Abstract
The molecular pathogenesis of Barrett's metaplasia (BM) of the esophagus is poorly understood. The change to an intestinal phenotype occurs on a background of esophagitis due to refluxing acid and bile. CDX1, an important regulator of normal intestinal development, was studied as a potential key molecule in the pathogenesis of BM. CDX1 mRNA and protein were universally expressed in all samples of BM tested but not in normal esophageal squamous or gastric body epithelia. This tissue-specific expression was attributable to the methylation status of the CDX1 promoter. Conjugated bile salts and the inflammatory cytokines TNF-α and IL-1β were all found to increase CDX1 mRNA expression in vitro. These effects were primarily mediated by NF-κB signaling but only occurred when the CDX1 promoter was unmethylated or partially methylated. The data suggest that CDX1 is a key molecule linking etiological agents of BM to the development of an intestinal phenotype. Although the initial trigger for CDX1 promoter demethylation is not yet identified, it seems likely that demethylation of its promoter may be the key to the induction and maintenance of CDX1 expression and so of the BM phenotype.
Keywords: colorectal carcinoma, deoxycytidine, gastroesophageal reflux disease, intestinal metaplasia, promoter methylation
Barrett's metaplasia (BM) describes the phenotypic change of esophageal squamous epithelium to a columnar and, in particular, an intestinal-type epithelium (1). This metaplastic change is of particular clinical importance because patients with such change have a 30- to 125-fold increased risk of developing esophageal adenocarcinoma (2). Although the molecular pathways that underlie the development of such neoplasms (reviewed in ref. 2) are much studied, little is known about the molecular changes that induce and maintain BM itself.
The intestinal phenotype in BM seems to be required for the development of neoplasia and so is usually considered to be the key to its definition (1). CDX1 and CDX2 are homeobox proteins that appear to play major roles in the development of intestinal epithelium in utero (3), suggesting that either, or both, may be important in the induction and maintenance of BM. This suggestion is supported by two main groups of studies. First, CDX1 and CDX2 mRNAs are consistently demonstrable in human gastric intestinal metaplasia (IM) (4) and in BM (5). Second, transgenic mouse studies have shown that ectopic expression of either CDX1 (6) or CDX2 (7, 8) in gastric epithelium is sufficient to induce IM.
The main etiological agent underlying BM is gastroesophageal reflux disease (2). Prolonged injury caused by the refluxate to esophageal squamous mucosa is assumed to induce esophagitis, followed by the development of metaplastic epithelium (1, 2). Both acid and bile salts, which are components of gastroesophageal refluxate, are likely candidates for activators of molecular pathways leading to BM. Such activation may either be a direct effect of these substances on epithelial cells or be due to the inflammatory milieu of reflux-related esophagitis. Two inflammatory mediators that are found abundantly in reflux esophagitis are the cytokines TNF-α and IL-1β (9, 10). Most available data suggest that CDX2 expression is down-regulated by etiological agents related to BM. Continuous culture of the colorectal carcinoma (CRC) cell line Caco2 at pH 5 for 3 days leads to reduced expression of CDX2 mRNA (11). Kapoor et al.¶ showed that a single pulse of acidified bile salts can down-regulate CDX2 protein expression in the CRC cell line LS174T. Similar protein down-regulation can also be induced by TNF-α in the CRC cell line HT29 (13). We have also confirmed that TNF-α down-regulates CDX2 mRNA expression in HT29 and in another CRC cell line, C32 (unpublished data). There are, however, no published reports on the effects on CDX1 expression of exposure to etiological agents related to BM.
It has been suggested that an epigenetic event in esophageal squamous epithelium may initiate BM (14). Loss of CDX1 expression in CRC has been shown to be directly attributable to promoter methylation (15, 16), suggesting that an epigenetic trigger for BM could involve CDX1. In contrast, our studies of four gastrointestinal tissues and four CRC cell lines failed to demonstrate any relation between CDX2 mRNA expression and CDX2 promoter methylation status (unpublished data). Further, Hinoi et al. (17) were unable to induce CDX2 expression in CRC cell lines through treatment with the demethylating agent 5-aza-2′-deoxycytidine (5aza2).
The following study is an attempt to demonstrate a molecular mediator that links etiological agents of BM to the induction and maintenance of the intestinal phenotype. In view of the data discussed above, we particularly focused on CDX1 and its promoter methylation as a potential mediator.
