Abstract
In the past two decades, studies of anatomy, behavior, and, most recently, DNA sequences have clarified the phylogeny of the ants at the subfamily and generic levels. In addition, a rich new harvest of Cretaceous and Paleogene fossils has helped to date the major evolutionary radiations. We collate this information and then add data from the natural history of the modern fauna to sketch a history of major ecological adaptations at the subfamily level. The key events appear to have been, first, a mid-Cretaceous initial radiation in forest ground litter and soil coincident with the rise of the angiosperms (flowering plants), then a Paleogene advance to ecological dominance in concert with that of the angiosperms in tropical forests, and, finally, an expansion of some of the lineages, aided by changes in diet away from dependence on predation, upward into the canopy, and outward into more xeric environments.
Keywords: ecology, evolution, phylogeny, sociobiology
Humanity lives in a world largely filled by prokaryotes, fungi, flowering plants, nematode worms, spiders, mites, and six ecological keystone insect groups: termites, hemipteran “true” bugs, phytophagan beetles, cyclorraphan flies, glossatan moths, and hymenopterans, the last comprising bees, wasps— and ants (1). Ants are especially notable among the insects for their ecological dominance as predators, scavengers, and indirect herbivores. Although the ≈11,000 species of the ant family (Formicidae) make up <2% of the known global insect fauna, they compose at least one-third of its biomass (2). In the Brazilian Amazon, as judged by one survey (3), the biomass of ants is approximately four times greater than that of all of the land vertebrates (mammals, birds, reptiles, and amphibians) combined.
The combination of advanced colonial life and worldwide environmental influence gives special significance to the evolutionary history of the ants. Recent phylogenetic analyses, when combined with data from a rich new harvest of Cretaceous and Paleogene fossils and the natural history of the modern fauna, permit a defensible reconstruction of the broad-scale ecological features of ant evolution.
The Earliest Ants
Ants evidently arose during the Cretaceous period at somewhat more than 100 million years ago. Their earliest known fossils fall into two groups. The first is the very primitive Mesozoic subfamily Sphecomyrminae. The second group consists of primitive members of the extant subfamily Formicinae and the poneromorph group of subfamilies, as recently divided (4), comprising the abundant and diverse Ponerinae and five other less prominent subfamilies.
The workers of Sphecomyrma, the best known sphecomyrmine genus, were a mosaic of ant and wasp traits (5, 6). Specimens have been found in deposits of Late Cretaceous amber (fossilized resin) in Asia, Siberia, and North America, hence across much of the northern supercontinent of Laurasia (7, 8).
Unlike the ants of later, Paleogene deposits, the sphecomyrmines are very rare. Among the many thousands of Cretaceous insect specimens examined from New Jersey, Canada, and Burma, only seven specimens have enough features preserved to be called definitively sphecomyrmine workers. Of the biology of these ancient ants it can be said only that they lived in mesic forests with rich floras and insect faunas. The exact timing and causes of their extinction in the final 10 or 20 million years of that era remain unknown (9, 10).
Early in the history of the sphecomyrmines, a wider radiation beyond the stem genus Sphecomyrma occurred. In addition to clearly derivative sphecomyrmine genera were a sphecomyrmine-ponerine intermediate (8), an apparent true poneromorph, a member of the advanced subfamily Formicinae (9), and a possible member of the subfamily Aneuretinae, considered to be precursors of the modern subfamily Dolichoderinae (10).
What appear to be the oldest certifiable ant fossils include Gerontoformica cretacica, from the Early Cenomanian to Uppermost Abian (Lower Cretaceous) amber of France, dated to ≈100 million years B.P. (11). Because of imperfect preservation, it cannot be placed with reference to fossil or existing subfamilies but evidently contains a mix of sphecomyrmine and more derivative traits. The Burmese amber (10), containing sphecomyrmines and a possible myrmeciine, may be of comparable or even somewhat older provenance.
