Abstract
The infected cell protein (ICP)0 enables gene expression and the replication of herpes simplex virus (HSV)-1 in cells infected at low multiplicities and enhances the expression of genes introduced into cells by transfection or infection. We report that a short sequence of ICP0 is similar to a sequence in the amino terminus of CoREST, a corepressor that exists in complexes with the repressor REST and histone deacetylases (HDACs) 1 or 2 to repress cellular gene expression. In wild-type-virus-infected cells, HDAC1 dissociates from the CoREST/REST complex, CoREST and HDAC1 are phosphorylated by a process mediated by viral protein kinases, and CoREST and HDAC1 are partially translocated to the cytoplasm. In cells infected with a virus mutant (ΔICP4), in which ICP0 accumulates, but post-α gene expression is blocked, HDAC1 is dissociated from the CoREST/REST complex, but translocation to the cytoplasm does not occur. After infection with a mutant virus from which ICP0 is deleted, the complex remains intact, but, under conditions of productive infection, the complex is partially translocated to the cytoplasm. These results suggest that, at low multiplicities of infection, ICP0 blocks CoREST-mediated silencing of viral genes by dissociation of HDAC1, whereas subsequent modifications and translocation of the components of the complex are the functions of other viral gene products made later in infection.
Keywords: derepression, infected cell protein 0, phosphorylation
Amajor function of infected cell protein (ICP)0 is to preclude the silencing of viral DNA. Thus, viral gene expression is significantly delayed in cells infected at low multiplicity with a viral mutant in which the wild-type α0 gene was replaced with a cDNA copy (1). In these cells, viral gene expression is accelerated by inhibitors of histone deacetylases (HDACs) such as sodium butyrate, Helminthosporium carbonum toxin, or trichostatin A (2). It was also noted that HDACs1 and 2, but not HDAC3, were posttranslationally modified between 3 and 6 h after infection by a process mediated by the viral US3 protein kinase (2). Other studies reported that ICP0 interacts physically with HDACs 4, 5, and 7 (3).
One hypothesis tested in the course of our studies is that to block silencing of the viral genome, ICP0 could have acquired a cellular sequence that mimics that of a host protein involved in gene repression. To our surprise, we detected significant similarity between the carboxyl-terminal amino acids 537–613 of ICP0 and those of amino acids 3–79 of CoREST (Fig. 1). This finding led us to investigate the behavior of CoREST and related proteins during herpes simplex virus (HSV) infection. CoREST (4) was identified initially as a corepressor for the RE1-silencing transcription factor REST (also called NRSF), a transcriptional repressor that binds, in nonneuronal cells, to a consensus sequence present in a large number of neuronal genes (5, 6). The CoREST complex contains, in addition to REST, HDACs 1 and 2 (7, 8), providing a mechanism by which CoREST can mediate silencing or repression. Even in neurons where REST is absent, CoREST is expressed to high levels and exists in complexes with HDACs 1 and 2 (9). Thus, CoREST is likely to constitute a predominant repressor mechanism in different cellular contexts. Although several functional domains in CoREST have been identified, including its repressor domains (8, 9), a role for the domain in CoREST that shares amino acid similarity to ICP0 has not yet been identified.
Fig. 1.
Alignment of amino acid sequences of ICP0 and CoREST. Identities, 23 of 77 (31%); positives, 43 of 77 (55%). Expect value = 3 × 10–6.
ICP0 is a 775-residue multifunctional α (immediate-early) protein encoded by three exons of the α0 gene. As reviewed in ref. 10, ICP0 is extensively posttranslationally modified by viral (UL13) and cellular (cdc2) protein kinases and is nucleotidylated by casein kinase II. The protein physically interacts with the translation-elongation factor 1δ, cyclin D3, the ubiquitin-conjugating enzyme UbcH3 (cdc34), the ubiquitin-specific protease 7, the transcriptional factor BML1, and a protein designated as p60. Δα0 mutants fail to replicate in experimental animal systems and either become arrested at post-α gene expression or replicate sluggishly in most cell lines exposed to low ratios of plaque-forming units (PFU) per cell. The defects in Δα0 mutants are largely overcome in cells infected at high multiplicities of infection. In transduced cells, ICP0 mediates the dispersal of nuclear structures known as ND10 and degrades PML, the protein responsible for the organization of ND10. However, HSV-1 replicates equally well in cell lines in which the ND10 structures are stabilized by transduction with promyelocytic leukemia protein or in cells in which the gene encoding this protein has been knocked out. As noted in detail in ref. 10, these studies indicate that ICP0 does not enable viral gene expression by mediating the degradation of ND10 structures. A significant clue to the role of ICP0 is the observation that it transactivates the expression of viral or cellular genes introduced by infection or transfection, even though it does not bind DNA directly and is not known to bind known transcriptional factors.