Materials and Methods
Tissue Samples and Cell Culture. Endoscopic tissue biopsies were collected prospectively after approval by the Oxfordshire Clinical Research Ethics Committee (Oxford). Because whole biopsies were used for DNA, RNA, or protein extraction without any epithelial cell enrichment process, the potential for tissue heterogeneity between samples should be considered when interpreting the following results. The sources of the CRC cell lines studied (C10, C32, CC07, HCA7, HCT116, HT29, and NCI-747) are outlined in ref. 16. Two esophageal squamous carcinoma cell lines (KYSE30 and OE21) were obtained from R. C. Fitzgerald (Hutchison/MRC Research Centre, Cambridge, U.K.). DNA and RNA were extracted from cell pellets or tissue samples by using the DNeasy or RNeasy kits (both from Qiagen, Valencia, CA).
Treatments and Transfections. Human recombinant TNF-α (Sigma) was dissolved in sterile distilled water, which was therefore used as a treatment control. Sterile PBS containing 1% BSA (Sigma) was used to dissolve the human recombinant IL-1β (Sigma) and also used as a treatment control. Cells grown to 60–70% confluence were incubated for 72 h in culture medium containing a final 5aza2 (Sigma) concentration of 5 μg/μl. The 5aza2 was dissolved in a 1:1 mixture of acetic acid (Sigma):distilled water, which was used as a treatment control. The signaling pathway inhibitors, BAY 11-7085 (NF-κB pathway; Affiniti, Nottingham, U.K.), PD90859 [extracellular signal-regulated kinase (ERK) pathway; Calbiochem], SB202190 [p38 mitogen-activated protein kinase (MAPK) pathway; Calbiochem], and SP600125 (c-Jun N-terminal kinase pathway; Calbiochem), were all dissolved in DMSO (Sigma), which was therefore used as a treatment control. All of the inhibitors were added to cultured cells in serum-free conditions for 1 h. Sufficient TNF-α or IL-1β (in serum-free medium) was then added, without removing the medium containing the inhibitor, to yield the desired final cytokine concentration.
Based on previous studies of the effects of acid exposure on cultured cells (18, 19), our acid exposure experiments were carried out by using hydrochloric acid in medium at a pH of 3.5 for 15 min to obtain maximum effect. The bile salt mixture used consisted of the salts of glycocholic acid (349 μM), taurocholic acid (503 μM), taurodeoxycholic acid (71 μM), and taurolithocholic acid (77 μM) (all from Sigma). These four bile salts were chosen because they have been found at the highest intraesophageal concentrations in in vivo studies of patients with BM (20). The relative proportions of the bile salts used matched those found in the in vivo studies. The total amount of each was adjusted to give a final total concentration of 1 mM. Higher total concentrations were found to be lethal to cultured cells.
The p65 expression vector (pMT2T-p65) and the corresponding empty vector were gifts from M. B. Loncar (University of Tübingen, Tübingen, Germany). Transient transfection of cell lines was carried out by using the LipofectAMINE PLUS system (Invitrogen) according to the manufacturer's protocol. The empty vector was used as a treatment control with each transfection.
CDX1 Promoter Bisulfite Sequencing. The methylation status of gene promoter CpGs is best analyzed by using direct sequencing after sodium bisulfite modification of target DNA (bisulfite sequencing) (21). Details of bisulfite sequencing for CDX1 are given in ref. 16. In brief, a 400-bp region upstream of exon 1 of CDX1 was amplified from bisulfite-modified DNA, and the PCR products were purified, cloned, and sequenced. As discussed in ref. 16, the CDX1 promoter is most likely to reside in the 200-bp 5′ flanking region.
RT-PCR. cDNA was synthesized from 2 μg of denatured RNA (65°C for 5 min) by incubation at 37°C for 60 min with final quantities or concentrations of 1 μM oligo(dTs), 4 units of Omniscript reverse transcriptase (Qiagen), 10 units of RNase inhibitor (Ambion, Austin, TX), and a 0.5 mM concentration of each dNTP. The primers used for CDX1 RT-PCR were as follows: forward, 5′-AGGACAAGTACCGCGTGGTCTA-3′; reverse, 5′-CCTCTGAACGTATGGAGGAGGA-3′. The primers used for CDX2 RT-PCR were as follows: forward, 5′-CAGTCGCTACATCACCATC-3′; reverse, 5′-AGAGTCCACGCTCCTCAT-3′. The primers used for β-actin RT-PCR were as follows: forward, 5′-ACACCTTCTACAATGAGC-3′; reverse, 5′-ACGTCACACTTCATGATG-3′. All RT-PCRs were carried out in a 25-μl PCR volume containing a 0.2 μM concentration of each primer, 0.5 units of AmpliTaq Gold (PerkinElmer) polymerase, 1.5 mM MgCl2, a 200 μM concentration of each dNTP, and 200 μg (CDX1 and CDX2) or 100 μg (β-actin) of cDNA. The cycling conditions used for these PCRs were a denaturation step of 95°C for 10 min, 30 (CDX1 and CDX2) or 25 (β-actin) cycles of 95°C for 45 s, 66°C (CDX1 and CDX2) or 56°C (β-actin) for 45 s, 72°C for 60 s, and a final extension step of 72°C for 10 min. All PCR products were separated by electrophoresis in 2% agarose gels and visualized by ethidium bromide staining.
