Abstract
Background
This review investigates the side effects of glucagon-like peptide-1 receptor agonists (GLP-1RAs) like liraglutide, semaglutide, and tirzepatide, medications known for their efficacy in promoting weight loss among individuals with obesity. The rationale is rooted in understanding the balance between their therapeutic benefits and associated risks.
Methods
This was a comprehensive clinical review, including systematic reviews, meta-analyses, randomized controlled trials (RCTs), and cohort studies. Data were extracted from databases such as PubMed, Scopus, Embase, MEDLINE, and Google Scholar, focusing on the tolerability, severity, and risks of these medications.
Results
GLP-1RAs demonstrated significant weight loss outcomes. In clinical trials, liraglutide showed a placebo-corrected weight loss of around 5 %, semaglutide 12 %, and tirzepatide 18 %. Common side effects were predominantly gastrointestinal, including nausea, diarrhea, constipation, and vomiting. Rare serious adverse events included gallbladder disorders and acute pancreatitis. In, addition, multiple studies identify new risks associated with GLP-1RAs including increased aspiration risk during anesthesia due to delayed gastric emptying and challenges with bowel preparation for colonoscopies.
Conclusion
While GLP-1RAs are effective in managing obesity, their use is associated with gastrointestinal side effects and rare but serious adverse events. The findings underscore the importance of individualized dosing and thorough patient assessment. Continuous research and vigilant monitoring are essential to optimize their safe use. Further studies are needed to refine guidelines, particularly regarding new concerns such as delayed gastric emptying and its implications for anesthesia.
Keywords: Cardiometabolic outcomes, GLP-1, Liraglutide, Semaglutide, Side effects, Tirzepatide
Graphical abstract
Highlights
-
•
Side Effect Profile: Predominantly mild to moderate gastrointestinal side effects with rare occurrences of serious events like gallbladder disorders and acute pancreatitis.
-
•
Individualized Treatment: Emphasizes the importance of individualized dosing and patient assessment to optimize safety and efficacy.
-
•
New Concerns: Identifies new risks including increased aspiration risk during anesthesia due to delayed gastric emptying and challenges with bowel preparation for colonoscopies.
-
•
Conclusion: While effective for obesity management, ongoing research and vigilant patient monitoring are essential to address and mitigate potential risks associated with GLP-1 RAs.
1. Introduction
Obesity is a chronic, relapsing, and multifactorial disease that is projected to affect around half of the United States population by 2030 [1]. Currently, it is approximated that 650 million adults and 340 million children and adolescents (5–19 years) live with obesity around the world [2]. In addition to the dramatically increasing prevalence, obesity has significant medical and financial implications at the individual and national levels. This disease increases the risk of adiposity-associated diseases including cardiovascular disease risk factors (e.g., hypertension, dyslipidemia, type-2 diabetes mellitus-T2D), cardiovascular disease itself, and cancer [3,4]. Financially, obesity contributes to $480.7 billion in direct health care costs in the United States and is estimated to result in an additional indirect cost of $1.24 trillion attributed to lost economic productivity [5].
Multiple interventions have been developed to promote weight loss including lifestyle interventions comprised of diet with or without exercise and/or behavioral therapy [6]; anti-obesity medications (AOMs) [7,8]; and endoscopic and surgical procedures [9]. AOM candidates include individuals with a body mass index (BMI) ≥30 kg/m2, or a BMI of 27–29.9 kg/m2 with weight-related comorbidities (e.g., T2D, hypertension, dyslipidemia, cardiovascular disease, etc.). Importantly, AOMs are particularly indicated for those who have not met clinical weight-loss goals of at least 5% of total body weight at three to six months with a comprehensive lifestyle intervention [10]. AOMs as an adjunct to lifestyle modification have shown to be more effective than lifestyle modification alone in terms of weight loss [11]. Although bariatric procedures show superior weight loss outcomes, AOMs are achieving remarkable weight loss outcomes while being less invasive. Hence, more providers are prescribing AOMs to enhance weight loss in adults with obesity, especially those who are at a high risk of undergoing bariatric procedures under anesthesia (e.g., adults with organ failure such as heart, liver, and renal failure) [12]. Amongst AOMs, glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and dual GLP-1 and glucose-dependent insulinotropic polypeptide receptor agonist (GLP-1/GIP RAs) have shown significant weight loss outcomes and are becoming increasingly popular among the AOM treatment options. The Food and Drug Administration (FDA) approved for the treatment of overweight and obesity the GLP-1 RA liraglutide in 2014 and semaglutide in 2021, and the dual GLP-1/GIP RA tirzepatide in 2023. Although these medications have shown remarkable weight loss outcomes in randomized clinical trials (RCTs) [[13], [14], [15]] and real-word studies [[16], [17], [18], [19], [20]], many concerns have been raised regarding the safety profile, tolerability, and health risks associated with these medications. In this review, we aim to assess and evaluate the weight loss outcomes, tolerability, side effects and risks linked to these medications. We also include an expert opinion (MDH) about their experience with patients tolerating these medications with the side effect profile.
2. Methods
In this clinical review, we included publications that included information about the tolerability, side effects and their severity, and risks of using liraglutide, semaglutide, and tirzepatide. We searched various databases including PubMed, Scopus, Embase, MEDLINE, and Google Scholar for related studies these database inceptions to December 1, 2023. Our search was limited to publications in the English language. Specifically, we assessed data from systematic reviews and meta-analyses, RCTs, and prospective and retrospective cohort studies. We also include an expert opinion in the fields of GLP-1 and GLP-1/GIP RAs and obesity to present a clinical point of view from the daily practice in weight management clinics.
