Abstract
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.
LIBRETTO-001 is a registrational phase I/II, single-arm, open-label study of selpercatinib in patients with RET (REarranged during Transfection)-activated cancers (ClinicalTrials.gov identifier: NCT03157128). We present long-term safety and efficacy from LIBRETTO-001 in patients with RET-mutant medullary thyroid cancer (MTC; n = 324) and RET fusion-positive thyroid cancer encompassing different histological subtypes (TC; n = 66). At the data cutoff of January 2023, the objective response rate was 82.5% among patients with cabozantinib/vandetanib-naïve MTC and 95.8% among patients with treatment-naïve TC. At a median follow-up time of 42.4 and 44.0 months in patients with cabozantinib/vandetanib-naïve and pretreated MTC, the median progression-free survival (PFS) was not reached and 41.4 months, respectively. At a median follow-up time of 24.9 and 30.4 months in patients with treatment-naïve and pretreated TC, the median PFS was not reached and 27.4 months, respectively. Three-year PFS rates were 75.2% and 87.3% among patients with cabozantinib/vandetanib-naïve MTC and treatment-naïve TC, respectively. Median PFS was similar to median duration of response for each patient group. The safety profile of selpercatinib was consistent with previous reports. With an additional follow-up of 37 months and 228 more patients from the last disclosure, selpercatinib continued to provide durable and robust responses in treatment-naïve and previously treated patients with RET-mutant MTC and RET fusion-positive TC.
INTRODUCTION
Selpercatinib, a first-in-class, highly selective, and potent RET (REarranged during Transfection) inhibitor, is currently approved in several regions around the world, including the United States, European Union, and Japan, for treatment of RET-mutant medullary thyroid cancer (MTC) and RET fusion-positive thyroid cancer (TC) encompassing different histological subtypes in adults and adolescents (age ≥12 years).1-3 These initial approvals were based on the clinical benefits reported in the LIBRETTO-001 trial (ClinicalTrials.gov identifier: NCT03157128) in which selpercatinib demonstrated high response rates and favorable toxicity in both treatment-naïve and previously treated patients.4 With over 3 years of additional follow-up and more than twice as many patients, we present the long-term results from safety and efficacy analyses of selpercatinib in patients with RET-activated MTC and TC from the LIBRETTO-001 clinical trial.
METHODS
Study Design
In the previously published phase I/II, open-label LIBRETTO-001 trial,4,5 patients received oral selpercatinib (capsule or liquid), in 28-day continuous cycles at doses of 20 mg once daily to 240 mg twice daily during the dose-escalation phase. The recommended 160 mg twice daily dose was used in phase II.4,5 Treatment continued until progressive disease, death, withdrawal of consent, or unacceptable toxicity. Patient enrollment required the identification of a prospective RET alteration (fusion or mutation). A positive germline DNA test for a RET mutation was acceptable for patients with MTC. Patients with RET-mutant MTC who were cabozantinib/vandetanib naïve were also required to have radiographic progressive disease within the previous 14 months. Before enrollment, the sponsor reviewed and confirmed the results of local molecular testing conducted in a certified laboratory using next-generation sequencing, fluorescence in situ hybridization, or polymerase chain reaction to determine RET alteration status. This central confirmation of the locally identified RET alteration was not required. This report includes patients with RET-mutant MTC and RET fusion-positive TC of any histologic type who were treatment-naïve or treated with prior systemic therapy. Prior exposure to a RET inhibitor was an exclusion criterion except for patients in cohort 6 (MTC: n = 8; TC: n = 1), who were excluded from the efficacy analyses but included in the safety analyses.
Efficacy and Safety Measures
Efficacy and safety assessments were performed in patients enrolled in the LIBRETTO-001 study as previously described.4,5 The primary end point was objective response rate (ORR). Responses were determined by an independent review committee of expert radiologists according to RECIST, version 1.1. Secondary end points included clinical benefit rate, progression-free survival (PFS), duration of response (DoR), and overall survival (OS). All responses necessitated validation through a subsequent consecutive scan obtained no less than 4 weeks after the initial scan indicating a response. Patients who were alive or lost to follow-up as of the data analysis cutoff date were censored. Safety was analyzed through adverse event (AE) reporting, graded according to the Common Terminology Criteria for Adverse Events, version 4.03.
Statistical Methods
The data cutoff date for this analysis was January 13, 2023. Confidence intervals (CIs) for response rates were calculated using the Clopper-Pearson method. DoR, PFS, and OS were estimated using the Kaplan-Meier method. The CI for the median survival time was derived following the method outlined by Brookmeyer and Crowley.6 The reverse Kaplan-Meier method was used to estimate median follow-up times. Median follow-up durations were provided for each efficacy end point to provide additional context. Additional statistical analysis methodology is reported in the Protocol (online only).
RESULTS
Patient Demographics and Follow-Up
Among the 837 patients enrolled from May 2017 to May 2022, 324 had RET-mutant MTC and 66 had RET fusion-positive TC. The baseline characteristics and demographics are presented in Table 1 and Data Supplement (Table S1, online only). Prior systemic therapy use is shown in the Data Supplement (Table S2). The median follow-up duration and range for each subgroup and each efficacy end point are presented in the Data Supplement (Table S3).
TABLE 1.
