Abstract
Increasing public interest has resulted in the widespread use of non-pharmaceutical cannabidiol (CBD) products. The sales of CBD products continue to rise, accompanied by concerns regarding unsubstantiated benefits, lack of product quality control, and potential health risks. Both animal and human studies have revealed a spectrum of toxicological effects linked to the use of CBD, including changes in organ weight, reproduction, liver function, blood pressure, and the immune system, as well as gastrointestinal discomfort, fatigue, and changes in appetite. This review centers on human-derived data, including clinical studies and in vitro investigations. The objective is to offer an overview of CBD-related hepatotoxicity, metabolism, and potential CBD-drug interactions, thereby providing insights into the current understanding of CBD’s impact on human health. It’s important to note that this review does not serve as a risk assessment but seeks to summarize available information to contribute to the broader understanding of potential toxicological effects of CBD on the liver.
Keywords: cannabidiol, CBD, toxicity, liver toxicity, metabolism
1. Introduction
Cannabidiol (CBD, 2-[(6R)-6-isopropenyl-3-methyl-2-cyclohexen-1-yl]-5-pentyl-1,3-benzene-diol) was first isolated from the plant Cannabis sativa in 1940. Its chemical structure was determined in 1963.1,2 The most abundant chemical component found in Cannabis sativa, responsible for its intoxicating effects, is delta-9-tetrahydrocannabinol (THC). Despite CBD and THC having similar chemical structures, CBD lacks intoxicating effects. The therapeutic potential of CBD has been the subject of intense research interest, resulting in a growing body of literature that suggests CBD may be a promising therapeutic agent for a wide range of conditions, including epilepsy, anxiety, depression, insomnia, chronic pain, cancer, and neurodegenerative disorders, such as Parkinson’s and Alzheimer’s disease.3–5
To date, two CBD-based drugs, under the tradenames Sativex and Epidiolex, have been approved by various regulatory authorities for specific, narrow conditions of use. Sativex (Nabiximols) is an oral spray consisting of a 1:1 ratio of THC and CBD, and has been approved since 2005 in Canada, the United Kingdom, and several other European countries as an adjunctive treatment for spasticity in people with multiple sclerosis.6,7 However, it is not currently approved for use in the United States. In 2018, the U.S. Food and Drug Administration (FDA) approved Epidiolex, a CBD-based oral solution for the treatment of seizures associated with two rare and severe forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome, in patients two years of age and older.8 Subsequently, in 2020, FDA approved the same Epidiolex oral solution for the treatment of seizures associated with tuberous sclerosis complex in patients one year of age and older.9 Epidiolex, branded under the alternative tradename “Epidyolex”, was approved by the European Medicines Agency (EMA) in 2019 for the same medical conditions.10
The increasing public interest in CBD’s potential utility has promoted the sales of non-pharmaceutical CBD products. The sales of CBD continue to increase annually in the United States and other countries.11 The 2019 International Cannabis Policy Study online survey reported that more than half of the participants (aged 16–65 years) in the United States (n = 30,288) and Canada (n = 15,042) claimed that they have used CBD products. Approximately 26% of respondents in the United States and 16% in Canada used CBD products in the past 12 months.12 However, issues, such as unproven beneficial effects and the lack of testing and product quality control guidelines, raise particular concern about consumer use of non-pharmaceutical CBD.13 Research in animals and in humans has shown that CBD may contribute to myriad toxicological effects, including changes in organ weight, fetal development, reproduction, liver function, blood pressure, immune system function, and genotoxicity.1,14,15 In humans, the most reported side effects from CBD include diarrhea, nausea, headache, fatigue, and changes of appetite and weight.16–20 Hepatic abnormalities have been documented in human studies, and CBD-associated liver injury is one of the FDA’s concerns regarding CBD.13
While multiple review articles on CBD’s toxicity potential have been published, and should be read to gain a more comprehensive insight into the topic,1,14,15,21 this review focuses mainly on the available human data, which includes clinical studies and in vitro studies using human-derived cellular systems. This literature review does not serve as a risk assessment; we only aimed to summarize the available data and information on hepatotoxicity, metabolism, and the potential for drug-drug interactions associated with the use of CBD.
2. Absorption, distribution, metabolism, and excretion (ADME)
The ADME profile of CBD varies considerably among species. This review summarizes the ADME profile for CBD mainly based on available human data; interspecies differences are discussed when considered relevant.
2.1. Absorption
CBD is a lipophilic compound that undergoes first-pass metabolism when ingested orally. A clinical trial conducted in the Netherlands on healthy adults showed that after a single oral dose of 1,500 mg, CBD is rapidly and extensively metabolized to 7-hydroxy-CBD (7-OH-CBD) and 7-carboxy-CBD (7-COOH-CBD) (Figure 1). A maximum CBD plasma concentration (Cmax) of 292.4 ng/mL was reached 4–5 hours after a single oral dose and the Cmax reached 1,385 ng/mL after multiple oral doses. The absolute bioavailability of CBD was low due to rapid and extensive first pass metabolism.22 A different study in humans similarly reported that, in healthy adults, plasma concentration peaked between 2–4 hours after ingestion of a single oral dose of 5–20 mg/kg CBD, declining to the lowest plasma levels 8 days after exposure. The authors also reported that plasma Cmax increased with increasing dose.23
Figure 1.
