Abstract
Corticotropin-releasing factor (CRF) and the closely related family of neuropeptides urocortins (Ucns) are ancient paracrine-signaling peptides secreted in both the central and peripheral neural circuits. CRF and Ucns released from the CNS (central) regulate a plethora of physiological processes that include food intake, inflammation, and bowel motility and permeability. In the gastrointestinal tract, CRF actions are largely proinflammatory, whereas the effects of the Ucn subtypes can be either pro- or antiinflammatory. Central (intracerebroventricular) or peripheral (i.p.) administration of CRF or Ucns inhibits gastric emptying and promotes colonic motility. To ascertain the role of peripherally expressed CRF and UcnII in gastrointestinal inflammation and motility, we generated ileum-specific phenotypic knockouts of these peptides by using RNA interference. Long dsRNA effectively silenced basal expression of CRF and UcnII in ileum. Control dsRNA or saline treatment did not affect CRF or UcnII expression. In an experimental model of toxin-induced intestinal inflammation, inhibition of CRF ablated the inflammatory response (measured by epithelial damage, mucosal edema, and neutrophil infiltration). UcnII dsRNA treatment did not alter the inflammatory response to toxin. Furthermore, ileal motility was increased after site-specific inhibition of both CRF and UcnII. Thus, we demonstrate that ileal-specific CRF promotes inflammation and both CRF and UcnII modulate bowel motility.
Keywords: corticotropin-releasing factor, long dsRNA, RNA interference
Corticotropin-releasing factor (CRF) and urocortin (Ucn) I, II, and III (also termed “stresscopins”) are structurally related neuropeptides secreted in both the CNS (central) and peripheral neural circuits (1–3). CRF and Ucns are paracrine-signaling peptides involved in the response of an organism to environmental changes, contributing to, and possibly linking, endocrine, behavioral, autonomic, and immune responses (4). CRF and UcnII are expressed in numerous sites in the CNS. Some of the sites that coexpress both CRF and UcnII include the paraventricular nucleus, the cortex, and the amygdala (1, 5). CRF and Ucns are also expressed in peripheral tissues, but their complete expression profile is unknown. Recently, CRF and UcnI have been identified in the heart, spleen, and thymus (6). CRF is also present in nerve fibers of the submucosal and myenteric plexus of rat duodenum (7). CRF and Ucns act by means of two G protein-coupled receptors, CRF-R1 and CRF-R2. CRF and UcnI can bind both CRF-R1 and CRF-R2, whereas UcnII and UcnIII are highly selective for the CRF-R2 receptor (8).
Both physical and psychological stress induce central and peripheral expression of CRF and Ucns. Central secretion of CRF and Ucns is associated with anxiety coping behavior. CRF is also the main regulator of the hypothalamic–pituitary–adrenal axis, whereby psychological and physical stress is rapidly communicated to the adrenal cortex, triggering release of corticosterone. Corticosterone release mediates the antiinflammatory effects of central CRF. In contrast, peripherally expressed CRF is largely proinflammatory. Little is known about the role of either centrally or peripherally expressed UcnII in inflammation. In human gastric and colonic mucosa, UcnI is involved in the regulation of local inflammatory responses and can have both anti- and proinflammatory effects (9, 10).
In animal models, psychological stress leads to increased colonic propulsion and decreased gastric emptying. Exogenous administration of CRF/Ucns in rats mimics the motility abnormalities associated with stress (11, 12). In rats, the route of administration (central vs. peripheral) of CRF and Ucns is correlated with the effect on gastrointestinal (GI) motility. For example, in rats, centrally (intracerebroventricularly) administered CRF and UcnII are potent inhibitors of gastric emptying, whereas UcnI or UcnIII has minimal effects. In contrast, peripheral injection (i.p.) of UcnI or UcnII inhibits gastric emptying to a greater extent than CRF. In mice, central CRF is a potent promoter of colonic motility. UcnI and UcnII can also enhance colonic motility but to a lesser degree than CRF, whereas UcnIII appears to have no effect on colonic motility (11, 13). These findings suggest that CRF and Ucns closely link central stress and peripheral GI tract reactivity. Of note, many studies of CRF and its receptors in stress-mediated intestinal dysfunction have been carried out by using CRF/Ucn peptide or receptor antagonists. Because CRF and Ucns bind and activate both CRF-R1 and CRF-R2, these studies could not isolate discrete differences between the ligands.
CRF and Ucns are involved in stress-induced inflammatory responses. The GI tract is laden with commensal flora. The presence of a few pathogenic bacteria can adversely affect GI physiology. These effects include tissue damage, altered immune response, enhanced motility, and epithelial permeability. Clostridium difficile is a pathogen that causes a wide range of GI illnesses, ranging from mild diarrhea to life-threatening colitis. C. difficile produces two toxins, toxin A (TxA) (enterotoxin) and toxin B (cytotoxin), which are mainly responsible for tissue destruction (14). The acute inflammatory reaction elicited by C. difficile TxA has been associated with increased mucosal CRF, CRF-R1, and CRF-R2 expression. The inflammatory response to TxA can be blunted by CRF receptor antagonists (15, 16). A recent study demonstrated that, after TxA exposure, the Crh–/– mice had decreased ileal inflammation as compared with wild-type mice (17). In these experiments, distinction between the roles of central vs. peripheral CRF in the regulation of acute inflammation could not be discerned.
