Abstract
After intravenous and oral administration of clarithromycin at a dose of 20 mg/kg of body weight to rats with diabetes mellitus induced by alloxan (DMIA) and diabetes mellitus induced by streptozotocin (DMIS), the area under the curve values were significantly smaller than those of respective control rats. The in vitro intrinsic clearance values for the disappearance of clarithromycin were significantly faster in both rats with DMIA and rats with DMIS than in control rats. The above data suggested that metabolism of clarithromycin increased in both types of diabetic rat due to an increase in the expression and mRNA level of CYP3A1(23) in the rats.
Animal models of insulin-dependent diabetes mellitus induced by administration of several chemicals, principally alloxan, streptozotocin, and zinc chelators, have been reported (22, 30). There are major differences in the diabetic effects of streptozotocin and alloxan (reference 30 and references therein). It is known that structural alterations in pancreatic beta cells (total degranulation) occur within 48 h after administration of streptozotocin and last up to 4 months. Alloxan causes a decrease in hepatic glycogen within 24 to 72 h, an effect that is partially reversible by insulin. Alloxan generally produces greater cytotoxicity owing to its conversion to anionic radicals.
For rats with diabetes mellitus induced by streptozotocin (DMIS), a decreased bile flow rate and altered bile compositions (3), hepatotoxicity (31), and impaired kidney function (18, 21) have been reported. Glucuronidation and sulfation were also profoundly affected by DMIS in rats (23). In rats with diabetes mellitus induced by alloxan (DMIA), kidney function was also impaired (12, 20).
A previous study (13) has shown that the expression of hepatic microsomal cytochrome P450 (CYP) 1A2, 2B1/2, 2E1, and 3A23 increased 2.8, 1.8, 3.0, and 1.5 times, respectively, in rats with DMIA compared to controls, whereas the expression of CYP2C11 decreased to 23% of the control value. Similarly, the expression of CYP1A2, 2B1/2, 2E1, and 3A23 also increased 3.1, 3.3, 2.8, and 1.9 times, respectively, in rats with DMIS compared to controls, whereas the expression of CYP2C11 decreased to 37% of the control value. The mRNA levels of CYP1A2, 2B1, 2B2, 2E1, and 3A23 increased 3.4, 1.9, 1.6, 4.3, and 1.6 times, respectively, in rats with DMIA compared to controls, whereas CYP2C11 decreased to 31% of the control value. Similarly, the mRNA levels of CYP1A2, 2B1, 2B2, 2E1, and 3A23 also increased 4.2, 3.9, 1.9, 3.6, and 2.2 times, respectively, in rats with DMIS compared to controls, whereas CYP2C11 decreased to 28% of the control value. Similar changes in some of the above-mentioned CYP isozymes have also been reported for rats with DMIA and/or DMIS (8, 15, 24-27, 32).
It has been reported that CYP3A1(23) is involved in the metabolism of clarithromycin in rats (14). Hence, it would be expected that pharmacokinetics of clarithromycin could be changed in rats with DMIA and DMIS due to an increase in the expression and mRNA level of CYP3A1(23) levels in the rats. The purpose of this study is to report the pharmacokinetic changes after intravenous and oral administration of clarithromycin at a dose of 20 mg/kg of body weight to rats with DMIA and DMIS in relation to CYP3A1(23) changes.
Chemicals (1, 14), the methods of induction of diabetes mellitus by alloxan (12, 20) or streptozotocin (21), pretreatment and surgical procedures for intravenous and oral administration (1, 14), procedures for intravenous (n = 12 and 11 for rats with DMIA and their control rats, respectively, and n = 8 and 7 for rats with DMIS and their control rats, respectively) and oral (n = 9 and 11 for rats with DMIA and their control rats, respectively, and n = 10 and 9 for rats with DMIS and their control rats, respectively) studies (1, 14), and high-performance liquid chromatography analysis of clarithromycin (1, 14) were similar to previously reported methods. The procedures for the measurement of the maximum rate of metabolism (Vmax), Km, and the intrinsic clearance (CLint) for the disappearance of clarithromycin in hepatic microsomal fractions were similar to previously reported methods (1). The livers of rats with DMIA and DMIS and their respective controls (n = 5 per group) were homogenized. Incubation was conducted with the above microsomal fraction (equivalent to 2 mg protein), a 10-μl aliquot of clarithromycin (to have substrate concentrations of 2, 5, 10, 20, 50, and 100 μM), and 50 μl (1 mM) of NADPH in a final volume of 500 μl (achieved by adding 0.1 M phosphate buffer, pH 7.4) in a water bath shaker kept at 37°C at a rate of 500 oscillations per min. All of the above microsomal incubation conditions were linear. The protocol of the animal study was approved by the Animal Care and Use Committee of the College of Pharmacy, Seoul National University.
