Abstract
Background:
Due to the heterogeneity of existing studies and wide range of human papilloma virus (HPV) prevalence in India, further research into the incidence of HR-HPV and its spectrum of genotypes is essential to develop screening policies. This study aimed to determine the incidence and demographic distribution of HR-HPV among cisgender female patients attending a tertiary care facility in North India.
Materials and Methods:
This study was conducted in the Department of Obstetrics and Gynaecology, SGRR Institute of Medical and Health Sciences, Dehradun, India. HPV-DNA test results of 653 female patients were assessed for HR-HPV positivity, genotyping, and age-based differences via Chi-square analysis.
Results:
Overall prevalence of HR-HPV was 4.90%, HPV-16 was 1.37%, HPV-18 was 0.76%, and HPV non-16,18 was 2.7%. In patients ≤ 50 years, prevalence of HPV-16 was 0.97%, HPV-18 was 0.38%, and HR-HPV non-16,18 was 2.71%. In patients > 50 years, prevalence of HPV-16 was 2.89%, HPV-18 was 2.17%, and HR-HPV non-16,18 was 2.89%. The difference in the prevalence of HPV-16,18 between patients ≤ and > 50 years was found to be highly statistically significant (P = 0.007485). The difference in the prevalence of total HR-HPV between patients ≤ and > 50 years was not found to be statistically significant (P = 0.059905).
Conclusion:
Our study’s finding of higher HR-HPV positivity rates in patients > 50 years emphasizes the need for continued HR-HPV-DNA-based screening of this cohort. With widespread use in post-menopausal patients, HPV screening can serve as an important armamentarium in the fight against cervical cancer.
Keywords: Cervical cancer screening, high-risk HPV, HPV-DNA test, HPV in older women, HPV prevalence
INTRODUCTION
A sexually transmitted pathogen, human papilloma virus (HPV) is considered the most significant risk factor for cervical cancer. Two strains HPV-16 and HPV-18 cause over 70% of all cervical cancer cases worldwide and are the most common genotypes in India.[1] With an estimated 604,127 cases and 341,831 deaths in 2020, cervical cancer ranks as the second most common cancer among women1 worldwide.[2] In India, cervical cancer is the second leading cause of cancer and cancer deaths among women aged 15-44 years; in 2020, an estimated 123,907 new cervical cancer cases were diagnosed and 77, 348 cervical cancer deaths occurred in India. The crude rate of cervical cancer in India ranges from 6.5 to 24.2 per 100,000 based on region.[2]
Today, the HPV-DNA test is the primary screening method for cervical cancer, with its higher sensitivity, specificity, and level of user-friendliness when compared to pap cytology and visual inspection with acetic acid.[3,4] The prevalence of HPV in India ranges from 2.3% to 36.9%.[5] Given this wide range of HPV prevalence and the heterogeneity of existing studies, further research into the incidence of high-risk HPV (HR-HPV) and the spectrum of HR-HPV genotypes in local areas is essential for medical institutions to develop policies to serve their communities. The present study was undertaken to determine the incidence and demographic distribution of HR-HPV among cisgender female patients attending a tertiary care facility in North India.
STUDY METHODOLOGY
Sample
This cross-sectional study was conducted from January 2021 to April 2022 in the Department of Obstetrics and Gynaecology at Shri Guru Ram Rai Institute of Medical and Health Sciences, Dehradun, India, after approval of the Institutional Review Board. The study population included 653 cisgender female patients who underwent HPV-DNA testing after informed consent. The inclusion criteria for the testing were (1) patients between 30 and 65 years; (2) asymptomatic patients undergoing routine cervical cancer screening; (3) patients with symptoms suggestive of cervical pathology, such as post-coital bleeding, contact bleeding, or those with a clinically suspicious cervix; or (4) those patients referred with abnormal cytology reports for triage purpose. Exclusion criteria for the study were (1) patients < 30 years of age, (2) patients > 65 years of age, and (3) those with clinically visible cervical cancer.
