acid, such as Pro, Lys, Tyr, and Val. The sum of the individual FAAs was significantly higher (P < 0.05) in HCA when compared to the control sample. Amino acid formation is mainly brought about by the action of proteolytic enzymes from the starter bacteria and the rennet which contribute to the breakdown of casein (Santos et al., 2003). Differences between control and HCA samples may be attributed to the nature of the matrices used, as HCA contains various proteins, peptides, and enzymes derived the side-stream flours. In fact, FAAs profile of HCA made with side-stream flour containing spent yeast, corresponds to those found in the literature (Podpora et al., 2016; Ibarruri et al., 2019) with the additional detection of Asn and GABA. Previously, fermentation of soybean by Rhizopus oligosporus led to a high yield of GABA (Rousta et al., 2023; Jaeger et al., 2024) while the production of asparagine (Asn) was also observed during the same fungal fermentation (Sulagna, 2020). HCA made with side-stream flour containing malt rootlets exhibited higher concentrations of FAAs, consistent with other literature findings (Bretträger et al., 2023). The above-mentioned studies justify also the presence of Asp, Asn, and Orn amino acids in HCA instead of the control sample. No trend was observed in the amino acid concentrations between the HCA made with and without rennet. In some cases (Ser, Asn, Leu, Tyr, Phe) amino acids increased with rennet addition, while in other cases (Asp, Gln) amino acids increased when rennet was not present. Calf rennet, commonly used in the dairy industry, has a high proteolytic activity in pH 5.2–6.6 and 35 °C (Liu et al., 2021). Only 0–15% of the rennet activity added to the milk is retained in the curd after manufacturing while the majority is lost in the whey. The coagulant activity depends on various factors such as the type of coagulant, the ratio of different enzymes in blends, the cooking temperature, the type of cheese, and the final cheese's moisture content (Sousa et al., 2001). Rhizopus oligosporus has been reported as an alternative solution to calf rennet due to its capability to produce acid, neutral, and alkaline proteases that report coagulation properties (Egbune et al., 2023). However, it has been found that natural inhibitors, like extracts from potatoes, barley, and soybeans, inactivate the actions of microbial proteases that are alkaline and neutral, but not acidic (Wang and Hesseltine, 1965). The acid proteases produced by Rhizopus oligosporus were found to have the maximum activity at 30–33oC and pH 5.0 (Lusiana et al., 2023; Usman et al., 2021). Thus, we can assume that different environments and incubation conditions lead to different behaviour of the enzymes, and as a result, to different proteolytic products. The formation of the distinctive flavor in cheeses is considered to be influenced by the amino acid fraction's composition and the relative amounts of each amino acid (Sousa et al., 2001). Glutamine that was significantly higher in HCA, enhance sweetness, while phenylalanine is a floral aroma precursor (Teter et al., 2020). Other FAAs found significantly higher in HCA compared to the control, such as alanine, lycine, serine and threonine are associated to sweet flavor, and glutamic acid and aspartic acid with sour (McSweeney, 1997).
5. Conclusions
The incorporation of fermented flours utilizing brewery's side-stream sources (spent yeast and malt rootlets) to produce semi-hard fresh hybrid cheeses could be feasible with up to 3.5% addition. Hybrid cheeses had acceptable sensorial and textural properties, while they showed self-coagulating properties. However, the addition of rennet enhanced the release of peptides but surprisingly didn't reflect to a proportionate release in FAAs. The fermentation process had a positive effect in minimizing the phytic acid content. Final HCA had broader area of produced peptides and concentration of specific FAAs compared to the dairy based analogue, which could be a result of proteolysis or carry-over from the side-stream flours that were previously fermented. Despite the positive outcomes, to facilitate incorporation of plant-based proteins with animal proteins requires changes in the cheese making protocols. Following the normal cheese making process, the coagulum showed comparable textural properties with the dairy based but after storage these properties were influenced negatively. Furthermore, the elevated number of Enterobacteriaceae in the HCA demonstrates that the microbiological quality of raw materials is poor, suggesting the importance of minimizing contaminations along their chain of production.
Data availability
Majority of the data are displayed in the main manuscript but also in the supplementary material. Further data will be made available on request.
Funding
The work for this publication has been undertaken as part of the SMART PROTEIN project https://smartproteinproject.eu/objectives/. This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No. 862957.