In this study, we determined the role of peripherally expressed CRF and UcnII in inflammation and bowel motility by inhibiting their basal expression in a site-specific fashion by using long dsRNA-mediated RNA interference (RNAi). First, we investigated their role in an experimental model of C. difficile TxA-induced inflammation by measuring the effects of TxA on tissue damage and intestinal fluid secretion. Second, we studied the effect of inhibition of CRF and UcnII on GI motility under basal or unstressed conditions by measuring the rate of passage of a locally administered fecal marker. Our results provide direct evidence that locally synthesized CRF is essential for initiation, augmentation, and perpetuation of acute GI inflammation after TxA exposure.
Materials and Methods
Animals and Experimental Design. Male Sprague–Dawley rats weighing 225–250 g (B & K, Fremont, CA, or Simonsen Laboratories, Gilroy, CA; design 1 and 2, respectively) were individually housed in hanging wire cages in temperature- and light-controlled rooms. Rats had ad libitum access to chow and water unless otherwise stated. All procedures were in accordance with the Committee on Animal Research at the University of California, San Francisco.
Cytokine Response to Naked Long dsRNA or Small Interfering RNA (siRNA) Treatment. There have been conflicting reports as to whether long dsRNA or siRNA (18–25 bp) evoke indiscriminate innate immune responses. Both types of dsRNA have been shown to induce expression of certain specific cytokines in cultured cells (18, 19). However, this response has not been measured in vivo. To address this question, we injected the ileal wall with 15 μg of either long dsRNA (>350–1,000 bp) or siRNA (20–25 bp) for CRF, UcnII, β-globin, or GFP. GFP and β-globin dsRNA served as nonspecific controls. Saline-injected rats served as additional controls. Three to 5 days after treatment, basal blood samples were collected by using the tail-nick method, and levels of 14 different cytokines were measured. In all subsequent experiments described below, long dsRNA was used.
Experimental Design 1: Inflammation Studies. Rats were injected with dsRNA in four treatment groups (n = 5–8): CRF, UcnII, GFP, and β-globin. Normal saline-injected rats served as additional controls. On day 0, rats were anesthetized with a mixture of ketamine and xylazine (75:1 mg per 100 g of body weight). The terminal ileum was identified and exteriorized. dsRNA (15 μg) was injected into the bowel wall of the distal ileum 3–4 cm proximal to the cecum. Ketoprofen (10 mg/kg s.c.) was given postoperatively. On day 4 or 6, a basal blood sample was taken by tail nick, and rats were fasted overnight with free access to water. The following morning, intestinal inflammation was induced by using the C. difficile TxA. Briefly, animals were anesthetized with isofluorane, the previous laparotomy incision was opened, and one 3- to 5-cm loop was created in the terminal ileum (encompassing the dsRNA injection site) (20). Tris·HCl buffer (50 mM, pH 7.4) or 5 μg of TxA in buffer was injected into the loop lumen in a 0.4-ml volume. Three hours later, rats were decapitated and trunk blood was collected. Ileal loops were removed. The weight and length of the loops and the volume of fluid secreted into the lumen were recorded. Ileal tissue was collected for histology, Western blotting, immunohistochemistry (IHC), and RNA isolation.
Experimental Design 2: Motility Studies. Rats were pretreated with long dsRNA as described in design 1. At the time of dsRNA injections, a silicon catheter (1.2 mm ID and 1.7 mm OD) was implanted 1 cm proximal to the injection site in the terminal ileum. The catheter was sutured to the ileal wall, s.c. tunneled, and exteriorized in the interscapular region. On days 3 and 4 after dsRNA treatment, 0.4 ml of Carmine red (1.2 g per 100 ml of saline) was injected into the catheter. Carmine red, a non-absorbable dye, is routinely used for measuring gut transit time in both humans and rodents (21–24). The number of times red mucus (or red-tinted fecal pellets) was excreted during a 5-h period after Carmine red injection was used to assess intestinal transit time and served as an indicator of intestinal motility.
Synthesis of dsRNA. Sense and antisense RNA were synthesized from cDNA inserts cloned in plasmid vectors by using a Mega-Script RNA kit (Ambion, Austin, TX) according to the manufacturer's specification and as described elsewhere (25). GFP and rat β-globin sequences were used as nonspecific dsRNA controls. siRNA was prepared by digesting 50 μg of long dsRNA with RNase III (Ambion), resulting in a pool of siRNA ranging from 18 to 25 bp in length.
cDNA Synthesis and RT-PCR. RNA was isolated from tissues homogenized in Stat60 buffer. Random hexamers were used to reverse transcribe cDNA from 1 μg of total RNA followed by a 30-cycle PCR with use of gene-specific primers. The resulting products were cloned into pTOPO vector (Invitrogen). UcnII-specific forward and reverse primer sequences corresponded to nucleotide numbers 13–31 and 365–384, respectively (GenBank accession no. AY044835). The N terminus of GFP was subcloned into pBlueScript SK vector after restriction enzyme digestion of the parent pEGFP plasmid sequences. CRF and β-globin plasmid constructs have been described elsewhere (26). RT-PCR using gene-specific primers was also used to assess the degree of mRNA degradation after dsRNA injections.