The fundamental finding reported in this study is that ICP0 mediates the dissociation of HDAC1 from the CoREST/REST complex. CoREST and HDAC1 are then phosphorylated and translocated to the cytoplasm in an HSV-1-dependent but ICP0-independent fashion.
Materials and Methods
Cells and Viruses. The origin and derivation of the wild-type HSV-1(F) strain and the recombinant mutants R7041 (ΔUS3), R7356 (ΔUL13), R7353 (ΔUS3/UL13), R7910 (Δα0), and d120 (ΔICP4) were described in refs. 11–15. The source and procedures for cultivation, maintenance, and infection of human embryonic lung (HEL) fibroblasts, HeLa, and SK-N-SH cells were described in ref. 16.
Nuclear–Cytoplasmic Fractionation. The fractionations were done by two methods. In the first, packed cells were suspended in 5 vol of buffer A (10 mM Tris, pH 8.0/1.5 mM MgCl2/10 mM KCl/0.5 mM DTT/1× proteinase-inhibitor mixture) and allowed to swell on ice for 10 min. The cells were then collected, resuspended in 2 vol of buffer A, and lysed by two strokes with a tight pestle in a Dounce homogenizer. The nuclei were rinsed in buffer A plus 0.05% Nonidet P-40, resuspended in 1 vol of buffer D (20 mM Tris, pH 8.0/420 mM NaCl/1.5 mM MgCl2/0.2 mM EDTA/0.5 mM DTT/25% glycerol/1× proteinase-inhibitor mixture), and disrupted by 10 strokes of a tight Dounce homogenizer. Both the cytoplasmic and nuclear extracts were dialyzed against immunoprecipitation (IP) buffer (20 mM Tris·HCl, pH 8.0/1 mM EDTA/0.5% Nonidet P-40/150 mM NaCl/2mMDTT/0.1 mM NaVO4/10 mM NaF/1× proteinase-inhibitor mixture) (Sigma) and cleared of insoluble proteins.
In the second procedure, mock-infected or infected HeLa cells were suspended in the IP buffer containing 0.1% Nonidet P-40. The nuclei were collected, suspended in the same buffer, and disrupted by sonication, and both the cytoplasmic and nuclear fractions were cleared of insoluble materials.
Coimmunoprecipitations. Nuclear and cytoplasmic fractions or total lysates of mock or infected cells in IP buffer were reacted with polyclonal Ab (anti-CoREST Ab supplied by G.M.) or anti-ICP0 Ab (14) overnight at 4°C. The immunoprecipitates were harvested with protein A Sepharose CL-4B (Amersham Pharmacia). The beads were rinsed three times with the IP buffer and once with IP buffer plus 0.1% SDS and then eluted with 1× SDS loading buffer.
Dephosphorylation Assay. Lysates (30 μg of protein) of mock-infected or 9-h-infected SK-N-SH cells in lysis buffer (10 mM Tris·HCl, pH 8.0/140 mM NaCl/1.5 mM MgCl2/1 mM DTT/0.5% Nonidet P-40/1× proteinase-inhibitor mixture) were reacted at 34°C for 30 min with 20 units of calf intestine alkaline phosphatase (CIAP) (Roche) in dephosphorylation buffer provided by the manufacturer. The reactions were stopped by the addition of SDS-loading buffer.
Immunoblotting. Electrophoretically separated proteins from lysates or immunoprecipitates were transferred from denaturing polyacrylamide gels to preequilibrated poly(vinylidene difluoride) membrane (Millipore) or nitrocellulose membrane (Schleicher & Schuell). The membranes were blocked in TBST (20 mM Tris, pH 7.5/150 mM NaCl/0.5% Tween 20) containing 5% nonfat dry milk and reacted at 4°C overnight with appropriate primary Ab in TBST/5% dry milk, rinsed, and reacted with secondary Ab (goat anti-rabbit (1:3,000) or goat anti-mouse peroxidase-conjugated Abs (1:1,000) from Sigma, developed with ECL Western blotting detection reagents (Amersham Pharmacia Biosciences) according to the manufacturer's instructions, scanned with Molecular Dynamics Storm 860 PhosphorImager, and quantitated with imagequant 5.0. The primary Abs were polyclonal Ab to CoREST (1:2,000) from Upstate Biotechnology (Lake Placid, NY), REST (1:500) derived by G.M., monoclonal Abs to ICP0 (H1112, 1:1,000) from the Rumbaugh– Goodwin Institute for Cancer Research (Plantation, FL), CoREST (1:250) from Transduction Laboratories (Lexington, KY), cdc2 (1:400) from Santa Cruz Biotechnology, and HDAC1 (1:1,000) from Upstate Biotechnology.