CRediT authorship contribution statement
Anastasia Palatzidi: Methodology, Formal analysis, Investigation, Writing – original draft. Olga Nikoloudaki: Methodology, Writing – original draft, Writing – review & editing, Supervision. Maria Garcia Torreiro: Resources. Carolina Matteucci: Resources. Giovanna Ferrentino: Investigation. Matteo Mario Scampicchio: Writing – review & editing, Resources. Raffaella Di Cagno: Conceptualization, Supervision. Marco Gobbetti: Funding acquisition, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors want to thank Prof. Emanuele Zannini for his valuable expert advice, insight and technical assistance. This work was partially supported by the Open Access funding provided by the Free University of Bozen-Bolzano, Italy.
Handling Editor: Dr. Xing Chen
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2024.100829.
Contributor Information
Anastasia Palatzidi, Email: apalatzidi@unibz.it.
Olga Nikoloudaki, Email: olga.nikoloudaki@unibz.it.
Maria Garcia Torreiro, Email: mgt@mogu.bio.
Carolina Matteucci, Email: caimatte94@gmail.com.
Giovanna Ferrentino, Email: Giovanna.Ferrentino@unibz.it.
Matteo Mario Scampicchio, Email: matteo.scampicchio@unibz.it.
Raffaella Di Cagno, Email: raffaella.dicagno@unibz.it.
Marco Gobbetti, Email: marco.gobbetti@unibz.it.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- AOAC Official Method 975.20 Salt in cheese - procedure. 1975. [DOI]
- AOAC Official Method 933.05 Fat in Cheese. 1933. [DOI]
- AOAC Official Method 935.42 Ash of cheese: Gravimetric method. 1935. [DOI]
- AOAC Official Method 994.12 Amino Acids in Feeds: Performic Acid Oxidation with Acid Hydrolysis-Sodium Metabisulfite Method. 1997. [DOI]
- Ali M.M.A., Sant'Ana A.S., Bavisetty S.C.B. Trends in Food Sci. And Technology. vol. 129. Elsevier Ltd; 2022. Sustainable preservation of cheese: advanced technologies, physicochemical properties and sensory attributes; pp. 306–326. [DOI] [Google Scholar]
- Awasthi M.K., Harirchi S., Sar T., Vigneswaran V.S., Rajendran K., Gómez-García R., Hellwig C., Binod P., Sindhu R., Madhavan A., Kumar A.N.A., Kumar V., Kumar D., Zhang Z., Taherzadeh M.J. vol. 360. Elsevier Ltd; 2022. Myco-biorefinery approaches for food waste valorization: present status and future prospects. (Bioresource Technology). [DOI] [PubMed] [Google Scholar]
- Badia-Olmos C., Laguna L., Haros C.M., Tárrega A. Techno-functional and rheological properties of alternative plant-based flours. Foods. 2023;12(7):1411. doi: 10.3390/foods12071411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainy E.M., Tosh S.M., Corredig M., Poysa V., Woodrow L. Varietal differences of carbohydrates in defatted soybean flour and soy protein isolate by-products. Carbohydr. Polym. 2008;72(4):664–672. doi: 10.1016/j.carbpol.2007.10.008. [DOI] [Google Scholar]
- Banerjee S., Banerjee S., Banerjee S., Das A., Bose S. Entrepreneurship with Microorganisms. Elsevier; 2024. Fungi in nutraceutical and baking purposes; pp. 143–161. [DOI] [Google Scholar]
- Bezerra T.K.A., de Oliveira Arcanjo N.M., Garcia E.F., Gomes A.M.P., do Egypto R.D.C.R., de Souza E.L., Madruga M.S. Effect of supplementation with probiotic lactic acid bacteria, separately or combined, on acid and sugar production in goat ‘coalho’ cheese. LWT. 2017;75:710–718. doi: 10.1016/j.lwt.2016.10.023. [DOI] [Google Scholar]
- Bintsis T. Lactic acid bacteria as starter cultures: an update in their metabolism and genetics. AIMS Microbiology. 2018;4(4):665–684. doi: 10.3934/microbiol.2018.4.665. AIMS Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobade H., Sharma S., Singh B. Food Formulation: Novel Ingredients and Processing Techniques. wiley; 2021. Effect of ingredient interactions on techno-functional properties; pp. 71–99. [DOI] [Google Scholar]
- Bretträger M., Sacher B., Gastl M., Becker T. J. Of the American Society of Brewing Chemists. Taylor and Francis Ltd; 2023. The black gap: understanding the potential roles of black fungal-derived enzymes in malting and brewing quality: a review. [DOI] [Google Scholar]