IHC and Western Analysis. Ileum or colon tissue was fixed in 4% paraformaldehyde and 30% sucrose, embedded in OCT compound (Tissue-Tek, Sakura Finetek, Torrance, CA), sectioned (4 μm), and thaw-mounted onto Superfrost Plus (Fisher, Pittsburgh) slides. CRF was localized by indirect immunofluorescence (FITC) as described (26). Protein extraction, polyacrylamide gel electrophoresis, and Western blot analysis were performed as described elsewhere (27). Antibodies for CRF receptors (Santa Cruz Biotechnology) were used at a dilution of 1:1,000. Secondary antibody (donkey anti-goat) was diluted in 5% nonfat milk (1:4,000). β-Actin (1:4,000, Sigma) antibody was used for normalization.
Histological Evaluation. Ileal tissue was stained with hematoxylin/eosin. The severity of inflammation was scored in a blinded fashion (by E.F.G.). The degree of (i) epithelial damage, (ii) hemorrhagic congestion and mucosal edema, and (iii) neutrophil infiltration was assessed as described (28). Each parameter was given a score of 0–7. Histological damage was calculated by adding scores from all these parameters.
Measurement of Plasma Hormones and Cytokines. Blood samples were centrifuged at 1,000 × g for 15 min at 4°C, and the plasma was aliquoted and stored at –80°C. Corticosterone levels (μg/dl) were measured by using an RIA kit (ICN) as described (29). An ELISA was used to measure circulating cytokine levels by using a multiplex rat cytokine panel at Linco Laboratories (St. Louis).
Statistical Analysis. Data were first analyzed by one-way ANOVA. A significant (P < 0.05) global effect of ANOVA was followed by post hoc tests of individual group differences (Fisher's probable least-squares difference test).
Results
Inhibition of Basal CRF and UcnII in Rat Ileum by dsRNA and Cytokine Response to dsRNA.
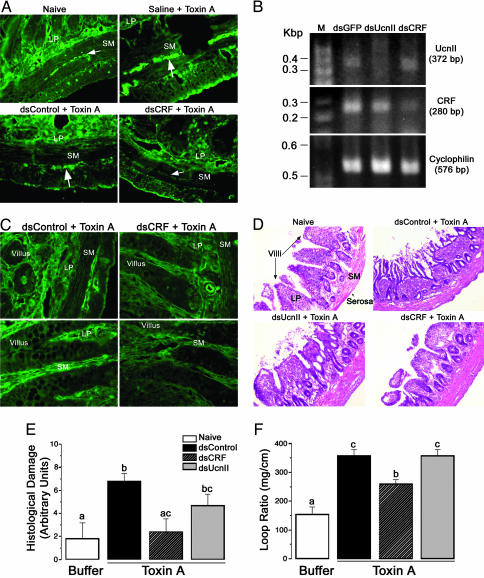
The expression pattern of CRF along the GI tract under basal or unstressed condition is incomplete. By using IHC, we determined that CRF is expressed in the nerve fibers of the submucosal plexus of the ileum (Fig. 1A), jejunum, and duodenum, but not the colon (data not shown). In the ileum, low levels of CRF were detected in the lamina propria and Paneth cells. No CRF was detectable in the epithelial cells (Fig. 1 A Left). Long dsRNA for CRF (dsCRF) and UcnII (dsUcnII) injected into the muscle wall effectively inhibited expression of basal CRF and UcnII mRNAs. By using RT-PCR, we first established that dsRNA effectively inhibited their cognate mRNAs. RT-PCR products for CRF or UcnII in groups treated with dsRNA for CRF or UcnII, respectively, were either very low or undetectable 5 days after dsRNA treatment (Fig. 1B). Both CRF and UcnII products were present in the GFP long dsRNA (dsGFP) group. Furthermore, UcnII mRNA was present in the dsCRF group and vice versa. Cyclophilin, a housekeeping gene, was detectable by RT-PCR in all three groups, further confirming that the lack of CRF or UcnII cDNA products in their respective dsRNA groups was due to specific degradation of cognate mRNA by RNAi (Fig. 1B). Next, we showed that, in addition to inhibiting mRNA, CRF dsRNA effectively silenced CRF protein expression in the nerve fibers of the submucosal plexus 5–7 days after dsRNA treatment as assessed by IHC. In control animals (dsGFP and saline), CRF expression was similar to that observed in naïve animals (Fig. 1 A). Thus, treatment with dsRNA effectively achieved an ileal-specific silencing of CRF or UcnII peptides.
Fig. 1.