Confocal Microscopy. SK-N-SH or HEL cells grown on four-well glass slides (Cell Line Associates, Newfield, NJ) were exposed to 10 PFU of wild-type or mutant viruses per cell for 1 h. After incubation at 37°C for time intervals indicated in Results, the cells were fixed in 4% paraformaldehyde and permeabilized with 0.2% Triton X-100, blocked in PBS containing 0.1% of Tween 20 (PBST) and 5% FBS, reacted with primary Abs at 4°C overnight, rinsed with PBST buffer, and reacted with FTIC-conjugated goat anti-rabbit (Sigma) and Texas-red-conjugated goat anti-mouse (Molecular Probes) secondary Abs, as stated in Results or legends to figures, and examined with a Zeiss confocal microscope.
Results
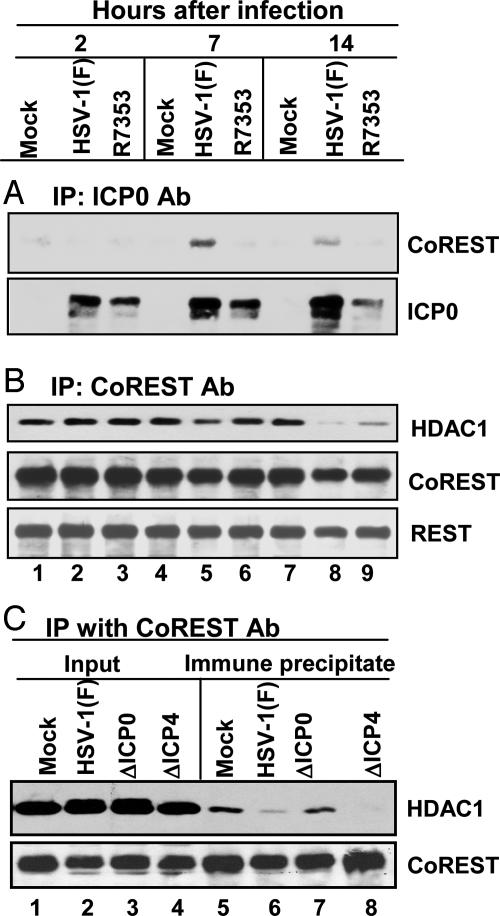
ICP0 Is in Complexes with CoREST in Wild-Type Virus-Infected Cells. Alignment of the primary amino acid sequence of ICP0 with all HDACs (1–10) and three known binding partners (RbAp48, mSin3A, and CoREST) revealed that 23 of the 77 amino acid residues of ICP0 between residues 537 and 613 were identical, and 43 of the 77 scored positive were conserved, with no gaps, from residues 3–79 of CoREST (Fig. 1). This similarity was conserved in ICP0 of HSV-2 but not in other orthologues (e.g., ICP0 of bovine herpesvirus 1). To determine whether ICP0 was in complexes with CoREST in infected cells, nuclear lysates obtained from HeLa cultures harvested 2, 7, or 14 h after infection with wild-type HSV-1(F) or the R7353 (ΔUS3/UL13) mutant were reacted with polyclonal Ab against ICP0 (Fig. 2A), CoREST (Fig. 2B), or HDAC1 (Fig. 2C). The immune precipitates were electrophoretically separated in denaturing polyacrylamide gels and reacted with Ab against CoREST, HDAC1, ICP0, or REST. The results were as follows.
Fig. 2.
Interactions between ICP0 and the components of the CoREST/REST/HDAC complex. Nuclear lysates from mock-infected, HSV-1(F)-infected, or R7353-infected HeLa cells harvested at 2, 7, or 14 h after infection were reacted with polyclonal Ab against ICP0 (A) or CoREST (B and C). The aliquots of the lysates and immunoprecipitates were electrophoretically separated in denaturing gels and reacted with anti-CoREST, anti-HDAC1, anti-REST, or anti-ICP0 Ab as shown. Input was 10% of the total protein used for immunoprecipitation.
Ab to ICP0 coprecipitated CoREST from lysates harvested 7 or 14 h after infection with wild-type but not mutant virus (Fig. 1 A). ICP0 accumulated to a large amount in SK-N-SH cells soon after infection (16). In these cells, complexes containing ICP0 and CoREST were detected in lysates harvested as early as 1 h after infection (data not shown). The failure to detect CoREST in immunocomplexes from lysates of ΔUS3/ΔUL13 mutant-virus-infected cells may reflect lower amounts of ICP0. CoREST was present, albeit in reduced amounts, in precipitates with ICP0 Ab from lysates of ΔUS3/ΔUL13 mutant-virus-infected SK-N-SH cells (data not shown). ICP0 Ab did not pull down HDAC1, and we failed to detect ICP0 in precipitates with anti-CoREST Ab (data not shown). The failure of the anti-CoREST Ab to pull down ICP0 may reflect a competition between the Ab and ICP0 for a specific site on CoREST or on a protein that links CoREST with ICP0.