Clinicopathologic Features in RET Fusion-Positive TC and RET-Mutant Medullary Thyroid Cancer (MTC)
| Characteristic | RET Fusion-Positive TC | RET-Mutant MTC | ||
|---|---|---|---|---|
| Treatment Naïve (n = 24)a | Previously Treated (n = 41) | Cabozantinib/Vandetanib Naïve (n = 143)b | Previously Treated (n = 152) | |
| Age, years, median (range) | 60.5 (20-84) | 58.0 (25-88) | 57.0 (15-87) | 58.0 (17-90) |
| Sex, No. (%) | ||||
| Female | 10 (41.7) | 23 (56.1) | 60 (42.0) | 55 (36.2) |
| Male | 14 (58.3) | 18 (43.9) | 83 (58.0) | 97 (63.8) |
| Race, No. (%) | ||||
| White | 18 (75.0) | 24 (58.5) | 124 (86.7) | 137 (90.1) |
| Asian | 1 (4.2) | 12 (29.3) | 8 (5.6) | 2 (1.3) |
| Black | 0 | 3 (7.3) | 2 (1.4) | 2 (1.3) |
| Native Hawaiian or other Pacific Islander | 0 | 0 | 1 (0.7) | 0 |
| American Indian or Alaska Native | 0 | 0 | 0 | 1 (0.7) |
| Other | 3 (12.5) | 2 (4.9) | 7 (4.9) | 10 (6.6) |
| Missing | 2 (8.3) | 0 | 1 (0.7) | 0 |
| Smoking status, No. (%) | ||||
| Never | 12 (50.0) | 28 (68.3) | NR | NR |
| Former | 10 (41.7) | 13 (31.7) | NR | NR |
| Current | 1 (4.2) | 0 | NR | NR |
| Missing | 1 (4.2) | 0 | NR | NR |
| ECOG performance status, No. (%) | ||||
| 0 | 14 (58.3) | 11 (26.8) | 69 (48.3) | 42 (27.6) |
| 1 | 9 (37.5) | 27 (65.9) | 68 (47.6) | 99 (65.1) |
| 2 | 1 (4.2) | 3 (7.3) | 6 (4.2) | 11 (7.2) |
| TC histologic subtype, No. (%) | ||||
| Papillary TC | 23 (95.8) | 31 (75.6) | — | — |
| Poorly differentiated TC | 1 (4.2) | 5 (12.2) | — | — |
| Anaplastic TC | 0 | 4 (9.8) | — | — |
| Hurthle cell TC | 0 | 1 (2.4) | — | — |
| MTC | 0 | 0 | 143 (100) | 152 (100) |
| No. of prior systemic regimens, (%) | ||||
| 0 | 6 (25.0) | 0 | 116 (81.1) | — |
| 1 | 10 (41.7) | 10 (24.4) | 22 (15.4) | 73 (48.0) |
| 2 | 3 (12.5) | 8 (19.5) | 5 (3.5) | 37 (24.3) |
| ≥3 | 5 (20.8) | 23 (56.1) | 0 | 42 (27.6) |
| Previous regimen,c No. (%) | ||||
| Chemotherapy | — | 8 (19.5) | 5 (3.5) | 16 (10.5) |
| Immunotherapy | — | 3 (7.3) | 5 (3.5) | 13 (8.6) |
| Multikinase inhibitor | — | 35 (85.4) | 9 (6.3) | 152 (100) |
| Otherd | 18 (75.0) | 30 (73.2) | 9 (6.3) | 16 (10.5) |
| RET fusion, No. (%) | ||||
| CCDC6 | 15 (62.5) | 25 (61.0) | — | — |
| NCOA4 | 7 (29.2) | 8 (19.5) | — | — |
| Other | 2 (8.3) | 7 (17.1) | — | — |
| Unknown | 0 | 1 (2.4) | — | — |
| RET mutation type, No. (%) | ||||
| M918T | — | — | 86 (60.1) | 99 (65.1) |
| Extracellular cysteine mutation | — | — | 34 (23.8) | 24 (15.8) |
| V804 M/L | — | — | 6 (4.2) | 8 (5.3) |
| Other | — | — | 17 (11.9) | 21 (13.8) |
| CNS metastases at baseline,e No. (%) | ||||
| Yes | 1 (4.2) | 12 (29.3) | 3 (2.1) | 11 (7.2) |
| No | 23 (95.8) | 29 (70.7) | 140 (97.9) | 141 (92.8) |
| Patients who received at least one dose of 160 mg twice daily, No. (%) | 23 (95.8) | 40 (97.6) | 141 (98.6) | 144 (94.7) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; MTC, medullary thyroid cancer; mTOR, mammalian target of rapamycin; NR, not reported; RET, REarranged during Transfection; TC, thyroid cancer; VEGF, vascular endothelial growth factor.
Treatment naïve refers to therapies other than radioactive iodine.
Cabozantinib/vandetanib naïve included treatment-naïve patients (n = 116) and patients who were not previously treated with cabozantinib/vandetanib (n = 27).
Patients may have received more than one prior systemic therapy.
Other prior systemic therapies included radioactive iodine, mTOR inhibitor, EGFR inhibitor, VEGF/VEGF receptor inhibitor, hormonal therapy, and selective RET inhibitor.
Includes both measurable and nonmeasurable CNS metastases.
RET-Mutant MTC
Three groups of patients with RET-mutant MTC were analyzed: (1) treatment-naïve patients (n = 116); (2) cabozantinib/vandetanib-naïve patients (may have received other systemic therapies; n = 143); and (3) patients previously treated with any multikinase inhibitor (MKI; n = 152). The treatment-naïve cohort (n = 116) was a subset of the cabozantinib/vandetanib-naïve cohort (n = 143); response outcomes were reported for patients in the cabozantinib/vandetanib-naïve cohort as outcomes were not meaningfully different between these two cohorts. Response outcomes are presented in Table 2, Figure 1, and the Data Supplement (Table S4 and Figs S1-S5).
TABLE 2.