CRF and UcnII inhibition by RNAi in rat ileum. Groups of rats (n = 4–6) were treated with long dsRNA for CRF (dsCRF), UcnII (dsUcnII), or nonspecific control (dsGFP) for 5–7 days. (A) CRF peptide is undetectable by IHC in the nerve fibers (arrows) of the submucosal (SM) plexus of rats treated with dsRNA for CRF, whereas other treatment groups continued to express CRF in the ileum. (B) Results from RT-PCR reveal that mRNA for CRF or UcnII is significantly inhibited by RNAi treatment, with no UcnII cDNA product amplifiable in dsUcnII groups or very low levels of CRF cDNA product in dsCRF group. Cyclophilin, a housekeeping gene, was detectable in all three groups, and its expression was unaffected by RNAi treatment. (A and C) CRF expression is induced 3 h after TxA treatment in the submucosa and lamina propria (LP). This induction is specifically blocked by 6 days of pretreatment with long dsRNA against CRF. CRF, but not UcnII, is proinflammatory. (D and E) TxA treatment markedly destroys tissue morphology in control or UcnII dsRNA groups (n = 4–6) as assessed by epithelial damage, edema, and neutrophil infiltration. Inhibition of CRF by dsRNA resulted in significant protection of tissue damage that appeared similar to buffer-treated naïve animals. ANOVA indicated a global significant effect [F (3, 12) = 5.5; P < 0.02]. (F) Reduced tissue damage also resulted in reduced fluid secretion in dsCRF animals as a measured loop ratio (weight of the loop/length). ANOVA indicated a global effect [F (4, 17) = 8; P < 0.001]. Different letters denote statistically significant differences among groups (P < 0.01). [Magnification: ×10 (A and D) and ×40 (C).]
We next established that treatment with either long dsRNA or siRNA in vivo did not elicit a nonspecific systemic immune response. Levels of 8 of 14 cytokines were within normal range in all treatment groups (Table 1). Treatment with siRNA resulted in a greater variability within the group than that seen with long dsRNA treatment (Table 1); however, the differences between the groups were not statistically significant. Effects of siRNA on inhibition of protein were shorter in duration as compared with long dsRNA (maximum of 4 days vs. 7–8 days, respectively). Because effects of long dsRNA persisted for a longer duration, we used long dsRNA for the studies reported here.
Table 1. Cytokine profile after long dsRNA or siRNA treatment.
| Treatment | GM-CSF* | IL-1α | MCP-1 | IL-2 | IL-6 | IL-12p70 | IFN-γ | IL-18 | GRO/KC |
|---|---|---|---|---|---|---|---|---|---|
| Naïve | <24.5 | <24.5 | 173.3 ± 63.8 | <24.5 | <24.5 | <24.5 | <24.5 | 186.3 ± 88.4 | 221.5 ± 49.7 |
| dsControl | <24.5 | <24.5 | 212.0 ± 37.8 | 75.0 ± 67.3 | 32.5 ± 1.1 | <24.5 | 61.3 ± 44.6 | 568.9 ± 476.1 | 224.5 ± 124.2 |
| dsCRF | <24.5 | 58.7 ± 45.6 | 171.3 ± 41.2 | 42.5 ± 12.0 | 300.8 ± 184.2 | 37.2 ± 16.9 | 117.5 ± 62.0 | 114.5 ± 65.0 | 223.8 ± 109.6 |
| dsUcnII | <24.5 | 43.3 ± 25.1 | 155.2 ± 26.1 | <24.5 | 46.2 ± 28.9 | 38.3 ± 18.4 | 90.7 ± 76.6 | 66.0 ± 30.7 | 115.5 ± 45.7 |
| siCRF | <24.5 | 259.4 ± 236.8 | 284.5 ± 61.0 | 46.9 ± 28.3 | 439.3 ± 375.8 | 32.5 ± 9.3 | 131.4 ± 54.6 | 311.3 ± 92.5 | 254.3 ± 49.6 |
| siControl | <24.5 | <24.5 | 212.0 ± 28.7 | 28.8 ± 0.4 | 32.5 ± 10.7 | <24.5 | 25.7 ± 1.6 | 243.3 ± 113.1 | 225.3 ± 52.4 |
Cytokine concentrations in basal plasma sample were measured (pg/ml). Long dsRNA or siRNA treatment had either no effect or a similar effect on cytokine levels (±SEM). si, small interfering; MCP, monocyte chemotatic protein 1; GRO/KC; KC is a chemokine and GROα is the murine homologue.
Levels of 8 of 14 cytokines (GM-CSF, IL-1β, IL-4, IL-5, IL-10, IL-12p70, and TNF-α) were within normal (basal) range (<24.5 pg/ml).
Inhibition of Basal CRF but Not UcnII Ablates TxA-Induced Acute Tissue Damage. To determine the precise role of peripherally synthesized CRF and UcnII in TxA-induced inflammation, dsRNA for CRF, UcnII, or GFP was injected into the ileal wall 4–6 days before TxA exposure. As anticipated, TxA induced CRF peptide expression in the nerve fibers of the submucosa, lamina propria, and the myenteric plexus in all treatment groups (Fig. 1 A). As predicted, CRF dsRNA treatment effectively prevented this induction of CRF expression in TxA-treated rats (Fig. 1 A and C). Interestingly, not only was CRF expression inhibited by dsCRF, but also the degree of ileal inflammation was markedly decreased in these animals. Blind numerical scoring of the degree of epithelial damage, tissue edema, and neutrophil infiltration of hematoxylin/eosin-stained sections demonstrated that dsCRF-treated animals resembled buffer controls (Fig. 1 D and E). In contrast to dsCRF, dsUcnII did not reduce inflammation (Fig. 1D). The severity of tissue damage paralleled ileal loop fluid secretion (expressed as a loop ratio). dsCRF animals had significantly decreased fluid secretion as compared with dsUcnII and dsGFP control (Fig. 1F).