Standard methods (10) were used to calculate the following pharmacokinetic parameters: the total area under the plasma concentration-time curve from time zero to infinity (AUC0-∞) (4); time-averaged total body clearance (CL), renal clearance (CLR), and nonrenal clearance (CLNR); total area under the first moment of the plasma concentration-time curve (AUMC0-∞); mean residence time (MRT); and the apparent volume of distribution at steady state (Vss) (11). The maximum plasma concentration (Cmax) and the time to reach a Cmax (Tmax) were directly read from the experimental data. The extent of absolute oral bioavailability (F) was estimated by dividing the AUC after oral administration by the AUC after intravenous administration. The harmonic mean method was used to calculate the mean values of Vss (5), terminal half-life (9), and each clearance (6).
The parametric unpaired t test or nonparametric Mann-Whitney rank sum test was performed after the tests for normality (Kolmogorov-Smirnov) and equal variance (Levene median) for each parameter using the SigmaStat program (Systat Software Inc., Richmond, CA). A P value of less than 0.05 was considered to be statistically significant. All results are expressed as means ± standard deviations.
In both rats with DMIA and rats with DMIS, the Vmax for the disappearance of clarithromycin was significantly faster (135 and 96.2% increases, respectively) than that in respective controls (Table 1), suggesting that the maximum velocity for the disappearance of clarithromycin was significantly faster in both kinds of diabetic rats. However, the Michaelis-Menten constant (Km) values were not significantly different for the two types of diabetic rats and their respective controls (Table 1), suggesting that the affinity of clarithromycin to the enzyme(s) is not changed in the diabetic rats. As a result, the clarithromycin CLints were significantly faster in rats with DMIA and DMIS (56.1 and 55.4% increases, respectively) (Table 1), suggesting that the disappearance of clarithromycin (mainly due to metabolism) increased in the diabetic rats.
TABLE 1.
Vmax, Km, and CLint values for the disappearance of clarithromycin in hepatic microsomes of rats with DMIA and DMIS and their respective control ratsa
| Parameter | Value for:
|
|||
|---|---|---|---|---|
| DMIA controls (n = 5) | Rats with DMIA (n = 5) | DMIS controls (n = 5) | Rats with DMIS (n = 5) | |
| Vmax (pmol/min/mg protein) | 121 ± 27.4 | 284 ± 141b | 185 ± 31.7 | 363 ± 150b |
| Km (μM) | 25.6 ± 6.30 | 36.6 ± 10.2 | 41.7 ± 7.03 | 56.2 ± 30.9 |
| CLint (μl/min/mg protein) | 4.83 ± 0.944 | 7.54 ± 1.82b | 4.46 ± 0.300 | 6.93 ± 1.11c |
Values shown are means ± standard deviations.
P < 0.05 compared with the control.
P < 0.01 compared with the control.