As expected, both REST and CoREST were in immunocomplexes from mock-infected cells by using CoREST Ab (Fig. 2B). Complexes precipitated by CoREST Ab from lysates harvested 14 h after infection with HSV-1(F) or R7353 mutant virus contained no HDAC1 or much less HDAC1 than precipitates from mock-infected cells (Fig. 2B). The loss of HDAC1 from immunocomplexes precipitated with anti-CoREST Ab from lysates of wild-type-virus-infected cells was reproducible. Moreover, immune complex precipitated from mock-infected or infected cells by a preimmune rabbit serum did not contain the REST protein (data not shown).
The role of ICP0 in determining the presence of HDAC1 in CoREST immune precipitates is apparent from results shown in Fig. 2C. In this experiment, the CoREST immunocomplexes from HSV-1(F) or ΔICP4 mutant-virus-infected cells contained little or no HDAC1. ΔICP4 mutant-virus-infected cells express predominantly α protein and accumulate large amounts of ICP0. In contrast, the amounts of HDAC1 precipitated by CoREST Ab from lysates of ΔICP0-mutant-infected cells was similar to that present in precipitates from lysates of mock-infected cells. The results of this experiment indicate that the dissociation of HDAC1 from the CoREST/REST complex does not require, de novo, post-α gene expression but does require ICP0.
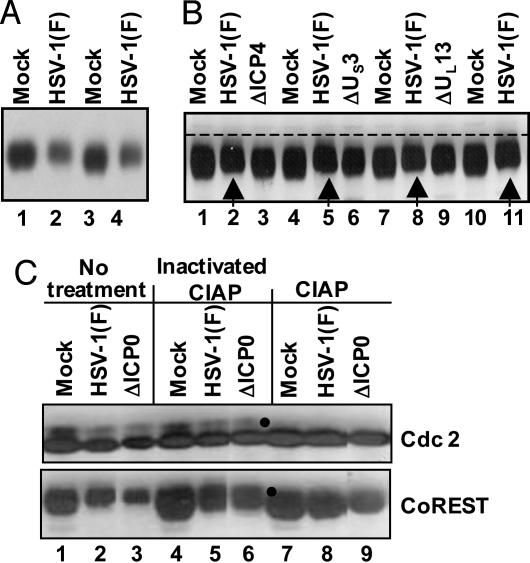
CoREST Is Posttranslationally Modified After Infection with HSV-1(F). As shown in Fig. 3A, the electrophoretic mobility of CoREST present in lysates of SK-N-SH cells harvested 24 h after infection with HSV-1 migrated slightly more slowly than in mock-infected cells. To determine whether the change in electrophoretic mobility of CoREST was mediated by ICP0 and reflected a phosphorylation of the protein, the experiment was repeated, except that aliquots of each sample either were left untreated or were treated with CIAP or with heat-inactivated CIAP before electrophoresis in denaturing gels. The electrophoretically separated proteins were reacted with Ab against cdc2 or CoREST. Cdc2 served as a positive control because earlier studies had established that this protein is phosphorylated in both mock-infected and infected cells (17). The results shown in Fig. 3C indicate that the CIAP was active because the slower-migrating form of cdc2 (marked with a filled circle) disappeared after treatment with CIAP (lanes 7–9) and that the electrophoretic mobility of CoREST present in lysates of either wild-type virus or ΔICP0 mutant-virus-infected cells could not be differentiated from that of mock-infected cells after treatment with CIAP. We conclude that CoREST is phosphorylated in infected cells independently of ICP0.
Fig. 3.
CoREST is phosphorylated in HSV-1(F)-infected cells. (A) Electrophoretically separated lysates of cells harvested 24 h after mock-infection or exposure to SK-N-SH cells were reacted with anti-CoREST Ab as described in Materials and Methods. Duplicate samples from infected cells were interspersed with samples from mock-infected cells to enable better visualization of small changes in electrophoretic mobility. (B) Electrophoretically separated lysates of mock-infected cells and cells infected with HSV-1(F), ΔICP4), ΔUS3, or ΔUL13 mutant viruses were reacted with anti-CoREST Ab. The arrows identify the bands formed by CoREST present in lysates of wild-type-virus-infected cells. (C) Lysates from mock-, HSV-1(F)-, or ΔICP0-mutant-virus-infected cells were reacted with dephosphorylation buffer alone (No treatment), heat-inactivated CIAP, or active CIAP and electrophoretically separated in denaturing gels and reacted to anti-CoREST or anti-cdc2 Ab. The filled circles indicate the disappearance of slower-mobilitybands of cdc2 and CoREST.
To test the role of viral gene products in the posttranslational modification of CoREST, the experiments were repeated with lysates of cells infected with mutant viruses lacking the viral protein kinases (ΔUS3 and ΔUL13) or the major regulatory protein ICP4 (ΔICP4). The posttranslational modification of CoREST required the expression of post-α genes and the presence of both viral protein kinases as indicated by the distinct gel mobilities compared with mock-infected cells (Fig. 3B). The results of these experiments do not resolve the question of whether both protein kinases are directly involved or the posttranslational modification of CoREST is mediated by an UL13-dependent posttranslational modification of the US3 protein kinase.
