Abstract
Evasion of the immune response is an integral part of the pathogenesis of glioma. In humans, important mechanisms of immune evasion include recruitment of regulatory T cells (Tregs) and polarization of macrophages towards an M2 phenotype. Canine glioma has a robust immune cell infiltrate that has not been extensively characterized. The purpose of this study was to determine the distribution of immune cells infiltrating spontaneous intracranial canine gliomas. Seventy-three formalin-fixed, paraffin-embedded tumor samples were evaluated using immunohistochemistry for CD3, forkhead box 3 (FOXP3), CD20, Iba1, calprotectin (Mac387), CD163, and indoleamine 2,3-dioxygenase (IDO). Immune cell infiltration was present in all tumors. Low-grade and high-grade gliomas significantly differed in the numbers of FoxP3+ cells, Mac387+ cells, and CD163+ cells (p = 0.006, 0.01, and 0.01, respectively). Considering all tumors, there was a significant increase in tumor area fraction of CD163 compared to Mac387 (p < 0.0001), and this ratio was greater in high-grade tumors than in low-grade tumors (p = 0.005). These data warrant further exploration into the roles of macrophage repolarization or Treg interference therapy in canine glioma.
Keywords: Astrocytoma, brain, dogs, cancer, immunohistochemistry, immunology, lymphocytes, macrophages, oligodendroglioma
Gliomas are a group of devastating brain tumors that affect both dogs and humans.1,3,11,50,64,69,72 They represent approximately 35% of primary intracranial tumors in the dog and are overrepresented in brachycephalic breeds such as the boxer, Boston terrier, and English and French bulldog.49,64,73 Glioma subtypes in humans include oligodendroglioma, astrocytoma and glioblastoma, and in dogs are oligodendroglioma, astrocytoma, and undefined glioma.39,44,49 Median survival for glioblastoma, the most common and most aggressive brain tumor in humans, is approximately 14 months with current standard of care (maximal surgical resection followed by radiation and temozolomide chemotherapy).70 Median survival for dogs with suspected glioma (based on imaging) receiving radiation therapy is similar, ranging from 9 to 14 months.5,7,67 Treatment failure in both species occurs due to the failure of local therapy, and neoplastic cells can survive within grossly normal brain parenchyma several centimeters from the main tumor mass.22,27,81 Therefore, novel therapies for these devastating cancers are desperately needed. One group of treatments that hold tremendous promise are immunotherapies, which have the potential to harness the immune system to seek out and destroy neoplastic cells in brain regions distant from the main tumor mass. An essential function of the immune system is to recognize and eliminate foreign cells. However, a hallmark of cancer is evasion of the immune system, and gliomas are particularly adept at such evasion.23,28,82 Important components of immune evasion by gliomas in humans include recruitment of regulatory T cells (Tregs) to the tumor and polarization of tumor-associated macrophages towards an M2 phenotype.54,56,83
The primary function of Tregs is to inhibit the functions of effector immune cells, which prevents the development of autoimmunity in healthy individuals. However, Tregs can be recruited and coopted by tumors, and play a role in glioma pathogenesis by suppressing the adaptive immune response to invading tumor cells.15,16,25,32,45,54,65 Indoleamine 2,3 dioxygenase (IDO) is an enzyme responsible for the catabolism of tryptophan, an amino acid essential to effector lymphocyte function.35,89 In addition to tryptophan depletion, tryptophan metabolites, including kynurenine, contribute to Treg expansion and effector T cell suppression.35,89 Studies in humans and experimental rodent models have shown that IDO is highly expressed in gliomas, contributes to intratumoral Treg expansion, is associated with survival, and may serve as a viable therapeutic target.29,35,78–80,89
Macrophages are also increasingly recognized as important in tumor immunology and can be polarized along a spectrum from an M1 to an M2 phenotype.2 M1 macrophages are associated with a pro-inflammatory, normoxic state, and have anti-tumor properties associated with longer survival in human glioblastoma patients, while M2 macrophages are associated with an anti-inflammatory, hypoxic state, with pro-tumor functions and shorter survival in these patients.21,56,83,87 The designation of macrophages as M1/pro-inflammatory or M2/anti-inflammatory would ideally be made using a test of cellular function. However, such tests are difficult to perform when evaluating samples from either human or veterinary patients and surrogate markers of function are frequently used. Calprotectin and CD163 have been used as markers of M1/pro-inflammatory and M2/anti-inflammatory macrophages (respectively) in a number of species, including dogs.9,13,20,31,53,76,77,85
Successful understanding of canine glioma pathogenesis and implementation of immune therapies will require characterization of the tumor immunological landscape within this species. However, such studies have been limited to date, particularly in regards to microglia, macrophages and IDO.1,18,55,63 In this study, our objectives were to characterize the infiltration of canine intracranial gliomas by T cells, B cells, Tregs, microglia, macrophages, M1-like macrophages, and M2-like macrophages, to compare immune cell infiltration between glioma subtypes and grades, and to determine if IDO is expressed in canine gliomas.
MATERIALS AND METHODS
Sample Preparation and Diagnostic Review
A search was performed for canine glioma cases from the pathology archives at North Carolina State University’s College of Veterinary Medicine spanning years 2006–2018. Case demographic data (age at sample collection, weight, sex) and time from death to necropsy examination were obtained from the medical records. Brains were fixed whole in 10% neutral buffered formalin (standard protocol of 48–72 hours), routinely processed for histology, and embedded in paraffin. Paraffin-embedded samples were sectioned at 5 μm, mounted on charged glass slides, stained with hematoxylin and eosin (H&E) or used for immunohistochemistry, and digitally scanned to an electronic database (Aperio AT Turbo, Leica Biosystems, Buffalo Grove, IL, USA).
A panel of four board-certified (American College of Veterinary Pathologists) veterinary anatomic pathologists (GAK, DEM, ADM, DAT) and one physician neuropathologist (CRM) independently reviewed the H&E-stained sections, as well as immunohistochemistry for oligodendrocyte transcription factor (Olig2), glial fibrillary acidic protein (GFAP), 2’,3’-cyclic-nucleotide 3’-phosphodiesterase (CNPase), and Ki-67. Pathologists diagnosed each case as oligodendroglioma, astrocytoma, undefined glioma, or other (non-glioma) and also determined grade (low-grade or high-grade) for cases diagnosed as glioma. Classification was based on diagnostic criteria from recently published consensus guidelines for canine glioma.39 Briefly, tumors were classified as oligodendroglioma or astrocytoma if > 80% of the tumor reflected characteristics typical of these neoplasms (Supplemental Figs. S1–S2). Undefined glioma was diagnosed if features of both neoplasms were present and the minority phenotype composed more than 20% of the tumor. Features that designated a high-grade status were geographic necrosis (Supplemental Fig. S3), microvascular proliferation (Supplemental Fig. S4), presence of mitoses, overt cellular or nuclear pleomorphism, or atypia. A majority diagnosis (agreement between ≥ 3 pathologists) was required to define each tumor with a glioma subtype and corresponding grade and to include further analysis for immune cell infiltration and IDO expression.
Case Details and Tumor Samples
A majority diagnosis of glioma subtype and grade was achieved for 73 cases, which included 9 low-grade oligodendrogliomas (12%), 8 low-grade astrocytomas (11%), 44 high-grade oligodendrogliomas (60%), 10 high-grade astrocytomas (14%), and 2 high-grade undefined gliomas (3%). Given the low prevalence of undefined gliomas, comparisons between tumor subtypes were limited to oligodendrogliomas and astrocytomas. Undefined gliomas were included in statistical analyses when comparing data across tumor grades. All tumor samples were collected at necropsy. The time from death until necropsy, which was available for 69 cases, ranged from 0–120 hours (0–24 hours, n = 24; 24–48 hours, n = 39; 48–72 hours, n = 4; 72–96 hours, n = 1; 96–120 hours, n = 1). The mean age of the cases was 8.2 years (range 2.9–14.5 years) and the mean body weight was 23.6 kg (range 3.7–50.9 kg). There were 39 spayed females, 28 castrated males, and 6 intact males represented.
