Abstract
Varroa mites (Varroa destructor) are ectoparasites of honey bees (Apis mellifera) and cause serious damage to bee colonies. The mechanism of how varroa mites kill honey bees remains unclear. We have addressed the effects of the mites on bee immunity and the replication of a picorna-like virus, the deformed wing virus (DWV). The expression of genes encoding three antimicrobial peptides (abaecin, defensin, and hymenoptaecin) and four immunity-related enzymes (phenol oxidase, glucose dehydrogenase, glucose oxidase, and lysozyme) were used as markers to measure the difference in the immune response. We have demonstrated an example of an ectoparasite immunosuppressing its invertebrate host with the evidence that parasitization significantly suppressed expression of these immunity-related genes. Given that ticks immunosuppress their vertebrate hosts, our finding indicates that immunosuppression of hosts may be a common phenomenon in the interaction and coevolution between ectoparasites and their vertebrate and invertebrate hosts. DWV viral titers were significantly negatively correlated with the expression levels of the immunity-related enzymes. All bees had detectable DWV. Mite-infested pupae developed into adults with either normal or deformed wings. All of the deformed-wing bees were greatly infected by DWV (≈106 times higher than varroa-infested but normal-winged bees). Injection with heat-killed bacteria dramatically promoted DWV titers (105 times in 10 h) in the mite-infested, normal-winged bees to levels similar to those found in mite-infested, deformed-wing bees. Varroa mites may cause the serious demise of honey bees by suppressing bee immunity and by boosting the amplification of DWV in bees exposed to microbes.
Keywords: antimicrobial peptides, immunosuppression, innate immunity, varroa mite, honey bee
Understanding the interactions among ectoparasites, pathogens, and hosts is of importance to both applied and basic science. It is well documented that some ectoparasites, especially ticks (1–4), immunosuppress their vertebrate hosts, and that the immunocompromised vertebrate hosts have been shown to be more susceptible to infectious diseases (5–8). Little is known, however, of how an ectoparasite affects the immunity and pathology of its invertebrate hosts. In this research, we used honey bees (Apis mellifera), the ectoparasitic varroa mites (Varroa destructor), and picorna-like deformed wing virus (DWV) as a model to examine these interactions.
Honey bees are important economic insects used in pollination of many fruits and crops (9). This service has been threatened by an outbreak of varroa mites, causing devastating harm to bee colonies in the United States (10, 11). Varroa mites parasitize pupae and adult bees and reproduce in the pupal brood cells (11). The main symptom of varroa infestation is to cause wing deformity in new adults. Some varroa-infested bees do not show obvious symptoms, but it is possible that they have been weakened. The mechanism of how varroa mites kill honey bees is unknown.
Previously, we have demonstrated that the varroa-infested bees had shorter life spans when challenged with live E. coli bacteria, a lower activity of glucose dehydrogenase (GLD) in the hemocytes, a higher number of damaged hemocytes, and the existence of putative viral particles in their hemocytes (12). Preliminary data indicate that the total level of antimicrobial peptides is reduced in varroa-infested bees as compared with mite-free bees (12). These results suggest a possible immuno-suppression of the bees by varroa mites. A reduction in the expression of any genes underlying the immune response may result in a depressed immune response in honey bees. If so, bee pathogens may obtain an opportunity to more effectively infect honey bees.
Insects have an innate immune system composed of cellular and humoral immune responses (13, 14), which are regulated by many immunity-related genes (15). The cellular response requires the participations of two critical enzymes, phenol oxidase (PO) (16, 17) and GLD (18). The function of PO is thought to be a part of the recognition system of foreignness in insect immunity (16), and PO is a key component of the primary immune response of arthropods (17). GLD is a nonmetabolic enzyme with temporal and special restrictions (18) and belongs to glucose-methanol-choline (GMC)-oxidoreductases (19). GLD is hypothesized to be required for killing of pathogens during an encapsulation reaction via oxidative free radicals and reacts with the quinones produced by PO (20).
In addition to PO and GLD, lysozyme (LYS) and glucose oxidase (GOX) are also important enzymes to insect immunity. LYS hydrolyzes β-(1,4)-glycosidic bonds in the peptidoglucan of bacterial cell wall. The expression of LYS genes is strongly induced by bacteria (21, 22). GOX catalyses the oxidation of β-d-glucose by molecular oxygen to d-glucono-1,5-lactone and hydrogen peroxide. GOX in honey bees is also a member of the GMC-oxidoreductases and may have arisen by gene duplication from GLD (K. Iida, D. R. Cavener, D.L.C.-F., and X.Y., unpublished work). In honey bees, GOX is expressed in the hypopharyngeal gland (23) and is secreted into larval food by the worker bees (24, 25), which provides a means to sterilize the food and is thought to prevent many larval diseases. Thus, GOX provides immunological protection at the colony level.