- Busetta G., Ponte M., Barbera M., Alfonzo A., Ioppolo A., Maniaci G., Guarcello R., Francesca N., Palazzolo E., Bonanno A., Moschetti G., Settanni L., Gaglio R. Influence of citrus essential oils on the microbiological, physicochemical and antioxidant properties of primosale cheese. Antioxidants. 2022;11(10) doi: 10.3390/antiox11102004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai K.F., Ng K.R., Samarasiri M., Chen W.N. Precision fermentation to advance fungal food fermentations. Curr. Opin. Food Sci. 2022 doi: 10.1016/j.cofs.2022.100881. [DOI] [Google Scholar]
- Chandra S., Singh S., Kumari D. Evaluation of functional properties of composite flours and sensorial attributes of composite flour biscuits. J. Food Sci. Technol. 2015;52:3681–3688. doi: 10.1007/s13197-014-1427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavan R.S., Jana A. Cheese substitutes: an alternative to natural cheese-A review. Int. J. of Food Sci., Technology & Nutrition. 2007;2(2):25–39. [Google Scholar]
- Chen M.T., Lu Y.Y., Weng T.M. Comparison of milk-clotting activity of proteinase produced by Bacillus subtilis var, natto and Rhizopus oligosporus with commercial rennet. Asian-Australasian J of Anim Sci. 2010;23(10):1369–1379. doi: 10.5713/ajas.2010.80694. [DOI] [Google Scholar]
- Chutrtong J., Bussabun T. Preparation of tempeh spore powder by freeze-drying. Int. J. of Bioengineering and Life Sci. 2014;8(1):40–43. 10.5281/zenodo.1326822. [Google Scholar]
- Cipolat-Gotet C., Cecchinato A., De Marchi M., Penasa M., Bittante G. Comparison between mechanical and near-infrared methods for assessing coagulation properties of bovine milk. J. Dairy Sci. 2012;95(11):6806–6819. doi: 10.3168/jds.2012-5551. [DOI] [PubMed] [Google Scholar]
- Costa C., Lucera A., Marinelli V., Del Nobile M.A., Conte A. Influence of different by-products addition on sensory and physicochemical aspects of Primosale cheese. J. Food Sci. Technol. 2018;55(10):4174–4183. doi: 10.1007/s13197-018-3347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pasquale I., Di Cagno R., Buchin S., De Angelis M., Gobbetti M. Microbial ecology dynamics reveal a succession in the core microbiota involved in the ripening of pasta filata caciocavallo pugliese cheese. Appl. Environ. Microbiol. 2014;80(19):6243–6255. doi: 10.1128/AEM.02097-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cagno R., Filannino P., Cavoski I., Lanera A., Mamdouh B.M., Gobbetti M. Bioprocessing technology to exploit organic palm date (Phoenix dactylifera L. cultivar Siwi) fruit as a functional dietary supplement. J. Funct.Foods. 2017;31:9–19. doi: 10.1016/j.jff.2017.01.033. [DOI] [Google Scholar]
- Domingues Galli B., Nikoloudaki O., Tonini S., Helal A., Di Cagno R., Gobbetti M., Tagliazucchi D. How starter cultures affect the peptidomic profile and bioactive activities of the Asiago-PDO cheese throughout ripening. Food Res. Int. 2023;167 doi: 10.1016/j.foodres.2023.112743. [DOI] [PubMed] [Google Scholar]
- Egbune E.O., Avwioroko O.J., Orororo O.C., Egbune O.U., Aganbi E., Anigboro A.A., Tonukari N.J. Rhizopus oligosporus alkaline protease in cassava fermentation: characterization and detergent potential. Biocatal. Agric. Biotechnol. 2023;54 doi: 10.1016/j.bcab.2023.102954. [DOI] [Google Scholar]
- Eldridge A.C., Black L.T., Wolf W.J. Carbohydrate composition of soybean flours carbohydrate composition of soybean flours, protein concentrates, and isolates. Food Chem. 1979;27(4) https://pubs.acs.org/sharingguidelines [Google Scholar]
- Erkan S.B., Gürler H.N., Bilgin D.G., Germec M., Turhan I. Production and characterization of tempehs from different sources of legume by Rhizopus oligosporus. LWT. 2020;119 doi: 10.1016/j.lwt.2019.108880. [DOI] [Google Scholar]
- European Commission . L338. Official J. of the European Union; 2005. pp. 1–26.https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32005R2073 (Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs). (accessed March 2024) [Google Scholar]