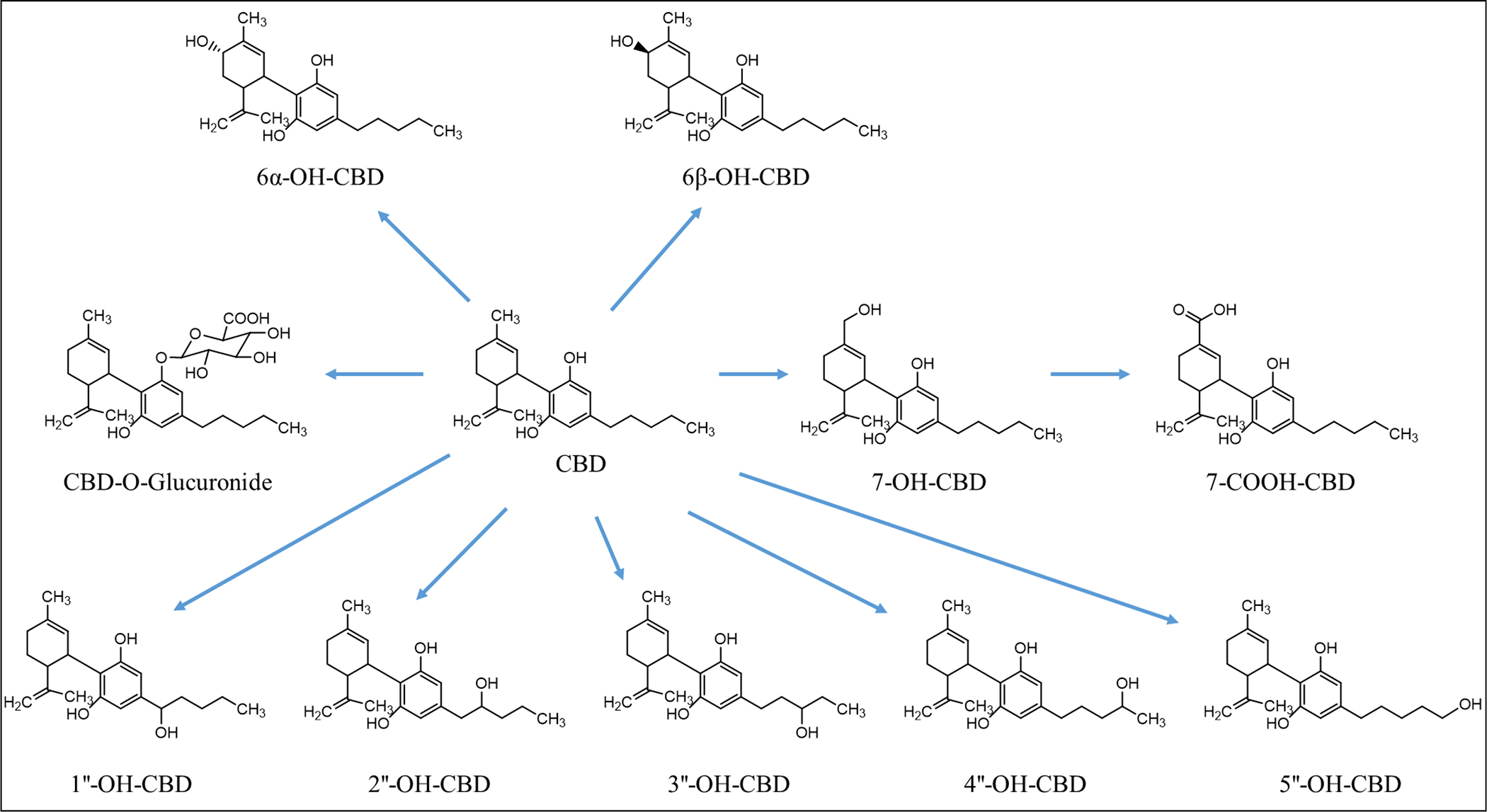
Structures of CBD and its metabolites
Some factors known to influence CBD absorption are food consumption and disease status. For example, high-fat meals increase the oral bioavailability of CBD, in turn increasing CBD plasma Cmax by ~5-fold in healthy adults.22 and by 14-fold in adult patients with refractory epilepsy.24 This increase in oral bioavailability could be linked to the lipophilic nature of CBD. Additionally, a clinical trial conducted in subjects with impaired liver functions suggested that liver status had little impact on CBD absorption, because CBD was absorbed rapidly in all groups regardless of the severity of the hepatic impairment. The time to maximum plasma concentration (Tmax) across all groups was 2–2.8 hours after a single oral dose of 200 mg CBD, which is similar to what has been discussed previously. However, the Cmax was 2-fold higher in individuals with severe hepatic impairment when compared to the healthy control group, indicating the importance of liver status.25
2.2. Distribution
No data have been reported for the tissue distribution of CBD in humans, and the data reported in animal studies are limited and equivocal. CBD and its metabolites demonstrate high affinity for plasma proteins. In human, up to 92% of CBD binds to plasma protein,26–28 and 10% is bound to red blood cells.3 In an animal study focusing on CBD detection in brain tissue, unchanged CBD and its metabolites were detected in the blood and the brain of rodents following oral, intraperitoneal, or intravenous administration of CBD.2 Intragastric administration of CBD to rats results in higher hepatic CBD concentrations, which were 30- to 50-fold higher than that what was detected in the brain.29 In contrast, a recent study did not detect CBD in the brain or liver of rats when an oral dose of 0.66 mg/kg CBD was administered for 90 days. CBD was, instead, detected in kidney and heart, and at higher levels in skin and muscle tissues.30
2.3. Metabolism
The metabolism of CBD and the production of its metabolites differ between humans and other simple monogastric species, such as mice, rats, dogs, and non-human primates.31,32 In female mice dosed orally with CBD, the circulating levels of 7-OH-CBD and 7-COOH-CBD were similar, while the circulating level of CBD was about 1.2-fold higher than the two metabolites, with the ratio varying by dose and sex.32 In female rats, the level of CBD was about 10-fold higher than 7-OH-CBD and was about 1.7-fold higher than 7-COOH-CBD, whereas in dogs, the levels of CBD are about 20-fold greater than 7-OH-CBD and 7-COOH-CBD.32 Interestingly, a drug misuse study involving rhesus monkeys and rats found that the metabolic profile of CBD was similar between the two species following intravenous administration.31 Based on the information outlined in the Epidiolex review package, 7-COOH-CBD occurs to a much greater extent (30-fold higher) than either unconjugated CBD or 7-OH-CBD in pregnant New Zealand White rabbits.32 Similar to rabbits, 7-COOH-CBD is the most abundant circulating CBD metabolite in human plasma, followed by CBD and 7-OH-CBD.22,32 Importantly, 7-COOH-CBD circulates at levels 50-fold greater than CBD in humans and represents at least 90% of the parent drug and metabolites measured in plasma, and 7-OH-CBD circulates at levels of approximately 50% of CBD in human plasma.32
Clinical trials in healthy volunteers and in children with epilepsy indicate that CBD is metabolized into several hydroxylated and carboxylated metabolites after oral administration. Consistently, 7-COOH-CBD and 7-OH-CBD are the two major metabolites that were detected in the plasma of clinical trial participants.22,33 In animal models of epilepsy, CBD and 7-OH-CBD demonstrated anticonvulsant properties, while the most abundant metabolite, 7-COOH-CBD was inactive for this endpoint.22,32 Glucuronidated CBD was detected in plasm of children with epilepsy.33