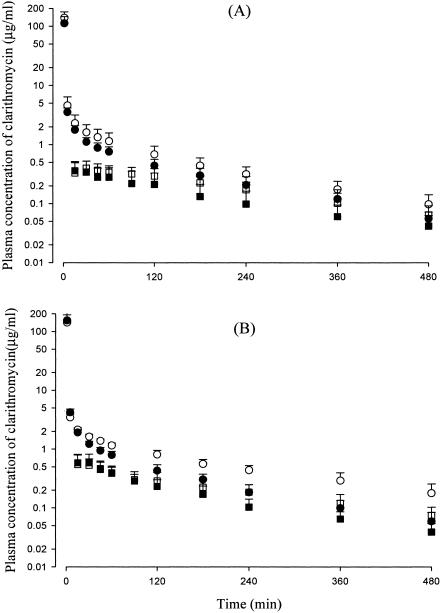
After intravenous administration of clarithromycin at a dose of 20 mg/kg to rats with DMIA and DMIS and their respective controls, the mean arterial plasma concentrations of clarithromycin declined in a polyexponential fashion for each group of rats, with lower levels in rats with DMIA (Fig. 1A) and DMIS (Fig. 1B) than in control rats. This resulted in significantly smaller AUC0-∞ values in the diabetic rats (26.1 and 24.5% decreases, respectively) than those in controls (Table 2). The smaller AUC values in rats with DMIA and DMIS could be due to significantly faster CLs (35.2 and 32.6% increases, respectively) in the rats (Table 2). The faster CL in the diabetic rats could be due to significantly faster CLNRs (43.8 and 34.0% increases, respectively), since CLRs were comparable for the diabetic rats and their respective controls (Table 2). Other pharmacokinetic parameters of clarithromycin, including the total amount of unchanged clarithromycin excreted in the urine up to 24 h (Ae0-24 h), listed in Table 2 were not significantly different for the diabetic rats and their controls, except that rats with DMIS have a significantly smaller terminal half-life (23.8% decrease), MRT (59.0% decrease), and Vss (35.8% decrease). Although the exact reason is not clear, the smaller Vss in rats with DMIS could not be due to a significant decrease in the free (unbound in plasma proteins) fraction of clarithromycin in the rats; the free fractions were 35.2 and 29.4% for rats with DMIS and their controls, respectively, at a clarithromycin concentration of 5 μg/ml using an equilibrium dialysis technique (2). Note that body weight gain decreased significantly in rats with DMIA (from 262 to 251 g) and DMIS (from 275 to 263 g) compared to controls (from 260 to 300 g and from 268 to 324 g, respectively) (Table 2).
FIG. 1.
Mean arterial plasma concentration-time profiles of clarithromycin after 1 min of intravenous infusion (circles) and oral administration (squares) at a dose of 20 mg/kg to rats with DMIA (filled shapes; n = 12 and 9 for intravenous and oral administration, respectively) and their control rats (open shapes; n = 11 for both intravenous and oral administration) (A) and rats with DMIS (filled shapes; n = 8 and 10 for intravenous and oral administration, respectively) and their control rats (open shapes; n = 7 and 9 for intravenous and oral administration, respectively) (B). Bars represent standard deviations.
TABLE 2.
Pharmacokinetic parameters of clarithromycin after a 1-min intravenous infusion at a dose of 20 mg/kg to rats with DMIA and DMIS and their respective control ratsa
| Parameter | Value for:
|
|||
|---|---|---|---|---|
| DMIA control rats (n = 11) | Rats with DMIA (n = 12) | DMIS control rats (n = 7) | Rats with DMIS (n = 8) | |
| Body weight (g) | ||||
| Initialb | 260 ± 7.07 | 262 ± 8.65 | 268 ± 8.59 | 275 ± 3.78 |
| Finalc | 300 ± 9.61 | 251 ± 21.2d | 324 ± 17.3 | 263 ± 18.1d |
| AUC0-∞ (μg · min/ml) | 502 ± 120 | 371 ± 66.2e | 576 ± 89.5 | 435 ± 87.4e |
| Terminal half-life (min) | 137 ± 39.7 | 138 ± 31.8 | 172 ± 79.1 | 131 ± 20.7f |
| MRT (min) | 93.7 ± 25.5 | 83.1 ± 26.6 | 163 ± 96.6 | 66.9 ± 18.2e |
| Vss (ml/kg) | 3,580 ± 1,260 | 4,390 ± 1,640 | 4,500 ± 2,350 | 2,890 ± 2,700f |
| CL (ml/min/kg) | 39.8 ± 9.23 | 53.8 ± 9.72e | 34.7 ± 4.82 | 46.0 ± 8.79f |
| CLR (ml/min/kg) | 6.78 ± 2.53 | 8.00 ± 3.79 | 7.23 ± 3.02 | 10.2 ± 1.65 |
| CLNR (ml/min/kg) | 31.5 ± 7.09 | 45.3 ± 7.17d | 26.5 ± 3.13 | 35.5 ± 7.66f |
| Ae0-24 h (% of dose) | 18.4 ± 4.38 | 16.0 ± 4.30 | 23.3 ± 7.68 | 22.4 ± 3.39 |
| GI24 h (% of dose) | 0.289 ± 0.147 | 0.166 ± 0.0825 | 0.496 ± 0.534 | 0.280 ± 0.107 |
Values shown are means ± standard deviations.
