Abstract
The activating NKG2D receptor plays a critical role in innate and adaptive immune responses by natural killer cells and subpopulations of T cells. The human receptor assembles with the DAP10 signaling dimer, and it is thought that one NKG2D homodimer pairs with a single DAP10 dimer by formation of two salt bridges between charged transmembrane (TM) residues. However, direct stoichiometry measurements demonstrated that one NKG2D homodimer assembles with four DAP10 chains. Selective mutation of one of the basic TM residues of NKG2D resulted in loss of two DAP10 chains, indicating that each TM arginine serves as an interaction site for a DAP10 dimer. Assembly of the hexameric structure was cooperative because this mutation also significantly reduced NKG2D dimerization. A monomeric NKG2D TM peptide was sufficient for assembly with a DAP10 dimer, indicating that the interaction between these proteins occurs in the membrane environment. Formation of a three-helix interface among the TM domains involved ionizable residues from all three chains, the TM arginine of NKG2D and both TM aspartic acids of the DAP10 dimer. The organization of the TM domains thus shows similarities to the T cell antigen receptor–CD3 complex, in particular to the six-chain assembly intermediate between T cell antigen receptor and the CD3δε and CD3γε dimers. Binding of a single ligand can thus result in phosphorylation of four DAP10 chains, which may be relevant for the sensitivity of NKG2D receptor signaling, in particular in situations of low ligand density.
Keywords: natural killer cells, stoichiometry, membrane biochemistry
The NKG2D receptor recognizes a group of ligands that represent distant relatives of MHC class I molecules, and expression of these ligands is induced or up-regulated by infected and transformed cells (1–8). In innate immune responses mediated by natural killer (NK) cells, NKG2D serves as a primary activating receptor and ligand binding triggers cytotoxicity and cytokine production. The receptor is also expressed by CD8 T cells and γδ T cells and provides important costimulatory signals in T cell-mediated adaptive immune responses by amplifying T cell cytokine production and proliferation (2, 3, 9, 10). The human NKG2D ligands MICA and MICB have a domain organization similar to MHC class I molecules (α1, α2, and α3 domains) but do not associate with β2-microglobulin or peptide (1). The NKG2D homodimer binds in a diagonal orientation across the surface of the α1 and α2 domains, and each receptor monomer contacts one of the long α-helices of the α1–α2 platform, reminiscent of the binding of T cell antigen receptor (TCR) αβ heterodimers to MHC/peptide complexes (11). The human NKG2D ligands UL binding proteins 1, 2, 3, and 4 and the murine ligands RAE-1α–ε, H60, and murine UL binding protein-like transcript 1 lack the membrane-proximal α3 domain and are quite dissimilar in sequence (4, 5, 12), but NKG2D nevertheless binds in a similar diagonal orientation to the α1–α2 domains of these proteins (13, 14).
The NKG2D receptor has recently attracted considerable attention because human MICA and MICB were found to be broadly expressed in various epithelial tumors, such as carcinomas of the lung, breast, and colon (15). In a mouse model of skin carcinogenesis, local application of carcinogens was shown to induce the expression of NKG2D ligands that can be recognized by resident γδ T cells (16). Inoculation of mice with tumor cell lines that had been transfected with RAE-1β and H60 expression vectors resulted in efficient NK cell-mediated killing of these cells. Interestingly, these mice were also protected from subsequent challenge with NKG2D ligand-negative parental tumor cells whereas such tumors grew rapidly in nonvaccinated mice. Immunity was specific for the tumor cell lines used in the initial vaccination and required CD8 memory T cells (17, 18). An escape mechanism for tumor cell lysis by NKG2D-positive effector cells was identified in human studies: tumor cells produced soluble MICA, which down-regulated NKG2D levels on tumor infiltrating and circulating T cells (19). NKG2D is also relevant in chronic inflammatory diseases by enhancing T cell proliferation and production of proinflammatory cytokines (20–23). In patients with rheumatoid arthritis, substantial numbers of CD4 T cells in peripheral blood and synovial fluid expressed NKG2D, and synovial cells expressed MICA. Expression of NKG2D could be induced in CD4 T cells from normal subjects by IL-15 and TNF-α, cytokines that are present in abundant quantities in the synovial fluid in rheumatoid arthritis (20). In the nonobese diabetic mouse model, the development of type 1 diabetes could be prevented by administration of a NKG2D antibody (23), indicating that this receptor represents a potential target for the treatment of autoimmune diseases.
Signals triggered by the NKG2D receptor are transmitted through the associated DAP10 dimer. The DAP10 dimer carries a pair of aspartic acid residues close to the center of the transmembrane (TM) domains, and a conserved arginine in the TM sequence of NKG2D is required for assembly with the DAP10 dimer. DAP10 forms a disulfide-linked homodimer and its cytoplasmic domain carries a YxxM motif that binds the p85 subunit of phosphatidylinositol-3 kinase after phosphorylation (3). The YxxM motif is also present in the cytoplasmic domain of CD28, and the phosphatidylinositol-3 kinase pathway is critical for the costimulatory function of this molecule in T cells (24). It is thought that one NKG2D homodimer assembles with one DAP10 dimer in the endoplasmic reticulum (ER); such a complex would have two conserved basic residues in the TM domains of NKG2D and two acidic residues in the TM segments of the DAP10 dimer. However, in recent work on the TCR–CD3 complex we demonstrated that the three basic TM residues of the TCR interact with the six acidic residues in the TM domains of the three signaling dimers through a distinctive arrangement. Assembly of TCR with each of the signaling dimers is based on the interaction of one basic TCR TM residue with a pair of acidic TM residues of the respective signaling dimer and, thus, results in the formation of a three-helix interface in the membrane (25). In addition, we have recently demonstrated that this three-helix motif is also relevant for the assembly of other activating immune receptors (26). Studies on model TM helices have shown that placement of an acidic residue within a hydrophobic sequence can result in dimer or trimer formation, possibly by hydrogen bonding between (partially) protonated carboxyl groups (27, 28). These results raised the question of whether the NKG2D receptor complex represents a hexamer in which each TM domain of NKG2D interacts with a DAP10 dimer. We have addressed this question by defining the stoichiometry of the NKG2D–DAP10 complex and the molecular interactions that guide receptor assembly.