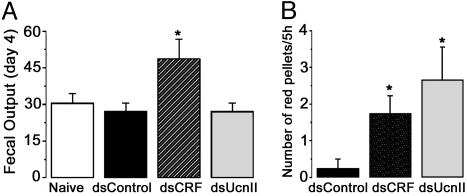
Inhibition of CRF and UcnII Increases Ileal Motility Under Basal Conditions. Peripheral CRF- and UcnII-related mechanisms are known to contribute to intestinal motility (29, 30). Silencing of CRF, but not UcnII, increased the total number of fecal pellets excreted over 24 h as measured daily for 4 days after dsRNA injections [ANOVA: F (2, 13) = 4.7; P < 0.03, Fig. 2A]. Interestingly, the total dry weight of fecal pellets collected in a 24-h period was similar in all treatment groups (data not shown). The increase in the number of pellets was more predominant in the lights-on period and before the diurnal rise of endogenous corticosterone concentrations. To further elucidate diurnal effects of corticosterone on ileal motility, animals were preinjected with dsRNA and implanted with ileal catheters (design 2). On the morning of days 3 and 4, Carmine red dye was injected into the catheter, and the intestinal transit time and fecal frequency were monitored for 5 h. In contrast to cumulative fecal output, both dsCRF and dsUcnII groups displayed an increased number of red mucus/pellets excreted in a 5-h period as compared with dsGFP controls [ANOVA: F (2, 8) = 5.2; P < 0.04; Fig. 2B]. No abnormal luminal fluid accumulation was discernible at the time of death, and hematoxylin/eosin staining displayed normal ileal histology in all groups. Enhanced expulsion of red dye was attributed to increased propulsion activity of ileum due to inhibition of basal CRF or UcnII.
Fig. 2.
CRF and UcnII inhibition modulate intestinal motility. (A) Inhibition of CRF, and not UcnII, results in cumulative increase in fecal output over 24 h. Fecal pellets were counted daily for up to 4 days after dsRNA treatment. In contrast to CRF, UcnII displays circadian rhythm in exerting effects on ileal motility. (B) Assessment of the number of times red mucus or red-tinted pellets were expelled within a 5-h time frame (soon after lights on and just before the circadian rise in endogenous corticosteroid levels) indicates that ileal motility is enhanced by inhibition of both CRF and UcnII in this period.
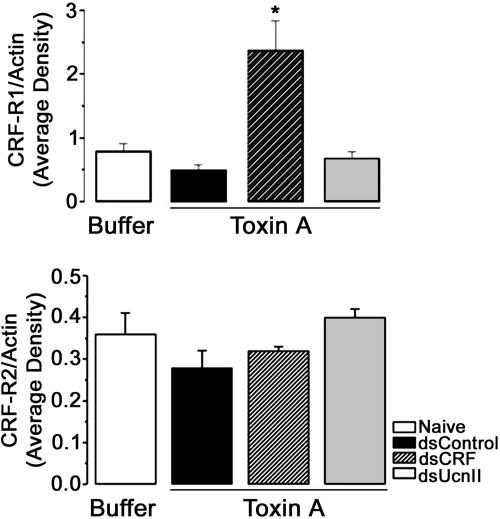
CFR Receptors Are Differentially Regulated by CRF and UcnII. We investigated whether specific inhibition of either CRF (that acts predominantly through CRF-R1) or UcnII [acting specifically through CRF-R2 (8)] would lead to increased expression of CRF-R1 or CRF-R2 as a means of compensation for the loss of ligand after TxA treatment. Western blot analysis of ileal tissue from buffer- or TxA-treated animals shows specific up-regulation of CRF-R1 receptor in rats treated with dsCRF (Fig. 3). Expression of CRF-R2 was similar in all treatment groups.
Fig. 3.
Effect of down-regulation of ligands on their (CRF) receptors. After TxA administration, CRF-R1 protein expression is specifically up-regulated in rats pretreated with dsRNA against CRF, whereas that of CRF-R2 is not different (as determined by Western blot analysis; n = 4–6 per group).
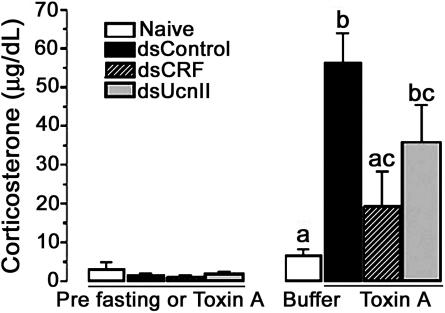
Plasma Corticosterone (B) Response to TxA Is Blunted in dsCRF-Treated Animals. To examine the effects of TxA-mediated inflammation on B release, we measured plasma B levels before and after exposure to TxA. Basal or pre-TxA concentrations of B were within normal range in all treatment groups. TxA exposure increased plasma B levels in dsUcnII and control animals, but not in dsCRF-treated animals, as compared with buffer-injected controls (Fig. 4).
Fig. 4.