CoREST and HDAC1 Are Translocated, in Part, to the Cytoplasm in HSV-1(F)-Infected Cells. Several lines of investigation indicate that CoREST and HDAC1 are translocated, at least in part, into the cytoplasm. The experiments and the results may be summarized as follows.
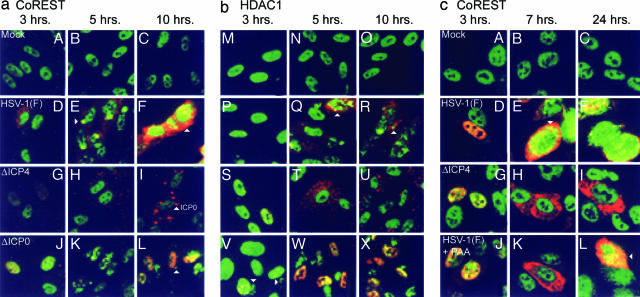
Confocal-microscopy-based studies of SK-N-SH cells or HEL fibroblasts infected with wild-type virus, fixed at different times after infection, and reacted with Abs to CoREST (FITC) and ICP0 (Texas red) indicated that, with time after infection, CoREST was, in part, translocated to the cytoplasm (Fig. 4 a D–F and c D–F). These results are in sharp contrast to the distribution of CoREST in mock-infected cells in which CoREST was predominantly found in the nuclei (Fig. 4 a A–C and c A–C).
Fig. 4.
Translocation of CoREST and HDAC1 from nuclei to cytoplasm. (a) HEL fibroblasts grown in four-well slides were fixed 3, 5, or 10 h after infection with HSV-1(F), ΔICP4, or ΔICP0 mutant viruses. The fixed slides were reacted with anti-CoREST (FITC) and anti-ICP0 (Texas red) Abs (A–I) or anti-CoREST (FITC) and anti-ICP8 (Texas red) (J–L) Abs. Images were collected in a Zeiss confocal microscope equipped with a ×63 objective. (b) Duplicate slides prepared as described for a were reacted with anti-HDAC1 in place of anti-CoREST (FITC) and either anti-ICP0 (M–U) or anti-ICP8 (V–X) (Texas red). (c) SK-N-SH cells grown on four-well slides were fixed at 3, 7, or 24 h after mock-infection or infection with HSV-1(F) or ΔICP4 mutant virus (A–I). One set of wells was infected with wild-type virus and maintained in medium containing phosphonoacetate (PAA, 300 μg/ml) (J–L). The cells were reacted with anti-CoREST Ab (FITC) and anti-ICP0 (Texas red). The arrowheads in aE, aF, and aL and in cE and cL point to cytoplasmic CoREST. The arrowheads in bQ, bR, and bV point to cytoplasmic HDAC1. The arrowhead in aI points to cytoplasmic aggregates of ICP0. Similar aggregates are present in corresponding images in b and c.
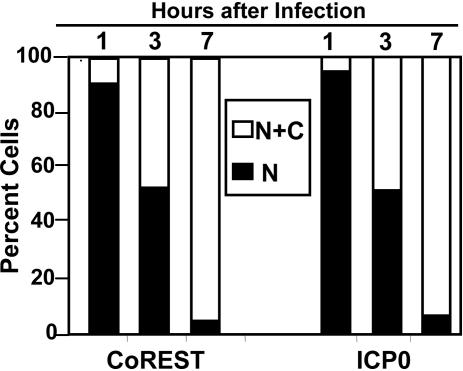
The results of a more detailed analysis are shown in Fig. 5. Earlier studies have shown that ICP0 is translocated from nucleus to cytoplasm between 5 and 9 h after infection (20). Because only a fraction of CoREST was translocated, we quantified the distributions of CoREST and ICP0 either in the nucleus only or in both nucleus and cytoplasm of cells infected with either wild-type or mutant virus. We examined ≈200 cells in contiguous fields of four-well cultures infected with each virus. As shown in Fig. 5, the distribution of cells containing CoREST in nuclei or in both nuclei and cytoplasm were virtually identical to the distribution of ICP0 in either nucleus only or both nucleus and cytoplasm. The results suggest the possibility that the molecular events that drive ICP0 from the nucleus to the cytoplasm may also affect the partial export of CoREST.
Fig. 5.
Localization of CoREST and ICP0. SK-N-SH cells grown on four-well slides were fixed at 1, 3, or 7 h after mock-infection or infection with HSV-1(F). The localization of ICP0 and CoREST from ≈200 cells for each infection was tabulated under the microscope. The numbers of cells with ICP0 or CoREST present in both nucleus and cytoplasm (N+C) or nucleus (N) are shown as the percentage of total counted cells.