In insects, two main NF-κB-like signaling pathways, the Imd and Toll pathways, may be activated upon detection of microbes (13, 14). These pathways are in the control of expression of genes encoding antimicrobial peptides for humoral response. The Toll pathway is responsible for defense against fungal and Gram-positive bacterial infections, whereas the Imd pathway is primarily involved in defense against Gram-negative bacteria and controls most of the antimicrobial peptides in Drosophila (13, 26). However, these two pathways interact, and an important antimicrobial peptide defensin is coregulated by both Imd and Toll pathways (27). Antimicrobial peptides are active at low concentrations and exhibit a potent and broad spectrum of activity. Most of these peptides act at the cell-wall membranes and act in synergy by attacking different components of the cell envelope (28, 29). In honey bees, at least four antimicrobial peptides (abaecin, defensin, apidaecin, and hymenoptaecin) are produced (30–33).
Varroa mites have been implicated in several viral diseases (11) and the outbreak of parasitic mite syndrome (34). Recently, it was demonstrated that varroa mites vector Kashmir bee virus (KBV) and activate the replication of KBV in bee pupae (35). DWV (GenBank accession number NC_004830) is the virus that is directly related to varroa mite infestation and that causes severe bee colony death (36, 37). It has been hypothesized that varroa mites are vectors or activators of several bee viruses (36–42).† Interestingly, a picorna-like virus termed Kakugo RNA, which has 98–99% homology with DWV, was identified in the brains of aggressive worker bees and was suggested to underlie the aggressive behavior of the guard bees (43).
In this study, we hypothesize that varroa mites may suppress expression of genes encoding antimicrobial peptides and immunity-related enzymes, as well as promote the replication of DWV. The expression of these genes was used as markers to measure the changes in the immunity of the bees infested by varroa mites, and the titers of DWV were used as a measure of changes in bee pathogen levels. This research may give insight into how an ectoparasite can affect the immunity and pathology of its invertebrate host and may lead to the development of methods to control varroa mite-related damage to honey bees.
Experimental Procedures
Collection of Honey Bees. All of the bees were from a colony kept in the University Park Apiary on the Pennsylvania State University campus. The colony was heavily infested with varroa mites. A frame with pupae of worker bees was removed from the colony when the bees were ready to emerge as adults. The adult bees were collected while emerging from the sealed brood cells. To measure the parasite load for each adult, the number of female adult mites present in each cell was recorded. The degree of wing deformity for each bee was measured with the following criteria: degree 0, all of the wings were normal without any noticeable deformity; degree 1, only one tip of a front wing had noticeable deformity, but the rest of the wing body was normal; degree 2, both front wing tips were deformed and also the width of wings (measured at the widest point) was slightly narrower than normal wings; degree 3, both front wings were shrunken and the width was >50% as compared with normal wings; degree 4, both front wings were heavily reduced, and the width was <50% of normal wings, but the wings were not thread-like (in addition, the bee body was smaller than normal bees); degree 5, at least one of the front wings was a thread-like structure or missing (in addition, the body was significantly smaller than normal bees).
The adult bees were divided into three groups according to mite infestation and wing deformity: normal-winged and mite-free (MF), mite-infested but normal-winged (NW), and mite-infested and deformed-wing (DW). All of the mites were removed from the infested bees, upon bee emergence.
Injection of E. coli into Adult Bees. Fresh E. coli (DH10B) cells were grown in LB liquid medium at 37°C in a shaking incubator overnight. The E. coli cells were pelleted and then washed three times in sterile bee saline (0.156 M NaCl/0.003 M KCl/0.002 M CaCl2). The cell density was measured spectrometrically at 600 nm by using a SpectraMax 250 (Molecular Devices) against a standard curve. The cells were killed by autoclaving the suspension at 121°C for 20 min.
Under a dissecting microscope, the bees were injected at 8.3 μl per bee with either sterile bee saline or heat-killed E. coli (or 1.9 × 105 cells per bee) by using a sterile microinjector. The microinjector was designed by Scott Camazine, using a manual micrometer drive (Newport Corp., Fountain Valley, CA) and a 1-ml sterile syringe (NORM-JECT) with a 30-G sterile needle by Becton Dickinson. New syringes were used for each treatment, and new needles were used for each bee. If bleeding occurred at the injection site, the bee was discarded. The microinjector was reproducible as demonstrated by gravimetric calibration (8.3 ± 0.03 μl). Before injection, an injection site (dorsal mesothoracic wing base) was treated with 70% ethanol. After injection, the bees were kept individually in sterile queen cages at 35°C and 50% relative humidity and were provided sugar and water; and after 10 h, the bees were immediately frozen at –80°C. A total of 84 bees were treated, with 12 bees in each group. As a control, 12 noninjected but similarly handled MF bees were used.
RNA Isolation. Total RNA was isolated from the frozen bees with TRIzol reagent (Life Technologies) and was treated with the RQ1 RNase-free DNase (M6101, Promega) according to the manufacturer's specifications.
Making cDNA. The total RNA concentration was determined spectrometrically (SmartSpec 300, Bio-Rad). Five micrograms of total RNA from each sample was used to make cDNA with the M-MLV Reverse Transcriptase kit (M1701, Promega) according to the manufacturer's specifications.
