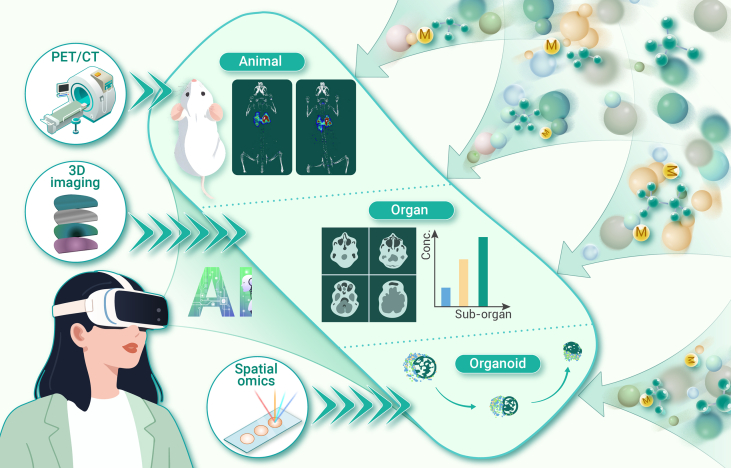
Vector effect of microplastic particles
The microplastic pollution issue is infamous for its enormous multidimensionality and the intricate combination arising from plastics themselves and “plastic-associated chemicals.” Interactions between microplastic particles with multiple chemicals can affect biological health and alter the toxicity of the primary environmental pollutants. These complex combinations raise great challenges to experimental design for toxicity evaluation, going beyond the investigation of individual or dual contaminations. Systematic research and an integrated visualization strategy are needed to decipher these biochemical processes and molecular mechanisms.1,2
The role of microplastic particles as vectors for the cycling and bioaccumulation of plastic-associated chemicals, especially hydrophobic organic chemicals, is a topic of much debate. Scientists propose the “microplastics vector effect,” namely that the desorption of chemicals from microplastics after particle uptake may promote chemical exposure and pose chemical risks to various organisms. These chemicals involve sorbed environmental pollutants (POPs, bisophenols, metals, etc.) and additives (brominated flame retardant, etc.). However, further ample evidence is required to clarify the transfer vector effect of microplastics into animals or for plastic additives like flame retardants. First, experimental designs need to consider the total chemical exposure from both the transport or uptake of microplastics and other parallel ingestion pathways. Second, a realistic chemical concentration gradient between plastics and organisms should be considered because these exogenous additives are subject to obvious dilution in the natural environment. More specifically, it would be inappropriate to investigate potential toxicity effects using in vitro models with extracts exhibiting a high plastic-to-water ratio. Third, it will be best to comprehensively evaluate the enrichment characteristics of microplastic particles and plastic-associated chemicals at the (sub-)organ level, including spatiotemporal distribution, and the correlation between contents and distributions. Above all, multiple factors should be taken into account in plastics analysis from different in vivo and in vitro models, including the dilution of pollutants, ingestion from various media, and spatial distribution.
Microplastome and its visualizations
To trans-spatiotemporally compare microplastic features and potential toxicity, it is necessary to consider their complexity and multidimensionality. For example, a similar composition of microplastics can reveal the homogeneity of their surrounding environmental conditions. A significant correlation between changes in the composition and content of microplastics from different specimens can indicate a variety of information. This correlation can demonstrate the potential transfer or exchange of microplastics between two sampling points for environmental specimens and also reveal the organ enrichment and uptake characteristics of microplastics within the organ network of model organisms. Most noteworthy, scientists propose the idea of the “microplastome,” defined as the collection of all plastic particles (<5 mm) and their related substances (such as chemicals and microbes). Scientists also propose holistic quantification and characterization in microplastics analysis to disclose their differences, connections, and effects in different ecosystem components.3 From this foundation, microplastome visualizations are proposed to provide the most direct evidence for investigating multiple barrier impairments and the occurrence of diseases induced by microplastic particles (Figure 1).
Figure 1.