Experimental Procedures
The assembly of human NKG2D and DAP10 was examined in an in vitro translation system by using ER microsomes, as described (25, 26). After assembly, membranes were solubilized with 0.5% digitonin, and radiolabeled proteins were immunoprecipitated by using streptavidin for proteins with a C-terminal streptavidin binding peptide (SBP) tag or antibodies directed against C-terminal hemagglutinin (HA) or protein C (PC) affinity tags. Quantification was performed by using a phosphorimager after SDS/PAGE and transfer of radiolabeled proteins to poly(vinylidene difluoride) membranes (for details, see Supporting Methods and Fig. 8, which are published as supporting information on the PNAS web site).
Results
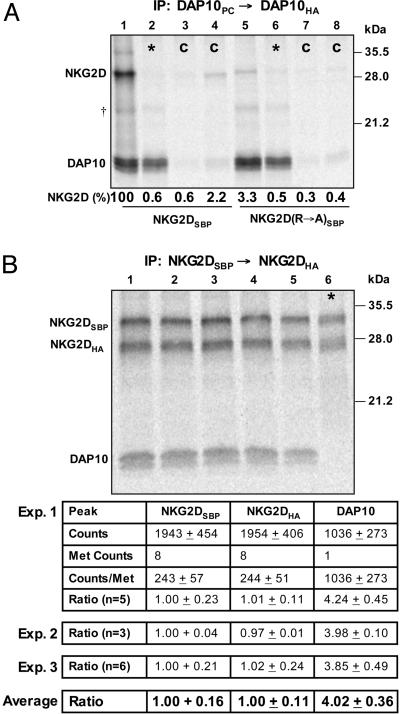
Stoichiometry of the NKG2D–DAP10 Complex. The assembly of human NKG2D and DAP10 was studied by using an in vitro translation system with ER microsomes because previous studies have shown that this system faithfully reproduces the assembly of TCR–CD3 and other membrane protein complexes (29, 30, 25). Assembly products of defined composition were isolated by using a sequential nondenaturing immunoprecipitation (snIP) technique with specialized affinity tags that permitted elution after the first IP by competition with biotin (SBP) or chelation of calcium with EDTA (PC) (25). To determine whether this approach was suitable for studying the interaction between NKG2D and DAP10, assembly reactions were set up with NKG2D and two DAP10 chains that carried PC or HA epitope tags. The DAP10 dimer and associated proteins were then isolated in a two-step snIP that targeted the PC and HA epitope tags (PC→HA). The observed interaction between the DAP10 dimer and NKG2D (Fig. 1A, lane 1) was specific based on control reactions in which the primary or secondary IP antibodies were replaced with isotype controls (Fig. 1A, lanes 3 and 4). Assembly occurred in the ER membrane because no complex was observed in mixing controls (Fig. 1A, asterisk in lane 2) in which DAP10 and NKG2D chains were translated in separate reactions, the contents of which were combined before solubilization and IP. In addition, the interaction was disrupted by mutation of the TM arginine of NKG2D to alanine (Fig. 1A, lane 5), consistent with studies in a cellular system (3). These experiments were performed with a DAP10 construct in which one of the two cysteine residues in the extracellular domain was mutated to alanine because small quantities of higher-order DAP10 complexes were observed under nonreducing conditions when both cysteine residues were present (Fig. 8). These experiments demonstrated that either one of the two cysteine residues was sufficient to ensure WT levels of covalent DAP10 dimers. This issue was relevant because such artifacts could otherwise bias the outcome of stoichiometry measurements.
Fig. 1.
Stoichiometry of the NKG2D–DAP10 complex. (A) An in vitro translation system with ER microsomes was used to study the assembly of NKG2D and DAP10. After assembly, membranes were solubilized with 0.5% digitonin, and 35S-labeled complexes were isolated in a two-step snIP that targeted the PC and HA epitope tags attached to the two DAP10 chains. In the mixing controls (*, lanes 2 and 6), DAP10 and NKG2D chains were translated separately, and the reactions were combined immediately before solubilization. In the antibody controls (C, lanes 3, 4, 7, and 8), the PC antibody (lanes 3 and 7) or the HA antibody (lanes 4 and 8) used in the first and second IP step, respectively, were replaced with species- and isotype-matched antibodies of irrelevant specificity. Assembly was abrogated by mutation of the TM arginine of NKG2D to alanine (NKG2D R→ A, lane 5). The ratio of NKG2D and DAP10 dimer was determined and expressed as the percentage relative to WT (lane 1). Data are representative of four experiments. †, A faint background band appeared at the indicated position in this and other experiments; this band was nonspecific because it was also observed in mixing controls. (B) The stoichiometry of the complex was directly determined by quantification of 35S-labeled NKG2D and DAP10 with a phosphorimager. The NKG2D dimer was isolated with a two-step snIP that targeted the SBP and HA tags attached to the two NKG2D chains, and the molar ratio between NKG2D and associated DAP10 was determined based on the known methionine content of the chains. Multiple parallel reactions were analyzed in each of the three experiments, and the average and standard deviation were calculated for all reactions. *, In each experiment, a mixing control was included to verify specificity. These experiments demonstrated that four DAP10 chains are present for each NKG2D dimer.