Real-Time PCR. The TaqMan probe system was used for real-time PCR. Optimal primers (forward, 5′-TGAACGGCAGGTGAAGATCTG-3′; reverse, 5′-GCTGTTTCTTCTTGTTCACTTTGC-3′) and probe (6-carboxyfluorescein-labeled 5′-TTCCAAAACCGGCGGGCAAA-3′ tetramethylrhodamine) for CDX1 real-time PCR were designed by using the software package primer express (Applied Biosystems). The primers were synthesized by the Cancer Research U.K. Oligonucleotide Laboratory, and the probe was synthesized by MWG Biotech (Ebersberg, Germany). Preoptimized primer/probe mixture (Yakima yellow dye-labeled, read through VIC channel) for GAPDH real-time PCR was purchased from Eurogentec (Brussels). The CAS-1200 robotics system (Corbett Research, Cambridge, U.K.) was used for preparation of master and sample reaction mixtures. Standard real-time cycling conditions were used for both CDX1 and GAPDH: 50°C for 2 min followed by 95°C for 10 min and then 40 cycles of 95°C for 15 s and 60°C for 1 min. The PCRs were performed on a Rotor-gene RG-3000 (Corbett Research), and the data were analyzed by using rotor-gene analysis 5.0 software (Corbett Research). Triplicates were run for each sample tested.
The following reaction mixture, in a total reaction volume of 25 μl, was used for CDX1: 12.5 μl of TaqMan master mix (Applied Biosystems), a 100 nM concentration of each primer, 200 nM probe, and 200 ng of cDNA. Based on the supplier's recommendations, the following reaction mixture, also in a total reaction volume of 25 μl, was used for GAPDH: 12.5 μl of TaqMan master mix (Applied Biosystems), a 300 nM concentration of each primer, 125 nM probe, and 50 ng of cDNA. When standard curves were run, the above conditions yielded amplification efficiencies close to the theoretical maximum for both CDX1 and GAPDH. These conditions also produced nearly equal amplification efficiencies for both the CDX1 and GAPDH PCRs, which justified the use of the ΔΔCT method for analyzing the data generated by these two PCRs (22).
Protein Lysate Preparation and Western Blotting. Whole-cell protein lysates were prepared from cell pellets or tissue samples with RIPA lysis buffer [150 mM NaCl/1% (wt/vol) Nonidet P-40/0.5% Na deoxycholate/0.1% SDS/50 mM Tris·Cl, pH 7.5] supplemented with a protease inhibitor mixture (Complete Mini tablets, Roche Diagnostics). Separate cytosol and nuclear protein lysates were prepared by using the Active Motif Nuclear Extract Kit (Active Motif Europe, Rixensart, Belgium), according to the manufacturer's protocol. For routine quantitation of proteins, the bicinchoninic acid procedure was used, following the manufacturer's protocol (Pierce). Protein samples (between 35 and 50 μg of protein) were denatured, separated by SDS/PAGE, and transferred overnight onto nitrocellulose membrane by using the MiniPROTEAN 3 apparatus (Bio-Rad). After blocking in PBS with 5% skimmed milk and 0.1% Tween 20 (Sigma), the membranes were incubated with primary antibody (see Table 1, which is published as supporting information on the PNAS web site). After washing, the membranes were incubated for 1 h in the appropriate secondary antibody. The blotted membranes were visualized by using the ECL Plus Western blotting detection system (Amersham Pharmacia).
