Abstract
Climate change has already triggered species distribution shifts in many parts of the world. Increasing impacts are expected for the future, yet few studies have aimed for a general understanding of the regional basis for species vulnerability. We projected late 21st century distributions for 1,350 European plants species under seven climate change scenarios. Application of the International Union for Conservation of Nature and Natural Resources Red List criteria to our projections shows that many European plant species could become severely threatened. More than half of the species we studied could be vulnerable or threatened by 2080. Expected species loss and turnover per pixel proved to be highly variable across scenarios (27-42% and 45-63% respectively, averaged over Europe) and across regions (2.5-86% and 17-86%, averaged over scenarios). Modeled species loss and turnover were found to depend strongly on the degree of change in just two climate variables describing temperature and moisture conditions. Despite the coarse scale of the analysis, species from mountains could be seen to be disproportionably sensitive to climate change (≈60% species loss). The boreal region was projected to lose few species, although gaining many others from immigration. The greatest changes are expected in the transition between the Mediterranean and Euro-Siberian regions. We found that risks of extinction for European plants may be large, even in moderate scenarios of climate change and despite inter-model variability.
Keywords: Intergovernmental Panel on Climate Change storylines, species extinction, species turnover, niche-based model
Recent rapid climate change is already affecting a wide variety of organisms (1, 2). Long-term data indicate that the anomalous climate of the past half-century is already affecting the physiology, distribution, and phenology of some species in ways that are consistent with theoretical predictions (3). Although natural climate variation and nonclimatic factors such as land transformation may well be responsible for some of these trends, human-induced climate and atmospheric change are the most parsimonious explanation for many (3, 4).
Several studies have modeled future species distributions at regional (5-8) and local scales (9, 10) and have extrapolated alarming extinction risks for the next century (11). However, few studies have considered the consequences of multiple climate-change scenarios (7, 8), which represent the outcome of different assumptions about the future (12). Using four representative scenarios and three different climate models (HadCM3, CGCM2, and CSIRO2), and a range of niche-based modeling techniques implemented in biomod (13), we develop predictions of the potential consequences for 1,350 plant species in Europe. The “future climate” we contrast with today's climate (averaged from 1961 to 1990) is the projected mean for the period from 2051 to 2080.
The “bioclimatic envelope” describes the conditions under which populations of a species persist in the presence of other biota as well as climatic constraints (6, 14). Future distributions are projected on the assumption that current envelopes reflect species' environmental preferences, which will be retained under climate change. This principle has strong support from studies demonstrating the evolutionary conservatism of ecological niches and the phylogenetic inertia of species across time scales (15, 16) and comparative biogeographical studies (17, 18). However, this approach also assumes instantaneous species-range change, it ignores physiological CO2 responses, and it does not capture details of population dynamics or biotic interactions nor the lags in spatial range shifts associated with processes of dispersal, establishment, and local extinction. To assess the sensitivity of projections to the most critical of these assumptions, we considered two contrasting assumptions about migration ability (7, 8, 11): either species are unable to disperse at all on the time scale considered (no migration), or they have no constraints to dispersal and establishment (universal migration). The reality for most species is likely to fall between these extremes, depending on their ability to migrate across fragmented landscapes (19). We calculated losses of climatically suitable areas (“species loss”) assuming no migration and gains (“species gain”) and turnover (“species turnover”) assuming universal migration.
Methods
Data Sources. Species' distribution data are available for 2,294 plants (20), comprising ≈20% of the total European flora, sampled between 1972 and 1996. Modeling was conducted by using available data for Europe on a 50 × 50 km grid. The mapped area comprises western, northern and southern Europe, but excludes most of the eastern European countries where recording effort was both less uniform and less intensive (21). After removing species with <20 records, we considered range responses of 1,350 plant species of Europe. We assume this sample can be taken as representative of the responses of European plant species to climate change because it includes most of the life forms and phytogeographic patterns found among plant species in Europe.
