Abstract
Thyroid gland metastases from nonthyroidal malignancies are extremely rare. The most common primary malignancies associated with metastasis to thyroid gland include renal cell carcinoma, colorectal carcinoma, lung cancer, and breast cancer. Metastasis to thyroid rarely arises from primary laryngeal cancer. The presence of metastasis to thyroid gland is invariable and associated with poor prognosis and thus, should be differentiated from primary thyroid malignancy. Hereby, we have one such case of metastasis to thyroid gland from laryngeal cancer diagnosed on 18F-fluorodeoxyglucose positron emission tomography/computed tomography scan.
Keywords: Fluorodeoxyglucose positron emission tomography/computed tomography scan, laryngeal cancer, thyroid gland metastases
A 65-year-old male presented with swelling on the left side of his neck. The swelling was insidious in onset and progressive in nature. Upon evaluation, the patient was found to have ulceroproliferative lesion involving the supraglottis extending to the oropharynx. Punch biopsy from the lesion and fine needle aspiration cytology (FNAC) from the left neck swelling were then performed. Histopathological examination revealed moderately differentiated squamous cell carcinoma of supraglottis with metastasis to cervical lymph nodes. Subsequently, 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F FDG-PET/CT) was performed which revealed hypermetabolic heterogeneously enhancing lesion in the left vallecula, left aryepiglottic, and glossoepiglottic fold extending to the floor of mouth suggestive of primary malignancy. In addition, there were multiple hypermetabolic cervical, mediastinal lymph nodes, right 6th rib lesion, and multiple soft-tissue lesions in bilateral lung fields as well as hypermetabolic hypodense lesion involving the right lobe of thyroid suggestive of metastatic disease [Figure 1]. FNAC from the thyroid lesion confirmed the presence of metastatic squamous cell carcinomatous deposit [Figure 2].
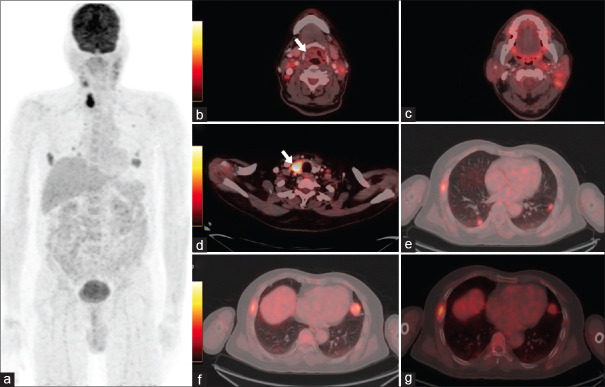
Figure 1.
Fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) scan performed postneoadjuvant chemoradiotherapy. Maximum intensity projection image showing hypermetabolic lesion in the neck-and-thorax region (a). Hybrid PET-CT images showing soft-tissue lesion in the right vallecula with no significant metabolic activity (white arrow) (b), Hypermetabolic multiple bilateral cervical lymph nodes (c), hypodense lesion in the right lobe of thyroid (white arrow) (d), soft-tissue lesion in bilateral lung fields (e and f) and lytic lesion involving the right 6rh rib (g) suggestive of metastatic disease
Figure 2.
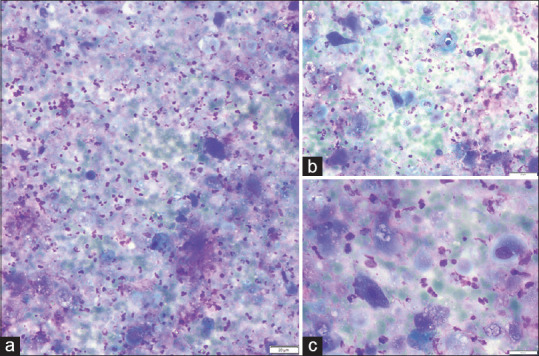
Cytology images of fine-needle aspiration from the thyroid nodule at (a) ×20, (b) ×40 and (c) ×80 magnification showing atypical squamous cells in a necrotic background
The patient then underwent local radiotherapy to the laryngeal lesion along with platinum-based chemotherapy (cisplatin+paclitaxel). Follow-up 18-F FDG-PET/CT performed 5-month postchemotherapy revealed regression in size and resolution of metabolic activity from ulceroproliferative supraglottic lesion, however, there was increase in size and metabolic activity of cervical and mediastinal lymph nodes, increase in metabolic activity of right 6th rib lesion as well as appearance of few new lung nodules and soft-tissue deposit along right crus of diaphragm and new lesion in the isthmus of thyroid, suggesting progressive nature of the disease [Figure 3].