In still other very early deposits have been found additional products of the initial ant radiation, which appear to be members or precursors of the bulldog ant subfamily Myrmeciinae, including the living “dawn ant” Nothomyrmecia macrops. The subfamily is today limited to Australia, with one very rare species of Myrmecia on New Caledonia. A possible but still uncertain myrmeciine or myrmeciine precursor, Cariridris bipetiolata, has been described from a single, poorly preserved specimen in the Santana Formation of Brazil, ≈110 million years old. What seem to us to be other myrmeciines are 10 rock fossils from the Upper Cretaceous Orapa deposits of Botswana (≈90 million years B.P.) (12). Several later myrmeciines of Paleogene age, Ameghinoia and Polanskiella of Argentina, Archimyrmex of the U.S. Green River Formation, and two species of Prionomyrmex recorded from the Baltic amber, bear witness to the spread of the subfamily around the world, followed by its retreat to Australia. It is reasonable to suppose that the Myrmeciinae diverged from the sphecomyrmine stem and spread extensively by Late Cretaceous or Eocene times (13, 14).
By Paleocene times, as evidenced in 10 specimens of ants from the presumed Paleocene amber of Sakhalin, dolichoderines and aneuretines (the latter with one contemporary species, Aneuretus simoni, surviving in Sri Lanka) had made their appearance alongside of poneromorphs, myrmeciines, and formicines (15).
By the Early to Middle Eocene, as revealed by the recently described Fushun amber fauna from northeastern China that is ≈50 million years or somewhat younger in age, the diversification of the major groups of ants was in full play (16). Among some 20 identifiable specimens of workers and queens is a variety of primitive ponerines and myrmicines as well as primitive members of the “formicoid” complex, comprising formicines and an assortment of what are either primitive dolichoderines, aneuretines, or both. Most also bear traits shared with their presumed sphecomyrmine or sphecomyrmine-like ancestors, including short mandibles with small numbers of teeth, circular or ovoid head shapes, and relatively unmodified middle body segments. Several sedimentary compression fossils of Early Eocene provenance recently discovered in British Columbia also represent relatively primitive poneromorphs (B. Archibald, personal communication).
Emergence of the Modern Fauna
The next glimpse into the history of the ants has come from a set of three specimens found in a middle Eocene amber deposit at Malvern, Arkansas, that represents three of the dominant subfamilies in the present-day fauna: Myrmicinae, Dolichoderinae, and Formicinae (17).
That the modern radiation had not only begun by Eocene times but was full-blown is disclosed by the Baltic amber fossils, which are Middle Eocene in age. In examining 10,988 specimens as part of their pioneering ant fossil studies, G. Mayr (18) and W. M. Wheeler (19) distinguished no fewer than 92 genera. Ants were among the dominant insects, and the species as a whole had a distinctly modern aspect, despite its great age of ≈45 million years.
Not surprisingly, the richly documented and younger amber of the Dominican Republic, which is Early Miocene in age (and thus roughly 20 million years old) has an even more modern composition. Of 38 genera and well defined subgenera identified (20), 34 have survived somewhere in the New World tropics to the present, although all of the species studied thus far are extinct. Of the surviving genera and subgenera, at least 22 persist on Hispaniola (Dominican Republic plus Haiti). Fifteen genera and subgenera have colonized the island since amber times, restoring the number to 37.
Origin and Phylogeny
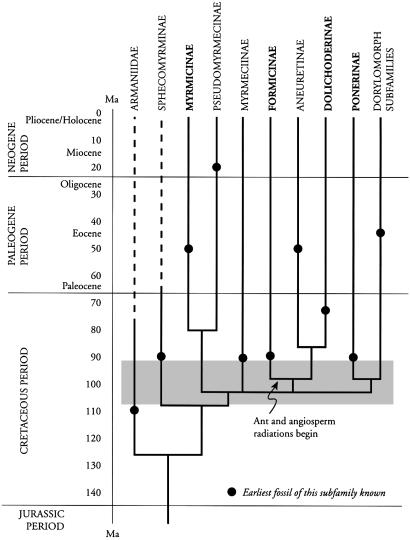
The phylogeny of ants has been the subject of several steadily improving reconstructions during the past half-century, starting with morphological data of living faunas (9, 14, 21–23), then advancing with additional morphological information supplemented by fossil evidence, as reviewed here, and, finally, by the addition of DNA sequence data (24, 25). Fig. 1 presents a simplified version of what we interpret to be the consensus among recent published reconstructions. The exact origin of the ants (family Formicidae), involving an ancestral form presumably close to Sphecomyrma, as well as the timing of the origin, remain to be clarified. One estimate from DNA divergence extrapolation places the origin of ants at ≈140 million years B.P. (ma) (26); another puts it at 185 ± 36 ma (95% confidence limits) (24).