Climate data were obtained from the Climatic Research Unit (www.cru.uea.ac.uk) and included mean annual, winter, and summer precipitation, mean annual temperature and minimum temperature of the coldest month (MTC), growing degree days (>5°) and an index of moisture availability (22). These variables were chosen because of their strong link with the physiology and growth of plant species (23, 24). For instance, MTC discriminates species based on their ability to assimilate soil water and nutrients, and continue cell division, differentiation and tissue growth at low temperatures (lower limit), and chilling requirements for processes such as bud break and seed germination (upper limit). The moisture index discriminates species through processes related to phenology, rooting strategy, leaf morphology, and xylem vulnerability to cavitation. However, because there is surprisingly little experimental work for any particular species to guide the choice of bioclimatically limiting variables, the variables are generic and represent a hypothetical minimum basic set for niche-based modeling. Climate data were averaged for the 1961-1990 period. The data were supplied on a 10-foot (1 ft = 0.3 m) grid covering Europe. They were aggregated by averaging to 50 × 50 km Universal Transverse Mercator (UTM) to match the species data grid. Niche-based models were calibrated on the 50 × 50 km UTM grid, and modeled species distributions were projected back onto the 10′ grid for current and future climate.
Future projections were derived by using climate model outputs made available through the Intergovernmental Panel on Climate Change (IPCC) Data Distribution Centre (ipcc-ddc.cru.uea.ac.uk).‡‡ The modeled climate anomalies were scaled based on four scenarios proposed by the IPCC (12). The A1 scenario describes a globalized world with rapid economic growth and global population that peaks in mid-century and declines thereafter and assumes rapid introduction of new and more efficient technologies. Concentrations of CO2 increase from 380 ppm in 2000 to 800 ppm in 2080, and temperature rises by 3.6 K (12). The A2 scenario describes a heterogeneous world with regionally oriented economic development. Per capita economic growth and technological change are slower than in the other scenarios. Global concentrations of CO2 increase from 380 ppm in 2000 to 700 ppm in 2080, and temperature rises by 2.8 K. The B1 scenario describes a convergent world with global population that peaks in mid-century and declines thereafter, as in A1, but with a rapid change toward a service and information economy and the introduction of clean and resource-efficient technology. Concentrations of CO2 increase from 380 ppm in 2000 to 520 ppm in 2080, and temperature rises by 1.8 K. The B2 scenario describes a world in which the emphasis is on local solutions to socioeconomic and environmental sustainability. It is a world with continuously increasing global population (at a rate lower than A2), intermediate levels of economic development, and less rapid and more diverse technological change than in the B1 and A1 scenarios. Concentrations of CO2 increase from 380 ppm in 2000 to 550 ppm in 2080, and temperature rises by 2.1 K (12).
We did not assess the impacts of land-use change, even though this factor will potentially compound the effects of climate change on species distributions (25). However, given the spatial extent and resolution of our data and the magnitude of climate change in most projections, the effect of land use would be most likely overridden by climate (26, 27).
Niche-Based Models of Species Climatic Envelops. We used the biomod framework, which capitalizes on several widely used niche-based modeling techniques (generalized linear models, generalized additive models, classification tree analysis, and artificial neural networks) to provide alternative spatial projections (13). For each climate change scenario, models relating species distributions to the seven bioclimatic variables were fitted by using biomod and projected into the future. Then, a consensus principal component analysis was run to explore central tendencies in projections and select the niche-based model representing the greatest commonality among projections (8).
There is increasing evidence that model projections can be extremely variable, and there remains a need to test the accuracy of models and to reduce uncertainties (8, 28, 29). One recent analysis has however provided the first test of the predictive accuracy of such models by using bird observed species' range shifts and climate change in two periods of the recent past (30). This work provides validation of niche-based models under climate change and demonstrated how uncertainty can be reduced by selecting the most consensual projections, as done in this study. We are therefore confident that this strategy provides a robust and defensible approach to species range projections for the purposes of conservation planning and biodiversity management.
To evaluate species extinction risks, we summed the number of pixels lost, potentially gained (under universal migration), or stable by each species for the different climate-change scenarios. We assigned each species to an International Union for Conservation of Nature and Natural Resources (IUCN) threat category (IUCN 2001). Those that were not listed were classified as lower risk, depending on the projected reduction in range size from present to 2080. Present and future range sizes (area of occupancy) were estimated from the number of pixels where species occurred. Loss in range size was calculated by subtracting future potential range size from present potential range size. In line with IUCN Red List criterion A3(c), the following thresholds were then used to assign a species to a threat category (IUCN 2001). Extinct is a species with a projected range loss of 100% in 50 or 80 years, critically endangered has a projected range loss of >80%, endangered has a projected range loss of >50%, and vulnerable has a projected range loss of >30%. Although this Red Listing approach is simplistic and considers only the effects of climate change, it provides a synthetic overview of species-specific threats due to climate change.