We then used this system to directly measure the stoichiometric relationship between the NKG2D and DAP10 chains (Fig. 1 B and C). To ensure that only intact NKG2D dimers would be isolated in the IP, we performed assembly reactions with two NKG2D chains that carried SBP or HA tags and performed a SBP→HA snIP for the NKG2D dimer. The [35S]methionine-labeled proteins were then separated by SDS/PAGE, transferred to a poly(vinylidene difluoride) membrane and quantitated by using a phosphorimager. The ratio between the NKG2DSBP, NKG2DHA, and DAP10 chains was calculated by taking the methionine content of each chain into account. These experiments clearly demonstrated that for each NKG2DSBP chain (defined as 1.0), one NKG2DHA and four DAP10 chains were present. In each experiment, several parallel reactions were performed so that experimental variation could be excluded as a potential source of error. Three independent experiments with a total of 14 identical assembly reactions demonstrated that this result was highly reproducible, a conclusion that was also supported by statistical analysis of the data. These results were also confirmed with a DAP10 construct in which the number of labeled positions was increased from one to three by addition of two methionine residues to the cytoplasmic tail (Fig. 9, which is published as supporting information on the PNAS web site). The NKG2D homodimer thus assembles with two DAP10 dimers.
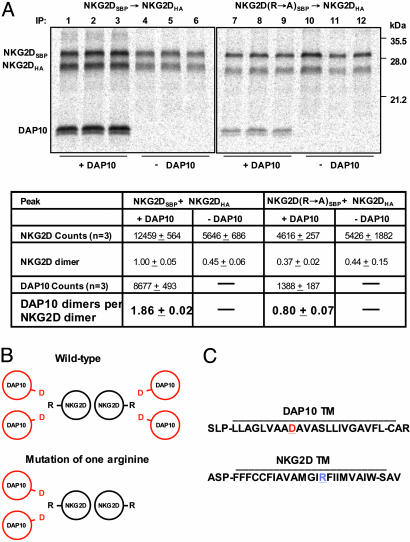
Cooperative Assembly of NKG2D with DAP10. In the experiments described above, only the NKG2D dimer was targeted in the IP, and it was thus surprising that almost all isolated NKG2D dimers had assembled with two DAP10 dimers. This result suggested that assembly is a cooperative process that strongly favors the formation of the complete receptor structure over partial complexes. Furthermore, the result suggested that a mixed NKG2D dimer in which the arginine in one of the TM domains had been mutated to alanine (R→A) would associate with a single DAP10 dimer. Mixed NKG2D dimers with a single TM arginine were created by replacing the NKG2DSBP chain with a SBP-tagged NKG2D R→A mutant, and dimers composed of this mutant and a HA-tagged NKG2D WT chain were isolated with a SBP→HA snIP (Fig. 2). The stoichiometry data in Fig. 2 are summarized as NKG2D dimers and DAP10 dimers rather than individual chains as in Fig. 1. The mixed NKG2D dimer still assembled with DAP10, but the ratio of DAP10 dimer per NKG2D dimer was reduced to 0.8 (Fig. 2 A, lanes 7–9), indicating that only a single DAP10 dimer could associate with this protein. These experiments were performed with a DAP10 chain in which two methionine residues were added to the cytoplasmic tail because the signal with the WT DAP10 chain was too weak for the mixed NKG2D dimer. Interestingly, the yield of isolated NKG2D dimer was also reduced substantially for the mixed dimer (37% relative to WT NKG2D dimer), indicating that two DAP10 binding sites are required for efficient NKG2D dimerization. This conclusion was supported by quantification of NKG2D dimers in reactions in which DAP10 was omitted (Fig. 2 A, lanes 4–6 and 10–12), given that the yield of WT and mixed mutant NKG2D dimer was reduced to ≈45% in the absence of DAP10. These results demonstrate that assembly with DAP10 promotes NKG2D dimer formation, although the NKG2D extracellular domain has a dimerization interface (31). These results can be explained by a model in which an individual NKG2D chain assembles with a DAP10 dimer such that all three ionizable TM residues are buried at a three-helix interface in the membrane. Efficient assembly of the intact receptor may then proceed by interactions between the NKG2D extracellular domains as well as the three-helix TM bundles. Because NKG2D and DAP10 dimers can assemble independently, the hexameric structure may also form by assembly of preformed dimers.
Fig. 2.