Translocation of CoREST requires post-α gene expression. Cells infected with the ΔICP4 mutant make predominantly, if not exclusively, α-proteins (21). In these cells, ICP0 is translocated to the cytoplasm very early after infection and aggregates into dense bodies shown in Fig. 4 a H and I and b T and U as dense punctate structures (20). In cells infected with the ΔICP4 mutant, CoREST was localized to the nucleus, and its distribution was not different from that of mock-infected cells.
ICP0 is not required for the partial translocation of CoREST to the cytoplasm. In this series of experiments, the HEL cells were grown in four-well slides, infected with the ΔICP0 mutant, fixed at times shown, and reacted with Ab to CoREST conjugated with FITC and Ab to ICP8 conjugated with Texas red. Unlike ICP0, ICP8 is a viral DNA-binding nuclear protein, and its synthesis signifies that the multiplicity of infection was sufficient to enable productive infection. In these cells, CoREST was still translocated to the cytoplasm (Fig. 4 a K and L).
CoREST translocation is delayed in cells exposed to inhibitors of viral DNA synthesis. Unlike the case for ICP0, inhibition of viral DNA synthesis with phosphonoacetate did not inhibit the translocation of CoREST (Fig. 4cL). However, CoREST was predominantly nuclear at 7 h after infection in the drug-treated cells (Fig. 4cK) at the time when CoREST was already present in the cytoplasm of most control infected cells (Figs. 4cE and 5).
Last, parallel studies on replicate slide cultures illustrated in Fig. 4b indicated that HDAC1 visualized by FITC is partially translocated to the cytoplasm. The requirements for translocation of HDAC1 were similar to those for CoREST (compare Fig. 4b with 4a).
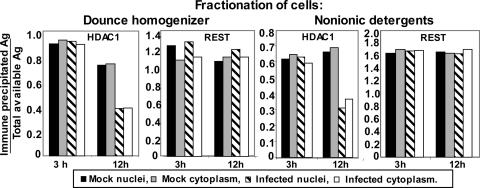
The Interaction of CoREST and REST Remains Stable Throughout Infection, Whereas the Interaction of HDAC1 with the CoREST/REST Complex Is Disrupted in both Nuclei and Cytoplasm. The experiments described below had two objectives. The first was to determine whether CoREST and REST remain stably associated during the first 12 h after infection. The second was to verify that HDAC1 is, at least in part, dissociated from the CoREST/REST complex. As illustrated in Fig. 2, HDAC1 was absent or diminished in amount in complexes precipitated by anti-CoREST Ab late in infection. To reduce the experimental error resulting from nuclear leakage from nuclei disrupted at late times of infection, experiments were performed in HeLa cells by using two different protocols for fractionation of the cells. From past experience, cell disruption by two strokes of a Dounce homogenizer results in a nuclear fraction contaminated by cytoplasmic proteins deriving from a small number of intact cells. Nonionic detergents may produce a more enriched nuclear preparation but at a cost of a loss of nuclear materials through disrupted nuclear pores or as a consequence of disruption of nuclear membranes. Both procedures were applied in this study, as described in Materials and Methods. The fractionated samples were reacted with anti-CoREST Ab, and samples of input as well as the precipitates were electrophoretically separated in denaturing gels and reacted with Ab against REST or HDAC1. The ratios of precipitated to total available HDAC1 or REST antigen at 3 and 12 h after infection with HSV-1(F) are shown in Fig. 6. The key findings are that the two fractionation methods yielded identical results. The amount of REST in immune precipitates did not change between early (3 h) and late (12 h) times after infection. In contrast, complexes precipitated late in infection from either nucleus or cytoplasm contained half the amount of HDAC1 present at early times. These results suggest that the dissociation of HDAC1 from the CoREST/REST complex takes place before the translocation of HDAC1 or CoREST to the cytoplasm.
Fig. 6.
Dissociation of HDAC1 from the CoREST/REST complex after HSV-1 infection. Replicated cultures of HeLa cells were mock- or HSV-1(F)-infected for 3 or 12 h and fractionated with either a Dounce homogenizer or nonionic detergents as described in Materials and Methods. The precipitates obtained with the anti-CoREST Ab from the nuclear and cytoplasmic fractions and samples of the lysates were individually electrophoretically separated in denaturing gels and reacted with Ab to HDAC1, REST, or CoREST. The amounts of CoREST, REST, or HDAC1 antigen in the nuclear or cytoplasmic fractions and in the precipitates were quantified with the Molecular Dynamics PhosphorImager Storm 860 fluorescence imager and imagequest 5 software. The ratios of precipitated REST or HDAC1 to total antigen (Ag) available for precipitation were plotted with excel software (Microsoft). Input was 10% of the lysate used for immunoprecipitation.