Take a typical organ and cell imaging for example to clarify microplastome visualizations
vivo imaging of diesel exhaust particulates in mice. Some images are reprinted from Lee et al.4 with permission from The Royal Society of Chemistry.
Spatiotemporal distribution of pollutants
The key issue with respect to microplastics is determining the concentration and extent to which they cause hazard to human health and the environment. To accurately estimate the potential risks of these particles, spatial distribution, enrichment, and translocation of microplastics must first be investigated. Some approaches have been applied for the qualitative and quantitative analyses of microplastics in biological specimens, including pyrolysis gas chromatography coupled with mass spectrometry (MS), liquid chromatography coupled with tandem MS, surface-enhanced Raman spectroscopy, etc. However, these ex vivo approaches cannot track the real-time transport behavior and in vivo biodistribution of microplastics. Although in vivo fluorescence imaging has been used to track microplastics in mice or cellular samples, this semi-quantitative approach is limited by its throughput. Above all, it is urgent to explore novel imaging approaches to visualize the organ distribution of microplastics and analyze their transfer fate.
Spatiotemporal variations and quantitative analysis of microplastics have an important role in revealing the potential toxicity of microplastics and plastic-associated chemicals across different exposure models, particularly to offspring health following prenatal exposure to pollutants. Noninvasive imaging techniques, such as in vivo positron emission tomography (PET) and single-photon emission computed tomography (SPECT), have been used to monitor the biodistribution of radiolabeled microplastics in different model organisms at the spatiotemporal level. For example, polystyrene micro-/nano-particles with amine-coated surfaces (positively charged and hydrophilic at physiological pH) can be modified with a DFO or DOTA chelator (negatively charged and hydrophilic) and radiolabeled with metallic radionuclides (such as 89Zr and 64Cu) to yield [89Zr]Zr-DFO-PS or [64Cu]Cu-DOTA-PS, respectively. Upon administration to mice, the spatial distributions of these radioplastics are tracked using PET, ultimately describing their in vivo behavior and quantitative variations. The results demonstrate that [64Cu]Cu-DOTA-PS accumulates in the liver and intestine within 1 h post-ingestion, while [89Zr]Zr-DFO-PS accumulates in the gastrointestinal tract and is eliminated through feces within 48 h post-ingestion. In addition, different administration approaches can simulate how microplastics enter model organisms.
Risk assessment
Exposure to microplastic particles causes a series of “omics” changes in organs, including the genome, epigenome, proteome, lipidome, and metabolome. Deciphering the spatiotemporal heterogeneity of omics data in (sub-)organs is the key to investigating pollutants’ potential toxicity. Biological information indicating the exposure risk is screened out and used as a toxic biomarker of pollutant exposure to evaluate the overall decomposition, digestion, and detoxification of pollutants in various organs. An integrated imaging method involving spatial omics and histomorphology is introduced to explore the organ toxicity of pollutants.5,6
To characterize the biodistribution and toxicity of microplastics simultaneously, we propose an in vivo imaging-guided MS technique to achieve the spatial distribution profiling of exogenous (such as microplastics and plastic-associated chemicals) and endogenous (such as metabolites, lipids, proteins, and peptides from different organs exposed to microplastics) substance molecules. Take microplastics-induced immunotoxicity for example; high-resolution reconstructions of multiple markers including protein and metabolites on tissue sections are obtained by using imaging mass cytometry and MS imaging after PET imaging. Then, the subsets of immune cells associated with microplastic exposure are screened, and the relationship between the immune cell phenotype and cellular metabolism is constructed to deduce the immunotoxicity characteristics caused by microplastic exposure.
Organ network for toxicity analysis
The rapid development of imaging technology has made it easier for us to achieve large amounts of visualizing data associated with the environmental and biological exposure of microplastics. It is imperative to use massive data to conduct microplastic analysis with a holistic perspective and reveal the distribution and transport patterns of microplastics as well as their overall impacts on the environment and organisms.