HPV-DNA test procedure
Specimen was collected by cytobrush. The lower portion of the brush (with the brush side down) was immersed in a vial containing specimen transport medium (0.05% sodium azide). Manual extraction of HPV-DNA was carried out from the specimen. QIAscreen® HPV polymerase chain reaction (PCR) test, a multiplex, real-time PCR-based assay directed against the E7 gene of 15 HR-HPV types, was used to detect three targets: HPV-16, HPV-18, and a pool of 13 other HR-HPV types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 67, and 68). Real-time PCR detection system (Rotor-Gene® Q) was used for this test. Results from the HPV-DNA tests for screening or triage of 653 patients were assessed for HR-HPV positivity and genotyping into HPV-16, HPV-18, or HR-HPV non-16,18.
Outcome measures
The outcome measures were total, age-specific, and type-specific prevalence of HR-HPV positivity. Total prevalence of HR-HPV was defined as the number of patients who tested positive for any strain of HR-HPV out of the total number of women tested during the study period. Age-specific prevalence of HR-HPV was defined as the number of patients who tested positive for HR-HPV in the age groups ≤ and > 50 years, out of the total number of women tested. Type-specific prevalence was defined as the number of HR-HPV-positive cases stratified by type (HPV 16, HPV 18, non-16/18) out of the total number of women tested in the study period.
Statistical analysis
The total and age-specific prevalence of each of these strains of HR-HPV were calculated. The Chi-square statistic and corresponding P value for the difference in the proportion of HPV-16 and HPV-18 between patients ≤ and > 50 years were calculated. Similarly, the Chi-square statistic and corresponding P value for the difference in the proportion of total HR-HPV (HPV-16, HPV-18, and HPV non-16,18) between patients ≤ and > 50 years were calculated. P values of less than 0.05 were considered statistically significant, and those of less than 0.01 were considered highly statistically significant. The approval from ethics committee has been obtained on date 25 January 2023.(Reference no. SGRR/IEC/01/23).
RESULTS
Sample demographics
A total number of 653 patients underwent the HPV-DNA test. The mean age of the patients was 44.78 ± 9.91 years. The results were denoted as HR-HPV negative or HR-HPV positive. HR-HPV positive results were further divided into HPV-16, HPV-18, and HPV non-16,18. These results were stratified by age ranges for further analysis.
Age distribution
This study found the overall prevalence of HR-HPV to be 4.90%. The prevalence of specific strains of HR-HPV was found as follows: HPV-16 was 1.37%, HPV-18 was 0.76%, and HPV non-16,18 was 2.7%. Table 1 depicts the prevalence of HPV-16, -18 and non-16,18 in the different age groups.
Table 1:
HR-HPV Prevalence in Sample by Age (<40, 41-50, 51-60, and >60 years)
| Age Group (years) | HPV Not Detected | HPV-16 | HPV-18 | HPV Non-16, 18 | Total |
|---|---|---|---|---|---|
| 30-40 | 247 (96.10%) | 0 (0.00%) | 0 (0.00%) | 10 (3.90%) | 257 |
| 41-50 | 247 (95.70%) | 5 (1.93%) | 2 (0.77%) | 4 (1.55%) | 258 |
| 51-60 | 82 (91.10%) | 3 (3.30%) | 3 (3.30%) | 2 (2.20%) | 90 |
| 60-65 | 45 (93.75%) | 1 (2.10%) | 0 (0.00%) | 2 (4.20%) | 48 |
| 621 | 9 | 5 | 18 | 653 |
As seen in Figure 1 and Table 2, in patients ≤ 50 years, HPV-16 was found to be positive in 5 (0.97%) patients, HPV-18 in 2 (0.38%) patients, and HR-HPV non-16,18 in 14 (2.71%) patients. In patients > 50 years, HPV-16 was found to be positive in 4 (2.89%), HPV-18 in 3 (2.17%), and HR-HPV non-16,18 in 4 (2.89%) patients.
Figure 1.