Efficacy in RET Fusion-Positive TC and RET-Mutant MTC
| Response | RET Fusion-Positive TC | RET-Mutant MTC | ||
|---|---|---|---|---|
| Treatment Naïve (n = 24)a | Previously Treated (n = 41) | Cabozantinib/Vandetanib Naïve (n = 143)b | Previously Treated (n = 152) | |
| Objective response rate by IRC,c % (95% CI) | 95.8 (78.9 to 99.9) | 85.4 (70.8 to 94.4) | 82.5 (75.3 to 88.4) | 77.6 (70.2 to 84.0) |
| Best overall response | ||||
| CR, No. (%) | 5 (20.8) | 5 (12.2) | 34 (23.8) | 19 (12.5) |
| PR, No. (%) | 18 (75.0) | 30 (73.2) | 84 (58.7) | 99 (65.1) |
| SD, No. (%) | 1 (4.2) | 6 (14.6) | 20 (14.0) | 25 (16.4) |
| PD, No. (%) | 0 | 0 | 2 (1.4) | 2 (1.3) |
| Not evaluable | 0 | 0 | 3 (2.1) | 7 (4.6) |
| Clinical benefit rate,d,e % (95% CI) | 100 (85.8 to 100) | 100 (91.4 to 100) | 94.4 (89.3 to 97.6) | 91.4 (85.8 to 95.4) |
| DoR | ||||
| Median (95% CI)f,g months | NE (42.8 to NE) | 26.7 (12.1 to NE) | NE (51.3 to NE) | 45.3 (33.6 to NE) |
| Patients with censored data, No. (%) | 21 (91.3) | 20 (57.1) | 87 (73.7) | 72 (61.0) |
| Rate of DoR at median follow-up time, % (95% CI) | 100 (100 to 100) | 45.6 (25.6 to 63.6) | 72.4 (62.2 to 80.3) | 55.7 (44.8 to 65.3) |
| Rate of DoR,g,h % (95% CI) | ||||
| 1 year | 100 (NE to NE) | 71.7 (52.4 to 84.2) | 91.4 (84.6 to 95.3) | 83.0 (74.6 to 88.8) |
| 2 years | 90.9 (50.8 to 98.7) | 50.7 (30.4 to 67.8) | 84.1 (75.9 to 89.7) | 66.4 (56.3 to 74.7) |
| 3 years | 90.9 (50.8 to 98.7) | 45.6 (25.6 to 63.6) | 76.7 (67.4 to 83.7) | 60.3 (49.8 to 69.3) |
| 4 years | NE (NE to NE) | 45.6 (25.6 to 63.6) | 67.6 (55.6 to 77.0) | 48.5 (36.2 to 59.7) |
| 5 years | — | — | NE (NE to NE) | 48.5 (36.2 to 59.7) |
| PFS | ||||
| Disease progression, No. (%) | 3 (12.5) | 16 (39.0) | 33 (23.1) | 53 (34.9) |
| Median (95% CI)f,g months | NE (44.2 to NE) | 27.4 (14.5 to NE) | NE (53.1 to NE) | 41.4 (30.2 to NE) |
| Patients with censored data, No. (%) | 21 (87.5) | 24 (58.5) | 104 (72.7) | 83 (54.6) |
| Rate of PFS at median follow-up time, % (95% CI) | 87.3 (56.4 to 96.8) | 49.5 (31.1 to 65.4) | 70.2 (60.9 to 77.8) | 47.8 (38.50 to 56.6) |
| Rate of PFS,g,h % (95% CI) | ||||
| 1 year | 95.2 (70.7 to 99.3) | 70.6 (53.2 to 82.6) | 91.1 (84.8 to 94.8) | 79.5 (71.8 to 85.3) |
| 2 years | 95.2 (70.7 to 99.3) | 57.1 (38.6 to 71.8) | 82.5 (74.8 to 88.0) | 64.9 (56.2 to 72.3) |
| 3 years | 87.3 (56.4 to 96.8) | 49.5 (31.1 to 65.4) | 75.2 (66.8 to 81.8) | 54.6 (45.6 to 62.8) |
| 4 years | 65.5 (17.5 to 90.2) | 49.5 (31.1 to 65.4) | 65.9 (55.1 to 74.8) | 45.9 (36.2 to 55.1) |
| 5 years | NE (NE to NE) | 49.5 (31.1 to 65.4) | NE (NE to NE) | 41.6 (31.1 to 51.7) |
| OS | ||||
| Median (95% CI)f,g months | NE (NE to NE) | NE (25.3 to NE) | NE (NE to NE) | 64.3 (48.3 to NE) |
| Patients with censored data, No. (%) | 23 (95.8) | 30 (73.2) | 128 (89.5) | 96 (63.2) |
| Rate of OS at median follow-up time, % (95% CI) | 94.4 (66.6 to 99.2) | 65.5 (46.0 to 79.4) | 88.8 (82.0 to 93.1) | 63.4 (54.7 to 70.9) |
| Rate of OS,g,h % (95% CI) | ||||
| 1 year | 100 (NE to NE) | 94.8 (80.7 to 98.7) | 99.3 (95.0 to 99.9) | 87.8 (81.3 to 92.1) |
| 2 years | 94.4 (66.6 to 99.2) | 76.4 (58.1 to 87.5) | 94.9 (89.7 to 97.5) | 76.6 (68.8 to 82.7) |
| 3 years | 94.4 (66.6 to 99.2) | 65.5 (46.0 to 79.4) | 89.7 (83.3 to 93.8) | 67.8 (59.4 to 74.8) |
| 4 years | 94.4 (66.6 to 99.2) | 65.5 (46.0 to 79.4) | 88.8 (82.0 to 93.1) | 60.7 (51.5 to 68.7) |
| 5 years | NE (NE to NE) | 65.5 (46.0 to 79.4) | 88.8 (82.0 to 93.1) | 57.1 (47.1 to 65.9) |
Abbreviations: CR, complete response; DoR, duration of response; IRC, independent review committee; MTC, medullary thyroid cancer; NE, not evaluable; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; RET, REarranged during Transfection; SD, stable disease; TC, thyroid cancer.
Treatment naïve refers to therapies other than radioactive iodine.
Cabozantinib/vandetanib naïve included treatment-naïve patients (n = 116) and patients who were not previously treated with cabozantinib/vandetanib (n = 27).
Objective response rate was defined as the proportion of patients with a best overall response of confirmed CR or PR.
95% CI was calculated using the Clopper-Pearson method.
Clinical benefit rate (%) was defined as the proportion of patients with a best overall response of a confirmed CR, PR, or SD lasting ≥16 weeks. SD was measured from the date of the first dose of selpercatinib until the criteria for PD were first met.
95% CIs were calculated using the Brookmeyer-Crowley method.
Estimate based on the Kaplan-Meier method.
95% CIs were calculated using the Greenwood formula.
FIG 1.