Results
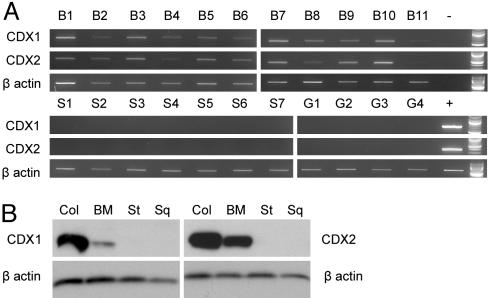
Tissue Expression of CDX1 and CDX2. CDX1 and CDX2 expression was evaluated in 11 cases of BM, 7 cases of normal esophageal squamous mucosa, and 4 cases of normal gastric body mucosa. CDX1 and CDX2 mRNAs were detected in all of the BM cases but in none of the normal gastric body or normal esophageal squamous samples (Fig. 1A). Western blotting also showed the presence of CDX1 and CDX2 protein in all three BM cases studied but in none of two normal gastric body or three esophageal squamous samples (Fig. 1B). The universal expression of CDX1 and CDX2 mRNA and protein in BM are consistent with a key role for either CDX1 or CDX2 in the induction and maintenance of the BM phenotype. However, as discussed in the introduction, the lack of methylation control of the expression of CDX2, and its down-regulation by etiological agents of BM, led us to focus on CDX1 as the primary basis for the induction and maintenance of BM.
Fig. 1.
CDX1 and CDX2 expression in gastrointestinal tissues. (A) CDX1, CDX2, and β-actin RT-PCRs performed on mRNA derived from normal esophageal squamous mucosal samples (S1–7), normal gastric mucosal samples (G1–4), and BM samples (B1–11). Positive (+) and water blank (–) controls and 100-bp ladders are all shown on the right side of the gels. (B) Examples of CDX1, CDX2, and β-actin Western blotting of lysates from normal colonic mucosa (Col), BM, normal gastric body mucosa (St), and normal esophageal squamous mucosa (Sq).
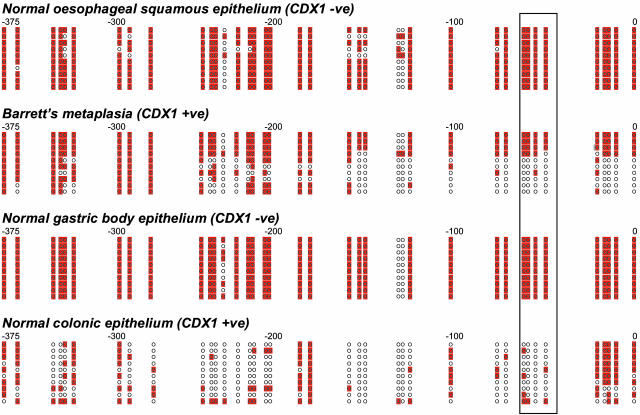
Regulation of Tissue-Specific CDX1 Expression. To investigate whether the differences in CDX1 expression were under promoter methylation control, bisulfite sequencing was carried out on DNA extracted from epithelium dissected from normal esophagus, normal colon, normal gastric body, and cases of BM. The CDX1 promoter region was completely methylated in normal squamous and gastric epithelia, in keeping with their lack of CDX1 mRNA or protein expression (Fig. 2). In contrast, the CDX1 promoter region in colonic epithelium was predominantly unmethylated. A majority of DNA clones from BM showed demethylated CDX1 promoter sequence, particularly involving the CpGs that appear to be critical for the control of CDX1 expression in CRC (16) (Fig. 2).
Fig. 2.
CDX1 promoter bisulfite sequencing of normal esophageal squamous epithelium, BM, normal gastric body epithelium, and normal colonic epithelium. For each tissue sample, relative location and methylation status of CDX1 promoter CpGs for 10 clones are shown. Each circle represents a CpG, and base positions relative to the CDX1 transcription start site are shown above each cluster of clones. Methylated CpGs are shaded. CpGs enclosed by the box (positions –53 to –65) represent those suggested to be crucial for transcriptional control (16).
These results support the hypothesis that CDX1 promoter methylation status determines the expression of CDX1 in normal and metaplastic tissues of the gastrointestinal tract.
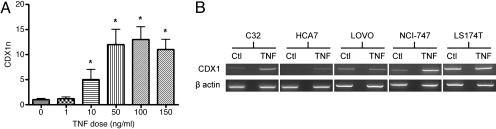
TNF-α, CDX1 mRNA Expression, and CDX1 Promoter Methylation Status. Previous studies have demonstrated that the CRC cell line HT29 responds to TNF-α treatment by showing, for example, an increase in IL-8 expression (23). HT29 was therefore used in initial studies to explore possible effects of TNF-α on CDX1 expression. HT29 expresses a low, although readily detectable, basal level of CDX1 mRNA, which was further increased by TNF-α at a dose of 10 ng/ml and higher, reaching a plateau at a dose of 50 ng/ml (Fig. 3A). This effect of TNF-α on CDX1 expression required at least 3 h of treatment with the cytokine.