To evaluate the percentage of extinctions for a given area, we summed the number of species lost (L) by pixel and related it to current species richness by pixel. The procedure was the same to assess the percentage of species gained (G) by pixel (under assumptions that species could reach a new suitable climate space). Percentage of species turnover by pixel, under the assumption of universal migration, is then given by T = 100 × (L + G)/(SR + G) where SR is the current species richness.
Results and Discussion
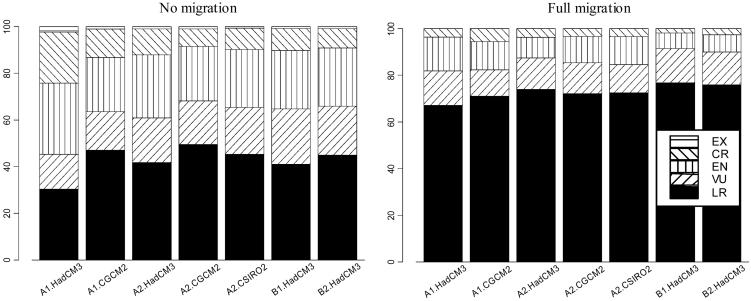
Many European species could be threatened by future climate change (Fig. 1). Under the assumption of no migration, more than half of the species we considered become vulnerable or committed to extinction by 2080. The impacts of climate change are, naturally, less under the universal migration because of the possibility for species to move across landscapes. Under the no-migration assumption and the most severe climate change scenario (A1-HadCM3), 22% of the species become critically endangered (>80% range loss), and 2% extinct by 2080. These numbers decrease for the other scenarios and climate models. Under the universal migration assumption, the results are, as expected, less severe. Under tA1-HadCM3, 67% of species would be classified as low risk, whereas under B1-HadCM3, 76% of the species would be at low risk.
Fig. 1.
Proportion of species classified according to the IUCN Red List assessment under two extremes assumptions about species migration. EX, extinct; CR, critically endangered; EN, endangered; VU, vulnerable; LR, lower risk.
Our results coincide with the direction of predictions made by Thomas et al. (11), although the magnitude of the risks we project is less [and note that we project distributions to 2080, whereas Thomas et al. (11) only projected to 2050].
Niche-based modeling does not address the proximate causes of species extinction. Nevertheless, any reduction in the potential geographic range of a species is likely to lead to an increased risk of local extinction (11). This conclusion is, in fact, the rationale for building IUCN Red Lists (31). A decrease in range size implies that smaller stochastic events affect a larger proportion of the species' total population, especially in fragmented landscapes. If a species becomes restricted to a few sites, then local catastrophic events (such as droughts or disease outbreaks) or an increase of land transformation by humans could easily cause the extinction of that species (32).
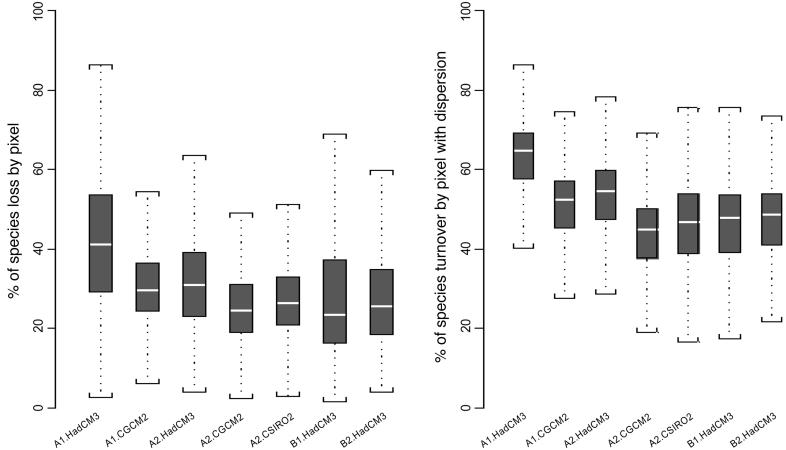
Rates of species' loss and turnover show great variation across scenarios (Fig. 2). In A1-HadCM3, the mean European temperature increases by up to 4.4 K, leading to a mean species loss of 42% and turnover of 63%. This scenario provides the widest range of variability across Europe for both species loss (2.5-86%) and turnover (22-90%). The percentage of species loss could exceed 80% in some areas, such as northcentral Spain and the Cevennes and Massif Central in France. B1-HadCM3 gives the lowest expected mean percentage of species loss (27%), reflecting the fact that this scenario has the lowest rate of increase in CO2 and temperature by 2080 (mean European temperature increase of 2.7 K). Other scenarios show intermediate mean rates of species loss (≈30%) and turnover (≈50%).