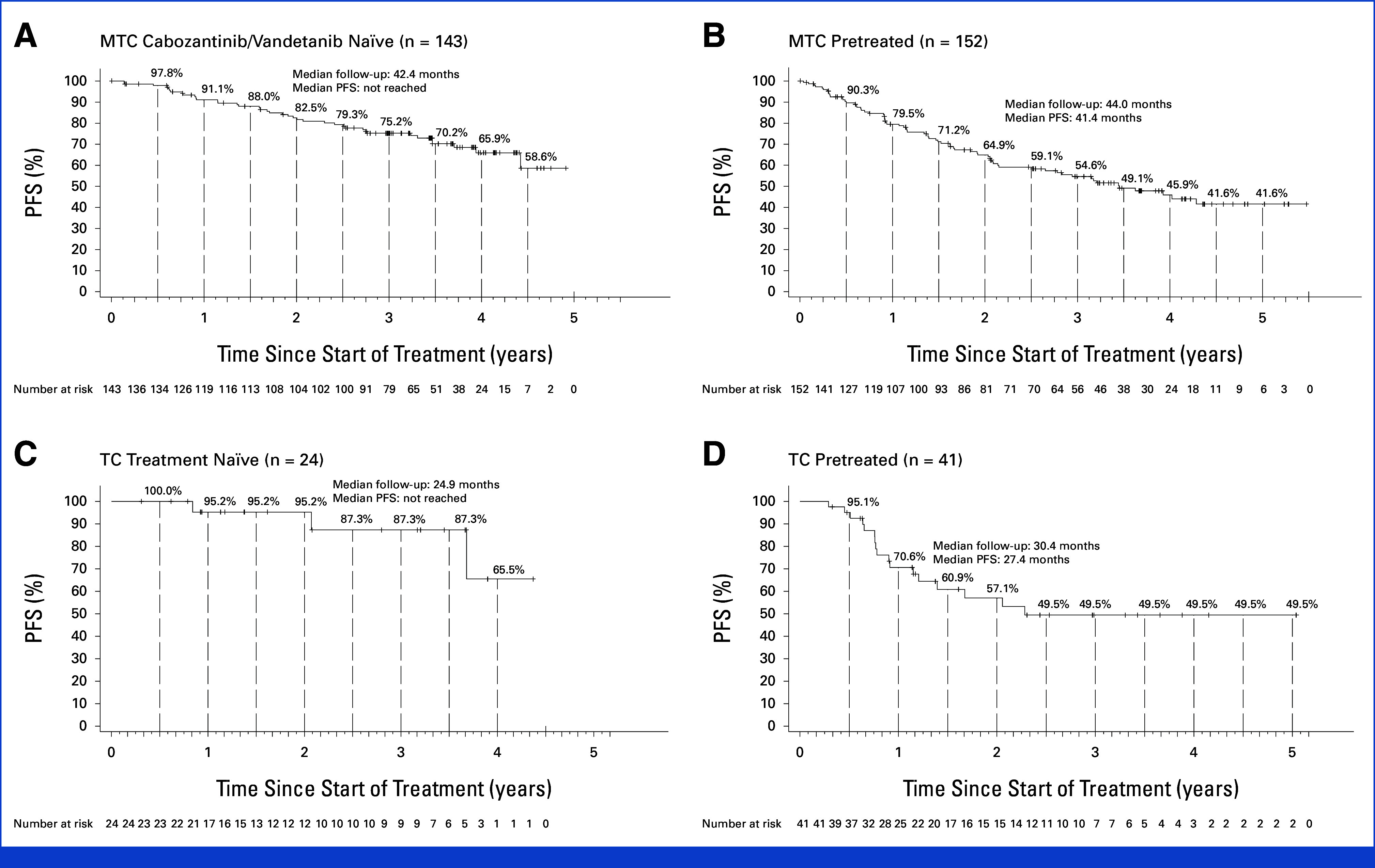
Long-term PFS with selpercatinib. Kaplan-Meier plots show PFS for the (A) RET-mutant MTC cabozantinib/vandetanib-naïve group, (B) RET-mutant MTC pretreated group, (C) RET fusion-positive TC treatment-naïve group, and (D) RET fusion-positive TC pretreated group. Tick marks indicate censored data. Eligible patients are defined as treated patients. Patients enrolled in phase II who discontinued selective RET inhibitor(s) because of intolerance were excluded. MTC, medullary thyroid cancer; PFS, progression-free survival; RET, REarranged during Transfection; TC, thyroid cancer.
The ORR was 84.5% (95% CI, 76.6 to 90.5) in treatment-naïve patients and 82.5% (95% CI, 75.3 to 88.4) in cabozantinib/vandetanib-naïve patients, with 25.9% and 23.8%, respectively, achieving a complete response (CR). The ORR was 77.6% (95% CI, 70.2 to 84.0) in patients previously treated with MKIs, with 12.5% of patients achieving a CR. In the overall MTC cohort (N = 295), ORR was 79.5% (95% CI, 72.9 to 85.0) in patients with an M918T RET mutation and 80.9% (95% CI, 72.3 to 87.8) in patients with other mutation types.
The median DoR was not reached (95% CI, 51.3 to not evaluable [NE]) in the cabozantinib/vandetanib-naïve group (median follow-up, 39.4 months). The median DoR in patients previously treated with MKIs (median follow-up, 38.3 months) was 45.3 months (95% CI, 33.6 to NE). At 4 years, 67.6% (95% CI, 55.6 to 77.0) of the cabozantinib/vandetanib-naïve group had responses that were ongoing. In the overall cohort, DoR was not reached in patients with an M918T RET mutation (95% CI, 51.3 to NE) or other mutation types (95% CI, 36.8 to NE).
At a median follow-up of 42.4 months, the median PFS was not reached (95% CI, 53.1 to NE) in the cabozantinib/vandetanib-naïve group. In patients previously treated with MKIs and with a follow-up of 44.0 months, the median PFS was 41.4 months (95% CI, 30.2 to NE). Three-year PFS rates were 75.2% (95% CI, 66.8 to 81.8) and 54.6% (95% CI, 45.6 to 62.8) in cabozantinib/vandetanib-naïve patients and patients previously treated with MKIs, respectively.
The median OS was not reached in the cabozantinib/vandetanib-naïve group (median follow-up, 44.6 months). In patients previously treated with MKIs with a median follow-up of 46.9 months, the median OS was 64.3 months. Three-year OS rates were 89.7% among cabozantinib/vandetanib-naïve patients and 67.8% among patients previously treated with MKIs.
RET Fusion-Positive TC
Two groups of patients with RET fusion-positive TC were analyzed: (1) systemic treatment-naïve patients (other than radioactive iodine therapy; n = 24) and (2) patients previously treated with any systemic therapy other than radioactive iodine (n = 41). Response outcomes are presented in Table 2, Figure 1, and the Data Supplement (Figs S1-S4).
The ORR was 95.8% (95% CI, 78.9 to 99.9) in treatment-naïve patients and 85.4% (95% CI, 70.8 to 94.4) in pretreated patients, with 20.8% and 12.2%, respectively, achieving a CR.
At a median follow-up of 17.8 months in the treatment-naïve group, the median DoR was not reached (95% CI, 42.8 to NE), and the 2-year response rate was 90.9%. In the pretreated group with a median follow-up of 33.9 months, the median DoR was 26.7 months (95% CI, 12.1 to NE), and the 4-year response rate was 45.6%.
The median PFS was not reached (95% CI, 44.2 to NE) in the treatment-naïve group at a median follow-up of 24.9 months and was 27.4 months (95% CI, 14.5 to NE; median follow-up, 30.4 months) in the pretreated group. Two-year PFS rates among patients in the treatment-naïve and pretreated groups were 95.2% and 57.1%, respectively.
At a median follow-up of 38.7 and 36.9 months, the median OS was not reached among treatment-naïve or pretreated groups, respectively. Three-year OS rates among patients in the treatment-naïve and pretreated groups were 94.4% and 65.5%, respectively.