Fig. 3.
TNF-α treatment of CRC cell lines. (A) CDX1 real-time PCR performed on HT29 cells treated with different doses of TNF-α for 5 h. The y axis (CDX1n) represents the amount of CDX1 normalized to GAPDH and relative to a calibrator (0 ng/ml sample). The error bars represent the calculated standard error of CDX1n for each sample. * denotes a significantly (at the 95% level) higher CDX1n of the sample compared with that of the calibrator. (B) CDX1 and β-actin RT-PCRs performed after TNF-α treatment of five CRC cell lines. Corresponding control-treated cell lines (Ctl) are also shown.
To test whether TNF-α could induce de novo expression of CDX1, four cell lines that normally lack detectable CDX1 mRNA (the CRC cell lines C10 and HCT116 and the esophageal squamous cell carcinoma cell lines KYSE30 and OE21) were treated with TNF-α at 50 ng/ml for 5 h. TNF-α treatment failed to induce any CDX1 mRNA expression in these four lines, all of which have a fully methylated CDX1 promoter, whereas HT29 has a completely unmethylated CDX1 promoter (ref. 16 and unpublished data). When four CRC cell lines with partially methylated CDX1 promoters [C32, CC07, HCA7, and NCI-747 (16)] were treated with TNF-α, all four showed an increase in CDX1 mRNA expression (all but CC07 are shown in Fig. 3B).
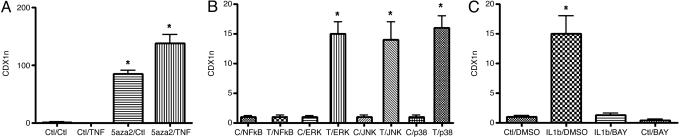
As additional confirmation that CDX1 promoter methylation status determines the effect of TNF-α on CDX1 expression, KYSE30 cells were treated with 5aza2 and then with TNF-α or a treatment control. In contrast to treatment with TNF-α alone, 5aza2 treatment alone induced de novo CDX1 mRNA expression in KYSE30 (Fig. 4A). However, TNF-α induced a further increase in CDX1 expression on top of the effects of 5aza2 treatment (Fig. 4A).
Fig. 4.
5aza2, signaling inhibitor, and cytokine treatments. (A) CDX1 real-time PCR performed on KYSE30 cells treated without 5aza2 or TNF-α, with 5aza2 or TNF-α, or with both. The calibrator used was the “control/control” treated sample. (B) CDX1 real-time PCR performed on four pairs of C32 cell cultures. Each pair was pretreated with an inhibitor of the NF-κB, ERK, c-Jun N-terminal kinase (JNK), or p38 MAPK signaling pathways. One of each pair of cultures was then treated with 50 ng/ml TNF-α (T) or control medium (C) for 5 h. The calibrator used for each pair was the control-treated sample. (C) CDX1 real-time PCR performed on C32 cells. The cells were pretreated with BAY11-7085 (BAY) or its carrier medium DMSO and then treated with 50 ng/ml IL-1β or control medium (Ctl). The calibrator used was the “control/DMSO” treated sample. The format of all three graphs is as for Fig. 3A.
The only exceptions to the relationship between methylation of the CDX1 promoter and TNF-α induction of CDX1 were two CRC cell lines (Fig. 3B); one cell line expressed a high basal level of CDX1 (LS174T), and the other expressed a high endogenous level of TNF-α mRNA (LOVO) (data not shown).
NF-κB Signaling and CDX1 Expression. The cellular effects of TNF-α are known to be mediated by a variety of signaling pathways, the best characterized of which include the NF-κB and the various MAPK pathways (24). To determine which of these pathways might mediate the TNF-α-induced increase in CDX1 expression, the cell line C32 was treated with selective inhibitors of these pathways followed by TNF-α. As shown in Fig. 4B, the NF-κB inhibitor BAY11-7085 completely abolished the TNF-α-induced increase in CDX1 expression, whereas this increase was not affected at all by any of the three MAPK pathway inhibitors.
The NF-κB signaling pathway is shared by TNF-α and IL-1β (24). Incubation of C32 cells in IL-1β for 5 h at concentrations of 50–100 ng/ml consistently increased CDX1 mRNA. This effect of IL-1β was completely abolished by pretreatment with BAY11-7085 (Fig. 4C).