- Feizollahi E., Mirmahdi R.S., Zoghi A., Zijlstra R.T., Roopesh M.S., Vasanthan T. Review of the beneficial and anti-nutritional qualities of phytic acid, and procedures for removing it from food products. Food Res. Int. 2021;143 doi: 10.1016/j.foodres.2021.110284. [DOI] [PubMed] [Google Scholar]
- Fiorino G.M., Tlais A.Z.A., Losito I., Filannino P., Gobbetti M., Di Cagno R. Triacylglycerols hydrolysis and hydroxy- and epoxy-fatty acids release during lactic fermentation of plant matrices: an extensive study showing inter- and intra-species capabilities of lactic acid bacteria. Food Chem. 2023;412 doi: 10.1016/j.foodchem.2023.135552. [DOI] [PubMed] [Google Scholar]
- Fox P.F., Mulvihill D.M. Casein. Food gels: Chapter. 1990;4:121–173. doi: 10.1007/978-94-009-0755-3_4. [DOI] [Google Scholar]
- Frederiksen P.D., Hammershøj M., Bakman M., Andersen P.N., Andersen J.B., Qvist K.B., Larsen L.B. Variations in coagulation properties of cheese milk from three Danish dairy breeds as determined by a new free oscillation rheometry-based method. Dairy Sci. Technol. 2011;91(3):309–321. doi: 10.1007/s13594-011-0018-5. [DOI] [Google Scholar]
- Fu W., Yano H. Development of “new” bread and cheese. Processes. 2020;8(12):1–23. doi: 10.3390/pr8121541. MDPI AG. [DOI] [Google Scholar]
- Galli B.D., Nikoloudaki O., Granehäll L., Carafa I., Pozza M., De Marchi M., Gobbetti M., Di Cagno R. Comparative analysis of microbial succession and proteolysis focusing on amino acid pathways in Asiago-PDO cheese from two dairies. Int. J. Food Microbiol. 2024;411 doi: 10.1016/j.ijfoodmicro.2023.110548. [DOI] [PubMed] [Google Scholar]
- Gaya P., Medina M., Nunez M. Accelerated decrease of Enterobacteriaceae counts during ripening of raw milk manchego cheese by lactic culture inoculation. J Food Prot. 1983;46(4):305–308. doi: 10.4315/0362-028X-46.4.305. [DOI] [PubMed] [Google Scholar]
- Genet B.M.L., Sedó Molina G.E., Wätjen A.P., Barone G., Albersten K., Ahrné L.M., Hansen E.B., Bang-Berthelsen C.H. Hybrid cheeses—supplementation of cheese with plant-based ingredients for a tasty, nutritious and sustainable food transition. Ferment. 2023;9(7) doi: 10.3390/fermentation9070667. MDPI. [DOI] [Google Scholar]
- Giammanco G.M., Pepe A., Aleo A., Agostino V.D., Milone S., Mammina C. Microbiological quality of Pecorino Siciliano “primosale” cheese on retail sale in the street markets of Palermo, Italy. New Microbiol. 2011;34(2):179–185. [PubMed] [Google Scholar]
- Giraffa G. Functionality of enterococci in dairy products. Int. J. Food Microbiol. 2003;88(2–3):215–222. doi: 10.1016/S0168-1605(03)00183-1. [DOI] [PubMed] [Google Scholar]
- Gobbetti M., De Angelis M., Di Cagno R., Mancini L., Fox P.F. Pros and cons for using non-starter lactic acid bacteria (NSLAB) as secondary/adjunct starters for cheese ripening. Trends in Food Sci. and Technology. 2015;45(2):167–178. doi: 10.1016/j.tifs.2015.07.016. Elsevier Ltd. [DOI] [Google Scholar]
- Grossmann L., McClements D.J. Trends in Food Sci. And Technology. vol. 118. Elsevier Ltd; 2021. The science of plant-based foods: approaches to create nutritious and sustainable plant-based cheese analogs; pp. 207–229. [DOI] [Google Scholar]
- Hinderink E.B.A., Boire A., Renard D., Riaublanc A., Sagis L.M.C., Schroën K., Bouhallab S., Famelart M.H., Gagnaire V., Guyomarc’h F., Berton-Carabin C.C. vol. 56. Elsevier Ltd; 2021. Combining plant and dairy proteins in food colloid design. (Current Opinion in Colloid and Interface Sci). [DOI] [Google Scholar]
- Ibarruri J., Cebrián M., Hernández I. Solid state fermentation of brewer's spent grain using Rhizopus sp. to enhance nutritional value. Waste Biomass Valorization. 2019;10(12):3687–3700. doi: 10.1007/s12649-019-00654-5. [DOI] [Google Scholar]
- Jaeger A., Arendt E.K., Zannini E., Sahin A.W. Brewer's spent yeast (BSY), an underutilized brewing by-product. Ferment. 2020;6(4) doi: 10.3390/fermentation6040123. MDPI. [DOI] [Google Scholar]