Immunohistochemistry
Slides were deparaffinized in xylene, and the tissues were rehydrated through graded ethanols. Antigen retrieval was performed using various buffers within a heated Decloaker® pressure chamber (Biocare Medical, Concord, CA, USA) or using an enzymatic technique, depending on antibody requirements (Supplemental Table S1). Endogenous peroxidase was blocked with 3% hydrogen peroxide, and non-specific binding was blocked by incubating slides with normal serum from the appropriate species. Immunohistochemistry was performed using primary antibodies against a variety of immune cell markers, and positive control tissue was canine in origin (Supplemental Table S1). Immune cell markers and their intended targets were CD3 (T cells), forkhead box 3 (FoxP3, Tregs), CD20 (B cells), Iba1 (macrophages/microglia). calprotectin (Mac387, M1-like macrophages), CD163 (M2-like macrophages), and IDO. Isotype-matched antibodies were used for negative control tissues.33 Antigen-antibody complexes were detected using 3,3-diaminobenzidine (DAB, Dako, Agilent, Santa Clara, CA). Slides were counterstained with hematoxylin, dehydrated in graded ethanols, cleared with xylenes and cover-slipped.
Immune Cell Infiltrate Analysis
The immunohistochemically labeled brain tumor slides were scanned at 200X magnification using a whole slide scanner (Hamamatsu Nanozoomer NDP.scan, Hamamatsu City, Japan) and imported into a database for quantitative image analysis, using commercially available software (Visiopharm Version 2020.05.0.7761, Visiopharm, Westminster, CO). A region of interest (ROI) was manually generated around the entire tumor within each section, excluding areas of geographic necrosis.
Immunolabeled cells were characterized by DAB binding and hematoxylin counterstaining. Labeled cells were segmented from negative cells, fibers, and background using an artificial intelligence machine learning decision forest classifier or by applying a threshold (the method was dependent on the cellular marker), and processing steps were performed to separate cells that were close together and count them as individual cells (Supplemental Figs. S5–S8). False positives based on the size of the cell of interest were removed, as was background staining and artifact.
For CD3, FoxP3, and CD20, cell counts were derived by dividing the total immunolabeled cell count by the total area of the defined ROI (cells/μm2). This was then converted to a standard high-powered light microscopy field (HPF) (400X, FN 22 mm, 0.237 mm2).47 Manual cell counts per 10 HPF in each tumor, as well as manual assessment of cellular and nuclear morphology of immunolabeled cells, were also performed as a means of quality control.
Although the algorithms for Iba1, Mac387, and CD163 were robust in identifying immunolabeled cells, frequent dense clustering of cells created difficulty in separating cells and establishing accurate cell counts. Therefore, in order to more accurately capture microglial and macrophage infiltration, area fractions were determined for Iba1, Mac387, and CD163 by dividing the immunolabeled area by the total ROI area. Manual counts, as well as manual assessment of cellular and nuclear morphology, of immunolabeled cells were performed as a means of quality control. In addition, the preponderant microglial phenotype (ramified, reactive, or amoeboid) was recorded manually for each tumor.
Assessment of IDO immunolabeling was performed manually. Cases were recorded as positive or negative with zero threshold.
Statistical Analysis
Descriptive statistics were generated for dog ages and weights. A D’Agostino-Pearson test for normality was used to determine that the cell counts and area fractions did not follow Gaussian distributions, and nonparametric tests were used to compare differences in cell populations across tumor subtypes and grades. Comparisons made between two groups were evaluated using a Mann-Whitney test, and comparisons between greater than two groups were evaluated using a Kruskal-Wallis test. Comparisons between paired samples (Mac387 and CD163) were evaluated using a Wilcoxon matched-pairs signed rank test. The correlation between FoxP3 and CD163 counts was performed using Spearman correlation testing. Statistical calculations were performed using commercially available software (Graphpad Prism, La Jolla, CA, USA), and a p value < 0.05 was considered to be significant.
Data Availability
The raw data analyzed in this study are not available as Supplemental Materials, though requests to the corresponding author can be made for those interested in further information.
RESULTS
Lymphocyte and IDO Immunohistochemistry
Subcellular localization of antibody immunolabeling was appropriate for all antibodies (membranous for CD3 and CD20; nuclear for FoxP3; cytoplasmic for IDO). Cells immunolabeled for each of the lymphocyte markers were detected in all tumors. CD3+ lymphocytes were generally dispersed throughout the tumors (Fig. 1), though they were also present in dense clusters around foci of microvascular proliferation (Fig. 2) and occasionally surrounding normal blood vessels. FoxP3+ lymphocytes were quite variable in number and these cells were generally scattered throughout the tumors (Fig. 3). They occasionally extended beyond the tumor margin into the adjacent brain but were not otherwise detected in normal brain (Fig. 4). CD20+ lymphocytes were scattered throughout the tumor or limited to perivascular aggregates (Fig. 5). Manual cell counts of lymphocyte subtypes concorded with the automated counts (data not shown).
Figures 1–6: High-grade oligodendroglioma, brain, dog. Immunohistochemistry.
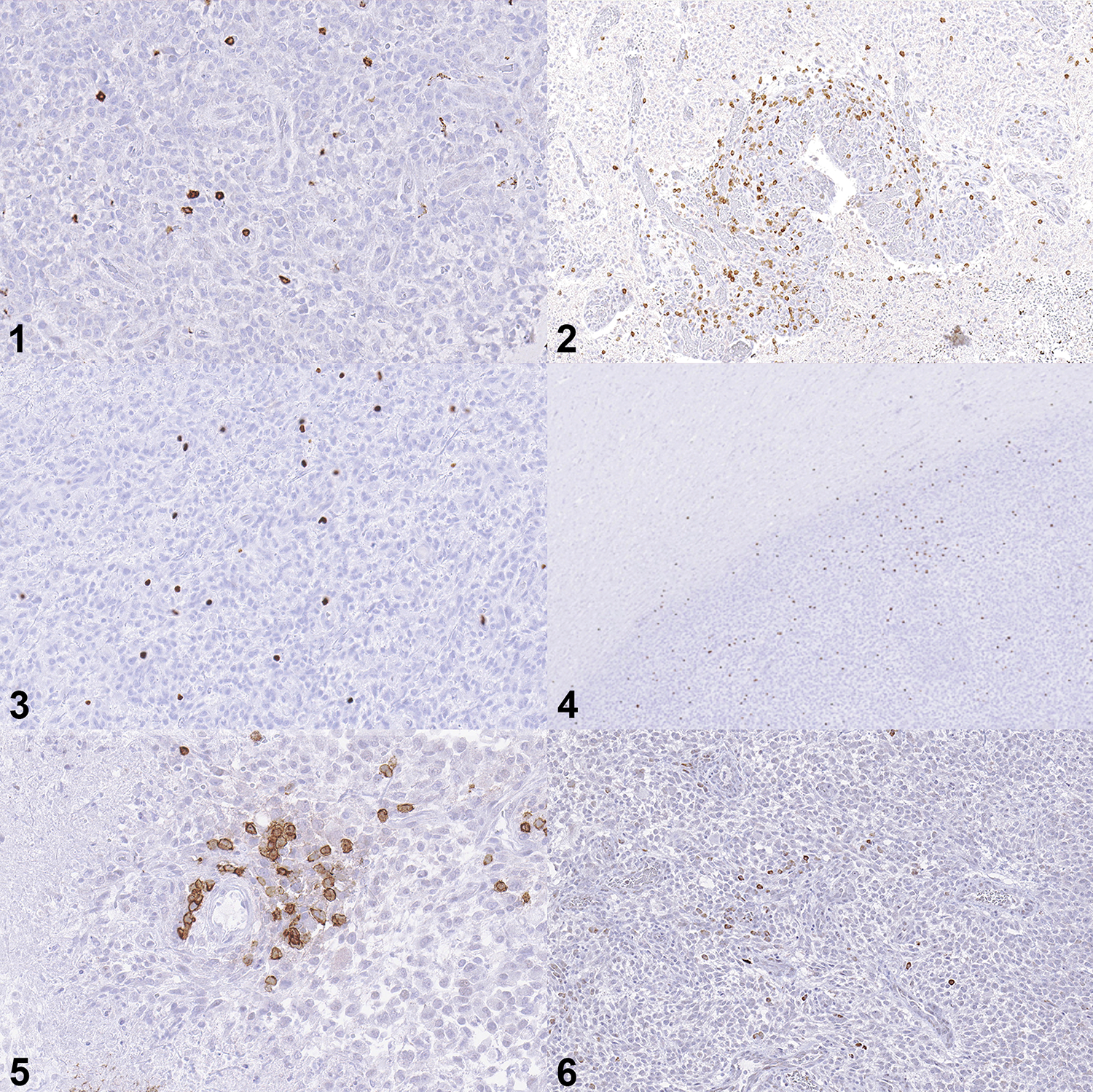
Figure 1: Moderate numbers of CD3-immunolabeled cells are scattered through the tumor.