In vitro studies suggest that CBD undergoes cytochrome P450 (CYP)-mediate phase I oxidation followed by glucuronidation via UDP-glucuronosyltransferase (UGT). A study using human liver microsomes demonstrated that CBD was metabolized to eight hydroxylated metabolites (6α-OH-, 6β-OH-,7-OH-, 1″-OH-, 2″-OH-, 3″-OH-, 4″-OH-, and 5″-OH-CBD) (Figure 1). Among them, 6α-OH-, 6β-OH-, 7-OH-, and 4″-OH-CBDs displayed relatively high abundance. Several CYPs, including CYP1A1, 2C19, 2D6, 3A4, and 3A5 are involved in the biotransformation of CBD; of which, CYP3A4 and CYP2C19 are considered the principal phase I enzymes based on correlation analyses and inhibition studies. CYP3A4 is involved in the formation of 6α-OH-CBD, 6β-OH-CBD, and 4-OH-CBD; and CYP2C19 is involved in the formation of 7-OH-CBD.34 Beers et al. studied CBD metabolism using human liver microsomes pooled from multiple donors, recombinant CYPs, and CYP-selective inhibitors, and found that in addition to CYP2C19, CYP2C9 also played a significant role in the formation of 7-OH-CBD.35 Using bacterial membranes (bactosomes) containing recombinant human CYPs or UGTs, a recent study by Havlasek et al. found that CBD was hydroxylated by several CYPs, of which CYP2C8, 2C19, and 2D6 played the major roles, while CYP3A4 played a minor role.36 Moreover, a recent study demonstrated that NAD+- and NADPH-dependent microsomal enzymes contribute to the formation of 7-COOH-CBD, with minor contributions by CYPs.37
In a study using human liver microsomes and 12 recombinant UGTs, CBD was found to undergo limited glucuronidation. It was concluded that UGT enzymes such as UGT1A9, 2B7, and 2B17 may be involved in the glucuronidation of CBD, but all formed a minimal amount of the glucuronidated CBD product.38 In the study by Havlasek et al., CBD was found to be glucuronidated mainly by UGT1A3, 1A7, 1A8, 1A9, and 2B7.36 Glucuronidation depends on upstream processing by enzymes such as CYP2C9 and CYP3A4, or hydroxylated metabolites of CBD.39
2.4. Excretion
Studies in animals and in humans have reported that a large portion of the administered CBD dose is excreted intact, and to a less extent, in the form of glucuronide conjugated. In an early clinical study in healthy volunteers, 16% and 33% of a single intravenous dose of 20 mg [3H] CBD was excreted unchanged within 72 hours in the urine and feces, respectively. The significant portion of unchanged CBD excreted in the feces (33%), suggesting that some of the CBD dose is not absorbed into the bloodstream and is instead eliminated from the body through the digestive system.2 In healthy adults, the plasma half-life (T1/2) is about 70 h after ingestion of a single oral dose of 5–20 mg/kg CBD, suggesting that 2–3 weeks are likely required to fully eliminate CBD.23
3. Toxicity
Common adverse effects include diarrhea, nausea, headache, fatigue, and changes in appetite and weight.16–20,40,41 A 4-week clinical trial was conducted in the Netherlands by Taylor et al.18 where volunteers were administered orally a 750 mg pharmaceutical formulation of highly purified CBD, twice daily; 97% of the study participants reported some adverse effects. The most common adverse events reported were diarrhea (63%), headache (50%), abdominal pain (47%), nausea (43%), and fatigue (33%). Other adverse events (>10%) included dizziness, somnolence, skin rash, myalgia, and eosinophilia. All reported adverse events were noted by the authors as either mild or moderate.18
Preclinical repeated-dosing oral toxicology studies in mice, rats, and dogs have reported elevated levels of liver enzymes, such as alanine aminotransferase (ALT) and alkaline phosphatase (ALP), and hepatocellular hypertrophy following oral doses of CBD.32
3.1. Liver toxicity in patients with epilepsy
In a multiple center clinical trial for evaluating long‐term safety and treatment effects of CBD in children and adult patients with treatment‐resistant epilepsies, hepatotoxicity was observed. Of 607 patients with a treatment duration of 48 weeks, 61 (10%) patients had ALT or aspartate aminotransferase (AST) greater than 3 times the upper limit of normal (ULN). Of these, 46 (75%) patients were concomitantly taking valproate,17 a known hepatotoxicant.42 Devinsky et al. also reported that elevated aminotransferase levels were observed in patients taking CBD oral solution at a dose of 20 mg per kg of body weight per day or placebo, in addition to standard antiepileptic treatment.43 Elevated aminotransferase levels (ALT or AST level >3 times ULN) led to the withdrawal of 3 patients in the treatment group and one patient in the placebo group; all of whom were taking sodium valproate. In the 9 patients who had elevated ALT or AST levels and continued in the trial, the levels returned to normal while they continued to receive CBD and sodium valproate. None of the observed elevations in liver enzymes met the criteria for drug induced liver injury (DILI) which include either: ALT ≥5 times ULN; ALP ≥2 times ULN; or ALT ≥3 times ULN and total bilirubin ≥2 times ULN (i.e., Hy’s law) and all patients were reported to recover.