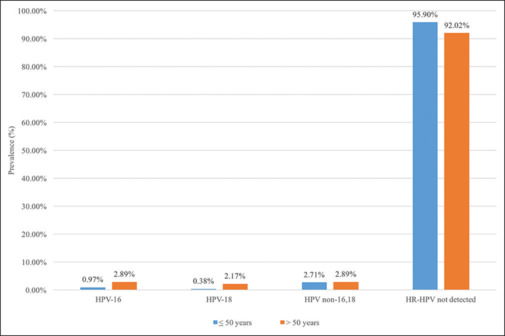
HPV Prevalence in Sample by Age (≤ 50, > 50 years)
Table 2:
HR-HPV Prevalence in Sample by Age (≤50, >50 years)
| Type of Infection | Age in Years |
||
|---|---|---|---|
| ≤50 | >50 | Total | |
| HPV-16 | 5 (.97%) | 4 (2.89%) | 9 (1.37%) |
| HPV-18 | 2 (.38%) | 3 (2.17%) | 5 (0.77%) |
| HR- HPV Non-16,18 | 14 (2.71%) | 4 (2.89%) | 18 (2.76%) |
| HPV-16, 18 detected | 7 (1.36%) | 7 (5.07%) | 14 (2.14%) |
| Total HR-HPV detected | 21 (4.08%) | 11 (7.97%) | 32 (4.90%) |
| HPV not detected | 494 (95.90%) | 127 (92.02%) | 621 (95.09%) |
| Total | 515 | 138 | 653 |
Table 3 shows that the difference in the prevalence of HPV-16,18 between patients ≤ and > 50 years was found to be highly statistically significant (X2 (1, N = 653) =7.1528, P = 0.007485).
Table 3:
Chi-Square Analysis for HPV-16, 18 Prevalence in Sample by Age (≤50, >50 years)
| Type of Infection | Age in Years |
||
|---|---|---|---|
| ≤50 | >50 | Total | |
| HPV-16,18 detected | 7 (11.04) [1.48] | 7 (2.96) [5.52] | 14 |
| Either HPV non-16,18 or HPV not detected | 508 (503.96) [0.03] | 131 (135.04) [0.12] | 639 |
| Total | 515 | 138 | 653 |
χ2 (1, n=653)=7.1528, P=0.007485.** The table above reports observed values, expected values in (), and the Chi-square statistics in []. **denotes P<0.01
Table 4 shows that the difference in the prevalence of total HR-HPV between patients ≤ and > 50 years was not found to be statistically significant (X2 (1, N = 653) = 3.54, P = 0.059905).
Table 4:
Chi-square analysis for total HR-HPV prevalence in sample by age (≤50, >50 years)
| Type of Infection | Age in Years |
||
|---|---|---|---|
| ≤50 | >50 | Total | |
| Total HR-HPV detected | 21 (25.24) [0.71] | 11 (6.76) [2.66] | 32 |
| Total HR-HPV not detected | 494 (489.76) [0.04] | 127 (131.24) [0.14] | 621 |
| Total | 515 | 138 | 653 |
χ2 (1, n=653)=3.54, P=0.059905. The table above reports observed values, expected values in (), and the Chi-square statistics in []
DISCUSSION
Our study’s finding of a 4.90% prevalence of HR-HPV is largely consistent with previous literature [Table 5]. In a study by Dutta et al. (2012),[6] the population prevalence of any HPV in a sample of 2501 women between 25 and 65 years in Eastern India was found to be 9.9%. This higher prevalence likely stems from their inclusion of both LR-HPV and HR-HPV subtypes. Similar to our combined prevalence of 2.14% for HPV-16,18, Dutta et al. (2012)[6] determined their combined prevalence of HPV 16,18 to be 2.0%. Nonetheless, Dutta et al. found the prevalence of HPV-16 to be 0.6% and HPV-18 to be 1.4%, in contrast to our values of HPV-16 as 1.37% and HPV-18 as 0.77%.