Figure 2: CD3-immunolabeled cells form an aggregate around a focus of glomeruloid vascular proliferation.
Figures 3: Moderate numbers of FoxP3-immunolabeled cells are present in the tumor.
Figure 4: FoxP3-immunolabeled cells extend slightly beyond the brain-tumor interface but are rare in normal brain distant from the mass.
Figure 5: Clusters of CD20-immunolabeled cells form a perivascular aggregate.
Figure 6: IDO-immunolabeled cells are sporadically scattered through this tumor.
All cells immunolabeled for IDO were morphologically consistent with neoplastic glial cells, and immunolabeled cells were detected in 8/73 tumors (11%), comprising 7/44 (16%) high-grade oligodendrogliomas and 1/10 (10%) high-grade astrocytomas. Immunolabeled cells were generally sporadic, although they occasionally occurred in clusters (Fig. 6). The distribution of FoxP3 cell counts per HPF in IDO+ tumors was 0.2, 0.3, 0.4, 0.9, 1.1, 2.1, 2.1, and 58.1. The tumor with 58.1 FoxP3+ cells per HPF was a high-grade astrocytoma, and the remainder of the tumors were high-grade oligodendrogliomas.
No significant differences were found in CD3+ cell counts between different tumor subtypes (p = 0.67) or grades (p = 0.10, Fig. 7). There was a significant difference in FoxP3+ lymphocyte counts between different tumor grades, with increased counts in high-grade tumors (Fig. 8, p = 0.006). There was no difference in FoxP3+ lymphocyte counts between oligodendrogliomas and astrocytomas (p = 0.33). No significant differences were found in CD20+ cell counts between different tumor subtypes or grades (Fig. 9, p = 0.14 and p = 0.18, respectively).
Figures 7–9: Numbers of CD3+, Foxp3+, and CD20+ cells in canine glioma. HG = high-grade, LG = low-grade, oligo = oligodendroglioma, astro = astrocytoma, undef = undefined glioma.
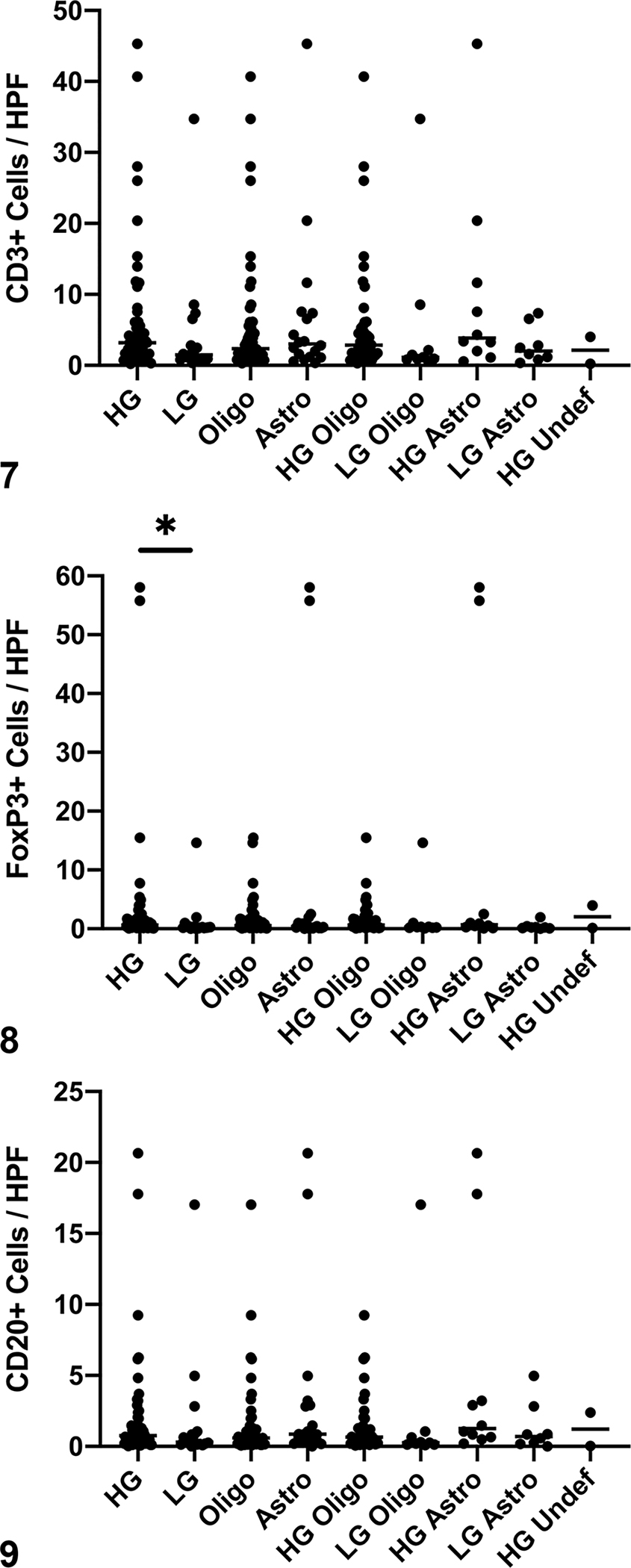
Figure 7: There are no differences in CD3+ cell counts between subtypes or grades of tumors. Bar = median.
Figure 8: High-grade tumors contain more FoxP3+ cells than do low-grade tumors (*, p = 0.006, Mann-Whitney test). There are no differences in FoxP3+ cell counts between tumor subtypes.
Figure 9: There are no significant differences in CD20+ cell counts between tumor subtypes or grade. Bar = median.
Microglia and Macrophage Immunohistochemistry
Subcellular localization of antibody immunolabeling was appropriate for all antibodies (cytoplasmic for Iba1 and Mac387; membranous for CD163). Cells immunolabeled for each of the macrophage markers were detected in all tumors.
Numerous Iba1-immunolabeled macrophages and microglia were scattered throughout the tumors. Ramified microglia had small cell bodies and multiple long, branching processes (Fig. 10). Reactive microglia were similar to ramified cells, though they had more robust cell bodies and diminished cell processes (Fig. 11). Amoeboid microglia and macrophages had even larger cell bodies with very small to undetectable cell processes (Fig. 12). The preponderant morphology recorded in these tumors was amoeboid, with much fewer, and almost equal numbers of ramified and reactive morphologies (Table 1). This was particularly true of high-grade tumors. In adjacent normal brain and at the brain-tumor interface, microglia generally exhibited a ramified phenotype, even if the majority phenotype within the tumor was amoeboid (Fig. 13). Mac387+ and CD163+ cells were scattered throughout the tumor and exhibited amoeboid morphologies (Fig.14 – 15).