Lo et al. conducted a systematic review and meta-analysis of clinical trial data published before February 2022, to investigate the possibility of an association between CBD use and DILI. DILI was defined using the criteria of American Association for the Study of Liver Disease and the American College of Gastroenterology’s guidelines. The results indicate that CBD is associated with liver enzyme elevation (odds ratio of 5.85) and DILI (odds ratio of 4.82), compared to the placebo. Based on the analysis, the authors noted that the risk of DILI was likely low at CBD doses of <300 mg/day; elevated liver enzymes and DILI were common adverse drug reactions associated with moderate-to-high doses of CBD. DILI induced by CBD was strongly associated with high-dose CBD (≥1000 mg/day) and the concomitant use of antiepileptic drugs, particularly sodium valproate. The pattern of liver injury was identified as hepatocellular in most cases, with some cases classified as a mixed type of hepatocellular and cholestatic.44
3.2. Liver toxicity in healthy adult volunteers
CBD-induced liver toxicity has also been observed in healthy adults. In an open-label phase I clinical trial, 16 healthy adult volunteers were given a therapeutic dose of CBD (1,500 mg/day, about 20 mg/kg bw/day in a 75 kg person) for approximately 3.5 weeks. The notable biochemical abnormalities were an increase in serum ALT, AST, and GGT (gamma-glutamyl transpeptidase). Out of 16 participants, 7 (44%) had peak serum ALT values greater than the ULN, and 5 (31%) met the international consensus criteria for DILI, with peak serum ALT values 5-times greater than the ULN. In this study, there was no clear correlation between CBD plasma concentrations and peak ALT elevations, but the authors surmised that interparticipant genetic variability likely contributed to differences in transaminase elevations. Patient risk factors for ALT elevations, such as CYP2C19 metabolizer status, were not identified in this study.45 In fact, to date, there have been no reports available regarding the effects of interindividual differences (i.e., genetic polymorphism) in CYP on CBD metabolism and how they impact the susceptibility to liver toxicity from CBD. Elevations in transaminases were also reported in a different clinical trial in healthy volunteers, which examined the withdrawal effects from CBD (1,500 mg/day) administration for 4 weeks. Of the 30 enrolled volunteers, two individuals (6.7%) experienced moderate DILI with increased transaminases and associated DILI symptoms, such as eosinophilia, chest pain, epigastric discomfort, regurgitation, and esophageal discomfort. In addition, increased transaminase levels were observed in 12 volunteers (40%), although the increased levels were less than 3-times greater than the ULN.18 Another open-label phase I clinical trial study investigating CBD-drug interactions also reported elevations of transaminases in participants. In this study, 16 healthy volunteers were given a single dose of caffeine (200 mg) on day 1 and then titrated CBD from 250 mg/day to 1,500 mg/day from days 3 – 11, and subsequently 1,500 mg/day from days 12 – 27. Out of the total volunteers, 6 (37.5%) discontinued treatment before the end of the trial due to elevated ALT and AST. One subject experienced a severe adverse event with ALT level greater than 8-times the ULN.19 Together, these data suggest that CBD can produce liver injury, in the absence of concomitant exposure to anti-epileptic medications like sodium valproate.
3.3. Liver toxicity in animals
Animal studies on CBD’s hepatotoxicity potential are mostly limited to CBD-containing cannabis extracts, which likely carry different pharmacological and toxicological properties than CBD itself. Ewing et al. investigated the hepatotoxicity of orally administered CBD-rich cannabis plant extract (CRCE) for different exposure durations in male B6C3F1 mice.46 The authors noted that dose of 246 mg/kg extract was equivalent to 20 mg/kg CBD per day, the maximum recommended human maintenance dose of CBD in Epidiolex. In the acute toxicity study, mice were dosed with a single oral dose of either 0, 246, 738, or 2,460 mg/kg of CRCE (equivalent to 0, 20, 60, and 200 mg CBD/kg/day) and observed for 24 h. Clinical biochemistry analyses revealed moderate, but statistically significant (p < 0.01–0.001), dose-dependent increases in both AST and ALT serum levels for the treatments of 738 mg/kg and 2,460 mg/kg. Administration of 2,460 mg/kg CBD led to marked elevations of total bilirubin (>20-fold, p < 0.001). CBD also caused a dose-dependent induction of several CYPs and UDP-glucuronosyltransferases (UGT), including Cyp1a1, 1a2, 2b10, 2e1, 3a4, and 3a11 and Ugt1a1 and 2a3. In a sub-acute toxicity study, animals were dosed with 61.5, 184.5, or 615 mg/kg (equivalent to 5, 15, and 500 mg CBD/kg/day) CRCE daily for 10 days. Histopathological evaluation identified hepatic cytoplasmic swelling in mice dosed with 184.5 and 615 mg/kg CBD. Clinical biochemistry analyses indicated that mice receiving 615 mg/kg CRCE (had significantly elevated total bilirubin, moderately high levels of ALT and AST, but no significant increased ALP or GGT. In contrast, no significant changes in these parameters were observed at lower CRCE doses. Gene expression analyses suggested that similar changes in CYP and UGT occurred in the sub-acute study as were observed in the acute study.
A recent study evaluated the toxicity of a Hemp-derived CBD isolate (purity = 99.08–101.46%) administered for either 14 or 90 days in male and female Sprague Dawley rats. Test animals were orally dosed with 0, 30, 70, or 150 mg/kg CBD for 14 days, or 0, 50, 80, 120, or 140 mg/kg CBD for 90 days. No treatment-related clinical chemistry changes were noted in either study, including changes in AST, ALT, ALP, and total bilirubin.47 However, liver histopathological examination showed a dose-dependent increase in hepatocellular hypertrophy in the 14-day study, which was consistent with an increase in liver weights. Similarly, hepatocellular hypertrophy was also noted in the 90-day study; this effect resolved after a 28-day recovery period where CBD administration was discontinued.
4. CBD-Drug Interactions
Drug-drug interactions (DDIs) occur when two or more medications are co-administered. The interactions between the drugs can alter their efficacy, pharmacokinetics, and adverse effects. For example, one drug may alter the absorption, distribution, and metabolism of another drug, leading to changes in its concentration and potentially its efficacy or safety profiles. CBD has been reported to be a substrate, an inhibitor, or an inducer of certain CYP enzymes,41,48 and thus it can impact the activity of enzymes that play a crucial role in the biotransformation of prescription drugs and xenobiotics, resulting in altered blood levels of those chemicals. This can potentially lead to a higher risk of adverse reactions or toxicity associated with the drugs or chemicals that have been absorbed. Regarding CBD-drug interactions, it is important to go beyond the common side effects reported, like dizziness and somnolence caused by co-administration of CBD and other sedating medications and consider the potential for DDIs that can affect metabolism or potentiate toxicity outcomes. To date, the available research has primarily focused on the evaluation of the effects of CBD on concomitantly administered drugs. However, it is important to note that other drugs may also affect the metabolism and toxicity of CBD. A number of review articles have summarized the interactions of CBD and antiepileptic drugs,49–51 anticoagulant and antiplatelet drugs,52 and anticancer drugs.53–55 A few noted cases are described below in detail.
Thai et al. investigated the drug interaction of CBD and caffeine in 16 healthy volunteers aged 18–60 years.19 Following coadministration of 200 mg caffeine and 750 mg CBD, there was a 15% increase in Cmax and 88% increase in AUC0‐t of caffeine, indicating an increase in caffeine bioavailability. Therefore, there is an enhanced potential of experiencing clinically significant adverse effects, such as insomnia, nervousness, restlessness, nausea, increased heart rate, headache, anxiety, and chest paint, for individuals who consume high doses of caffeine over a short period in conjunction with CBD.