Fig. 1.
A schematic of the evolution of ants (Formicidae) at the subfamily level. Minor subfamilies are omitted. The four most diverse, abundant, and geographically widespread living subfamilies are bold-faced. The “dorylomorphs” are driver and army ants (Dorylinae) and an array of other, smaller subfamilies related to them. The schematic is based on our interpretation of the consensus of recent phylogenetic reconstructions (8, 9, 23–26). The Armaniidae were a Cretaceous sister group of the Formicidae that were believed not to be social; i.e., they had no anatomically distinct worker class (7).
The Ponerine Paradox
What was the fate of the first evolutionary radiation of the ants? The Sphecomyrminae evidently vanished by the end of the Mesozoic. The myrmeciines retreated to Australia and New Caledonia, and the aneuretines retreated to Sri Lanka. But the myrmicines, formicines, and dolichoderines, together with the ponerines (the dominant poneromorph subfamily), not only flourished, but spread worldwide as ecologically pre-eminent insect groups. The history of the Ponerinae is of special interest in this story. Evidently monophyletic and comprising three tribes and 25 genera (4), the ponerines are as variable overall in anatomical characteristics and patterns of colony organization as any of the other subfamilies. Yet, despite being with the Formicinae and Myrmeciinae members of the oldest documented living phylogenetic assemblage and despite having achieved such prolific diversification and geographic breadth, they remain oddly primitive in their general social organization (27).
Queens and workers of ponerine species are much closer in size than are the castes in the “higher” ant subfamilies Myrmicinae, Dolichoderinae, and Formicinae and army ant subfamilies.
Most ponerine queens have relatively low fertility, seldom producing more than five eggs each day.
Corresponding to the low degree of queen fecundity, colony sizes of ponerines are small.
Young ponerine queens of many, perhaps most, species start new colonies independently but not claustrally. That is, they leave their natal nest, mate, and construct a nest of their own or find a preformed cavity. But then, like some wasps and other poneromorph ants, they forage outside the nest to obtain at least part of the food with which they rear the first brood of workers.
With a few, sometimes striking exceptions (28, 29), workers of ponerine species forage alone and do not use odor trails or other pheromone signals to recruit nestmates to food sources they encounter.
True oral trophallaxis, the exchange of regurgitated food among adults and between workers and larvae, is wide-spread in the higher subfamilies Myrmicinae, Dolichoderinae, and Formicinae. It is evidently much less so in the Ponerinae and, to date, has been observed only in the closely related genus Ponera and Hypoponera (30), with liquid otherwise transferred from drops held between the mandibles (31).
This, then, is the ponerine paradox: globally successful yet socially primitive. The puzzle might be partially resolved if the more advanced ant subfamilies can be shown to be derived from a ponerine stock or, put in the more exact language of cladistics, the paradox is partly soluble if the ponerines prove to be polyphyletic (of multiple origins). But even if such proves to be the case (contrary to the opinion of systematists who consider the ponerines overall monophyletic), there likely remain diverse modern subgroups that are surely monophyletic, such as the very large and global tribe Ponerini. So the paradox would not be truly resolved by taxonomic splitting.
The full and definitive solution to the ponerine paradox and other mysteries of ant evolution requires more information from paleontology and ecology than now exists. In the interim, and in the hope of stimulating research in the critical areas, we offer the following combined phylogenetic and ecological “dynastic-succession” hypothesis (under the “Dynastic-Succession Hypothesis” heading below), consistent with existing knowledge (commentary added above and below heading).
Possibly toward the end of Cretaceous times, but more likely during Paleocene and Early Eocene times, after the end-of-Mesozoic extinction spasm of many plant and animal groups, the ponerines underwent most of the adaptive radiation in taxonomic tribes and adaptive types that persist to the present time.
The fossil record of this interval, spanning some 30 million years, is still poorly represented for ants, preventing an exact placement of the ponerine radiation in geological history.
During this primary expansion, the ponerines became entrenched worldwide as arthropod predators, especially in warm-temperate and tropical mesic forests. They also evolved to favor ground and leaf-litter sites. In effect, they pre-empted this array of opportunities, partially blocking the sites from the later, otherwise more successful radiations of the advanced subfamilies Dolichoderinae and Formicinae. Of the “big four” in present-day diversity and geographic reach, only the Myrmicinae rivaled the Ponerinae in invasion of the forest-floor predator niches. Either the myrmicine radiation coincided with the peak of the ponerine expansion or followed close behind it.