Figures 10–15: High-grade oligodendroglioma, brain, dog. Immunohistochemistry.

Figure 10: Iba1-immunolabeled cells have small cell bodies with long processes, consistent with a ramified morphology.
Figure 11: Iba1-immunolabeled cells have medium-sized cell bodies with shortened processes, consistent with a reactive morphology.
Figure 12: Iba1-immunolabeled cells have robust cell bodies with short to absent processes, consistent with an amoeboid morphology.
Figure 13: Iba1-immunolabeled macrophages and microglia within the tumor exhibit an amoeboid morphology (right), which transitions towards a ramified morphology as the brain-tumor interface is approached (center). Macrophages and microglia within normal brain exhibit a ramified morphology (left).
Figure 14: Mac387 immunohistochemistry. There are low numbers of Mac387-immunolabeled cells scattered through the tumor consistent with M1 like macrophages. Inset: high magnification.
Figure 15: Large numbers of CD163-immunolabeled cells are scattered through the tumor consistent with M2-like macrophages. Inset: high magnification.
Table 1:
Predominance of ramified, reactive and amoeboid macrophage morphologies in 73 canine gliomas. The data show the number of tumors based on the predominant macrophage morphology. Amoeboid morphology predominates when considering all tumors, driven mainly by high-grade oligodendrogliomas. Low-grade tumors and astrocytomas have similar frequencies of ramified, reactive, and amoeboid morphologies
| Ramified | Reactive | Amoeboid | |
|---|---|---|---|
| Oligodendroglioma | 5 | 11 | 37 |
| Astrocytoma | 6 | 3 | 8 |
| High-Grade Undefined Glioma | 0 | 0 | 2 |
| Low-Grade Glioma | 6 | 4 | 7 |
| High-Grade Glioma | 5 | 10 | 40 |
| Low-Grade Oligodendroglioma | 3 | 2 | 4 |
| High-Grade Oligodendroglioma | 2 | 9 | 33 |
| Low-Grade Astrocytoma | 3 | 3 | 3 |
| High-Grade Astrocytoma | 3 | 1 | 5 |
| All | 11 | 14 | 48 |
Iba1+ macrophages/microglia were present in high numbers, but there were no significant differences in Iba1 labeling between different tumor subtypes or grades (p = 0.43 and p = 0.46, respectively; Fig. 16). Both the Mac387 and CD163 area fractions were significantly larger in high-grade than in low-grade tumors (p = 0.01 for both markers, Fig. 17–18) and were driven by the differences between high and low-grade oligodendrogliomas (p = 0.0006 for Mac387 and p = 0.02 for CD163), with no such difference within astrocytic tumors (p = 0.97 for Mac387 and p = 0.36 for CD163). However, no differences between oligodendrogliomas and astrocytomas were found for Mac387+ or CD163+ area fractions (p = 0.74 and 0.79, respectively). Manual cell counts of microglia and macrophage subtypes concorded with the automated counts (data not shown).
Figures 16–19. Quantification of immunolabeling for immune cells based on area fraction in canine glioma. HG = high-grade, LG = low-grade, oligo = oligodendroglioma, astro = astrocytoma, undef = undefined glioma.

Figure 16: There are no significant differences in Iba1 area fraction between different tumor subtypes or grade. Bar = median.
Figure 17: High-grade tumors have a higher Mac387 area fraction than low-grade tumors (*, p = 0.01, Mann-Whitney test). High-grade oligodendrogliomas have a higher Mac387 area fraction than low-grade-tumors (**, p = 0.0006, Mann-Whitney test). Bar = median.
Figure 18: High-grade tumors have a higher CD163 area fraction than low-grade tumors (*, p = 0.01, Mann-Whitney test). High-grade oligodendrogliomas have a higher CD163 area fraction than low-grade oligodendrogliomas (**, p = 0.02, Mann-Whitney test). Bar = median.
Figure 19: CD163 area fraction is significantly higher than Mac387 area fraction in high-grade tumors versus low-grade tumors (*, p = 0.005, Mann-Whitney test). Bar = median.
Considering all tumors, CD163+ area fraction was significantly greater than Mac387+ area fraction (p < 0.0001) (mean ratio CD163:Mac387 = 21.45, median 8.49, range 0.09 – 120.10, standard deviation 28.33). This ratio was greater in high-grade tumors compared to low-grade tumors (p = 0.005, Fig. 19), but it did not differ between tumor subtypes (p = 0.12, Fig. 19). There was a weak correlation between FoxP3 counts and CD163 counts (r = 0.49, p < 0.0001).
DISCUSSION
This study is the largest and most comprehensive characterization of inflammatory infiltrates in canine glioma to date, adding to the few previous studies in the dog.1,18,55,63 In this study, we found robust infiltration of Iba1+ microglia and macrophages into gliomas, with smaller numbers of CD3+ and CD20+ lymphocytes. FoxP3+ cells were found in all cases but were more numerous in high-grade tumors. Most Iba1+ microglia/macrophages exhibited an amoeboid morphology, particularly in high-grade gliomas. Both Mac387+ and CD163+ macrophages were found in greater numbers in high-grade versus low-grade gliomas, driven mainly by the difference in oligodendroglial tumors. However, CD163+ cell numbers were greater than Mac387+ counts in all tumors, a discrepancy that became wider in high-grade gliomas. We found a correlation, albeit a weak one, between CD163+ cell counts and FoxP3+ cell counts. Only a small subset of these gliomas was found to express IDO, consisting primarily of high-grade oligodendrogliomas.
We found CD3+ T cells in all tumors, both dispersed throughout the neoplasms and clustered around normal and glomeruloid vasculature, consistent with previous studies of canine gliomas.55,63 Pi Castro et al. found higher numbers of T cells in high-grade tumors55, a finding we did not replicate. One potential reason for this discrepancy is use of a different cell counting methodology, as Pi Castro et al. manually counted regions with the highest concentrations of immunolabeled cells55, while we analyzed entire tumor sections via computerized image analysis. Although present in lower numbers, we also found CD20+ B cells in all tumors, while these prior studies have only identified B cells in a subset of canine gliomas.55,63 Although we did see some individual B cells scattered throughout tumors, a prominent perivascular pattern was noted, as in these other studies. Differences in cell counting methodologies may also explain some of these B cell discrepancies. There is literature that demonstrates a relationship between B cells and T cells in experimental glioma models, with B cells serving as antigen-presenting cells and involved in the immune system’s response to glioma.8 Further work is necessary to explore such a relationship in the dog.
T cell infiltration is also a consistent feature of human gliomas.36,37,43,58,59,68 Most studies have focused on T cells, with less investigation of B cells and fewer of these cells identified when analyzed, although this has not been the case in all reports.86 Further subset analysis in several human studies have shown a predominance of CD8+ cells within this T cell subset, suggesting an attempt at a cytotoxic response that is ultimately ineffective.37,84 A lack of antibodies suitable for use in formalin-fixed, paraffin-embedded tissue sections has hampered further subset analysis in canine tumors, although one study of gliomas has shown a predominance of CD8+ cells with smaller numbers of CD4+ cells using in-situ hybridization.18 Several analyses have found an association between T cell infiltration of gliomas and longer survival times in human patients,37,38,43 although contradictory results were found in other studies.59 Such discrepancies are likely related to discrete T cell subsets with variable functions within these larger cell populations.