3. Results
3.1. Weight loss outcomes
3.1.1. Mean weight loss (Table 1)
Table 1.
Weight loss outcomes of GLP-1RA and GLP-1/GIP RA in randomized clinical trials.
| GLP-1RA | 3 months | 6 months | 9 months | 12 months |
|---|---|---|---|---|
| Liraglutide 3.0 mg | 6 % | 8 % | 9 % | 9 % |
| Semaglutide 2.4 mg | 6 % | 10 % | 14 % | 15 % |
| Tirzepatide 15 mg | 8 % | 15 % | 18 % | 20 % |
GLP-1 and GLP-1/GIP RAs have demonstrated significant weight loss outcomes in RCTs and real-world studies.
In the Satiety and Clinical Adiposity Liraglutide Evidence (SCALE) clinical trial, liraglutide resulted in up to 8.0 ± 6.7 % (8.4 ± 7.3 kg) of body weight loss at 56 weeks compared to a mean of 2.6 ± 5.7 % (2.8 ± 6.5 kg) in the placebo group with a placebo corrected weight reduction of approximately 5 % [21]. In the Semaglutide Treatment Effect in People with Obesity (STEP) trial in adults without diabetes, semaglutide 2.4 mg showed superior weight loss outcomes of 14.9 % compared to 2.4 % with the placebo group with a placebo corrected weight loss of around 12 % [22]. As for tirzepatide 15 mg, the SURMOUNT-1 trial presented a mean weight loss of 20.9 % while the placebo group had 3.1 % of weight loss, reflecting a placebo-corrected weight loss of 18 %, approximately. In fact, all three tirzepatide doses (5, 10, and 15 mg) were superior to placebo [[13], [14], [15]].
3.1.2. Categorical weight loss outcomes
Similarly, a greater proportion of participants lost ≥5 %, ≥10 %, ≥15 % and ≥20 % of body weight with tirzepatide and semaglutide compared to Ref. [21]glutide. In the pivotal RCTs of these AOMs, liraglutide 3 mg daily, semaglutide 2.4 mg weekly, and tirzepatide 15 mg weekly in adults without diabetes, 63.2 % vs 86.4 % vs 91 % of participants lost ≥5 % of body weight, respectively; 33.1 % vs 69.1 % vs 83.5 % of participants lost ≥10 % of body weight, respectively; and 14.4 % vs 50.5 % vs 70.6 % of participants lost ≥15 % of body weight. A greater proportion of participants lost ≥20 % of body weight with tirzepatide 15 mg (56.7 %) compared to semaglutide 2.4 mg (32.0 %). Importantly, in the tirzepatide trial, 36 % of participants achieved a body weight loss of ≥25 % at week 72 after starting the AOM [14,23,24].
3.1.3. Glycemic control and cardiometabolic variables
Despite the different weight loss achieved by these AOMs, all trials have demonstrated improvement in glycemic control and cardiometabolic variables. There was a significantly greater reduction in glycated hemoglobin, fasting glucose, systolic and diastolic blood pressure with the three GLP-1RA as compared to the placebo groups. In addition, fasting lipid levels have also shown remarkably greater improvement with liraglutide, semaglutide and tirzepatide compared to placebo [14,23,24].
In fact, in the Semaglutide Effects on Cardiovascular Outcomes in People with Overweight or Obesity (SELECT) trial, major cardiovascular events (i.e., nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes nonfatal myocardial infarction or nonfatal stroke) occurred in 6.5 % of participants in the semaglutide group compared to 8.0 % of participants in the placebo group (hazard ratio, 0.80; 95 % confidence interval [CI], 0.72 to 0.90; P < 0.001) [25].
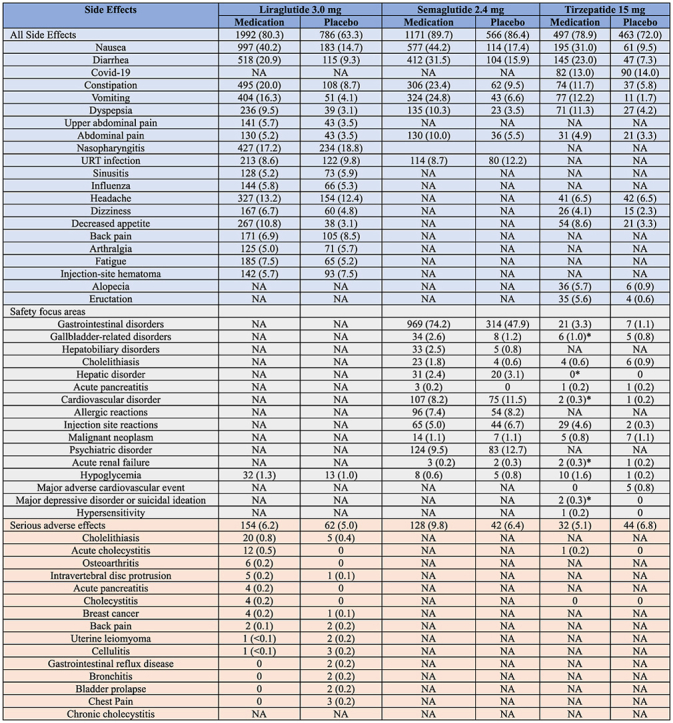
3.2. Side effects and tolerability (Table 2)
Table 2.