CRF or UcnII dsRNA differentially alters TxA-mediated rise in corticosterone (B) concentrations. Basal prefasting or pre-TxA B concentrations were not significantly different among the groups. TxA treatment, however, increased B concentrations significantly in UcnII and control dsRNA-treated rats [ANOVA: F (3, 14) = 7.58; P < 0.01]. B levels in CRF dsRNA-treated rats were not significantly elevated or different from naïve buffer-treated controls. Different letters denotes statistically significant differences.
Discussion
Our results provide direct evidence that (i) both long dsRNA and siRNA are effective in silencing gene expression in peripheral tissues and neither evokes a nonspecific immune response in vivo, (ii) both CRF and UcnII are synthesized locally in the ileum under basal conditions, (iii) ileal-specific CRF is essential for propagation of proinflammatory effects of C. difficile TxA, and (iv) local CRF and UcnII modulate ileal motility.
We generated site-specific “knockouts” of CRF or UcnII in the ileum by using RNAi. RNAi acts by targeting and degrading mRNA. Hence, for RNAi to be effective, both CRF and UcnII must be synthesized at the site of RNAi delivery. RNAi treatment did not have nonspecific effects on expression of other genes because expression of cyclophilin was evident and unaltered in all treatment groups. Furthermore, expression of UcnII, structurally related to CRF, was evident in the dsCRF group and vice versa. Expression of CRF-R1 in the cecum from all treatment groups was unaffected (data not shown). These results argue that effects of RNAi were confined to terminal ileum (an area of ≈5–6 cm) and RNAi did not affect the adjacent parts of the intestine.
Knowledge of the peripheral distributions and functions of CRF and Ucn isoforms is incomplete. The presence of CRF-immunoreactive peptide within nerve fibers of the myenteric plexus, submucosal blood vessels, and lamina propria of rat duodenum has been reported (7). We now show that CRF and UcnII are expressed in the nerve fibers of the ileal submucosa, myenteric plexus, and lamina propria under basal conditions.
Interestingly, dsCRF also confirmed a potent effect of local CRF inhibition on acute inflammation. Even when dsCRF injection preceded TxA exposure by up to 7 days, there was no histologic evidence of inflammation, and ileal fluid secretion was reduced 3 h after TxA exposure (the time of killing) in the dsCRF-treated animals. Other reports show that intestinal inflammation is associated with changes in the local expression and signaling of CRF and its receptors (16). Macrophages and lymphocytes are known to synthesize CRF in response to stress (29, 30). Thus, the possibility exists that the local inflammatory cells were also exposed to dsCRF during the ileal muscle layer injection, contributing to the absence of an inflammatory reaction. Thus, CRF appears to be pivotal for the initiation and/or propagation of acute inflammation after ileal TxA exposure. In contrast to CRF, specific UcnII down-regulation did not alter the course of TxA-induced inflammation. It has been reported that peripheral administration of Ucn is more effective in suppressing inflammation and cytokine release than CRF (31). Thus, the final outcome of activation of CRF/CRF-R1 or UcnII/CRF-R2 paracrine circuit on inflammation appears to be highly organ/tissue-specific and context-dependent.
Expression of CRF-R1 and CRF-R2 mRNA levels is increased (as assessed by RT-PCR) in response to TxA-mediated ileitis in CRF-intact animals (16). By using RNAi, it was possible to directly address the response of these receptors in TxA-challenged tissue in the absence of ileal CRF or UcnII. TxA treatment induced CRF-R1 receptor expression specifically in the dsCRF-preinjected animals. Surprisingly, CRF-R2 receptor levels were not significantly different in any treatment group, although they tended to be low in dsCRF and high in dsUcnII animals. These results suggest that the differential up-regulation of CRF R1/R2 may also depend on their ligand rather than TxA treatment as suggested (16). In this regard, CRF and UcnI have been shown to regulate transcription of CRF-R1 in a positive-feedback loop in both central and peripheral cells (32, 33). It is also possible that the message up-regulation for CRF-R2 precedes increase in protein levels. Further investigation is required to understand the regulation of receptor expression during inflammation.
Corticosterone (B) is released in response to stress and pain and has been suggested to reduce GI inflammatory response to TxA treatment (34). In contrast, Crh–/– mice, which are unable to hypersecrete B in response to stress, also have decreased inflammatory response to TxA (17). Before initiation of TxA treatment, rats were fasted overnight, and fasting is known to increase plasma B levels (35). In our study, ileal-specific CRF or UcnII down-regulation did not alter this B response to fasting (data not shown); thus, it seems unlikely that B levels at the time of TxA administration were different. After TxA, B levels correlated with the extent of tissue damage over time in all animals, most likely because the decreased severity of inflammation in the dsCRF animals decreased their abdominal pain and systemic stress. Moreover, peripheral release of proinflammatory cytokines can also directly or indirectly (by means of the hypothalamic–pituitary–adrenal axis) increase B release from the adrenals, and release of these cytokines may be altered after TxA treatment.
Ileal-specific inhibition of CRF resulted in increased number of fecal pellets over 24 h. The difference in number of pellets excreted between controls and CRF or UcnII dsRNA-treated animals was more pronounced during the morning phase, corresponding to the period when circulating B levels are at their nadir. This finding is not surprising because CRF, Ucn I, and UcnII expression is regulated by B (36, 37). It appears that CRF or CRF-dependent circuits in the ileum act to decrease local motility, which is in contrast to its effect on colonic motility (11, 13). Thus, CRF and UcnII effects on GI motility can be either inhibitory or stimulatory, depending on the region of GI tract and the specific pathways (signals to musculature vs. neurons) that are activated or repressed (17, 38, 39). Further study of the signals that interact with CRF and Ucn will help resolve this issue.