Measured just before injection of alloxan, streptozotocin, or respective vehicles.
Measured just before starting the experiment.
P < 0.001 compared with the control.
P < 0.01 compared with the control.
P < 0.05 compared with the control.
After oral administration of clarithromycin at a dose of 20 mg/kg in rats with DMIA and DMIS and their respective controls, absorption of clarithromycin was rapid and almost complete; clarithromycin was detected in plasma from the first blood sampling time (16 min), and the plasma concentration reached its peak at 21.7 to 53.2 min for all groups of rats (Fig. 1). The total amounts of unchanged clarithromycin recovered from the entire gastrointestinal tract at 24 h (GI24 h) were 0.363 to 1.36% of the oral dose for all groups of rats (Table 3). However, F was considerably low (19.7 to 22.5% for all groups of rats), indicating that the first-pass effect of clarithromycin was considerable (Table 3). After reaching a Cmax, the plasma concentrations of clarithromycin declined in a monoexponential fashion for each group of rats, with considerably lower levels in rats with DMIA (Fig. 1A) and DMIS (Fig. 1B) than controls. This resulted in significantly smaller AUC0-∞ values in rats with DMIA and DMIS (33.7 and 29.0% decreases, respectively) than those in controls (Table 3). Note that body weight gain also decreased significantly in rats with DMIA (from 252 to 234 g) and DMIS (from 257 to 236 g) compared to controls (from 248 to 271 g and from 248 to 286 g, respectively) (Table 3).
TABLE 3.
Pharmacokinetic-parameters of clarithromycin after oral administration at a dose of 20 mg/kg to rats with DMIA and DMIS and their respective control ratsa
| Parameter | Value for:
|
|||
|---|---|---|---|---|
| DMIA control rats (n = 11) | Rats with DMIA (n = 9) | DMIS control rats (n = 9) | Rats with DMIS (n = 10) | |
| Body weight (g) | ||||
| Initialb | 248 ± 7.17 | 252 ± 6.12 | 248 ± 5.65 | 257 ± 10.6 |
| Finalc | 271 ± 7.69 | 234 ± 8.94d | 286 ± 20.4 | 236 ± 20.4d |
| AUC0-∞ (μg · min/ml) | 113 ± 33.8 | 74.9 ± 34.7e | 121 ± 44.1 | 85.9 ± 24.4e |
| Terminal half-life (min) | 190 ± 104 | 214 ± 88.4 | 173 ± 44.0 | 112 ± 43.4e |
| Cmax (μg/ml) | 0.462 ± 0.113 | 0.414 ± 0.171 | 0.607 ± 0.205 | 0.655 ± 0.232 |
| CLR (ml/min/kg) | 14.3 ± 5.21 | 15.4 ± 6.10 | 16.0 ± 10.3 | 24.2 ± 11.9 |
| Tmax (min) | 53.2 ± 52.1 | 36.7 ± 26.1 | 21.7 ± 10.9 | 27.0 ± 15.5 |
| Ae0-24 h (% of dose) | 8.58 ± 2.54 | 6.46 ± 4.05 | 10.4 ± 3.45 | 11.5 ± 3.74 |
| GI24 h (% of dose) | 0.363 ± 0.437 | 1.23 ± 1.07 | 0.752 ± 1.02 | 1.36 ± 1.25 |
| F (%) | 22.5 | 20.2 | 21.0 | 19.7 |
Values shown are means ± standard deviations.
Measured just before injection of alloxan, streptozotocin, or respective vehicles.
Measured just before starting the experiment.
P < 0.001 compared with the control.
P < 0.05 compared with the control.
