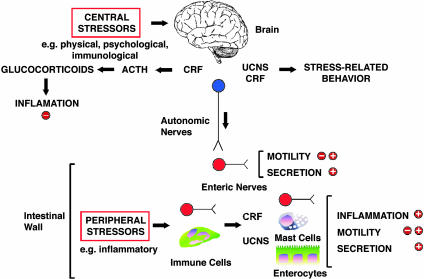
Most of us have experienced at first hand the effects of stress on our digestive systems. As early as 1833, Beaumont (1) described that fear and anger influenced acid secretion from the stomach of his patient Alexis St. Martin, a Canadian trapper with a permanent gastric fistula caused by a gunshot wound. The impact of psychological, physical, and immunological stressors on gastrointestinal secretion, motility, epithelial permeability, and inflammation is now thoroughly documented, and stress has a major influence on digestive diseases (refs. 2–4 and references therein). Most studies concern central mechanisms whereby a stressful event perceived by the brain triggers neuronal and hormonal reflexes that influence the gut. However, a report by la Fleur et al. in this issue of PNAS (5) makes the exciting observation that the intestine produces the same stress peptides that are present in the central nervous system. A local stressor, in this case a bacterial toxin that is the principal cause of antibiotic-induced colitis and diarrhea, results in the local generation and action of stress peptides that mediate inflammation without involving the central nervous system (Fig. 1). These peptides also regulate the transit of digested material through the intestine under normal conditions. This intrinsic stress response mechanism may mediate altered digestive processes that accompany physical and chemical insults to the intestine and could contribute to poorly understood disorders, such as inflammatory bowel disease and irritable bowel syndrome, for which stress exacerbates the symptoms.
Fig. 1.
Stress-induced alterations in gastrointestinal functions. Central stressors induce release of CRF, ACTH, and glucocorticoids, which have antiinflammatory actions. Centrally released CRF and Ucns activate parasympathetic nerves, which in turn innervates enteric neurons within the gut wall to stimulate or inhibit motility (depending on the region) and stimulate secretion. Peripheral stressors induce local release of CRF and Ucns, possibly from enteric neurons and immune cells. Peripherally derived CRF may act on enteric nerves, mast cells, and enterocytes to induce inflammation and control motility and secretion.
The study focuses on the corticotropin-releasing factor (CRF) and urocortin (Ucn) family of neuropeptides (reviewed in refs. 3, 4, and 6). CRF and UcnI, -II, and -III are structurally related peptides that interact with two CRF receptors (CRF-Rs). CRF preferentially interacts with CRF-R1 over CRF-R2, UcnII interacts similarly with both receptors, and UcnII and UcnIII interact only with CRF-R2. These peptides and their receptors are expressed in distinct regions of the brain, where they mediate the effects of psychological, physical, and immunological stressors on hormonal responses, anxiety, mood, feeding behavior, and gastrointestinal functions. During stress, CRF from the hypothalamus stimulates secretion of adrenocorti-cotrophic hormone (ACTH) from the pituitary, which in turn releases glucocorticoids from the adrenal gland. CRF is thus a crucial link in the hypothalamic–pituitary–adrenal axis that mediates neuroendocrine stress responses (Fig. 1). Central CRF and Ucns also control activity of autonomic nerves and thereby mediate the effects of central stressors on gastrointestinal motility and secretion (Fig. 1). However, the gut has its own nervous system, the enteric nervous system or “little brain,” which can regulate gastrointestinal functions independently of central control. Enteric nerves and other cells in the gut express CRF, Ucns, and their receptors, although in some cases the precise sites remain to be determined. Systemically administered CRF and Ucns also affect gastrointestinal motility and mucosal functions presumably by activating receptors in the gut wall. Because the intestine expresses stress peptides and receptors, could an intrinsic stress response system mediate the effects of local stressors on the digestive tract?