Several studies have identified a pharmacokinetic interaction with clobazam, an anticonvulsant and anti-anxiety drug, and CBD. In a study of 13 children taking clobazam with purified CBD, the mean plasma level of N-desmethylclobazam was significantly increased after administration of CBD compared to the pre-CBD baseline.56 These increased blood levels ultimately led to a reduction of clobazam dose due to the sedative effects of high blood levels. This interaction might have been caused by CBD’s potent inhibition of CYP2C19, the enzyme primarily responsible for the metabolism of N-desmethylclobazam. CBD’s inhibitory effect on CYP2C19 was confirmed in an in vitro study using recombinant CYP2C19.57 More recently, the inhibitory effect of CBD on CYPs was studied in 18 healthy adults who were administered cannabinoids and an oral CYP probe drug cocktail. This study showed that oral CRCE inhibited several CYPs, with the inhibition potency order being CYP2C19 > CYP2C9 > CYP3A > CYP1A2 (Figure 2). By contrast, CYP2D6 appeared to be unaffected.58 Unfortunately, the use of CRCE in this study does not allow the separation between the effects of CBD alone and those of the other cannabinoids found in CRCE.
Figure 2.
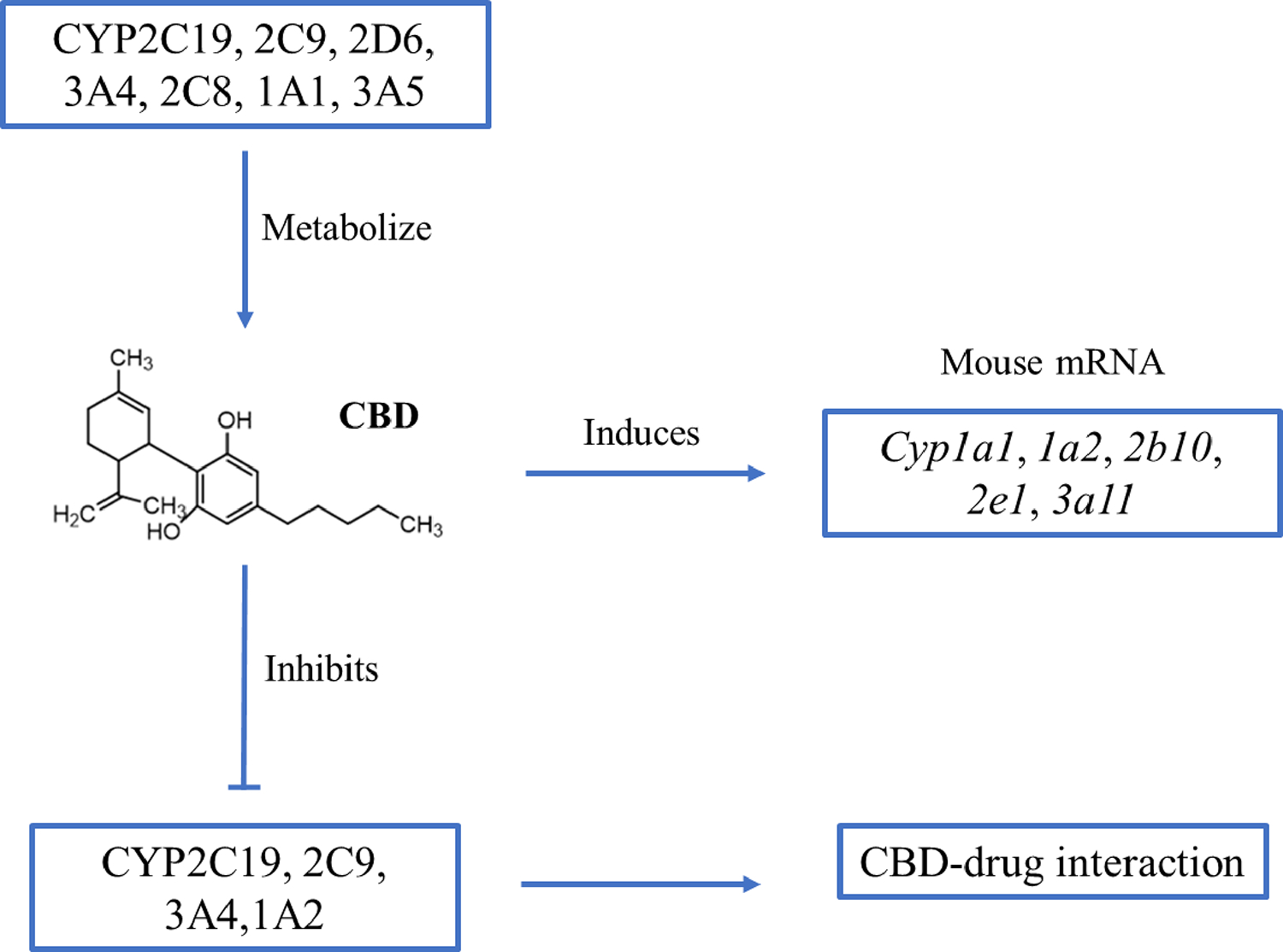
Interactions between CBD and cytochrome P450 enzymes
Gaston et al. studied the interactions between CBD and commonly used antiepileptic drugs in 39 adults and 42 pediatric patients by measuring blood levels of antiepileptic drugs.59 With increasing oral CBD dose, significant increases in serum levels of N-desmethylclobazam, topiramate, eslicarbazepine, zonisamide, and rufinamide were observed. The authors suspected the observed interaction could be due, in part, to CBD’s inhibitory action on CYP enzymes involved in the metabolism of these drugs. Additionally, as noted above, patients taking valproate and CBD concomitantly showed increased ALT and AST levels compared to patients not taking valproate, and no significant change in serum levels of valproate was observed with increasing CBD dose.
One case report describes a clinically significant interaction between CBD, specifically the Epidiolex formulation, and warfarin, the most used oral anticoagulant.60 In their study, a 44-year-old Caucasian male with Marfan Syndrome enrolled in an open-label program for compassionate use of CBD for the management of treatment-resistant epilepsy. At the time of enrollment, the patient was taking warfarin (7.5 mg daily with a goal International Normalized Ratio (INR) of 2–3). Due to its narrow therapeutic index, monitoring INR is required to measure the effectiveness of warfarin. In this individual, a non-linear increase in the INR was noted with increasing dose of CBD (5–35 mg/kg/day), and warfarin dosage adjustments were made to maintain an acceptable INR level. This observation likely occurred due to competitive phase I metabolism, where CYP2C9 and 3A4 are the dominant enzymes for warfarin hydroxylation; CBD is a substrate for both of there CYPs. Thus, CBD use may increase warfarin plasma concentrations and INR, potentially increasing the risk of bleeding complications.