The diets of by far the most known ponerines consist primarily or exclusively of fresh insect and other arthropod prey, supplemented by the scavenging of arthropods newly killed by other causes. Many ponerine species are, moreover, specialized predators (22, 27).
Ants foraging as solitary huntresses have small colonies, a necessity imposed by the relative paucity of their food, and the more so when they specialize on particular kinds of prey. Predation as a way of life and small colony size, in turn, render other social traits simple and hence “primitive.” Foraging tends to be solitary, recruitment and alarm simple, and worker subcastes few to none; workers are prepared to reproduce on their own in the absence of the queen, and brood care tends to be relatively unorganized.
The ponerines and other poneromorphs do well as predators and also as occupants of nest sites on forest floors in warm regions around the world (32). The favored sites are small spaces in the many dimensions of the litter, including the interiors of rotting logs and stumps, tree limbs and twigs lying on the ground, clusters of dead leaves, masses of bryophytes, and the root systems of living trees, shrubs, and herbaceous plants.
The ponerines hold their own in this complex and nutrient-rich environment. In samples recently analyzed from forested localities around the world (32), poneromorphs (mostly ponerines) composed 22.2% of the species and 12.4% of the specimens, compared with 10.6% of the species and 12.9% of the specimens for the formicines and a relatively paltry 1.1% of the species and 0.5% of the specimens for the dolichoderines. But these three subfamilies of the “big four” were dwarfed by the myrmicines, which made up 65.2% of the species and 73.7% of the specimens. Clearly, the myrmicines, many of which (such as members of the tribes Dacetini and Basicerotini) are specialized predators like the ponerines, are the rulers of the world's forest litter.
The soil and ground litter of the world's angiosperm forests, and especially tropical forests, comprise the habitat with the highest density and species diversity of ants. Because all of the subfamilies of ants since their origins in the mid-Cretaceous have living representatives, save the Sphecomyrminae and Formiciinae (the latter were giant ants, not to be confused with Formicinae), and most of the genera originating since the late Eocene also have them, it is reasonable to suppose that the tropical forest soil and ground litter has always played the same role. This habitat is reasonably interpreted to be the headquarters of ant evolution, from which major ant groups have spread into other habitats or, in a great many cases, failed to do so.
The picture changes radically for the ponerines and most other poneromorphs, for example, in habitats away from the tropical and warm-temperate forests. They are notably scarce in cool-temperate forests, deserts, and arid grasslands. (In contrast, one other poneromorph subfamily, the Ectatomminae, has done well in these peripheral habitats, especially in Australia.)
The picture changes in equal degree across a few vertical meters in the forests and thence on up into the forest canopy. In the Amazonian and Bornean forests, for example, subfamily dominance is nearly flipped upside down. There, the formicines and dolichoderines have risen sharply in numbers relative to the myrmicines, while the ponerines have dropped to very low levels (33, 34).
The great majority of the canopy ant species appear to be specialized for arboreal life, as opposed to being invaders from ground nests. Oddly, the arboreal ants, as a whole, are so abundant and possess such a large part of the animal biomass as to be inviable if they lived exclusively as predators and scavengers. Although tropical arboreal ants are, in general, highly efficient in these roles (35), there are not enough herbivorous insects available as immediate sources of protein to support them. This additional paradox now appears solved: It has been learned that in the Amazon forest, at least a large fraction of the ant populations of the canopy are not primarily predators but “cryptic herbivores,” subsisting on the liquid exudates of hemipterous sap-feeding insects, including scale insects and treehoppers (36, 37). They further tend many of these insects as a kind of cattle, protecting them from parasites and predators. Extrafloral nectaries are yet another significant plant-based source of nutrition.
Around the world, in all major habitats, a large fraction of the species of dolichoderines and formicines have invested their biology heavily in symbioses with aphids, mealybugs, and other homopterous insects. Myrmicines have done so to lesser degree, and ponerines almost never. Although quantitative data are lacking, these disparities are commonly observed in natural history studies, and it seems clear that the dolichoderines and formicines have benefited in the growth of their biomass and diversity through coevolution with symbiotic homopterans. These observations lead to the final part of the dynastic-succession hypothesis.