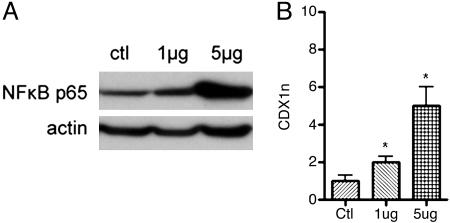
To confirm that NF-κB signaling has a direct effect on CDX1 expression, the active NF-κB subunit (p65) was transiently transfected into C32 cells. Western blots of nuclear protein lysates confirmed that the transfection had successfully induced nuclear signaling of NF-κB p65 (Fig. 5A). The C32 cells showed a clear dose-response-related increase of CDX1 mRNA expression after p65 transfection (Fig. 5B).
Fig. 5.
Transfection of C32 cells with NF-κB p65. The cell line was transfected with 1 μg or 5 μg of NF-κB p65 expression vector or with empty vector as a treatment control (ctl). (A) Western blotting of nuclear lysates confirmed successful activation of NF-κB signaling as indicated by increased nuclear expression of NF-κB p65 protein (β-actin was used as a loading control). (B) CDX1 real-time PCR showed that C32 cells responded to the NF-κB p65 transfection-mediated expression with an increase in CDX1 mRNA expression. The format of the graph is as for Fig. 3A. The calibrator used was the “control” transfected sample.
These overall results strongly imply that NF-κB signaling is the primary mediator of the TNF-α- and IL-1β-induced effects on CDX1 expression.
Effects of Acid and Bile Salts on CDX1 Expression. Acid and bile salts may affect CDX1 expression in esophageal epithelial cells indirectly by triggering an inflammatory response and so increasing local levels of TNF-α and IL-1β. However, it has been suggested that at least acid may have direct effects on epithelial cells, mediated by MAPK signaling pathways (18). We therefore tested the effects of acid and bile salts on CDX1 expression in C32 cells.
CDX1 mRNA expression in C32 cells was increased after 5-h exposure to bile salts or to acidified bile salts but not to acid alone (Fig. 6B). As reported in ref. 18, acid treatment did activate the ERK pathway (Fig. 6A). However, the effect of bile salt exposure on CDX1 expression in C32 cells was associated with NF-κB p65 nuclear signaling, with no evidence of ERK pathway activation (Fig. 6A).
Fig. 6.
Treatment of C32 cells with either acid (pH 3.5) or bile salts or both. Mock-treated C32 cells (ctl) served as a control. (A) Western blotting of nuclear lysates showed that bile salts alone, or acidified bile salts, activated NF-κB signaling. (B) The treatments were also associated with an increase in CDX1 mRNA expression as assessed by real-time PCR. The format of the graph is as for Fig. 3A. The calibrator used was the control treated sample.
Treatment of OE21 cells with acid and/or bile salts showed similar activation of NF-κB p65 signaling (either with bile salts alone or with acidified bile salts) but no induction of CDX1 mRNA expression (data not shown). The difference between OE21 and C32 is presumably due to the fact that OE21 has a fully methylated CDX1 promoter, whereas that of C32 is only partially methylated. This lack of response of OE21 cells is similar to the failure of TNF-α to induce de novo CDX1 mRNA expression when the CDX1 promoter is fully methylated.
Discussion
Our data suggest that CDX1 is the molecular link between the etiological agents that cause BM and the induction of an intestinal phenotype and that NF-κB signaling plays the key role in activating CDX1 expression. This signaling, however, only affects CDX1 expression if the CDX1 promoter is either unmethylated or partially methylated, as is expected on the basis that promoter methylation prevents access to regulatory transcription factors (25). We suggest, therefore, that CDX1 promoter demethylation is the key trigger for the development of BM. Gene-specific methylation or demethylation must be based on the recognition of the relevant DNA sequence, which can only be achieved, as far as is known at present, by either DNA binding transcription factors or by small noncoding RNAs. Both have been shown to cause demethylation (26, 27). It seems possible that prolonged conditions of gastroesophageal reflux disease, and hence NF-κB signaling, could trigger demethylation by one or another of these mechanisms. There are at least four potential NF-κB subunit binding sites in the promoter 5′UTR region of CDX1 and preliminary data (not shown) indicate that the NF-κB p65 subunit does bind to the CDX1 promoter and so can activate CDX1 transcription directly. We have not, however, so far been able to show that prolonged incubation of cell lines with methylated CDX1 promoters, in the presence of TNF-α or bile salts, leads to de novo CDX1 expression or to demethylation of the CDX1 promoter. Clearly, the conditions for such a switch need to be further explored.