Safety
Treatment-emergent AEs, serious AEs (SAEs) regardless of causality, and AEs deemed related to selpercatinib by the investigator are shown in the Data Supplement (Tables S5 and S6). The most common (≥5%) grade ≥3 treatment-emergent AEs were hypertension (MTC, 21.6%; TC, 15.2%) and increased ALT (MTC, 9.0%; TC, 6.1%). Grade ≥3 prolonged QT interval was observed in 4.3% and 4.5% of patients with MTC and TC, respectively. The most common grade ≥3 treatment-related AEs included hypertension (MTC, 14.5%; TC, 6.1%) and increased ALT (MTC, 7.4%; TC, 3.0%; Data Supplement, Table S5). The most common SAEs were pneumonia (4.6%) in patients with MTC and abdominal pain and confusional state (both 4.5%) in patients with TC (Data Supplement, Table S6). Ascites (1.5%) was the most common treatment-related SAE in patients with MTC. Abdominal pain, hyperbilirubinemia, vomiting, cholestasis, and lymphopenia (one patient each) were the observed treatment-related SAEs in patients with TC.
DISCUSSION
Here, we report the long-term safety and efficacy data in patients with TC from the LIBRETTO-001 trial. Importantly, this disclosure builds on the initial publication for this patient population with a follow-up of over 3 years, demonstrating durability of response and describing long-term safety data with selpercatinib therapy.4 Selpercatinib continued to demonstrate a potent response (with almost 70% of responses ongoing in cabozantinib/vandetanib-naïve patients at 4 years) and a consistent safety profile as previously described in this population of patients with RET-activated TC.
In the initial report from LIBRETTO-001 (N = 162; patients enrolled from May 2017 through June 2019), among cabozantinib/vandetanib-pretreated (n = 55) and treatment-naïve (n = 88) patients with RET-mutant MTC, ORRs were 69% and 73% with 1-year PFS rates of 82% and 92%, respectively.4 In patients with RET fusion-positive TC (n = 19), the ORR was 79% and PFS at 1 year was 64%.4 The current analysis demonstrates continued marked efficacy in a larger population of patients with MTC and TC. Moreover, selpercatinib demonstrates a favorable ORR, durable responses, and a tolerable safety profile both in patients with MTC and TC, without cumulative or late toxic effects.
MKIs, currently used as first-line treatment for MTC (cabozantinib and vandetanib) and papillary TC (lenvatinib and sorafenib), show modest efficacy and limited durability of responses. This is in part due to AEs, acquired resistance, and limited potency due to nonselectivity.7-9 These data provide additional support to current guidelines10 that recommend selpercatinib treatment for MTC and TC on identification of a RET alteration. Furthermore, we observed more favorable outcomes in patients with treatment-naïve RET-activated MTC and TC compared with previously treated patients. These data should be interpreted with caution since this analysis was not designed to compare outcomes between these groups of patients. However, recently disclosed data from the phase 3 LIBRETTO-531 trial showed superior PFS and treatment failure–free survival with first-line treatment with selpercatinib compared with cabozantinib or vandetanib in patients with RET-mutant MTC.11 The AE profile reported here is also consistent with that reported in the LIBRETTO-531 trial, with notable AEs such as liver enzyme elevation, prolonged QT interval as documented on an ECG, and hypertension.11 More importantly, the safety profile remains unchanged despite longer treatment with selpercatinib.
In conclusion, selpercatinib continued to demonstrate durable and potent efficacy with a consistent safety profile in a larger number of patients with RET-mutant MTC and RET fusion-positive TC, regardless of line of therapy, after longer follow-up. Testing for RET alterations in MTC and TC should be performed before initiating systemic therapy to identify patients who will benefit from RET inhibition with selpercatinib treatment.12
ACKNOWLEDGMENT
We thank the patients and their families and caregivers, as well as the investigators and their personnel for their participation in the study. We thank David Hyman and Boris Lin for their insights, guidance, and critical revisions of the manuscript files and Pavan ML and Rafael Heard for their data visualization assistance. Medical writing support was provided by Ira Ayene and Preethi Govindarajan, and editorial support was provided by Adrienne Schreiber of Syneos Health and funded by Eli Lilly and Company in accordance with Good Publication Practice (2022) guidelines (https://www.ismpp.org/gpp-2022).
Lori J. Wirth
Consulting or Advisory Role: Merck, Eisai, Lilly, Bayer, Exelixis, Coherus Biosciences, METIS Precision Medicine, Tome Biosciences, EMD Serono, Ellipses Pharma, Illumina, Nested
Research Funding: Checkmate Pharmaceuticals (Inst), Lilly (Inst), Eisai (Inst), Novartis (Inst)
Other Relationship: PDS Biotechnology
Marcia S. Brose
Honoraria: Bayer, Eisai, Lilly
Consulting or Advisory Role: Bayer, Eisai, Loxo, Exelixis, Lilly, AADi
Research Funding: Loxo (Inst), Lilly (Inst)
Vivek Subbiah
Consulting or Advisory Role: Loxo/Lilly, Relay Therapeutics (Inst), Pfizer (Inst), Roche (Inst), Bayer (Inst), Incyte (Inst), Novartis (Inst), Pheon Therapeutics (Inst), Abbvie (Inst), Illumina, AADi, Foundation Medicine
Research Funding: Novartis (Inst), GlaxoSmithKline (Inst), NanoCarrier (Inst), Northwest Biotherapeutics (Inst), Genentech/Roche (Inst), Berg Pharma (Inst), Bayer (Inst), Incyte (Inst), Fujifilm (Inst), PharmaMar (Inst), D3 Oncology Solutions (Inst), Pfizer (Inst), Amgen (Inst), Abbvie (Inst), Multivir (Inst), Blueprint Medicines (Inst), LOXO (Inst), Vegenics (Inst), Takeda (Inst), Alfasigma (Inst), Agensys (Inst), Idera (Inst), Boston Biomedical (Inst), Inhibrx (Inst), Exelixis (Inst), Amgen (Inst), Turning Point Therapeutics (Inst), Relay Therapeutics (Inst)