Cooperative assembly of the hexameric NKG2D–DAP10 complex. (A) NKG2D dimers composed of two WT chains (lanes 1–6) or of one WT and one mutant chain (substitution of TM arginine by alanine, R→ A) (lanes 7–12) were isolated by a two-step snIP that targeted the SBP and HA tags attached to the two NKG2D chains. The DAP10 construct used for this experiment had two additional methionine residues (total of three methionine residues, compared with one for WT DAP10 used in Fig. 1) to increase the signal. The stoichiometry measurements are presented here for NKG2D dimers and DAP10 dimers, rather than individual chains as in Fig. 1. Mutation of one of the TM arginine residues in the NKG2D dimer reduced the number of associated DAP10 dimers from 1.86 for the WT NKG2D dimer (lanes 1–3) to 0.8 (lanes 7–9), indicating that only a single DAP10 dimer was bound to the mixed NKG2D dimer. Furthermore, the yield of the mixed NKG2D dimer was reduced to 37% (lanes 7–9) relative to WT (100%, lanes 1–3). In reactions without DAP10 (lanes 4–6 and 10–12), the yield of NKG2D dimer was reduced to a level similar to the NKG2D dimer with one mutated chain. Data are representative of three experiments. (B) Graphical representation of the TM domains (as simplified helical wheels) for the six-chain complex formed by WT NKG2D dimer, compared with the four-chain complex formed by the mixed NKG2D dimer in which one of the TM arginine residues (R) has been mutated to alanine (A). The aspartic acid (D) residues in the TM domains of the DAP10 dimer are indicated in red and positioned at the interface with the NKG2D TM domains. (C) Predicted TM sequences and flanking segments for NKG2D and DAP10.
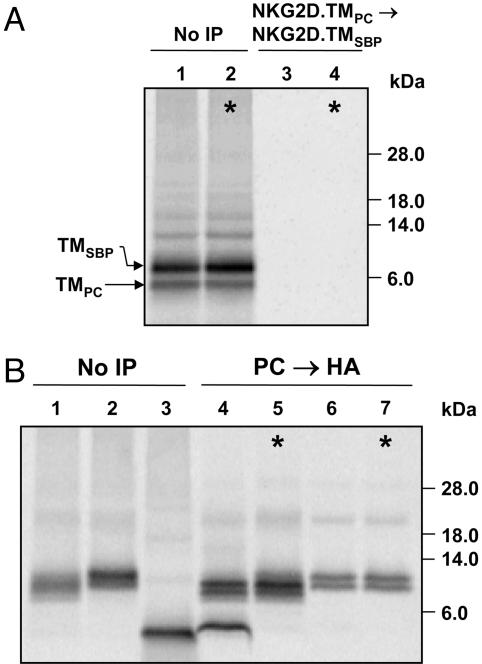
The NKG2D TM Domain Is Sufficient for Interaction with a DAP10 Dimer. We next aimed to define the minimal unit required for the interaction between NKG2D and DAP10 and created a construct that represented the NKG2D TM domain (NKG2D.TM) and five N- and C-terminal-flanking NKG2D residues for proper membrane insertion; in addition, this protein carried an N-terminal methionine and a C-terminal VSV epitope tag. In an important control experiment, we demonstrated that this NKG2D.TM peptide is monomeric (Fig. 3A). The NKG2D.TM peptide formed a three-chain complex with DAP10 because it was coprecipitated in a two-step snIP that targeted the PC- and HA-tagged DAP10 chains (Fig. 3B, lane 4). This interaction was specific based on the mixing control (Fig. 3B, lane 5) and required the TM aspartic acid of DAP10 (Fig. 3B, lane 6).
Fig. 3.
The TM domain of NKG2D is sufficient for assembly of a three-chain complex. A construct representing only the NKG2D TM domain and flanking residues (NKG2D.TM) was used to define the minimal interaction site. (A) The NKG2D.TM protein was monomeric, as shown by two-step PC→ SBP snIP analysis of translation reactions with SBP- and PC-tagged NKG2D.TM domains (lane 3). Data are representative of two experiments. (B) The monomeric NKG2D.TM domain formed a three-chain complex with the DAP10 dimer, as shown by two-step PC→ HA snIP targeting the epitope tags attached to the two DAP10 chains (lane 4). The interaction was disrupted by mutation of the TM aspartic acid of DAP10 (D→ A, lane 6). The low-molecular-weight TM peptides shown here and in Fig. 4 were resolved on 12% NuPAGE [bis(2-hydroxyethyl)amino]tris(hydroxymethyl)methane gels under reducing conditions. Data are representative of six experiments.
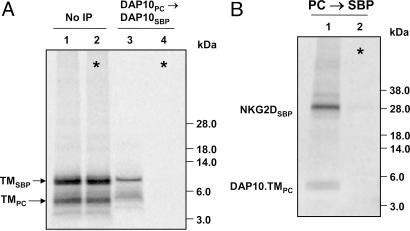
Localization of the interaction to the TM domains was further substantiated by experiments in which the extracellular and cytoplasmic domains of DAP10 were truncated to the membrane-spanning and membrane-proximal segments by using a similar design as described above for NKG2D.TM. These SBP- and PC-tagged DAP10 truncation mutants formed a DAP10 dimer (Fig. 4A) and assembled with full-length NKG2D (Fig. 4B). These DAP10 proteins lacked the cysteine residues that form the interchain disulfide bonds, confirming the conclusion from the experiment shown in Fig. 8 that the cysteine residues promote dimer formation and/or stability but are not essential for DAP10 dimerization. A single NKG2D TM domain is thus sufficient for assembly with a DAP10 dimer, explaining how the WT NKG2D dimer can assemble with two DAP10 dimers.
Fig. 4.