Fig. 2.
Estimated percentage of species loss and turnover. Upper extreme, upper quartile, median, lower quartile, and lower extreme are represented for each box.
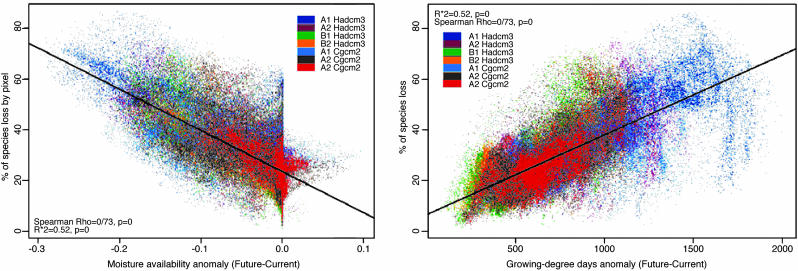
The relationship between the modeled percentage of species loss and the anomalies for the two most significantly correlated bioclimatic variables, growing-degree days (representing accumulated warmth) and a moisture availability index, was used to uncover the potential causes of variations in predicted changes in plant diversity across regions within and across scenarios (Fig. 3). The strong consistent linear relationship across scenarios indicates that projected species loss from our models could be estimated from these two predictors. The Spearman rank-correlation values for the separate univariate relationships were 0.73 and 0.65, respectively. Multiple-linear regression by using these two predictors explains 60% of the variance across scenarios. The temperature of the coldest month, although being an important predictor of distributions for many species (6), did not show a strong relationship with species loss overall and was therefore not used in this analysis.
Fig. 3.
Relationships between the percentage of species loss and anomalies of moisture availability and growing-degree days. The colors correspond to different climate change scenarios.
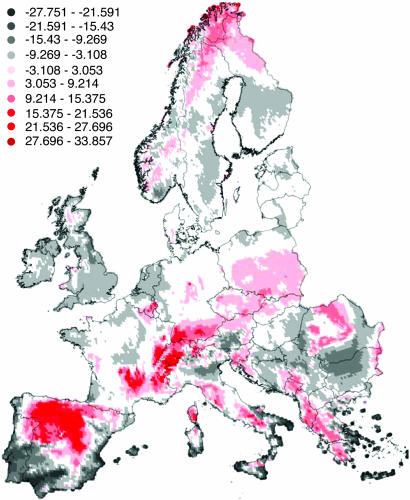
Regional deviations from the inferred relationship (positive and negative residuals) can be interpreted as indications of particularly high or low species vulnerability, because of ecological and historical characteristics of the flora, and/or specific environmental conditions (Fig. 4). An excess of species loss (red color) is shown for mountain regions (mid-altitude Alps, mid-altitude Pyrenees, central Spain, French Cevennes, Balkans, Carpathians). Severe climatic conditions have occurred in mountains over evolutionary times, promoting highly specialized species with strong adaptation to the limited opportunities for growth and survival (33). The narrow habitat tolerances of the mountain flora, in conjunction with marginal habitats for many species, are likely to promote higher rates of species loss for a similar climate anomaly than in any other part of Europe (34). By contrast, the southern Mediterranean and part of the Pannonian regions have a negative residual for species loss (gray color). Both regions are characterized by hot and dry summers and are occupied by species that tolerate strong heat and drought. Under the scenarios used here, these species are likely to continue to be well adapted to future conditions.
Fig. 4.
Regional projections of the residuals from the multiple regression of species loss against growing-degree days and moisture availability. Red colors indicate an excess of species loss; gray colors indicate a deficit.