Real-Time PCR. Real-time quantitative PCR was performed by using the Brilliant SYBR Green QPCR Core Reagent kit (Stratagene). Specific primers were designed with primer express software (Applied Biosystems) for the three antimicrobial peptides, four immunity-related enzymes, DWV, and a housekeeping gene as follows: defensin (5′-TGTCGGCCTTCTCTTCATGG-3′ and 5′-TGACCTCCAGCTTTACCCAAA-3′), hymenoptaecin (5′-ATATCCCGACTCGTTTCCGA-3′ and 5′-TCCCAAACTCGAATCCTGCA-3′), abaecin (5′-AGATCTGCACACTCGAGGTCTG-3′ and 5′-TCGGATTGAATGGTCCCTGA-3′), PO (5′-AATCCATTACCTGA A AT TGATGCT TAT-3′ and 5′-TA ATCT TCCA ACTA AT TCATACGCTCT T-3′), GLD (5′-CTGCACA ACCACGTCTCGTT-3′ and 5′-ACCGCCGAAGAAGATTTGG-3′), GOX (5′-GAGCGAGGTTTCGAATTGGA-3′ and 5′-GTCGTTCCCCCGAGATTCTT-3′), LYS (5′-ACACGGTTGGTCACTGGTCC-3′ and 5′-GTCCCACGCTTTGAATCCCT-3′), DWV (5′-GACAAAATGACGAGGAGATTGTT-3′ and 5′-CAACTACCTGTAATGTCGTCGTGTT-3′), and β-actin (5′-ATGCCAACACTGTCCTTTCTGG-3′ and 5′-GACCCACCAATCCATACGGA-3′). The PCR reaction contained 1× core PCR buffer, 2 mM MgCl2, 0.8 mM dNTP mix, 8% glycerol, 3% DMSO, 300 nM reference dye, 0.5× SYBR Green I dye (Stratagene), 0.05 units/μl SureStart Taq DNA polymerase (Stratagene), 100 nM both forward and reverse primers, and an appropriate volume of Milli-Q water (Millipore) to bring the total volume to 25 μl. The PCR measurements were duplicated on two separate plates. The PCR reactions were carried out in an ABI PRISM 7700 sequence detection system (with sds 1.9.1 software package, Applied Biosystems) by using the following settings: 1 cycle of a 10-min preincubation at 95°C, and 40 cycles of amplification with 30 sec of denaturing at 95°C, 1 min of annealing at 59°C, and a 1-min extension at 72°C. Relative quantifications were calculated by using the comparative CT method against the expression levels of the housekeeping gene β-actin in the respective samples. The expression levels of β-actin were highly similar in different groups of bees and treatments. Melting curves, DNA agarose gels, and DNA sequencing were used to confirm the specificity of PCR amplifications.
Statistical Data Analyses. The statview 5.0.1 statistical package (SAS Institute, Cary, NC) was used to analyze the data by ANOVA and correlation analyses.
Results
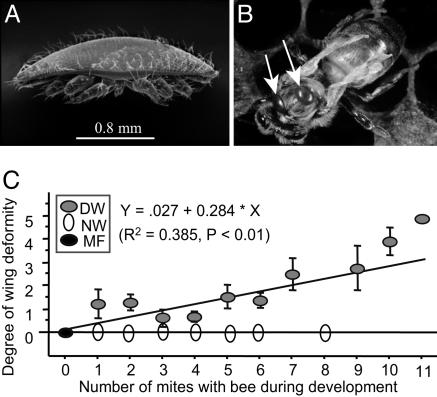
Relationship Between the Degree of Wing Deformity and Mite Density. The typical symptom of varroa mite infestation in honey bees is wing deformity (Fig. 1 A and B), and the wing deformity was only found in bees infested by varroa mites. However, heavy mite infestation did not always cause wing deformity (Fig. 1C). About 50% of the bees infested as pupae with varroa mites had wing deformity. Among the bees with deformed wings, the degree of wing deformity was significantly related to the varroa mite density (Fig. 1C). This indicated that the varroa infestation was necessary to cause wing deformity in honey bees but was not sufficient to cause wing deformity alone.
Fig. 1.
The relationship between bee wing deformity and the density of varroa mites. (A) A scanning electron microscope photograph of a female varroa mite. (B) A newly emerged worker bee with a typical symptom of deformed wings with two mites on her thorax as the white arrows indicate. (C) Linear regression analysis for the relationship between the degree of wing deformity and the number of mites per cell (mean ± SEM). Three groups of newly emerged worker bees were distinguished: DW, NW, and MF. (A is used with the permission of Scott Camazine; B is used with the permission of Maryann Frazier.)