- Jaeger A., Nyhan L., Sahin A.W., Zannini E., Arendt E.K. Lactic acid fermentation as a valorising agent for brewer's spent yeast—improving the sensory quality and nutritional potential. Ferment. 2024;10(1) doi: 10.3390/fermentation10010054. [DOI] [Google Scholar]
- Kerby C., Vriesekoop F. An overview of the utilisation of brewery by-products as generated by british craft breweries. Beverages. 2017;3(2) doi: 10.3390/beverages3020024. [DOI] [Google Scholar]
- Kim I.S., Kim C.H., Yang W.S. Physiologically active molecules and functional properties of soybeans in human health-a current perspective. Int. J. of Molecular Sci.s. 2021;22(8) doi: 10.3390/ijms22084054. MDPI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna C. Solid-state fermentation systems - an overview. Crit. Rev. Biotechnol. 2005;25(1–2):1–30. doi: 10.1080/07388550590925383. [DOI] [PubMed] [Google Scholar]
- Ktenioudaki A., Alvarez-Jubete L., Smyth T.J., Kilcawley K., Rai D.K., Gallagher E. Application of bioprocessing techniques (sourdough fermentation and technological aids) for brewer's spent grain breads. Food Res. Int. 2015;73:107–116. doi: 10.1016/j.foodres.2015.03.008. [DOI] [Google Scholar]
- Kuchroo C.N., Fox P.F. Soluble nitrogen in Cheddar cheese: comparison of extraction procedures. Milchwissenschaft. 1982;37(6):331–335. [Google Scholar]
- Kühl S., Schäfer A., Kircher C., Mehlhose C. Beyond the cow: consumer perceptions and information impact on acceptance of precision fermentation-produced cheese in Germany. Future Foods. 2024;100411 doi: 10.1016/j.fufo.2024.100411. [DOI] [Google Scholar]
- Kyriakopoulou K., Keppler J.K., Van Der Goot J., Boom R.M. Alternat. To meat and dairy. Annual Review of Food Scienc. and Technology. 2021;12(1):29–50. doi: 10.1146/annurev-food-062520-101850. [DOI] [PubMed] [Google Scholar]
- Landfeld A., Novotna P., Houska M. Influence of the amount of rennet, calcium chloride addition, temperature, and high-pressure treatment on the course of milk coagulation. Czech J. Food Sci. 2002;20(6):237–244. doi: 10.17221/3537-CJFS. [DOI] [Google Scholar]
- Langenaeken N.A., De Schepper C.F., De Schutter D.P., Courtin C.M. Carbohydrate content and structure during malting and brewing: a mass balance study. J. of the Institute of Brewing. 2020;126(3):253–262. doi: 10.1002/jib.619. [DOI] [Google Scholar]
- Lapčíková B., Lapčík L., Valenta T., Chvatíková M. Plant-based emulsions as dairy cream alternatives: comparison of viscoelastic properties and colloidal stability of various model products. Foods. 2024;13 doi: 10.3390/foods13081225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law B.A., Tamime A.Y. Copyright © 2010 Blackwell Publishing Ltd; 2011. Technology of Cheesemaking. Society of Dairy Technology Second Edition, 512. [DOI] [Google Scholar]
- Leahu A., Ropciuc S., Ghinea C. Plant-based milks: alternatives to the manufacture and characterization of ice cream. Applied Sci. (Switzerland) 2022;12 doi: 10.3390/app12031754. [DOI] [Google Scholar]
- Liu X., Wu Y., Guan R., Jia G., Ma Y.C., Zhang Y. Food Res. Int. vol. 149. Elsevier Ltd; 2021. Advances in research on calf rennet substitutes and their effects on cheese quality. [DOI] [PubMed] [Google Scholar]
- López M., García V., Rovira S., Teruel R., Boutoial K., Rodríguez J., Roa I., López M.B. Effect of vegetable coagulant, microbial coagulant and calf rennet on physicochemical, proteolysis, sensory and texture profiles of fresh goats cheese. Dairy Sci. Technol. 2012;92(6):691–707. doi: 10.1007/s13594-012-0086-1ï. [DOI] [Google Scholar]
- Lusiana R., Poernomo A.T., Syahrani A. Fibrinolytic protease production: impact of initial pH and temperature in solid-state fermentation by Rhizopus microsporus var. oligosporus FNCC 6010. Pharmacy & Pharmaceutical Sci.s J. 2023;10(3):290–299. doi: 10.20473/jfiki.v10i32023.290-299. [DOI] [Google Scholar]