One important subgroup that has emerged is the Treg subset, traditionally defined as CD4+CD25+FoxP3+ lymphocytes and often identified in tissue by immunohistochemical labeling of FoxP3. Our study identified FoxP3+ cells in all canine gliomas examined. Though intratumoral FoxP3+ cells were generally low in number, these counts were quite variable, with robust infiltration in a number of tumors. We found greater Treg counts in high-grade tumors, consistent with another recent study of canine glioma, though that study found higher numbers of FoxP3+ cells in astrocytomas when compared to oligodendrogliomas, a finding not replicated here.55 Interestingly, in our study, the two highest Treg cell counts were from dogs with high-grade astrocytomas, and it is possible that our findings may be impacted by a limited number of astrocytomas in this study cohort. The presence of Tregs in all gliomas examined in our study and in this previous study contrasts with a study of canine intracranial meningiomas, in which only 39% of tumors had FoxP3+ cells identified via manual cell counting.6
Tregs inhibit immune system effector cells, impairing their attacks on tumor cells, and their recruitment from peripheral blood to the tumor is thought to play a role in glioma pathogenesis in humans.15,16,25,32,45,54,65 Though high-grade gliomas contain more Tregs than low-grade gliomas in humans, there are conflicting data regarding the effect of the magnitude of Treg infiltration on prognosis.30,32,71 Additional studies have demonstrated that the proportion of T effector cells to Tregs is more important than absolute numbers of either subset to T effector cell function and ultimately to prognosis.17,61 Similar studies are warranted in dogs, but are hampered by a lack of antibodies suitable for immunohistochemistry in formalin-fixed, paraffin-embedded tissue. Alternative methods such as in-situ hybridization and flow cytometry may facilitate such studies, especially with prospective collection of fresh or frozen tissue.18 Though the association of Treg infiltration with prognosis has not yet been investigated in canine glioma, the links between increased intratumoral Tregs and poor prognoses have been established in other canine cancers, including oral malignant melanoma, oral squamous cell carcinoma, pulmonary adenocarcinoma, malignant mammary carcinoma, and intestinal lymphoma.10,36,46,60
We detected expression of IDO with immunohistochemistry in only 11% of canine gliomas in this study, all of which were high grade tumors. This enzyme converts tryptophan to kynurenine, is highly expressed in human glioma, and is involved in activation of Tregs.14,51,78,88,89 In human glioma, high IDO expression has been associated with decreased survival in glioblastoma, and inhibition of IDO has been shown to be synergistic with other therapies in the treatment of glioma.29,35,78–80,88 We could not identify any relationship between IDO expression and Tregs, as the number of Tregs in IDO-expressing tumors was highly variable. Our data suggest that IDO may not play a significant role in the pathogenesis of canine glioma, though studies using other detection techniques may be necessary to further explore such a hypothesis.
Resident microglia and macrophages account for 5–10% of cells in the normal CNS41, though they can comprise up to 30% of cells in gliomas.38,40,57 We found robust infiltration of tumors by Iba1+ microglia and macrophages, which has been documented in other studies of canine brain tumors including gliomas, meningiomas, and choroid plexus tumors.1,6,12,63 Macrophages and microglia demonstrated a predominantly amoeboid phenotype in most gliomas, and this was particularly true in high grade oligodendrogliomas, with higher relative numbers of ramified and reactive microglial phenotypes in lower grade tumors and astrocytomas (Table 1). The changes in morphology of microglia from ramified through activated to amoeboid phenotypes are generally believed to correlate to a transition from a resting state to a more activated one in which the cells acquire more phagocytic properties.19,24,52,63 However, the true functional capabilities of these tumor-associated microglia and macrophages are unclear in the setting of canine intracranial gliomas.
We attempted to contribute to the emerging body of knowledge surrounding the immune response to these tumors by evaluating infiltration of cells immunolabeled for markers considered to represent relative polarization of macrophages towards an M1 or an M2 phenotype. This M1-M2 paradigm is an oversimplification, as macrophages exist along a continuum of function between these two extremes4 and cells can switch between M1 and M2 polarization.2 Mac387 is considered to be a marker of M1-polarized macrophages associated with a pro-inflammatory, normoxic, anti-tumor state, while M2-polarized macrophages, identified in part by CD163 expression, are associated with an anti-inflammatory, hypoxic, pro-tumor state.26,56,66,83,87,90,91 Other canine studies also have utilized these markers to identify macrophages with proposed M1/pro-inflammatory (Mac387) or M2/anti-inflammatory (CD163) functions.9,13,20,31,53,62,76,77,85
In our study, the CD163+ area fraction was greater than the Mac387+ area fraction, with a mean ratio of 21.45 (median 8.49) across all gliomas, suggesting general polarization of tumor-associated macrophages towards an M2-like phenotype. This polarization was even more apparent in high grade gliomas (mean CD163:Mac387 ratio = 22.67). These findings mirror several studies of human gliomas, which have demonstrated an increased M2-macrophage infiltrate in gliomas48 that becomes more prominent with higher tumor grades74,92 and has been associated with shorter survival in some studies.21,42,74
Previous studies of canine glioma have documented the presence of either Mac387+ or CD163+ cells, although not ratios between the two.1,63 Sloma et al. demonstrated relatively sparse numbers of Mac387+ macrophages within canine oligodendrogliomas and determined that most of these cells were in the intravascular compartment.63 Indeed, our counts of Mac387+ cells likely overestimated the numbers of cells within tumor parenchyma, as the automated platform used did not distinguish these from intravascular cells. Other studies have demonstrated that Mac387 can immunolabel neutrophils in dogs.75 However, the cellular and nuclear morphologies of Mac387+ cells in our study were not consistent with neutrophils and these cells are consistent with macrophages. Amin et al. demonstrated infiltration of CD163+ cells in a small number of canine gliomas (n=11) but did not examine a potential M1 phenotype.1 Our study supports the hypothesis that macrophages within canine glioma are polarized towards an M2-like phenotype, and that this polarization is greater in high-grade tumors. We also found a correlation between FoxP3+ cell counts and CD163+ cell counts in our study, suggesting a relationship between these cell types, as demonstrated in humans.4 Such a relationship warrants further study in canine tumors.
There are a number of limitations associated with this study. Dogs received a variety of different antemortem treatments, including glucocorticoids, non-steroidal anti-inflammatory drugs, or radiation therapy, which were not always possible to document due to the retrospective nature of the study. It is possible that these treatments affected the immune cell infiltrate within some of the tumors. The retrospective nature of the sample identification also led to some variation in the time from death to necropsy, as well as tissue fixation time. However, we believe that the effects of this variation were minimal due to similar death to necropsy intervals in most cases and the use of a standard fixation time. Additionally, immunolabeled cells were seen in all tumors. Intravascular monocytes, which are typically Mac387+,63 could not be excluded from automated digital counting and may have spuriously increased cell counts. However, the automated method allowed for unbiased analysis of all tumor tissue on a slide (with the intentional exclusion of geographic regions of necrosis), instead of the standard selection of certain high-power fields, which is subject to inaccuracies due to sampling errors and investigator bias.61 Another limitation is the use of single markers for Tregs (FoxP3), M1-like macrophages (Mac387) and M2-like macrophages (CD163). A panel of immune labels is likely to be more accurate in identifying immune cell subtypes and might include CD4, CD25, CD62L, CTLA-4, GITR for Tregs,16,17 ADAM10, ADAM17, iNOS, TNF-α, IL-1β, and IL-12 for M1 polarization, and CD206, CCL13, MMP-9, MMP14, IL-10, IL-6, and TGF-β for M2 polarization.18,21,26,34,42,48,87 Ultimately, the most relevant assessment of both lymphocyte and macrophage phenotype would be a functional one, and this aspect of immune-glioma interactions in dogs awaits further study.
Our ultimate goal is to further knowledge that will help predict determine prognosis and guide appropriate therapies in dogs with gliomas. As canine glioma is increasingly recognized as a relevant model of the human condition1,3, this work may have implications for the design of future studies and clinical trials investigating novel glioma therapeutics in both species.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Ms. Eli Ney of the National Toxicology Program at the National Institute of Environmental Health Sciences (NIEHS/NTP) for assistance with figure preparation, the NIEHS/NTP histology and immunohistochemistry core laboratories for technical support, and Drs. Daven Jackson-Humbles and Charan Ganta of the NIEHS/NTP for providing internal review of the manuscript.