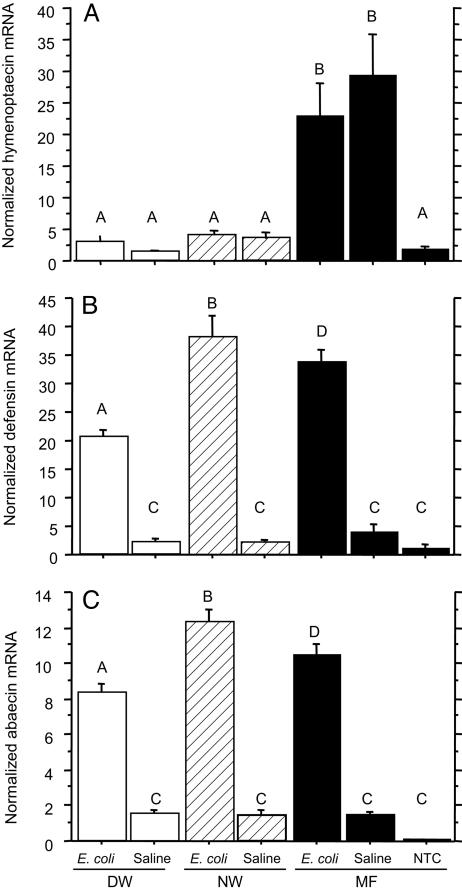
Effects of Varroa Infestation on Expression of the Genes Encoding Antimicrobial Peptides. Varroa infestation suppresses the mRNA level of hymenoptaecin. In the mite-free (MF) bees, the mRNA levels of hymenoptaecin were significantly elevated by injection of either saline or heat-killed E. coli, as compared with the nontreatment control (NTC) bees (Fig. 2A). However, the mRNA levels of hymenoptaecin in injected mite-infested bees, including both the DW and NW bees, were significantly lower than those of injected MF bees. Moreover, the hymenoptaecin mRNA levels of the injected mite-infested bees were not significantly different from those of nontreatment MF bees. These results demonstrated that the expression of hymenoptaecin gene was significantly suppressed in mite-infested bees. Comparing DW and NW bees, there was no significant difference in the mRNA levels of hymenoptaecin, indicating that the symptom of wing deformity was not linked to the expression level of hymenoptaecin gene. Varroa infestation suppresses expression of genes encoding defensin and abaecin in DW bees. In response to saline and E. coli injections, the patterns of gene expression of defensin and abaecin had the same trend (Fig. 2 B and C). The three groups of bees responded differently to the injection of saline as compared with E. coli injection. Saline injection did not activate the expression of defensin and abaecin in all of the three groups of bees, but E. coli injection activated the expression of these two antimicrobial peptides in all bee groups. Upon E. coli challenge, the DW bees had significantly lower mRNA levels of defensin and abaecin than the normal-winged bees (i.e., the MF and NW bees). This indicated that gene expression of defensin and abaecin was suppressed in the deformed-wing bees but not solely by mite infestation.
Fig. 2.
The effect of varroa infestation on the expression of genes encoding the antimicrobial peptides in newly emerged worker bees: DW, NW, and MF. The bees were either injected with bee saline or heat-killed E. coli suspended in the bee saline. NTC refers to the nontreatment control (i.e., the MF bees without injection). All of the values shown are mean ± SEM. (A) Varroa infestation suppressed the expression of hymenoptaecin gene. The bars with different letters are significantly different (ANOVA, P < 0.0001; pairwise comparison with Fisher's protected least significant difference (PLSD), P ≤ 0.0003). (B) Varroa infestation suppressed the expression of defensin gene in the DW bees injected with bacteria. The bars with different letters are significantly different (ANOVA, P < 0.0001; pairwise comparison with Fisher's PLSD, P ≤ 0.049). (C) Varroa infestation suppressed the expression of abaecin gene in the DW bees injected with the bacteria. The bars with different letters are significantly different (ANOVA, P < 0.0001; pairwise comparison with Fisher's PLSD, P ≤ 0.0029).
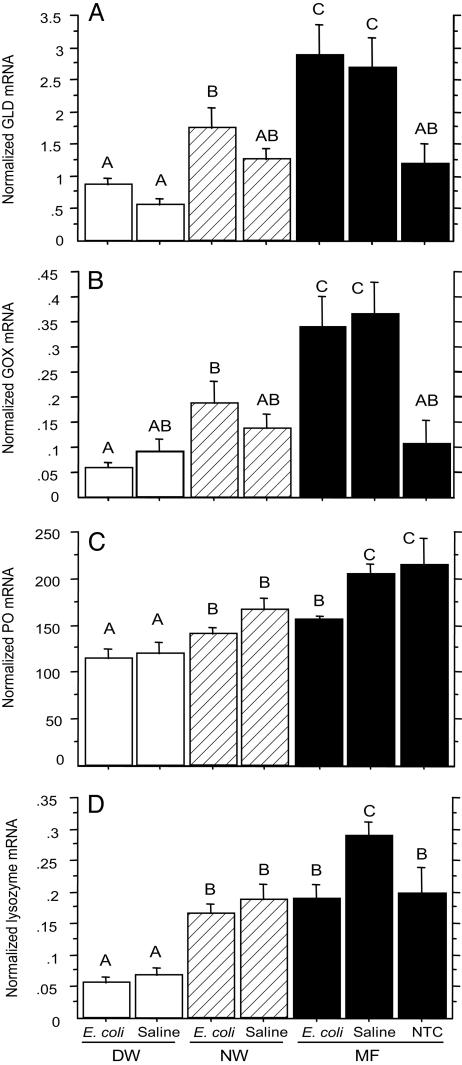
Effects of Varroa Infestation on Immunity-Related Enzymes. Varroa infestation suppresses expression of genes encoding GLD and GOX. The patterns of gene expression of GLD and GOX also had a similar trend (Fig. 3 A and B). In response to both saline and E. coli injections, the GLD and GOX mRNA levels of the mite-infested bees were significantly reduced as compared with those of injected MF bees. This indicated that varroa infestation suppressed the transcriptional levels of GLD and GOX genes. However, within the group of mite-infested bees, in response to E. coli injection, the DW bees had significantly lower GLD and GOX mRNA levels than the NW bees. This meant that expression of GLD and GOX genes was related to the symptom of wing deformity when the bees were exposed to a bacterial challenge. In response to the injections, the mRNA levels of GLD and GOX of the MF bees were significantly elevated; however, the GLD and GOX mRNA levels of the injected mite-infested bees were not significantly different from those of the NTC bees. This also indicated that the GLD and GOX gene expression was suppressed in the mite-infested bees.