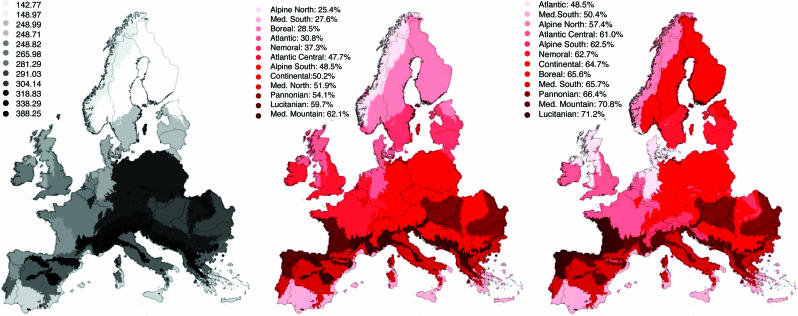
We finally present mean percentages of species loss and turnover by environmental zones (M. Metzger, unpublished data) with the A1-HadCM3 scenario of maximum change to best illustrate the spatial patterns (Fig. 5). The major spatial patterns are similar over all scenarios. The northern Mediterranean (52%), Lusitanian (60%) and Mediterranean mountain (62%) regions are the most sensitive regions; the Boreal (29%), northern Alpine (25%), and Atlantic (31%) regions are consistently less sensitive. Species turnover shows a somewhat different pattern. The Boreal region could, in principle, gain many species from further south, leading to a high species turnover (66%). The Pannonian region could also theoretically gain eastern Mediterranean species and has a calculated turnover of 66%. Thus, these regions stand to lose a substantial part of their plant species diversity, and (in time) to show a major change in floristic composition. Projected species turnover peaks at the transition between the Mediterranean and continental regions (Fig. 5) with extirpation of Euro-Siberian species and expansion for Mediterranean or Atlantic species. Southern Fennoscandia is also an area of high potential turnover with the loss of boreal species and gain of Euro-Siberian species.
Fig. 5.
Spatial sensitivity of plant diversity in Europe ranked by biogeographic regions. Mean percentage of current species richness (Left) and species loss (Center) and turnover (Right) by environmental zones under the A1-HadCM3 scenario.
These results cannot be taken as precise forecasts given the uncertainties in climate change scenarios, the coarse spatial resolution of the analysis (35), and uncertainties in the modeling techniques used (8, 29). The relatively coarse grid scale of our study may hide potential refuges for species and environmental heterogeneity that could enhance species survival, especially in mountain areas where our estimation of risks of extinctions could be overestimated. On the other hand, landscape fragmentation could increase the vulnerability of these refuges to fire or other disturbances, which in combination with the lack of propagule flow, could compromise the survival of remnant populations. There are also major uncertainties due to lags associated with biotic processes. The recognized time scales for assigning species IUCN Red List categories are not suited to evaluating the consequences of slow-acting but persistent threats. We have substituted a time scale of 80 years (instead of 20) for critically endangered, endangered and vulnerable, respectively, over which to assess declines. The extent of species losses may be overestimated, because the plasticity of species and the survival of species in favorable microhabitats is not considered. However, even if the numbers are overestimated, patterns across regions may stand (e.g., the ranking of region in terms of vulnerability to loss). Species loss does not necessarily imply the immediate loss of a species from a site, rather it may imply a potential lack of reproductive success and recruitment that will tend to extinction on a longer time scale (36). Migration rates are likely to be species-specific, and resulting biotic interactions in “no-analogue” assemblages may alter species' realized niches.
Land use and associated habitat fragmentation are likely to generally inhibit migration rates (19). Further, future species distributions will likely be influenced by other environmental factors than changing climate. The current atmospheric CO2 concentration exceeds any experienced during the past 20 million years (12). Plant physiological responses, including growth responses to increased atmospheric CO2 and changes in wateruse efficiency, are expected to ameliorate the response of some plant functional types to climate change (37). On the other hand, nitrogen deposition, the enhanced potential for invasion by exotic species, or the promotion of more competitive native species may change competitive interactions in plant communities, yielding novel patterns of dominance and ecosystem function (38).
Despite uncertainties, our findings provide illustration of the potential importance and the likely direction of climate change effects. From a conservation perspective, a proportion of European plant species could become vulnerable. The strong positive relationship between projected species loss and changes in bioclimatic variables implies that action to reduce greenhouse gas emissions would also mitigate climate-change effects on plant diversity. However, even under the least severe scenario considered, the risks to biodiversity appear to be considerable. Different regions are expected to respond differently to climate change, with the greatest vulnerability in mountain regions and the least in the southern Mediterranean and Pannonian regions. Recent observations (39) and predictions (9) corroborate our conclusion regarding the climatic sensitivity of species in European mountain areas. We have also identified a broad transition zone where the greatest species mixing is likely to occur. During the Quaternary period, this region acted both as a crossing point and a refuge zone for Boreo-alpine and Euro-Siberian species (40). This transition zone will be a strategic region for plant-species conservation in a changing climate.