- Lynch K.M., Steffen E.J., Arendt E.K. Brewers' spent grain: a review with an emphasis on food and health. J. of the Institute of Brewing. 2016;122(4):553–568. doi: 10.1002/jib.363. John Wiley and Sons Inc. [DOI] [Google Scholar]
- Mäkinen O.E., Wanhalinna V., Zannini E., Arendt E.K. Foods for special dietary needs: non-dairy plant-based milk substitutes and fermented dairy-type products. Crit. Rev. Food Sci. Nutr. 2016;56:339–349. doi: 10.1080/10408398.2012.761950. [DOI] [PubMed] [Google Scholar]
- Marcus A., Fox G. Fungal biovalorization of a brewing industry byproduct, brewer's spent grain: a review. Foods. 2021;10(9) doi: 10.3390/foods10092159. MDPI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson G.V., de Castro R.J.S., Belleville M.P., Hubinger M.D. Spent brewer's yeast as a source of high added value molecules: a systematic review on its characteristics, processing and potential applications. World J. of Microbiology and Biotechnology. 2020;36(7) doi: 10.1007/s11274-020-02866-7. Springer. [DOI] [PubMed] [Google Scholar]
- McSweeney P.L.H. The flavour of milk and dairy products: III. Cheese: taste. Int. J. Dairy Technol. 1997;50(4):123–128. doi: 10.1111/j.1471-0307.1997.tb01752.x. [DOI] [Google Scholar]
- Mejri W., Bornaz S., Sahli A. Formulation of non-fat yogurt with β-glucan from spent brewer's yeast. J. of Hyg. Eng. and Design. 2014;8:163–173. https://api.semanticscholar.org/CorpusID:7471227 [Google Scholar]
- Nájera A.I., Nieto S., Barron L.J.R., Albisu M. A review of the preservation of hard and semi-hard cheeses: quality and safety. Int. J. of Environmental Res. and Public Health. 2021;18(18) doi: 10.3390/ijerph18189789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neylon E., Arendt E.K., Lynch K.M., Zannini E., Bazzoli P., Monin T., Sahin A.W. Rootlets, a malting by-product with great potential. Ferment. 2020;6(4) doi: 10.3390/fermentation6040117. MDPI. [DOI] [Google Scholar]
- Neylon E., Arendt E.K., Zannini E., Sahin A.W. Fermentation as a tool to revitalise brewers' spent grain and elevate techno-functional properties and nutritional value in high fibre bread. Foods. 2021;10(7) doi: 10.3390/foods10071639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neylon E., Arendt E.K., Zannini E., Sahin A.W. Fundamental study of the application of brewers spent grain and fermented brewers spent grain on the quality of pasta. Food Struct. 2021;30 doi: 10.1016/j.foostr.2021.100225. [DOI] [Google Scholar]
- Neylon E., Nyhan L., Zannini E., Monin T., Münch S., Sahin A.W., Arendt E.K. Food ingredients for the future: in-depth analysis of the effects of lactic acid bacteria fermentation on spent barley rootlets. Ferment. 2023;9(1) doi: 10.3390/fermentation9010078. [DOI] [Google Scholar]
- Neylon E., Nyhan L., Zannini E., Sahin A.W., Arendt E.K. From waste to taste: application of fermented spent rootlet ingredients in a bread system. Foods. 2023;12(7) doi: 10.3390/foods12071549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norazlina M.R., Jahurul M.H.A., Hasmadi M., Mansoor A.H., Norliza J., Patricia M., Ramlah George M.R., Noorakmar A.W., Lee J.S., Fan H.Y. Trends in blending vegetable fats and oils for cocoa butter alternative application: a review. Trends Food Sci. Technol. 2021 doi: 10.1016/j.tifs.2021.07.016. [DOI] [Google Scholar]
- Ogrodowczyk A.M., Drabińska N. Crossroad of tradition and innovation - the application of lactic acid fermentation to increase the nutritional and health-promoting potential of plant-based food products - a review. Polish J. of Food and Nutrition Sci.s. 2021;71(2):107–134. doi: 10.31883/pjfns/134282. Polish Academy Sci.s. Institute of Animal Reproduction and Food Res. [DOI] [Google Scholar]
- Olanipekun B., Adelakun O. Nutritional and microbiological attributes of soybean(Glycine max) during fermentation with Rhizopus oligosporus. Food Sci. and Quality Management. 2015;39:111–119. https://api.semanticscholar.org/CorpusID:83021355 [Google Scholar]