Table 5:
Prevalence of HPV in various studies
| Study | Country | Sample Size | Any HPV | HR-HPV | HPV-16/18 | HPV-16 | HPV-18 |
|---|---|---|---|---|---|---|---|
| Aggarwal et al. (2006)[7] | India | 472 | 36.8% | 8.26% | -- | 3.18% | 4.66% |
| Dutta et al. (2012)[6] | India | 2501 | 9.9% | -- | 2.0% | 0.6% | 1.4% |
| Moosa et al. (2014)[8] | Bahrain | 571 | 9.8% | -- | 1.5% | 1.1% | 0.4% |
| Sarma et al. (2013)[9] | India | 226 | 9.73% | -- | 8.0% | 5.3% | 2.7% |
| Tang et al. (2021)[10] | China | 12,053 | 10.16% | 8.52% | -- | 2.19% | -- |
| Present study (2022) | India | 653 | -- | 4.90% | 2.14% | 1.37% | 0.77% |
In a meta-analysis of 194 studies across five continents, Bruni et al. (2010)[11] found the overall worldwide prevalence of HPV to be 11.7% and the prevalence in Southern Asia (India) to be 7.1%. Again, the higher prevalence in Southern Asia compared to our study is likely the result of the study’s inclusion of LR-HPV, as well as HR-HPV. Even more, Bruni et al.’s[11] inclusion of women <25 years could further inflate estimated prevalence, as cohorts <25 years and ≥45 years associate with the two age-specific HPV prevalence distribution peaks. Inclusion of younger patients in their study could also explain the higher prevalence found for HPV-16 (2.5%) and HPV-18 (1.4%) in Asia as compared to our study.
Another North Indian study similarly reported a higher HR-HPV prevalence of 8.26% in a sample of 472 patients attending the gynecology outpatient department. The sample prevalence of HPV-16 was 3.18%, HPV-18 was 4.66%, and simultaneous HPV-16,18 infection was 0.42%.[7] These higher values also can be explained by the study’s inclusion of patients ≥19 years.
In Bahrain, Moosa et al. (2014)[8] found the prevalence of total HPV in their sample of 571 women to be 9.8%; again, their study’s higher prevalence likely stems from their inclusion of LR-HPV and patients ≥ 20 years undergoing routine cervical screening, as well as those ≥ 16 years attending post-natal check-ups. Despite inclusion of younger patients, their prevalence calculations of HPV-16 (1.1%) and HPV-18 (0.4%) are comparable to our calculations of HPV-16 and HPV-18.
Other studies in India have focused on patients with cervical cancer. In a study conducted in Tamil Nadu, India, 246 patients with cervical cancer were found to have an overall HPV prevalence of 81.4%.[12] In comparison, a large meta-analysis of any HPV type distribution in South Asia found a 94.6% HPV positivity rate in patients with invasive cervical cancer.[13]
The prevalence of HR-HPV strains in women of the age group 20-30 years was found to be 71% (95% CI, 59 to 83) in those with abnormal cytology and 32% (95% CI, 22 to 41) in those with normal cervical cytology.[14] Nonetheless, the clinical utility of testing for HPV in this demographic is doubtful given that HPV prevalence is high in women <30 years, and up to 90% of these infections clear spontaneously over the course of one to two years.[15,16]
Furthermore, current Federation of Obstetric and Gynaecological Societies of India (FOGSI) cervical cancer screening guidelines indicate that screening with HPV-DNA tests should begin from the age of 30 years, as screening of younger patients is associated with higher rates of unnecessary treatment of findings that would naturally regress.[17] Because our study included only patients 30 years or above, our research allows for a more accurate prediction of the prevalence of HPV infections that are more likely to persist and turn cancerous. This methodology is thus a positive aspect of our study when compared with numerous other studies that have included a younger age group and thus report higher HPV prevalence rates.