FUNDING
The work presented in this manuscript was funded by North Carolina State University’s College of Veterinary Medicine, the NIEHS/NTP, and Charles River Laboratories.
Footnotes
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, or publication of this article.
Contributor Information
Gregory A. Krane, National Institute of Environmental Health Sciences: National Toxicology Program, Cellular and Molecular Pathology Branch, Research Triangle Park, NC, USA North Carolina State University, College of Veterinary Medicine, Department of Molecular Biomedical Sciences, Raleigh, NC, USA; Charles River Laboratories, Shrewsbury, MA.
Carly A. O’Dea, Charles River Laboratories, Durham, NC, USA
David E. Malarkey, National Institute of Environmental Health Sciences: National Toxicology Program, Cellular and Molecular Pathology Branch, Research Triangle Park, NC, USA
Andrew D. Miller, Cornell University, College of Veterinary Medicine, Department of Biomedical Sciences, Section of Anatomic Pathology, Ithaca, NY, USA
C. Ryan Miller, University of Alabama at Birmingham, Department of Pathology, Division of Neuropathology, O’Neal Comprehensive Cancer Center, Comprehensive Neuroscience Center, Birmingham, AL, USA.
Debra A. Tokarz, Experimental Pathology Laboratories Inc., Research Triangle Park, NC, USA
Heather L. Jensen, National Institute of Environmental Health Sciences: National Toxicology Program, Cellular and Molecular Pathology Branch, Research Triangle Park, NC, USA
Kyathanahalli S. Janardhan, Abbvie, North Chicago, IL, USA
Keith R. Shockley, National Institute of Environmental Health Sciences: Division of Intramural Research, Biostatistics and Computational Biology Branch, Research Triangle Park, NC, USA
Norris Flagler, National Institute of Environmental Health Sciences: National Toxicology Program, Cellular and Molecular Pathology Branch, Research Triangle Park, NC, USA.
Brittani A. Rainess, North Carolina State University, College of Veterinary Medicine, Comparative Neuroimmunology and Neuro-oncology Laboratory, Raleigh, NC, USA
Christopher L. Mariani, North Carolina State University, College of Veterinary Medicine, Comparative Neuroimmunology and Neuro-oncology Laboratory, Raleigh, NC, USA North Carolina State University, College of Veterinary Medicine, Department of Clinical Sciences, Raleigh, NC, USA.
REFERENCES
- 1.Amin SB, Anderson KJ, Boudreau CE, et al. Comparative Molecular Life History of Spontaneous Canine and Human Gliomas. Cancer Cell. 2020;37: 243–257.e247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Annovazzi L, Mellai M, Bovio E, Mazzetti S, Pollo B, Schiffer D. Microglia immunophenotyping in gliomas. Oncology letters. 2018;15: 998–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bentley RT, Ahmed AU, Yanke AB, Cohen-Gadol AA, Dey M. Dogs are man’s best friend: in sickness and in health. Neuro-oncology. 2017;19: 312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11: 889–896. [DOI] [PubMed] [Google Scholar]
- 5.Bley CR, Sumova A, Roos M, Kaser-Hotz B. Irradiation of brain tumors in dogs with neurologic disease. J Vet Intern Med. 2005;19: 849–854. [DOI] [PubMed] [Google Scholar]
- 6.Boozer LB, Davis TW, Borst LB, Zseltvay KM, Olby NJ, Mariani CL. Characterization of immune cell infiltration into canine intracranial meningiomas. Vet Pathol. 2012;49: 784–795. [DOI] [PubMed] [Google Scholar]
- 7.Brearley MJ, Jeffery ND, Phillips SM, Dennis R. Hypofractionated radiation therapy of brain masses in dogs: a retrospective analysis of survival of 83 cases (1991–1996). J Vet Intern Med. 1999;13: 408–412. [DOI] [PubMed] [Google Scholar]
- 8.Candolfi M, Curtin JF, Yagiz K, et al. B cells are critical to T-cell-mediated antitumor immunity induced by a combined immune-stimulatory/conditionally cytotoxic therapy for glioblastoma. Neoplasia (New York, NY 2011;13: 947–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter WJ, Crispin SM, Gould DJ, Day MJ. An immunohistochemical study of uveodermatologic syndrome in two Japanese Akita dogs. Vet Ophthalmol. 2005;8: 17–24. [DOI] [PubMed] [Google Scholar]
- 10.Carvalho MI, Pires I, Prada J, Gregório H, Lobo L, Queiroga FL. Intratumoral FoxP3 expression is associated with angiogenesis and prognosis in malignant canine mammary tumors. Vet Immunol Immunopathol. 2016;178: 1–9. [DOI] [PubMed] [Google Scholar]
- 11.Connolly NP, Shetty AC, Stokum JA, et al. Cross-species transcriptional analysis reveals conserved and host-specific neoplastic processes in mammalian glioma. Scientific reports. 2018;8: 1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalton MF, Stilwell JM, Krimer PM, Miller AD, Rissi DR. Clinicopathologic Features, Diagnosis, and Characterization of the Immune Cell Population in Canine Choroid Plexus Tumors. Front Vet Sci. 2019;6: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Döring AK, Junginger J, Hewicker-Trautwein M. Cruciate ligament degeneration and stifle joint synovitis in 56 dogs with intact cranial cruciate ligaments: Correlation of histological findings and numbers and phenotypes of inflammatory cells with age, body weight and breed. Vet Immunol Immunopathol. 2018;196: 5–13. [DOI] [PubMed] [Google Scholar]
- 14.Du L, Xing Z, Tao B, et al. Both IDO1 and TDO contribute to the malignancy of gliomas via the Kyn-AhR-AQP4 signaling pathway. Signal Transduct Target Ther. 2020;5: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Andaloussi A, Lesniak MS. CD4+ CD25+ FoxP3+ T-cell infiltration and heme oxygenase-1 expression correlate with tumor grade in human gliomas. J Neurooncol. 2007;83: 145–152. [DOI] [PubMed] [Google Scholar]
- 16.El Andaloussi A, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro-oncology. 2006;8: 234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fecci PE, Mitchell DA, Whitesides JF, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer research. 2006;66: 3294–3302. [DOI] [PubMed] [Google Scholar]
- 18.Filley A, Henriquez M, Bhowmik T, et al. Immunologic and gene expression profiles of spontaneous canine oligodendrogliomas. J Neurooncol. 2018;137: 469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franco-Bocanegra DK, McAuley C, Nicoll JAR, Boche D. Molecular Mechanisms of Microglial Motility: Changes in Ageing and Alzheimer’s Disease. Cells. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gelberman RH, Linderman SW, Jayaram R, et al. Combined Administration of ASCs and BMP-12 Promotes an M2 Macrophage Phenotype and Enhances Tendon Healing. Clin Orthop Relat Res. 2017;475: 2318–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gjorgjevski M, Hannen R, Carl B, et al. Molecular profiling of the tumor microenvironment in glioblastoma patients: correlation of microglia/macrophage polarization state with metalloprotease expression profiles and survival. Bioscience reports. 2019;39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glas M, Rath BH, Simon M, et al. Residual tumor cells are unique cellular targets in glioblastoma. Annals of Neurology. 2010;68: 264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorsi HS, Malicki DM, Barsan V, et al. Nivolumab in the Treatment of Recurrent or Refractory Pediatric Brain Tumors: A Single Institutional Experience. Journal of pediatric hematology/oncology. 2019;41: e235–e241. [DOI] [PubMed] [Google Scholar]