Other Relationship: Medscape, Clinical Care Options
Francis Worden
Honoraria: Merck Sharp & Dohme, Eisai, Bristol Myers Squibb, Regeneron, Exelixis, EMD Serono
Consulting or Advisory Role: Merck, Loxo, Bristol Myers Squibb, eisai, Bayer, Regeneron, Exelixis, Sun Pharma Advanced Research Company, Sun Pharma Advanced Research Company, Sun Pharma Advanced Research Company, Sun Pharma Advanced Research Company, Sun Pharma Advanced Research Company, Adlai Nortye, Coherus Biosciences, Coherus Biosciences
Research Funding: Merck (Inst), Eisai (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), CUE Biopharma (Inst), Loxo/Lilly (Inst)
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Bayer, Sun Pharma Advanced Research Company
Ben Solomon
Honoraria: AstraZeneca, Merck Sharp & Dohme (Inst), Roche/Genentech, Pfizer (Inst), Amgen (Inst)
Consulting or Advisory Role: Bristol Myers Squibb (Inst), Merck Sharp & Dohme, AstraZeneca (Inst), Pfizer (Inst), Roche/Genentech (Inst), Amgen, Lilly, BeiGene, Takeda, GlaxoSmithKline (Inst), Janssen (Inst), GlaxoSmithKline
Research Funding: Sanofi (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Bruce Robinson
Leadership: Cochlear, Mayne Pharma
Stock and Other Ownership Interests: Cochlear, Mayne Pharma
Consulting or Advisory Role: Loxo, Eisai
Speakers' Bureau: Eisai
Travel, Accommodations, Expenses: Eisai
Julien Hadoux
Honoraria: ITM Isotope Technologies Munich (Inst), AAA/Endocyte/Novartis (Inst), HRA Pharma (Inst)
Consulting or Advisory Role: Eisai Europe, Lilly, Roche, PharmaMar, HRA Pharma, Bayer (Inst)
Research Funding: Novartis (Inst), Lilly (Inst)
Travel, Accommodations, Expenses: Roche
Pascale Tomasini
Consulting or Advisory Role: AstraZeneca, Roche, Bristol Myers Squibb Foundation, Takeda, Amgen, Janssen
Travel, Accommodations, Expenses: Bristol Myers Squibb/Pfizer, AstraZeneca, Takeda
Daniela Weiler
Honoraria: Lilly, MSD Oncology, Roche, Roche
Consulting or Advisory Role: Lilly, MSD
Speakers' Bureau: Lilly
Barbara Deschler-Baier
Research Funding: Lilly (Inst)
Expert Testimony: Lilly
Daniel S.W. Tan
Honoraria: Takeda (Inst), Novartis (Inst), Roche (Inst), Pfizer (Inst)
Consulting or Advisory Role: Merck (Inst), AstraZeneca (Inst), Roche (Inst), Pfizer (Inst), Amgen (Inst), DKSH (Inst), Bayer (Inst), Genmab, Zymeworks
Research Funding: Novartis (Inst), GlaxoSmithKline (Inst), AstraZeneca (Inst), ACM Biolabs (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Pfizer, Boehringer Ingelheim, Roche
Patricia Maeda
Employment: Bayer, Lilly
Stock and Other Ownership Interests: Bayer, Lilly
Travel, Accommodations, Expenses: Lilly
Yan Lin
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Ravinder Singh
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Research Funding: Lilly
Travel, Accommodations, Expenses: Lilly
Theresa Bayt
Employment: Lilly
Stock and Other Ownership Interests: Lilly, Lilly
Alexander Drilon
Stock and Other Ownership Interests: Treeline Biosciences, mBrace
Honoraria: Pfizer, Loxo/Bayer/Lilly, IASLC, Helsinn Therapeutics, BeiGene, Remedica, Remedica, TP Therapeutics, Verastem, Ignyta/Genentech/Roche, AstraZeneca, Liberum, Lungevity, NIH, PER, OncLive/MJH Life Sciences, Clinical Care Options/NCCN, Lung Cancer Research Foundation, Associazione Italiana Oncologia Toracica (AIOT), Chugai Pharma, Sirio Libanes Hospital, Answers in CME, Research to Practice, i3 Health, RV Mais
Consulting or Advisory Role: Ignyta, Loxo, AstraZeneca, Pfizer, Blueprint Medicines, Genentech/Roche, BeiGene, Hengrui Therapeutics, Exelixis, Bayer, Tyra Biosciences, Takeda/Millennium, BerGenBio, MORE Health, Lilly, Abbvie, 14ner Oncology/Elevation Oncology, Monopteros Therapeutics, Novartis, EMD Serono/Merck, Repare Therapeutics, Melendi, Archer, Nuvalent, Inc, Janssen, Amgen, Merus, Axis Pharma, Medscape, Liberum, Med Learning, PeerView, EPG Health, Journal of the National Comprehensive Cancer Network, Ology Medical Education, Ology Medical Education, Clinical Care Options, Clinical Care Options, touchIME, Entos, Prelude Therapeutics, Applied Pharmaceutical Science, Treeline Biosciences, Monte Rosa Therapeutics, EcoR1 Capital
Research Funding: Foundation Medicine
Patents, Royalties, Other Intellectual Property: Wolters Kluwer (Royalties for Pocket Oncology), Osimertinib Selpercatinib
Other Relationship: Merck, GlaxoSmithKline, Teva, Taiho Pharmaceutical, Pfizer, PharmaMar, Puma Biotechnology, Pfizer, Merus, Boehringer Ingelheim
Philippe A. Cassier
Consulting or Advisory Role: OSE Immunotherapeutics, Bristol Myers Squibb/Celgene, Boehringer Ingelheim, Brenus Pharma, Scenic Biotech
Research Funding: Novartis (Inst), Roche/Genentech (Inst), Lilly (Inst), Blueprint Medicines (Inst), AstraZeneca (Inst), Abbvie (Inst), Bristol Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Taiho Pharmaceutical (Inst), Toray Industries (Inst), Transgene (Inst), Loxo (Inst), GlaxoSmithKline (Inst), Innate Pharma (Inst), Janssen (Inst), Boehringer Ingelheim (Inst), Daiichi Sankyo/UCB Japan (Inst), Adlai Nortye (Inst), Alligator Bioscience (Inst), Amgen (Inst), C4 Therapeutics (Inst), Debiopharm Group (Inst), Exelixis (Inst), Incyte (Inst), ITeos Therapeutics (Inst), OSE Immunotherapeutics (Inst), Molecular Partners (Inst), Pierre Fabre (Inst), Relay Therapeutics (Inst), Sotio (Inst), Tango Therapeutics (Inst)
Travel, Accommodations, Expenses: Roche, OSE Immunotherapeutics, Novartis
Uncompensated Relationships: ReACT Therapeutics
No other potential conflicts of interest were reported.
SUPPORT
Supported by Loxo Oncology, a wholly owned subsidiary of Eli Lilly and Company. A.D. was supported by grants from the National Cancer Institute/National Institutes of Health (P30CA008748, 1R01CA251591001A1, 1R01CA273224-01), Happy Lungs, and Lungevity.