In summary, we show that both CRF and UcnII are synthesized locally in the terminal ileum, and inhibition of CRF by RNAi protects ileal tissue from tissue damage against TxA.
Local release of CRF may also be critical for activation of stress response in the local immune cells. This finding suggests that the local release of CRF after stress may trigger, as well as exacerbate, an inflammatory response, perhaps contributing to the pathophysiology of inflammatory bowel disease.
Acknowledgments
We thank Dr. C. Pothoulakis (Harvard Medical School, Boston) for his generous gift of C. difficile TxA and for his invaluable advice and useful discussions. We thank Dr. W. Vale (The Salk Institute, La Jolla, CA) for his gift of the CRF antibody and Dr. M. Million (University of California, Los Angeles) for his assistance in setting up motility studies. We thank Drs. M. F. Dallman and M. Adams (University of California, San Francisco) for critical reading of the manuscript.
Author contributions: S.E.l.F., E.C.W., and A.B. designed research; S.E.l.F., E.C.W., P.S.I., E.F.G., and A.B. performed research; A.B. contributed new reagents/analytic tools; S.E.l.F., E.C.W., P.S.I., E.F.G., and A.B. analyzed data; and S.E.l.F., E.C.W., and A.B. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CRF, corticotropin-releasing factor; Ucn, urocortin; RNAi, RNA interference; GI, gastrointestinal; TxA, toxin A; siRNA, small interfering RNA; IHC, immunohistochemistry.
See Commentary on page 7409.
References
- 1.Reyes, T. M., Lewis, K., Perrin, M. H., Kunitake, K. S., Vaughan, J., Arias, C. A., Hogenesch, J. B., Gulyas, J., Rivier, J., Vale, W. W. & Sawchenko, P. E. (2001) Proc. Natl. Acad. Sci. USA 98, 2843–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaughan, J., Donaldson, C., Bittencourt, J., Perrin, M. H., Lewis, K., Sutton, S., Chan, R., Turnbull, A. V., Lovejoy, D., Rivier, C., et al. (1995) Nature 378, 287–292. [DOI] [PubMed] [Google Scholar]
- 3.Lewis, K., Li, C., Perrin, M. H., Blount, A., Kunitake, K., Donaldson, C., Vaughan, J., Reyes, T. M., Gulyas, J., Fischer, W., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 7570–7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grigoriadis, D. E., Lovenberg, T. W., Chalmers, D. T., Liaw, C. & De Souze, E. B. (1996) Ann. N.Y. Acad. Sci. 780, 60–80. [DOI] [PubMed] [Google Scholar]
- 5.Swanson, L. W., Sawchenko, P. E., Rivier, J. & Vale, W. W. (1983) Neuroendocrinology 36, 165–186. [DOI] [PubMed] [Google Scholar]
- 6.Baigent, S. M. & Lowry, P. J. (2000) J. Mol. Endocrinol. 25, 43–52. [DOI] [PubMed] [Google Scholar]
- 7.Wolter, H. J. (1984) Biochem. Biophys. Res. Commun. 122, 381–387. [DOI] [PubMed] [Google Scholar]
- 8.Coste, S. C., Murray, S. E. & Stenzel-Poore, M. P. (2001) Peptides (Tarrytown, NY) 22, 733–741. [DOI] [PubMed] [Google Scholar]
- 9.Chatzaki, E., Charalampopoulos, I., Leontidis, C., Mouzas, I. A., Tzardi, M., Tsatsanis, C., Margioris, A. N. & Gravanis, A. (2003) J. Clin. Endocrinol. Metab. 88, 478–483. [DOI] [PubMed] [Google Scholar]
- 10.Kohno, M., Kawahito, Y., Tsubouchi, Y., Hashiramoto, A., Yamada, R., Inoue, K. I., Kusaka, Y., Kubo, T., Elenkov, I. J., Chrousos, G. P., et al. (2001) J. Clin. Endocrinol. Metab. 86, 4344–4352. [DOI] [PubMed] [Google Scholar]
- 11.Martinez, V., Wang, L., Rivier, J. E., Vale, W. & Tache, Y. (2002) J. Pharmacol. Exp. Ther. 301, 611–617. [DOI] [PubMed] [Google Scholar]
- 12.Million, M., Maillot, C., Saunders, P., Rivier, J., Vale, W. & Tache, Y. (2002) Am. J. Physiol. 282, G34–G40. [DOI] [PubMed] [Google Scholar]
- 13.Martinez, V., Wang, L., Million, M., Rivier, J. & Tache, Y. (2004) Peptides (Tarrytown, NY) 25, 1733–1744. [DOI] [PubMed] [Google Scholar]
- 14.Wilkins, T. D. (1987) Gastroenterology 93, 389–391. [DOI] [PubMed] [Google Scholar]