5. Summary
While CBD shows promise as a therapeutic agent, especially for certain medical conditions like treatment-resistant epilepsy, it is important to consider the potential adverse effects and the need for further research to ensure its safe and effective use. With regard to the potential liver toxicity of CBD, animal studies have shown that CBD can induce liver injury, hepatocellular hypertrophy, and changes in liver enzymes. Similar observations have been reported in some clinical studies involving patients with treatment-resistant epilepsies, both in children and adults. Elevated levels of liver enzymes, such as ALT, were observed in some patients, particularly when CBD was co-administered with other drugs, such as sodium valproate. However, hepatotoxicity was also observed in healthy volunteers without concomitant medications, suggesting that CBD’s effects on the liver may be the result of direct drug-induced damage to the hepatocytes.
Despite the breadth of data discussed in this review, there are knowledge gaps that need to be addressed. As reported, most data are from clinical studies, so further research, especially mechanistic studies using in vitro and in vivo models are needed to understand fully the metabolism, liver toxicity, and potential drug-drug interactions of CBD. General toxicity testing in cells, particularly human-relevant cell models,61,62 is of importance for evaluating CBD’s toxicity. Molecular and biochemical approaches to elucidate the in-depth mechanisms of CBD’s cytotoxicity should also be implemented. To date, there are a limited number of studies that provide mechanistic insight into CBD toxicities, such as CBD-inhibited mitochondrial function in cultured cells.63 Recently, we found that cell cycle disturbances, apoptosis, and ER stress are associated with CBD-induced hepatic cytotoxicity (unpublished data). Identification of variations in CYP enzymes that are responsible for CBD’s metabolism could help indicate potential for CBD-drug interactions, and in turn, help assess CBD risks at individual levels.
Acknowledgments
The authors express their gratitude to Dr. Laura Ewing for conducting the literature search and to Drs. Luisa Camacho and Xiaoqing Guo for their valuable critical review.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Disclaimer: This article reflects the views of the authors and does not necessarily reflect those of the U.S. Food and Drug Administration. Any mention of commercial products is for clarification only and is not intended as approval, endorsement, or recommendation.
References
- [1].Huestis MA, Solimini R, Pichini S, et al. Cannabidiol Adverse Effects and Toxicity. Curr Neuropharmacol. 2019;17:974–989. doi: 10.2174/1570159X17666190603171901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ujvary I, Hanus L. Human Metabolites of Cannabidiol: A Review on Their Formation, Biological Activity, and Relevance in Therapy. Cannabis Cannabinoid Res. 2016;1:90–101. doi: 10.1089/can.2015.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Devinsky O, Cilio MR, Cross H, et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55:791–802. doi: 10.1111/epi.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hill AJ, Williams CM, Whalley BJ, Stephens GJ. Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacol Ther. 2012;133:79–97. doi: 10.1016/j.pharmthera.2011.09.002. [DOI] [PubMed] [Google Scholar]
- [5].The National Academies of Sciences E, Medicine. . The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press, 2017. https://nap.nationalacademies.org/catalog/24625/the-health-effects-of-cannabis-and-cannabinoids-the-current-state. 2017. doi: 10.3390/nu14102152. [DOI] [PubMed] [Google Scholar]
- [6].EMA . Sativex: Available at: https://www.ema.europa.eu/en/documents/pip-decision/p/0316/2016-ema-decision-2-december-2016-acceptance-modification-agreed-paediatric-investigation-plan/delta-9-tetrahydrocannabinol-sativex-emea-000181-pip01-08-m03_en.pdf. 2016. doi.
- [7].EMC. SSativex Oromucosal Spray. SmPC www.medicines.org.uk/emc/product/602/smpc 2020. doi.
- [8].FDA. FDA Approves First Drug Comprised of an Active Ingredient Derived from Marijuana to Treat Rare, Severe Forms of Epilepsy. In. https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-comprised-active-ingredient-derived-marijuana-treat-rare-severe-forms Safety of CBD in Humans – A Literature Review. https://www.fda.gov/media/152317/download. 2018. doi.
- [9].FDA. FDA Approves New Indication for Drug Containing an Active Ingredient Derived from Cannabis to Treat Seizures in Rare Genetic Disease | FDA.https://www.fda.gov/news-events/press-announcements/fda-approves-new-indication-drug-containing-active-ingredient-derived-cannabis-treat-seizures-rare. 2020. doi. [Google Scholar]
- [10].EMA. Epidyolex: https://www.ema.europa.eu/en/medicines/human/EPAR/epidyolex. 2019. doi.
- [11].Smith T, Majid F, Eckl V, Reynolds CM. Herbal supplement sales in US increase by record-breaking 17.3% in 2020. HerbalGram. 2021;131:52–65. doi. [Google Scholar]
- [12].Goodman S, Wadsworth E, Schauer G, Hammond D. Use and Perceptions of Cannabidiol Products in Canada and in the United States. Cannabis Cannabinoid Res. 2022;7:355–364. doi: 10.1089/can.2020.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].FDA. What You Need to Know (And What We’re Working to Find Out) About Products Containing Cannabis or Cannabis-derived Compounds, Including CBD. https://www.fda.gov/consumers/consumer-updates/what-you-need-know-and-what-were-working-find-out-about-products-containing-cannabis-or-cannabis. 2020. doi.