Discussion
In nonneuronal cells, CoREST forms a complex with REST and HDACs 1 and 2 (7, 8). On the basis of a short sequence conserved in both ICP0 and CoREST, we examined the interaction of these proteins in the context of infected cells. The results presented in this report show several sequential or parallel interactions of viral products with the CoREST/REST/HDAC complex. Specifically, (i) in infected cells, ICP0 is present in a complex with CoREST. We have not established whether the presence of ICP0 in the complex is related to the shared sequence. (ii) Earlier studies have shown that HDAC1 and HDAC2 are posttranslationally modified by a process mediated by US3 protein kinase (2). Here, we report that CoREST was phosphorylated by a process requiring both US3 and UL13 protein kinases and that this modification did not take place in the absence of post-α gene expression. (iii) HDAC1 and CoREST have been shown to be translocated, at least in part, to the cytoplasm. Because the ratio of CoREST and REST in immune complexes precipitated by anti-CoREST Ab from both nuclei and cytoplasm remained constant throughout infection, we may infer that REST is also translocated to the cytoplasm. The translocation did not take place in cells infected with the ΔICP4 mutant but occurred under all conditions tested in which post-α genes were expressed. (iv) HDAC1 was dissociated from the CoREST/REST complex in wild-type-virus-infected cells and in cells infected with the ΔICP4 mutant. Cells infected with this mutant accumulate large amounts of α proteins, including ICP0 (21). Although only the results of studies on HDAC1 are shown here, other experiments showed that HDAC2 was also dissociated and translocated to the cytoplasm in a similar fashion (data not shown).
We conclude from these observations that dissociation of HDAC1 from the CoREST/REST complex takes place early in the absence of β (early) or γ (late) proteins. At later times, viral protein kinases mediate posttranslational modification of CoREST and HDAC1. Finally, molecular events still later in infection mediated by viral gene products whose synthesis was partially dependent on ongoing viral DNA synthesis mediated the translocation of a portion of CoREST/REST and HDAC1 to the cytoplasm.
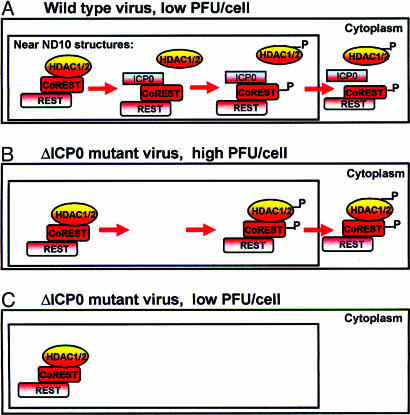
In cells infected with one or more PFU of wild-type virus per cell, viral gene expression is sequentially ordered from α to β to γ (21). In the absence of ICP0, at low multiplicities of infection, α genes are transactivated by the viral transactivator VP16 (α-TIF) brought into the cell by the infecting virus (21) but post-α genes are silenced. A clue to the role of ICP0 in blocking the silencing of post-α genes may be deduced from the results presented in this report and from the observation that, early in infection, both viral DNA and the newly made ICP0 colocalize with the ND10 nuclear structures (10, 18, 19, 22). The model, illustrated in part in Fig. 7 and that we propose, is that colocalization of wild-type virus genome and the newly made ICP0 at the ND10 structures serves two objectives. The first, illustrated in Fig. 7A is to dissociate the HDAC1/2 from the CoREST/ REST complex at that site and block the silencing of post-α gene expression. The second objective, reviewed in detail in ref. 10 and most likely performed sequentially, is to disperse ND10 components and degrade the protein PML, thereby blocking the IFN-mediated host response to infection. The viral protein kinases, but particularly US3, mediate the posttranslational modification of HDAC1/2 and CoREST, and products made still later in infection mediate the translocation of these proteins to the cytoplasm. Exposure of cells to high amounts of ΔICP0 mutant virus per cell results in productive infection. In these cells, the likelihood of post-α gene expression is enhanced because of large numbers of viral genomes per cell. In these cells HDAC1/2 remains complexed with CoREST, and REST and the entire repressor complex is translocated, in part, to the cytoplasm (Fig. 7B). The maintenance of the CoREST/REST/HDAC1 repressor complex in cells infected with the ΔICP0 mutant is consistent with a role for ICP0 in the dissociation of the complex. Last, the model predicts that, in cells exposed to low particle ratios of the ΔICP0 mutant virus particles per cell, the absence of post-α gene expression precludes the posttranslational modification and export of the CoREST/REST/HDAC1/2 repressor complex from the nucleus.
Fig. 7.
A model of the role of ICP0 in the initiation of viral post-α gene expression. (A) Newly made ICP0 and entering HSV DNA colocalize in the vicinity of ND10 nuclear structures. ICP0 mediates the displacement of HDAC1 from the CoREST/REST complex, enabling post-α gene expression. Post-α gene products mediate the posttranslational modification of HDAC1/2 and CoREST, which are then exported to the cytoplasm. The representation of the HDAC/CoREST/REST repressor complex follows the model proposed by Ballas et al. (9). (B) In cells infected at high ratios of ΔICP0 virions per cell, HDAC/CoREST/REST complex remains intact, but the complex is exported to the cytoplasm. (C) In the absence of post-α gene expression, this process does not occur in cells infected at low ΔICP0 particle-to-cell ratios.