In both rats with DMIA and rats with DMIS, the CLs of clarithromycin were significantly faster than those in respective controls (Table 2). Similar results have also been reported. For example, in rats with DMIS, the CLs of atenolol enantiomers (17), digoxin (31), aminopyrine (33), rose bengal (29), cefoperazone and cephradine (19), phenol red, procainamide ethobromide, ouabain, taurocholic acid (28), theophylline (13), and cefazolin (18) were significantly faster than in control rats. In rats with DMIA, the CL of theophylline was significantly faster (34.8% increase) and the AUC0-∞ values of cefotaxime (16) and chlorzoxazone (15) were significantly smaller than in control rats. The CL changes of drugs in rats with DMIS or DMIA mentioned above were mainly explained by impaired renal function and changes in biliary or renal excretion. However, CL changes of drugs in rats with DMIS or DMIA due to CYP isozyme changes have not been reported, except for chlorzoxazone (due to an increase in CYP2E1, since hydroxychlorzoxazone was mainly formed via CYP2E1 in rats) (16) and theophylline (13). In rats with DMIA or DMIS, the AUC0-∞ values of theophylline and one of its metabolites, 1,3-dimethyluric acid, were significantly smaller and greater, respectively, since 1,3-dimethyluric acid was formed via CYP1A2 and 2E1 in rats (13).
It has been reported that CYP3A1(23) is involved in the metabolism of clarithromycin in rats (14), and the expression and mRNA level of CYP3A1(23) increased in rats with DMIA and DMIS (13). An increased CYP3A level has also been reported for diabetic rats (24, 27). Hence, it would be expected that the pharmacokinetics of clarithromycin could be changed due to CYP3A1(23) induction. This could be supported by the significantly smaller AUC0-∞ values of clarithromycin after both intravenous (Table 2) and oral (Table 3) administration of clarithromycin to rats with DMIA and DMIS. Moreover, the CLints based on in vitro hepatic microsomal study were significantly faster in rats with DMIA and DMIS than those in controls (Table 1). It has been shown that different substrates of CYP3A4, a homologous human form of CYP3A1(23), differently modulate the metabolic kinetics of CYP3A4 by changing the P450 conformation. The heterotropic effects for CYP3A4 were observed with alpha-naphthoflavone compounds. In the present study, we found that the Km values for control rats and diabetic rats were comparable to each other, indicating that enzyme activation was not responsible for the pharmacokinetic alterations of clarithromycin. Our observation that the mRNA and protein levels of CYP3A1(23) were both increased in diabetic rats rather strongly supports the conclusion that the induction of CYP3A1(23) by diabetes contributed to the metabolic changes in clarithromycin.
CYP2C11 is known to be male specific, whereas CYP2C12 is female specific. Among the cytochrome P450 isozymes induced by diabetes, only CYP2E1 is female dominant. Since CYP3A1(23) is the enzyme responsible for clarithromycin metabolism, we do not expect that the pharmacokinetic alterations of clarithromycin are sex specific.
Although the absorption of clarithromycin was rapid and almost complete, the F values were 19.7 to 22.5% for all groups of rats (Table 2), indicating that the first-pass effect of clarithromycin could be considerable. Marked first-pass metabolism of clarithromycin after oral administration has also been reported for humans (7).
In conclusion, after intravenous and oral administration of clarithromycin at a dose of 20 mg/kg to rats with DMIA and DMIS, the AUC0-∞ values were significantly smaller than controls, and this could be due to induction of CYP3A1(23) in the rats. The changes in the pharmacokinetics of clarithromycin (especially the AUC0-∞ and CLs) were not different for rats with DMIA and rats with DMIS.
Acknowledgments
This study was supported in part by Korea Research Foundation (2001-042-F00115).