- Olivares-Galván S., Marina M.L., García M.C. Trends in Food Sci. And Technology. vol. 127. Elsevier Ltd; 2022. Extraction of valuable compounds from brewing residues: malt rootlets, spent hops, and spent yeast; pp. 181–197. [DOI] [Google Scholar]
- Peralta G.H., Wolf I.V., Perotti M.C., Bergamini C.V., Hynes E.R. Formation of volatile compounds, peptidolysis and carbohydrate fermentation by mesophilic lactobacilli and streptoccocci cultures in a cheese extract. Dairy Sci. Technol. 2016;96(5):603–621. doi: 10.1007/s13594-016-0291-4. [DOI] [Google Scholar]
- Picciotti U., Massaro A., Galiano A., Garganese F. Cheese fortification: review and possible improvements. Food Reviews Int. 2022;38(S1):474–500. doi: 10.1080/87559129.2021.1874411. Taylor and Francis Ltd. [DOI] [Google Scholar]
- Podpora B., Swiderski F., Sadowska A., Rakowska R., Wasiak-Zys G. Spent brewer's yeast extracts as a new component of functional food. Czech J. of Food Sci.s. 2016;34(6):554–563. doi: 10.17221/419/2015-CJFS. [DOI] [Google Scholar]
- Pua A., Tang V.C.Y., Goh R.M.V., Sun J., Lassabliere B., Liu S.Q. Ingredients, processing, and fermentation: addressing the organoleptic boundaries of plant-based dairy analogues. Foods. 2022;11(6):875. doi: 10.3390/foods11060875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putri A.M.H., Waluyo J., Setiawan A.A.R. Carbon footprint analysis of modern and traditional tempeh production in Indonesia. AIP Conf. Proc. 2018;2024 doi: 10.1063/1.5064296. [DOI] [Google Scholar]
- Rachwał K., Waśko A., Gustaw K., Polak-Berecka M. Utilization of brewery wastes in food industry. PeerJ. 2020;8:E9427–E9428. doi: 10.7717/peerj.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasha Mohamed K., Abou-Arab E.A., Gibriel A.Y., Rasmy N.M., Abu-Salem F.M. Effect of legume processing treatments individually or in combination on their phytic acid content. Afr. J. Food Sci. Technol. 2011;2(2):36–46. [Google Scholar]
- Rizzello C.G., Cassone A., Di Cagno R., Gobbetti M. Synthesis of angiotensin I-converting enzyme (ACE)-inhibitory peptides and γ-aminobutyric acid (GABA) during sourdough fermentation by selected lactic acid bacteria. J. Agric. Food Chem. 2008;56(16):6936–6943. doi: 10.1021/jf800512u. [DOI] [PubMed] [Google Scholar]
- Rouch D.A., Roginski H., Britz M.L., Roupas P. Determination of a nitrogen conversion factor for protein content in Cheddar cheese. Int. Dairy J. 2008;18:216–220. doi: 10.1016/j.idairyj.2007.07.004. [DOI] [Google Scholar]
- Rousta N., Aslan M., Yesilcimen Akbas M., Ozcan F., Sar T., Taherzadeh M.J. Critical Reviews in Food Sci. And Nutrition. Taylor and Francis Ltd; 2023. Effects of fungal based bioactive compounds on human health: review paper. [DOI] [PubMed] [Google Scholar]
- Santos W.C., Souza M.R., Cerqueira M.M.O.P., Glória M.B.A. Bioactive amines formation in milk by Lactococcus in the presence or not of rennet and NaCl at 20 and 32 C. Food Chem. 2003;81(4):595–606. doi: 10.1016/S0308-8146(02)00502-2. [DOI] [Google Scholar]
- Shahryari Z., Niknezhad S.V. Current Developments in Biotechnology and Bioengineering: Filamentous Fungi Biorefinery. Elsevier; 2022. Production of industrial enzymes by filamentous fungi; pp. 293–323. [DOI] [Google Scholar]
- Shams El Din A., El-Dardiry A., Abo Srea M.M., Refaey M.M.M. Influence of added cassava flour (manihot esculenta) on the properties of kareish cheese. J. of Food and Dairy Sci. 2022;13(1):25–31. doi: 10.21608/jfds.2022.121818.1038. [DOI] [Google Scholar]
- Short E.C., Kinchla A.J., Nolden A.A. Plant-based cheeses: a systematic review of sensory evaluation studies and strategies to increase consumer acceptance. Foods. 2021;10(4) doi: 10.3390/foods10040725. MDPI AG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa M.J., Ardo Y., Mcsweeney P.L.H. Advances in the study of proteolysis during cheese ripening. Int. Dairy J. 2001;11(4–7):327–345. doi: 10.1016/S0958-6946(01)00062-0. [DOI] [Google Scholar]