Interaction of DAP10 TM peptides with NKG2D. (A) DAP10 TM peptides formed a dimer, based on two-step PC→ SBP snIP of translation reactions with PC- and SBP-tagged DAP10.TM chains (lane 3). Data are representative of three experiments. (B) The DAP10.TM protein assembled with full-length NKG2D because the complex could be isolated by two-step snIP for the PC and SBP tags attached to DAP10.TM and NKG2D, respectively. *, Mixing controls demonstrated that assembly occurred in ER membranes. Data are representative of two experiments.
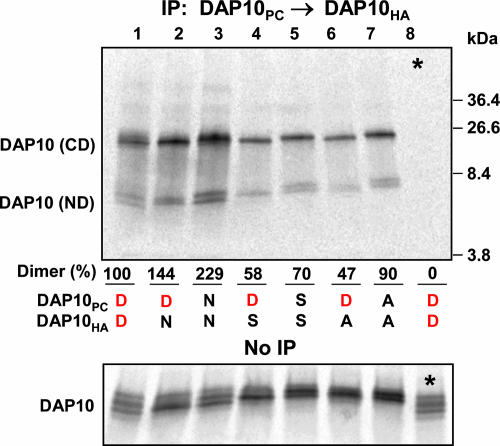
Structural Requirements for the Assembly of NKG2D with DAP10. The acidic residues of the DAP10 dimer may be located at or near the DAP10 dimer interface, and this functional pair may represent the interaction site for the basic TM arginine of NKG2D. We therefore examined the effect of mutating one or both aspartic acid residues on DAP10 dimer formation (Fig. 5) and assembly with NKG2D (Fig. 6). DAP10 dimers with mutation of one or both aspartic acid residues to asparagine, serine, or alanine were isolated in a two-step PC→HA snIP. The DAP10 TM aspartic acids were not required for dimerization as mutation of both aspartic acids to alanine (Fig. 5, lane 7) did not substantially reduce the yield of DAP10 dimer relative to WT (Fig. 5, lane 1). However, substitution of one or both aspartic acids by asparagine (DN and NN combinations; Fig. 5, lanes 2 and 3, respectively) significantly increased DAP10 dimer formation, suggesting that these polar residues are located at or near the DAP10 dimer interface.
Fig. 5.
Evidence for the localization of the aspartic acid pair at or near the DAP10 dimer interface. DAP10 dimers in which one or both TM aspartic acids (D) were mutated to asparagine (N), serine (S), or alanine (A) were isolated by two-step snIP that targeted the PC and HA tags attached to WT or mutant chains. Covalent and noncovalent DAP10 dimers (CD and ND, respectively) are indicated, and the total amount of DAP10 dimer was compared for each combination relative to WT (100%, lane 1). Mutation of one or both TM aspartic acids by asparagine (DN and NN combinations in lanes 2 and 3, respectively) increased the yield of DAP10 dimers, whereas substitution of one aspartic acid by alanine (DA combination, lane 6) yielded the lowest level of DAP10 dimers. *, Lane 8 represents the mixing control for the WT combination. The upper gel was run under nonreducing conditions, and the lower gel was run under reducing conditions. Data are representative of four experiments.
Fig. 6.
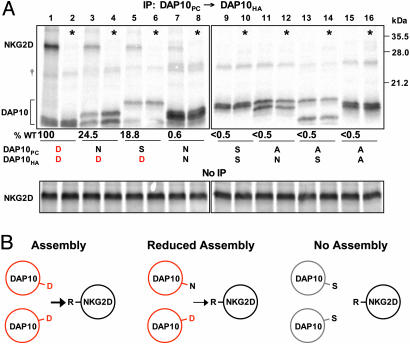
Both TM aspartic acids of the DAP10 dimer contribute to assembly with NKG2D. (A) Assembly reactions were performed with NKG2D and with PC- and HA-tagged WT and mutant DAP10 chains, and DAP10 dimers were targeted by two-step PC→ HA snIP. Radiolabeled NKG2D and DAP10 chains were quantitated and expressed as the ratio of NKG2D/DAP10 relative to WT (lane 1, 100%). Analysis of an aliquot of the reaction not subjected to IP demonstrated equal amounts of NKG2D in each reaction. Substitution of one of the aspartic acid residues by asparagine (DN combination, lane 3) or serine (DS combination, lane 5) substantially reduced the amount of associated NKG2D. The individual DAP10 chains with mutation of the TM aspartic acid migrated differently from the WT chain. *, Alternate lanes represent mixing controls in which DAP10 and NKG2D chains were translated separately. Data are representative of three experiments.
Assembly experiments with these DAP10 mutants and NKG2D (Fig. 6) demonstrated that substitution of even one of the aspartic acids in the DAP10 dimer by asparagine substantially reduced the interaction with NKG2D (Fig. 6A, lane 3), although formation of the DAP10 dimer was enhanced. Assembly with NKG2D was reduced to a similar level when one aspartic acid was mutated to a smaller polar residue (serine, DS combination; Fig. 6A, lane 5) and was abrogated by substitution of both aspartic acid residues with polar or nonpolar amino acids (Fig. 6A, lanes 9, 11, 13, and 15). These experiments demonstrated that both aspartic acids of the DAP10 dimer participate in the assembly with NKG2D. The assembly of the NKG2D–DAP10 complex thus shows striking similarities to the TCR–CD3 complex (25), even though NKG2D and TCR have no sequence homology and represent type II and type I membrane proteins, respectively.