Acknowledgments
M.B.A. thanks T. Lathi and R. Lampinen for providing a digital version of Atlas Florae Europaeae. We thank M. Erhard (Potsdam-Institute for Climate Impact Research, Potsdam, Germany) for repackaging and aggregating original climate data into the Atlas Florae Europaeae grid and M. Metzger for his environmental classification of Europe. This research was funded by the integrated projects of the European Commission's FP5 Advanced Terrestrial Ecosystem Analysis and Modeling (EVK2-CT-2000-00075) and FP6 Assessing Large-Scale Environmental Risks with Tested Methods (GOCE-CT-2003-506675).
Author contributions: W.T. and S.L. designed research; W.T. performed research; W.T., S.L., M.B.A., M.T.S., and I.C.P. contributed new reagents/analytic tools; W.T. and M.B.A. analyzed data; and W.T., S.L., M.B.A., M.T.S., and I.C.P. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: IUCN, International Union for Conservation of Nature and Natural Resources.
Footnotes
Mitchell, T. D., Carter, T. R., Jones, P. D., Hulme, M. & New, M. (2004) A Comprehensive Set of High-Resolution Grids of Monthly Climate for Europe and the Globe: The Observed Record (1901-2000) and 16 Scenarios (2001-2100) (Tyndall Centre for Climate Change Res., Norwich, U.K.), Working Paper 55.
References
- 1.Edwards, M. & Richardson, A. J. (2004) Nature 430, 881-884. [DOI] [PubMed] [Google Scholar]
- 2.Parmesan, C. (1996) Nature 382, 765-766. [Google Scholar]
- 3.Hughes, L. (2000) Trends Ecol. Evol. 15, 56-61. [DOI] [PubMed] [Google Scholar]
- 4.Parmesan, C. & Yohe, G. (2003) Nature 421, 37-42. [DOI] [PubMed] [Google Scholar]
- 5.Erasmus, B. F. N., Van Jaarsweld, A. S., Chown, S. L., Kshatriya, M. & Wessels, K. J. (2002) Glob. Change Biol. 8, 679-693. [Google Scholar]
- 6.Huntley, B., Berry, P. M., Cramer, W. & McDonald, A. P. (1995) J. Biogeogr. 22, 967-1001. [Google Scholar]
- 7.Peterson, A. T., Ortega-Huerta, M. A., Bartley, J., Sanchez-Cordero, V., Soberon, J., Buddemeier, R. H. & Stockwell, D. R. B. (2002) Nature 416, 626-629. [DOI] [PubMed] [Google Scholar]
- 8.Thuiller, W. (2004) Glob. Change Biol. 10, 2020-2027. [Google Scholar]
- 9.Gottfried, M., Pauli, H., Reiter, K. & Grabherr, G. (1995) Divers. Distrib. 5, 241-251. [Google Scholar]
- 10.Guisan, A. & Theurillat, J.-P. (2000) Integr. Assess. 1, 307-320. [Google Scholar]
- 11.Thomas, C. D., Cameron, A., Green, R. E., Bakkenes, M., Beaumont, L. J., Collingham, Y. C., Erasmus, B. F. N., Ferreira de Siquiera, M., Grainger, A., Hannah, L., et al. (2004) Nature 427, 145-148. [DOI] [PubMed] [Google Scholar]
- 12.Intergovernmental Panel on Climate Change (2001) Climate Change 2001: Impacts, Adaptation, and Vulnerability: Contribution of Working Group II to the Third Asssessment Report of the IPCC (Cambridge Univ. Press, Cambridge, U.K.).