- 24.Graeber MB, Streit WJ. Microglia: biology and pathology. Acta Neuropathol. 2010;119: 89–105. [DOI] [PubMed] [Google Scholar]
- 25.Grauer OM, Nierkens S, Bennink E, et al. CD4+FoxP3+ regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress antiglioma immune responses in vivo. Int J Cancer. 2007;121: 95–105. [DOI] [PubMed] [Google Scholar]
- 26.Guadagno E, Presta I, Maisano D, et al. Role of Macrophages in Brain Tumor Growth and Progression. International journal of molecular sciences. 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halperin EC, Burger PC, Bullard DE. The fallacy of the localized supratentorial malignant glioma. Int J Radiat Oncol Biol Phys. 1988;15: 505–509. [DOI] [PubMed] [Google Scholar]
- 28.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144: 646–674. [DOI] [PubMed] [Google Scholar]
- 29.Hanihara M, Kawataki T, Oh-Oka K, Mitsuka K, Nakao A, Kinouchi H. Synergistic antitumor effect with indoleamine 2,3-dioxygenase inhibition and temozolomide in a murine glioma model. Journal of neurosurgery. 2016;124: 1594–1601. [DOI] [PubMed] [Google Scholar]
- 30.Heimberger AB, Kong LY, Abou-Ghazal M, et al. The role of tregs in human glioma patients and their inhibition with a novel STAT-3 inhibitor. Clin Neurosurg. 2009;56: 98–106. [PubMed] [Google Scholar]
- 31.Herrmann I, Gotovina J, Fazekas-Singer J, et al. Canine macrophages can like human macrophages be in vitro activated toward the M2a subtype relevant in allergy. Dev Comp Immunol. 2018;82: 118–127. [DOI] [PubMed] [Google Scholar]
- 32.Jacobs JF, Idema AJ, Bol KF, et al. Prognostic significance and mechanism of Treg infiltration in human brain tumors. J Neuroimmunol. 2010;225: 195–199. [DOI] [PubMed] [Google Scholar]
- 33.Janardhan KS, Jensen H, Clayton NP, Herbert RA. Immunohistochemistry in Investigative and Toxicologic Pathology. Toxicol Pathol. 2018;46: 488–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kennedy BC, Showers CR, Anderson DE, et al. Tumor-associated macrophages in glioma: friend or foe? Journal of oncology. 2013;2013: 486912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kesarwani P, Prabhu A, Kant S, et al. Tryptophan Metabolism Contributes to Radiation-Induced Immune Checkpoint Reactivation in Glioblastoma. Clin Cancer Res. 2018;24: 3632–3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JH, Hur JH, Lee SM, Im KS, Kim NH, Sur JH. Correlation of Foxp3 positive regulatory T cells with prognostic factors in canine mammary carcinomas. Vet J. 2012;193: 222–227. [DOI] [PubMed] [Google Scholar]
- 37.Kim YH, Jung TY, Jung S, et al. Tumour-infiltrating T-cell subpopulations in glioblastomas. Br J Neurosurg. 2012;26: 21–27. [DOI] [PubMed] [Google Scholar]
- 38.Kmiecik J, Poli A, Brons NH, et al. Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J Neuroimmunol. 2013;264: 71–83. [DOI] [PubMed] [Google Scholar]
- 39.Koehler JW, Miller AD, Miller CR, et al. A Revised Diagnostic Classification of Canine Glioma: Towards Validation of the Canine Glioma Patient as a Naturally Occurring Preclinical Model for Human Glioma. J Neuropathol Exp Neurol. 2018;77: 1039–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kushchayev SV, Kushchayeva YS, Wiener PC, Scheck AC, Badie B, Preul MC. Monocyte-derived cells of the brain and malignant gliomas: the double face of Janus. World neurosurgery. 2014;82: 1171–1186. [DOI] [PubMed] [Google Scholar]
- 41.Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39: 151–170. [DOI] [PubMed] [Google Scholar]
- 42.Lisi L, Ciotti GM, Braun D, et al. Expression of iNOS, CD163 and ARG-1 taken as M1 and M2 markers of microglial polarization in human glioblastoma and the surrounding normal parenchyma. Neuroscience letters. 2017;645: 106–112. [DOI] [PubMed] [Google Scholar]
- 43.Lohr J, Ratliff T, Huppertz A, et al. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-β. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17: 4296–4308. [DOI] [PubMed] [Google Scholar]
- 44.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131: 803–820. [DOI] [PubMed] [Google Scholar]
- 45.Lowther DE, Hafler DA. Regulatory T cells in the central nervous system. Immunol Rev. 2012;248: 156–169. [DOI] [PubMed] [Google Scholar]
- 46.Maeda S, Ohno K, Fujiwara-Igarashi A, Uchida K, Tsujimoto H. Changes in Foxp3-Positive Regulatory T Cell Number in the Intestine of Dogs With Idiopathic Inflammatory Bowel Disease and Intestinal Lymphoma. Vet Pathol. 2016;53: 102–112. [DOI] [PubMed] [Google Scholar]
- 47.Meuten DJ, Moore FM, George JW. Mitotic Count and the Field of View Area: Time to Standardize. Vet Pathol. 2016;53: 7–9. [DOI] [PubMed] [Google Scholar]
- 48.Mignogna C, Signorelli F, Vismara MF, et al. A reappraisal of macrophage polarization in glioblastoma: Histopathological and immunohistochemical findings and review of the literature. Pathology, research and practice. 2016;212: 491–499. [DOI] [PubMed] [Google Scholar]
- 49.Miller AD, Miller CR, Rossmeisl JH. Canine Primary Intracranial Cancer: A Clinicopathologic and Comparative Review of Glioma, Meningioma, and Choroid Plexus Tumors. Frontiers in oncology. 2019;9: 1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitchell D, Chintala S, Fetcko K, et al. Common Molecular Alterations in Canine Oligodendroglioma and Human Malignant Gliomas and Potential Novel Therapeutic Targets. Frontiers in oncology. 2019;9: 780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitsuka K, Kawataki T, Satoh E, Asahara T, Horikoshi T, Kinouchi H. Expression of indoleamine 2,3-dioxygenase and correlation with pathological malignancy in gliomas. Neurosurgery. 2013;72: 1031–1038; discussion 1038–1039. [DOI] [PubMed] [Google Scholar]
- 52.Morioka T, Baba T, Black KL, Streit WJ. Response of microglial cells to experimental rat glioma. Glia. 1992;6: 75–79. [DOI] [PubMed] [Google Scholar]
- 53.Nolte A, Junginger J, Baum B, Hewicker-Trautwein M. Heterogeneity of macrophages in canine histiocytic ulcerative colitis. Innate Immun. 2017;23: 228–239. [DOI] [PubMed] [Google Scholar]
- 54.Ooi YC, Tran P, Ung N, et al. The role of regulatory T-cells in glioma immunology. Clin Neurol Neurosurg. 2014;119: 125–132. [DOI] [PubMed] [Google Scholar]
- 55.Pi Castro D, Jose-Lopez R, Fernandez Flores F, et al. Expression of FOXP3 in Canine Gliomas: Immunohistochemical Study of Tumor-Infiltrating Regulatory Lymphocytes. J Neuropathol Exp Neurol. 2019. [DOI] [PubMed] [Google Scholar]
- 56.Robinson S, Cohen M, Prayson R, Ransohoff RM, Tabrizi N, Miller RH. Constitutive expression of growth-related oncogene and its receptor in oligodendrogliomas. Neurosurgery. 2001;48: 864–873; discussion 873–864. [DOI] [PubMed] [Google Scholar]
- 57.Roggendorf W, Strupp S, Paulus W. Distribution and characterization of microglia/macrophages in human brain tumors. Acta Neuropathol. 1996;92: 288–293. [DOI] [PubMed] [Google Scholar]
- 58.Rossi ML, Hughes JT, Esiri MM, Coakham HB, Brownell DB. Immunohistological study of mononuclear cell infiltrate in malignant gliomas. Acta Neuropathol. 1987;74: 269–277. [DOI] [PubMed] [Google Scholar]
- 59.Rossi ML, Jones NR, Candy E, et al. The mononuclear cell infiltrate compared with survival in high-grade astrocytomas. Acta Neuropathol. 1989;78: 189–193. [DOI] [PubMed] [Google Scholar]