Discussion
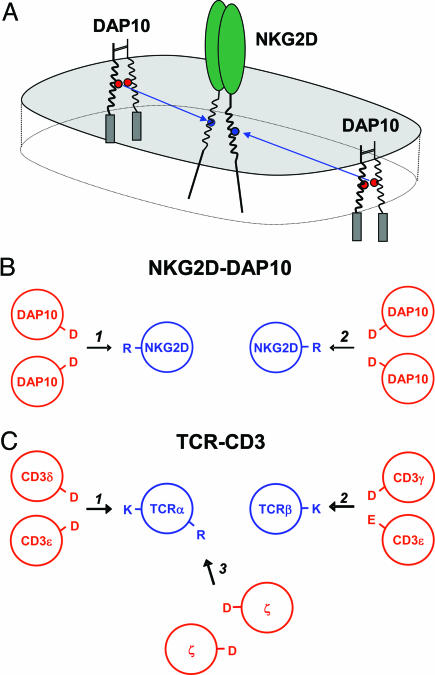
These results demonstrate that the activating human NKG2D receptor assembles into a hexameric structure based on the interaction of each NKG2D TM domain with a DAP10 dimer. This conclusion is based on four major findings: (i) Direct stoichiometry measurements showed that four DAP10 chains associate with a NKG2D dimer; (ii) selective mutation of one of the TM arginine residues of NKG2D eliminated the binding site for one of the two DAP10 dimers; (iii) a monomeric TM peptide of NKG2D assembled with a DAP10 dimer; and (iv) ionizable residues within the TM domains of the three interacting chains were critical for assembly. Assembly of the hexameric structure can thus be described as the formation of two three-helix structures in the membrane between one TM domain of NKG2D and both TM domains of the interacting DAP10 dimer. The arrangement of the TM helices thus shows similarities to the TCR–CD3 complex, in particular to the six-chain assembly intermediate between TCR and the CD3δε and CD3γε dimers (Fig. 7).
Fig. 7.
The assembly process that leads to the formation of a hexameric NKG2D–DAP10 receptor complex. (A) The data demonstrate that each TM domain of human NKG2D assembles with one DAP10 dimer and that this interaction involves both TM aspartic acids of DAP10 (red circles) and the TM arginine (blue circles). (B and C) The arrangement of the TM domains in the hexameric NKG2D–DAP10 structure (B) thus shows similarities to an assembly intermediate of the TCR–CD3 complex (C) that lacks the ζ–ζ dimer.
TCR recognition of MHC/peptide ligands is exquisitely sensitive, and only a few copies of an agonist MHC/peptide ligand (possibly only one or two copies) on the surface of target cells are sufficient to trigger the receptor (32, 33). The sensitivity of TCR recognition is even more surprising given the short half-life (typically <10 s) of TCR interactions with MHC/peptide (34) and has been ascribed to the presence of three signaling modules that permit the assembly of large signaling complexes on tyrosine residues phosphorylated by Src kinases. The presence of four, rather than two, DAP10 chains in a NKG2D–DAP10 complex may be relevant for sensitive detection of NKG2D ligands, in particular when they are expressed at low densities. The sensitivity of NKG2D ligand recognition is important in several biological settings, such as immune evasion by viral pathogens and tumor cells. Immune responses mediated by NK cells and CD8 T cells are important in the control of cytomegalovirus (CMV), and human and murine CMV encode viral proteins (UL16 for human CMV and gp40 for murine CMV) that retain NKG2D ligands in intracellular compartments and thus impair antiviral NK cell and CD8 T cell responses (35–38). Recognition of tumor cells that express NKG2D ligands can be impaired by tumor-derived soluble MICA, which reduces NKG2D surface expression on tumor-infiltrating and circulating T cells (19, 39). The strength of the signal transmitted through the NKG2D receptor thus has a major impact on NK cell and CD8 T cell function in these diseases.
The data also strongly suggest that assembly into a hexameric structure is a common feature of activating immune receptors that represent homodimers with a basic residue in each TM domain (Table 1). The data on NKG2D unequivocally demonstrate that each of the two basic TM residues interacts with a separate signaling dimer, and the structural motif responsible for assembly is remarkably similar to the TCR–CD3 complex (Fig. 7). This group of receptors includes members of both the C-type lectin (murine Ly49D and Ly49H and murine NKR–P1C) and Ig families (human NKp46), and these proteins are primarily expressed by NK cells and in some cases subpopulations of T cells (40–42). Within this group of receptors, the murine NKG2D and Ly49H receptors are of particular interest. The activating Ly49H is critical for NK cell recognition in C57BL/6 mice that are resistant to murine cytomegalovirus, and Ly49H recognition of a viral protein encoded by the m157 gene triggers NK cell activation through the associated DAP12 dimer (43). The murine NKG2D gene encodes two splice variants, and the long isoform (NKG2D-L) only assembles with DAP10 as human NKG2D. However, the short isoform (NKG2D-S) is expressed by activated NK cells and can assemble with DAP10 and DAP12 (44, 45). Based on the data on human NKG2D described here, it is thus possible that a hexameric NKG2D complex is assembled in activated murine NK cells, which incorporates one DAP10 and one DAP12 dimer. Formation of such a receptor structure could be functionally relevant because the two dimers initiate distinct signaling cascades: DAP12 activates the Syk/ZAP70 pathway, and DAP10 signals through the phosphatidylinositol-3 kinase pathway (42)
Table 1. Activating homodimeric receptors with two basic TM residues.