Our study also shows that the prevalence values of HPV-16, 18 are greater among patients >50 years with high statistical significance. This high prevalence is consistent with the second age-specific HPV prevalence peak that Bruni et al.[11] found for women ≥45 years. These results are also similar to a study by Sarma et al. (2013)[9] in Guwahati, where researchers found an increased prevalence of HPV infection due to persistent infection in women >50 years. In a study of 12,053 patients in the Hengyang district of the Hunan province in China, the authors found higher prevalence values of any HPV and HR-HPV infection among patients >50 years, similar to our results.[10]
The reason for this second peak in older patients has yet to be established conclusively, as researchers consider reinfection or reactivation as possible explanations.[18] Current evidence seems to favor reactivation. Gravitt et al. (2013)[19] enrolled a cohort of 843 women aged 35–60 years and analyzed for a potential interaction among age, lifetime number of sex partners, and oncogenic HPV infection. They found that the population attributable risk for oncogenic HPV infection due to ≥5 lifetime sex partners was higher among older women (87.2%) compared to younger women (28.0%), suggesting that older women may be at risk for HPV reactivation. It is also suggested that the infection is acquired during teenage or early adulthood years and remains in a dormant state for years. With immunosenescence, lower levels of persistent HPV may then reactivate, leading to the second bimodal peak.[18]
In India, estimates for annual new cases of cervical cancer cases in 2020 peak at 16,024 in the age range of 55-59. For women around the age of 50 years, age-specific cervical cancer rates in India reach 45% and continue increasing in a sigmoidal curve with a plateau around the age range of 75-79.[2] Similarly, in another recent study of 1678 cases of cervical carcinoma conducted at Tata Memorial Institute, India, the median age at detection of cervical cancer was reported to be 53 years, with 60% of the total cases belonging to the age group >50 years.[20] The five-year overall survival at 70.1% for patients aged >50 years was found to be lower than the 76% five-year overall survival for patients ≤50 years of age.[20]
The World Health Organization has recommended that women between 35 and 45 years in resource-limited settings be screened at least once in their lifetime to detect and treat cervical lesions before they progress into cervical cancer.[21] The authors agree with the importance of screening in this younger age group. In addition, we found a high prevalence of HR-HPV in patients >50 years in our study, and numerous studies have reported the peak incidence of cervical cancer in India in participants >50 years, along with a reduced survival in this age group.
Based on this data and on the physiological recession of the transformation zone in menopause, we would like to emphasize continued screening in this older age group. While such organized screening programs are being developed, we suggest that practitioners utilize older patients’ outpatient visits to perform HPV-based screening. High-risk HPV positivity in these patients can be an important, highly sensitive tool to signal underlying pre-malignant and malignant endo-cervical pathologies, which can easily be missed on naked eye examination.[22]
The limitations of our study include the inherent biases associated with it being a cross-sectional study. The authors suggest a possibility of participation bias, as the study was conducted in patients presenting to the Gynecologic Outpatient Department with various complaints, and HPV-DNA testing was performed in an opportunistic manner. The prevalence found in this study group cannot be assumed to accurately estimate the HPV-DNA positivity rates in the general population. The authors recommend larger population-based studies to further establish the HPV positivity rates in older women.
CONCLUSION
This study demonstrated that HPV prevalence and its genotype distribution at a tertiary care institute in a hilly state of North India were largely in accordance with the present situation of HPV prevalence in India and Southern Asia. We would like to emphasize continued HR-HPV-DNA-based screening of patients ≥50 years, based on the higher HR-HPV positivity rates found in this age group in our study. If low-cost HPV-DNA sequencing is available, we can extend its use beyond the target age group of 35-45 years to the biologically significant second peak age group of cervical cancer. With commitment to widespread use in post-menopausal communities, HPV screening can serve as an important armamentarium in the fight against cervical cancer.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
While sample language specifies patients’ gender identity as cisgender females, in other places the terms “women” and “female” are used according to the literature to which they refer.
REFERENCES
- 1.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 2.Bruni L, Albero G, Serrano B, Mena M, Collado JJ, Gómez D, et al. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre) Human Papillomavirus and Related Diseases in India. Summary Report 10 March 2023 [Google Scholar]