The Dynastic-Succession Hypothesis
The K/P (Cretaceous/Paleogene, or end-of-Mesozoic), or K/T (Cretaceous/Tertiary, as commonly denoted) extinction event may have terminated the sphecomyrmine ants, or these insects could have vanished before the event, but anatomically primitive ponerines soldiered on. During the reassembly and continued expansion of the flowering plants, which replaced much of the old gymnosperm flora worldwide, forest litter became more complex. (In all respects, especially structural but also chemical and microclimatic, the litter of angiosperm forests is much better for ant colonies than that of gymnosperms.) Insects inhabiting the soil, ground litter, and vegetation of the forests and savannas grew correspondingly diverse and abundant. In Paleocene and Early Eocene times, the ponerines experienced an adaptive radiation, and some of the genera appearing back then have survived to the present time.
During, or perhaps more precisely, toward the end of the ponerine expansion, and probably no later than the Early Eocene, the myrmicines began their own radiation. They became formidable competitors of the ponerines for both prey and nest sites. In time, they equaled and then surpassed the ponerines in biomass and diversity. Many also added seeds and elaiosomes to their diets and, at least partly as a result of these important new sources of oils and carbohydrates, were able to expand more effectively into deserts and dry grasslands.
Most importantly, some of the myrmicines added symbioses with homopterans to their repertory: largely, scale insects and treehoppers in tropical and warmtemperate vegetation, aphids in cooltemperate vegetation, and mealybugs underground everywhere. Similar symbioses were contracted with the caterpillars of honeydew-secreting butterflies.
Dolichoderines and formicines also diversified, perhaps with the myrmicines but more likely later, in Early to Middle Eocene times. They were less successful than the ponerines and myrmicines in the forest litter environment, having been preempted there by these two groups, but more successful at creating homopteran symbioses. However, they were able to penetrate environments less available to predators, including cool-temperate climates and tropical forest canopies. Their success is reflected in their high levels of abundance in amber (especially worker specimens) and rock fossils (winged specimens), as would be expected from a preponderance in arboreal habitats.
In a phrase, the breakout of the dolichoderines and formicines, and to some extent that of the myrmicines, was due to a change in diet. This shift, in turn, was aided by the rising dominance of angiosperms over much of the land environment, an expansion that began in the Cretaceous and culminated in the Paleocene and Eocene. It was furthered by the expansion of the honeydew-producing homopterans and lepidopterans, groups also favored by the angiosperm dominance.
Future Research
The ecological history of the ants through geological time, culminating in the profusion of complexly social creatures around us today, must be accounted one of the great epics of evolution. Its unfolding, however, can still be glimpsed only in fragments. Large gaps remain in the fossil record, especially across the critical period of major radiation extending from the late Cretaceous into the Paleogene. Of equal consequence, the life cycles and natural history of the vast majority of living species, which still bear the indelible stamp of this history, remain unexplored.
Acknowledgments
We thank B. Archibald, D. Grimaldi, M. Kaspari, C. Saux, T. Schultz, and M. S. Wang for their invaluable help during the preparation of this article.
References
- 1.Grimaldi, D. (2000) Proc. XXI Int. Congr. Entomol. 1, xix–xxvii. [Google Scholar]
- 2.Wilson, E. O. (1990) Success and Dominance in Ecosystems: The Case of the Social Insects (Ecology Institute, Oldendorf/Luhe, Germany).