| Receptor | Signaling dimer | Protein family |
|---|---|---|
| Human | ||
| NKG2D | DAP10 | C-type lectin |
| NKp46 | FCγ, ζ | Immunoglobulin |
| Mouse | ||
| NKG2D | DAP10 | C-type lectin |
| NKR-P1C | Fcγ | C-type lectin |
| Ly49D | DAP12 | C-type lectin |
| Ly49H | DAP12 | C-type lectin |
A number of receptors that are primarily expressed by human and/or murine NK cells represent homodimers with a basic residue in each TM domain and may thus form a hexameric structure. The human NKp46 receptor can assemble with the Fcγ or ζ-ζ signaling dimers (40), and two-step IP experiments demonstrated that it forms a homodimer (data not shown). The mouse Ly49H and Ly49D receptors assemble with DAP12 (41), whereas the mouse NKR-P1C receptor assembles with Fcγ (42). The stoichiometry described for the NKG2D-DAP10 complex may thus be relevant for several receptors from the C-type lectin and immunoglobulin families.
The activating NKG2D receptor thus assembles into a hexameric structure based on the same fundamental mechanism as the TCR–CD3 complex. The cooperativity of the assembly process permits efficient assembly of this receptor structure, which is important for NK cell and T cell function in viral infections and autoimmune diseases, and the recognition of transformed cells.
Supplementary Material
Acknowledgments
This work was supported by a National Institutes of Health Grant R01 AI054520 (to K.W.W.).
Author contributions: K.W.W. designed research; D.G., M.E.C., and J.F. performed research; and K.W.W. wrote the paper.
Abbreviations: NK, natural killer; ER, endoplasmic reticulum; TM, transmembrane; TCR, T cell antigen receptor; SBP, streptavidin-binding peptide; PC, calcium-dependent protein C epitope tag antibody; HA, hemagglutinin; IP, immunoprecipitation; snIP, sequential nondenaturing IP.
References
- 1.Bahram, S., Bresnahan, M., Geraghty, D. E. & Spies, T. (1994) Proc. Natl. Acad. Sci. USA 91, 6259–6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer, S., Groh, V., Wu, J., Steinle, A., Phillips, J. H., Lanier, L. L. & Spies, T. (1999) Science 285, 727–729. [DOI] [PubMed] [Google Scholar]
- 3.Wu, J., Song, Y., Bakker, A. B., Bauer, S., Spies, T., Lanier, L. L. & Phillips, J. H. (1999) Science 285, 730–732. [DOI] [PubMed] [Google Scholar]
- 4.Cerwenka, A., Bakker, A. B., McClanahan, T., Wagner, J., Wu, J., Phillips, J. H. & Lanier, L. L. (2000) Immunity 12, 721–727. [DOI] [PubMed] [Google Scholar]
- 5.Cosman, D., Mullberg, J., Sutherland, C. L., Chin, W., Armitage, R., Fanslow, W., Kubin, M. & Chalupny, N. J. (2001) Immunity 14, 123–133. [DOI] [PubMed] [Google Scholar]
- 6.Vivier, E., Tomasello, E. & Paul, P. (2002) Curr. Opin. Immunol. 14, 306–311. [DOI] [PubMed] [Google Scholar]
- 7.Cerwenka, A. & Lanier, L. L. (2003) Tissue Antigens 61, 335–343. [DOI] [PubMed] [Google Scholar]
- 8.Raulet, D. H. (2003) Nat. Rev. Immunol. 3, 781–790. [DOI] [PubMed] [Google Scholar]
- 9.Groh, V., Rhinehart, R., Randolph-Habecker, J., Topp, M. S., Riddell, S. R. & Spies, T. (2001) Nat. Immunol. 2, 255–260. [DOI] [PubMed] [Google Scholar]
- 10.Jamieson, A. M., Diefenbach, A., McMahon, C. W., Xiong, N., Carlyle, J. R. & Raulet, D. H. (2002) Immunity 17, 19–29. [DOI] [PubMed] [Google Scholar]
- 11.Li, P., Morris, D. L., Willcox, B. E., Steinle, A., Spies, T. & Strong, R. K. (2001) Nat. Immunol. 2, 443–451. [DOI] [PubMed] [Google Scholar]
- 12.Carayannopoulos, L. N., Naidenko, O. V., Fremont, D. H. & Yokoyama, W. M. (2002) J. Immunol. 169, 4079–4083. [DOI] [PubMed] [Google Scholar]
- 13.Li, P., McDermott, G. & Strong, R. K. (2002) Immunity 16, 77–86. [DOI] [PubMed] [Google Scholar]
- 14.McFarland, B. J., Kortemme, T., Yu, S. F., Baker, D. & Strong, R. K. (2003) Structure (Cambridge, Mass.) 11, 411–422. [DOI] [PubMed] [Google Scholar]
- 15.Groh, V., Rhinehart, R., Secrist, H., Bauer, S., Grabstein, K. H. & Spies, T. (1999) Proc. Natl. Acad. Sci. USA 96, 6879–6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girardi, M., Oppenheim, D. E., Steele, C. R., Lewis, J. M., Glusac, E., Filler, R., Hobby, P., Sutton, B., Tigelaar, R. E. & Hayday, A. C. (2001) Science 294, 605–609. [DOI] [PubMed] [Google Scholar]