- [14].Gingrich J, Choudhuri S, Cournoyer P, Downey J, Muldoon Jacobs K. Review of the oral toxicity of cannabidiol (CBD). Food Chem Toxicol. 2023;176:113799. doi: 10.1016/j.fct.2023.113799. [DOI] [PubMed] [Google Scholar]
- [15].Madeo G, Kapoor A, Giorgetti R, Busardo FP, Carlier J. Update on Cannabidiol Clinical Toxicity and Adverse Effects: a Systematic Review. Curr Neuropharmacol. 2023. doi: 10.2174/1570159X21666230322143401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Iffland K, Grotenhermen F. An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res. 2017;2:139–154. doi: 10.1089/can.2016.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Szaflarski JP, Bebin EM, Comi AM, et al. Long-term safety and treatment effects of cannabidiol in children and adults with treatment-resistant epilepsies: Expanded access program results. Epilepsia. 2018;59:1540–1548. doi: 10.1111/epi.14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Taylor L, Crockett J, Tayo B, Checketts D, Sommerville K. Abrupt withdrawal of cannabidiol (CBD): A randomized trial. Epilepsy Behav. 2020;104:106938. doi: 10.1016/j.yebeh.2020.106938. [DOI] [PubMed] [Google Scholar]
- [19].Thai C, Tayo B, Critchley D. A Phase 1 Open-Label, Fixed-Sequence Pharmacokinetic Drug Interaction Trial to Investigate the Effect of Cannabidiol on the CYP1A2 Probe Caffeine in Healthy Subjects. Clin Pharmacol Drug Dev. 2021;10:1279–1289. doi: 10.1002/cpdd.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].FDA. Safety of CBD in Humans – A Literature Review. https://www.fda.gov/media/152317/download. 2019. doi.
- [21].Scopetti M, Morena D, Manetti F, et al. Cannabinoids and Brain Damage: A Systematic Review on a Frequently Overlooked Issue. Curr Pharm Biotechnol. 2023;24:741–757. doi: 10.2174/1389201023666220614145535. [DOI] [PubMed] [Google Scholar]
- [22].Taylor L, Gidal B, Blakey G, Tayo B, Morrison G. A Phase I, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose, Multiple Dose, and Food Effect Trial of the Safety, Tolerability and Pharmacokinetics of Highly Purified Cannabidiol in Healthy Subjects. CNS Drugs. 2018;32:1053–1067. doi: 10.1007/s40263-018-0578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Perkins D, Butler J, Ong K, et al. A Phase 1, Randomised, Placebo-Controlled, Dose Escalation Study to Investigate the Safety, Tolerability and Pharmacokinetics of Cannabidiol in Fed Healthy Volunteers. Eur J Drug Metab Pharmacokinet. 2020;45:575–586. doi: 10.1007/s13318-020-00624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Birnbaum AK, Karanam A, Marino SE, et al. Food effect on pharmacokinetics of cannabidiol oral capsules in adult patients with refractory epilepsy. Epilepsia. 2019;60:1586–1592. doi: 10.1111/epi.16093. [DOI] [PubMed] [Google Scholar]
- [25].Taylor L, Crockett J, Tayo B, Morrison G. A Phase 1, Open-Label, Parallel-Group, Single-Dose Trial of the Pharmacokinetics and Safety of Cannabidiol (CBD) in Subjects With Mild to Severe Hepatic Impairment. J Clin Pharmacol. 2019;59:1110–1119. doi: 10.1002/jcph.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Elmes MW, Kaczocha M, Berger WT, et al. Fatty acid-binding proteins (FABPs) are intracellular carriers for Delta9-tetrahydrocannabinol (THC) and cannabidiol (CBD). J Biol Chem. 2015;290:8711–21. doi: 10.1074/jbc.M114.618447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li Y, Wu Q, Li X, et al. In vitro effects of cannabidiol and its main metabolites in mouse and human Sertoli cells. Food Chem Toxicol. 2022;159:112722. doi: 10.1016/j.fct.2021.112722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tayo B, Taylor L, Sahebkar F, Morrison G. A Phase I, Open-Label, Parallel-Group, Single-Dose Trial of the Pharmacokinetics, Safety, and Tolerability of Cannabidiol in Subjects with Mild to Severe Renal Impairment. Clin Pharmacokinet. 2020;59:747–755. doi: 10.1007/s40262-019-00841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Siemens AJ, Walczak D, Buckley FE. Characterization of blood disappearance and tissue distribution of [3H]cannabidiol. Biochem Pharmacol. 1980;29:462–4. doi: 10.1016/0006-2952(80)90532-8. [DOI] [PubMed] [Google Scholar]
- [30].Polanska HH, Petrlakova K, Papouskova B, et al. Safety assessment and redox status in rats after chronic exposure to cannabidiol and cannabigerol. Toxicology. 2023;488:153460. doi: 10.1016/j.tox.2023.153460. [DOI] [PubMed] [Google Scholar]
- [31].Gray RA, Heal DJ, Maguire DR, et al. Preclinical Assessment of the Abuse Potential of Purified Botanical Cannabidiol: Self-Administration, Drug Discrimination, and Physical Dependence. J. Pharmacol. Exp. Ther. 2022;382:54. doi: 10.1124/jpet.121.000988. [DOI] [PubMed] [Google Scholar]
- [32].FDA. Non-Clinical Review: Application number 210365Orig1s000. Non-Clinical Review: Application number 210365Orig1s000. 2017. doi. [Google Scholar]
- [33].Sempio C, Almaraz-Quinones N, Jackson M, et al. Simultaneous Quantification of 17 Cannabinoids by LC-MS-MS in Human Plasma. J Anal Toxicol. 2022;46:383–392. doi: 10.1093/jat/bkab030. [DOI] [PubMed] [Google Scholar]
- [34].Jiang R, Yamaori S, Takeda S, Yamamoto I, Watanabe K. Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes. Life Sci. 2011;89:165–70. doi: 10.1016/j.lfs.2011.05.018. [DOI] [PubMed] [Google Scholar]
- [35].Beers JL, Fu D, Jackson KD. Cytochrome P450-Catalyzed Metabolism of Cannabidiol to the Active Metabolite 7-Hydroxy-Cannabidiol. Drug Metab Dispos. 2021;49:882–891. doi: 10.1124/dmd.120.000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Havlasek J, Vrba J, Zatloukalova M, et al. Hepatic biotransformation of non-psychotropic phytocannabinoids and activity screening on cytochromes P450 and UDP-glucuronosyltransferases. Toxicol Appl Pharmacol. 2023:116654. doi: 10.1016/j.taap.2023.116654. [DOI] [PubMed] [Google Scholar]