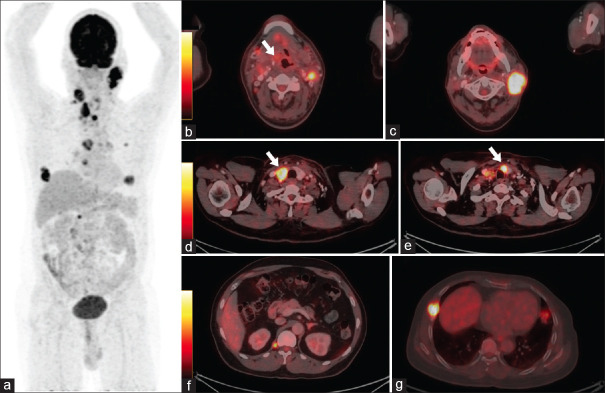
Figure 3.
Follow-up 18F-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) performed post 6 cycles of adjuvant chemotherapy. (a) MIP image showing hypermetabolic lesion in the neck and thorax showing increase in size and metabolic activity with the appearance of few new hypermetabolic foci. Hybrid PET-CT images showing mildly hypermetabolic soft-tissue lesion in the right vallecula (white arrow) (b), Increase in size and metabolic activity of bilateral cervical lymph nodes (c), hypodense lesion in the right lobe of thyroid (white arrow) (d) and lytic lesion involving the right 6rh rib (g) and lung nodules (not shown). There is appearance of new lesions involving the isthmus of thyroid (white arrow) (e), and right crus of diaphragm (f) and mediastinal lymph nodes (not shown), overall suggestive of progressive disease
Despite the significant prevalence of primary thyroid cancer, metastases to the thyroid gland are reported infrequently with an incidence of approximately 0.36%.[1,2] The most frequent sources of metastases to the thyroid include renal cell carcinoma, followed by lung, colorectal, and breast carcinomas with laryngeal carcinomas being rarely reported.[3,4] Metastasis to thyroid gland represents a rare entity and ponders a poor prognosis irrespective of the site of the primary disease with median overall survival as low as 10 months.[5] However, when suspected, it is essential to differentiate it from primary synchronous/metachronous thyroid cancer as well as from contiguous spread from adjacent metastatic disease or adjoining primary malignancy. Imaging findings, histopathology, as well as immunohistochemistry/immunocytochemistry, should be cautiously reviewed before arriving at the final diagnosis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Tang Q, Wang Z. Metastases to the thyroid gland: What can we do? Cancers (Basel) 2022;14:3017. doi: 10.3390/cancers14123017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nixon IJ, Coca Pelaz A, Kaleva AI, Triantafyllou A, Angelos P, Owen RP, et al. Metastasis to the thyroid gland: A critical review. Ann Surg Oncol. 2017;24:1533–9. doi: 10.1245/s10434-016-5683-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Momin SM, Hamal M, Chaston N, Nissanka Jayasuriya E, Al Lami A. Thyroid metastasis from caecal adenocarcinoma. Cureus. 2022;14:e24120. doi: 10.7759/cureus.24120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens TM, Richards AT, Bewtra C, Sharma P. Tumors metastatic to thyroid neoplasms: A case report and review of the literature. Patholog Res Int. 2011;2011:238693. doi: 10.4061/2011/238693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gormley M, Creaney G, Schache A, Ingarfield K, Conway DI. Reviewing the epidemiology of head and neck cancer: Definitions, trends and risk factors. Br Dent J. 2022;233:780–6. doi: 10.1038/s41415-022-5166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]