REFERENCES
- 1.Ahn, C. Y., E. J. Kim, J. W. Kwon, S. J. Chung, S. G. Kim, C.-K. Shim, and M. G. Lee. 2003. Effects of cysteine on the pharmacokinetics of intravenous clarithromycin in rats with protein-calorie malnutrition. Life Sci. 73:1783-1794. [DOI] [PubMed] [Google Scholar]
- 2.Bae, S. K., W.-S. Chung, E. J. Kim, J. K. Rhee, J. W. Kwon, W. B. Kim, and M. G. Lee. 2004. Pharmacokinetics, blood partition, and protein binding of DA-7867, a new oxazolidinone. Biopharm. Drug Dispos. 25:127-135. [DOI] [PubMed] [Google Scholar]
- 3.Carnovale, C. E., R. A. Marinelli, and E. A. Rodriguez Garay. 1986. Bile flow decrease and altered bile composition in streptozotocin-treated rats. Biochem. Pharmacol. 35:2625-2628. [DOI] [PubMed] [Google Scholar]
- 4.Chiou, W. L. 1978. Critical evaluation of potential error in pharmacokinetic studies using the linear trapezoidal rule method for the calculation of the area under the plasma level-time curve. J. Pharmacokinet. Biopharm. 6:539-546. [DOI] [PubMed] [Google Scholar]
- 5.Chiou, W. L. 1979. New calculation method of mean apparent drug volume of distribution and application to rational dosage regimen. J. Pharm. Sci. 68:1067-1069. [DOI] [PubMed] [Google Scholar]
- 6.Chiou, W. L. 1980. New calculation method of mean total body clearance of drugs and its application to dosage regimens. J. Pharm. Sci. 69:90-91. [DOI] [PubMed] [Google Scholar]
- 7.Chu, S. Y., R. Deaton, and J. Cavanaugh. 1992. Absolute bioavailability of clarithromycin after oral administration in humans. Antimicrob. Agents Chemother. 36:1147-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong, Z. G., J. Y. Hong, O. A. Ma, D. C. Li, J. Bullock, F. J. Gonzalez, S. S. Park, H. V. Gelboin, and C. S. Yang. 1988. Mechanism of induction of cytochrome P-450ac (P-450j) in chemically induced and spontaneously diabetic rats. Arch. Biochem. Biophys. 263:29-35. [DOI] [PubMed] [Google Scholar]
- 9.Eatman, F. B., W. A. Colburn, H. G. Boxenbaum, H. N. Posmanter, R. E. Weinfeld, R. Ronfeld, L. Weissman, J. D. Moore, M. Gibaldi, and S. A. Kaplan. 1977. Pharmacokinetics of diazepam following multiple dose oral administration to healthy human subjects. J. Pharmacokinet. Biopharm. 5:481-494. [DOI] [PubMed] [Google Scholar]
- 10.Gibaldi, M., and D. Perrier. 1982. Pharmacokinetics, 2nd ed. Marcel Dekker, Inc., New York, N.Y.
- 11.Kim, S. H., Y. M. Choi, and M. G. Lee. 1993. Pharmacokinetics and pharmacodynamics of furosemide in protein-calorie malnutrition. J. Pharmacokinet. Biopharm. 21:1-17. [DOI] [PubMed] [Google Scholar]
- 12.Kim, S. H., W. B. Kim, and M. G. Lee. 1998. Pharmacokinetics of a new carbapenem, DA-1131, after intravenous administration to rats with alloxan-induced diabetes mellitus. Biopharm. Drug Dispos. 19:303-308. [DOI] [PubMed] [Google Scholar]
- 13.Kim, Y. C. 2005. Pharmacokinetic changes of drugs in rats with diabetes mellitus with respect to CYP isozymes changes. Ph.D. thesis. Seoul National University, Seoul, Korea.
- 14.Lee, A. K., J. H. Lee, J. W. Kwon, W. B. Kim, S. G. Kim, S. H. Kim, and M. G. Lee. 2004. Pharmacokinetics of clarithromycin in rats with acute renal failure induced by uranyl nitrate. Biopharm. Drug Dispos. 25:273-282. [DOI] [PubMed] [Google Scholar]
- 15.Li, L., and Y. Zhang. 1998. Changes of CYP2E1 activity in diabetic rat model. Yaoxue Xuebao 33:891-895. (In Chinese.) [PubMed] [Google Scholar]
- 16.Li, Q. Z., and C. L. Zhang. 1995. The pharmacokinetics and pharmacodynamics of cefotaxime in experimental diabetic rats. Yaoxue Xuebao 30:495-499. (In Chinese.) [PubMed] [Google Scholar]
- 17.Mehvar, R. 1991. Effect of experimental diabetes mellitus on the pharmacokinetics of atenolol enantiomers in rats. J. Pharm. Sci. 80:207-211. [DOI] [PubMed] [Google Scholar]
- 18.Nadai, M., H. Yoshizumi, T. Kuzuya, T. Hasegawa, I. Johno, and S. Kitazawa. 1990. Effects of diabetes on disposition and renal handling of cefazolin in rats. J. Pharmacol. Exp. Ther. 18:565-570. [PubMed] [Google Scholar]
- 19.Nakashima, E., R. Matsushita, M. Takeda, T. Nakanishi, and F. Ichimura. 1992. Comparative pharmacokinetics of cefoperazone and cephradine in untreated streptozotocin diabetic rats. Drug Metab. Dispos. 20:730-735. [PubMed] [Google Scholar]
- 20.Park, J. H., W. I. Lee, W. H. Yoon, Y. D. Park, J. S. Lee, and M. G. Lee. 1998. Pharmacokinetic and pharmacodynamic changes of furosemide after intravenous and oral administration to rats with alloxan-induced diabetes mellitus. Biopharm. Drug Dispos. 19:357-364. [DOI] [PubMed] [Google Scholar]
- 21.Park, J. M., C. H. Moon, and M. G. Lee. 1996. Pharmacokinetic changes of methotrexate after intravenous administration to streptozotocin-induced diabetes mellitus rats. Res. Commun. Mol. Pathol. Pharmacol. 93:343-352. [PubMed] [Google Scholar]
- 22.Pickup, J. C., and G. Williams. 1991. Textbook of diabetes, vol. 1. Blackwell Scientific Publications, Oxford, United Kingdom.