- 13.Thuiller, W. (2003) Glob. Change Biol. 9, 1353-1362. [Google Scholar]
- 14.Sykes, M. T., Prentice, I. C. & Cramer, W. (1996) J. Biogeogr. 23, 203-233. [Google Scholar]
- 15.Martinez-Meyer, E., Peterson, A. T. & Hargrove, E. C. (2004) Glob. Ecol. Biogeogr. 13, 305-314. [Google Scholar]
- 16.Peterson, A. T., Soberon, J. & Sanchez-Cordero, V. (1999) Science 285, 1265-1267. [DOI] [PubMed] [Google Scholar]
- 17.Beerling, D. J., Huntley, B. & Bailey, J. P. (1995) J. Veg. Sci. 6, 269-282. [Google Scholar]
- 18.Huntley, B., Bartlein, P. J. & Prentice, I. C. (1989) J. Biogeogr. 16, 551-560. [Google Scholar]
- 19.Higgins, S. I., Lavorel, S. & Revilla, E. (2003) Oikos 101, 354-366. [Google Scholar]
- 20.Jalas, J. & Suominen, J. (1972-1996) Altas Florae Europaeae (Comm. Mapping Flora Europe Societas Biologica Fennica, Vanamo, Helsinki).
- 21.Williams, P. H., Humphries, C., Araújo, M. B., Lampinen, R., Hagemeijer, W., Gasc, J.-P. & Mitchell-Jones, T. (2000) Belg. J. Entomol. 2, 21-46. [Google Scholar]
- 22.Gerten, D., Schaphoff, S., Haberlandt, U., Lucht, W. & Sitch, S. (2004) J. Hydrol. (Amersterdam) 286, 249-270. [Google Scholar]
- 23.Bartlein, P. J., Prentice, I. C. & Webb, T. (1986) J. Biogeogr. 13, 35-57. [Google Scholar]
- 24.Prentice, I. C., Cramer, W., Harrison, S. P., Leemans, R., Monserud, R. A. & Solomon, A. M. (1992) J. Biogeogr. 19, 117-134. [Google Scholar]
- 25.Travis, J. M. J. (2003) Proc. R. Soc. London Ser. B 270, 467-473. [Google Scholar]
- 26.Pearson, R. G., Dawson, T. P. & Liu, C. (2004) Ecography 27, 285-298. [Google Scholar]
- 27.Thuiller, W., Araújo, M. B. & Lavorel, S. (2004) J. Biogeogr. 31, 353-361. [Google Scholar]
- 28.Araújo, M. B., Pearson, R. G., Thuiller, W. & Erhard, M. (2005) Glob. Change Biol., in press.
- 29.Thuiller, W., Araújo, M. B., Pearson, R. G., Whittaker, R. J., Brotons, L. & Lavorel, S. (2004) Nature 430, 34. [DOI] [PubMed] [Google Scholar]
- 30.Araújo, M. B., Whittaker, R. J., Ladle, L. & Erhard, M. (2005) Glob. Ecol. Biogeogr., in press.
- 31.International Union for Conservation of Nature and Natural Resources (2001) IUCN Red List Categories and Criteria (Int. Union Conserv. Nat. Nat. Resour. Species Survival Commission, Cambridge, U.K.) Version 3.1.
- 32.Lawton, J. H. & May, R. M. (1995) Extinction Rates (Oxford Univ. Press, Oxford).
- 33.Körner, C. (1999) Alpine Plant Life (Springer, Berlin).
- 34.Thuiller, W., Lavorel, S. & Araújo, M. B. (2005) Glob. Ecol. Biogeogr., in press.
- 35.Weaver, A. J. & Zwiers, F. W. (2000) Nature 407, 571-572. [DOI] [PubMed] [Google Scholar]
- 36.Chuine, I., Cambon, G. & Comtois, P. (2000) Glob. Change Biol. 6, 1-11. [Google Scholar]
- 37.Sitch, S., Smith, B., Prentice, I. C., Arneth, A., Bondeau, A., Cramer, W., Kaplan, J. O., Levis, S., Lucht, W., Sykes, M. T., et al. (2003) Glob. Change Biol. 9, 161-185. [Google Scholar]
- 38.Craine, J. M., Reich, P. B., Tilman, G. D., Ellsworth, D., Fargione, J., Knops, J. & Naeem, S. (2003) Ecol. Letters 6, 623-630. [Google Scholar]
- 39.Walther, G.-R., Post, E., Convey, P., Menzel, A., Parmesan, C., Beebee, T. J. C., Fromentin, J.-M., Hoegh-Gudberg, O. & Bairlein, F. (2002) Nature 416, 389-395. [DOI] [PubMed] [Google Scholar]
- 40.Taberlet, P. & Cheddadi, R. (2002) Science 297, 2009-2010. [DOI] [PubMed] [Google Scholar]