- 3.Fittkau, E. J. & Klinge, H. (1973) Biotropica 5, 2–14. [Google Scholar]
- 4.Bolton, B. (2003) Mem. Am. Entomol. Inst. 71, 1–103. [Google Scholar]
- 5.Wilson, E. O., Carpenter, F. M. & Brown, W. L. (1967) Science 157, 1038–1040. [DOI] [PubMed] [Google Scholar]
- 6.Wilson, E. O. (1985) Paleobiology 13, 44–55. [Google Scholar]
- 7.Dlussky, G. M. (1983) Paleontol. J. 17, 65–78. [Google Scholar]
- 8.Grimaldi, D. A., Agosti, D. & Carpenter, J. M. (1997) Am. Mus. Novitates 3208, 1–43. [Google Scholar]
- 9.Grimaldi, D. A. & Agosti, D. (2000) Proc. Natl. Acad. Sci. USA 97, 13678–13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimaldi, D. A., Engel, M. S. & Nascimbene, P. C. (2002) Am. Mus. Novitates 3361, 1–71. [Google Scholar]
- 11.Nel, A., Perrault, G., Perrichot, V. & Néraudeau, D. (2004) Geol. Acta 2, 23–29. [Google Scholar]
- 12.Dlussky, G. M., Brothers, D. J. & Rasnitsyn, A. P. (2004) Insect Syst. Evol. 35, 1–13. [Google Scholar]
- 13.Baroni Urbani, C. (2000) Ecol. Geol. Helv. 93, 471–480. [Google Scholar]
- 14.Ward, P. S. & Brady, S. G. (2003) Invert. Syst. 17, 361–386. [Google Scholar]
- 15.Dlussky, G. M. (1988) Paleontol. J. 22, 50–61. [Google Scholar]
- 16.Hong, Y.-C. (2002) Amber Insects of China (Beijing Scientific and Technological Publishing House/Henan Scientific and Technological Publishing House, Beijing), in 2 vols.
- 17.Wilson, E. O. (1985) Psyche (Cambridge, MA) 92, 205–216. [Google Scholar]
- 18.Mayr, G. (1868) Beit. Natur. Preussens Königliche Physikal.-Ökonom. Gesells. Königsberg 1, 1–102. [Google Scholar]
- 19.Wheeler, W. M. (1914) Schrift. Physikal.-Ökonom. Gesells. Königsberg 55, 1–142. [Google Scholar]
- 20.Wilson, E. O. (1985) Science 229, 265–267. [DOI] [PubMed] [Google Scholar]
- 21.Brown, W. L. (1954) Insectes Soc. 1, 21–31. [Google Scholar]
- 22.Hölldobler, B. & Wilson, E. O. (1990) The Ants (Harvard Univ. Press, Cambridge, MA).
- 23.Baroni Urbani, C., Bolton, B. & Ward, P. S. (1992) Syst. Entomol. 17, 301–329. [Google Scholar]
- 24.Crozier, R. H., Jermiin, L. S. & Chiotis, M. (1997) Naturwissenschaften 84, 22–23. [Google Scholar]
- 25.Astruc, C., Julien, J. F., Errard, C. & Lenoir, A. (2004) Mol. Phylogenet. Evol. 31, 880–893. [DOI] [PubMed] [Google Scholar]
- 26.Brady, S. G. (2003) Proc. Natl. Acad. Sci. USA 100, 6575–6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peeters, C. (1997) in Social Behavior in Insects and Arachnids, eds. Choe, J. C. & Crespi, B. J. (Cambridge Univ. Press, New York), pp. 372–391.
- 28.Baroni Urbani, C. (1993) Insectes Soc. 40, 233–250. [Google Scholar]
- 29.Witte, V. & Maschwitz, U. (2002) J. Insect Behav. 15, 195–217. [Google Scholar]
- 30.Hashimoto, Y., Yamauchi, K. & Hasegawa, E. (1995) Insect Soc. 42, 137–144. [Google Scholar]
- 31.Hölldobler, B. (1985) Isr. J. Entomol. 19, 89–99. [Google Scholar]
- 32.Ward, P. S. (2000) in Ants: Standard Methods for Measuring and Monitoring Biodiversity, eds. Agosti, D., Majer, J. D., Alonso, L. E. & Schultz, T. R. (Smithsonian Institution, Washington, D.C.), pp. 99–121.
- 33.Brühl, G. A., Gunsalam, G., Edward, K. & Linsenmair, K. E. (1998) J. Trop. Ecol. 14, 285–292. [Google Scholar]
- 34.Wilson, E. O. (1987) Biotropica 19, 245–251. [Google Scholar]
- 35.Floren, A., Biun, A. & Linsenmair, E. (2002) Oecologia 131, 137–144. [DOI] [PubMed] [Google Scholar]
- 36.Tobin, J. E. (1995) in Forest Canopies, eds. Lowman, M. D. & Nadkarni, N. M. (Academic, New York), pp. 129–147.
- 37.Davidson, D. W., Cook, S. C., Snelling, R. R. & Chua, T. H. (2003) Science 300, 969–972. [DOI] [PubMed] [Google Scholar]