Fig. 3.
The effects of varroa infestation on the expression of genes encoding the immunity-related enzymes in newly emerged worker bees: DW, NW, and MF. The bees were either injected with bee saline or heat-killed E. coli suspended in the saline. NTC refers to the nontreatment control (i.e., the MF bees without injection). All of the values shown are mean ± SEM. (A) The expression of GLD gene was suppressed in the mite-infested bees. The bars with different letters are significantly different (ANOVA, P < 0.0001; pairwise comparison with Fisher's PLSD, P ≤ 0.0159). (B) The expression of GOX gene was suppressed in the varroa-infested bees. The bars with different letters are significantly different (ANOVA, P < 0.0001; pairwise comparison with Fisher's PLSD, P ≤ 0.0386). (C) Varroa infestation suppressed the expression of PO gene. The bars with different letters are significantly different (ANOVA, P < 0.0001; pairwise comparison with Fisher's PLSD, P ≤ 0.0495). (D) Varroa infestation suppressed the expression of lysozyme gene. The bars with different letters are significantly different (ANOVA, P < 0.0001; pairwise comparison with Fisher's PLSD, P ≤ 0.0069).
Varroa infestation suppresses the expression of genes encoding PO and LYS. The patterns of gene expression of PO and LYS follow a similar trend (Fig. 3 C and D). In response to both E. coli and saline injections, the PO and LYS mRNA levels of the DW bees were significantly lower than those of NW bees. This indicated that wing deformity was linked to the gene expression of PO and LYS. Between the groups of mite-infested and MF bees, upon the challenge of saline injections, the PO and LYS mRNA levels of the former were significantly lower than those of the latter. This indicated that mite infestation suppressed the expression of PO and LYS genes when the bees were challenged with saline injections. However, when the bees were challenged with E. coli, a significant reduction in PO and LYS mRNA levels was only seen in the DW bees. Deformed-wing bees had significantly lower PO and LYS gene expression levels as compared with normal-winged bees.
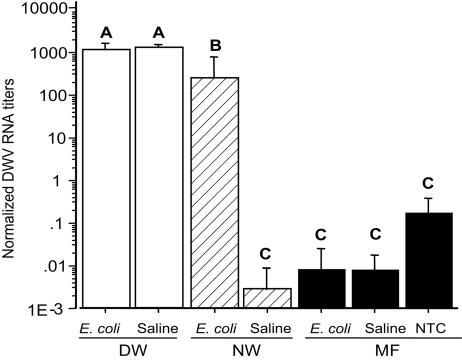
Deformed-Wing Bees Are Heavily Infested by DWV, and Varroa Infestation Boosts the Amplification of DWV. All of the MF bees carried a low level of DWV viral RNA; however, neither saline injection nor E. coli injection significantly changed the titers of DWV viral RNA in these MF bees (Fig. 4). The deformed-wing bees had an extremely high level of DWV viral RNA as compared with the normal-winged bees. For instance, the DWV viral RNA levels of the saline-injected DW bees were ≈106 times higher than those of the saline-injected NW bees. In the DW bees, the levels of DWV viral RNA of the saline-injected bees were not significantly different from those of E. coli-injected bees. In the mite-infested NW bees, when challenged with E. coli, DWV replication was significantly boosted in 10 h (≈105 times higher than in the saline-injected NW bees) to levels similar to those found in the DW bees, which was not seen in the MF bees. These data indicated that the high level of replication of DWV in honey bees needed two components: varroa mite infestation and microbial challenge.
Fig. 4.
The effect of varroa infestation and microbial exposure on DWV genomic RNA levels (mean ± SEM) in newly emerged worker bees: DW, NW, and MF. Deformed-wing bees are heavily infected by DWV, and varroa infestation and microbial exposure boost the replication of DWV (note that the y axis is a log scale). The bees were either injected with bee saline or heat-killed E. coli suspended in the saline. NTC refers to the nontreatment control (i.e., the MF bees without injection). The bars with different letters are significantly different (ANOVA, P < 0.0001; pairwise comparison with Fisher's PLSD, P ≤ 0.042).