Side effects associated with liraglutide, semaglutide, and tirzepatide.
| Side Effects | Liraglutide 3.0 mg |
Semaglutide 2.4 mg |
Tirzepatide 15 mg |
|||
|---|---|---|---|---|---|---|
| Medication | Placebo | Medication | Placebo | Medication | Placebo | |
| All Side Effects | 1992 (80.3) | 786 (63.3) | 1171 (89.7) | 566 (86.4) | 497 (78.9) | 463 (72.0) |
| Nausea | 997 (40.2) | 183 (14.7) | 577 (44.2) | 114 (17.4) | 195 (31.0) | 61 (9.5) |
| Diarrhea | 518 (20.9) | 115 (9.3) | 412 (31.5) | 104 (15.9) | 145 (23.0) | 47 (7.3) |
| Covid-19 | NA | NA | NA | NA | 82 (13.0) | 90 (14.0) |
| Constipation | 495 (20.0) | 108 (8.7) | 306 (23.4) | 62 (9.5) | 74 (11.7) | 37 (5.8) |
| Vomiting | 404 (16.3) | 51 (4.1) | 324 (24.8) | 43 (6.6) | 77 (12.2) | 11 (1.7) |
| Dyspepsia | 236 (9.5) | 39 (3.1) | 135 (10.3) | 23 (3.5) | 71 (11.3) | 27 (4.2) |
| Upper abdominal pain | 141 (5.7) | 43 (3.5) | NA | NA | NA | NA |
| Abdominal pain | 130 (5.2) | 43 (3.5) | 130 (10.0) | 36 (5.5) | 31 (4.9) | 21 (3.3) |
| Nasopharyngitis | 427 (17.2) | 234 (18.8) | NA | NA | ||
| URT infection | 213 (8.6) | 122 (9.8) | 114 (8.7) | 80 (12.2) | NA | NA |
| Sinusitis | 128 (5.2) | 73 (5.9) | NA | NA | NA | NA |
| Influenza | 144 (5.8) | 66 (5.3) | NA | NA | NA | NA |
| Headache | 327 (13.2) | 154 (12.4) | NA | NA | 41 (6.5) | 42 (6.5) |
| Dizziness | 167 (6.7) | 60 (4.8) | NA | NA | 26 (4.1) | 15 (2.3) |
| Decreased appetite | 267 (10.8) | 38 (3.1) | NA | NA | 54 (8.6) | 21 (3.3) |
| Back pain | 171 (6.9) | 105 (8.5) | NA | NA | NA | NA |
| Arthralgia | 125 (5.0) | 71 (5.7) | NA | NA | NA | NA |
| Fatigue | 185 (7.5) | 65 (5.2) | NA | NA | NA | NA |
| Injection-site hematoma | 142 (5.7) | 93 (7.5) | NA | NA | NA | NA |
| Alopecia | NA | NA | NA | NA | 36 (5.7) | 6 (0.9) |
| Eructation | NA | NA | NA | NA | 35 (5.6) | 4 (0.6) |
| Safety focus areas | ||||||
| Gastrointestinal disorders | NA | NA | 969 (74.2) | 314 (47.9) | 21 (3.3) | 7 (1.1) |
| Gallbladder-related disorders | NA | NA | 34 (2.6) | 8 (1.2) | 6 (1.0)∗ | 5 (0.8) |
| Hepatobiliary disorders | NA | NA | 33 (2.5) | 5 (0.8) | NA | NA |
| Cholelithiasis | NA | NA | 23 (1.8) | 4 (0.6) | 4 (0.6) | 6 (0.9) |
| Hepatic disorder | NA | NA | 31 (2.4) | 20 (3.1) | 0∗ | 0 |
| Acute pancreatitis | NA | NA | 3 (0.2) | 0 | 1 (0.2) | 1 (0.2) |
| Cardiovascular disorder | NA | NA | 107 (8.2) | 75 (11.5) | 2 (0.3)∗ | 1 (0.2) |
| Allergic reactions | NA | NA | 96 (7.4) | 54 (8.2) | NA | NA |
| Injection site reactions | NA | NA | 65 (5.0) | 44 (6.7) | 29 (4.6) | 2 (0.3) |
| Malignant neoplasm | NA | NA | 14 (1.1) | 7 (1.1) | 5 (0.8) | 7 (1.1) |
| Psychiatric disorder | NA | NA | 124 (9.5) | 83 (12.7) | NA | NA |
| Acute renal failure | NA | NA | 3 (0.2) | 2 (0.3) | 2 (0.3)∗ | 1 (0.2) |
| Hypoglycemia | 32 (1.3) | 13 (1.0) | 8 (0.6) | 5 (0.8) | 10 (1.6) | 1 (0.2) |
| Major adverse cardiovascular event | NA | NA | NA | NA | 0 | 5 (0.8) |
| Major depressive disorder or suicidal ideation | NA | NA | NA | NA | 2 (0.3)∗ | 0 |
| Hypersensitivity | NA | NA | NA | NA | 1 (0.2) | 0 |
| Serious adverse effects | 154 (6.2) | 62 (5.0) | 128 (9.8) | 42 (6.4) | 32 (5.1) | 44 (6.8) |
| Cholelithiasis | 20 (0.8) | 5 (0.4) | NA | NA | NA | NA |
| Acute cholecystitis | 12 (0.5) | 0 | NA | NA | 1 (0.2) | 0 |
| Osteoarthritis | 6 (0.2) | 0 | NA | NA | NA | NA |
| Intravertebral disc protrusion | 5 (0.2) | 1 (0.1) | NA | NA | NA | NA |
| Acute pancreatitis | 4 (0.2) | 0 | NA | NA | NA | NA |
| Cholecystitis | 4 (0.2) | 0 | NA | NA | 0 | 0 |
| Breast cancer | 4 (0.2) | 1 (0.1) | NA | NA | NA | NA |
| Back pain | 2 (0.1) | 2 (0.2) | NA | NA | NA | NA |
| Uterine leiomyoma | 1 (<0.1) | 2 (0.2) | NA | NA | NA | NA |
| Cellulitis | 1 (<0.1) | 3 (0.2) | NA | NA | NA | NA |
| Gastrointestinal reflux disease | 0 | 2 (0.2) | NA | NA | NA | NA |
| Bronchitis | 0 | 2 (0.2) | NA | NA | NA | NA |
| Bladder prolapse | 0 | 2 (0.2) | NA | NA | NA | NA |
| Chest Pain | 0 | 3 (0.2) | NA | NA | NA | NA |
| Chronic cholecystitis | NA | NA | NA | NA | NA | NA |
A significant portion of participants have experienced side effects in the RCTs and real-world studies. In clinical trials, adverse and serious adverse events are presented by their preferred terms from the Medical Dictionary for Regulatory Activities. Mild side effects were defined as easily tolerated, causing minimal discomfort, and not interfering with everyday activities; moderate adverse events as side effects that cause sufficient discomfort and interfere with daily activities; and severe events as those that prevent daily activities.