- 15.Kawahito, Y., Sano, H., Mukai, S., Asai, K., Kimura, S., Yamamura, Y., Kato, H., Chrousos, G. P., Wilder, R. L. & Kondo, M. (1995) Gut 37, 544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wlk, M., Wang, C. C., Venihaki, M., Liu, J., Zhao, D., Anton, P. M., Mykoniatis, A., Pan, A., Zacks, J., Karalis, K. & Pothoulakis, C. (2002) Gastroenterology 123, 505–515. [DOI] [PubMed] [Google Scholar]
- 17.Anton, P. M., Gay, J., Mykoniatis, A., Pan, A., O'Brien, M., Brown, D., Karalis, K. & Pothoulakis, C. (2004) Proc. Natl. Acad. Sci. USA 101, 8503–8508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sledz, C. A., Holko, M., de Veer, M. J., Silverman, R. H. & Williams, B. R. (2003) Nat. Cell Biol. 5, 834–839. [DOI] [PubMed] [Google Scholar]
- 19.Bridge, A. J., Pebernard, S., Ducraux, A., Nicoulaz, A. L. & Iggo, R. (2003) Nat. Genet. 34, 263–264. [DOI] [PubMed] [Google Scholar]
- 20.Castagliuolo, I., Keates, A. C., Wang, C. C., Pasha, A., Valenick, L., Kelly, C. P., Nikulasson, S. T., LaMont, J. T. & Pothoulakis, C. (1998) J. Immunol. 160, 6039–6045. [PubMed] [Google Scholar]
- 21.Monnikes, H., Schmidt, B. G. & Tache, Y. (1993) Gastroenterology 104, 716–723. [DOI] [PubMed] [Google Scholar]
- 22.Read, N. W., Miles, C. A., Fisher, D., Holgate, A. M., Kime, N. D., Mitchell, M. A., Reeve, A. M., Roche, T. B. & Walker, M. (1980) Gastroenterology 79, 1276–1282. [PubMed] [Google Scholar]
- 23.Enck, P., Merlin, V., Erckenbrecht, J. F. & Wienbeck, M. (1989) Gut 30, 455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McClure, R. J. & Newell, S. J. (1999) Arch. Dis. Child. 80, F54–F58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhargava, A., Dallman, M. F., Pearce, D. & Choi, S. (2004) Brain Res. Brain Res. Protoc. 13, 115–125. [DOI] [PubMed] [Google Scholar]
- 26.Bhargava, A., Fullerton, M. J., Myles, K., Purdy, T. M., Funder, J. W., Pearce, D. & Cole, T. J. (2001) Endocrinology 142, 1587–1594. [DOI] [PubMed] [Google Scholar]
- 27.Pothoulakis, C., Castagliuolo, I., LaMont, J. T., Jaffer, A., O'Keane, J. C., Snider, R. M. & Leeman, S. E. (1994) Proc. Natl. Acad. Sci. USA 91, 947–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhargava, A., Meijer, O. C., Dallman, M. F. & Pearce, D. (2000) J. Neurosci. 20, 3129–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karalis, K., Sano, H., Redwine, J., Listwak, S., Wilder, R. L. & Chrousos, G. P. (1991) Science 254, 421–423. [DOI] [PubMed] [Google Scholar]
- 30.Stephanou, A., Jessop, D. S., Knight, R. A. & Lightman, S. L. (1990) Brain Behav. Immun. 4, 67–73. [DOI] [PubMed] [Google Scholar]
- 31.Agnello, D., Bertini, R., Sacco, S., Meazza, C., Villa, P. & Ghezzi, P. (1998) Am. J. Physiol. 275, E757–E762. [DOI] [PubMed] [Google Scholar]
- 32.Konishi, S., Kasagi, Y., Katsumata, H., Minami, S. & Imaki, T. (2003) Endocr. J. 50, 21–36. [DOI] [PubMed] [Google Scholar]
- 33.Parham, K. L., Zervou, S., Karteris, E., Catalano, R. D., Old, R. W. & Hillhouse, E. W. (2004) Endocrinology 145, 3971–3983. [DOI] [PubMed] [Google Scholar]
- 34.Castagliuolo, I., Karalis, K., Valenick, L., Pasha, A., Nikulasson, S., Wlk, M. & Pothoulakis, C. (2001) Am. J. Physiol. 280, G539–G545. [DOI] [PubMed] [Google Scholar]
- 35.Dallman, M. F., Akana, S. F., Bhatnagar, S., Bell, M. E., Choi, S., Chu, A., Horsley, C., Levin, N., Meijer, O., Soriano, L. R., et al. (1999) Endocrinology 140, 4015–4023. [DOI] [PubMed] [Google Scholar]
- 36.Chen, A., Vaughan, J. & Vale, W. W. (2003) Mol. Endocrinol. 17, 1622–1639. [DOI] [PubMed] [Google Scholar]
- 37.Kageyama, K., Bradbury, M. J., Zhao, L., Blount, A. L. & Vale, W. W. (1999) Endocrinology 140, 5651–5658. [DOI] [PubMed] [Google Scholar]
- 38.Heitkemper, M. M. & Marotta, S. F. (1983) Am. J. Physiol. 244, G58–G64. [DOI] [PubMed] [Google Scholar]
- 39.Oriaku, E. T. & Soliman, K. F. (1986) Arch. Int. Pharmacodyn. Ther. 280, 136–144. [PubMed] [Google Scholar]