One of the obstacles to determining the specific role of gut-derived CRF and Ucns is the difficulty of selectively antagonizing these peptides within the intestinal wall. When injected centrally or systemically, agonists or antagonists could affect many cell types expressing CRF-Rs, and the generation of mice that lack CRF, Ucns, or their receptors in particular populations of intestinal cells is a daunting task. To circumvent these difficulties, la Fleur et al. (5) used RNA interference (RNAi) to silence the expression of CRF and UcnII. RNAi depends on the cellular uptake and processing of double-stranded RNA (dsRNA) into small interfering RNAs (siRNAs) of 21–23 nt by the RNase III enzyme Dicer. siRNAs pair with cognate mRNA, resulting in degradation and gene silencing (7). Injection of long sequences of CRF or UcnII dsRNA into the wall of the rat ileum led to a prominent down-regulation of peptide and mRNA within 4–6 days. The down-regulation was tissue-specific, being confined to the ileum, and circulating levels of corticosterone were unchanged, confirming a lack of effect on central CRF. A potential drawback is that long dsRNA can induce an IFN response and exert nonspecific effects. However, CRF and UcnII dsRNA were specific for the selected peptide without affecting expression of unrelated genes, and β-globin or GFP dsRNA, used as controls, had no effect (5). Cytokines, including IFN, were at similar levels in peripheral blood in animals receiving CRF, UcnII, or GFP dsRNA or in animals treated with a mixture of CRF siRNAs, arguing against a systemic inflammatory response. This powerful approach could be used for the tissue-specific silencing of other gut mediators, although it will be important to assess tissue cytokine levels to exclude local inflammation.
The effects of CRF and UcnII silencing on intestinal inflammation and motility were investigated (5). To induce a local inflammatory stress, toxin A from the bacterium Clostridium difficile, the cause of diarrhea and colitis after antibiotic therapy, was injected into the ileal lumen. In animals treated with control dsRNA, toxin A caused up-regulation of CRF in intestinal nerves, shedding of enterocytes, massive neutrophil recruitment, and extensive fluid secretion into the lumen. Remarkably, CRF dsRNA, which inhibited up-regulation of CRF, prevented epithelial damage and inflammation and suppressed fluid secretion. UcnII dsRNA had no effect. Thus, a local stressor, toxin A, induces the synthesis and release of CRF by enteric neurons, which acts within the gut itself to cause inflammation. The results are in line with reports that CRF antagonism and deletion prevent toxin A-induced ileitis (8, 9). However, in those studies it was not possible to determine whether CRF from the central or enteric sources was involved. What is clear is that, whereas central CRF is antiinflammatory because of the release of glucocorticoids, enteric CRF is proinflammatory but by unknown mechanisms. To study motility, the investigators monitored the excretion of fecal pellets and measured the transit of a dye through the uninflamed intestine (5). Silencing of ileal CRF increased the frequency of defecation, and CRF and UcnII dsRNA accelerated intestinal transit. Thus, under normal circumstances, enteric CRF and UcnII dampen intestinal motility, perhaps by modulating the peristaltic reflex. Indeed, peripheral CRF delays gastric emptying and small intestinal transit and accelerates colonic transit, possibly by acting on enteric nerves (3, 4).
Corticotropin-releasing factor antagonists may be useful antiinflammatory drugs.
The discovery of a local stress response system in the gut not only adds to our understanding of similar systems in other tissues, such as the skin (10), but also raises intriguing questions. Does the system mediate intestinal responses to other local stressors? What is the source of intestinal CRF and what controls its release? Although CRF is found in enteric nerves, it is also produced by immune cells, which could be an important source in inflamed tissues (9). How does CRF exert its proinflammatory and antipropulsive effects? CRF-Rs are expressed by enteric neurons and epithelial cells and in the lamina propria (11), and the levels are up-regulated in the inflamed intestine (8). Additional studies are required to define the precise sites of receptor expression in normal and diseased states, and tissue-specific silencing of CRF-R1 and CRF-R2 by RNAi could identify the functionally important receptors. One CRF target could be enteric nerves containing the neuropeptide substance P, an essential mediator of toxin A ileitis (12). CRF and substance P colocalize in ileal nerves, and CRF deletion prevents toxin A-induced up-regulation of substance P (9). Thus, neuronal CRF secreted in response to toxin A could stimulate substance P release to induce inflammation. CRF and UcnII may control release of other enteric neurotransmitters to control peristalsis. CRF could also induce inflammation through effects on mast cells, because mast cells mediate the effects of stress and CRF on intestinal motility, secretion, and epithelial permeability (13, 14). Perhaps the most intriguing question is whether these mechanisms contribute to gastrointestinal disorders. The study by La Fleur et al. (5) adds to a body of evidence that peripheral CRF is proinflammatory and CRF antagonists may be useful antiinflammatory drugs (8, 9). Stress peptides may also contribute to irritable bowel syndrome, a common disorder characterized by altered bowel habits and abdominal pain, which can be worsened by stress. Central and systemic CRF and Ucns induce the symptoms of this syndrome, and antagonists blunt the effects of stress on the gut (3, 4). A CRF-R1 antagonist also suppresses the colonic motility and pain responses to colonic distension and stimulation of the rectal mucosa in patients with irritable bowel syndrome (15). Additional mechanistic studies, such as that of la Fleur et al., are clearly warranted to further our understanding of the intestinal stress response system in diseases and the physiological regulation of digestion.