- 17.Cerwenka, A., Baron, J. L. & Lanier, L. L. (2001) Proc. Natl. Acad. Sci. USA 98, 11521–11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diefenbach, A., Jensen, E. R., Jamieson, A. M. & Raulet, D. H. (2001) Nature 413, 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groh, V., Wu, J., Yee, C. & Spies, T. (2002) Nature 419, 734–738. [DOI] [PubMed] [Google Scholar]
- 20.Groh, V., Bruhl, A., El-Gabalawy, H., Nelson, J. L. & Spies, T. (2003) Proc. Natl. Acad. Sci. USA 100, 9452–9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hue, S., Mention, J. J., Monteiro, R. C., Zhang, S., Cellier, C., Schmitz, J., Verkarre, V., Fodil, N., Bahram, S., Cerf-Bensussan, N. & Caillat-Zucman, S. (2004) Immunity 21, 367–377. [DOI] [PubMed] [Google Scholar]
- 22.Meresse, B., Chen, Z., Ciszewski, C., Tretiakova, M., Bhagat, G., Krausz, T. N., Raulet, D. H., Lanier, L. L., Groh, V., Spies, T., et al. (2004) Immunity 21, 357–366. [DOI] [PubMed] [Google Scholar]
- 23.Ogasawara, K., Hamerman, J. A., Ehrlich, L. R., Bour-Jordan, H., Santamaria, P., Bluestone, J. A. & Lanier, L. L. (2004) Immunity 20, 757–767. [DOI] [PubMed] [Google Scholar]
- 24.Kane, L. P. & Weiss, A. (2003) Immunol. Rev. 192, 7–20. [DOI] [PubMed] [Google Scholar]
- 25.Call, M. E., Pyrdol, J., Wiedmann, M. & Wucherpfennig, K. W. (2002) Cell 111, 967–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng, J., Garrity, D., Call, M. E., Moffett, H. & Wucherpfennig, K. W. (2005) Immunity, 122, 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gratkowski, H., Lear, J. D. & DeGrado, W. F. (2001) Proc. Natl. Acad. Sci. USA 98, 880–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou, F. X., Merianos, H. J., Brunger, A. T. & Engelman, D. M. (2001) Proc. Natl. Acad. Sci. USA 98, 2250–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bijlmakers, M. J., Benaroch, P. & Ploegh, H. L. (1994) EMBO J. 13, 2699–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huppa, J. B. & Ploegh, H. L. (1997) J. Exp. Med. 186, 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolan, D. W., Teyton, L., Rudolph, M. G., Villmow, B., Bauer, S., Busch, D. H. & Wilson, I. A. (2001) Nat. Immunol. 2, 248–254. [DOI] [PubMed] [Google Scholar]
- 32.Sykulev, Y., Joo, M., Vturina, I., Tsomides, T. J. & Eisen, H. N. (1996) Immunity 4, 565–571. [DOI] [PubMed] [Google Scholar]
- 33.Irvine, D. J., Purbhoo, M. A., Krogsgaard, M. & Davis, M. M. (2002) Nature 419, 845–849. [DOI] [PubMed] [Google Scholar]
- 34.Matsui, K., Boniface, J. J., Steffner, P., Reay, P. A. & Davis, M. M. (1994) Proc. Natl. Acad. Sci. USA 91, 12862–12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krmpotic, A., Busch, D. H., Bubic, I., Gebhardt, F., Hengel, H., Hasan, M., Scalzo, A. A., Koszinowski, U. H. & Jonjic, S. (2002) Nat. Immunol. 3, 529–535. [DOI] [PubMed] [Google Scholar]
- 36.Dunn, C., Chalupny, N. J., Sutherland, C. L., Dosch, S., Sivakumar, P. V., Johnson, D. C. & Cosman, D. (2003) J. Exp. Med. 197, 1427–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rolle, A., Mousavi-Jazi, M., Eriksson, M., Odeberg, J., Soderberg-Naucler, C., Cosman, D., Karre, K. & Cerboni, C. (2003) J. Immunol. 171, 902–908. [DOI] [PubMed] [Google Scholar]
- 38.Wu, J., Chalupny, N. J., Manley, T. J., Riddell, S. R., Cosman, D. & Spies, T. (2003) J. Immunol. 170, 4196–4200. [DOI] [PubMed] [Google Scholar]
- 39.Salih, H. R., Rammensee, H. G. & Steinle, A. (2002) J. Immunol. 169, 4098–4102. [DOI] [PubMed] [Google Scholar]
- 40.Pessino, A., Sivori, S., Bottino, C., Malaspina, A., Morelli, L., Moretta, L., Biassoni, R. & Moretta, A. (1998) J. Exp. Med. 188, 953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith, K. M., Wu, J., Bakker, A. B., Phillips, J. H. & Lanier, L. L. (1998) J. Immunol. 161, 7–10. [PubMed] [Google Scholar]
- 42.Lanier, L. L. (2005) Ann. Rev. Immunol. 23, 225–274. [DOI] [PubMed] [Google Scholar]
- 43.Arase, H., Mocarski, E. S., Campbell, A. E., Hill, A. B. & Lanier, L. L. (2002) Science 296, 1323–1326. [DOI] [PubMed] [Google Scholar]
- 44.Diefenbach, A., Tomasello, E., Lucas, M., Jamieson, A. M., Hsia, J. K., Vivier, E. & Raulet, D. H. (2002) Nat. Immunol. 3, 1142–1149. [DOI] [PubMed] [Google Scholar]
- 45.Gilfillan, S., Ho, E. L., Cella, M., Yokoyama, W. M. & Colonna, M. (2002) Nat. Immunol. 3, 1150–1155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.