CLINICAL TRIAL INFORMATION
NCT03157128 (LIBRETTO)
L.J.W., M.S.B., and V.S. contributed equally to this work.
DATA SHARING STATEMENT
Eli Lilly and Company provides access to all individual data collected during the trial, after anonymization, with the exception of pharmacokinetic, genomic, or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org. A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.23.02503.
AUTHOR CONTRIBUTIONS
Conception and design: Lori J. Wirth, Vivek Subbiah, Patricia Maeda, Theresa Bayt, Philippe A. Cassier
Financial support: Daniela Weiler
Administrative support: Vivek Subbiah, Daniela Weiler, Philippe A. Cassier
Provision of study materials or patients: Lori J. Wirth, Vivek Subbiah, Ben Solomon, Bruce Robinson, Julien Hadoux, Pascale Tomasini, Daniela Weiler, Barbara Deschler-Baier, Daniel S.W. Tan, Alexander Drilon, Philippe A. Cassier
Collection and assembly of data: Lori J. Wirth, Marcia S. Brose, Vivek Subbiah, Ben Solomon, Bruce Robinson, Julien Hadoux, Pascale Tomasini, Daniela Weiler, Barbara Deschler-Baier, Daniel S.W. Tan, Patricia Maeda, Theresa Bayt, Alexander Drilon, Philippe A. Cassier
Data analysis and interpretation: Lori J. Wirth, Marcia S. Brose, Vivek Subbiah, Francis Worden, Ben Solomon, Bruce Robinson, Julien Hadoux, Pascale Tomasini, Daniel S.W. Tan, Patricia Maeda, Yan Lin, Ravinder Singh, Theresa Bayt, Alexander Drilon, Philippe A. Cassier
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Durability of Response With Selpercatinib in Patients With RET-Activated Thyroid Cancer: Long-Term Safety and Efficacy From LIBRETTO-001
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Lori J. Wirth
Consulting or Advisory Role: Merck, Eisai, Lilly, Bayer, Exelixis, Coherus Biosciences, METIS Precision Medicine, Tome Biosciences, EMD Serono, Ellipses Pharma, Illumina, Nested
Research Funding: Checkmate Pharmaceuticals (Inst), Lilly (Inst), Eisai (Inst), Novartis (Inst)
Other Relationship: PDS Biotechnology
Marcia S. Brose
Honoraria: Bayer, Eisai, Lilly
Consulting or Advisory Role: Bayer, Eisai, Loxo, Exelixis, Lilly, AADi
Research Funding: Loxo (Inst), Lilly (Inst)
Vivek Subbiah
Consulting or Advisory Role: Loxo/Lilly, Relay Therapeutics (Inst), Pfizer (Inst), Roche (Inst), Bayer (Inst), Incyte (Inst), Novartis (Inst), Pheon Therapeutics (Inst), Abbvie (Inst), Illumina, AADi, Foundation Medicine
Research Funding: Novartis (Inst), GlaxoSmithKline (Inst), NanoCarrier (Inst), Northwest Biotherapeutics (Inst), Genentech/Roche (Inst), Berg Pharma (Inst), Bayer (Inst), Incyte (Inst), Fujifilm (Inst), PharmaMar (Inst), D3 Oncology Solutions (Inst), Pfizer (Inst), Amgen (Inst), Abbvie (Inst), Multivir (Inst), Blueprint Medicines (Inst), LOXO (Inst), Vegenics (Inst), Takeda (Inst), Alfasigma (Inst), Agensys (Inst), Idera (Inst), Boston Biomedical (Inst), Inhibrx (Inst), Exelixis (Inst), Amgen (Inst), Turning Point Therapeutics (Inst), Relay Therapeutics (Inst)
Other Relationship: Medscape, Clinical Care Options
Francis Worden
Honoraria: Merck Sharp & Dohme, Eisai, Bristol Myers Squibb, Regeneron, Exelixis, EMD Serono
Consulting or Advisory Role: Merck, Loxo, Bristol Myers Squibb, eisai, Bayer, Regeneron, Exelixis, Sun Pharma Advanced Research Company, Sun Pharma Advanced Research Company, Sun Pharma Advanced Research Company, Sun Pharma Advanced Research Company, Sun Pharma Advanced Research Company, Adlai Nortye, Coherus Biosciences, Coherus Biosciences
Research Funding: Merck (Inst), Eisai (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), CUE Biopharma (Inst), Loxo/Lilly (Inst)
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Bayer, Sun Pharma Advanced Research Company
Ben Solomon
Honoraria: AstraZeneca, Merck Sharp & Dohme (Inst), Roche/Genentech, Pfizer (Inst), Amgen (Inst)
Consulting or Advisory Role: Bristol Myers Squibb (Inst), Merck Sharp & Dohme, AstraZeneca (Inst), Pfizer (Inst), Roche/Genentech (Inst), Amgen, Lilly, BeiGene, Takeda, GlaxoSmithKline (Inst), Janssen (Inst), GlaxoSmithKline
Research Funding: Sanofi (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Bruce Robinson
Leadership: Cochlear, Mayne Pharma
Stock and Other Ownership Interests: Cochlear, Mayne Pharma
Consulting or Advisory Role: Loxo, Eisai
Speakers' Bureau: Eisai
Travel, Accommodations, Expenses: Eisai
Julien Hadoux
Honoraria: ITM Isotope Technologies Munich (Inst), AAA/Endocyte/Novartis (Inst), HRA Pharma (Inst)
Consulting or Advisory Role: Eisai Europe, Lilly, Roche, PharmaMar, HRA Pharma, Bayer (Inst)
Research Funding: Novartis (Inst), Lilly (Inst)
Travel, Accommodations, Expenses: Roche
Pascale Tomasini
Consulting or Advisory Role: AstraZeneca, Roche, Bristol Myers Squibb Foundation, Takeda, Amgen, Janssen
Travel, Accommodations, Expenses: Bristol Myers Squibb/Pfizer, AstraZeneca, Takeda
Daniela Weiler
Honoraria: Lilly, MSD Oncology, Roche, Roche
Consulting or Advisory Role: Lilly, MSD
Speakers' Bureau: Lilly
Barbara Deschler-Baier
Research Funding: Lilly (Inst)
Expert Testimony: Lilly
Daniel S.W. Tan
Honoraria: Takeda (Inst), Novartis (Inst), Roche (Inst), Pfizer (Inst)
Consulting or Advisory Role: Merck (Inst), AstraZeneca (Inst), Roche (Inst), Pfizer (Inst), Amgen (Inst), DKSH (Inst), Bayer (Inst), Genmab, Zymeworks
Research Funding: Novartis (Inst), GlaxoSmithKline (Inst), AstraZeneca (Inst), ACM Biolabs (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Pfizer, Boehringer Ingelheim, Roche
Patricia Maeda
Employment: Bayer, Lilly
Stock and Other Ownership Interests: Bayer, Lilly
Travel, Accommodations, Expenses: Lilly
Yan Lin