Correlations Among the Gene Expression Levels of the Enzymes, Antimicrobial Peptides, and the Viral RNA Titers of DWV. The expression of the three antimicrobial peptides was not all correlated (Table 1). The expression of abaecin gene was significantly positively correlated to that of defensin gene (correlation coefficient of 0.971), which suggested that the two antimicrobial peptides share the same regulatory pathway. However, the expression of hymenoptaecin was not correlated to either abaecin or defensin. This suggested that hymenoptaecin uses a different regulatory pathway than abaecin and defensin.
Table 1. Correlation matrix of normalized titers of DWV, mRNA levels of antimicrobial peptides, and immunity-related enzymes of adult honey bees in all treatments.
| DWV | LYS | PO | GOX | GLD | Hym | Def | Aba | |
|---|---|---|---|---|---|---|---|---|
| Aba | -0.007 | -0.142 | -0.217 | 0.191 | 0.300** | -0.061 | 0.971*** | 1 |
| Def | -0.113 | -0.052 | -0.156 | 0.281* | 0.379*** | -0.035 | 1 | |
| Hym | -0.197 | 0.294* | 0.327** | 0.365** | 0.225 | 1 | ||
| GLD | -0.454*** | 0.546*** | 0.261* | 0.892*** | 1 | |||
| GOX | -0.570*** | 0.590*** | 0.270* | 1 | ||||
| PO | -0.570*** | 0.462*** | 1 | |||||
| LYS | -0.675*** | 1 | ||||||
| DWV | 1 |
β-actin was used to normalize the data with the comparative CT method. Hym, hymenoptaecin; Def, defensin; Aba, abaecin. Statistical significance of correlation: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
The expression of the genes encoding the four immunity-related enzymes (GLD, GOX, PO, and LYS) was significantly positively correlated. This suggested that these four enzymes share common transcriptional regulation in the newly emerged adults. Among the enzymes, the expression of GLD and GOX genes was highly positively correlated (correlation coefficient of 0.892), which may be explained by the finding that the bee GOX gene may have originated from duplication of the GLD gene (K. Iida, D. R. Cavener, D.L.C.-F., and X.Y., unpublished work).
The gene expression of these four enzymes was differently correlated to the expression of the three antimicrobial peptides. The expression of GLD gene was correlated to that of abaecin and defensin genes, the expression of GOX gene was correlated to that of defensin and hymenoptaecin genes, and the expression of PO and LYS genes was only correlated to hymenoptaecin gene.
The correlations among the titers of DWV viral RNA and the expression of these immunity-related genes were all negative, but only the correlations with expression of the four immunity-related enzymes were significant. This suggested that DWV titers were related to depressed cellular immune responses or enzyme-based immunity and not to antimicrobial peptide-related immunity. This finding may be explained by the fact that abaecin, defensin, and hymenoptaecin are only known to be antibacterial peptides, the functions of which have not been previously linked to an antiviral response in insects.
Discussion and Conclusion
This work demonstrated that varroa infestation suppressed immunity in honey bees by reducing the transcription of genes encoding antimicrobial peptides and immunity-related enzymes. Not only the DW bees but also the asymptomatic NW bees had impaired immunity due to the ectoparasitization. To our knowledge, this is the first example of an ectoparasite immunosuppressing its invertebrate host. Given that ticks immunosuppress their vertebrate hosts (1–4), ectoparasites not only immunosuppress their vertebrate hosts but also immunosuppress their invertebrate hosts. Thus, immunosuppression of the hosts may be a common phenomenon in the interaction and coevolution between ectoparasites and their vertebrate and invertebrate hosts.
The replication of DWV in honey bees provides another example that a bacterial factor stimulates replication of some viruses. It is documented that bacterial infections induce the replication of HIV as measured by increases in plasma HIV RNA titers (44–47). The increased replication of DWV in honey bees needs two components, varroa mite parasitization and exposure to a bacterial factor. This microbial challenge may naturally exist, because bacterial colonies are found on the varroa feeding sites in some bee pupae (48). DWV may rapidly replicate in these mite-parasitized, bacteria-exposed pupae, causing them to develop into DW bees. This requirement of a microbial factor for DWV replication may also help explain the results of a statewide extensive survey in Pennsylvania (10) and experiments in South America (49), where bee colonies treated with antibiotics survived significantly better than the untreated colonies. The antibiotics might have controlled the bacteria in the bee colonies, and as a result, one of the components for DWV replication may have been removed or depressed. The finding that all DW bees contained high level of DWV suggested that increased DWV in bees played a significant role in the collapse of bee colonies. This is consistent with the short survival time (<48 h) of DW bees (12).
We propose that varroa mites may cause collapse of honey bee colonies as follows. Varroa mite infestation may reduce the expression of genes encoding antimicrobial peptides and immunity-related enzymes, eventually leading to depressed bee immunity for both cellular and humoral immune responses. Thus, the bee colonies may become more susceptible to various bee pathogens, especially DWV.