3.2.1. Common side effects
In the liraglutide pivotal trial, 80.3 % of participants taking liraglutide experienced adverse events compared to 63.3 % of participants on placebo. However, it is important to note that adverse events reported in clinical trials do not indicate causality. Most of the experienced adverse events (94 %) were labeled as mild or moderate in severity. The majority of side effects were gastrointestinal-related, including nausea (40.2 %), diarrhea (20.9 %), constipation (20.0 %), and vomiting (16.3 %). In fact, gastrointestinal adverse events led to medication discontinuation in 6.4 % of participants compared to 0.7 % in the placebo group only. Other adverse events are documented in Table 2. Most participants experienced nausea in the first 4–8 weeks, and this percentage progressively decreased with time. In fact, 24.7 % participants experienced nausea at week 4, 14.7 % at week 8, and 5.5 % at week 56 [13].
For semaglutide, the incidence of side effects was a similar among participants in the treatment (89.7 %) and placebo groups (86.4 %) experienced side effects, irrespective of causality. Similar to liraglutide, gastrointestinal adverse events (e.g., nausea, diarrhea, vomiting, and constipation) were most commonly reported (74.2 % of participants taking semaglutide). Importantly, the majority of these side effects were considered mild-moderate and transient and did not require discontinuation. The majority of participants experienced the side effects within 20 weeks of starting semaglutide [14].
With tirzepatide, compared to placebo, 78.9–81.8 % of participants on tirzepatide reported at least one adverse event compared (vs. 72.0 %), which is also irrespective of causality. The majority of adverse events were also gastrointestinal. In addition, most side effects were transient, mild to moderate in severity, and were experienced primarily during the dose-escalation period of tirzepatide. Most side effects occurred within the first 8–16 weeks of starting the medication [15].
3.2.2. Serious side effects
In RCTs, serious adverse events were statistically higher in the liraglutide group (6.2 %) compared to the placebo group (5.0 %) [13]. Similarly, participants taking semaglutide experienced a higher number of serious adverse events (9.8 %) compared to participants taking placebo (6.4 %). In fact, this difference was mainly attributed to gastrointestinal side effects (1.4 % vs 0 %) and hepatobiliary disorders (1.3 % vs 0.2 %) [14]. Consequently, the group taking semaglutide had a higher discontinuation percentage of the medication (7.0 % vs 3.1 %). In addition, 5.1–6.3 % participants taking tirzepatide compared to 6.8 % participants in the placebo group reported serious adverse events.
In a recent investigation involving a randomized sample of 16 million patients from the PharMetrics Plus database (IQVIA), the utilization of liraglutide and semaglutide, when compared to bupropion-naltrexone, was linked to an elevated risk of pancreatitis (adjusted HR, 9.09 [95 % CI, 1.25–66.00]), bowel obstruction (HR, 4.22 [95 % CI, 1.02–17.40]), and gastroparesis (HR, 3.67 [95 % CI, 1.15–11.90) but not biliary disease (HR, 1.50 [95 % CI, 0.89–2.53]) [26].
3.2.2.1. Gallbladder-related disorders
In participants taking liraglutide, the majority of documented severe adverse events were gallbladder-related (cholelithiasis, 0.8 % and acute cholecystitis, 0.5 %). The reported weight loss was greater in participants who experienced gallbladder related events as compared to the total population. A recent meta-analysis concludes that GLP-1 RAs were linked to a higher risk of gallbladder or biliary diseases, particularly when used at higher doses, for extended periods, and specifically for weight loss [27]. Most participants (78 %) in the liraglutide group who reported cholelithiasis or cholecystitis underwent an elective cholecystectomy. Out of these individuals, 84 % continued their assigned course after recovery without encountering further complications [13]. In participants taking semaglutide, more gallbladder-related disorders were reported compared to participants taking placebo (2.6 % vs 1.2 %, respectively). The majority of these events were due to cholelithiasis [14]. The incidence of cholelithiasis was similar among the three doses groups of tirzepatide and placebo groups (1.1 % in 5 mg, 1.4 % in 10 mg, 0.6 % in 15 mg, and 0.9 % in placebo groups). However, acute cholecystitis was more frequently demonstrated in the tirzepatide groups than in the placebo group (0.2–0.6 % vs 0 %). Importantly, the incidence of cholecystitis was relatively low (≤0.6 % of participants on tirzepatide) [15].