- 60.Sakai K, Maeda S, Yamada Y, et al. Association of tumour-infiltrating regulatory T cells with adverse outcomes in dogs with malignant tumours. Vet Comp Oncol. 2018;16: 330–336. [DOI] [PubMed] [Google Scholar]
- 61.Sayour EJ, McLendon P, McLendon R, et al. Increased proportion of FoxP3+ regulatory T cells in tumor infiltrating lymphocytes is associated with tumor recurrence and reduced survival in patients with glioblastoma. Cancer immunology, immunotherapy : CII. 2015;64: 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Silveira TL, Veloso ES, Gonçalves INN, et al. Cyclooxygenase-2 expression is associated with infiltration of inflammatory cells in oral and skin canine melanomas. Vet Comp Oncol. 2020. [DOI] [PubMed] [Google Scholar]
- 63.Sloma EA, Creneti CT, Erb HN, Miller AD. Characterization of Inflammatory Changes Associated with Canine Oligodendroglioma. Journal of comparative pathology. 2015;153: 92–100. [DOI] [PubMed] [Google Scholar]
- 64.Snyder JM, Shofer FS, Van Winkle TJ, Massicotte C. Canine intracranial primary neoplasia: 173 cases (1986–2003). J Vet Intern Med. 2006;20: 669–675. [DOI] [PubMed] [Google Scholar]
- 65.Sonabend AM, Rolle CE, Lesniak MS. The role of regulatory T cells in malignant glioma. Anticancer Res. 2008;28: 1143–1150. [PubMed] [Google Scholar]
- 66.Soulas C, Conerly C, Kim WK, et al. Recently infiltrating MAC387(+) monocytes/macrophages a third macrophage population involved in SIV and HIV encephalitic lesion formation. Am J Pathol. 2011;178: 2121–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spugnini EP, Thrall DE, Price GS, Sharp NJ, Munana K, Page RL. Primary irradiation of canine intracranial masses. Vet Radiol Ultrasound. 2000;41: 377–380. [DOI] [PubMed] [Google Scholar]
- 68.Stavrou D, Anzil AP, Weidenbach W, Rodt H. Immunofluorescence study of lymphocytic infiltration in gliomas. Identification of T-lymphocytes. J Neurol Sci. 1977;33: 275–282. [DOI] [PubMed] [Google Scholar]
- 69.Stoica G, Kim HT, Hall DG, Coates JR. Morphology, immunohistochemistry, and genetic alterations in dog astrocytomas. Vet Pathol. 2004;41: 10–19. [DOI] [PubMed] [Google Scholar]
- 70.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352: 987–996. [DOI] [PubMed] [Google Scholar]
- 71.Thomas AA, Fisher JL, Rahme GJ, et al. Regulatory T cells are not a strong predictor of survival for patients with glioblastoma. Neuro-oncology. 2015;17: 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thomas R, Duke SE, Wang HJ, et al. ‘Putting our heads together’: insights into genomic conservation between human and canine intracranial tumors. Journal of neuro-oncology. 2009;94: 333–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Truve K, Dickinson P, Xiong A, et al. Utilizing the Dog Genome in the Search for Novel Candidate Genes Involved in Glioma Development-Genome Wide Association Mapping followed by Targeted Massive Parallel Sequencing Identifies a Strongly Associated Locus. PLoS Genet. 2016;12: e1006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vidyarthi A, Agnihotri T, Khan N, et al. Predominance of M2 macrophages in gliomas leads to the suppression of local and systemic immunity. Cancer Immunol Immunother. 2019;68: 1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Villiers E, Baines S, Law AM, Mallows V. Identification of acute myeloid leukemia in dogs using flow cytometry with myeloperoxidase, MAC387, and a canine neutrophil-specific antibody. Vet Clin Pathol. 2006;35: 55–71. [DOI] [PubMed] [Google Scholar]
- 76.Vrolyk V, Wobeser BK, Al-Dissi AN, Carr A, Singh B. Lung Inflammation Associated With Clinical Acute Necrotizing Pancreatitis in Dogs. Vet Pathol. 2017;54: 129–140. [DOI] [PubMed] [Google Scholar]
- 77.Wagner A, Junginger J, Lemensieck F, Hewicker-Trautwein M. Immunohistochemical characterization of gastrointestinal macrophages/phagocytes in dogs with inflammatory bowel disease (IBD) and non-IBD dogs. Vet Immunol Immunopathol. 2018;197: 49–57. [DOI] [PubMed] [Google Scholar]
- 78.Wainwright DA, Balyasnikova IV, Chang AL, et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18: 6110–6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wainwright DA, Chang AL, Dey M, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20: 5290–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wainwright DA, Lesniak MS. Menage a trois: Sustained therapeutic anti-tumor immunity requires multiple partners in malignant glioma. Oncoimmunology. 2014;3: e28927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wallner KE, Galicich JH, Krol G, Arbit E, Malkin MG. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 1989;16: 1405–1409. [DOI] [PubMed] [Google Scholar]
- 82.Wang X, Guo G, Guan H, Yu Y, Lu J, Yu J. Challenges and potential of PD-1/PD-L1 checkpoint blockade immunotherapy for glioblastoma. Journal of experimental & clinical cancer research : CR. 2019;38: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wei J, Wu A, Kong LY, et al. Hypoxia potentiates glioma-mediated immunosuppression. PloS one. 2011;6: e16195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang I, Han SJ, Sughrue ME, Tihan T, Parsa AT. Immune cell infiltrate differences in pilocytic astrocytoma and glioblastoma: evidence of distinct immunological microenvironments that reflect tumor biology. Journal of neurosurgery. 2011;115: 505–511. [DOI] [PubMed] [Google Scholar]
- 85.Yarnall BW, Chamberlain CS, Hao Z, Muir P. Proinflammatory polarization of stifle synovial macrophages in dogs with cruciate ligament rupture. Vet Surg. 2019;48: 1005–1012. [DOI] [PubMed] [Google Scholar]
- 86.Yasuda K, Alderson T, Phillips J, Sikora K. Detection of lymphocytes in malignant gliomas by monoclonal antibodies. J Neurol Neurosurg Psychiatry. 1983;46: 734–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zeiner PS, Preusse C, Golebiewska A, et al. Distribution and prognostic impact of microglia/macrophage subpopulations in gliomas. Brain pathology (Zurich, Switzerland). 2019;29: 513–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhai L, Ladomersky E, Dostal CR, et al. Non-tumor cell IDO1 predominantly contributes to enzyme activity and response to CTLA-4/PD-L1 inhibition in mouse glioblastoma. Brain, behavior, and immunity. 2017;62: 24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhai L, Ladomersky E, Lenzen A, et al. IDO1 in cancer: a Gemini of immune checkpoints. Cell Mol Immunol. 2018;15: 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang M, Ding L, Wang X, et al. Circulating CD14(+)CD163(+)CD115(+) M2 monocytes are associated with the severity of new onset severe acute pancreatitis in Chinese patients. Int Immunopharmacol. 2018;57: 181–189. [DOI] [PubMed] [Google Scholar]
- 91.Zhang ML, Jiang YF, Wang XR, et al. Different phenotypes of monocytes in patients with new-onset mild acute pancreatitis. World J Gastroenterol. 2017;23: 1477–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu C, Kros JM, van der Weiden M, Zheng P, Cheng C, Mustafa DA. Expression site of P2RY12 in residential microglial cells in astrocytomas correlates with M1 and M2 marker expression and tumor grade. Acta Neuropathol Commun. 2017;5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data analyzed in this study are not available as Supplemental Materials, though requests to the corresponding author can be made for those interested in further information.