Acknowledgments
We thank Dr. Deborah Grove in the Nucleic Acid Facility at Pennsylvania State University for helping with the real-time PCR experiments, Dr. Scott Camazine for helping to initiate this research and for designing the microinjector, Dr. Joachim de Miranda for providing E. coli DH10B strain, and Drs. Gary Felton and Fred Gildow for reviewing the manuscript. This work was supported by the Graduate Student Competitive Grant sponsored by the College of Agricultural Sciences at Pennsylvania State University and by a grant from the Pennsylvania Department of Agriculture.
Author contributions: X.Y. and D.L.C.-F. designed research; X.Y. performed research; X.Y. and D.L.C.-F. analyzed data; and X.Y. and D.L.C.-F. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: DW, mite-infested and deformed-wing; DWV, deformed wing virus; GLD, glucose dehydrogenase; GOX, glucose oxidase; LYS, lysozyme; MF, normal-winged and mite-free; NW, mite-infested but normal-winged; PO, phenol oxidase.
Footnotes
Ball, B. V. (1989) Meeting of the EC Expert's Group, November 28–30, 1988, Udine, Italy, pp. 241–244.
References
- 1.Schoeler, G. B. & Wikel, S. K. (2001) Ann. Trop. Med. Parasitol. 95, 755–771. [DOI] [PubMed] [Google Scholar]
- 2.Wikel, S. K. & Alarcon-Chaidez, F. J. (2001) Vet. Parasitol. 101, 275–287. [DOI] [PubMed] [Google Scholar]
- 3.Wikel, S. K. (1999) Int. J. Parasitol. 29, 851–859. [DOI] [PubMed] [Google Scholar]
- 4.Waxman, L., Smith, D. E., Arcuri, K. E. & Vlasuk, G. P. (1990) Science 248, 593–596. [DOI] [PubMed] [Google Scholar]
- 5.Titus, R. G. & Ribeiro, J. M. C. (1988) Science 239, 1306–1308. [DOI] [PubMed] [Google Scholar]
- 6.Jones, L. D., Hodgson, E. & Nuttall, P. A. (1989) Arch. Virol. 1, Suppl., 227–234. [Google Scholar]
- 7.Edwards, J. F., Higgs, S. & Beaty, B. J. (1998) J. Med. Entomol. 35, 261–265. [DOI] [PubMed] [Google Scholar]
- 8.Ellis, J. A. & Demartini, J. C. (1985) Vet. Immunol. Immunopathol. 8, 93–106. [DOI] [PubMed] [Google Scholar]
- 9.Morse, R. A. & Calderone, N. W. (2000) Bee Culture 128, 1–15. [Google Scholar]
- 10.Finley, J., Camazine, S. & Frazier, M. (1996) Am. Bee J. 136, 805–808. [Google Scholar]
- 11.Sammataro, D., Gerson, U. & Needham, G. (2000) Annu. Rev. Entomol. 45, 519–548. [DOI] [PubMed] [Google Scholar]
- 12.Yang, X. (2004) Ph. D. thesis (Pennsylvania State Univ., University Park), pp. 1–270.
- 13.Hoffmann, J. A. (2003) Nature 426, 33–38. [DOI] [PubMed] [Google Scholar]
- 14.Osta, M. A., Christophides, G. K., Vlachou, D. & Kafatos, F. C. (2004) J. Exp. Biol. 207, 2551–2563. [DOI] [PubMed] [Google Scholar]
- 15.Christophides, G. K., Zdobnov, E., Barillas-Mury, C., Birney, E., Blandin, S., Blass, C., Brey, P. T., Collins, F. H., Danielli, A., Dimopoulos, G., et al. (2002) Science 298, 159–165. [DOI] [PubMed] [Google Scholar]
- 16.Ashida, M. & Yamazaki, H. I. (1990) in Molting and Metamorphosis, eds. Ohnishi, E. & Ishizaki, H. (Japan. Sci. Soc. Press), pp. 239–265.
- 17.Decker, H. & Jaenicke, E. (2004) Dev. Comp. Immunol. 28, 673–687. [DOI] [PubMed] [Google Scholar]
- 18.Cox-Foster, D. L., Schonbaum, C. P., Murtha, M. T. & Cavener, D. R. (1990) Genetics 124, 873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whetten, R., Organ, E., Krasney, P., Cox-Foster, D. & Cavener, D. (1988) Genetics 120, 475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox-Foster, D. L. & Stehr, J. E. (1994) J. Insect Physiol. 40, 235–250. [Google Scholar]
- 21.Hultmark, D. (1996) in Lysozymes: Model Enzymes in Biochemistry and Biology, ed. Jollès, P. (Birkhauser, Basel), pp. 87–102.