3.2.2.2. Acute pancreatitis
Overall, ten participants taking liraglutide experienced pancreatitis compared to one in the placebo group. Out of these ten individuals, 9 were graded as mild and 5 had gallstone-related pancreatitis [13]. With semaglutide, mild pancreatitis was reported in three participants taking semaglutide and all recovered during the trial period. In these participants, all had a personal medical history of acute pancreatitis and/or gallstones [14]. As for tirzepatide, there were four reported cases evenly distributed across treatment groups, including the placebo group (1 case in each of 5 mg, 10 mg, 15 mg, and placebo group). None of these events were graded as severe [15].
Multiple recent systematic reviews and meta-analyses show that although there is a statistically significant increased risk of elevation of pancreatic enzymes associated with GLP-1-based agents, there is no significance difference in development of acute pancreatitis or pancreatic cancer between individuals on GLP-1 RAs compared to placebo [[28], [29], [30]].
3.2.2.3. Cardiovascular parameters risks
The mean resting pulse was demonstrated to be increased with all three medication by the end of the trial (change of 2.5, 3.5, and 2.6 with liraglutide 3.0 mg, semaglutide 2.4 mg,and tirzepatide 15 mg, respectively) [[13], [14], [15]].
In the participants taking liraglutide, cardiac arrhythmias were similar compared to placebo despite tachycardia being higher in liraglutide group (0.6 events per 100 patient-years of exposure vs. 0.1 events per 100 patient-years of exposure). However, the majority of these cases were considered non-serious [13].
3.2.2.4. Mental health assessment
There was no clinically significant difference in terms of mental health assessments (e.g., psychiatric disorders, depression, or suicidal behavior) in participants taking liraglutide, semaglutide, or tirzepatide compared to participants on placebo [[13], [14], [15]]. In fact, some studies show improvement in mental health in patients using GLP-1 RA [31]. On the other hand, there has been also some cases of reported deterioration in mood with GLP1-RA [31].
3.2.2.5. Hypoglycemia
Spontaneous hypoglycemia was reported by 1.3 % vs 1.0 %, 0.6 % vs 0.8 %, and 1.6 % vs 0.2 % in the liraglutide, semaglutide and tirzepatide (15 mg) groups compared to their respective placebo groups. None of these events were considered to be serious [[13], [14], [15]]. However, hypoglycemia remains a possible risk that should be taken into account when prescribing this medication in patients treated with insulin secretagogues or insulin, as shown in previous studies [32].
3.2.2.6. Neoplasm
There was no difference in the incidence of benign and malignant neoplasms in liraglutide, semaglutide, or tirzepatide groups compared to placebo. Importantly, there were no reports of medullary thyroid carcinoma or C-cell hyperplasia in individuals randomized to liraglutide, semaglutide, or tirzepatide group and there was no clinically significant increase in serum calcitonin concentrations associated with the use of these medications [[13], [14], [15]]. Interestingly, more female participants in the liraglutide group compared to placebo (9 vs 3) had a diagnosis of malignant and premalignant breast neoplasms. Most of these women had above-average weight loss reported [13].
3.2.2.7. Death
Overall, there has not been an association between the use of GLP-1RA and death [[13], [14], [15]]. In fact, multiple studies have been demonstrating the survival benefit of these medications [33,34]. In one meta-analysis, the risk of all-cause mortality was decreased by 11 % in patients using GLP1-RA (RR 0.89, 95 % CI 0.81–0.99) [33].
4. Discussion: clinical expert
GLP-1 and dual GLP-1/GIPRAs have been proven to be effective for the treatment of overweight and obesity. As with all pharmacologic agents, side effects can occur. Most side effects of GLP-1 and GLP-1/GIP RAs involve gastrointestinal symptoms, are mild-to-moderate, and improve with time. While concerns have been raised about serious side effects (e.g., gastrointestinal paralysis, suicide, and medullary thyroid cancer), these are rare. Importantly the side effect profile of these medications should be put in the right context. Obesity is one of the most prevalent diseases worldwide. Obesity is also associated with a substantial risk of developing cardiometabolic risk factors, cardiovascular disease, and cardiovascular death. Similarly, obesity is a risk factor for various cancers including breast, ovarian, uterine, esophageal, gastric, hepatic, pancreatic, and renal, among others. Considering that cardiovascular disease and cancer are the leading causes of death among adults globally, the benefits of these medications outweigh the risks for most adults living with overweight and obesity [35].
Like for any other pharmacologic intervention, there are individuals in whom the benefits of these medications do not outweigh the risks (e.g., adults with a history of medullary thyroid cancer). To identify these individuals, a detailed medical history should be obtained, and a thorough physical examination should be performed. It is of utmost importance that these medications are taken under the supervision of a health care professional trained and knowledgeable on the use of these medications. In this way, patients can be exhaustively presented the evidence about these medications' effectiveness while also being counseled about potential side effects taking into consideration their current health. Shared-decision-making should be undertaken based on the information gathered and the evidence presented. The goal is to provide an effective treatment for adults living with obesity while considering each individual's context of life, values, and preferences.