- 3.Rajaram S, Gupta B. Screening for cervical cancer: Choices and dilemmas. Indian J Med Res. 2021;154:210–20. doi: 10.4103/ijmr.IJMR_857_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gravitt PE, Paul P, Katki HA, Vendantham H, Ramakrishna G, Sudula M, et al. Effectiveness of VIA, Pap, and HPV DNA testing in a cervical cancer screening program in a peri-urban community in Andhra Pradesh, India. PLoS One. 2010;5:e13711. doi: 10.1371/journal.pone.0013711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruni L, Albero G, Serrano B, Mena M, Gómez D, Muñoz J, et al. ICO/IARC information centre on HPV and cancer (HPV Information Centre) Human Papillomavirus and Related Diseases in the World. Summary Report 17 June 2019. Available from: https://www.hpvcentre.net/statistics/reports/XWX.pdf . [Last accessed on 2024 Apr 21] [Google Scholar]
- 6.Dutta S, Begum R, Mazumder Indra D, Mandal SS, Mondal R, Biswas J, et al. Prevalence of human papillomavirus in women without cervical cancer: A population-based study in Eastern India. Int J Gynecol Pathol. 2012;31:178–83. doi: 10.1097/PGP.0b013e3182399391. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal R, Gupta S, Nijhawan R, Suri V, Kaur A, Bhasin V, et al. Prevalence of high-risk human papillomavirus infections in women with benign cervical cytology: A hospital based study from North India. Indian J Cancer. 2006;43:110–6. doi: 10.4103/0019-509x.27932. [DOI] [PubMed] [Google Scholar]
- 8.Moosa K, Alsayyad AS, Quint W, Gopala K, DeAntonio R. An epidemiological study assessing the prevalence of human papillomavirus types in women in the Kingdom of Bahrain. BMC Cancer. 2014;14:905. doi: 10.1186/1471-2407-14-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarma U, Mahanta J, Borkakoty BJ, Talukdar KL, Gogoi R, Yadav K. Demographic characteristic of HPV infection in women - A hospital based study from Guwahati, India. Natl J Med Res. 2013;3:1–4. [Google Scholar]
- 10.Tang SY, Liao YQ, Hu Y, Shen HY, Wan HP, Wu YM. HPV prevalence and genotype distribution among women from Hengyang District of Hunan Province, China. Front Public Health. 2021;9:710209. doi: 10.3389/fpubh.2021.710209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: Meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202:1789–99. doi: 10.1086/657321. [DOI] [PubMed] [Google Scholar]
- 12.Vinodhini K, Shanmughapriya S, Sanmugham S, Senthikumar G, Das BC, Natarajaseenivasan K. Prevalence of high-risk HPV and associated risk factors in cases of cervical carcinoma in Tamil Nadu, India. Int J Gynaecol Obstet. 2012;119:253–6. doi: 10.1016/j.ijgo.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Bhatla N, Lal N, Bao YP, Ng T, Qiao YL. A meta-analysis of human papillomavirus type-distribution in women from South Asia: Implications for vaccination. Vaccine. 2008;26:2811–7. doi: 10.1016/j.vaccine.2008.03.047. [DOI] [PubMed] [Google Scholar]
- 14.Deleré Y, Schuster M, Vartazarowa E, Hänsel T, Hagemann I, Borchardt S, et al. Cervicovaginal self-sampling is a reliable method for determination of prevalence of human papillomavirus genotypes in women aged 20 to 30 Years. J Clin Microbiol. 2011;49:3519–22. doi: 10.1128/JCM.01026-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winer RL, Hughes JP, Feng Q, Xi LF, Cherne S, O’Reilly S, et al. Early natural history of incident type-specific human papillomavirus infections in newly sexually active women. Cancer Epidemiol Biomark Prev. 2011;20:699–707. doi: 10.1158/1055-9965.EPI-10-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gy H, Bierman, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:432–8. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 17.FOGSI GCPR. Screening and Treatment of Preinvasive Lesions of Cervix and HPV Vaccination. GOGSI Gynaecologic Oncology Committee; 2018 [Google Scholar]
- 18.Brown DR, Weaver B. Human papillomavirus in older women: New infection or reactivation? J Infect Dis. 2013;207:211–2. doi: 10.1093/infdis/jis662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gravitt PE, Rositch AF, Silver MI, Marks MA, Chang K, Burke AE, et al. A cohort effect of the sexual revolution may be masking an increase in human papillomavirus detection at menopause in the United States. J Infect Dis. 2013;207:272–80. doi: 10.1093/infdis/jis660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balasubramaniam G, Gaidhani RH, Khan A, Saoba S, Mahantshetty U, Maheshwari A. Survival rate of cervical cancer from a study conducted in India. Indian J Med Sci. 2021;73:203–11. [Google Scholar]
- 21.Indicator Metadata Registry Details. Available from: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/3240 . [Last accessed on 2022 Jul 19] [Google Scholar]
- 22.Nofech-Mozes S, Khalifa MM, Ismiil N, Dubé V, Saad RS, Sun P, et al. Detection of HPV-DNA by a PCR-based method in formalin-fixed, paraffin-embedded tissue from rare endocervical carcinoma types. Appl Immunohistochem Mol Morphol. 2010;18:80–5. doi: 10.1097/PAI.0b013e3181ae7240. [DOI] [PubMed] [Google Scholar]