Transgenic mouse studies, in which ectopic expression of CDX1 in the gastric epithelium induces an intestinal phenotype (6), support the hypothesis that CDX1 alone may induce a complete intestinal phenotype in other tissues such as the esophagus. We have preliminary data suggesting that CDX1 expressed in primary cultured esophageal squamous cells by transient transfection induces expression of A33 (unpublished data), a glycoprotein whose expression is essentially limited to intestinal epithelium (28). There are also published in vitro data showing that CDX1 can regulate directly the expression of other gut-related molecules, such as MUC2 (29) and pancreatitis associated protein I (30). It is clearly possible that CDX1 cooperates with other molecules in both inducing and maintaining a complete intestinal phenotype. CDX2 is an obvious candidate for such cooperation in view of its putative role in normal intestinal epithelial development (3) and its ability to induce intestinal metaplasia in the murine stomach (7, 8).
In conclusion, based on our findings and other previously published data on CDX1 and CDX2, we propose that BM, and probably other intestinal metaplasias (IMs), are triggered by injurious agents that can activate either directly or indirectly (by means of, for example, TNF-α and IL-1β) the expression of CDX1 through NF-κB signaling. This, in turn, initiates the development of the intestinal phenotype. Drugs that target cytokine and/or NF-κB signaling or that are generally antiinflammatory, such as nonsteroidal antiinflammatory drugs, may therefore have a role in preventing BM and other forms of IM and thus adenocarcinomas of the esophagus and stomach. Interestingly, both carcinomas show loss of CDX1 expression (12), although this loss of expression may relate to putative tumor suppressor activity of the protein (15, 16). The key event that enables and maintains the expression of CDX1 in BM is presumed to be the demethylation of the CDX1 promoter by gene-specific mechanisms that remain to be elucidated.
Supplementary Material
Acknowledgments
We thank Drs. R. C. Fitzgerald and L. J. Hardie for their advice. This work was funded by Cancer Research UK, CORE (formerly the Digestive Disorders Foundation), and the Jean Shanks Foundation.
Abbreviations: 5aza2, 5-aza-2′-deoxycytidine; BM, Barrett's metaplasia; CRC, colorectal carcinoma; MAPK, mitogen-activated protein kinase; ERK, extracellular signal-regulated kinase.
Footnotes
Kapoor, N., Yu, L. & Bodger, K. (2004) Gut 53, Suppl. iii, A15 (abstr.).
References
- 1.Nandurkar, S. & Talley, N. J. (1999) Am. J. Gastroenterol. 94, 30–40. [DOI] [PubMed] [Google Scholar]
- 2.Wild, C. P. & Hardie, L. J. (2003) Nat. Rev. Cancer 3, 676–684. [DOI] [PubMed] [Google Scholar]
- 3.Freund, J. N., Domon-Dell, C., Kedinger, M. & Duluc, I. (1998) Biochem. Cell Biol. 76, 957–969. [DOI] [PubMed] [Google Scholar]
- 4.Eda, A., Osawa, H., Yanaka, I., Satoh, K., Mutoh, H., Kihira, K. & Sugano, K. (2002) J. Gastroenterol. 37, 94–100. [DOI] [PubMed] [Google Scholar]
- 5.Eda, A., Osawa, H., Satoh, K., Yanaka, I., Kihira, K., Ishino, Y., Mutoh, H. & Sugano, K. (2003) J. Gastroenterol. 38, 14–22. [DOI] [PubMed] [Google Scholar]
- 6.Mutoh, H., Sakurai, S., Satoh, K., Osawa, H., Hakamata, Y., Takeuchi, T. & Sugano, K. (2004) Gut 53, 1416–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mutoh, H., Hakamata, Y., Sato, K., Eda, A., Yanaka, I., Honda, S., Osawa, H., Kaneko, Y. & Sugano, K. (2002) Biochem. Biophys. Res. Commun. 294, 470–479. [DOI] [PubMed] [Google Scholar]