Acknowledgments
I thank M. Dallman (University of California, San Francisco), Y. Tache (University of California, Los Angeles), and C. Pothoulakis (Harvard University, Cambridge, MA) for helpful discussions.
See companion article on page 7647.
References
- 1.Beaumont, W. (1959) Experiments and Observations on the Gastric Juice and the Physiology of Digestion (Dover, New York). [DOI] [PubMed]
- 2.Tache, Y., Martinez, V., Million, M. & Wang, L. (2001) Am. J. Physiol. 280, G173–G177. [DOI] [PubMed] [Google Scholar]
- 3.Martinez, V., Wang, L., Million, M., Rivier, J. & Tache, Y. (2004) Peptides 25, 1733–1744. [DOI] [PubMed] [Google Scholar]
- 4.Tache, Y., Martinez, V., Wang, L. & Million, M. (2004) Br. J. Pharmacol. 141, 1321–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.la Fleur, S. E., Wick, E. C., Idumalla, P. S., Grady, E. F. & Bhargava, A. (2005) Proc. Natl. Acad. Sci. USA 102, 7647–7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bale, T. L. & Vale, W. W. (2004) Annu. Rev. Pharmacol. Toxicol. 44, 525–557. [DOI] [PubMed] [Google Scholar]
- 7.Wang, Q. & Carmichael, G. G. (2004) Microbiol. Mol. Biol. Rev. 68, 432–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wlk, M., Wang, C. C., Venihaki, M., Liu, J., Zhao, D., Anton, P. M., Mykoniatis, A., Pan, A., Zacks, J., Karalis, K. & Pothoulakis, C. (2002) Gastroenterology 123, 505–515. [DOI] [PubMed] [Google Scholar]
- 9.Anton, P. M., Gay, J., Mykoniatis, A., Pan, A., O'Brien, M., Brown, D., Karalis, K. & Pothoulakis, C. (2004) Proc. Natl. Acad. Sci. USA 101, 8503–8508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slominski, A., Wortsman, J., Pisarchik, A., Zbytek, B., Linton, E. A., Mazurkiewicz, J. E. & Wei, E. T. (2001) FASEB J. 15, 1678–1693. [DOI] [PubMed] [Google Scholar]
- 11.Chatzaki, E., Crowe, P. D., Wang, L., Million, M., Tache, Y. & Grigoriadis, D. E. (2004) J. Neurochem. 90, 309–316. [DOI] [PubMed] [Google Scholar]
- 12.Castagliuolo, I., Riegler, M., Pasha, A., Nikulasson, S., Lu, B., Gerard, C., Gerard, N. P. & Pothoulakis, C. (1998) J. Clin. Invest. 101, 1547–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castagliuolo, I., Lamont, J. T., Qiu, B., Fleming, S. M., Bhaskar, K. R., Nikulasson, S. T., Kornetsky, C. & Pothoulakis, C. (1996) Am. J. Physiol. 271, G884–G892. [DOI] [PubMed] [Google Scholar]
- 14.Soderholm, J. D., Yang, P. C., Ceponis, P., Vohra, A., Riddell, R., Sherman, P. M. & Perdue, M. H. (2002) Gastroenterology 123, 1099–1108. [DOI] [PubMed] [Google Scholar]
- 15.Sagami, Y., Shimada, Y., Tayama, J., Nomura, T., Satake, M., Endo, Y., Shoji, T., Karahashi, K., Hongo, M. & Fukudo, S. (2004) Gut 53, 958–964. [DOI] [PMC free article] [PubMed] [Google Scholar]