Although the data is limited, it is important to highlight that there are certain interventions that can be considered to minimize the incidence and intensity of side effects [36]. For instance, the dosing should always start at the lowest doses recommended for each medication. Dose titration has been established for all these medications, with liraglutide titration occurring every week and semaglutide and tirzepatide titrations occurring every four weeks, until the maximum dose is reached. Importantly, dose escalation should be individualized, considering the patient's health context and goals, the effectiveness of the medication at the individual level, and most importantly, the incidence and severity of side effects. Individuals who develop side effects that affect activities of daily living may benefit from dose de-escalation if not at the lowest dose, and/or staying on a certain dose for longer than what is recommended (e.g., more than one week of liraglutide and more than four weeks for semaglutide or liraglutide).
Notably, as these medications become increasingly effective and their use continues to rise, we will continue to learn about side effects that have yet to be reported. For instance, a new concern has been raised about the risk of aspiration in patients undergoing anesthesia due to the effect of these medications on gastric emptying [[37], [38], [39]]. Despite adequate fasting period, several case reports have attributed aspiration in patients undergoing anesthesia to taking GLP-1RA for weight loss. In a prospective study including 20 participants undergoing ultrasound evaluation after an 8-h fast, residual gastric contents were demonstrated in most patients on semaglutide, which may have major implications for aspiration risk during anesthetic care [40]. Many experts have recommended withholding the medication for at least three half-lives (approximately 88 % clearance of the drug) prior to the scheduled procedure [41]. In the case of semaglutide, this would equate to a three-week duration. As for individuals using GLP-1RA for T2D, it is advisable to consult with an endocrinologist to assess the potential risks and benefits of discontinuing the medication at least three half-lives before the planned procedure. If it is not feasible to suspend GLP-1RAs for a duration of at least three half-lives, a rapid sequence induction in instances where general anesthesia is necessary might be considered. This approach aims to minimize the risk of gastric contents aspiration [41]. The American College of Gastroenterology reported that in patients using GLP-1 RAs who have adhered to standard perioperative protocols and lack symptoms such as nausea, vomiting, dyspepsia, or abdominal distention, the recommendation is to proceed with upper and/or lower endoscopy. For those exhibiting symptoms indicative of possible retained gastric contents, transabdominal ultrasonography may be employed to assess the stomach, contingent on clinical expertise and equipment availability; however, evidence supporting this practice is limited. In cases where delaying endoscopy poses clinical risks, rapid-sequence intubation is a consideration, though its feasibility may be restricted in ambulatory or office-based settings. Additionally, when feasible, transitioning patients to a liquid diet the day before sedated procedures is suggested as an alternative strategy, preserving GLP-1 RA use and aligning with a holistic preprocedural management approach for similar conditions [42]. As more of these cases are reported, we must develop evidence-based guidelines to promote the safe use of GLP-1 and GLP-1/GIP RAs.
Importantly, the utilization of GLP-1Ras has also been linked to a significantly lower quality of bowel preparation, leading to a notable increase in the requirement for repeat colonoscopies. To enhance preprocedural planning for outpatient colonoscopies, it is crucial to comprehend the cumulative impact of medications that may impede gastric emptying. This understanding is essential for determining appropriate measures and counseling strategies [43].
In addition, reports of depression and suicidality prompted the European Medicines Agency to review around 150 cases of possible self-injury and suicidal thoughts associated with liraglutide and semaglutide. Although it is unclear if the medications caused these incidents, similar issues have been reported with other AOMs. While liraglutide, semaglutide, and tirzepatide labels include warnings about monitoring for depression or suicidal thoughts, other GLP-1RA lack this warning. Most clinical trials for GLP-1RA excluded individuals with a history of depression or suicidal behavior. The specific reasons for this exclusion are not fully clear. Monitoring and caution are advised when prescribing these drugs, particularly for individuals with a history of suicidality [44]. Importantly, a recent study concludes that by applying the Bradford Hill criteria and accounting for confounding factors, there was no evidence of a causal relationship between GLP-1 RAs and suicidality [45]. Similarly, real word studies also demonstrate that GLP1-RA (e.g., semaglutide) do not pose a higher risk of suicidal ideation compared to non-GLP-1 receptor agonist anti-obesity or anti-diabetes medications [46].
5. Conclusion
GLP-1 and GLP-1/GIP RAs have demonstrated substantial weight loss outcomes in both clinical trials and real-world studies. Despite their efficacy, concerns regarding safety, tolerability, and potential health risks persist. Gastrointestinal adverse events, such as nausea and diarrhea, were common, leading to discontinuation in some cases. Serious adverse events, though rare, were reported, with pancreatitis and gallbladder-related disorders among the notable concerns. However, the benefits of these medications, including improved glycemic control and cardiometabolic variables, seem to outweigh the risks for most adults with obesity, considering the associated risks of cardiovascular diseases and various cancers (e.g., esophageal, colorectal, endometrial [35]). Expert opinions emphasize the importance of careful patient selection, shared decision-making, and individualized dosing to balance the benefits and risks. Ongoing research is crucial to uncover and address potential side effects, such as the impact on gastric emptying and the need for precautionary measures during anesthesia. As the use of these medications continues to rise, evidence-based guidelines are essential to ensure their safe and effective utilization in the management of obesity.Takeaways.
-
F0B7
Effective Weight Loss: GLP-1 RAs like liraglutide, semaglutide, and tirzepatide lead to significant weight loss, with tirzepatide being the most effective.