- 8.Silberg, D. G., Sullivan, J., Kang, E., Swain, G. P., Moffett, J., Sund, N. J., Sackett, S. D. & Kaestner, K. H. (2002) Gastroenterology 122, 689–696. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald, R. C., Onwuegbusi, B. A., Bajaj-Elliott, M., Saeed, I. T., Burnham, W. R. & Farthing, M. J. (2002) Gut 50, 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamaguchi, M., Fujiwara, Y., Takashima, T., Hayakawa, T., Sasaki, E., Shiba, M., Watanabe, T., Tominaga, K., Oshitani, N., Matsumoto, T., et al. (2003) Digestion 68, 189–197. [DOI] [PubMed] [Google Scholar]
- 11.Faller, G., Dimmler, A., Rau, T., Spaderna, S., Hlubek, F., Jung, A., Kirchner, T. & Brabletz, T. (2004) J. Pathol. 203, 904–908. [DOI] [PubMed] [Google Scholar]
- 12.Silberg, D. G., Furth, E. E., Taylor, J. K., Schuck, T., Chiou, T. & Traber, P. G. (1997) Gastroenterology 113, 478–486. [DOI] [PubMed] [Google Scholar]
- 13.Kim, S., Domon-Dell, C., Wang, Q., Chung, D. H., Di Cristofano, A., Pandolfi, P. P., Freund, J. N. & Evers, B. M. (2002) Gastroenterology 123, 1163–1178. [DOI] [PubMed] [Google Scholar]
- 14.Seery, J. P. (2002) J. Cell Sci. 115, 1783–1789. [DOI] [PubMed] [Google Scholar]
- 15.Suh, E. R., Ha, C. S., Rankin, E. B., Toyota, M. & Traber, P. G. (2002) J. Biol. Chem. 277, 35795–35800. [DOI] [PubMed] [Google Scholar]
- 16.Wong, N. A. C. S., Britton, M. P., Choi, G. S., Stanton, T. K., Bicknell, D. C., Wilding, J. L. & Bodmer, W. F. (2004) Proc. Natl. Acad. Sci. USA 101, 574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinoi, T., Loda, M. & Fearon, E. R. (2003) J. Biol. Chem. 278, 44608–44616. [DOI] [PubMed] [Google Scholar]
- 18.Souza, R. F., Shewmake, K., Terada, L. S. & Spechler, S. J. (2002) Gastroenterology 122, 299–307. [DOI] [PubMed] [Google Scholar]
- 19.Morgan, C., Alazawi, W., Sirieix, P., Freeman, T., Coleman, N. & Fitzgerald, R. (2004) Am. J. Gastroenterol. 99, 218–224. [DOI] [PubMed] [Google Scholar]
- 20.Nehra, D., Howell, P., Williams, C. P., Pye, J. K. & Beynon, J. (1999) Gut 44, 598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frommer, M., McDonald, L. E., Millar, D. S., Collis, C. M., Watt, F., Grigg, G. W., Molloy, P. L. & Paul, C. L. (1992) Proc. Natl. Acad. Sci. USA 89, 1827–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Applied Biosystems (1997) User Bulletin No. 2: ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA), p. 14.
- 23.Vallee, S., Fouchier, F., Bremond, P., Briand, C., Marvaldi, J. & Champion, S. (2003) Biochem. Biophys. Res. Commun. 305, 831–839. [DOI] [PubMed] [Google Scholar]
- 24.MacEwan, D. J. (2002) Cell. Signalling 14, 477–492. [DOI] [PubMed] [Google Scholar]
- 25.Jubb, A. M., Bell, S. M. & Quirke, P. (2001) J. Pathol. 195, 111–134. [DOI] [PubMed] [Google Scholar]
- 26.Lin, I. G. & Hsieh, C. L. (2001) EMBO Rep. 2, 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imamura, T., Yamamoto, S., Ohgane, J., Hattori, N., Tanaka, S. & Shiota, K. (2004) Biochem. Biophys. Res. Commun. 322, 593–600. [DOI] [PubMed] [Google Scholar]
- 28.Sakamoto, J., Kojima, H., Kato, J., Hamashima, H. & Suzuki, H. (2000) Cancer Chemother. Pharmacol. 46, Suppl., S27–S32. [DOI] [PubMed] [Google Scholar]
- 29.Mesquita, P., Jonckheere, N., Almeida, R., Ducourouble, M. P., Serpa, J., Silva, E., Pigny, P., Silva, F. S., Reis, C., Silberg, D., et al. (2003) J. Biol. Chem. 278, 51549–51556. [DOI] [PubMed] [Google Scholar]
- 30.Moucadel, V., Soubeyran, P., Vasseur, S., Dusetti, N. J., Dagorn, J. C. & Iovanna, J. L. (2001) Eur. J. Cell Biol. 80, 156–163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.