This model, based on the studies reported here, may be the underpinning of the role of ICP0 as a promiscuous transactivator. It is conceivable, for example, that ICP0 colocalizes with DNA introduced into cells by infection or transfection and blocks its silencing by the CoREST/REST/HDAC1 repressor complex.
Acknowledgments
This work was supported, in part, by National Institutes of Health Grant NS22518 (to G.M.) and National Cancer Institute Grants CA87661, CA83939, CA71933, CA78766, and CA88860 (to B.R.). G.M. is an Investigator of the Howard Hughes Medical Institute.
Author contributions: B.R. designed research; H.G. and Y.L. performed research; G.M. contributed new reagents/analytic tools; G.M. analyzed data; and B.R. wrote the paper.
Abbreviations: CIAP, calf intestine alkaline phosphatase; HDAC, histone deacetylase; HEL, human embryonic lung; HSV, herpes simplex virus; ICP, infected cell protein; IP, immunoprecipitation; PFU, plaque-forming units.
References
- 1.Poon, A. P., Silverstein, S. J. & Roizman, B. (2002) J. Virol. 76, 9744–9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poon, A. P., Liang, Y. & Roizman, B. (2003) J. Virol. 77, 12671–12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lomonte, P., Thomas, J., Texier, P., Caron, C., Khochbin, S. & Epstein, A. L. (2004) J. Virol. 78, 6744–6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andres, M. E., Burger, C., Peral-Rubio, M. J., Battaglioli, E., Anderson, M. E., Grimes, J., Dallman, J., Ballas, N. & Mandel, G. (1999) Proc. Natl. Acad. Sci. USA 96, 9873–9878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chong, J. A., Tapia-Ramirez, J., Kim, S., Toledo-Aral, J. J., Zheng, Y., Boutros, M. C., Altshuller, Y. M., Frohman, M. A., Kraner, S. D. & Mandel, G. (1995) Cell 80, 949–957. [DOI] [PubMed] [Google Scholar]
- 6.Schoenherr, C. J. & Anderson, D. J. (1995) Science 267, 1360–1363. [DOI] [PubMed] [Google Scholar]
- 7.Humphrey, G. W., Wang, Y., Russanova, V. R., Hirai, T., Qin, J., Nakatani, Y. & Howard, B. H. (2001) J. Biol. Chem. 276, 6817–6824. [DOI] [PubMed] [Google Scholar]
- 8.You, A., Tong, J. K., Grozinger, C. M. & Schreiber, S. L. (2001) Proc. Natl. Acad. Sci. USA 98, 1454–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballas, N., Battaglioli, E., Atouf, F., Andres, M. E., Chenoweth, J., Anderson, M. E., Burger, C., Moniwa, M., Davie, J. R., Bowers, W. J., et al. (2001) Neuron 31, 353–365. [DOI] [PubMed] [Google Scholar]
- 10.Hagglund, R. & Roizman, B. (2004) J. Virol. 78, 2169–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ejercito, P. M., Kieff, E. D. & Roizman, B. (1968) J. Gen. Virol. 2, 357–364. [DOI] [PubMed] [Google Scholar]
- 12.Post, L. E., Mackem, S. & Roizman, B. (1981) Cell 24, 555–565. [DOI] [PubMed] [Google Scholar]
- 13.Purves, F. C., Ogle, W. O. & Roizman, B. (1993) Proc. Natl. Acad. Sci. USA 90, 6701–6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawaguchi, Y., Van Sant, C. & Roizman, B. (1997) J. Virol. 71, 7328–7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeLuca, N. A., McCarthy, A. M. & Schaffer, P. A. (1985) J. Virol. 56, 558–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu, H. & Roizman, B. (2003) Proc. Natl. Acad. Sci. USA 100, 8963–8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Advani, S. J., Brandimarti, R., Weichselbaum, R. R. & Roizman, B. (2000) J. Virol. 74, 8–15. [PMC free article] [PubMed] [Google Scholar]
- 18.Maul, G. G., Ishov, A. M. & Everett, R. D. (1996) Virology. 217, 67–75. [DOI] [PubMed] [Google Scholar]
- 19.Mullen, M. A., Gerstberger, S., Ciufo, D. M., Mosca, J. D. & Hayward, G. S. (1995) J. Virol. 69, 476–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez, P., Van Sant, C. & Roizman, B. (2001) J. Virol. 75, 3832–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roizman, B. and Knipe, D. M. (2001) in Fields Virology, eds. Knipe, D. M., Howley, P., Griffin, D. E., Lamb, R. A., Martin, M. A., Roizman, B. & Straus, S. E. (Lippincott Williams & Wilkins, New York), 4th Ed., pp. 2399–2459.
- 22.Lopez, P., Jacob, R. J. & Roizman, B. (2002) J. Virol. 76, 9355–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]