Metabolic interactions between organs have co-evolved with multicellular organisms to maintain metabolic homeostasis and ensure that organisms have high adaptability to cope with internal and external stimuli. We have noted that significant changes in organ metabolism occurred during the overall processes exposed to pollutants. However, these complex interaction networks among molecules across the organ-organ or cell-cell contexts remain unanswered. The molecular network strategy provides a suitable approach to help us mine the toxic patterns behind the phenotypic data. We propose molecule interactions based on MS imaging data, namely iMS data-driven multiscale network analysis (iMS2Net) using a prenatal PM2.5-exposed mouse fetus model. We also define five network models to uncover PM2.5-induced metabolic variation in cell pixels and organ-specific patterns. With the evolution of AI, the automatic identification, classification, and organ distribution of microplastics based on machine learning have become more robust. Based on the physical and chemical composition characteristics of microplastics in different scenarios, we speculate that machine learning models are developed to correlate the composition characteristics of microplastics with environmental variables, organ distributions, and metabolic networks, respectively, which can help us to elucidate the dynamic and adaptive processes of the organism in response to environmental pollution, as well as the occurrence and development of systemic diseases.
In summary, attributing toxic effects to a specific source is challenging because microplastics can transfer from water to the atmosphere, soils, and animals, and vice versa. Various and combined pathways by which microplastics enter organisms usually occur simultaneously. To clarify their potential toxicity, we suggest comprehensively surveying the vector effect of microplastic particles and using microplastic extracts from actual samples based on their compositional information to simulate a more true-to-exposure setting. By establishing AI models for overall spatial distribution and concentration and generating an associated organ network, we can assess and predict the risk of microplastics in multiple samples.
Acknowledgments
The work was supported by the National Natural Science Foundation of China (22176195, 82127801, 22104120, 22376212, and 31901040), the National Key R&D Program of China (2022YFF0705003), the Guangdong Province Zhu Jiang Talents Plan (2021QN02Y028), the Shenzhen Key Laboratory of Precision Diagnosis and Treatment of Depression (ZDSYS20220606100606014), the Shenzhen Medical Research Fund (D2301001), the Three Qin Talents Introduction Program for Youths of Shaanxi Province, the “Young Talent Support Plan” of Xi’an Jiaotong University, the Guangdong Science and Technology Department (2021B1212030004), and Fundamental Research Funds for the Central Universities (xtr052023008).
Declaration of interests
The authors declare no competing interests.
Published Online: August 12, 2024
Contributor Information
Chao Zhao, Email: chao.zhao@siat.ac.cn.
Zongwei Cai, Email: zwcai@hkbu.edu.hk.
References
- 1.Koelmans A.A., Redondo-Hasselerharm P.E., Nor N.H.M., et al. Risk assessment of microplastic particles. Nat. Rev. Mater. 2022;7:138–152. [Google Scholar]
- 2.Lim X. Microplastics are everywhere-but are they harmful? Nature. 2021;593:22–25. doi: 10.1038/d41586-021-01143-3. [DOI] [PubMed] [Google Scholar]
- 3.Li C.C., Li X.Y., Bank M.S., et al. The “microplastome”-a holistic perspective to capture the real-world ecology of microplastics. Environ. Sci. Technol. 2024;58:4060–4069. doi: 10.1021/acs.est.3c08849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee C.H., Shim H.E., Song L., et al. Efficient and stable radiolabeling of polycyclic aromatic hydrocarbon assemblies: in vivo imaging of diesel exhaust particulates in mice. Chem. Commun. 2019;55:447–450. doi: 10.1039/c8cc08304e. [DOI] [PubMed] [Google Scholar]
- 5.Zhao C., Guo L., Dong J.Y., et al. Mass spectrometry imaging-based multi-modal technique: The next-generation of biochemical analysis strategy. Innovation. 2021;2(4):100151. doi: 10.1016/j.xinn.2021.100151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou P., Xiao Y., Zhou X., et al. Mapping spatiotemporal heterogeneity in multifocal breast tumor progression by noninvasive ultrasound elastography-guided mass spectrometry imaging strategy. JACS Au. 2024;4:465–475. doi: 10.1021/jacsau.3c00589. [DOI] [PMC free article] [PubMed] [Google Scholar]