- 23.Price, V. F., and D. J. Jollow. 1986. Strain differences in susceptibility of normal and diabetic rats to acetaminophen hepatotoxicity. Biochem. Pharmacol. 35:687-695. [DOI] [PubMed] [Google Scholar]
- 24.Raza, H., I. Ahmed, M. S. Lakhani, A. K. Sharma, D. Pallot, and W. Montague. 1996. Effect of bitter melon (Momordica charantia) fruit juice on the hepatic cytochrome P450-dependent monooxygenases and glutathione S-transferases in streptozotocin-induced diabetic rats. Biochem. Pharmacol. 52:1639-1642. [DOI] [PubMed] [Google Scholar]
- 25.Song, B. J., T. Matsunaga, J. P. Hardwick, S. S. Park, R. L. Veech, C. S. Yang, H. V. Gelboin, and F. J. Gonzalez. 1987. Stabilization of cytochrome P450j messenger ribonucleic acid in the diabetic rat. Mol. Endocrinol. 1:542-547. [DOI] [PubMed] [Google Scholar]
- 26.Thummel, K. E., and J. B. Schenkman. 1990. Effects of testosterone and growth hormone treatment on hepatic microsomal P450 expression in the diabetic rat. Mol. Pharmacol. 37:119-129. [PubMed] [Google Scholar]
- 27.Verrecchia, A., and A. Guaitani. 1993. Insulin-mimetic effects of vanadate in preventing the increase of P450IIIA and P450IA subfamily proteins in streptozotocin-diabetic rats. Acta Diabetol. 30:128-131. [DOI] [PubMed] [Google Scholar]
- 28.Watkins, J. B., III, and T. P. Dykstra. 1987. Alterations in biliary excretory function by streptozotocin-induced diabetes. Drug Metab. Dispos. 15:177-183. [PubMed] [Google Scholar]
- 29.Watkins, J. B., III, and H. Noda. 1986. Biliary excretion of organic anion in diabetic rats. J. Pharmacol. Exp. Ther. 239:467-473. [PubMed] [Google Scholar]
- 30.Watkins, J. B., III, and R. A. Sanders. 1995. Diabetes mellitus-induced alterations of hepatobiliary function. Pharmacol. Rev. 47:1-23. [PubMed] [Google Scholar]
- 31.Watkins, J. B., III, and S. E. Sherman. 1992. Long-term diabetes alters the hepatobiliary clearance of acetaminophen, bilirubin and digoxin. J. Pharmacol. Exp. Ther. 260:1337-1343. [PubMed] [Google Scholar]
- 32.Yamazoe, Y., N. Murayama, M. Shimada, K. Yamauchi, and R. Kato. 1989. Cytochrome P450 in livers of diabetic rats: regulation by growth hormone and insulin. Arch. Biochem. Biophys. 268:567-575. [DOI] [PubMed] [Google Scholar]
- 33.Zysset, T., and C. Tlach. 1987. Altered liver function in diabetes: model experiments with aminopyrine in the rat. J. Pharmacol. Exp. Ther. 240:271-276. [PubMed] [Google Scholar]