- Sternberg M. Microbial rennets. Adv. Appl. Microbiol. 1976;20:135–157. doi: 10.1016/S0065-2164(08)70111-4. [DOI] [Google Scholar]
- Stone H., Sidel J., Oliver S., Woolsey A., Singleton R.C. In: Descriptive Sensory Analysis in Practice. Gacula M.C., editor. 2004. Sensory evaluation by quantitative descriptive analysis. [DOI] [Google Scholar]
- Sulagna G. Nanyang Technol. University; Singapore: 2020. Ameliorating Nutritional Properties of Soybean Waste (Okara) through Biofermentation Using Rhizopus Oligosporus. Doctoral thesis. [DOI] [Google Scholar]
- Teter A., Barłowska J., Król J., Brodziak A., Rutkowska J., Litwińczuk Z. Volatile compounds and amino acid profile of short-ripened farmhouse cheese manufactured from the milk of the White-Backed native cow breed. LWT. 2020;129 doi: 10.1016/j.lwt.2020.109602. [DOI] [Google Scholar]
- Thakur M.S., Karanth N.G., Nand K. Production of fungal rennet by Mucor miehei using solid state fermentation. Appl. Microbiol. Biotechnol. 1990;32:409–413. doi: 10.1007/BF00903774. [DOI] [Google Scholar]
- Tlais A.Z.A., Kanwal S., Filannino P., Albiac M.A., Gobbetti M., Di Cagno R. Effect of sequential or ternary starters-assisted fermentation on the phenolic and glucosinolate profiles of sauerkraut in comparison with spontaneous fermentation. Food Res. Int. 2022;156 doi: 10.1016/j.foodres.2022.111116. [DOI] [PubMed] [Google Scholar]
- Tornadijo M.E., García M.C., Fresno J.M., Carballo J. Study of Enterobacteriaceae during the manufacture and ripening of san simón cheese. Food Microbiol. 2001;18(5):499–509. doi: 10.1006/fmic.2001.0423. [DOI] [Google Scholar]
- Tsakona S., Kopsahelis N., Chatzifragkou A., Papanikolaou S., Kookos I.K., Koutinas A.A. Formulation of fermentation media from flour-rich waste streams for microbial lipid production by Lipomyces starkeyi. J. Biotechnol. 2014;189:36–45. doi: 10.1016/j.jbiotec.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Usman A., Mohammed S., Mamo J. Production, optimization, and characterization of an acid protease from a filamentous fungus by solid-state fermentation. Int J Microbiol. 2021;2021 doi: 10.1155/2021/6685963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verni M., Rizzello C.G., Coda R. Fermentation biotechnology applied to cereal industry by-products: nutritional and functional insights. Front. Nutr. 2019;6(42) doi: 10.3389/fnut.2019.00042. Frontiers Media S.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinicius De Melo Pereira G., De Carvalho Neto D.P., Junqueira A.C.D.O., Karp S.G., Letti L.A., Magalhães Júnior A.I., Soccol C.R. A review of selection criteria for starter culture development in the food fermentation industry. Food Reviews Int. 2020;36(2):135–167. doi: 10.1080/87559129.2019.1630636. Taylor and Francis Inc. [DOI] [Google Scholar]
- Walstra P., Walstra P., Wouters J.T.M., Geurts T.J. second ed. CRC Press; Boca Raton: 2005. Dairy Sci. And Technology; p. 808. [DOI] [Google Scholar]
- Wang H.L., Hesseltine C.W. Studies on the extracellular proteolytic enzymes of Rhizopus oligosporus. Can. J. Microbiol. 1965;11(4):727–732. doi: 10.1139/m65-096. [DOI] [PubMed] [Google Scholar]
- Waters D.M., Kingston W., Jacob F., Titze J., Arendt E.K., Zannini E. Wheat bread biofortification with rootlets, a malting by-product. J. Sci. Food Agric. 2013;93(10):2372–2383. doi: 10.1002/jsfa.6059. [DOI] [PubMed] [Google Scholar]
- Yu L.J., Ngadi M., Raghavan G.S.V. Effect of temperature and pulsed electric field treatment on rennet coagulation properties of milk. J. Food Eng. 2009;95(1):115–118. doi: 10.1016/j.jfoodeng.2009.04.013. [DOI] [Google Scholar]
- Zwietering M.H., Jongenburger I., Rombouts F.M., Van’t Riet K.J.A.E.M. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990;56(6):1875–1881. doi: 10.1128/aem.56.6.1875-1881.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Majority of the data are displayed in the main manuscript but also in the supplementary material. Further data will be made available on request.
Data will be made available on request.