-
F0B7
Side Effects: Common side effects include nausea and vomiting, with rare but serious risks like pancreatitis and gallbladder issues, requiring careful patient monitoring.
-
F0B7
Emerging Risks: Concerns about gastric emptying and anesthesia risks are rising, necessitating ongoing research and updated guidelines for safe use.
Author contribution
WG: Writing - Original Draft, Writing - Review & Editing; MDH: Writing - Review & Editing.
Source of funding
Beyond payment to the research staff by the Mayo Clinic, this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of artificial intelligence (AI) and AI-assisted technologies
During the preparation of this work the authors did not use AI.
Contributor Information
Wissam Ghusn, Email: wissamghusn7@gmail.com.
Maria D. Hurtado, Email: Hurtado.mariadaniela@mayo.edu.
References
- 1.Ward Z.J., et al. Projected U.S. State-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381(25):2440–2450. doi: 10.1056/NEJMsa1909301. [DOI] [PubMed] [Google Scholar]
- 2.Sørensen T.I.A., Martinez A.R., Jørgensen T.S.H. Epidemiology of obesity. Handb Exp Pharmacol. 2022;274:3–27. doi: 10.1007/164_2022_581. [DOI] [PubMed] [Google Scholar]
- 3.Apovian C.M. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22(7 Suppl):s176–s185. [PubMed] [Google Scholar]
- 4.Busebee B., et al. Obesity: a review of pathophysiology and classification. Mayo Clin Proc. 2023;98(12):1842–1857. doi: 10.1016/j.mayocp.2023.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waters H., Graf M. The health and economic costs of excess weight. Milken Institute; Santa Monica, California: 2018. America's obesity crisis. [Google Scholar]
- 6.Cifuentes L., et al. Phenotype tailored lifestyle intervention on weight loss and cardiometabolic risk factors in adults with obesity: a single-centre, non-randomised, proof-of-concept study. EClinicalMedicine. 2023;58 doi: 10.1016/j.eclinm.2023.101923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De la Rosa A., et al. A comparison between weight loss outcomes with anti-obesity medications before and during Covid-19 pandemic at a tertiary weight management center. Obesity Pillars. 2022;4 doi: 10.1016/j.obpill.2022.100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghusn W., Hurtado M.D., Acosta A. Weight-centric treatment of type 2 diabetes mellitus. Obes Pillars. 2022;4 doi: 10.1016/j.obpill.2022.100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghusn W., et al. Diabetes mellitus Remission in Patients with BMI > 50 kg/m(2) after bariatric surgeries: a real-world multi-centered study. Obes Surg. 2023;33(6):1838–1845. doi: 10.1007/s11695-023-06622-2. [DOI] [PubMed] [Google Scholar]
- 10.Tchang B.G., et al. Pharmacologic treatment of overweight and obesity in adults. 2015;3:3–5. [Google Scholar]
- 11.Scheen A.J. The future of obesity: new drugs versus lifestyle interventions. Expert Opin Investig Drugs. 2008;17(3):263–267. doi: 10.1517/13543784.17.3.263. [DOI] [PubMed] [Google Scholar]
- 12.Smit-Fun V., Buhre W.F. The patient with chronic heart failure undergoing surgery. Curr Opin Anaesthesiol. 2016;29(3):391–396. doi: 10.1097/ACO.0000000000000335. [DOI] [PubMed] [Google Scholar]
- 13.Pi-Sunyer X., et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 14.Wilding J.P.H., et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 15.Jastreboff A.M., et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205–216. doi: 10.1056/NEJMoa2206038. [DOI] [PubMed] [Google Scholar]
- 16.Ghusn W., et al. Weight loss outcomes associated with semaglutide treatment for patients with overweight or obesity. JAMA Netw Open. 2022;5(9) doi: 10.1001/jamanetworkopen.2022.31982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haase C.L., et al. Use of liraglutide 3.0 mg for weight management in a real-world setting in Switzerland. Obes Facts. 2021;14(5):568–576. doi: 10.1159/000518325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghusn W., et al. Weight loss and cardiovascular disease risk outcomes of semaglutide: a one-year multicentered study. Int J Obes. 2024;48(5):662–667. doi: 10.1038/s41366-023-01456-5. [DOI] [PubMed] [Google Scholar]
- 19.Ghusn W., et al. The association between previous use of anti-obesity medication and semaglutide weight loss outcomes. Diabetes. Obes Metabol. 2024;26(6):2167–2175. doi: 10.1111/dom.15523. [DOI] [PubMed] [Google Scholar]
- 20.Ghusn W., et al. Weight loss outcomes with semaglutide based on diabetes severity using the individualized metabolic surgery score. eClinicalMedicine. 2024;72 doi: 10.1016/j.eclinm.2024.102625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pi-Sunyer X., et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 22.Wilding J.P.H., et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 23.Jastreboff A.M., et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205–216. doi: 10.1056/NEJMoa2206038. [DOI] [PubMed] [Google Scholar]
- 24.Bays H., et al. Liraglutide 3.0 mg for weight management: weight-loss dependent and independent effects. Curr Med Res Opin. 2017;33(2):225–229. doi: 10.1080/03007995.2016.1251892. [DOI] [PubMed] [Google Scholar]
- 25.Lincoff A.M., et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389(24):2221–2232. doi: 10.1056/NEJMoa2307563. [DOI] [PubMed] [Google Scholar]