- [37].Beers JL, Authement AK, Isoherranen N, Jackson KD. Cytosolic Enzymes Generate Cannabinoid Metabolites 7-Carboxycannabidiol and 11-Nor-9-carboxytetrahydrocannabinol. ACS Med Chem Lett. 2023;14:614–620. doi: 10.1021/acsmedchemlett.3c00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mazur A, Lichti CF, Prather PL, et al. Characterization of human hepatic and extrahepatic UDP-glucuronosyltransferase enzymes involved in the metabolism of classic cannabinoids. Drug Metab Dispos. 2009;37:1496–504. doi: 10.1124/dmd.109.026898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Harvey DJ, Mechoulam R. Metabolites of cannabidiol identified in human urine. Xenobiotica. 1990;20:303–20. doi: 10.3109/00498259009046849. [DOI] [PubMed] [Google Scholar]
- [40].Brown JD, Winterstein AG. Potential Adverse Drug Events and Drug-Drug Interactions with Medical and Consumer Cannabidiol (CBD) Use. J Clin Med. 2019;8. doi: 10.3390/jcm8070989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].EPIDIOLEX®, EPIDIOLEX® drug label information. 2023. [Google Scholar]
- [42].Ezhilarasan D, Mani U. Valproic acid induced liver injury: An insight into molecular toxicological mechanism. Environ Toxicol Pharmacol. 2022;95:103967. doi: 10.1016/j.etap.2022.103967. [DOI] [PubMed] [Google Scholar]
- [43].Devinsky O, Cross JH, Laux L, et al. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N Engl J Med. 2017;376:2011–2020. doi: 10.1056/NEJMoa1611618. [DOI] [PubMed] [Google Scholar]
- [44].Lo LA, Christiansen A, Eadie L, et al. Cannabidiol-associated hepatotoxicity: A systematic review and meta-analysis. J Intern Med. 2023. doi: 10.1111/joim.13627. [DOI] [PubMed] [Google Scholar]
- [45].Watkins PB, Church RJ, Li J, Knappertz V. Cannabidiol and Abnormal Liver Chemistries in Healthy Adults: Results of a Phase I Clinical Trial. Clin Pharmacol Ther. 2021;109:1224–1231. doi: 10.1002/cpt.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ewing LE, Skinner CM, Quick CM, et al. Hepatotoxicity of a Cannabidiol-Rich Cannabis Extract in the Mouse Model. Molecules. 2019;24:1694. doi: 10.3390/molecules24091694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Henderson RG, Lefever TW, Heintz MM, et al. Oral toxicity evaluation of cannabidiol. Food Chem Toxicol. 2023;176:113778. doi: 10.1016/j.fct.2023.113778. [DOI] [PubMed] [Google Scholar]
- [48].Zendulka O, Dovrtelova G, Noskova K, et al. Cannabinoids and Cytochrome P450 Interactions. Curr Drug Metab. 2016;17:206–26. doi: 10.2174/1389200217666151210142051. [DOI] [PubMed] [Google Scholar]
- [49].MacDonald E, Adams A. In The Use of Medical Cannabis with Other Medications: A Review of Safety and Guidelines - An Update; Ottawa (ON); 2019. [PubMed] [Google Scholar]
- [50].Miziak B, Walczak A, Szponar J, Pluta R, Czuczwar SJ. Drug-drug interactions between antiepileptics and cannabinoids. Expert Opin Drug Metab Toxicol. 2019;15:407–415. doi: 10.1080/17425255.2019.1605355. [DOI] [PubMed] [Google Scholar]
- [51].Rong C, Carmona NE, Lee YL, et al. Drug-drug interactions as a result of co-administering Delta(9)-THC and CBD with other psychotropic agents. Expert Opin Drug Saf. 2018;17:51–54. doi: 10.1080/14740338.2017.1397128. [DOI] [PubMed] [Google Scholar]
- [52].Greger J, Bates V, Mechtler L, Gengo F. A Review of Cannabis and Interactions With Anticoagulant and Antiplatelet Agents. J Clin Pharmacol. 2020;60:432–438. doi: 10.1002/jcph.1557. [DOI] [PubMed] [Google Scholar]
- [53].Opitz BJ, Ostroff ML, Whitman AC. The Potential Clinical Implications and Importance of Drug Interactions Between Anticancer Agents and Cannabidiol in Patients With Cancer. J Pharm Pract. 2020;33:506–512. doi: 10.1177/0897190019828920. [DOI] [PubMed] [Google Scholar]
- [54].Buchtova T, Lukac D, Skrott Z, et al. Drug-Drug Interactions of Cannabidiol with Standard-of-Care Chemotherapeutics. Int J Mol Sci. 2023;24. doi: 10.3390/ijms24032885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Guedon M, Le Bozec A, Brugel M, et al. Cannabidiol-drug interaction in cancer patients: A retrospective study in a real-life setting. Br J Clin Pharmacol. 2023. doi: 10.1111/bcp.15701. [DOI] [PubMed] [Google Scholar]
- [56].Geffrey AL, Pollack SF, Bruno PL, Thiele EA. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia. 2015;56:1246–51. doi: 10.1111/epi.13060. [DOI] [PubMed] [Google Scholar]
- [57].Jiang R, Yamaori S, Okamoto Y, Yamamoto I, Watanabe K. Cannabidiol is a potent inhibitor of the catalytic activity of cytochrome P450 2C19. Drug Metab Pharmacokinet. 2013;28:332–8. doi: 10.2133/dmpk.dmpk-12-rg-129. [DOI] [PubMed] [Google Scholar]
- [58].Bansal S, Zamarripa CA, Spindle TR, et al. Evaluation of Cytochrome P450-Mediated Cannabinoid-Drug Interactions in Healthy Adult Participants. Clin Pharmacol Ther. 2023. doi: 10.1002/cpt.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gaston TE, Bebin EM, Cutter GR, et al. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia. 2017;58:1586–1592. doi: 10.1111/epi.13852. [DOI] [PubMed] [Google Scholar]
- [60].Grayson L, Vines B, Nichol K, Szaflarski JP, Program UC. An interaction between warfarin and cannabidiol, a case report. Epilepsy Behav Case Rep. 2018;9:10–11. doi: 10.1016/j.ebcr.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Chen S, Wu Q, Li X, et al. Characterization of cytochrome P450s (CYP)-overexpressing HepG2 cells for assessing drug and chemical-induced liver toxicity. J Environ Sci Health C Toxicol Carcinog. 2021;39:68–86. doi: 10.1080/26896583.2021.1880242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Li X, Li Y, Ning KG, et al. The expression of Phase II drug-metabolizing enzymes in human B-lymphoblastoid TK6 cells. J Environ Sci Health C Toxicol Carcinog. 2022;40:106–118. doi: 10.1080/26896583.2022.2044242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Olivas-Aguirre M, Torres-Lopez L, Valle-Reyes JS, et al. Cannabidiol directly targets mitochondria and disturbs calcium homeostasis in acute lymphoblastic leukemia. Cell Death Dis. 2019;10:779. doi: 10.1038/s41419-019-2024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