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Ravinder Singh
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Research Funding: Lilly
Travel, Accommodations, Expenses: Lilly
Theresa Bayt
Employment: Lilly
Stock and Other Ownership Interests: Lilly, Lilly
Alexander Drilon
Stock and Other Ownership Interests: Treeline Biosciences, mBrace
Honoraria: Pfizer, Loxo/Bayer/Lilly, IASLC, Helsinn Therapeutics, BeiGene, Remedica, Remedica, TP Therapeutics, Verastem, Ignyta/Genentech/Roche, AstraZeneca, Liberum, Lungevity, NIH, PER, OncLive/MJH Life Sciences, Clinical Care Options/NCCN, Lung Cancer Research Foundation, Associazione Italiana Oncologia Toracica (AIOT), Chugai Pharma, Sirio Libanes Hospital, Answers in CME, Research to Practice, i3 Health, RV Mais
Consulting or Advisory Role: Ignyta, Loxo, AstraZeneca, Pfizer, Blueprint Medicines, Genentech/Roche, BeiGene, Hengrui Therapeutics, Exelixis, Bayer, Tyra Biosciences, Takeda/Millennium, BerGenBio, MORE Health, Lilly, Abbvie, 14ner Oncology/Elevation Oncology, Monopteros Therapeutics, Novartis, EMD Serono/Merck, Repare Therapeutics, Melendi, Archer, Nuvalent, Inc, Janssen, Amgen, Merus, Axis Pharma, Medscape, Liberum, Med Learning, PeerView, EPG Health, Journal of the National Comprehensive Cancer Network, Ology Medical Education, Ology Medical Education, Clinical Care Options, Clinical Care Options, touchIME, Entos, Prelude Therapeutics, Applied Pharmaceutical Science, Treeline Biosciences, Monte Rosa Therapeutics, EcoR1 Capital
Research Funding: Foundation Medicine
Patents, Royalties, Other Intellectual Property: Wolters Kluwer (Royalties for Pocket Oncology), Osimertinib Selpercatinib
Other Relationship: Merck, GlaxoSmithKline, Teva, Taiho Pharmaceutical, Pfizer, PharmaMar, Puma Biotechnology, Pfizer, Merus, Boehringer Ingelheim
Philippe A. Cassier
Consulting or Advisory Role: OSE Immunotherapeutics, Bristol Myers Squibb/Celgene, Boehringer Ingelheim, Brenus Pharma, Scenic Biotech
Research Funding: Novartis (Inst), Roche/Genentech (Inst), Lilly (Inst), Blueprint Medicines (Inst), AstraZeneca (Inst), Abbvie (Inst), Bristol Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Taiho Pharmaceutical (Inst), Toray Industries (Inst), Transgene (Inst), Loxo (Inst), GlaxoSmithKline (Inst), Innate Pharma (Inst), Janssen (Inst), Boehringer Ingelheim (Inst), Daiichi Sankyo/UCB Japan (Inst), Adlai Nortye (Inst), Alligator Bioscience (Inst), Amgen (Inst), C4 Therapeutics (Inst), Debiopharm Group (Inst), Exelixis (Inst), Incyte (Inst), ITeos Therapeutics (Inst), OSE Immunotherapeutics (Inst), Molecular Partners (Inst), Pierre Fabre (Inst), Relay Therapeutics (Inst), Sotio (Inst), Tango Therapeutics (Inst)
Travel, Accommodations, Expenses: Roche, OSE Immunotherapeutics, Novartis
Uncompensated Relationships: ReACT Therapeutics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Duke ES, Bradford D, Marcovitz M, et al. : FDA approval summary: Selpercatinib for the treatment of advanced RET fusion-positive solid tumors. Clin Cancer Res 29:3573-3578, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Medicines Agency : Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 7-10 December 2020. https://www.ema.europa.eu/en/news/meeting-highlights-committee-medicinal-products-human-use-chmp-7-10-december-2020
- 3.Pharmaceuticals and Medical Devices Agency : New drugs approved in FY 2021. https://www.pmda.go.jp/files/000250462.pdf
- 4.Wirth LJ, Sherman E, Robinson B, et al. : Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med 383:825-835, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drilon A, Subbiah V, Gautschi O, et al. : Selpercatinib in patients with RET fusion-positive non-small-cell lung cancer: Updated safety and efficacy from the registrational LIBRETTO-001 phase I/II trial. J Clin Oncol 41:385-394, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brookmeyer R, Crowley J: A confidence interval for the median survival time. Biometrics 38:29-41, 1982 [Google Scholar]
- 7.Fleeman N, Houten R, Chaplin M, et al. : A systematic review of lenvatinib and sorafenib for treating progressive, locally advanced or metastatic, differentiated thyroid cancer after treatment with radioactive iodine. BMC Cancer 19:1209, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brose MS, Bible KC, Chow LQM, et al. : Management of treatment-related toxicities in advanced medullary thyroid cancer. Cancer Treat Rev 66:6473, 2018 [DOI] [PubMed] [Google Scholar]
- 9.Gild ML, Bullock M, Tsang V, et al. : Challenges and strategies to combat resistance mechanisms in thyroid cancer therapeutics. Thyroid 33:682-690, 2023 [DOI] [PubMed] [Google Scholar]
- 10.Haddad RI, Bischoff L, Ball D, et al. : Thyroid carcinoma, version 2.2022, NCCN clinical practice guidelines in Oncology. J Natl Compr Canc Netw 20:925-951, 2022 [DOI] [PubMed] [Google Scholar]
- 11.Hadoux J, Elisei R, Brose MS, et al. : Phase 3 trial of selpercatinib in advanced RET-mutant medullary thyroid cancer. N Engl J Med 389:1851-1861, 2023 [DOI] [PubMed] [Google Scholar]
- 12.Belli C, Penault-Llorca F, Ladanyi M, et al. : ESMO recommendations on the standard methods to detect RET fusions and mutations in daily practice and clinical research. Ann Oncol 32:337-350, 2021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Eli Lilly and Company provides access to all individual data collected during the trial, after anonymization, with the exception of pharmacokinetic, genomic, or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org. A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.23.02503.