- 26.Sodhi M., et al. Risk of gastrointestinal adverse events associated with glucagon-like peptide-1 receptor agonists for weight loss. JAMA. 2023;330(18):1795–1797. doi: 10.1001/jama.2023.19574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He L., et al. Association of glucagon-like peptide-1 receptor agonist use with risk of gallbladder and biliary diseases: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2022;182(5):513–519. doi: 10.1001/jamainternmed.2022.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shihab H.M., et al. Risk of pancreatic adverse events associated with the use of glucagon-like peptide-1 receptor agonist and dipeptidyl peptidase-4 inhibitor drugs: a systematic review and meta-analysis of randomized trials. World Journal of Meta-Analysis. 2015;3(6):254–283. [Google Scholar]
- 29.Pinto L.C., et al. Glucagon-like peptide-1 receptor agonists and pancreatic cancer: a meta-analysis with trial sequential analysis. Sci Rep. 2019;9(1):2375. doi: 10.1038/s41598-019-38956-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Storgaard H., et al. Glucagon-like peptide-1 receptor agonists and risk of acute pancreatitis in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19(6):906–908. doi: 10.1111/dom.12885. [DOI] [PubMed] [Google Scholar]
- 31.Arillotta D., et al. GLP-1 receptor agonists and related mental health issues; insights from a range of social media platforms using a mixed-methods approach. Brain Sci. 2023;13(11) doi: 10.3390/brainsci13111503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Z., et al. Hypoglycemia following the use of glucagon-like peptide-1 receptor agonists: a real-world analysis of post-marketing surveillance data. Ann Transl Med. 2021;9(18):1482. doi: 10.21037/atm-21-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterson S.C., Barry A.R. Effect of glucagon-like peptide-1 receptor agonists on all-cause mortality and cardiovascular outcomes: a meta-analysis. Curr Diabetes Rev. 2018;14(3):273–279. doi: 10.2174/1573399813666170414101450. [DOI] [PubMed] [Google Scholar]
- 34.Ghusn W., et al. Weight loss and cardiovascular disease risk outcomes of semaglutide: a one-year multicentered study. Int J Obes. 2024;48:662–667. doi: 10.1038/s41366-023-01456-5. [DOI] [PubMed] [Google Scholar]
- 35.Wang L., et al. Glucagon-like peptide 1 receptor agonists and 13 obesity-associated cancers in patients with type 2 diabetes. JAMA Netw Open. 2024;7(7) doi: 10.1001/jamanetworkopen.2024.21305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorgojo-Martínez J.J., et al. Clinical recommendations to manage gastrointestinal adverse events in patients treated with glp-1 receptor agonists: a multidisciplinary expert consensus. J Clin Med. 2022;12(1) doi: 10.3390/jcm12010145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gulak M.A., Murphy P. Regurgitation under anesthesia in a fasted patient prescribed semaglutide for weight loss: a case report. Can J Anaesth. 2023;70(8):1397–1400. doi: 10.1007/s12630-023-02521-3. [DOI] [PubMed] [Google Scholar]
- 38.Klein S.R., Hobai I.A. Semaglutide, delayed gastric emptying, and intraoperative pulmonary aspiration: a case report. Can J Anaesth. 2023;70(8):1394–1396. doi: 10.1007/s12630-023-02440-3. [DOI] [PubMed] [Google Scholar]
- 39.Marroquin-Harris M., Olesnicky B. Aspiration risk with glucagon-like peptide 1 (GLP-1) agonists. Anaesthesia. 2023;78(12):1524. doi: 10.1111/anae.16099. 1524. [DOI] [PubMed] [Google Scholar]
- 40.Sherwin M., et al. Influence of semaglutide use on the presence of residual gastric solids on gastric ultrasound: a prospective observational study in volunteers without obesity recently started on semaglutide. Can J Anaesth. 2023;70(8):1300–1306. doi: 10.1007/s12630-023-02549-5. [DOI] [PubMed] [Google Scholar]
- 41.Jones P.M., Hobai I.A., Murphy P.M. Anesthesia and glucagon-like peptide-1 receptor agonists: proceed with caution. Canadian Journal of Anesthesia/Journal canadien d'anesthésie. 2023;70(8):1281–1286. doi: 10.1007/s12630-023-02550-y. [DOI] [PubMed] [Google Scholar]
- 42.Hashash J.G., Thompson C.C., Wang A.Y. AGA rapid clinical practice update on the management of patients taking GLP-1 receptor agonists prior to endoscopy: communication. Clin Gastroenterol Hepatol. 2024;42:221–224. doi: 10.1016/j.cgh.2023.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Yao R., et al. Effect of glucagon-like PEPTIDE-1 receptor agonists on bowel preparation for colonoscopy. Am J Gastroenterol. 2023;119(6):1154–1157. doi: 10.14309/ajg.0000000000002564. [DOI] [PubMed] [Google Scholar]
- 44.Ruder K. As semaglutide’s popularity soars, rare but serious adverse effects are emerging. JAMA. 2023;32:112–114. doi: 10.1001/jama.2023.16620. [DOI] [PubMed] [Google Scholar]
- 45.McIntyre R.S., et al. The association between glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and suicidality: reports to the Food and Drug Administration Adverse Event Reporting System (FAERS) Expert Opin Drug Saf. 2024;23(1):47–55. doi: 10.1080/14740338.2023.2295397. [DOI] [PubMed] [Google Scholar]
- 46.Wang W., et al. Association of semaglutide with risk of suicidal ideation in a real-world cohort. Nat Med. 2024;30(1):168–176. doi: 10.1038/s41591-023-02672-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