- 22.Gillespie, J. P., Kanost, M. R. & Trenczek, T. (1997) Annu. Rev. Entomol. 42, 611–643. [DOI] [PubMed] [Google Scholar]
- 23.Ohashi, K., Natori, S. & Kubo, T. (1999) Eur. J. Biochem. 265, 127–133. [DOI] [PubMed] [Google Scholar]
- 24.Sano, O., Kunikata, T., Kohno, K., Iwaki, K., Ikeda, M. & Kurimoto, M. (2004) J. Agric. Food Chem. 52, 15–20. [DOI] [PubMed] [Google Scholar]
- 25.Santos, K. S., dos Santos, L. D., Mendes, M. A., de Souza, B. M., Malaspina, O. & Palma, M. S. (2005) Insect Biochem. Mol. Biol. 35, 85–91. [DOI] [PubMed] [Google Scholar]
- 26.Lemaitre, B., Kromer-Metzger, E., Michaut, L., Nicolas, E., Meister, M., Georgel, P., Reichhart, J. M. & Hoffmann, J. A. (1995) Proc. Natl. Acad. Sci. USA 92, 9465–9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Gregorio, E., Spellman, P. T., Tzou, P., Rubin, G. M. & Lemaitre, B. (2002) EMBO J. 21, 2568–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lockey, T. D. & Ourth, D. O. (1996) Eur. J. Biochem. 236, 263–271. [DOI] [PubMed] [Google Scholar]
- 29.Morishima, I., Horiba, T., Iketani, M., Nishioka, E. & Yamano, Y. (1995) Dev. Comp. Immunol. 19, 357–363. [DOI] [PubMed] [Google Scholar]
- 30.Casteels, P., Ampe, C., Jacobs, F., Vaeck, M. & Tempst, P. (1989) EMBO J. 8, 2387–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casteels, P., Ampe, C., Riviere, L., Van Damme, J., Elicone, C., Fleming, M., Jacobs, F. & Tempst, P. (1990) Eur. J. Biochem. 187, 381–386. [DOI] [PubMed] [Google Scholar]
- 32.Fujiwara, S., Imai, J., Fujiwara, M., Yaeshima, T., Kawashima, T. & Kobayashi, K. (1990) J. Biol. Chem. 265, 11333–11337. [PubMed] [Google Scholar]
- 33.Casteels, P., Ampe, C., Jacobs, F. & Tempst, P. (1993) J. Biol. Chem. 268, 7044–7054. [PubMed] [Google Scholar]
- 34.Shimanuki, H., Calderone, N. W. & Knox, D. A. (1994) Am. Bee J. 134, 827–828. [Google Scholar]
- 35.Shen, M. (2003) Ph.D. thesis (Pennsylvania State Univ., University Park), pp. 1–180.
- 36.Bailey, L. & Ball, B. V. (1991) Honey Bee Pathology (Academic, San Diego), 2nd Ed., pp. 1–108.
- 37.Chen, Y. P., Higgins, J. A. & Feldlaufer, M. F. (2005) Appl. Environ. Microbiol. 71, 436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ongus, J. R., Peters, D., Bonmatin, J. M., Bengsch, E., Vlak, J. M. & van Oers, M. M. (2004) J. Gen. Virol. 85, 3747–3755. [DOI] [PubMed] [Google Scholar]
- 39.Bowen-Walker, P. L., Martin, S. J. & Gunn, A. (1999) J. Invertebr. Pathol. 73, 101–106. [DOI] [PubMed] [Google Scholar]
- 40.Camazine, S. & Liu, T. P. (1997) J. Invertebr. Pathol. 71, 177–178. [DOI] [PubMed] [Google Scholar]
- 41.Allen, M. F., Ball, R. F., White, R. F. & Antoniw, J. F. (1986) J. Apic. Res. 15, 100–105. [Google Scholar]
- 42.Ball, B. V. (1988) in Africanized Honey Bees and Bee Mites, eds. Needham, G. R., Page, R. E., Delfindo-Baker, M. & Bowmans, C. E. (Horwood, Chichester, U.K.), pp. 457–461.
- 43.Fujiyuki, T., Takeuchi, H., Ono, M., Ohka, S., Sasaki, T., Nomoto, A. & Kubo, T. (2004) J. Virol. 78, 1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sulkowski, M. S., Chaisson, R. E., Karp, C. L., Moore, R. D., Margolick, J. B. & Quinn, T. C. (1998) J. Infect. Dis. 178, 1642–1648. [DOI] [PubMed] [Google Scholar]
- 45.Equils, O., Faure, E., Thomas, L., Bulut, Y., Trushin, S. & Arditi, M. (2001) J. Immunol. 166, 2342–2347. [DOI] [PubMed] [Google Scholar]
- 46.Ghassemi, M., Asadi, F. K., Andersen, B. R. & Novak, R. M. (2000) AIDS Res. Hum. Retroviruses 16, 435–440. [DOI] [PubMed] [Google Scholar]
- 47.Rotchford, K., Strum, A. W. & Wilkinson, D. (2000) Sex. Transm. Dis. 27, 243–248. [DOI] [PubMed] [Google Scholar]
- 48.Kanbar, G. & Engels, W. (2003) Parasitol. Res. 90, 349–354. [DOI] [PubMed] [Google Scholar]
- 49.Roman, G. & Moncecchi, A. G. (1988) Veterinaria Argentina 48, 675–685. [Google Scholar]